Abstract
Neonatal abstinence syndrome (NAS) which is observed in 55–94% of the newborns from opioids-taking mothers produces deleterious neurological symptoms. Various pharmacological therapies have been investigated in neonates with NAS. This article reviews all studies on NAS treatment to analyze the duration of treatment, length of hospitalization and possible drug adverse effects. The search was limited to the randomized clinical trials which examined the treatments of neonates with NAS. Scientific databases including PubMed, Cochrane Library, ISI Web of Science, Embase and Scopus were systematically searched. Retrieved articles were reviewed by two researchers and evaluated using the JADAD scoring system. Finally, the treatment duration, hospitalization length and drug side-effects were extracted. Methadone, buprenorphine and clonidine were found more effective than morphine. Diluted tincture of opium (DTO) in combination with phenobarbital or clonidine was significantly more effective than DTO alone. Clonidine was a significantly better adjunctive therapy than phenobarbital in reducing morphine treatment days. No significant difference was observed between morphine and DTO effectiveness. Deciding the optimal regimen to manage symptomatic NAS, as a single or an adjunct therapy is not possible based on the literature, due to the low quality, small size and short-term treatment considered in the published studies.
Graphical abstract.
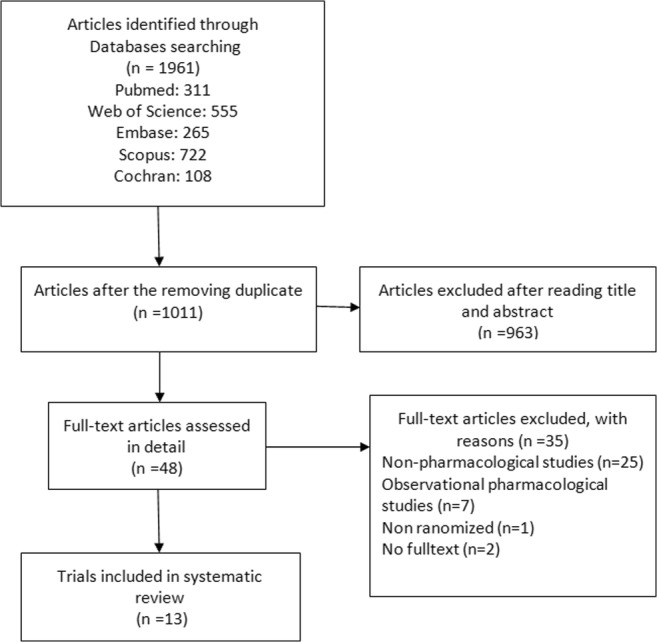
Process of selecting trials included in the present systematic review
Keywords: Neonatal abstinence syndrome, Neonatal withdrawal syndrome, Neonatal passive addiction, Opioid, Opium, Methadone, Morphine, Clonidine, Withdrawal
Introduction
The prevalence of nonmedical use of opioids is increasing. In the US, it is estimated that nearly 1% of the pregnant women actually use opioids during pregnancy [1]. Neonatal abstinence syndrome (NAS) is defined as “sudden discontinuation of fetal exposure to chemicals/drugs used/abused by the mother during pregnancy”. This syndrome is observed in 55–94% of infants born from opioid-dependent mothers [2]. Infants with NAS may present deleterious and even life-threatening features including neurological hyperexcitability (insomnia, irritability, hypertonia, hyperreflexia, tremors, and seizures), gastrointestinal symptoms (vomiting, diarrhea, and feeding disturbances) and sympathetic/parasympathetic deregulation (sweating, fever, tachypnea, and congestion) [2]. The time of NAS symptom onset is variable ranging from hours to 8 days post-delivery [3]. NAS is also associated with costly hospitalizations [4].
The majority of symptomatic newborns require pharmacological support for safe opioid weaning [3]. Nevertheless, no single drug has been evidenced as the most appropriate therapy to treat NAS. Although the American Academy of Pediatrics (AAP) has indicated opioids as first-line treatment of NAS, their use remains controversial in the neonate [1]. Alternative therapies include sedative drugs and/or centrally acting α2-agonists such as clonidine. The present article reviews all major studies published in the medical literature on NAS treatment aiming to analyze the duration of treatment, the length of hospitalization and the possible drug adverse effects.
Methods
Search strategy
The search was limited to the randomized clinical trials which examined the treatments of neonates with NAS. Scientific databases including PubMed, Cochrane Library, ISI Web of Science, Embase and Scopus were systematically searched for articles published until 29 August 2018, using combinations of the following keywords: (“Neonatal Abstinence Syndrome” OR “Neonatal Withdrawal Syndrome” OR “Neonatal Passive Addiction” OR “Opioid” OR “Opiates” OR “Heroin” OR “Hydromorphone” OR “oxycodone” OR “Opium” OR “Methadone” OR “Morphine” OR “Tramadol”) AND (“Management” OR “Therapy” OR “Therapeutics” OR “Treatment” OR “Detoxification). The reference lists of the retrieved publications were thoroughly checked to complete the search.
Inclusion/exclusion criteria
Randomized Clinical trials that investigated the drug effects on NAS were included in this systematic review. There was no limitation for the control group. Duplicate and non-English articles were excluded.
Data extraction
The articles were reviewed by two researchers. First, related articles were identified based on the title and abstract. Then, full-text articles were downloaded and evaluated independently by two researchers. The references cited in the retrieved articles as well as the review articles published in this field were reviewed to find relevant studies.
Outcome measures
Outcome measures were the “type of treatment”, “duration of treatment”, “length of hospitalization” and “drug side-effects”.
Quality assessment
To evaluate the quality of the trials, the Jadad scale which examines three important items [5] namely, randomization (description and adjustment), blinding (description and adjustment), report of withdrawals and the cause of loss to follow up, was used. Total score of Jadad ranges from 3 to 5 points. In the present work, two reviewers independently assessed the articles and calculated Jadad score. Any disagreements were resolved via consensus or by consulting with a third party. Intention-to-treat analysis was also reviewed and reported.
Data analysis
Due to the heterogeneity of the studies, it was not possible to perform meta-analysis and retrieved data were reported using a qualitative approach.
Results
We identified 1961 publications in our preliminary search. After excluding duplicate studies, 1011 articles remained. Of them, 963 articles were excluded based on the title and abstract and finally 48 full texts were reviewed. Here, 13 original articles were included in the final review. The PRISMA flow chart of this systematic review is presented in Fig. 1. The quality assessment of these articles is presented in Table 1. Their characteristics including the author’s name, time of study, design (with details), type of opioid exposure in utero, the intervention and control groups, assessment tool of NAS severity and the study primary outcome of are shown in Table 2.
Fig. 1.
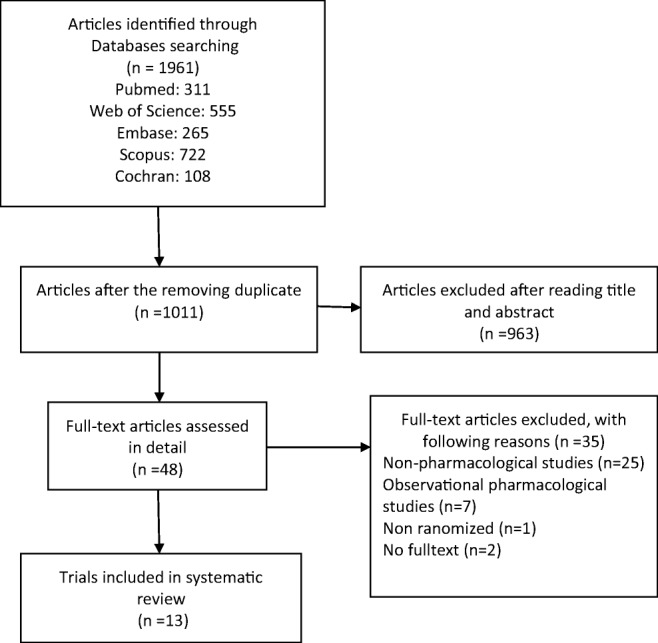
Process of selecting trials included in the present systematic review
Table 1.
The quality assessment of included studies in this systematic review
| First author, year | Registration site/ code | Randomization | Blinding | Report of loss to follow up | Intention-to-treat analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mention randomization | appropriate Method | Inappropriate Method | Mention blinding | appropriate Method | Inappropriate Method | ||||
| Davis, 2018 [6] | + | + | – | + | + | – | + | + | |
| Kraft, 2017 [7] | + | – | – | + | + | – | + | + | |
| Nayeri, 2015 [8] |
IRCT.ir IRCT201406239568N8 |
+ | + | – | – | – | – | + | ? |
| Bada, 2015 [1] | + | + | – | + | + | – | + | ? | |
| Brown, 2014 [9] | – | + | + | – | + | + | – | + | + |
| Surran, 2013 [10] | – | + | + | – | – | – | – | + | ? |
| Kraft, 2011 [11] | + | + | – | – | – | – | + | + | |
| Agthe, 2009 [2] | + | + | – | + | + | – | + | + | |
| Kraft, 2008 [12] | + | + | – | – | – | – | + | ? | |
| Langenfeld,2005 [13] | – | + | + | – | + | + | – | + | ? |
| Coyle, 2005 [14] | – | + | – | – | + | + | – | + | ? |
| Jackson,2004 [15] | – | + | + | – | + | + | – | + | ? |
| Coyle, 2002 [] | – | + | – | – | + | + | – | + | ? |
+: Present; −: Absent;?: Intention-to-treat not reported
Table 2.
Systematic review of the randomized studies regarding the neonatal abstinence syndrome
| Author, year | Study design | No. of patients (Int. /Cont.) | Type of Opioid exposure in uterus | Intervention | Control | Drop out (%) (Int. /Cont.) | Assessment tool | Primary outcome | Results |
|---|---|---|---|---|---|---|---|---|---|
| Davis, 2018 [6] | Multi centric, randomized, double-blind, clinical trial | 59/59 | Buprenorphine/Methadone/opioids for pain decreasing in pregnancy | methadone | Morphine | 1.7/0 | Finnegan | LOS | methadone was associated with decreased mean number of days for LOS by 14% (P = .046) |
| Kraft, 2017 [7] | single-site, randomized, double-blind, clinical trial | 33/30 | Methadone/ buprenorphine | sublingual buprenorphine | oral morphine | 9/6.7 |
MOTHER NAS scale |
Median length of treatment | shorter duration in Int. group vs. Cont. group [15 d vs. 28 d(p < 0.001)] |
| Nayeri, 2015 [8] | Multi centric randomized, open-label, clinical trial | 30/30 | Opium/heroin/methadone/cocaine/methamphetamine | Phenobarbital | Oral Morphine | 0/0 | Finnegan | Mean length of treatment | Non Sig. difference for the duration of treatment between Int. group (8.5 ± 4 d) vs. Cont. (8.5 ± 5 d) (P = 0.9) |
| Bada, 2015 [1] | randomized, double-blind clinical trial | 15/16 | Methadone, Buprenorphine, Oxycodone, hydrocodone Benzodiazepines | morphine | clonidine | 0/0 | Finnegan | Median length of Treatment | Longer duration of treatment in Int. group (39 d) Vs. Cont. (28 d) (p = 0.02) |
| Brown, 2014 [9] | single-site, randomized, double-blind clinical trial | 15/16 | Methadone/ buprenorphine | methadone | morphine | 0/0 | modified Finnegan | Median length of opioid treatment | shorter duration in Int. group vs. Cont. group [14 d vs. 21 d (p = 0.008)] |
| Surran, 2013 [10] | single site, open-label, randomized clinical trial | 34/34 | Methadone/ Buprenorphine/ Oxycodone | Clonidine+ morphine | Phenobarbital +morphine | 6/0 | Modified Finnegan | Median length of morphine treatment | Longer duration of treatment in clonidine Vs. Phenobarbital group [18.2 d Vs. 13.6 d, p = 0.037)] |
| Kraft, 2011 [11] | single site, open-label, Randomized, clinical trial | 12/12 | Buprenorphine/ Methadone | Sublingual buprenorphine | Morphine | 0/0 | MOTHER NAS score | Mean length of treatment | shorter duration in Int. group vs. Cont. group [23 ± 12 d vs. 38 ± 14 d, (p = 0.01)] |
| Agthe, 2009 [2] | Multi centric, double-blind, randomized, clinical trial | 40/40 |
Methadone/ Heroin/ Cocaine |
oral clonidine/ DTO* | placebo/DTO* | 0/0 | modified Finnegan | median length of treatment | shorter duration in Int. group vs. Cont. group [11 d vs. 15 d (p = 0.02)] |
| Kraft, 2008 [12] | open-label, randomized, clinical trial | 13/13 | opioids | sublingual buprenorphine | Opium solution | 8/0 | modified Finnegan | safety, tolerability, | Length of treatment (22d Vs. 32d), length of stay (27d Vs. 38d) were not significantly different |
| Langenfeld, 2005 [13] | Randomized double-blind clinical trial | 16/17 | Morphine/ Methadone | tincture of opium | Morphine | 0/0 | Finnegan |
1.Length of treatment 2.total dose of tincture of opium needed |
Length of treatment wasn’t different significantly (26.9 d Vs.29.8 d) |
| Coyle, 2005 [14] | partially Randomized, clinical trial | 17/15 | methadone | phenobarbital/ DTO* | placebo/ DTO* | 0/0 | Finnegan |
infant neurobehaviour during the first three weeks of life. LOS |
improved orientation, quality of movement, lower total stress/ abstinence score (p < 0.05) Shorter duration in Int. group Vs. control [39.6 ± 25 d Vs. 69.8 ± 31 d (p < 0.01)] |
| Jackson, 2004 [15] | Double blind, randomized clinical trial | 41/34 | opioids | morphine | phenobarbital | 0/0 | Lipsitz | Median length of treatment | Shorter duration in Int. group Vs. control [8 d vs. 12 d (p = 0.02)] |
| Coyle 2002, [16] | partially randomized, clinical trial | 10/10 | Methadone/ heroin | Phenobarbital/ DTO* | Placebo/ DTO* | 0/0 | Finnegan | duration of hospitalization | Shorter duration in Int. group Vs. control [38 d vs. 79 d (P < 0 .001)] |
LOS length of stay, Int. Intervention, Cont. Control, vs. versus, d day, DTO Diluted tincture of opium
*DTO: 0.4 mg/mL morphine
Methadone vs. morphine
Treatment with methadone was associated with 14% reduction in the mean number of hospitalization days (p = 0.046) and 16% reduction in the length of treatment (p = 0.02) in comparison to morphine [6]. In another study, methadone- compared to morphine-treated newborns had significantly shorter duration of treatment (p = 0.008) [9].
Phenobarbital vs. morphine
No significant differences between oral phenobarbital and morphine were reported in one study regarding the treatment duration (p = 0.9) and hospital length of stay (p = 0.7) [8]. Conversely, shorter treatment duration following oral morphine versus phenobarbital (p = 0.02) was reported in a second study [15]. Interestingly, in both studies, no significant differences in terms of need for adjuvant therapy were observed between the two groups.
Morphine vs. diluted tincture of opium (DTO)
No significant differences in the treatment duration and hospitalization length were reported between the newborns treated with DTO vs. morphine [13].
Buprenorphine vs. morphine
The outcomes of buprenorphine vs. morphine were compared showing significantly shorter length of treatment (p < 0.001) and hospital stay (p < 0.001) in the buprenorphine-treated newborns [7]. Moreover, 15% versus 23% of the newborns in the buprenorphine and morphine groups required supplemental phenobarbital, respectively; nonetheless, this difference was not significant (p = 0.36). In another study comparing the same treatments, the durations of treatment (p = 0.01) and hospital stay l (p = 0.05) were significantly shorter in the buprenorphine vs. the morphine group [11]. Besides, 25% vs. 8.3% of the newborns in the buprenorphine and morphine groups required phenobarbital, respectively. No statistical comparisons between the groups were performed.
Buprenorphine vs. opium solution
Sublingual buprenorphine and DTO treatment did not result in significantly different effects on the length of treatment (p = 0.077) and hospital stay (p = 0.068) [12].
Clonidine vs. morphine
The duration of treatment (p = 0.02) and post-discharge treatment (p = 0.005) significantly decreased in the clonidine- compared to the morphine-treated newborns; nevertheless, the length of hospital stay did not vary significantly between the groups [1].
Clonidine /morphine vs. phenobarbital/morphine
Clonidine was compared with phenobarbital when co-administered as adjuvant therapy along with morphine to reduce NAS treatment days [10]. Significantly longer duration of treatment (p = 0.037) was observed in the clonidine (18.2 days) compared to phenobarbital group (13.6 days).
Clonidine/ DTO vs. placebo/DTO
NAS newborns orally received DTO before being randomized to two groups to receive clonidine vs. placebo as adjuvant therapy. In the clonidine group, the median duration of treatment was reduced by 27% compared to the placebo group (p = 0.02). Importantly, 12.5% of treatment failure was seen in the placebo group while no treatment failure was observed in the clonidine group (p = 0.05) [2].
DTO vs. DTO/phenobarbital
About 48% decrease in the length of hospital stay (p < 0.001) was reported with DTO/phenobarbital as compared to DTO alone to treat NAS [16]. Additionally, DTO/phenobarbital-treated newborns spent less time presenting severe withdrawal (p < 0.04). In another study, treatment with DTO alone significantly improved orientation and resulted in smoother movements in comparison to DTO/phenobarbital treatment (p < 0.05) [14]. The newborns were significantly more interactive. Additionally, length of hospital stay was significantly shorter in the DTO/phenobarbital-treated newborns compared to those who received DTO alone (p < 0.01).
With respect to the serious adverse effects observed following administration of the above-noted treatments, methadone could induce apnea, lethargy and hypothermia [6]; morphine could induce inguinal hernia [7]; buprenorphine caused supraglottoplasty, reflux/poor feeding (probably not related) [11], and generalized seizures [12]; and clonidine/DTO induced supraventricular tachycardia (SVT). Furthermore, three clonidine/DTO-treated infants died within 2 months after being discharged from the hospital. Deaths were reported to be due to myocarditis, sudden infant death syndrome (SIDS), and methadone overdose; nevertheless, according to the FDA, deaths were not likely caused by clonidine [12].
Non-serious adverse reactions of treatments used against NAS reported by the clinical trials discussed in this systematic review, are shown in Table 3.
Table 3.
The side-effects of the drugs reported in the articles of this systematic review
| Drug | Adverse effects | Ref | Drug | Adverse effects | Ref |
|---|---|---|---|---|---|
| Methadone |
Shallow breathing Bradycardia Oxygen desaturation Lethargy Poor feeding Hypothermia Emesis |
[6] | Buprenorphine |
Anemia Skin condition Gastro-intestinal condition Cough Tachycardia Umbilical granuloma |
[7] |
|
CMV infection* Aminoaciduria* Clavicle fracture* Elevated transaminases * |
[11] | ||||
| Morphine |
Shallow breathing Bradycardia Oxygen desaturation Lethargy Poor feeding Hypothermia Emesis |
[6] | |||
| mild fungal paronychia * |
[11] [12] |
||||
| Phenobarbital |
poor feeding mild respiratory depression |
[10] | |||
| Clonidine/DTO | – | ||||
| Opium solution | – | ||||
| DTO | – | ||||
|
Skin condition Gastro-intestinal condition Respiratory infection Urinary tract infection Oral thrush* conjunctivitis* reflux* |
[7] | ||||
*probably unrelated in to intervention; Ref, Reference number; CMV, Cytomegalovirus; DTO, Tincture of opium
Discussion
In 2005, an estimate of 250,000–300,000 females were reported to be intravenous drug abusers in the US and 75–90% of these abusers were of child bearing ages [14]. It was also estimated that almost 1% of the pregnant women take opioids [1]. Interestingly, fetal exposure to methadone during pregnancy poses a 60–80% risk of NAS development [14]. However, despite the increasing number of NAS in neonates, no optimal therapeutic approach has been established.
NAS pathophysiology is complex. The mesolimbic dopaminergic pathway is crucially involved in the development of physical dependency to opioids. In this regard, it was shown that in morphine abstinence syndrome, decrease in serotonin and mesolimbic dopamine lead to continued physical symptoms [17]. Following fetal exposure to opioids, the inhibitory effect of opioids on the noradrenergic neuronal pathways is suddenly abolished at birth, thus augmenting the noradrenergic activities [1].
In the present review article, we found that the efficacy of methadone, buprenorphine and clonidine for NAS treatment was higher than that of morphine. Also, a combination of diluted tincture of opium (DTO) and phenobarbital or clonidine was significantly more effective than DTO alone. Clonidine was a significantly better adjunctive therapy than phenobarbital in reducing morphine treatment days. Nevertheless, no significant difference was observed between morphine and DTO effectiveness.
Opioid use to treat NAS remains controversial, although recommended as first-line agents by the AAP due to their ability to rapidly control withdrawal symptoms [15]. The effectiveness of four different opioids namely buprenorphine, methadone, morphine and DTO as well as clonidine and phenobarbital, used in trials investigating NAS treatment has been discussed in our study.
Buprenorphine is a partial opioid receptor agonist responsible for ceiling effects. High affinity and slow dissociation from opioid-receptors enable buprenorphine to block the effects of other opioids taken after buprenorphine, characteristics that may explain its higher effectiveness in NAS as compared to short-acting opioids [7, 18]. The bioavailability of buprenorphine is around 48 and 31% following intranasal and sublingual administrations, respectively. Its oral bioavailability is very low [19]. Observed half-time ranges from 2.2 h following IV administration to 37 h following sublingual administration [20]. Its partial effects on opioid receptors causes less respiratory depression, sedation, withdrawal syndrome, and arrhythmia compared to methadone [21]. However, buprenorphine side-effects include hyperthermia, tachycardia, blood pressure increment [18], constipation, headache, nausea, urinary retention, and sedation [22]; rarely, it may induce idiosyncratic hepatotoxic reactions [23]. Similarly to buprenorphine, treatment of NAS with methadone reduced the mean number of hospitalization and treatment days in comparison to morphine. Methadone is a fat-soluble opioid rapidly absorbed by oral route. Methadone binds and activates the opioid receptors leading to analgesia, euphoria, constipation, sedation, respiratory depression, nausea, and miosis. Methadone oral bioavailability differs widely among patients with different genetic background; however, its average oral bioavailability is approximately 60–70%, nearly two times higher than that of buprenorphine. The half-life of methadone ranges from 15 to 55 h [24]. Besides constipation, respiratory depression, nausea, miosis, and impaired judgment, heart failure, and cardiac arrhythmias [21] have been reported as rare side-effects of long-term methadone administration.
Morphine is a full opioid receptor agonist. It also binds and inhibits GABA inhibitory interneurons. Morphine presents a variety of effects such as analgesia, anxiolysis, euphoria, sedation, constipation, nausea, vomiting, respiratory depression, and gastrointestinal system smooth muscle contraction [25, 26].
Hyperalgesia [27] and rhabdomyolysis-induced acute renal failure [28] were rarely reported after morphine administration, especially following high-dose opioid therapy. The bioavailability of morphine is approximately 30% and it has a half-life of about 2–4 h [26].
There has been a growing tendency towards the administration of non-opioid drugs due to the concerns of potential deleterious consequences of prolonged opioid exposure in the newborns who are in the early stages of their brain development [1]. Clonidine and phenobarbital have been regularly used as adjunct drugs. Phenobarbital, which causes sedation by activation of gamma-amino butyric acid (GABA) receptors in the central nervous system, has been used to treat NAS due to the frequent combination of benzodiazepine and opioid abuse [22]. Phenobarbital binds GABA-receptors and increases synaptic inhibition resulting in elevation of seizure threshold. Phenobarbital also inhibits calcium channels and reduces the excitatory neurotransmitter release. The sedative and hypnotic effects of phenobarbital are attributed to calcium channel inhibition in the midbrain [29]. The side-effects of phenobarbital include incoordination, impaired balance, and drowsiness. The bioavailability of phenobarbital is approximately 95% [30] and its half-life decreases by increasing age as it has a half-life of 79 h in adults but 110 h in children [31].
Similarly, clonidine was shown to be a satisfactory alternative for morphine. Clonidine is a α2-adrenergic agent which suppresses noradrenergic activity and alleviates opiate withdrawal signs and symptoms. In this context, stimulation of α2-receptors by clonidine inhibits the secretion of noradrenaline leading to reduction in sympathetic tone, blood pressure and heart rate [32]. The bioavailability of clonidine is 75–95% and 60–70% for oral and transdermal administration, respectively [33]. The half-life is around 6–20 h in subjects with normal renal function. However, in those with impaired renal function the elimination half-time increases to 18–41 h. The elimination half-life is also dose-dependent and increases at higher doses [32]. Both hypo- and hypertension, bradycardia, hypothermia, arrhythmia, coma, and death were reported following clonidine abuse [34]. Long-term exposure to opioids increases opioid receptors in the locus coeruleus which includes groups of noradrenergic cells. Opioid exposure inhibits adenylate cyclase activity which reduces cyclic adenosine monophosphate concentration. This reduction augments the potassium efflux in parallel with reductions in calcium influx, both inhibiting brain noradrenergic activities [1]. Clonidine is able to suppress the increase in noradrenergic activity when opioid exposure is terminated [1]. Although the co-administration of clonidine with opioids to NAS newborns decreases the duration of therapy, concerns with postnatal exposure to opioids remain [1].
Drug combination was also highly suggested to improve the results in NAS newborn, due to their multiple drug exposure in utero. The active ingredient of DTO is morphine, with 1 mg DTO containing 0.04 mg morphine [35]. Addition of phenobarbital to DTO resulted in shorter hospital stay and therefore decreased hospital charges per patient, compared to DTO alone [23]. In comparison to the newborns treated with only DTO, those treated with the two agents showed more marked improvements, higher levels of movements and less anxiety during the first three weeks after delivery. Though both groups improved over time, newborns getting the two-drug treatment more rapidly exhibited improvement in their neurobehavioral conditions [14].
NAS newborns frequently require dose increase to reach the control of their symptoms [9]. Therefore, evaluation of drug exposure in pregnancy based on the individual characteristics is mandatory to optimize drug response in NAS. Considering the side-effects of the conventional approaches used to treat NAS, future researches should be oriented on the assessment of new pharmacological therapies.
Our analysis clearly showed that studies investigating the pharmacological management of NAS have been performed on small sample sizes with single-center designs, thus limiting their power and external validity. Almost all included studies had not reported intention-to-treatment analysis. Additionally, some studies did not exclude preterm neonates and/or were based on rather low-quality methods.
In conclusion, it is difficult to acknowledge which therapy is optimal to treat NAS newborns based on the available literature. High-quality studies with large sample sizes are urgently required to improve the management of these neonates and limit further morbidities.
Acknowledgments
The authors thank Vice chancellor of Research, Mashhad University of Medical Sciences, Iran for their support.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Highlights
• Although Neonatal abstinence syndrome (NAS) produces deleterious neurological symptoms with considerable emotional and economic burden to the society, no definite approach has been suggested for its treatment.
• The effectiveness of methadone, buprenorphine and clonidine was found to be superior to that of morphine in the treatment of NAS.
• Diluted tincture of opium (DTO) in combination with either phenobarbital or clonidine was significantly more effective than administered alone.
• There were conflicting findings regarding the comparative effectiveness of phenobarbital versus morphine to treat NAS.
• Clonidine was significantly better than phenobarbital as adjunctive therapy in reducing morphine treatment days in NAS.
• No significant difference was observed between morphine and DTO effectiveness.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Masumeh Ghazanfarpour and Mona Najaf Najafi contributed equally to this work.
References
- 1.Bada HS, Sithisarn T, Gibson J, Garlitz K, Caldwell R, Capilouto G, Li Y, Leggas M, Breheny P. Morphine versus clonidine for neonatal abstinence syndrome. Pediatrics. 2015;135(2):e383–e391. doi: 10.1542/peds.2014-2377. [DOI] [PubMed] [Google Scholar]
- 2.Agthe AG, Kim GR, Mathias KB, Hendrix CW, Chavez-Valdez R, Jansson L, Lewis TR, Yaster M, Gauda EB. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics. 2009;123(5):e849–e856. doi: 10.1542/peds.2008-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz L, Xiao R, Brown ER, Sommers E. Auricular acupressure augmentation of standard medical management of the neonatal narcotic abstinence syndrome. Med Acupunct. 2011;23(3):175–186. doi: 10.1089/acu.2011.0818. [DOI] [Google Scholar]
- 4.Winhusen T, Wilder C, Wexelblatt SL, Theobald J, Hall ES, Lewis D, van Hook J, Marcotte M. Design considerations for point-of-care clinical trials comparing methadone and buprenorphine treatment for opioid dependence in pregnancy and for neonatal abstinence syndrome. Contemp Clin Trials. 2014;39(1):158–165. doi: 10.1016/j.cct.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 6.Davis JM, Shenberger J, Terrin N, Breeze JL, Hudak M, Wachman EM, Marro P, Oliveira EL, Harvey-Wilkes K, Czynski A, Engelhardt B, D’Apolito K, Bogen D, Lester B. Comparison of safety and efficacy of methadone vs morphine for treatment of neonatal abstinence syndrome: a randomized clinical trial. JAMA Pediatr. 2018;172(8):741–748. doi: 10.1001/jamapediatrics.2018.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft WK, Adeniyi-Jones SC, Chervoneva I, Greenspan JS, Abatemarco D, Kaltenbach K, Ehrlich ME. Buprenorphine for the treatment of the neonatal abstinence syndrome. N Engl J Med. 2017;376:2341–2348. doi: 10.1056/NEJMoa1614835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayeri F, Sheikh M, Kalani M, Niknafs P, Shariat M, Dalili H, et al. Phenobarbital versus morphine in the management of neonatal abstinence syndrome, a randomized control trial. BMC Pediatr. 2015;15(1):15. doi: 10.1186/s12887-015-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MS, Hayes MJ, Thornton LM. Methadone versus morphine for treatment of neonatal abstinence syndrome: a prospective randomized clinical trial. J Perinatol. 2014;35(4):278–283. doi: 10.1038/jp.2014.194. [DOI] [PubMed] [Google Scholar]
- 10.Surran B, Visintainer P, Chamberlain S, Kopcza K, Shah B, Singh R. Efficacy of clonidine versus phenobarbital in reducing neonatal morphine sulfate therapy days for neonatal abstinence syndrome. A prospective randomized clinical trial. J Perinatol. 2013;33(12):954–959. doi: 10.1038/jp.2013.95. [DOI] [PubMed] [Google Scholar]
- 11.Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. J Addict. 2011;106(3):574–580. doi: 10.1111/j.1360-0443.2010.03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraft WK, Gibson E, Dysart K, Damle VS, Larusso JL, Greenspan JS, et al. Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial. Pediatrics. 2008;122(3):601–607. doi: 10.1542/peds.2008-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langenfeld S, Birkenfeld L, Herkenrath P, Müller C, Hellmich M, Theisohn M. Therapy of the neonatal abstinence syndrome with tincture of opium or morphine drops. Drug Alcohol Depend. 2005;77(1):31–36. doi: 10.1016/j.drugalcdep.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Coyle MG, Ferguson A, Lagasse L, Liu J, Lester B. Neurobehavioral effects of treatment for opiate withdrawal. Arch Dis Child Fetal Neonatal Ed. 2005;1(90):73–74. doi: 10.1136/adc.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson L, Ting A, McKay S, Galea P, Skeoch C. A randomized controlled trial of morphine versus phenobarbitone for neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):300–304. doi: 10.1136/adc.2003.033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coyle MG, Ferguson A, Lagasse L, Oh W, Lester B. Diluted tincture of opium (DTO) and phenobarbital versus DTO alone for neonatal opiate withdrawal in term infants. J.Pediatr. 2002;140(5):561–564. doi: 10.1067/mpd.2002.123099. [DOI] [PubMed] [Google Scholar]
- 17.Diana M, Muntoni AL, Pistis M, Melis M, Gessa GL. Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J Neurosci. 1999;11(3):1037–1041. doi: 10.1046/j.1460-9568.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 18.Ilbäck N-G, Siller M, Stålhandske T. Effects of buprenorphine on body temperature, locomotor activity and cardiovascular function when assessed by telemetric monitoring in rats. Lab Anim. 2008;42(2):149–160. doi: 10.1258/la.2007.06002e. [DOI] [PubMed] [Google Scholar]
- 19.Welsh C, Valadez-Meltzer A. Buprenorphine: a (relatively) new treatment for opioid dependence. Psychiatry (Edgmont) 2005;2(12):29. [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Biotechnology Information. PubChem Compound Database; CID=644073, https://pubchem.ncbi.nlm.nih.gov/compound/644073. Accessed 1 Nov 2018.
- 21.Behzadi M, Joukar S, Beik A. Opioids and cardiac arrhythmia: a literature review. Med Princ Pract. 2018;27(5):401–414. doi: 10.1159/000492616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volpe DA, Xu Y, Sahajwalla CG, Younis IR, Patel V. Methadone metabolism and drug-drug interactions: in vitro and in vivo literature review. J Pharm Sci. 2018;107(12):2983–2991. doi: 10.1016/j.xphs.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Bogenschutz MP, Abbott PJ, Kushner R, Tonigan JS, Woody GE. Effects of buprenorphine and hepatitis C on liver enzymes in adolescents and young adults. J Addict Med. 2010;4(4):211–216. doi: 10.1097/ADM.0b013e3181c4e27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown R, Kraus C, Fleming M, Reddy S. Methadone: applied pharmacology and use as adjunctive treatment in chronic pain. Postgrad Med J. 2004;80(949):654–659. doi: 10.1136/pgmj.2004.022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong D, Flood P, Diaz G. The side effects of morphine and hydromorphone patient-controlled analgesia. Anesth Analg. 2008;107(4):1384–1389. doi: 10.1213/ane.0b013e3181823efb. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Biotechnology Information. PubChem Compound Database; CID=5288826, https://pubchem.ncbi.nlm.nih.gov/compound/5288826.. Accessed 1 Nov 2018.
- 27.Wilson GR, Reisfield GM. Morphine hyperalgesia: a case report. Am J Hosp Palliat Care. 2003;20(6):459–461. doi: 10.1177/104990910302000608. [DOI] [PubMed] [Google Scholar]
- 28.Shen C, Hung C, Wu C, Huang H, Ho W. Rhabdomyolysis-induced acute renal failure after morphine overdose--a case report. Acta Anaesthesiol Sin. 1999;37(3):159–162. [PubMed] [Google Scholar]
- 29.Lewis CB, Adams N. Phenobarbital. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Jan 2018. Available at https://www.ncbi.nlm.nih.gov/books/NBK532277. Accessed 1 Nov 2018.
- 30.Nelson E, Powell JR, Conrad K, Likes K, Byers J, Baker S, et al. Phenobarbital pharmacokinetics and bioavailability in adults. J Clin Pharmacol. 1982;22(2–3):141–148. doi: 10.1002/j.1552-4604.1982.tb02662.x. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Biotechnology Information. PubChem Compound Database; CID=4763, https://pubchem.ncbi.nlm.nih.gov/compound/4763. Accessed 1 Nov 2018.
- 32.National Center for Biotechnology Information. PubChem Compound Database; CID=2803, https://pubchem.ncbi.nlm.nih.gov/compound/2803. Accessed 1 Nov 2018.
- 33.Lowenthal D, Matzek K, MacGregor T. Clinical pharmacokinetics of clonidine. Clin Pharmacokinet. 1988;14(5):287–310. doi: 10.2165/00003088-198814050-00002. [DOI] [PubMed] [Google Scholar]
- 34.Chiu S, Campbell K. Clonidine for the treatment of psychiatric conditions and symptoms: a review of clinical effectiveness, safety, and guidelines [internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018 Feb 21. Available at https://www.ncbi.nlm.nih.gov/books/NBK531717. Accessed 1 Nov 2018. [PubMed]
- 35.Liu T, Lewis T, Gauda E, Gobburu J, Ivaturi V. Mechanistic population pharmacokinetics of morphine in neonates with abstinence syndrome after oral administration of diluted tincture of opium. J Clin Pharmacol. 2016;56(8):1009–1018. doi: 10.1002/jcph.696. [DOI] [PMC free article] [PubMed] [Google Scholar]


