Abstract
Objective
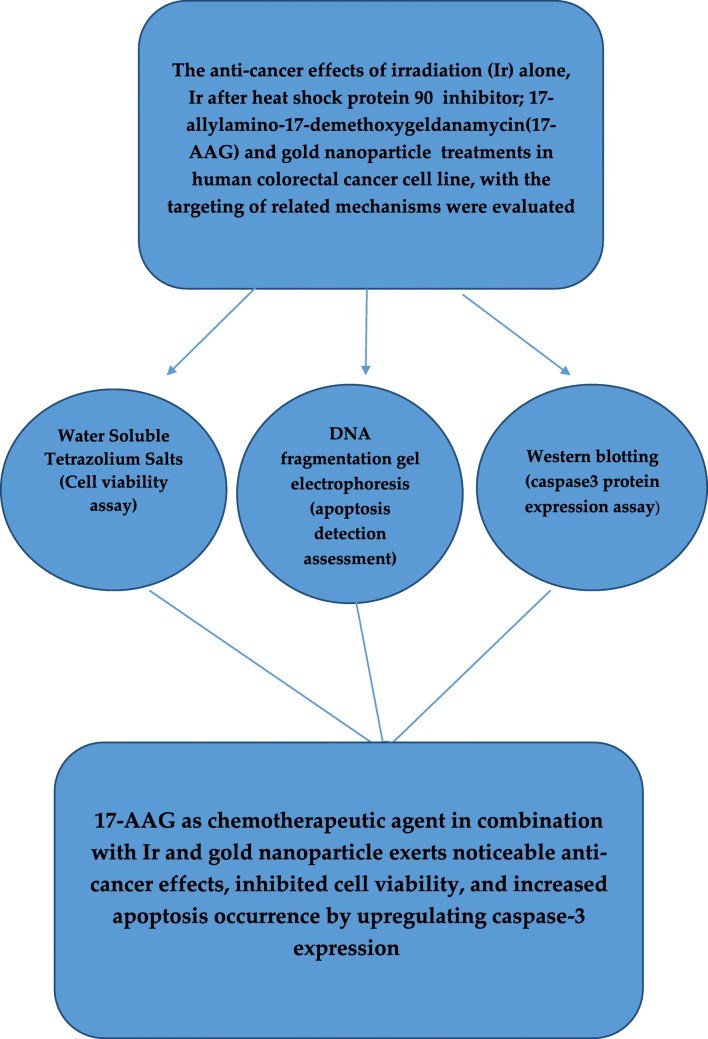
The present study evaluated the anti-cancer effects of irradiation (Ir) alone, Ir after heat shock protein 90 inhibitor; 17-allylamino-17-demethoxygeldanamycin (17-AAG) and gold nanoparticle (GNP) treatments in human colorectal cancer cell line (HCT-116), with the targeting of related mechanisms.
Methods
Water-soluble tetrazolium salt-1 assay was utilized to study the cytotoxic effects of 17-AAG, GNP, Ir in single and combination cases on the cell viability of HCT-116 cells. The cells were examined with DNA fragmentation electrophoresis and evaluated for apoptosis induction. Caspase-3 expression as a critical apoptosis element in protein level was detected by western blotting.
Results
Treatment with 17-AAG in a dose dependent manner for 24 h inhibited the cellular viability of HCT-116 cells. GNP at a dose of 70 μM had the lowest cytotoxic effects and was thus selected for combination treatment studies. Based on the results, GNP at a dose of 70 μM did not have a significant effect on cellular viability of HCT-116. In contrast, the evaluation of double and triple combinations, GNP with Ir (2 Gy of 6 MV X-ray radiation) and 17-AAG in double combinations induced significant cytotoxicity. Both DNA damage pattern and caspase-3 protein upregulation were present in Ir,GNP/17-AAG,GNP and Ir,17-AAG combinations compared to single treatments. Furthermore, in the three combination of GNP,Ir,17-AAG, radiosensitization effects (increased caspase-3 expression) occurred with a minimum concentration of 17-AAG.
Conclusion
According to the results of this study, 17-AAG as chemotherapeutic agent in combination with Ir and GNP exerts noticeable anti-cancer effects, inhibited cell viability, and increased apoptosis occurrence by upregulating caspase-3 expression. It is suggested that these combinations should be more evaluated as a promising candidate for colorectal cancer treatment.
Graphical abstract.
Anti-cancer effects of chemotherapeutic agent; 17-AAG, in combined with gold nanoparticles and irradiation in human colorectal cancer cells
Keywords: Colorectal cancer, HCT-116 cells, Irradiation, 17-AAG, Gold nanoparticle
Introduction
Colorectal cancer is a major cause of cancer incidence and mortality. Recently, numerous drug combinations have been used in its treatment, which has led to better response rates; however, drug resistance is unavoidable [1]. Radiotherapy has been used as one of the usual therapeutic strategy in the treatment of various cancers including colorectal cancer. Although appropriate response toward radiotherapy is achieved in the primary phase of cancer treatments, large numbers of patients have encountered cancer radiation resistance [2]. The evaluations of radiation oncology based on methods that improve the radio sensitizing effects are applicable. One of the major problems of radiotherapy is its lack of selectivity. Therefore, the application of metal nanoparticles may assist in increasing the contrast between the tumor and soft tissues, and subsequently provide radiosensitizing effects [3]. The application of gold nano particle (GNP) in radiotherapy may enhance treatment outcomes. According to previous research, the use of GNP in radiotherapy is to increase radiation dose against cancer cells. Indeed, in the study of colorectal cancer cells, the synergistic effects of PEGylated GNP in kilo-voltage & mega-voltage radiation were presented [4]. GNP may act as a bio-inert agent in colorectal cancer cells by increasing its radiosensitization [5]. On the other hand, chemotherapy is a common treatment for colorectal cancer in patients with lymph node involvement and metastatic cases. Chemotherapy resistance persists as a major challenge in the cure of metastatic cancers [6].
Apoptosis is a process of programmed cell death that happens in different cell phases. Apoptosis plays a key role in the evaluation of response to the number of cytotoxic and tumor cell destruction [7]. Caspases are considered as crucial apoptosis mediators. Among them, caspase-3 is the main protease activator and plays a major role in programmed cell death (apoptosis) [8]. In colorectal cancer growth, the balance among the cell growth and apoptosis rates is impaired gradually. Current report showed that impaired apoptosis might be a main factor in the development of colorectal cancer and its unsuccessful response to chemotherapy and radiation [9].
Novel chemotherapeutic treatment strategies including combination therapies may be effective in colorectal cancer treatment [10]. Heat shock protein 90 (HSP90) inhibitors are considered as an efficient therapeutic approach in cancer targeted therapy [11]. 17-allylamino-17-demethoxygeldanamycin (17-AAG) is a HSP90 inhibitor that exerts cytotoxic and apoptotic effects on cancer cells [12, 13].
According to a related study, blocking HSP90 inhibits the invasive characteristics of colon cancer cells [14]. In this regards, cytotoxic effects of 17-AAG in combination with other chemotherapeutic agents were studied in few previous surveys in colorectal cancer cells [15–17]. Nonetheless, based on our knowledge, there are not any studies which evaluated the effects of 17-AAG in combination with other anti-cancer therapies including radiotherapy and GNP (as possible radio-sensitizer and chemo-sensitizer) in colorectal cancer cells. Therefore, we design the cases of toxicity evaluation after the administration of 17-AAG and GNP in combination with irradiation (Ir) in colorectal cancer cells in relation to cancer cell death and apoptosis regulation.
Indeed, this study aimed to investigate the effectiveness of in vitro gold-based nanoparticle radiosensitization in combination with 17-AAG on the HCT-116 human colorectal cancer cell line.
Materials and methods
Experimental design
In the present study, we evaluate the anti-cancer activity of a newly developed chemotherapeutic agent; 17-AAG and GNP in combined treatments with Ir in human colorectal cancer cell line (HCT-116), with the targeting of possible mechanisms. We assess the therapeutic potential of these combinations in different concentrations of 17-AAG. The Ir dose (2 Gy of 6 MV X-ray radiation) were similar in all single and combined treatments. After plotting dose response curve, we selected the GNP concentration, which had minimal cytotoxic effects versus to other examined concentrations to decrease primary cytotoxicity of GNP alone. Also, low concentration of 17-AAG selected for these purpose in combinatorial cases (In-vitro). Cell viability was determined using Water-soluble tetrazolium salt-1 (WST-1) assay in HCT-116 different treatments. As a mechanistic approach, we looked at the role of apoptosis pathway, which is critically involved in the cell death. The apoptosis pathway induction was evaluated by different methods including DNA fragmentation using gel electrophoresis, and caspase-3 protein expression assay.
Chemicals and drugs
Human colorectal cancer cell line, HCT-116 provided by Pasteur institute (Iran, Tehran). Cells were maintained at 37 °C in incubator with 5% CO2 in Dulbecco’s Modified Eagle’s Medium, 10% fetal bovine serum and 1% Pen-Strep.
GNP was purchased from R&D department of Nanobon Company (Tehran, Iran). This company prepared folic-acid-GNP, with this procedure; an aqueous solution of HAuCl4 (Au; ppm) and trinatrium citrate was heated and vigorously stirred for about two hours until the color of the solution turned to deep red. After the solution cooled, a solution of folic-acid (in ethanol) was added under ultrasonic probe and sonicated. The formation of folic-acid - GNP was verified by spectrophotometer, transmission electronic microscope, Fourier transform infrared spectroscopy .
Prior to the administration of different concentrations of 17-AAG (40, 25, 20, 10, 5, 2.5 and 1 nM), GNP (200, 100, 70 and 35 μM), 1 × 104 HCT-116 cells were plated for 24 h.
Based on Chou reports [18], dose-effect curves could be plotted for each drug alone. Indeed each drug has a different potency [the Dm (IC50) value] and different shape of the dose-effect curve.
The Dm (IC50) can be obtained from the median-effect equation using computer software (CompuSyn) [18].
To calculate IC50, we used the series of dose-effect data (17-AAG concentrations; 40, 25, 20,10,5, 2.5 and 1 nM) and growth inhibition were evaluated. IC50 will correspond to the concentration of 17-AAG that leads to a loss of 50% of cellular viability. IC50 of 17-AAG was calculated with the CompuSyn.
For the assessment of cytotoxic effects of GNP and 17-AAG, dose response curve was plotted based on the results obtained from cell viability assay [(WST-1)TAKARA(Japan)]. From the dose response curve, GNP at a dose of 70 μM had the lowest toxicity effects on treated cells. This dose (70 μM) was considered for further analysis in combination cases.
1 × 104 HCT-116 cells were irradiated at a single dose rate of 2 Gy in a field size of 10 cm × 15 cm and source-to-surface distance of 100 cm at room temperature with a clinical linear accelerator machine (Elekta Compact 6 MV) at the Radiotherapy Unit, Emam Khomeini Hospital, Urmia, Iran. A Plexiglass sheet (1 cm) (water equivalent) was placed on the upper surface of the plate.
From the WST-1 results,the lower concentrations of 17-AAG (9.45 nM) in double combination and 4.72 nM in triple combinations with GNP (70 μM) were chosen and exposed to radiation(2 Gy of 6 MV X-ray radiation) for further analysis by western blotting and DNA fragmentation electrophoresis.
DNA fragmentation using gel electrophoresis
DNA fragmentation assessment was performed for HCT-116 cell cultures treated with 17-AAG, GNP and Ir in single, double and triple combinations for various treatment cases during 24 h as described. 1 × 106 cells were seeded in 6-well plates. Then, for the analysis of DNA fragmentation, using gel electrophoresis, we utilized the protocol described by Samarghandian et al. [19] with some modifications. Cells were trypsinized and washed with phosphate-buffered saline. Additionally, floating cells were calculated. In order to extract DNA, lysates were prepared using lysis buffer (10 mM Tris (pH 7.4), 150 mM NaCl, 10 mM ethylenediaminetetraacetic acid and 0.5% Triton X-100). Then lysates were mixed and centrifuged. DNA was isolated by phenol:chloroform:isoamyl alcohol and detected by 1.5% agarose gel electrophoresis technique under UV light.
Statistical analysis
The one-way ANOVA analysis of variance was used to analyse the cytotoxicity, and caspase3 protein expression levels. Data was presented as mean ± standard deviation of different independent experiments. For multiple comparison between groups, Post Hoc Tests [Tukey (HSD)] was used. Statistical differences were considered at p < 0.05.
Results
Impacts of Ir, 17-AAG and GNP in single and combination treatments cases on cell viability of HCT-116
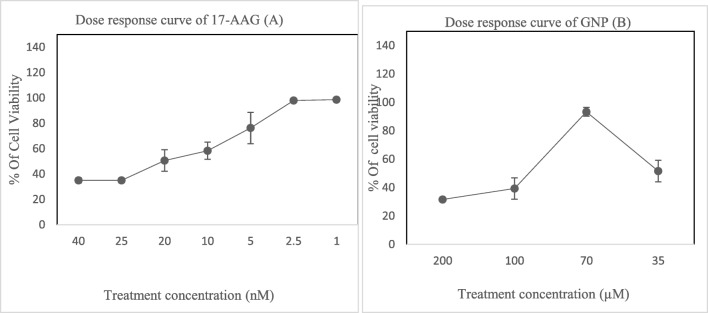
In order to study the cytotoxicity, cell viability was detected by WST-1 assay (treatment with 17-AAG at 40, 25,20,10,5,2.5 and 1 nM concentrations) after 24 h. Cytotoxic effects were presented in a dose dependent manner in HCT-116 cells. GNP cytotoxic effects on the HCT-116 were evaluated in 35, 70, 100, and 200 μM at 24 h. From the results of this study, WST-1 analyses showed that at 70-μM concentration of GNP, there were lower cytotoxic effects compared to other examined doses. The dose-response curve of 17-AAG and GNP are presented in Fig. 1.
Fig. 1.
Cytotoxic effects of 17-AAG (a) and GNP (b) based on WST-1 test in single exposures with different concentrations (24 h) in HCT-116 cells. Data represented as mean ± standard deviation
IC50 value of 17-AAG determined based on dose response curves from the WST-1 test and calculated using CompuSyn software. The amount of IC50 of 17-AAG after 24 h was 18.66 ± 1.96 nM.
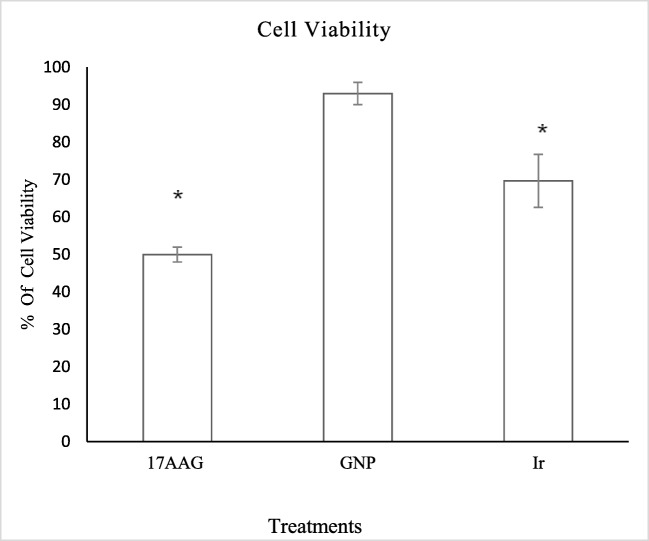
Results from WST-1 assay presented in Fig. 2 show that after 24 h, there was significant differences in cell viability between single Ir treated cells and control (p < 0.05). At a concentration of 70 μM GNP, there was no significant difference in comparison to the control (p > 0.05).
Fig. 2.
Results of WST-1 from single treatments including 17-AAG in concentration of 18.9 nM, GNP in 70 μM and x-Ray Ir in 2 Gy. *significant differences in compared to untreated control cells (p < 0.05)
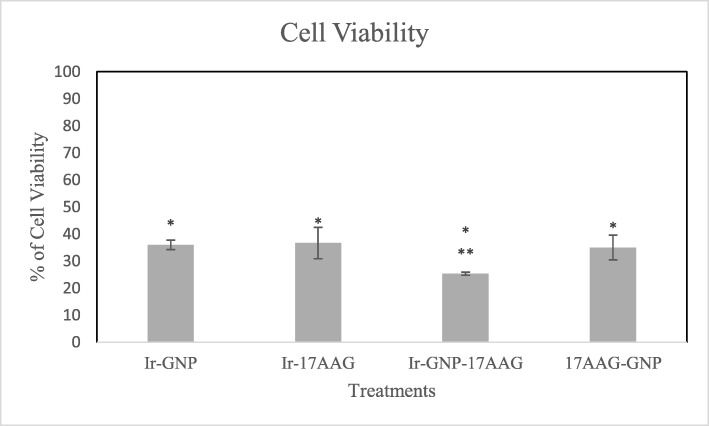
As presented in Fig. 3, in the study of combination cases, data indicated that Ir,GNP and Ir,17-AAG combinations induced higher cytotoxic effects compared to single treatments (p < 0.05). Therefore, it could be concluded that at low concentrations, 17-AAG (9.45 nM) and GNP (70 μM) act as radiosensitizers in combination with Ir. Also, in the case of 17-AAG,GNP significant decreased cellular viability was observed as compared to single treatments (p < 0.05).
Fig. 3.
WST-1 results (Cell viability assay) from double treatments (GNP,Ir/Ir,17-AAG/ GNP,17-AAG in the concentration of 70 μM of GNP, 9.45 nm of 17-AAG and x-Ray Ir in 2 Gy) and triple combination of Ir,GNP,17-AAG (in the concentration of 70 μM of GNP, 4.72 nm of 17-AAG and x-Ray Ir in 2 Gy). * Significant differences in compared to single drug treatments (p < 0.05). ** Significant differences in compared to double treatments [Ir,GNP/ Ir,17-AAG (p < 0.05)]
In triple combination of GNP,Ir,17-AAG after 24 h, 17-AAG at concentration of 4.72 nM resulted in higher cellular viability inhibition compared to the double combination of 17-AAG at 9.45 nm (Ir,17-AAG). Furthermore, the anti-proliferative effects of GNP (70 μM) with x-ray Ir double combination were lower than GNP,Ir,17-AAG in triple combination (p < 0.05).
DNA fragmentation assay of HCT-116 exposed to 17-AAG, Ir and GNP in single and combination treatment cases of HCT-116
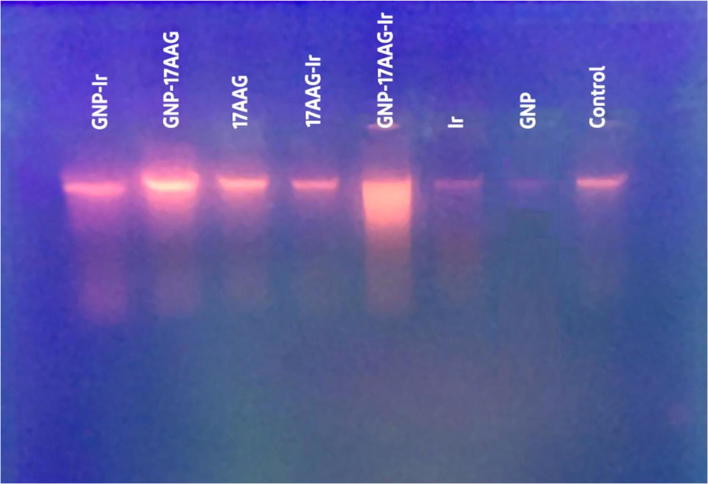
To evaluate the possible cell death mechanism of Ir, GNP and 17-AAG in different combinatorial treatments, DNA fragmentation test, as an apoptosis detection method was used. The cells were treated for 24 h and then, the extracted DNA was assessed on agarose gel electrophoresis. According to our results, the patterns of DNA damage (Fig. 4) was detected in cells after 24 h of various combinatorial treatments including double combinations (Ir,17-AAG/Ir,GNP/GNP,17-AAG) and triple combination (Ir,GNP,17-AAG). Our findings suggest that 17-AAG and GNP could be considered as radiosentisizers, which induce DNA damage and apoptosis in HCT-116 cells.
Fig. 4.
Results of DNA fragmentation gel electrophoresis in HCT-116 (treated to 17-AAG,Ir and GNP in single and combined cases). HCT-116 cells treated with 17-AAG (18.9, 9.45 and 4.72 nM concentrations in single, double and triple treatments respectively), GNP in 70 μM and 2Gy of x-Ray Ir for 24 h. DNA fragmentation pattern was evaluated on gel (1.5%) and visulized by UV
Western blot analysis of caspase-3 protein expression of HCT-116 exposed to 17-AAG, Ir and GNP in single and combination treatments
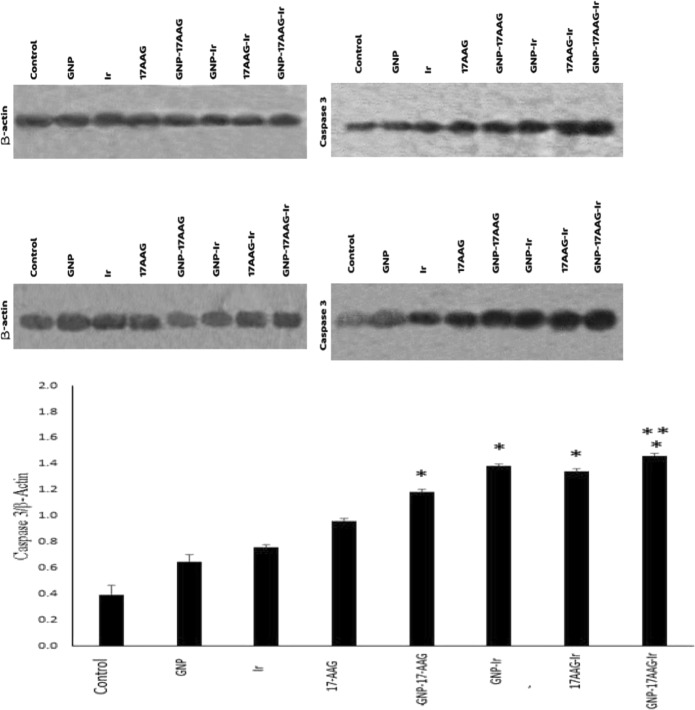
Caspase-3 protein was prepared in cell homogenates and evaluated by western blotting analysis for protein expression determination. Results from the western blotting assay are presented in Fig. 5.
Fig. 5.
Effect of 17-AAG, GNP and Ir in single and combined cases on the expression of caspase-3 in HCT-116 cells. In treatments including 17-AAG, the 18.9 nM for single treatment, the 9.45 nM for double and 4.72 nM for triple combination treatments were used. Results shown as caspase-3 to β-actin ratio (mean ± standard deviation) of separate tests. The beta-actin utilized to normalize the equal volume of protein loaded. Presented data is representative of two separate tests. *significant differences to single treatments(p < 0.05). ** significant differences to double combination [GNP,17-AAG (p < 0.05)]
A one-way ANOVA analysis of variance and Post hoc tests to analyses the caspase-3 protein expression levels were performed and data shows that after 24 h, there were significant increased caspase-3 protein levels in all single, double and triple combinations of Ir, GNP and 17-AAG compared to the untreated control cells (p < 0.05). According to our results, caspase-3 protein expression was induced by 17-AAG and GNP as radiosentisizer agents in double and triple combinations after 24 h.
Indeed, in all double combinations, caspase-3 expression levels (Ir,17-AAG/Ir,GNP/GNP,17-AAG) increased significantly in comparison to each single treatment (p < 0.05).
Triple combination treated cells (GNP at a dose of 70 μM in combination with 17-AAG at 4.72 nM, and 2 Gy of 6 MV X-ray Ir) had higher caspase-3 levels in compared to double combinations cases including Ir,17-AAG and Ir,GNP (p > 0.05). In addition, elevated caspase-3 level in triple combination had significant difference versus GNP, 17-AAG treated cases (p < 0.05).
Among the double combinations, there was higher caspase-3 upregulation in the case of GNP, Ir compared to GNP,17-AAG (P = 0.05). Further analysis showed that Ir, GNP, 17-AAG triple combination had higher caspase-3 levels compared to each single treatment (p < 0.05).
Discussion
In the current study, we showed cell death and apoptosis induction with 17-AAG and GNP in combination with Ir in human colorectal cancer cell line. Based on our findings, the low concentrations of 17-AAG in double and triple combinations led to higher cellular toxicity, caspase-3 upregulation and subsequently potential apoptosis induction. Definitely, these cellular toxic effects in HCT-116 are most likely through the activation of caspase-3 as the major apoptosis element.
To evaluate the effects of Ir and 17-AAG combined to GNP, we selected the concentration of GNP which exhibited minimal inherently cytotoxicity in single treatment. We assumed GNP as sensitizer agent in radio and chemotherapy with Ir and 17- AAG respectively.
Previous data reported that, GNP increases the sensitivity of biomolecules to radiation by changing the sensitivity of the objective biomolecules [20].
According to a previous study, 17-AAG as HSP90 inhibitor in HeLa cells increased radiosensitivity [21] and this was in agreement with our findings. In this study, the combination of 17-AAG at a low dose led to higher cytotoxic effects and apoptosis induction compared to single treatments.
Musha et al. indicated that 17-AAG with x-rays exerted synergistic effects on cell toxicity by weakening the G2/M inhibition promoted by x-rays [22] which is also in agreement with this study.
Additionally, in another study, combined exposures to 17-AAG and celecoxib increased the treatment effectiveness of ionizing radiation in cancer cells including colon cancer cells [23]. According to our results, 17-AAG induces caspase-3 expression and exerts higher cytotoxicity when administered with Ir. it seems that, 17-AAG and Ir acts in cooperative manner.
It could be said that, 17-AAG that inhibit HSP90, could increase HCT-116 cell susceptibility against Ir. This data displays that altered HSP90 function exerted colorectal cancer cell cytotoxicity and radiosensitization, proposing a potential therapeutic efficacy of 17-AAG in combination with Ir.
In a double combined treatment of Ir,GNP we obtained higher cytotoxicity effects and caspase-3 protein expression compared to single Ir. Therefore, our data show the radiosensitizing effects of GNP when combined with Ir.
In a similar study, the injection of Tio-GNPs to colorectal tumors in combination with x-rays (source was at 4 mA, 80 kV) decreased cell growth. Also, the use of tiopronin-coated GNPs (Tio-GNPs) with radiation led to decreased cell viability in HCT-116 [24]. The results of this study are in agreement with us.
Other researchers evaluated the impacts of GNP on radiosensitivity of colorectal cancer cell line (HT-29) when used with 18 MV x-rays. This data confirmed the possible effectiveness of GNPs with radiation therapy by utilizing high MV photons [25]. It has also been reported that the effects of GNP on HT-29 colorectal cancer is related to increased radiosensitization of MV X-ray energy. The radiosensitizing effects may be because of enhanced absorbed Ir dose [5].
Nowadays, theoretical reports have shown that GNP can be considered as radiosensitizers to improve radiotherapy outcomes due to photon-induced emission of photo-/Auger electrons [26]. Reports have also indicated that the effects of GNP radiosensitization are modulated by mitochondrial function [27]. A previous study established that GNP had noticeable effects on mitochondry, as manifested by the oxidation of mitochondrial membrane protein oxidation, cardiolipin and interruption of mitochondrial membrane [28]. Based on these data, and the fact that the depolarisation of mitochondria is related to cellular deterioration, including apoptosis [28], it could be related to describe our findings on apoptosis induction as shown in the Ir,GNP double combination.
Overall, in the present study, we examined whether combined treatment with 17-AAG and GNP could increase the radiosensitivity of HCT-116 cells. To the best of our knowledge, we did not find any similar study as regards the effects of triple combinations on colorectal cancer cell lines and our study was the first. According to our findings, combined treatment with 17-AAG at a very low concentration (4.72 nm) and GNP could increase the cytotoxic effects. These observed impacts were obtained at minimal concentrations of 17-AAG while a triple combination of 17-AAG, GNP and Ir showed higher anticancer impacts compared to double combinations of Ir, GNP and Ir, 17-AAG and single treatment at a higher concentration of 17-AAG. Therefore, triple combination elicited caspase-3 up regulation and subsequently, apoptosis induction at the given concentrations. GNP greater radiosensitizing impacts when combined with 17-AAG and Ir in comparison with Ir-GNP and Ir-17AAG were present.
Conclusion
It could be deduced that 17-AAG in combination cases acts in cooperative manner with GNP and Ir and these combinations showed significant anti-proliferative effects. Induction of cell death including apoptosis could be considered as potential therapeutic approaches in these cases. However, treatment of cancer using GNP is still needs further investigations and the cell death via apoptosis needs to be optimized by further regulating agents. These data proposed a promising approach to colorectal cancer treatment which show the efficacy of the 17-AAG as newly developed chemotherapeutic drug combined with GNP with targeting cell death activation. 17-AAG was demonstrated to be a feasible radiation-sensitive agent for radiotherapy in the presence of GNP, which suggest experimental combinations for further verifications of increments in GNP-mediated doses of biological interest. In conclusion, GNP can cause higher efficacy of anti-cancer treatments. However, future experiments are needed to assess the effects of these combinations.
Acknowledgements
Authors would like to thank Dr. Zeynali for practical support.
Abbreviation
- HSP90
Heat shock protein 90
- 17-AAG
17-allylamino-17-demethoxygeldanamycin
- GNP
Gold nanoparticle
- WST-1
Water-soluble tetrazolium salt-1
- Ir
Irradiation
Author contributions
Zhino Moradi performed the tests.
Mahshid Mohammadian was the second instructor of the experiments, interpreted the data, and wrote the manuscript draft.
Hassan Saberi participated in experimental procedures.
Meysam ebrahimifar participated in initial study concepts and prepared manuscript draft.
Zeinab Mohammadi contributed to the experimental procedures and participated in manuscript draft writing.
Mahnaz Ebrahimpour contributed to the experimental procedures.
Zhaleh Behrouzkia, was the principal instructor of the experiments and wrote the manuscript draft,
Funding
The research funding sources provided from Urmia University of Medical Sciences,Iran.
Data availability
Available upon the request.
Compliance with ethical standards
Competing interests
The authors declare that they have no conflict of interests.
All authors approved the final manuscript version.
Ethics approval
Not applicable. This article does not contain any studies with human subjects or animals conducted by authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhino Moradi, Email: zhinomoradi13770@gmail.com.
Mahshid Mohammadian, Email: Mohammadian_mahshid1365@yahoo.com.
Hassan Saberi, Email: H_saberi37@yahoo.com.
Meysam Ebrahimifar, Email: ebrahimifar67@gmail.com.
Zeinab Mohammadi, Email: mohammadi.moamma70@gmail.com.
Mahnaz Ebrahimpour, Email: m.ebrahimpoor71@gmail.com.
Zhaleh Behrouzkia, Phone: +989146620912, Email: zhalehkia@yahoo.com.
References
- 1.Temraz S, Mukherji D, Alameddine R, Shamseddine A. Methods of overcoming treatment resistance in colorectal cancer. Crit Rev Oncol Hematol. 2014;89(2):217–230. doi: 10.1016/j.critrevonc.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Jin H, Gao S, Guo H, Ren S, Ji F, Liu Z, et al. Re-sensitization of radiation resistant colorectal cancer cells to radiation through inhibition of AMPK pathway. Oncol Lett. 2016;11(5):3197–201. [DOI] [PMC free article] [PubMed]
- 3.Rosa S, Connolly C, Schettino G, Butterworth KT, Prise KM. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017;8(1):2. doi: 10.1186/s12645-017-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hau H, Khanal D, Rogers L, Suchowerska N, Kumar R, Sridhar S, et al. Dose enhancement and cytotoxicity of gold nanoparticles in colon cancer cells when irradiated with kilo- and mega-voltage radiation. Bioeng Transl Med. 2016;1(1):94–102. [DOI] [PMC free article] [PubMed]
- 5.Saberi A, Shahbazi-Gahrouei D, Abbasian M, Fesharaki M, Baharlouei A, Arab-Bafrani Z. Gold nanoparticles in combination with megavoltage radiation energy increased radiosensitization and apoptosis in colon cancer HT-29 cells. Int J Radiat Biol. 2017;93(3):315–323. doi: 10.1080/09553002.2017.1242816. [DOI] [PubMed] [Google Scholar]
- 6.Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8(1):57–84. doi: 10.1177/1758834015614530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azizi M, Ghourchian H, Yazdian F, Dashtestani F, AlizadehZeinabad H. Cytotoxic effect of albumin coated copper nanoparticle on human breast cancer cells of MDA-MB 231. PLoS ONE. 2017;12(11):e0188639. [DOI] [PMC free article] [PubMed]
- 8.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 9.Abraha AM, Ketema EB. Apoptotic pathways as a therapeutic target for colorectal cancer treatment. World J Gastrointest Oncol. 2016;8(8):583–591. doi: 10.4251/wjgo.v8.i8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammadi A, Yaghoobi MM, GholamhoseynianNajar A, Kalantari-Khandani B, Sharifi H, Saravani M. HSP90 inhibitor enhances anti-proliferative and apoptotic effects of celecoxib on HT-29 colorectal cancer cells via increasing BAX/BCL-2 ratio. Cell Mol Biol (Noisy-le-grand) 2016;62(12):62–67. doi: 10.14715/cmb/2016.62.12.11. [DOI] [PubMed] [Google Scholar]
- 11.Powers MV, Valenti M, Miranda S,Maloney A, Eccles SA, Thomas G, et al. Mode of cell death induced by the HSP90 inhibitor 17-AAG (tanespimycin) is dependent on the expression of pro-apoptotic BAX. Oncotarget. 2013;4(11):1963–75. [DOI] [PMC free article] [PubMed]
- 12.Zhao X, Wang J, Xiao L, Xu Q, Zhao E, Zheng X, Zheng H, Zhao S, Ding S. Effects of 17-allylamino-17-demethoxygeldanamycin on the induction of apoptosis and cell cycle arrest in HCT-116 cells. Oncol Lett. 2017;14(2):2177–2185. doi: 10.3892/ol.2017.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammadian M, Zeynali S, Azarbaijani AF, Khadem Ansari MH, Kheradmand F. Cytotoxic effects of the newly-developed chemotherapeutic agents 17-AAG in combination with oxaliplatin and capecitabine in colorectal cancer cell lines. Res Pharm Sci. 2017;12(6):517–525. doi: 10.4103/1735-5362.217432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser C, Lang SA, Kainz S, Gaumann A, Fichtner-Feigl S, Koehl GE, Schlitt HJ, et al. Blocking heat shock protein-90 inhibits the invasive properties and hepatic growth of human colon cancer cells and improves the efficacy of oxaliplatin in p53-deficient colon cancer tumors in vivo. Mol Cancer Ther. 2007;6(11):2868–2878. doi: 10.1158/1535-7163.MCT-07-0410. [DOI] [PubMed] [Google Scholar]
- 15.Davis LE, Rakitina TV, Vasilevskaya IA, O’Dwyer PJ. 17-AAG enhances cytotoxicity of the oxaliplatin/fluorouracil combination in colon cancer cell lines. Cancer Res. 2005;65(9):145.
- 16.Rakitina TV, Vasilevskaya IA, O’Dwyer PJ. Additive interaction of Oxaliplatin and 17-Allylamino-17-demethoxygeldanamycin in Colon Cancer cell lines results from inhibition of nuclear factor κB signaling. Cancer Res. 2003;63(24):8600–8605. [PubMed] [Google Scholar]
- 17.Zeynali-Moghaddam S, Mohammadian M, Kheradmand F, Fathi-Azarbayjani A, Rasmi Y, Esna-Ashari O, et al. A molecular basis for the synergy between 17-allylamino-17-demethoxy geldanamycin with Capecitabine and irinotecan in human colorectal cancer cells through VEFG and MMP-9 gene expression. Gene. 2019;684:30–8. [DOI] [PubMed]
- 18.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 19.Samarghandian S, Shabestari MM. DNA fragmentation and apoptosis induced by safranal in human prostate cancer cell line. Indian J Urol. 2013;29(3):177–183. doi: 10.4103/0970-1591.117278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao X, Huang C, Chen X, Yi Z, Sanche L. Chemical Radiosensitivity of DNA induced by gold nanoparticles. J Biomed Nanotechnol. 2015;11(3):478–485. doi: 10.1166/jbn.2015.1922. [DOI] [PubMed] [Google Scholar]
- 21.Enomoto A, Fukasawa T, Takamatsu N, Ito M, Morita A, Hosoi Y, Miyagawa K. The HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin modulates radiosensitivity by downregulating serine/threonine kinase 38 via Sp1 inhibition. Eur J Cancer. 2013;49(16):3547–3558. doi: 10.1016/j.ejca.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Musha A, Yoshida Y, Takahashi T, Ando K, Funayama T, Kobayashi Y, Negishi A, Yokoo S, Nakano T. Synergistic effect of heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin and X-rays, but not carbon-ion beams, on lethality in human oral squamous cell carcinoma cells. J Radiat Res. 2012;53(4):545–550. doi: 10.1093/jrr/rrs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y-M, Pyo H. Cooperative enhancement of Radiosensitivity after combined treatment of 17-(Allylamino)-17-Demethoxygeldanamycin and celecoxib in human lung and Colon Cancer cell lines. DNA Cell Biol. 2012;31(1):15–29. doi: 10.1089/dna.2011.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi M, Paquette B, Thippayamontri T, Gendron L, Guérin B, Sanche L. Increased radiosensitivity of colorectal tumors with intra-tumoral injection of low dose of gold nanoparticles. Int J Nanomedicine. 2016;11:5323–5333. doi: 10.2147/IJN.S97541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zabihzadeh M, Hoseini-Ghahfarokhi M, Bayati V, Teimoori A, Ramezani Z, Assarehzadehgan MA, et al. Enhancement of radio-sensitivity of colorectal cancer cells by gold nanoparticles at 18 MV energy. Nanomed J. 2018;5(2):111–20.
- 26.Ngwa W, Kumar R, Sridhar S, Korideck H, Zygmanski P, Cormack RA, Berbeco R, Makrigiorgos GM. Targeted radiotherapy with gold nanoparticles: current status and future perspectives. Nanomedicine (Lond) 2014;9(7):1063–1082. doi: 10.2217/nnm.14.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghita M, McMahon SJ, Taggart LE, Butterworth KT, Schettino G, Prise KM. A mechanistic study of gold nanoparticle radiosensitisation using targeted microbeam irradiation. Sci Rep. 2017;7:44752. doi: 10.1038/srep44752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taggart LE, McMahon SJ, Currell FJ, Prise KM, Butterworth KT. The role of mitochondrial function in gold nanoparticle mediated radiosensitisation. Cancer Nanotechnol. 2014;5(1):5. doi: 10.1186/s12645-014-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon the request.