Abstract
Purpose
Cisplatin, one of the most effective anticancer drugs, is known to cause undesirable adverse effects, including immunotoxicity. Echinacea purpurea is an important medicinal plant with immunostimulatory and anti-inflammatory activities. We have investigated the protective effect of an herbal formulation (Immulant) containing E. purpurea extract against cisplatin-induced immunotoxicity in rats.
Methods
Forty mature albino rats were randomized into four groups (10 rats/group). Control (group 1) animals were subjected to intraperitoneal (i.p.) injection of saline solution (0.2 ml) once every 3 days. Group 2 animals received cisplatin (3.5 mg/kg, i.p.) once every 3 days for successive 2 weeks. Group 3 rats received oral Immulant (150 mg/kg) once daily for 2 weeks. Group 4 animals received oral Immulant treatment as in group 3 in addition to cisplatin as in group 2. Serum level of total protein and albumin, total and differential leukocytic count, phagocytic activity of monocytes, humoral activity and splenic histopathology and immunohistochemistry were used as diagnostic markers of immunotoxicity.
Results
Cisplatin induced marked inhibition of cellular immunity as exhibited by significant decrease of leukocytic count, lymphocyte percentage and phagocytic activity with marked increase in neutrophil percentage. Humoral immunity represented by marked inhibition in total protein and γ-globulin concentration and significant inhibition in antibody titer against Mycoplasma gallisepticum were recorded. Histopathological and immunohistochemical observation of the spleen of cisplatin-treated rats revealed obvious pathological findings of marked depletion and degeneration of lymphoid tissue. Co-oral administration of Immulant resulted in substantial improvement of various immunotoxicological indices compared to cisplatin control.
Conclusion
The herbal medicine Immulant is an immunostimulant which could be used to treat the immunotoxic effects of cisplatin.
Graphical abstract.
Cisplatin (CP) is a highly effective antineoplastic DNA alkylating agent. CP induces free radical production causing an oxidative damage.Cisplatin induced marked inhibition in cellular and humoral immunityEchinacea purpurea (Immulant) is a powerful anticytotoxic agent against cisplatin toxicity.
Keywords: Echinacea purpurea, Immulant, Cisplatin, Immunotoxicity, Rats
Introduction
Cisplatin (also known as cisplatinum or cis-diamminedichloroplatinum) is a platinum-containing compound which inhibits synthesis of RNA, DNA and protein in cells. Cisplatin is one of the most effective anticancer drugs used for the treatment of various oncologic diseases, including testicular, cervical, ovarian, mammary, head and neck, esophageal, lung, and brain cancers [1]. Despite its effective anticancer efficacy, cisplatin exerts many undesirable adverse effects, including myelosuppression, hepatotoxicity, nephrotoxicity, spermiotoxicity, and immunotoxicity [2]. The toxic effects of cisplatin are attributed to several factors, such as peroxidation of the cell membrane, DNA damage, mitochondrial dysfunction, inhibition of protein synthesis, and ability to affect host immune response [3]. Immunotoxicity could be the result of direct or indirect action of a chemical on the immune system, causing a suppression or activation of the immune response. Compromised immune response can result in suppression of host resistance to infectious agents as well as tumor cells. Most anticancer drugs are found to suppress hematopoiesis in bone marrow and cause myelosuppression and lymphocytopenia, resulting in reduction or inhibition of lymphocytic responses [4]. Cisplatin has a cytotoxic effect on immune cells when they are rapidly dividing. Treatment of ovarian cancer patients with cisplatin caused reduction in the number of T-helper cells with an increase in the number of T-suppressor/cytotoxic cells [5].
Medicinal plants are known to possess various biological and pharmacological activities that have immense value in pharmaceutical industry for the discovery and development of drugs with appreciable efficacy and acceptable side effects. Echinacea purpurea (family: Asteraceae) is an important medicinal plant with various pharmacological properties [6]. E. purpurea was found to exhibit significant antioxidant, anti-inflammatory, and immunoregulatory activities [6]. Several active constituents, such as flavonoids, caffeic acid derivatives (alkamides), essential oils, and poly acetylenes, were isolated from this plant. Among various phytocomponents, alkamides were found to possess immunoregulatory effects [7]. Nevertheless, limited information is available on the potential of E. purpurea in combating immunotoxicity of chemotherapeutic drugs, such as cisplatin. Therefore, the purpose of the present study was to investigate the protective effect of a formulation containing E. purpurea extract (Immulant) against cisplatin-induced immunotoxicity in rats.
Materials and methods
Chemicals
Cisplatin was precured in the form of Unistin vial (EIMC United Pharmaceutical, Cairo, Egypt). E. purpurea extract (in the form of Immulant syrup, 16.7 mg/ml) was obtained from Arab Company for Pharmaceuticals and Medicinal Plants (Cairo, Egypt). All other chemicals and reagents used in this study were of analytical grade and obtained from local firms.
Animals
Forty mature male albino rats (about 3 months old, weighing 180–200 g) were obtained from the animal house, Faculty of Veterinary Medicine, Cairo University, Egypt. The rats were kept under appropriate environment of temperature (25 ± 2 °C), humidity (60–70%), and light (12-h dark/light cycles), with free access to a commercial balanced diet and tap water ad libitium.
Experimental design
All animal procedures were conducted according to the protocol approved by the Institutional Animal Care and Use Committee at Cairo University, Cairo, Egypt (IACUC, CU-II-F-10-19). After a 2-week acclimatization period, the rats were randomly divided into four groups (10 rats/group) in separate plastic cages and subjected to various treatments as follows. Group 1: Control group was injected intraperitoneally (i.p.) once every 3 days with 0.2 ml saline solution. Group 2: Cisplatin group was injected i.p. with cisplatin (3.5 mg/kg) once every 3 days for successive 2 weeks. The dose of cisplatin was selected based on a previous study [8]. Group 3: Immulant control rats in this group were orally administered Immulant (150 mg/kg) once daily for consecutive 2 weeks following the procedure of Zhai et al. [9]. Group 4: Immulant and cisplatin group was given orally once daily Immulant (150 mg/kg) as in group 3. Additionally, cisplatin (3.5 mg/kg) was administered as in group 2 in animals 30 min following Immulant administration. The experimental dose for Imuulant was selected based on conversion of therapeutic human dose to animal dose according to the method described previously [10, 11]. After 2 weeks, rats of all experimental groups were inoculated subcutaneously with 0.5 ml log10 50% embryo infectious doses (EID50) of M. gallisepticum (National Health Institute, Doki, Giza, Egypt).
Sample collection
Ten days after inoculation of M. gallisepticum, the rats were anesthetized by ether inhalation and a midline thoraco-abdominal incision was performed to expose their viscera. Blood samples were collected in heparinized sterile tubes via direct cardiac puncture. The samples were used for total and differential leukocytic count and measurement of phagocytic activity, while the sera were collected and stored in a freezer at −20 °C for subsequent assessment by immunoassay. Splenic tissues of rats were excised, cleaned from their surroundings, washed with normal saline, and subjected to histopathological and immunohistochemical examination.
Hematological and biochemical analysis
Total and differential leukocytic count was performed following the methods of Schalm et al. [12]. Total protein and albumin concentration were measured by the adapted methods of Gornallet al. [13] and Doumaset al. [14], respectively. Electrophoretic patterns of serum proteins (α-, β, and γ-globulin fractions) were carried out according to the method of Kaplan and Saveloy [15].
Assessment of phagocytic activity
Phagocytic activity of peripheral blood monocytes employing Candida albicans was measured as described previously [16, 17]. Peripheral blood mononuclear cells were separated using Ficoll-Hypaque density gradient was carried out according to the procedure of Boyum [18]. Subsequently, the mononuclear cell layer was collected, washed and re-suspended in RPMI-1640 medium supplemented with 10% fetal calf serum, and viability was recorded according to Hanks and Waalace [19]. The phagocytic percentage and index were calculated using the following formulas:
Measurement of antibodies for humoral activity
Serum samples of all groups were examined for humoral activity using M. gallisepticum Antibody Test Kit (BioChek, Westbrook, Maine, USA).
Histopathological studies
Spleen from all treated groups were carefully dissected out and quickly fixed in aqueous Bouin′s solution, dehydrated in ascending grades of ethyl alcohol, cleaned using xylene, impregnated in paraffin wax, and sections of 5–7 μm thickness were prepared. The deparaffinized sections were stained with Harri’s hematoxylin and eosin (H&E) as well as Crossmon’s trichrome stain following Gomori’s reticulin method according to Bancroft and Gamble [20]. Histological sections were examined under a light microscope and images were captured by a high-definition microscopic camera (Leica Microsystems, Wetzlar, Germany).
Immunohistochemical examination
Immunohistochemistry for detection of caspase-3 was performed using paraffin sections mounted on coated glass slides. Antigen retrieval was performed in citrate buffer (pH 6.0) using microwave digestion (2 cycles of 12 min each). Subsequently, endogenous peroxidase was blocked using 0.05% H2O2 for 30 min. Following incubation with normal horse serum (1:20 dilution), the slides were incubated with primary antibodies (AB3623, rabbit anti-active caspase-3, from Merck Millipore, Billerica, MA, USA) overnight at 4 °C.After that, the sections were treated with secondary antibodies associated with streptavidin-biotin-peroxidase complex. Diamino-benzidine was used as a chromogen. All tissue sections were counter-stained with hematoxylin. The sections were then washed with phosphate buffered saline following each step. Negative controls were used using non-immune serum instead of the primary or secondary antibodies as per the technique of Ramos-Vara [21].
Statistical analysis
Results were expressed as mean ± standard error of mean (SEM). Comparison of means was performed by one-way analysis of variance (ANOVA) followed by Student’s t test. Values of P less than 0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Hematological results
Table 1 presents results on total and differential leukocyte count in various groups. There was a significant decrease in total leukocyte (leukopenia) and lymphocyte counts (lymphocytopenia) as well as increase in neutrophil count (neutrophilia) in rats treated with cisplatin in comparison with control animals. In the group that received E. purpura extract in the form of Immulant in addition to cisplatin, all aforementioned parameters were reversed compared to cisplatin alone. In rats that received Immulant only, the results are similar to those in control group.
Table 1.
Effects of Immulant on various hematological parameters in control and cisplatin-treated rats
| Groups Parameters | Control | Cisplatin | Immulant | Cisplatin+Immulant |
|---|---|---|---|---|
| Leukocytes (×103) | 10.2 ± 0.15 | 6.3 ± 0.42a | 9.9 ± 0.61 | 8.9 ± 0.46c |
| Neutrophil (%) | 23.6 ± 1.2 | 29 ± 1.3a | 24.6 ± 2.02 | 25.6 ± 1.45c |
| Lymphocyte (%) | 65.0 ± 2.6 | 55.8 ± 1.3a | 64.0 ± 2.09 | 62.0 ± 1.15c |
| Monocytes (%) | 11.0 ± 1.34 | 12.4 ± 0.6 | 11.2 ± 0.71 | 12 ± 0.92 |
| Eosinophil (%) | 1.1 ± 0.05 | 2.1 ± 0.25 | 0.8 ± 0.02 | 1.8 ± 0.31 |
| Basophil (%) | 0.8 ± 0.02 | 0.4 ± 0.01b | 0.5 ± 0.02 | 0.3 ± 0.02 |
| Total protein (mg/dl) | 7.55 ± 0.17 | 6.4 ± 0.01a | 8.13 ± 0.01 | 7.3 ± 0.1 |
| Albumin (mg/dl) | 4.61 ± 0.04 | 4.07 ± 0.06 | 5.15 ± 0.03 | 4.49 ± 0.01 |
| α-Globulin (mg/dl) | 0.62 ± 0.03 | 0.59 ± 0.02 | 0.81 ± 0.01 | 0.57 ± 0.01 |
| β-Globulin (mg/dl) | 0.30 ± 0.01 | 0.29 ± 0.02 | 0.51 ± 0.03 | 0.28 ± 0.01 |
| γ-Globulin (mg/dl) | 2.14 ± 0.09 | 0.59 ± 0.02b | 2.3 ± 0.04 | 1.96 ± 0.03c |
Values are expressed as mean ± SEM. aP˂0.05 and bP˂0.01 compared to control. cP˂0.05 compared to cisplatin
Serum proteins
Data recorded in Table 1 reveals significant decrease in mean value of serum total protein in rats that received cisplatin in comparison with control group. Also, significant decrease was observed in γ-globulin concentration in cisplatin-treated rats, while no changes were recorded in, α- and β-globulin concentration in this group in comparison with the control. Significant improvement was observed in γ-globulin in cisplatin plus Immulant group compared to cisplatin alone group and the values were nearly similar to those of control group. In Immulant-treated group, electrophoretic pattern of all serum proteins was similar to those of control rats.
Immunological results
Table 2 shows the immune response (Ab titers and phagocytic activity) against M. gallisepticum in various rat groups. In cisplatin-treated group, the values of Ab titers, phagocytic index and percentage showed significant reduction in comparison with the control group. Administration of Immulant before cisplatin treatment caused significant improvement in the immunological parameters in comparison with the cisplatin group. In Immulant-treated group, the Ab titers, phagocytic index and percentage were higher than the control, but the results were not statistically significant.
Table 2.
Effect of Immulant on various immunological parameters in control and cisplatin-treated rats
| Control | Cisplatin | Immulant | Cisplatin +Immulant | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab Titers | Phagocytic | Ab Titers | Phagocytic | Ab Titers | Phagocytic | Ab Titers | Phagocytic | ||||
| % | index | % | index | % | index | % | index | ||||
| 800 ± 8.12 | 56 ± 3.15 | 0.24 ± 0.04 | 644 ± 7.11a | 20 ± 2.76a | 0.19 ± 0.01a | 874 ± 11.9 | 61.5 ± 2.71 | 0.27 ± 0.02 | 720 ± 10.45b | 37.8 ± 2.83c | 0.21 ± 0.02c |
Values are expressed as mean ± SEM. aP˂0.05 compared to control. bP˂0.05 and cP˂0.01 compared to cisplatin
Histological observations
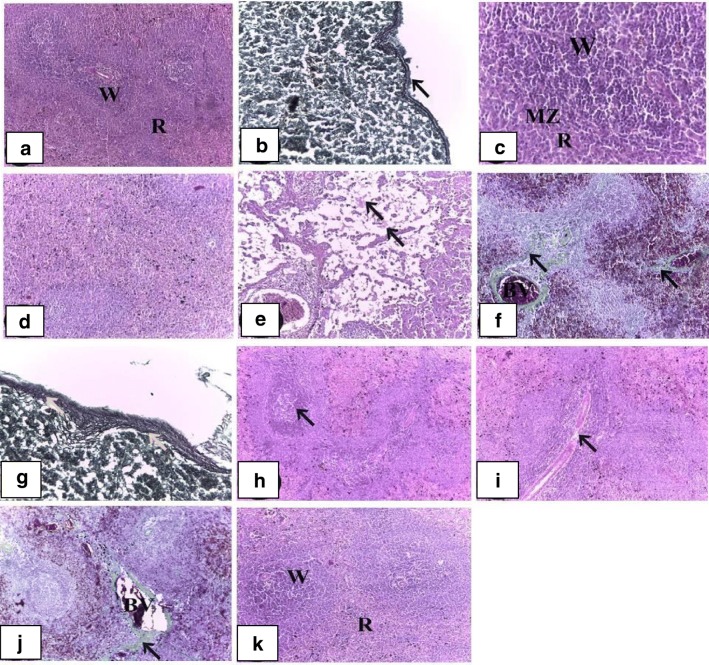
Examination of the spleen sections of control rats showed normal architecture (Fig. 1a). It contained white and red pulps surrounded by a capsule of dense connective tissue (Fig. 1b). White pulp was consisted of lymphoid nodules with the central artery located eccentrically. Lymphoid nodules of white pulp separated from red pulp with well visible marginal zone (Fig. 1c). Red pulp was composed of splenic cords and sinusoids. The spleen sections from rats treated with cisplatin only revealed obvious pathological changes, including disorganization of lymphoid follicles, hyperplasia in white pulp (Fig. 1d), depletion of lymphocytes in red pulp with edema, and some necrotic cells in white and red pulps (Fig. 1e). Increased hemosiderosis as well as fibrosis in the red pulp and some lymphoid follicles were also noticed. Additionally, dilated and congested blood vessels were observed (Fig. 1f). Thickening in the reticular fiber of the capsule and the trabeculae was also noticed (Fig. 1g). Immulant plus cisplatin rat group showed hypocellularity in the red pulp with the appearance of germinal center (Fig. 1h) and depletion of periarteriolar lymphatic sheath (Fig. 1i). Additionally, it decreased dilated and congested splenic vein and blood sinusoids, reduced hemosiderin deposition and restored the normal architecture of the tissue (Fig. 1j). Immulant-treated spleen simulated spleen of control group (Fig. 1k).
Fig. 1.
Histopathological findings on spleens from various experimental groups. a A section of spleen of the control group showing normal histological architecture into white (W) and red pulps (R) (H&E; ×100). b A section of spleen of the control group showing thin reticular fiber in the capsule (arrow) (Gomori’s reticulin; ×400). c A section of spleen of the control group showing white pulp (W) separated from red pulp (R) with well visible marginal zone (MZ) (H&E; ×400). d A section of spleen of the cisplatin-treated group showing disorganization of lymphoid follicles (H&E; ×400). e A section of spleen of the cisplatin-treated group showing depletion of lymphocytes in red pulp with edema (arrow), and some necrotic cells in white and red pulps (H&E; ×100). f A section of spleen of the cisplatin-treated group showing increasing of hemosiderosis, fibrosis (arrow) in the red pulp and some lymphoid follicles, dilated and congested blood vessels (BV) (Crossmon’s trichrome; ×400). g A section of spleen of the cisplatin-treated group showing thickening in the reticular fiber of the capsule and the trabeculae (Gomori’s reticulin; ×400). h A section of spleen of the cisplatin- and Immulant-treated group showing hypocellularity of the red pulp and germinal center (arrow). (H&E; ×100). i A section of spleen of the cisplatin- and Immulant-treated group showing depletion of periarterial lymphatic sheath (arrow). (H&E; ×100). j A section of spleen of the cisplatin- and Immulant-treated group showing decrease dilated and congested blood vessels (BV) and collagen fibers (arrow). (Crossmon’s trichrome; ×100). k A section of spleen of the Immulant-treated group showing white (W) and red pulps (R) (H&E; ×100)
Immunohistochemical observations
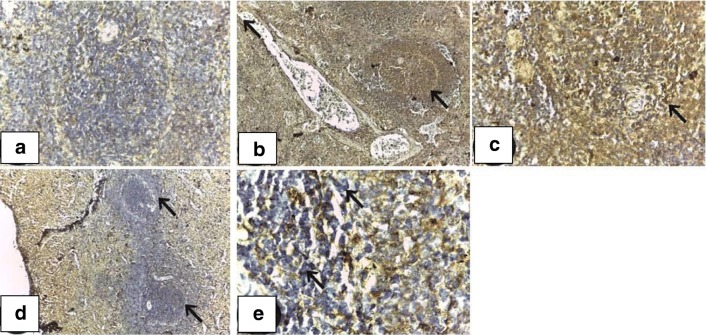
Negative caspase-3 immunohistochemical reactivity was observed in the spleen of control group (Fig. 2a).In cisplatin-treated group, intense immunoreaction in the white and red pulp revealed apoptotic events within the lymphoid follicles and to lesser extent, the periarteriolar lymphoid sheath (PALS) (Fig. 2b, c). Mild or negative immunoreactivity was detected in cisplatin- and Immulant-treated group (Fig. 2d). Immulant-treated group resembled control spleen (Fig. 2e).
Fig. 2.
Immunohistochemical profile of caspase-3 expression in spleen in various experimental groups. a A section of spleen of the control group showing negative caspase-3 immunoreactivity (caspase-3; ×100). b A section of spleen of the cisplatin-treated group showing intense caspase-3 reaction expressed in white and red pulp (caspase-3; ×100). c A section of spleen of the cisplatin-treated group showing intense caspase-3 reaction expressed in white and red pulp (caspase-3; ×400). d A section of spleen of the cisplatin-and Immulant-treated group showing mild to negative caspase-3 immunohistochemical expression (caspase-3; ×100). e A section of spleen of the Immulant-treated group showing negative caspase-3 immunohistochemical expression (caspase-3; ×1000)
Discussion
Immunotoxicity is the adverse effect of most cytotoxic chemotherapy on the immune system, prompting a suppression of the immune response. Cisplatin is one of the most effective drugs used for chemotherapy of various cancers. Nevertheless, the clinical utility of this drug is limited due to many adverse effects, including severe impact on the immune system. In this study, administration of cisplatin to mature rats resulted in marked immunotoxic effects represented by leukopenia, lymphocytopenia, neutrophilia as well as decrease in total protein, γ-globulin, antibody titers and phagocytic activity (percentage and index). Bone marrow is susceptible to damage by cytotoxic drugs since they cause neutropenia, lymphopenia, thrombocytopenia, and anemia [22]. Parket al. [23] revealed that cyclophosphamide used in various malignancies produced immunosuppressive effect as hematopoietic dysfunction, which manifests leukopenia, lymphopenia, anemia and thrombocytopenia. Our results were also supported by the previous data of Awadallah et al. [24] who indicated that administration of therapeutic doses of cisplatin to adult male rabbits resulted in leukopenia, lymphocytopenia, and neutrophilia.
Cisplatin induced immunosuppression and suppressed both humoral and cell-mediated immune response [25]. The hematotoxic effect of cisplatin may be attributed to its myelotoxicity as it binds with cellular DNA, affecting cell replication. Moreover, cisplatin produces reactive oxygen species and triggers apoptosis of the circulating blood and bone marrow cells [26]. This is in contrast with Markovic et al. [27] who reported that cisplatin increased the number of leukocytes due to the consequence of infection and inflammation.
In this study, exposure of male rats to cisplatin resulted in significant decrease in total protein and γ-globulin concentration. Based on a previous study, immunotoxic effect of cisplatin was manifested by significant decrease in antibody titers and phagocytic activity against M. gallisepticum [28]. Our results are supported by Jílek et al. [29] who reported reduction of γ-globulin in blood possibly due to the cytotoxic effect of cisplatin on the immunocompetent cells, especially B lymphocytes and plasma cells. Li et al. [30] showed that cisplatin can induce immunosuppressive effects through inhibition of T cell activity. Additionally, Kouchi et al. [31] reported that cisplatin blocked splenic T lymphocyte function more than splenic B lymphocyte function.
The reduction in antibody titers may be attributed to the direct effect of cisplatin on the immune system of rats. This speculation is supported by the histopathological examination of spleen of treated rats which revealed marked depletion and degeneration of lymphoid tissue. The immunotoxic effect of cisplatin was consistent with the mechanism of action of cisplatin which is a bifunctional alkylating agent. It is known to produce DNA strand cross-linking and subsequent inability of DNA to separate during cell division, resulting in cell death [32]. The current study noticed hyperplasia in the white pulp and depletion of lymphocytes in the red pulp in agreement with other investigators who observed reduced the extramedullary hematopoiesis in the red pulp with prominent apoptotic bodies in the lymphoid follicle [33–35]. Cytotoxic drugs suppress the immune response which can lead to diminished host resistance to infectious agents or tumor cells [36]. Immunotoxicity may parallel alterations in the weight of lymphoid organs and/or changes in the normal microscopic architecture [37]. However, splenic immunosuppression may attribute to the decreased lymphatic cells numbers in the spleen and other immune organs [37, 38]. Meneze sand colleagues [39] indicated that all extensive injuries were repaired with collagen fibers which may lead to the fibrosis as observed here. Crăciun and Paşca [40] reported that cisplatin had a significant toxic effect in both central (thymus) and peripheral (spleen) lymphoid organs.
Natural products, including bioactive phytochemicals, can augment the effectiveness of chemotherapeutic drugs and reduce their toxic effects. Natural herbal products can be used as immunomodulators to enhance the host defence mechanism against the immunotoxicity of chemotherapy. Such substances might provide immunoprotection against cisplatin toxicity [41]. Omega-3 fatty acids, such as eicosapentaenoic acid and docosahexaenoic acid, are polyunsaturated fatty acids. Omega-3 fatty acids have been found to exert immunomodulatory effects due to their ability to reduce interleukin-6 levels [42]. Glucans are present in the bran of grasses, such as barley, oats and wheat, and exhibit strong protective activities against the immunosuppressive effects of mutagenic agents, such as cisplatin. Glucans stimulate immune response through their ability to trap free radicals which are released during the biotransformation of mutagens [43]. Gallic acid, a triphenolic compound present in various fruits and plants, has been considered as an effective antioxidant agent. Gallic acid has been found to enhance the immune function against the immunotoxicity of cisplatin [44].
This study investigates the protective effect of Immulant (an herbal formulation containing an extract of medicinal plant E. purpurea) against the immunotoxicity of cisplatin in mature male rats. Animals that received cisplatin plus Immulant showed significant protective effect against hemotoxic and immunotoxic effect of cisplatin. These protective effects were manifested by increased percentage of total leukocytes, lymphocytes and decreased percentage of neutrophil. Immulant is frequently used as a dietary supplement to improve the function of immune system. It is used as herbal medicine to treat malignant tumors, respiratory infections, and various other inflammatory conditions [45]. Immulant is known to activate non-specific cellular and humoral immunity as well as the complement system [46]. Immulant has also been found to stimulate the immune system by increasing the production and activation of leukocytes, lymphocytes, monocytes, and cytokines [47].
The immunostimulation of Immulant may be linked to its polysaccharide fraction which has been found to stimulate the activity of macrophage and several other functions linked to cytokine production [48]. Various phenolic compounds and alkamides were found to exhibit antiviral and antifungal properties [49]. Immulant contains alkamides, caffeic acid esters, polysaccharide, and polyacetylenes [50]. Purified polysaccharide isolated from E. purpurea extract elicited an immuno-stimulatory effect when immune cells were exposed to this natural agent [51]. Several animal and human studies reported stimulation of neutrophil and macrophage phagocytic functions by E. purpurea [46].
Co-administration of Immulant with cisplatin induced marked improvement in the histopathological and histochemical changes in the spleen of treated rats. This observed effect may be due to the antioxidant properties of various constituents (e.g., polysaccharides and flavonoids) present in the E. purpurea extract.
The precise mechanisms of the observed immunoprotective effects of Immulantare not known at this time and require additional in-depth investigation. Various phytoconstituents present in E. purpurea, such as caffeic acid derivatives, alkamides, flavonoids, essential oils and polyacetylenes, are known to activate the non-specific cellular and humoral by increasing the production and activation of leukocytes, lymphocytes, monocytes and cytokines [52]. These components also modulate the immune response by macrophage phagocytosis, pro-inflammatory cytokine production, activation of NK cell activity, enhancement of B cell response, increased T cell proliferation and elevated production of T cell cytokines [52]. Hence, it is plausible that Immulant may confer immunoprotection through multifactorial immunomodulatory effects which could be achieved through chemical synergy of various bioactive constituents.
Conclusions
In conclusion, the results of this study showed that cisplatin treatment induced a substantial immune damage in rats and pretreatment with Immulant provided protective effect against cisplatin-induced immunotoxicity. However, further clinical investigation is warranted to understand the full potential of Immulant as an adjunct to cisplatin therapy.
Acknowledgements
The authors express their sincere thanks to Prof. Sheren Ghalebfor review of the manuscript.
Authors’ contributions
All authors participated in the design, interpretation of the study, analysis of the data, and review of the manuscript.
Compliance with ethical standards
Ethics approval
All animal studies were performed in accordance with the procedure approved by the Institutional Animal Care and Use Committee of Cairo University (IACUC, CU-II-F-10-19).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amr Reda Zaki, Email: amrzaki2030@yahoo.com.
Anupam Bishayee, Email: abishayee@lecom.edu, Email: abishayee@gmail.com.
References
- 1.Baek SM, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK. Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J Lab Clin Med. 2003;142:178–186. doi: 10.1016/S0022-2143(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 2.Hasaaan I, Chibber S, Naseem I. Ameliorative effect of riboflavin on the cisplatin induced nephrotoxicity and hepatotoxicity under photoillumination. Food Chem Toxicol. 2010;48:2050–2058. doi: 10.1016/j.fct.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000;57:1229–1235. doi: 10.1007/PL00000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zandvoort A, Lodewijk ME, Klok PA, Dammers PM, Kroese FG, Timens W. Slow recovery of follicular B cells and marginal zone B cells after chemotherapy: implications for humoral immunity. Clin Exp Immunol. 2001;124:172–179. doi: 10.1046/j.1365-2249.2001.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onsrud M, Bosnes V, Grahm I. Cis-platinum as adjunctive to surgery in early stage ovarian carcinoma: effects on lymphoid cell subpopulations. Gynecol Oncol. 1986;23:323–328. doi: 10.1016/0090-8258(86)90133-2. [DOI] [PubMed] [Google Scholar]
- 6.Lee TT, Chen CL, Shieh ZH, Lin JC, Yu B. Study on antioxidant activity of Echinacea purpurea L. extracts and its impact on cell viability. Afr J Biotechnol. 2009;8:5097–5105. [Google Scholar]
- 7.Matthias A, Banbury L, Bone KM, Leach DN, Lehmann RP. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008;79:53–58. doi: 10.1016/j.fitote.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Chen L, Huang G, He D, He J, Xu W, Zou C, Zong F, Li Y, Chen B, Wu S, Zhao W, Wu J. Klotho sensitizes human lung Cancer cell line to cisplatin via PI3k/Akt pathway. PLoS One. 2013;8:573–591. doi: 10.1371/annotation/5fa9cfb4-9964-4586-845d-d8205f318d68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai Z, Liu Y, Wu L, Senchina DS, Wurtele ES, Murphy PA, Kohut ML, Cunnick JE. Enhancement of innate and adaptive immune functions by multiple Echinacea species. J Med Food. 2007;10:423–434. doi: 10.1089/jmf.2006.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paget GE, Barnes JM. Toxicity tests. In: Laurence DR, Bachorach AL, editors. Evaluation of drug activities: Pharmacomertrics. New York: Academic Press; 1964. pp. 133–166. [Google Scholar]
- 11.Zink T, Chaffin J. Herbal ‘Health’ products: what family physicians need to know. Am Fam Physician. 1998;58:1133–1140. [PubMed] [Google Scholar]
- 12.Schalm OW, Jain NE, Caroll EJ. Veterinary haematology. 3. Philadelphia: Lea and Febiger; 1975. [Google Scholar]
- 13.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 14.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan A, Saveloy J. Evaluation of a cellulose-acetate electrophoresis system for serum fractionation. Clin Chem. 1965;11:937. [PubMed] [Google Scholar]
- 16.Anthony TWC, Twin KML, Erin MW, Michael EM. Phagocytic and killing capacities of uterine derived leukocytes from mares resistant and susceptible to chronic endometritis. Am J Vet Res. 1985;46:1938–1940. [PubMed] [Google Scholar]
- 17.Chu Y, Dietert RR. Monocyte function in chicken with hereditary dystrophy. J Poult Sci. 1989;68:226–232. doi: 10.3382/ps.0680226. [DOI] [PubMed] [Google Scholar]
- 18.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl. 1968;21:77–89. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- 19.Hanks DH, Waalace OH. Determination of cell viability. Proc Soc Exp Biol Med. 1985;98:183–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- 20.Bancroft JD, Gamble M. Theory and practice of histological techniques. 6. Edinburgh, London and New York: Churchill Livingston; 2007. [Google Scholar]
- 21.Ramos-Vara JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42:405–426. doi: 10.1354/vp.42-4-405. [DOI] [PubMed] [Google Scholar]
- 22.Wijermans PW, Gerrits WB, Haak HL. Severe immunodeficiency in patients treated with fludarabine monophosphate. Eur J Haematol. 1993;50:292–296. doi: 10.1111/j.1600-0609.1993.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 23.Park KP, Lee BC, Lee JS, Cho MH. Angelica gigas Nakai extract ameliorates the effects of cyclophosphamide on immunological and hematopoietic dysfunction in mice. J Med Plant Res. 2014;8:657–663. doi: 10.5897/JMPR2013.5288. [DOI] [Google Scholar]
- 24.Awadallah AM, Moussa FI, Abad El Hameed N. Treatment of cisplatin Haematotoxicity with Lasix or selenium or both in adult male rabbits. Pak J Biol Sci. 2001;4:89–93. doi: 10.3923/pjbs.2001.89.93. [DOI] [Google Scholar]
- 25.Pieretti M, Hopenhayn-Rich C, Khattar NH, Cao Y, Huang B, Tucker TC. Heterogeneity of ovarian cancer: relationships among histological group, stage of disease, tumor markers, patient characteristics, and survival. Cancer Investig. 2002;20:11–23. doi: 10.1081/CNV-120000361. [DOI] [PubMed] [Google Scholar]
- 26.Gale RP. Antineoplastic chemotherapy myelosuppression: mechanisms and new approaches. Exp Hematol. 1985;13:3–7. [PubMed] [Google Scholar]
- 27.Markovic SD, Zˇizic JB, Djacic DS, Obradovic AD, MG C’u, Cvetkovic DM, Ordevic NZ, Ognjanovic BA, ˇStajn AS. Alteration of oxidative stress parameters in red blood cells of rats after chronic in vivo treatment with cisplatin and selenium. Arch Biol Sci. 2011;63:991–999. doi: 10.2298/ABS1104991M. [DOI] [PubMed] [Google Scholar]
- 28.Bhoopendra K, Nitesh K. Immunotoxicity of lambda-Cyhalothrin in Wistar albino rats. International Journal of Toxicological and Pharmacological Research. 2014;6:47–56. [Google Scholar]
- 29.Jílek P, Dvoráková J, Turecková J, Procházková J. The effect of platinum cytostatics on delayed-type hypersensitivity in mice. Neoplasma. 1989;36:659–665. [PubMed] [Google Scholar]
- 30.Li XB, Schluesener HJ. Therapeutic effects of cisplatin on rat experimental autoimmune encephalomyelitis. Arch Immunol Ther Exp. 2006;54:51–53. doi: 10.1007/s00005-006-0005-3. [DOI] [PubMed] [Google Scholar]
- 31.Kouchi Y, Maeda Y, Ohuchida A, Ohsawa M. Immunotoxic effect of low dose cisplatin in mice. J Toxicol Sci. 1996;21:227–233. doi: 10.2131/jts.21.4_227. [DOI] [PubMed] [Google Scholar]
- 32.Hegab S, MutawaS A. Immunopathogenesis of Bebeet′s disease. Clin Immunol. 2000;96:174–186. doi: 10.1006/clim.2000.4901. [DOI] [PubMed] [Google Scholar]
- 33.Kuper CF, Schuurman HJ, Vos JG. Pathology in immunotoxicology. In: Burleson GR, Dean JH, Munson AE, editors. Methods in immunotoxicology. New York: Wiley-Liss; 1995. pp. 397–436. [Google Scholar]
- 34.Kuper CF, Harleman JH, Richter-Reichhelm HB, Vos JG. Histopathologic approaches to detect changes indicative of immunotoxicity. Toxicol Pathol. 2000;28:454–466. doi: 10.1177/019262330002800317. [DOI] [PubMed] [Google Scholar]
- 35.Kuper CF, deHeer E, Van Loveren H, Vox JG. Immune system. In: Haschek W, Rousseaux CG, Wallig MA, editors. Handbook of toxicological pathology. San Diego: Academic Press; 2002. pp. 585–646. [Google Scholar]
- 36.Descotes J. Methods of evaluating immunotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:249–259. doi: 10.1517/17425255.2.2.249. [DOI] [PubMed] [Google Scholar]
- 37.Pearse G, Pietersma A, Cunliffe J, Foster JR, Turton J, Derbyshire N, Randall KJ. Time-course study of the immunotoxic effects of the anticancer drug chlorambucil in the rat. Toxicol Pathol. 2009;37:887–901. doi: 10.1177/0192623309347907. [DOI] [PubMed] [Google Scholar]
- 38.Monfared AL, Jaafari A, Sheibani M. Histological and histometrical evidences for phenol immunotoxicity in mice. Comp Clin Pathol. 2014;23:529–553. doi: 10.1007/s00580-012-1645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, Zin WA, Rocco PR. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol. 2005;98:1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 40.Crăciun C, Paşca C. Structural and ultrastructural data on side effects of cisplatin in spleen, kidney and liver of rats. Acta Metallomica – MEEMB. 2014;11:9–22. [Google Scholar]
- 41.Tuorkey MJ. Cancer therapy with phytochemicals: present and future perspectives. Biomed Environ Sci. 2015;28:808–819. doi: 10.1016/S0895-3988(15)30111-2. [DOI] [PubMed] [Google Scholar]
- 42.Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, et al. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14:2434–2439. doi: 10.3748/wjg.14.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani MS, Bellini MF, Angeli JP, Oliveira RJ, Silva AF, Ribeiro LR. Beta-glucans in promoting health: prevention against mutation and cancer. Mutat Res. 2008;658:154–161. doi: 10.1016/j.mrrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Badhani B, Sharma N, Kakkar R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Advances. 2015;5:27540–27557. doi: 10.1039/C5RA01911G. [DOI] [Google Scholar]
- 45.Barrett B. Medicinal properties of Echinacea: a critical review. Phytomedicine. 2003;10:66–86. doi: 10.1078/094471103321648692. [DOI] [PubMed] [Google Scholar]
- 46.Cundell DR, Matrone MA, Ratajczak P, Pierce JD., Jr The effect of aerial parts of Echinacea on the circulating white cell levels and selected immune functions of the aging male Sprague-Dawley rat. Int Immunopharmacol. 2003;3:1041–1048. doi: 10.1016/S1567-5769(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 47.Kim LS, Waters RF, Burkholder PM. Immunological activity of larch arabinogalactan and Echinacea: a preliminary, randomized, double-blind, placebo-controlled trial. Altern Med Rev. 2002;7:138–149. [PubMed] [Google Scholar]
- 48.Echinacea BR. Biological effects and active principals, phytomedicines of Europe: chemistry and biological activity. In: Lawson LD, Bauer R, editors. ACS symposium series. Washington, DC: American Chemical Society; 1998. pp. 140–157. [Google Scholar]
- 49.Chen Y, Fu T, Tao T, Yang I, Chang Y, Kim L. Macrophage activating effect of new alkamides from the roots of Echinacea species. J Nat Prod. 2005;68:773–776. doi: 10.1021/np040245f. [DOI] [PubMed] [Google Scholar]
- 50.Merali S, Bnns S, Paulin-Levasseur M, Ficker C, Smith M, Baum BR. Antifungal and anti-inflammatory activity of the genus Echinacea. Pharm Biol. 2003;41:412–420. doi: 10.1076/phbi.41.6.412.17828. [DOI] [Google Scholar]
- 51.Wills RB, Bone K, Morgan M. Herbal products: active constituents, modes of action and quality control. Nutr Res Rev. 2000;13:47–77. doi: 10.1079/095442200108729007. [DOI] [PubMed] [Google Scholar]
- 52.Thygesen L, Thulin J, Mortensen A, Skibsted LH, Molgaard P. Antioxidant activity of cichoric acid and alkamides from Echinacea purpurea, alone and in combination. Food Chem. 2007;101:74–81. doi: 10.1016/j.foodchem.2005.11.048. [DOI] [Google Scholar]