Abstract
Background
Duloxetine and pregabalin are among the most widely used medications in the treatment of patients with fibromyalgia syndrome (FM).
Objectives
To add to the very few lines of evidence that exist on the comparative safety and efficacy of these two medications.
Methods
In this open-label randomized clinical trial, outpatient women, who were diagnosed with FM based on American College of Rheumatology 2010 criteria, and had an age range of 18–65 years old were assigned to either duloxetine 30-60 mg or pregabalin 75-150 mg per day for 4 weeks. Patients were excluded in cases of having used duloxetine, pregabalin, gabapentin, or antidepressants within 12 weeks prior to the study, having had a history of comorbid medical conditions that could provoke chronic pain, or having had comorbid neuropsychiatric disorders, except for major depressive/anxiety disorders. Primary outcomes were between-group differences in mean score changes from baseline to end point for Widespread Pain Index (WPI) and Beck Depression Inventory-II. Secondary outcomes were the same statistical estimates, but for Fibromyalgia Impact Questionnaire-Revised and 12-Item Short Form Survey. Descriptive statistics and independent samples t-test were the main methods of analysis. (www.irct.ir; IRCT2016030626935N1).
Results
Among all the scales, only WPI scores improved with a statistically significant difference between the two treatment arms, favoring duloxetine (Mean difference in score change − 2.32, 95% CI, −4.46 to − 0.18; p = 0.034; Cohen’s d 0.53 95% CI, 0.04 to 1.02). Drop out rate and cumulative incidence of nausea was significantly higher in the duloxetine arm compared to the pregabalin arm.
Conclusion
This study provides further evidence on higher efficacy of duloxetine compared to pregabalin for the treatment of pain in patients with fibromyalgia. Future comprehensive pragmatic clinical trials are warranted.
Keywords: Clinical trial, Fibromyalgia, Duloxetine, Pregabalin
Introduction
Fibromyalgia syndrome (FM) is a debilitating disorder, defined as chronic widespread musculoskeletal pain in the absence of identified organic abnormalities, accompanied by a variety of other symptoms, including but not limited to depression/anxiety, sleep disturbances, fatigue, and cognitive dysfunction [1]. It is estimated that 1–10% of the general population is affected by FM [2], with an approximate women/men ratio of 9:1 and some clinical differences in symptomatology between the two genders [3]. Alongside chronic pain as the main defining feature of patients with FM, mental symptoms and in particular depressive symptoms have been prominent enough to provoke a debate on classification of FM as a somatoform disorder [4]. According to previous studies, between 22% and 90% of patients with FM are affected by major depressive disorder (MDD) [5, 6], and several overlapping neurobiological findings as well as shared risk factors are documented for both conditions [5–12].
Treatment of FM remains a major challenge. Several non-pharmacological approaches as well as more than 40 pharmacological compounds have been the subject of research and clinical practice for the treatment of FM around the world [13, 14], with varied recommendations by international guidelines. Among the available treatments, duloxetine and pregabalin are the most widely prescribed medications to patients with FM and are the only similarly approved drugs by both Food and Drug Administration and Health Canada organizations [15]. The two drugs have different mechanisms of action; duloxetine is a serotonin norepinephrine reuptake inhibitor, while pregabalin is a gamma amino butyric acid (GABA) analogue that interacts with voltage-gated calcium channels in the central nervous system. It also appears that duloxetine and pregabalin differ in terms of improving FM symptoms, adverse events, and adherence to treatment [16–18]. To the best of our knowledge, comparison of these two drugs has been limited to only a single recent RCT with head-to-head comparison of the duloxetine and pregabalin [18], and indirect comparison through systematic reviews/meta-analyses on placebo-controlled randomized clinical trials (RCTs) of each drug [16, 17]. Further research on the direct comparison of the two drugs are prudent to cover the knowledge gaps in differential patients’ responses to these medications and defining specific indications for their clinical use.
We aimed at comparing duloxetine and pregabalin for feasibility and efficacy in treating women with FM, using an RCT design. Improvements in pain and depressive symptoms were primary outcomes of interest. We also compared the two drugs for improvements in fibromyalgia overall impact and quality of life, as well as incidence of adverse events.
Methods
Design and setting
In this open-label randomized clinical trial, patients with FM were assigned to either Cymbalta® (duloxetine) or Lyrica® (pregabalin) treatment for 4 weeks in an academic outpatient rheumatology clinic, Razi Hospital, Guilan University of Medical Sciences, from May 2016 through March 2017. The study protocol was approved by the ethics committee of Iran University of Medical Sciences (code: 25371–109,861) in accordance with World Medical Association’s code of ethics (Declaration of Helsinki, revised in Brazil 2013), and registered at an ICMJE-recognized registry of clinical trials (IRCT2016030626935N1; www.irct.ir). Written informed consent was obtained from all patients and if necessary, their caregivers. Participants were able to withdraw from the study at any time, and they were clearly informed that their relationship with the healthcare provider would not be affected.
Participants
Women diagnosed with FM, based on the American College of Rheumatology (ACR) 2010 criteria [19], were considered for study screening. Patients were eligible if they were aged between 18 and 65 and were willing to participate in the study. They were excluded if they had a history of taking certain drugs within a specified period prior to the study enrollment: duloxetine, pregabalin, gabapentin, or antidepressants within the last 12 weeks; monoamine oxidase inhibitors within the last 14 days; muscle relaxants, steroids, opioid analgesics, or benzodiazepines within the last week; injection of analgesics to painful areas within the last month. It was also required that patients: were not pregnant or breast feeding and did not intend to become pregnant during the trial; did not have other comorbid medical conditions that could provoke chronic pain such as malignancies, multiple major surgeries, recent traumatic injuries, or rheumatologic diseases other than FM; did not have concurrent neurological or psychiatric disorders except anxiety/depressive disorders; did not have occupations that demanded high level of concentration or alertness; were not known to have chronic liver diseases, severe renal failure, or uncontrolled narrow-angle glaucoma; and finally, had no history of hypersensitivity to trial medications.
Interventions
Patients initially received either duloxetine (30 mg per day) or pregabalin (75 mg per day). By the time of follow-up clinic visit at week 1, medications were titrated up to 60 mg duloxetine, once daily and 75 mg pregabalin, twice daily (150 mg per day) if the patient was tolerant and no serious adverse events were observed. During the next 3 weeks, there were no clinic visits planned; but, patients could come to the clinic in person for any concern. The study rheumatologist was available to answer patients’ phone calls, and medication doses were titrated down in case of new adverse events or intolerance. To monitor adherence to treatment, pill counts were used and were checked with individual patients as well as their caregivers or companions. In case pill counts exceeded the expected numbers, or non-adherence was reported by the patient or caregivers, the issue was explored in detail. Psychoactive/sedative or pain medications other than trial medications, or cognitive behavioral therapy were not given during the trial.
Outcomes and measures
Primary outcomes were defined as the mean difference in score change for Widespread Pain Index (WPI) (19) and Beck Depression Inventory-II (BDI-II) [20] at baseline and week 4, between the two treatment arms. Secondary outcomes included the mean difference in change of sub-scores and total score for Fibromyalgia Impact Questionnaire-Revised (FIQ-R) [21] and 12-Item Short Form Survey (SF-12) [22], as well as the difference in cumulative incidence of adverse events.
The WPI measures the number of painful areas (score range = 0–19) on the patient’s body over the last week prior to the assessment. The BDI-II is composed of 21 questions, each scored 0–3 (sum = 0–63), with higher scores indicting more severe depressive symptoms over the past two weeks prior to the assessment. The FIQ-R contains 3 domains of Activity Level (9 questions), Overall Impact (2 questions), and Intensity of Symptoms (10 questions) with each question scaled from 0 to 10, where higher score indicates higher severity of symptoms. A total score of 0–100 is calculated by adding up a third of the Activity Level, the raw Overall Impact score, and one half of the Intensity of Symptoms score. SF-12 measures 8 different concepts of quality of life through 12 questions, which are then aggregated in Physical Component Summary (PCS) and Mental Component Summary (MCS) scores, ranging from 0 (lowest level of health) to 100 (highest level of health), using a specific statistical method [23]. Valid Persian versions of all scales were available [24–27].
Each patient’s diagnosis with FM was confirmed by one of the authors (B.G.P), as an expert rheumatologist with more than 10 years of experience in diagnosis and management of FM. Questionnaires were mainly completed by the patients. Two well-trained general practitioners were available to provide patients with instructions on how to answer the questions, provide further explanations on specific questions, or provide assistance with filling the forms.
Safety and adverse events
Each patient underwent a thorough detailed medical history assessment, clinical examinations, and paraclinical evaluations before treatment assignment. At baseline visit, the patients and their caregivers were requested to record any unexpected symptoms or signs during the trial and to report it at follow-up clinic visits or through telephone calls. Patients were visited after one week to probe possible adverse events, using a comprehensive checklist and to provide an opportunity to ask their questions and discuss their concerns. The rheumatologist offered consult to patients on any emergent adverse events, whether it was a predicted adverse reaction or unpredicted signs/symptoms reported by the patients, and made a joint decision with the patient on continuation, discontinuation, or dose adjustment of medication based on severity, seriousness, and relatedness to the trial medications. The adverse event was considered related to the trial medications if it emerged after the start of the trial and was known as a pregabalin/duloxetine-related side effect on the manual provided by the company or according to the accredited scientific references).
Sample size
Two sample sizes were calculated separately using WPI and BDI-II as primary outcomes, and the larger one was chosen for this study. Expert opinion as well as published clinical trials of duloxetine and pregabalin [18, 28] were the basis for estimation of effect sizes and variances. Mean difference in score change of 2.5 for WPI with a standard deviation of 3.5 was used. Considering a type I error of 5%, and a type II error of 20%, a primary sample size of 60 (30 patients in each treatment arm) was calculated.
Randomization, allocation concealment, and blinding
Using computerized random number generation and permuted blocks of four, participants were randomized to either duloxetine or pregabalin treatment arm in a 1:1 ratio by an independent person. Initially, 30 patients were assigned to each treatment arm, and recruitment was further continued as patients dropped out of the study before treatment completion. Once there were 39 patients in each arm, 31 patients in the pregabalin arm had already completed the trial. From this point, abstract randomization was continued, and patients would enter the duloxetine arm if they were supposed to receive duloxetine based on the randomization number. Otherwise, if the randomization number indicated that the patient is supposed to receive the pregabalin, the patient would not enter the study. This was an open-label clinical trial with no allocation concealment or blinding. In fact, it was not practical to blind patients and healthcare providers since both duloxetine and pregabalin were from international brands, compliant with World Health Organization on Good Manufacture Practice.
Statistical analysis
The IBM SPSS statistics 19.0.0 (IBM Corporations) was used for all analyses. Analysis was carried out using complete cases. Shapiro-Wilk test of normality was the basis for deciding on measures of central tendency as well as tests for between group comparisons. Continuous and categorical data were reported as mean (standard deviation (SD) or 95% confidence intervals (95%CI)), median (range), or count (%). Cohen’s d (CI95%) was the choice measure of effect size. Baseline characteristics (e.g. demographics, history of any diagnosed comorbid condition), trial medication dosing strategy during the study, change in scale scores and adverse events were compared using independent samples t-test, Mann-Whitney U test, or Freeman-Halton extension of Fisher’s exact test, where appropriate. Degree of freedom was corrected if assumption of equality of variances was violated. No correction in p values for multiple testing was considered, as it could have masked important differences between the two treatment arms, especially in terms of the comparison of mean score changes. In other words, in this clinical trial of two effective and approved drugs for FM, we were interested in finding and discussing any difference between the two drugs. Also, we mainly focused on the patterns of response to treatment, rather than sole p values in our interpretation of the results.
Results
Participants and baseline characteristics
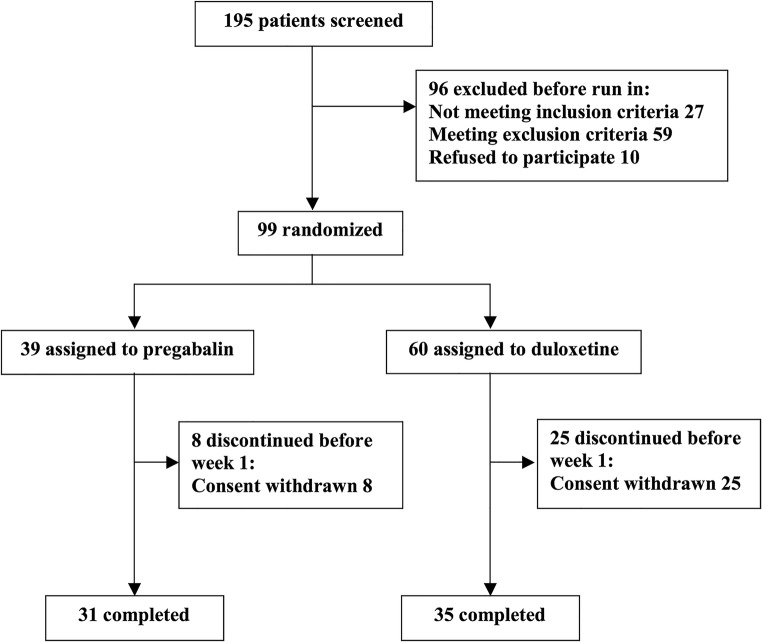
Out of the 195 screened patients, 99 were randomized to either duloxetine (n = 60) or pregabalin (n = 39), with 35 and 31 patients meeting the endpoint visit at week 4 in each of the duloxetine and pregabalin arms, respectively (Fig. 1). A significant difference in dropout rate was observed between the two treatment arms (Duloxetine: N (%) = 25(41.67), Pregabalin: N (%) = 8(20.51), p value = 0.024), where most drop outs happened in the first week of the trial. We did not observe any case of significant non-adherence among those who completed the study. No significant difference was detected between the two arms in terms of baseline socio-demographic characteristics, duration of illness, comorbidities, or medication dosing strategy (Table 1).
Fig. 1.
Flow diagram of patients with fibromyalgia
Table 1.
Characteristics of women with fibromyalgia
| Mean (SD), Median (Range), or Count (%) | |||
|---|---|---|---|
| Duloxetine (n = 35) | Pregabalin (n = 31) | P value | |
| Age, years | 41.60 (9.02) | 43.10 (7.78) | 0.476 |
| Marital Status, n% | 0.713 | ||
| • Single | 4 (11.43) | 4 (12.9) | |
| • Married | 31 (88.57) | 26 (83.87) | |
| • Divorced/widow | 0 (0) | 1 (3.23) | |
| Level of Education, n% | 0.878 | ||
| • Primary/Elementary | 12 (34.29) | 10 (32.26) | |
| • High school diploma | 15 (42.86) | 15 (48.39) | |
| • University degree | 8 (22.86) | 6 (19.35) | |
| Occupational Status, n% | 0.355 | ||
| • Householder | 31 (88.57) | 24 (77.42) | |
| • Employed | 4 (11.43) | 6 (19.35) | |
| • Student | 0 (0) | 1 (3.23) | |
| Duration of illness, months | 24 (0–240) | 36 (0–240) | 0.193 |
| Comorbid diseases/disorders, n% | 19 (54.29) | 22 (70.97) | 0.168 |
| Trial medication dose, n% | 0.352 | ||
| • Single dose | 4 (11.43) | 6 (19.35) | |
| • single➔double | 29 (82.86) | 21 (67.74) | |
| • single➔double➔single | 2 (5.71) | 4 (12.9) | |
Measurement scales
Mean baseline scores were significantly higher in the pregabalin group compared to the duloxetine group for FIQ-R activity level subscale (p = 0.037), intensity of symptoms subscale (p = 0.011), and total score (p = 0.010). Furthermore, there was a non-significant trend toward lower scores in the pregabalin arm for baseline mean MCS score. All the scale scores improved during the trial in both treatment arms (Table 2). The only scale score that improved with a statistically significant difference between the two treatment arms was the WPI score, as a primary outcome that favored duloxetine with a moderate effect size (Mean difference in score change − 2.32, 95% CI, −4.46 to − 0.18; p = 0.034; Cohen’s d 0.53 95% CI, 0.04 to 1.02). No significant difference was detected for BDI-II, FIQ-R, or SF-12 between the two treatment arms.
Table 2.
Change in scale scores from baseline to endpoint in women with fibromyalgia
| Mean (SD) | Mean (SD) | Mean (CI 95%) | Independent samples t-test | Cohen’s d (CI 95%) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Baseline to endpoint | Difference in change | t (df) | p value | |||
| Widespread Pain Index | ||||||||
| Duloxetine, n = 35 | 7.71 (3.67) | 3.69 (2.68) | −4.03 (4.05) | −2.32 (−4.46 to −0.18) | −2.16 (64) | 0.034 | 0.53 (0.04 to 1.02) | |
| Pregabalin, n = 31 | 8.03 (3.74) | 6.32 (5.01) | −1.71 (4.67) | |||||
| p value | 0.729 | |||||||
| Beck Depression Inventory II | ||||||||
| Duloxetine, n = 35 | 17.00 (9.27) | 11.65 (9.56) | −5.26 (7.49) | 1.35 (−3.06 to 5.76) | 0.61 (64) | 0.543 | −0.15 (−0.63 to 0.33) | |
| Pregabalin, n = 31 | 20.10 (11.43) | 13.48 (9.28) | −6.61 (10.20) | |||||
| p value | 0.229 | |||||||
| Fibromyalgia Impact Questionnaire-Revised | ||||||||
|
Activity Level (adjusted) |
Duloxetine, n = 35 | 15.07 (6.92) | 9.79 (7.81) | −5.28 (7.71) | 2.38 (−1.06 to 5.82) | 1.38 (64) | 0.171 | −0.34 (−0.83 to 0.14) |
| Pregabalin, n = 31 | 18.37 (5.44) | 10.71 (6.49) | −7.66 (6.03) | |||||
| p value | 0.037 | |||||||
| Overall Impact | Duloxetine, n = 35 | 10.97 (6.85) | 6.34 (6.70) | −4.63 (6.48) | 1.82 (−1.51 to 5.15) | 1.09 (64) | 0.278 | −0.27 (−0.75 to 0.22) |
| Pregabalin, n = 31 | 13.81 (4.92) | 7.35 (6.00) | −6.45 (7.06) | |||||
| p value | 0.056 | |||||||
| Intensity of Symptoms (adjusted) | Duloxetine, n = 35 | 28.87 (9.10) | 19.11 (12.10) | −9.76 (11.70) | −0.39 (−6.01 to 5.24) | −0.14 (64) | 0.891 | 0.03 (−0.45 to 0.52) |
| Pregabalin, n = 31 | 34.29 (7.54) | 24.92 (11.57) | −9.37 (11.10) | |||||
| p value | 0.011 | |||||||
| Total | Duloxetine, n = 35 | 54.91 (20.20) | 35.25 (24.90) | −19.66 (21.85) | 3.82 (−6.36 to 13.99) | 0.75 (64) | 0.456 | −0.19 (−0.67 to 0.30) |
| Pregabalin, n = 31 | 66.46 (13.86) | 42.98 (20.71) | −23.48 (19.19) | |||||
| p value | 0.008 | |||||||
| 12-Item Short Form Survey | ||||||||
| Physical Component Summary | Duloxetine, n = 35 | 36.96 (23.31) | 54.96 (22.07) | 17.65 (22.32) | 4.54 (−6.04 to 15.13) | 0.86 (64) | 0.394 | −0.21 (−0.70 to 0.27) |
| Pregabalin, n = 31 | 34.88 (16.12) | 47.98 (19.92) | 13.10 (20.18) | |||||
| p value | 0.671 | |||||||
| Mental Component Summary | Duloxetine, n = 34 | 56.69 (24.33) | 63.97 (22.51) | 6.02 (21.02) | −4.74 (−15.99 to 6.50) | −0.84 (63) | 0.402 | 0.21 (−0.27 to 0.70) |
| Pregabalin, n = 31 | 45.77 (27.21) | 56.53 (21.91) | 10.77 (23.96) | |||||
| p value | 0.092 | |||||||
Adverse events
Most adverse events occurred during the first and second week of the trial (Table 3). Overall incidence of nausea was significantly higher in the duloxetine arm compared to the pregabalin arm (Table 3). Although we registered higher incidence of constipation, dry mouth, headache, insomnia, and hot flashes in the duloxetine arm, no statistical significance was detected. Furthermore, some patients in the duloxetine arm experienced blurred vision, decreased appetite, and generalized weakness, while the patients in the pregabalin arm did not report these adverse events. In contrast, higher incidence of dizziness, light headedness, and drowsiness was reported by patients in the pregabalin arm, with no significant difference between the two treatment arms. Other adverse events like palpitation, tremor, decreased sexual desire, and bloating were scarce and mainly reported in the duloxetine arm.
Table 3.
Incidence of adverse events in women with fibromyalgia
| Number of Patients | Number (%) of Patients | Overall P value | |||||
|---|---|---|---|---|---|---|---|
| Adverse event | Week 1 | Week 2 | Week 3 | Week 4 | Overall, counting each patient once | ||
| Nausea |
Duloxetine (n = 35) |
5 | 7 | 2 | 2 | 12 (34.29) | 0.014 |
|
Pregabalin (n = 31) |
2 | 1 | 0 | 0 | 3 (9.68) | ||
| Vomiting |
Duloxetine (n = 35) |
1 | 0 | 0 | 0 | 1 (2.86) | 0.747 |
|
Pregabalin (n = 31) |
0 | 1 | 0 | 0 | 1 (3.23) | ||
| Bloating |
Duloxetine (n = 35) |
1 | 1 | 1 | 1 | 1 (2.86) | 0.735 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Abdominal pain |
Duloxetine (n = 35) |
0 | 1 | 1 | 1 | 1 (2.86) | 0.747 |
|
Pregabalin (n = 31) |
1 | 1 | 0 | 0 | 1 (3.23) | ||
| Constipation |
Duloxetine (n = 35) |
7 | 8 | 5 | 5 | 11 (31.43) | 0.062 |
|
Pregabalin (n = 31) |
4 | 3 | 3 | 3 | 4 (12.9) | ||
| Dry mouth |
Duloxetine (n = 35) |
3 | 5 | 2 | 2 | 6 (17.14) | 0.077 |
|
Pregabalin (n = 31) |
1 | 0 | 0 | 0 | 1 (3.23) | ||
| Bitter taste in the mouth |
Duloxetine (n = 35) |
1 | 1 | 1 | 1 | 1 (2.86) | 0.735 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Blurred vision |
Duloxetine (n = 35) |
1 | 2 | 0 | 0 | 2 (5.71) | 0.355 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Palpitation |
Duloxetine (n = 35) |
1 | 0 | 0 | 0 | 1 (2.86) | 0.735 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Tremor |
Duloxetine (n = 35) |
1 | 1 | 1 | 1 | 1 (2.86) | 0.735 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Headache |
Duloxetine (n = 35) |
3 | 7 | 4 | 3 | 8 (22.86) | 0.063 |
|
Pregabalin (n = 31) |
2 | 1 | 0 | 0 | 2 (6.45) | ||
| Dizziness |
Duloxetine (n = 35) |
4 | 4 | 2 | 2 | 6 (17.14) | 0.207 |
|
Pregabalin (n = 31) |
8 | 2 | 3 | 3 | 10 (32.26) | ||
| Lightheadedness |
Duloxetine (n = 35) |
1 | 2 | 1 | 1 | 2 (5.71) | 0.305 |
|
Pregabalin (n = 31) |
4 | 1 | 1 | 1 | 4 (12.9) | ||
| Disequilibrium |
Duloxetine (n = 35) |
1 | 1 | 0 | 0 | 1 (2.86) | 0.747 |
|
Pregabalin (n = 31) |
1 | 0 | 0 | 0 | 1 (3.23) | ||
| Drowsiness |
Duloxetine (n = 35) |
6 | 4 | 2 | 2 | 7 (20.00) | 0.276 |
|
Pregabalin (n = 31) |
7 | 4 | 3 | 2 | 10 (32.26) | ||
| Insomnia |
Duloxetine (n = 35) |
4 | 3 | 1 | 1 | 6 (17.14) | 0.200 |
|
Pregabalin (n = 31) |
1 | 1 | 1 | 0 | 2 (6.45) | ||
| Hot flashes |
Duloxetine (n = 35) |
4 | 2 | 2 | 2 | 4 (11.43) | 0.080 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Decreased sexual desire |
Duloxetine (n = 35) |
1 | 1 | 1 | 1 | 1 (2.86) | 0.735 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Decreased appetite |
Duloxetine (n = 35) |
2 | 2 | 1 | 1 | 3 (8.57) | 0.170 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Generalized weakness |
Duloxetine (n = 35) |
3 | 2 | 2 | 2 | 3 (8.57) | 0.170 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
| Urinary Frequency |
Duloxetine (n = 35) |
1 | 1 | 1 | 1 | 1 (2.86) | 0.735 |
|
Pregabalin (n = 31) |
0 | 0 | 0 | 0 | 0 (0) | ||
Discussion
According to primary results, baseline scores were significantly higher in pregabalin group compared to the duloxetine group for FIQ-R activity level subscale, intensity of symptoms subscale, and total score (Table 2). Moreover, there was a trend in baseline mean MCS score toward, with lower scores in the pregabalin arm compared to the duloxetine arm. In such situations, it is not recommended that the two treatment arms be compared in terms of mean score change from baseline to endpoint, due to the potentially large regression-to-mean effect, which may introduce bias to p values and distort conclusions. In other words, it is probable that the group with the higher baseline score shows a greater reduction in scores, or the group with lower baseline scores demonstrates a greater increase in scores, depending on the direction of score changes that indicate improvement. However, because there was no significant difference between the two treatment arms for score improvement in any of the abovementioned subscales of FIQ-R or MCS, we decided to report the results for comparing mean score changes between duloxetine and pregabalin arms for all scales, and then focus on patterns of score changes.
For BDI-II, FIQ-R total score, activity level subscale, and overall impact subscale, patients in the pregabalin arm had higher baseline scores compared to the duloxetine arm and experienced more reduction in scores. Also regarding the MCS, patients in the pregabalin arm had lower baseline scores and experienced a higher increase in scores during the trial. These patterns are compliant with regression-to-mean effect. Since we did not detect any significant differences between the two arms for these measures, we were unable to conclude that the two treatments were different in improving the scales’ scores, which mainly measure mental symptoms or overall impact of FM. However, interestingly, WPI and PCS scores did not follow this pattern. In fact, patients in the duloxetine arm had lower baseline WPI scores but demonstrated more reduction in WPI scores compared to the pregabalin arm. Also, they had higher baseline PCS scores, yet experienced a higher increase in PCS scores compared with patients who received pregabalin during the trial. Furthermore, while patients in the duloxetine arm had lower scores for FIQ-R intensity of symptoms subscale, compared to the pregabalin arm, both groups demonstrated similar improvements. These later patterns are opposite to regression-to-mean effect and imply superiority of duloxetine in improving the scores for these later three scales, which measure pain and physical symptoms. Taking all the abovementioned points together, we hypothesized that duloxetine is more effective on physical symptoms, in particular pain, than pregabalin, but similarly effective on mental health outcomes, such as depressive symptoms and mental health quality of life.
To the best of our knowledge, only one recently published RCT has carried out a head-to-head comparison of duloxetine and pregabalin for treatment of patients with FM [18]. Consistent with our findings regarding treatment efficacy, Gilron et al. [18] reported superiority of duloxetine to pregabalin in pain improvement, but insignificant differences in mental health or quality of life improvement between the two treatments. They also reported superiority of pregabalin to duloxetine in sleep improvement, which was not considered as a separate outcome in our study. Also, the two previous large-scale meta-analyses on placebo-controlled randomized trials of duloxetine, pregabalin, and milnacipran in FM, reported superiority of duloxetine to pregabalin for pain improvement [16, 17]. Furthermore, one of these meta-analyses reported superiority of duloxetine to pregabalin for improving depression [17], but superiority of pregabalin to duloxetine for fatigue [17].
Regarding adverse events, we observed less incidence of nausea in the pregabalin arm, compared to the duloxetine arm. Similarly, Gilron et al. [18] reported significantly less nausea and insomnia, and a previous meta-analysis indicated significantly less nausea, headache, and diarrhea for pregabalin compared to duloxetine [17]. Consistent with these findings, we observed a prominently higher rate of consent withdrawal in the duloxetine arm, compared to the pregabalin arm. Similarly, results by Gilron et al. and the two meta-analyses [16–18] tended to favor higher withdrawal due to adverse events in duloxetine compared with pregabalin; but, did not conclude statistical significance. As a potential explanation for variance of withdrawal rate between these studies, we can point out the differences in medication dose, interventions for increasing adherence, concomitant consumption of other medications, as well as the study population of interest.
Concerning methodological aspects, it is noteworthy to mention that the study by Gilron et al. [18] used a cross-over design alternating drug assignment between the treatment arms to reduce inter-subject confounding effects. In addition, the study was well-blinded. In comparison to that study, we excluded patients who consumed analgesics from one week prior to the study to the end of the trial. It was also required that patients in our study had not received pregabalin or duloxetine in a long period (3-months) before the study started. In this manner, we could estimate almost pure effects of the two discussed medications with no potential drug-drug interactions or carry-over effects. This issue is especially important, considering the prominently higher rates of drug-drug interactions and drug-condition interactions in patients receiving duloxetine, than those treated with pregabalin [29, 30], which may in turn affect treatment outcomes and introduce unwanted bias to the results. Our study provided us with more powerful means for the comparison of dropout rates between the two medications, regarding both the sample size and potential order effect. While we could compare a considerable number of patients receiving either duloxetine or pregabalin with no order effect from the beginning of the study, the study by Gilron et al. could account for head-to-head comparison of only about 10 patients in each of the duloxetine and pregabalin treatments from the beginning of their study. Interestingly, in the study by Gilron et al. [18], only 1 patient in the pregabalin group withdrew in the first period, and no patient in the pregabalin group dropped out later; but, 3 patients in the duloxetine group withdrew from the trial during the second and third periods, which implies potential order effect. Another difference to be mentioned is that we used ACR 2010 criteria for patient recruitment, but the mentioned study used ACR 1990.
This study was subject to some limitations. First, the sample was relatively small and highly selected using several exclusion criteria, which necessitates caution when generalizing the results to the target population of patients with FM. Second, the follow-up period was relatively short and therefore hindered our assessment for long-term safety and efficacy of the trial medications. Third, this was an open-label study with potential risks of bias, in particular observer bias. However, the fact that we used self-reported WPI and BDI-II for depression as primary outcomes lessens the concern of observer bias. Of note, most of the patients in this study were new-cases of FM with no previous exposure to duloxetine or pregabalin, which further reduced the risk of bias. Fourth, there would potentially be loss of some degree of variation in outcomes due to a high number of dropouts in both treatment arms. In other words, dropouts in clinical trials are a potential source for enrichment of cases, who are better responders to treatment. While we did not measure outcome among dropouts, the fact that they mostly reported adverse outcomes as the reason for withdrawing their consents lessens this concern. Fifth, we administered pregabalin up to 150 mg/day in patients, based on routine standard practice at our clinic. Previous trials and meta-analyses have reported different outcomes for the 300 and 150 mg doses of pregabalin, regarding safety and efficacy. Sixth, it was not practical to blind patients and healthcare providers, since any intervention by drug companies in Iran to make duloxetine and pregabalin similar in appearance or color would risk their quality.
Future randomized clinical trials for comparing duloxetine and pregabalin will benefit from longer follow-up periods, larger sample sizes, and comparison of different medication doses. Additionally, amongst combinations of different medications used for treatment of patients with FM as a common practice, duloxetine-pregabalin combination therapy is a relatively new approach that requires further investigation. So far, only one placebo-controlled clinical trial has compared duloxetine-pregabalin combination therapy with each medication alone, which yielded promising results regarding both safety and efficacy [18].
In conclusion, we provided further evidence for higher efficacy of duloxetine compared with pregabalin monotherapy to reduce pain in patients with fibromyalgia. Nevertheless, adverse events, adherence to treatment, and drug-drug interactions should be considered when deciding to tailor duloxetine or pregabalin to an individual patient. Future comprehensive pragmatic clinical trials are warranted to illuminate comparative effectiveness, safety, and healthcare costs of patients with fibromyalgia, who are treated with duloxetine and pregabalin, or a combination of the two.
Acknowledgements
We would like to offer our special thanks to Dr. Nahid Kianmehr from Iran University of Medical Sciences for helping the authors with patient recruitment procedures, and Ms. Kimia Ziafat from University of British Columbia for language editing of the manuscript.
Funding
This study was supported by Iran University of Medical Sciences (Grant No. 2470). The academic institution had no role in design, conduct, data collection, analysis, data interpretation, manuscript preparation, review, or decision to submit this paper for publication.
Compliance with ethical standards
Conflict of interest
The authors of this manuscript declare that they have no competing interests.
Ethical approval
All the procedures performed were approved by the ethics committee of Iran University of Medical Sciences (code: 25371–109,861) in accordance with the World Medical Association’s code of ethics (Declaration of Helsinki, revised in Brazil 2013).
Informed consent
Written informed consent was obtained from all patients and if necessary their caregivers. It was clearly explained for the participants that their relationship with the healthcare provider would not be affected should they chose to withdraw from the study at any time.
Research data policy
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ali Bidari and Ehsan Moazen-Zadeh contributed equally in this study.
References
- 1.Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol. 2011;7(9):518–527. doi: 10.1038/nrrheum.2011.98. [DOI] [PubMed] [Google Scholar]
- 2.Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17(8):356. doi: 10.1007/s11916-013-0356-5. [DOI] [PubMed] [Google Scholar]
- 3.Yunus MB, Inanici F, Aldag JC, Mangold RF. Fibromyalgia in men: comparison of clinical features with women. J Rheumatol. 2000;27(2):485–490. [PubMed] [Google Scholar]
- 4.Häuser W, Henningsen P. Fibromyalgia syndrome: a somatoform disorder? Eur J Pain. 2014;18(8):1052–1059. doi: 10.1002/j.1532-2149.2014.00453.x. [DOI] [PubMed] [Google Scholar]
- 5.Pae C-U, Luyten P, Marks DM, Han C, Park S-H, Patkar AA, Masand PS, van Houdenhove B. The relationship between fibromyalgia and major depressive disorder: a comprehensive review. Curr Med Res Opin. 2008;24(8):2359–2371. doi: 10.1185/03007990802288338. [DOI] [PubMed] [Google Scholar]
- 6.Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression. Pain Res Treat. 2012;2012. 10.1155/2012/486590. [DOI] [PMC free article] [PubMed]
- 7.Meeus M, Nijs J, Hermans L, Goubert D, Calders P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: peripheral and central mechanisms as therapeutic targets? Expert Opin Ther Targets. 2013;17(9):1081–1089. doi: 10.1517/14728222.2013.818657. [DOI] [PubMed] [Google Scholar]
- 8.Cordero MD, de Miguel M, Carmona-López I, Bonal P, Campa F, Moreno-Fernández AM. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol Lett. 2010;31(2). [PubMed]
- 9.Becker S, Schweinhardt P. Dysfunctional neurotransmitter systems in fibromyalgia, their role in central stress circuitry and pharmacological actions on these systems. Pain Res Treat. 2012;2012:741746. doi: 10.1155/2012/741746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–129. doi: 10.1016/j.neuroscience.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci. 2009;14:5291–5338. doi: 10.2741/3598. [DOI] [PubMed] [Google Scholar]
- 12.Thiagarajah AS, Guymer EK, Leech M, Littlejohn GO. The relationship between fibromyalgia, stress and depression. Int J Clin Rheumatol. 2014;9(4):371–384. doi: 10.2217/ijr.14.30. [DOI] [Google Scholar]
- 13.Chinn S, Caldwell W, Gritsenko K. Fibromyalgia pathogenesis and treatment options update. Curr Pain Headache Rep. 2016;20(4):25. doi: 10.1007/s11916-016-0556-x. [DOI] [PubMed] [Google Scholar]
- 14.Shakiba M, Moazen-Zadeh E, Noorbala AA, Jafarinia M, Divsalar P, Kashani L, et al. Saffron (Crocus sativus) versus duloxetine for treatment of patients with fibromyalgia: a randomized double-blind clinical trial. Avicenna J Phytomed. 2018;8(6):513–523. [PMC free article] [PubMed] [Google Scholar]
- 15.Kia S, Choy E. Update on treatment guideline in fibromyalgia syndrome with focus on pharmacology. Biomedicines. 2017;5(2):E20. doi: 10.3390/biomedicines5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YH, Song GG. Comparative efficacy and tolerability of duloxetine, pregabalin, and milnacipran for the treatment of fibromyalgia: a Bayesian network meta-analysis of randomized controlled trials. Rheumatol Int. 2016;36(5):663–672. doi: 10.1007/s00296-016-3468-5. [DOI] [PubMed] [Google Scholar]
- 17.Häuser W, Petzke F, Sommer C. Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. J Pain. 2010;11(6):505–521. doi: 10.1016/j.jpain.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Gilron I, Chaparro LE, Tu D, Holden RR, Milev R, Towheed T, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532–1540. doi: 10.1097/j.pain.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 21.Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The revised fibromyalgia impact questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009;11(4):R120. doi: 10.1186/ar2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Keller SD, Kosinski M. Sf-12: how to score the Sf-12 Physcial and mental health summary scales. Lincoln: QualityMetric Incorporated; 1998. [Google Scholar]
- 24.Bidari A, Hassanzadeh M, Parsa BG, Kianmehr N, Kabir A, Pirhadi S, et al. Validation of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia in an Iranian population. Rheumatol Int. 2013;33(12):2999–3007. doi: 10.1007/s00296-013-2829-6. [DOI] [PubMed] [Google Scholar]
- 25.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck depression inventory-second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21(4):185–192. doi: 10.1002/da.20070. [DOI] [PubMed] [Google Scholar]
- 26.Parsa BG, Maafi AA, Haghdoost A, Arabi Y, Khojamli M, Chatrnour G, et al. The validity and reliability of the Persian version of the revised fibromyalgia impact questionnaire. Rheumatol Int. 2014;34(2):175–180. doi: 10.1007/s00296-013-2929-3. [DOI] [PubMed] [Google Scholar]
- 27.Montazeri A, Vahdaninia M, Mousavi SJ, Omidvari S. The Iranian version of 12-item short form health survey (SF-12): factor structure, internal consistency and construct validity. BMC Public Health. 2009;9(1):341. doi: 10.1186/1471-2458-9-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasser K, Kivitz AJ, Maricic MJ, Silver DS, Silverman SL. Twice daily versus once nightly dosing of Pregabalin for fibromyalgia: a double-blind randomized clinical trial of efficacy and safety. Arthritis Care Res. 2014;66(2):293–300. doi: 10.1002/acr.22111. [DOI] [PubMed] [Google Scholar]
- 29.Ellis JJ, Sadosky AB, Ten Eyck LL, Cappelleri JC, Brown CR, Suehs BT, et al. Impact of potential pregabalin or duloxetine drug–drug interactions on health care costs and utilization among Medicare members with fibromyalgia. Clinicoecon Outcomes Res. 2014;6:389–699. doi: 10.2147/CEOR.S66759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston SS, Udall M, Cappelleri JC, Johnson BH, Shrady G, Chu B-C, Silverman SL. Potential drug–drug and drug–condition interactions among fibromyalgia patients initiating Pregabalin or duloxetine: prevalence and health care expenditure impact. Pain Med. 2014;15(8):1282–1293. doi: 10.1111/pme.12330. [DOI] [PubMed] [Google Scholar]