ABSTRACT
Objectives
Orthotopic liver transplantation (OLT) can require substantial usage of blood products. Higher rates of transfusion have been associated with increased length of hospital stay, higher rates of infection, graft failure, and mortality. This study was a retrospective analysis to assess the impact of quality improvement interventions in OLT.
Methods
Data collection included demographics, preoperative and intraoperative data, blood utilization, and cost data. Statistical analysis was performed using R software.
Results
Total blood product utilization was reduced by approximately 50%. Statistically significant decreases were noted in blood product usage in the intraoperative and first 48-hour postoperative utilization, the number of OLTs using fewer than five RBC units, length of hospital stay, and cost.
Conclusions
This study showed successful implementation of quality improvement team interventions to reduce blood utilization during OLT. Reduced transfusion significantly correlated with decreased length of hospital stay and cost.
Keywords: Transfusion practice, Orthotopic liver transplant, Quality improvement
Orthotopic liver transplantation (OLT) can require up to 10 times as many units of blood products as a heart transplant.1-3 This substantial blood usage in OLT has been attributed to multiple factors, including patient preexisting comorbidities, liver failure or splenomegaly, intraoperative and postoperative bleeding, the presence of low or dysfunctional platelets, clot lysis, enhanced proteolysis, graft dysfunction, and technical failures.4-8 Higher rates of transfusion have been associated with increased length of hospital stay, higher rates of infection, graft failure, and mortality.9-11 Although it is not clear if blood transfusion represents an independent risk factor for the abovementioned outcomes, research showed that it is associated with defined infectious and noninfectious complication risks,12-14 some of which appear increased in the OLT setting.15 Based on this knowledge, restrictive transfusion practice and multidisciplinary approaches to reduce blood utilization are recommended to prevent avoidable patient harm and to decrease cost.16-18 In addition, despite the myriad factors that affect the bleeding risk and the variability of transfusion practice in OLT,19,20 some researchers found that transfusion-free surgery is increasingly feasible.21
In a previous study, we described how we provide blood transfusion support in liver transplantation22 through a multidisciplinary team that includes anesthesiologists, surgeons, blood bank technologists, and transfusion medicine specialists. Transfusion decisions are directed by anesthesiologists and based on a patient’s observed clinical bleeding and/or signs of hemodynamic instability in conjunction with pre- and intraoperative laboratory data, such as hemoglobin (Hb), platelet count, fibrinogen, prothrombin time (PT), international normalized ratio (INR), and activated partial thromboplastin time (aPTT). Laboratory data are used to identify potential causes of bleeding rather than an immediate trigger for transfusion. Transfusion medicine specialists are not directly involved in the ordering process or perioperative blood management. However, they oversee the testing, release blood products, and assist with quality improvement expertise.
United Network for Organ Sharing assessment of blood utilization revealed that RBC usage during OLT at our institution was among the highest nationwide. A Liver Transplant Review Committee (LTRC) was appointed to investigate the reasons for this high utilization and to implement quality improvement interventions to reduce it. This committee, consisting of OLT surgeons, anesthesiologists, nurses, quality improvement personnel, and transfusion medicine physicians, met regularly to discuss aspects of practice and opportunities for improvement. This team introduced a number of interventions over a period of 6 months. A retrospective study was approved to analyze the improvement in the blood utilization, cost reduction, and possible predictive factors for increased blood usage in the context of the implemented interventions. In this article, we report the results of that analysis, including cost analysis and quality improvement data, spanning a period of 3 years. The main purpose of this retrospective study was to determine if blood utilization and OLT cost followed specific quality improvement interventions.
Materials and Methods
This study of transfusion in OLT took place from July 1, 2010, to July 31, 2013. The study sample consisted of 324 liver transplant surgeries performed in two hospitals: 75% performed at Vanderbilt University Medical Center (hospital 1) and 25% performed at the Veterans Administration of Tennessee Valley Health Care System (hospital 2). Approval for the study was obtained from both the Vanderbilt Internal Review Board (IRB) and the LTRC. The LTRC met regularly during the study period. Initial discussions included review of benchmark OLT blood utilization studies from American and Canadian transplant groups and the literature on predictors of blood utilization in OLT. Initial assessment included data collection from the electronic medical records of the patient population with the International Classification of Diseases, Ninth Revision (ICD-9) procedure code 50.59 (orthotopic liver transplant) specified as the primary procedure. Patients younger than 18 years were excluded from the study.
In 2011, comparison with hospitals having more than 50 cases revealed that our institution had the fourth highest OLT blood usage nationwide. The LTRC undertook multiple direct and indirect interventions aimed to decrease blood utilization during the OLT procedure, including (1) designation of blood transfusion “champion” anesthesiologists and nurses who monitored and reduced blood utilization in OLT operating rooms; (2) introduction of the cell saver; (3) adherence to a standardized safe surgical technique; (4) requesting surgeons to consider mindfully “slowing down,” especially during critical operative moments of OLTs, with greater attention to hemostasis23; (5) adherence to standard laboratory transfusion triggers for transfusions of fresh-frozen plasma (FFP) (PT >14 seconds; aPTT >35 seconds), Hb (<8 g/dL), and fibrinogen (<180 mg/dL) whenever these measurements were received from the laboratory; (6) better communication with anesthesia over the progress of the case; and (7) organization of regular educational meetings among physicians, nurses, and staff. The goal of the meetings was to improve utilization rates and implement enhancing strategies. Implementation of these changes took place over the course of 6 months (from January 1, 2012, to June 30, 2012; designated as period 2).
All OLT operations were performed in standard piggyback fashion in which the hepatic veins were clamped and the vena cava was not interrupted. Blood transfusions were administered intraoperatively based on patients’ bleeding status in the surgical field. All patients were discharged with hemoglobin in the normal range (above 8 mg/dL) per surgical discharge protocol.
A retrospective study was approved by the IRB to assess and compare the effects of the quality improvement interventions in the preintervention period (July 1, 2010, to December 31, 2011; designated as period 1) with the postintervention period (July 1, 2012, to July 31, 2013; designated as period 3). We selected the candidate dependent variables based on their biological and clinical representativeness (face validity) and included the following factors: patient data (age, race, sex, height, weight, disease etiology, previous abdominal surgeries), preoperative Model for End-Stage Liver Disease (MELD) scores, preoperative laboratory values (Hb, platelet count, INR for prothrombin activity, and fibrinogen), various intraoperative data (attending surgeon and anesthesiologist, length of surgery, warm and cold ischemia time, cell saver volume), intraoperative and 48-hour postoperative utilization of blood components, and clinical outcome data (30-day and 1-year graft survival, retransplant, spontaneous bacterial peritonitis and portal vein thrombosis occurrence, and length of hospital stay). In addition, the cost of blood and related testing (referred to as blood cost) and total OLT cost were extracted by diagnosis-related groups (DRGs) for OLT per case. Blood utilization charges and total OLT charges were extracted for DRG-005 (with major comorbidities) and DRG-006 (without major medical comorbidities). Blood cost included, as mentioned above, the cost of blood products and processing and blood testing (blood type, antibody screen, crossmatch, and transfusion reaction workup, if any).
Statistical Analysis
All statistical analyses were performed in R (open source, www.r-project.org) version 3.4.2. The original scores violated the assumption of normality; therefore, the difference between periods (ie, period 1 vs period 3) was evaluated based on several nonparametric tests, as follows: (1) Mann-Whitney U test for continuous variables, (2) Pearson χ2 test and Fisher exact test for categorical variables, and (3) Scheirer-Ray-Hare test followed by Mann-Whitney U test for both continuous and categorical variables. Box-and-whisker plots were used to show the median, minimum, and maximum values, as well as the interquartile range for each group. Moreover, kernel density curves were plotted to estimate the population distribution based on the sample data. The amount of missing data was relatively small (ie, <4%). Missing values of the continuous variables were imputed using multiple imputation by chained equations package in R.24 In addition, missing values of the categorical variable values were excluded from analysis. All tests were two tailed, and a P value less than .05 was considered statistically significant.
Results
Demographic and Patient Primary Diagnoses
Demographic and surgical characteristics of the study population are presented in Table 1. The population was 84% white and 70% male. There were no statistically significant differences in the demographic variables among any of the study periods. However, more patients with previous abdominal surgery were present in period 1 (P = .001; odds ratio [OR], 2.96; confidence interval [CI], 1.42-6.56). Overall, 30% of patients had cirrhosis due to hepatitis C virus, 23% hepatoma and/or hepatocellular carcinoma, 15% fatty liver cirrhosis, 10% alcoholic cirrhosis, and 22% other (including cryptogenic cirrhosis, autoimmune cirrhosis, primary sclerosing cholangitis, primary biliary cirrhosis, biliary atresia, α1 antitrypsin deficiency, primary oxalosis, Wilson disease, hemochromatosis, Budd-Chiari syndrome, and cirrhosis due to hepatitis B or B and C). No statistically significant differences between diagnoses occurrence were found between study groups except for cirrhosis due to hepatitis C, which was more common preintervention (30.1% vs 13.6%, P < .001). For other potential confounders, such as the presence of incidental tumor at the time of transplant, which could potentially lead to more bleeding, the differences between pre- and postintervention study times were not statistically significant.
Table 1.
Characteristics of the Patient Population Over the Three Study Periodsa
| Variable | Period 1 (n = 156) | Period 2 (n = 43) | Period 3 (n = 125) |
|---|---|---|---|
| Age, mean ± SD, y | 55.74 ± 9.03 | 55.12 ± 9.41 | 57.38 ± 8.67 |
| Male | 102 (65.38) | 33 (76.74) | 93 (76.00) |
| Race | |||
| White | 125 (83.89) | 36 (83.72) | 109 (87.20) |
| African American | 13 (8.72) | 5 (11.63) | 10 (8.00) |
| Asian/Alaskan/Indian | 1 (0.67) | 0 | 2 (1.60) |
| Unknown | 10 (6.71) | 2 (4.65) | 4 (3.20) |
| Weight, mean ± SD, kg | 85.82 ± 18.53 | 89.86 ± 20.61 | 88.71 ± 16.59 |
| Height, mean ± SD, cm | 173.30 ± 10.34 | 175.40 ± 10.17 | 174.80 ± 9.05 |
aValues are presented as number (%) unless otherwise indicated.
Preoperative Data
Preoperative variables are presented in Table 2. There were no statistically significant differences in the preoperative variables (age, sex, height, weight, total Hb, INR, platelet count, and MELD) in any of the three study periods.
Table 2.
Pre- and Intraoperative Variablesa
| Variable | Period 1 (n = 156) | Period 2 (n = 43) | Period 3 (n = 125) | P Value, Period 1 vs 3 |
|---|---|---|---|---|
| Preoperative variables | ||||
| Hemoglobin, g/dL | 10.59 ± 2.05 | 10.56 ± 2.32 | 10.43 ± 2.32 | .42 |
| Platelet count, 109/L | 74.69 ± 49.16 | 83.77 ± 57.28 | 74.24 ± 41.46 | .30 |
| INR | 1.92 ± 0.77 | 1.87 ± 0.46 | 1.95 ± 0.71 | .42 |
| Fibrinogen, mg/dL | 196.60 ± 99.64 | 217 ± 105.91 | 197.60 ± 95.32 | .29 |
| MELD score | 19.69 ± 8.81 | 20.84 ± 8.75 | 20.41 ± 9.11 | .37 |
| Previous abdominal surgery, No. (%) | 38 (24.68) | 5 (11.63) | 12 (9.92) | .015 |
| Intraoperative variables | ||||
| Surgery time, h | 5.06 ± 1.15 | 5.57 ± 1.35 | 5.46 ± 1.24 | 2.60E-16 |
| Warm ischemia time, min | 31.13 ± 7.83 | 34.09 ± 7.45 | 36.17 ± 14.92 | 1.91E-06 |
| Cold ischemia time, h | 5.88 ± 2.68 | 5.82 ± 2.41 | 5.38 ± 2.95 | 2.00E-02 |
| Cell saver volume, mL | 570.64 ± 1,315 | 1,324 ± 1,981.06 | 1,349 ± 1,794.56 | 2.60E-16 |
INR, international normalized ratio; MELD, Model for End-Stage Liver Disease.
aValues are presented as mean ± SD unless otherwise indicated.
Intraoperative Data
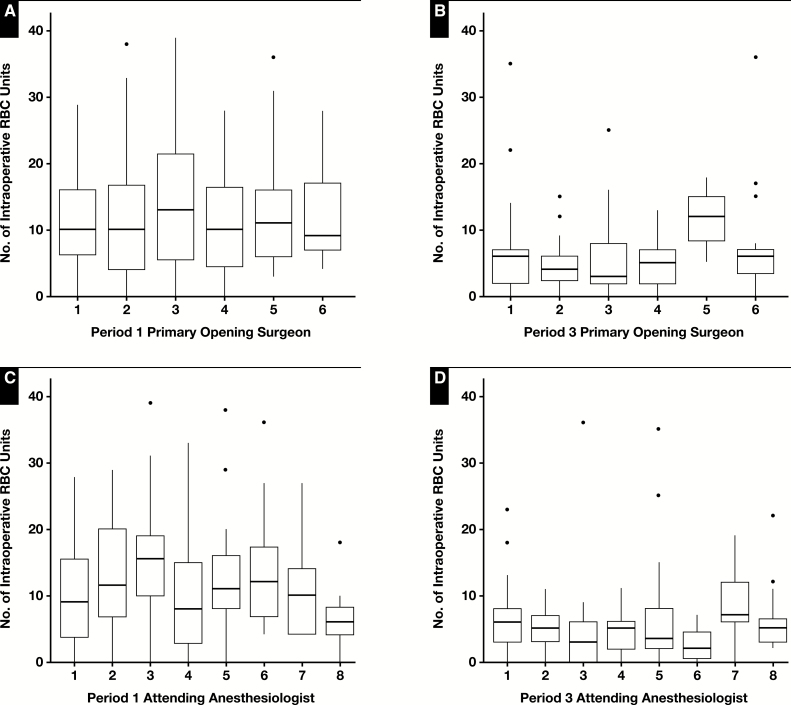
Box-and-whisker plots of the intraoperative RBC usage by individual anesthesiologists and opening surgeons are illustrated in Figure 1. Intraoperative usage of FFP and cryoprecipitate, as well as RBC utilization within the first 48 hours and all blood product utilization within the first 48 hours, was significantly associated with the anesthesiologist. Mean intraoperative RBC usage among all anesthesiologists was 11.3 ± 16.58 units.
Figure 1.
Box-and-whisker plots of the RBC intraoperative usage by opening surgeons (A, B) and anesthesiologists (C, D). The surgeons and anesthesiologists were independent of each other and not paired teams. The median (horizontal line within each box), the 25th percentile (lower edge of the box), and the 75th percentile (upper edge of the box) are displayed. The whiskers (vertical lines) indicate the minimum and maximum values within the data set. The length of the box represents the interquartile range (IQR). The dots indicate the outliers. Some of the outliers are not shown.
Intraoperative variables are illustrated in Table 2. From period 1 to period 3, surgery time and warm ischemia time slightly increased (from 5.06 ± 1.15 hours to 5.46 ± 1.24 hours and 31.13 ± 7.83 minutes to 36.17 ± 14.92 minutes, respectively), whereas cold ischemia time slightly decreased (from 5.89 ± 2.68 hours to 5.38 ± 2.95 hours). These changes paralleled an increase in cell saver volume from 570.64 ± 1,315.36 mL to 1,349 ± 1,794.56 mL.
Blood Utilization
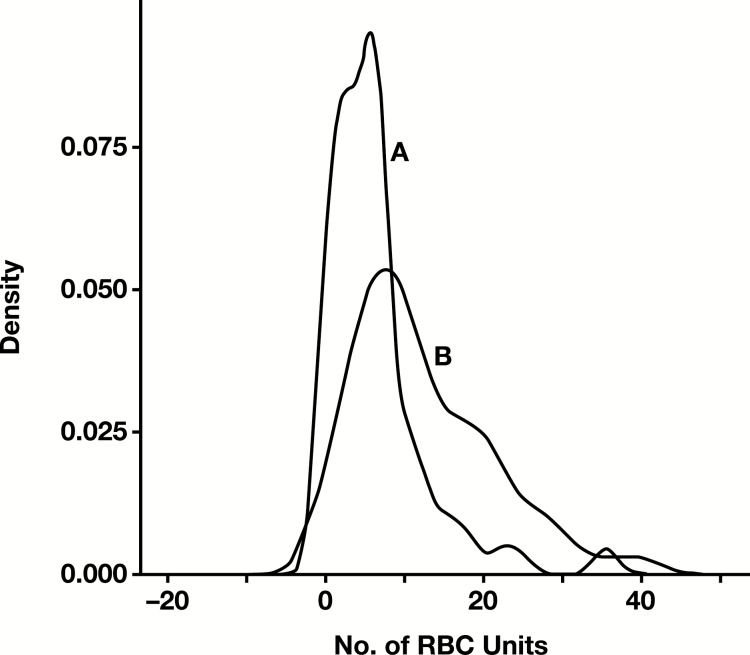
Table 3 shows that total blood product utilization was reduced by about 50% (from 49.26 to 25.15 units) following the quality improvement interventions between periods 1 and 3 of the study. The reduction in blood utilization was statistically significant for all blood products (P < .05) except for platelets (intraoperatively) and cryoprecipitate (during the first 48 hours postoperatively). Intraoperative RBC usage was also decreased by 50%. Kernel density curves showing the reduction in the intraoperative RBC usage pre- and postintervention are illustrated in Figure 2.
Table 3.
Blood Utilization
| Variable | Period 1 (n = 156), Mean ± SD | Period 2 (n = 43), Mean ± SD | Period 3 (n = 125), Mean ± SD | P Value, Period 1 vs 3 |
|---|---|---|---|---|
| Intraoperative | ||||
| RBC, units | 14.25 ± 17.42 | 12.95 ± 25.66 | 7.14 ± 9.20 | 5.78E-11 |
| FFP, units | 16.78 ± 15.92 | 17.93 ± 22.14 | 10.88 ± 11.72 | 3.46E-06 |
| Platelets, units | 1.47 ± 2.25 | 1.63 ± 2.06 | 1.10 ± 1.37 | .07 |
| Cryoprecipitate, doses | 0.43 ± 0.69 | 0.97 ± 1.30 | 0.74 ± 1.01 | .007 |
| All blood products, units | 32.93 ± 34.66 | 33.48 ± 49.80 | 19.85 ± 22.09 | 1.70E-07 |
| Postoperative | ||||
| RBC, units | 8.31 ± 14.71 | 4.67 ± 7.08 | 2.68 ± 3.86 | 4.07E-06 |
| FFP, units | 6.19 ± 11.90 | 3.37 ± 5.85 | 1.94 ± 3.20 | 8.40E-03 |
| Platelets, units | 1.58 ± 2.79 | 0.77 ± 1.65 | 0.56 ± 1.19 | 7.33E-05 |
| Cryoprecipitate, doses | 0.25 ± 0.60 | 0.13 ± 0.40 | 0.12 ± 0.35 | 1.20E-01 |
| All blood products 48 hours, units | 16.32 ± 29.30 | 8.94 ± 14.29 | 5.30 ± 7.84 | 3.70E-05 |
| All blood products, units | 49.26 ± 50.63 | 42.42 ± 58.99 | 25.15 ± 25.76 | 9.10E-08 |
Figure 2.
Kernel density estimation of the reduction in intraoperative RBC usage preintervention (A) and postintervention (B).
The number of liver transplants using more than five RBC units intraoperatively decreased by approximately 30% (82.69% preintervention vs 59.2% postintervention, P = .01).
Outcome Data
The average length of stay decreased from 14.35 ± 13.02 days in period 1 to 11.46 ± 9.45 days in period 3, and this decrease was statistically significant (P = .03). The 30-day graft survival was not statistically significant between the two groups.
Cost Data
The total blood cost was calculated as the sum of the cost of blood products and related processing and testing per case. This was reduced postintervention by 42.06% (from $7,404 ± $3,166 to $4,290 ± $1,583) for OLT with major comorbidities (DRG-005) and by 49.99% (from $3,939 ± $3,094 to $1,970 ± $837) for OLT without major comorbidities (DRG-006). The total OLT cost was also reduced by 24.09% (from $90,614 ± $33,466 to $68,785 ± $11,625) for OLT with major comorbidities (DRG-005) and by 9.41% (from $52,726 ± $10,728 to $47,762 ± $4,038) for OLT without major comorbidities (DRG-006). The total blood cost fraction of the total OLT cost also decreased from 8.17% to 6.23% and from 7.47% to 4.12% for OLT with and without major comorbidities, respectively.
Other Findings
Portal vein thrombosis (P = .01; OR, 3.22; 95% CI, 1.11-11.39) and spontaneous bacterial peritonitis (P < .001; OR, 4.64; 95% CI, 1.93-12.90) were decreased in the postintervention period.
Discussion
This retrospective study assessed the aggregate effects of multiple quality improvement interventions and showed successful reduction in blood product utilization and cost. We studied a total of 44 variables in 324 consecutive OLT cases performed at hospitals 1 and 2 over a period of 3 years, divided into a preintervention period (period 1), intervention period (period 2), and postintervention period (period 3). The decrease in blood utilization was significant for most blood products, especially intraoperative RBCs. The introduction of the cell saver significantly correlates with the decrease in blood transfusions. The blood volume used via cell saver was almost 40% greater in the postintervention period. The reduction in intraoperative RBC usage and total blood product utilization, as well as the increases in cell saver volume, likely reflected the effectiveness of this intervention, implemented in period 2. Other measures, such as education and team consensus, may have also played an important role, but they were not quantified.
The statistically significant association between reduced blood product usage and decreased postintervention total blood cost is expected. The total blood cost represents less than 10% of the total OLT cost across all periods, but this fraction is also decreased in the postintervention period by 2% to 3%, and it is possible that the decrease in OLT cost is associated with decreased length of hospital stay. The association of the decreased incidence of portal vein thrombosis and spontaneous bacterial peritonitis with the postintervention period is not clear; these are likely confounding factors.
Massicotte et al25 examined in a retrospective study 20 variables in 206 successive liver transplants from cadaveric donors over a period of 52 months, including patient data (age, sex, height, diagnosis, history of abdominal surgery, previous liver transplant, starting Hb, starting INR, starting platelet count, central venous pressure, body temperature, and Pugh and MELD scores) and operative factors (surgeon, anesthesiologist, surgeon work shift, clamping time, duration of cold ischemia). Like us, they found that demographic data did not correlate with transfusion requirements. Only seven variables (starting INR, starting platelet count, starting Hb value, MELD score, Pugh score, and diagnosis) in univariate analysis were correlated with increased transfusion rate. In contrast, this study shows that decreased intraoperative RBC usage did not correlate with preoperative Hb, platelet count, INR, and MELD. We agree that the predicting factors are difficult to interpret, and in addition, our study was not designed to provide a comprehensive assessment or predictors of blood transfusion but instead was a quality improvement endeavor. Nevertheless, a comparison with their transfusion rates suggests that there are additional opportunities for improvement at our institution. For example, the best transfusion rates in our study (period 3) were greater than their reported mean intraoperative RBC usage of 2.8 ± 3.5 RBC units per case. However, other studies show intraoperative RBC transfusion rates similar to ours, in the range of 6 ± 3.7 RBC units.26
The main limitation of our study derives from its retrospective design. Although retrospective designs are subject to numerous threats to both internal and external validity that alter the interpretation and generalizability of the results,24 we believe that this approach does not significantly affect the results of our study. This limitation is shared by most studies reported in the literature that identify transfusion practices of OLT. A second limitation lies in the inability to tease apart the contributions of individual surgical factors potentially affecting blood utilization since these interventions were introduced at different times during intervention period 2. It is known that surgical technical factors are associated with bleeding and transfusion requirements. In addition, numerous surgical techniques and associated approaches, including venovenous bypass, autologous blood transfusion, volume expansion, use of cauterization, and the use of the piggyback transplantation method, can be associated with decreased blood product usage.6 The impact of mindful “slowing down” in surgery with “cognitive refocusing” to increase attention during critical moments of operative practice has also been well described.23 The series of quality improvement interventions implemented to decrease the number of transfusions resulted in a significant reduction in blood utilization from 13 RBC units down to about 6 units. The clinical outcomes are not causally attributed to reduced transfusion; rather, the interventions cumulatively resulted in a reduction in blood transfusions.
Another limitation is the lack of a comprehensive list of preoperative risk factors (ie, ascites, preoperative bilirubin, and creatinine and urea levels) that were found to predict the transfusion requirements in OLT in some studies26-28 or documentation of pharmacological means to minimize blood loss (aprotinin, epsilon-aminocaproic acid, tranexamic acid, or others). However, other authors found that variables such as bilirubin and creatinine were not statistically significant,29 and the role of some drugs for reducing blood requirements remains controversial.30,31 Moreover, our study was not directly aimed at comprehensive identification of specific predictors for transfusion requirements during OLT but rather at improving the group practice and reduction in blood utilization. It is not clear why decreased rates of portal vein thrombosis and spontaneous bacterial peritonitis were found in the postintervention period. Further studies aimed at defining the role of comorbidities may perhaps elucidate this. Overall, this study shows that multidisciplinary teams can significantly reduce the RBC and total blood product utilization during OLT.
Conclusions
This study showed successful implementation of quality improvement interventions by a multidisciplinary team to improve blood utilization during OLT. Reduced transfusions were associated with decreased cost and length of hospital stay but did not affect the 1-year survival.
References
- 1. Sarkar RS, Philip J, Yadav P. Transfusion medicine and solid organ transplant—update and review of some current issues. Med J Armed Forces India. 2013;69:162-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis JH, Bontempo FA, Cornell F, et al. Blood use in liver transplantation. Transfusion. 1987;27:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porte RJ. Coagulation and fibrinolysis in orthotopic liver transplantation: current views and insights. Semin Thromb Hemost. 1993;19:191-196. [DOI] [PubMed] [Google Scholar]
- 4. Spence RK, Maurer J. Transfusion requirements in liver transplantation. Emedicine Updated.2006. [Google Scholar]
- 5. de Boer MT, Molenaar IQ, Hendriks HG, et al. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22:265-275. [DOI] [PubMed] [Google Scholar]
- 6. Feltracco P, Brezzi M, Barbieri S, et al. Blood loss, predictors of bleeding, transfusion practice and strategies of blood cell salvaging during liver transplantation. World J Hepatol. 2013;5:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olcese VA, Tuttle-Newhall B. Transfusion requirements in liver transplantation http://emedicine.medscape.com/article/431573-overview#showall. Accessed October 26, 2018.
- 8. Rana A, Petrowsky H, Hong JC, et al. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. J Am Coll Surg. 2013;216:902-907. [DOI] [PubMed] [Google Scholar]
- 9. Pereboom IT, de Boer MT, Haagsma EB, et al. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108:1083-1091. [DOI] [PubMed] [Google Scholar]
- 10. Ramos E, Dalmau A, Sabate A, et al. Intraoperative red blood cell transfusion in liver transplantation: influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003;9:1320-1327. [DOI] [PubMed] [Google Scholar]
- 11. Williamson LM, Lowe S, Love EM, et al. Serious hazards of transfusion (SHOT) initiative: analysis of the first two annual reports. BMJ. 1999;319:16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garraud O, Filho LA, Laperche S, et al. The infectious risks in blood transfusion as of today—a no black and white situation. Presse Med. 2016;45:303-311. [DOI] [PubMed] [Google Scholar]
- 13. Shander A, Lobel GP, Javidroozi M. Transfusion practices and infectious risks. Expert Rev Hematol. 2016;9:597-605. [DOI] [PubMed] [Google Scholar]
- 14. Benson AB, Burton JR Jr, Austin GL, et al. Differential effects of plasma and red blood cell transfusions on acute lung injury and infection risk following liver transplantation. Liver Transpl. 2011;17:149-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025-2035. [DOI] [PubMed] [Google Scholar]
- 16. Ad N, Holmes SD, Patel J, et al. The impact of a multidisciplinary blood conservation protocol on patient outcomes and cost after cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:597-605.e1. [DOI] [PubMed] [Google Scholar]
- 17. Timpa JG, O’Meara LC, Goldberg KG, et al. Implementation of a multidisciplinary bleeding and transfusion protocol significantly decreases perioperative blood product utilization and improves some bleeding outcomes. J Extra Corpor Technol. 2016;48:11-18. [PMC free article] [PubMed] [Google Scholar]
- 18. Danielson CF, Filo RS, O’Donnell JA, et al. Institutional variation in hemotherapy for solid organ transplantation. Transfusion. 1996;36:263-267. [DOI] [PubMed] [Google Scholar]
- 19. Allanki SD. Transfusion practice in orthotopic liver transplantation. Indian J Crit Care Med. 2009;13:120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massicotte L, Beaulieu D, Roy JD, et al. MELD score and blood product requirements during liver transplantation: no link. Transplantation. 2009;87:1689-1694. [DOI] [PubMed] [Google Scholar]
- 21. Jabbour N, Gagandeep S, Mateo R, et al. Transfusion free surgery: single institution experience of 27 consecutive liver transplants in Jehovah’s Witnesses. J Am Coll Surg. 2005;201:412-417. [DOI] [PubMed] [Google Scholar]
- 22. Zubair AC, Thorp K, Eichbaum Q. How we provide blood transfusion support in two large US liver transplant programs. Transfusion. 2016;56:1938-1943. [DOI] [PubMed] [Google Scholar]
- 23. Moulton CA, Regehr G, Lingard L, et al. Slowing down to stay out of trouble in the operating room: remaining attentive in automaticity. Acad Med. 2010;85:1571-1577. [DOI] [PubMed] [Google Scholar]
- 24. Trochim W. Research Methods: The Concise Knowledge Base. Mason, OH: Thomson; 2005. [Google Scholar]
- 25. Massicotte L, Sassine MP, Lenis S, et al. Transfusion predictors in liver transplant. Anesth Analg. 2004;98:1245-1251. [DOI] [PubMed] [Google Scholar]
- 26. Makroo RN, Walia RS, Aneja S, et al. Preoperative predictors of blood component transfusion in living donor liver transplantation. Asian J Transfus Sci. 2013;7:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deakin M, Gunson BK, Dunn JA, et al. Factors influencing blood transfusion during adult liver transplantation. Ann R Coll Surg Engl. 1993;75:339-344. [PMC free article] [PubMed] [Google Scholar]
- 28. McCluskey SA, Karkouti K, Wijeysundera DN, et al. Derivation of a risk index for the prediction of massive blood transfusion in liver transplantation. Liver Transpl. 2006;12:1584-1593. [DOI] [PubMed] [Google Scholar]
- 29. Solves P, Carpio N, Moscardo F, et al. Transfusion management and immunohematologic complications in liver transplantation: experience of a single institution. Transfus Med Hemother. 2015;42:8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalmau A, Sabaté A, Acosta F, et al. Tranexamic acid reduces red cell transfusion better than epsilon-aminocaproic acid or placebo in liver transplantation. Anesth Analg. 2000;91:29-34. [DOI] [PubMed] [Google Scholar]
- 31. Kaspar M, Ramsay MA, Nguyen AT, et al. Continuous small-dose tranexamic acid reduces fibrinolysis but not transfusion requirements during orthotopic liver transplantation. Anesth Analg. 1997;85:281-285. [DOI] [PubMed] [Google Scholar]