Abstract
Background
Chemotherapy, as one of the main approaches of cancer treatment, is accompanied with several adverse effects, including chemotherapy-induced peripheral neuropathy (CIPN). Since current methods to control the condition are not completely effective, new treatment options should be introduced. Medicinal plants can be suitable candidates to be assessed regarding their effects in CIPN. Current paper reviews the available preclinical and clinical studies on the efficacy of herbal medicines in CIPN.
Methods
Electronic databases including PubMed, Scopus, and Cochrane library were searched with the keywords “neuropathy” in the title/abstract and “plant”, “extract”, or “herb” in the whole text. Data were collected from inception until April 2018.
Results
Plants such as chamomile (Matricaria chamomilla L.), sage (Salvia officinalis L.), cinnamon (Cinnamomum cassia (L.) D. Don), and sweet flag (Acorus calamus L.) as well as phytochemicals like matrine, curcumin, and thioctic acid have demonstrated beneficial effects in animal models of CIPN via prevention of axonal degeneration, decrease in total calcium level, improvement of endogenous antioxidant defense mechanisms such as superoxide dismutase and reduced glutathione, and regulation of neural cell apoptosis, nuclear factor-ĸB, cyclooxygenase-2, and nitric oxide signaling. Also, five clinical trials have evaluated the effect of herbal products in patients with CIPN.
Conclusions
There are currently limited clinical evidence on medicinal plants for CIPN which shows the necessity of future mechanistic studies, as well as well-designed clinical trial for further confirmation of the safety and efficacy of herbal medicines in CIPN.
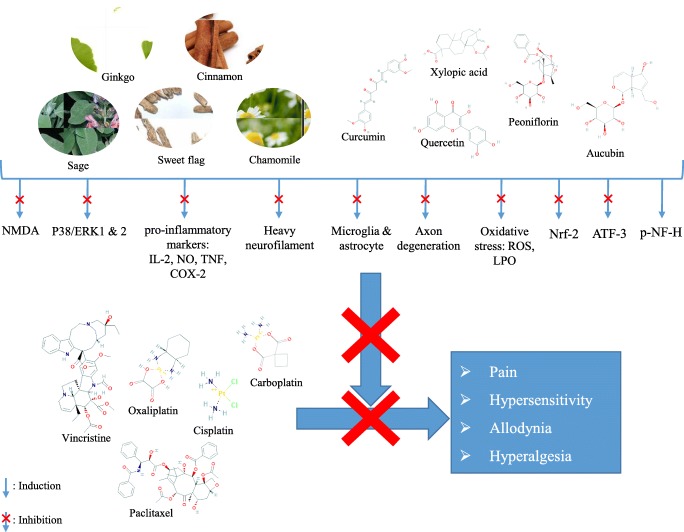
Graphical abstract.
Schematic mechanisms of medicinal plants to prevent chemotherapy-induced neuropathy: NO: nitric oxide, TNF: tumor necrosis factor, PG: prostaglandin, NF-ĸB: nuclear factor kappa B, LPO: lipid peroxidation, ROS: reactive oxygen species, COX: cyclooxygenase, IL: interleukin, ERK: extracellular signal-related kinase, X: inhibition, ↓: induction.
Keywords: Pain, Neuropathy, Phytotherapy, Chemotherapeutic agents, Inflammation, Phytochemicals, Medicinal plants, Clinical studies
Introduction
Peripheral neuropathy is a series of different medical conditions with various etiology, pathology and severity. Based on etiological, genetic, or pathological characteristics, peripheral neuropathy can be classified into hereditary or acquired neuropathy, acute and chronic neuropathy, single and multiple mono-neuropathy, symmetric poly-neuropathy and radiculopathy [1, 2].
Direct trauma, prolonged pressure on a nerve, chronic diseases like diabetes, as well as drugs with neurological side effects are amongst the most popular causes of peripheral neuropathy [3]. In severe cases, the disease can be fatal or have permanent symptoms which can have negative impacts on patients’ quality of life [1, 2, 4].
One of the predisposing factors which can lead to peripheral neuropathy is chemotherapy. Chemotherapy is a popular treatment modality in many types of cancer [5]; however, due to neurotoxicity of some chemotherapeutic agents, peripheral neuropathy is one of the major side effects [6]. Statistics have shown that from 10% to 20% (in 2002) and up to 48% (in 2014) of all cancer patients have experienced chemotherapy-induced peripheral neuropathy (CIPN) [7, 8]. CIPN can be recognized by short-term sensory symptoms and long-term motor weaknesses [5]. Type of chemotherapy and its cumulative dose are the key factors in the occurrence of CIPN [9]. Platinum agents, vinca alkaloids, and taxanes have more potential to cause CIPN [6, 7, 10].
Various treatments such as tricyclic antidepressants, gabapentin and its newer analogue pregabalin, lamotrigine, topical baclofen, ketamine and acetyl-L-carnitine are currently used to manage this type of neuropathy; though, there is no proven evidence from these medical treatments to prevent or cure CIPN. Despite some undeniable positive effects of the above mentioned agents, in several cases they were not better than placebo in attenuating pain and improving neuropathic symptoms which shows the need for further investigations regarding an effective agent to control the condition [4, 6, 11].
Since ancient times, medicinal plants were used due to their positive effects on various neurological and psychiatric diseases such as Parkinson’s disease, Alzheimer’s disease, epilepsy, insomnia and depression. New scientific evidences also support the effectiveness of plant-derived natural agents for the management of neurological disorders in preclinical and clinical studies [12–14]. Thus, medicinal plant can be introduced as new options for the management of CIPN in the future.
The aim of this study is to review preclinical and clinical evidences regarding the effectiveness of medicinal plants and their isolated phytochemicals in CIPN.
Search strategy
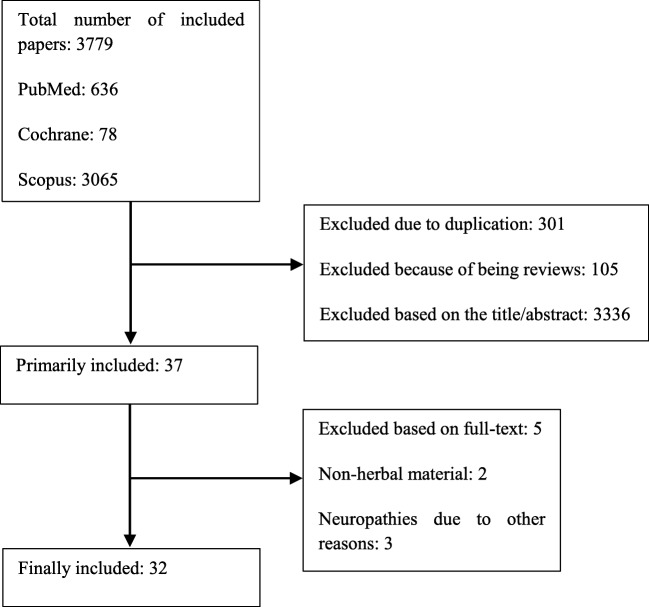
Electronic databases including PubMed, Scopus, and Cochrane library were searched with the keywords “neuropathy” in the title/abstract and “plant”, “extract”, or “herb” in the whole text. Data were collected from inception until April 2018. Only papers with English full-text were included in our study. Studies on the neuropathies due to causes other than chemotherapy were excluded. Studies on the other side effects of chemotherapy were also excluded. From total of 3379 studies, final number of 32 papers, including 27 animal studies and 5 clinical trials, were retrieved (Fig. 1). Results of the final included article are summarized in Tables 1, 2, and 3.
Fig. 1.
Flow diagram of study selection process
Table 1.
Animal studies on the effect of medicinal plants in chemotherapy-induced neuropathy
| Plant/ phytochemical name | Animal model | Dosage | Outcome | Possible mechanisms of action | Reference |
|---|---|---|---|---|---|
| Matricaria chamomilla/ hydroalcoholic extract | Formalin-induced pain & cisplatin-induced neuropathy in NMRI male mice | 25 mg/kg, IP | ↓First & second phase pain & inflammation, significantly better anti-inflammatory effect than morphine |
Anti-inflammatory, antispasmodic, ↓PG synthesis, ↓pro-inflammatory cytokines production |
[15] |
| Salvia officinalis/ hydroalcoholic extract | Formalin-induced pain & vincristine-induced neuropathy in NMRI male mice | 100 mg/kg, IP | ↓Second phase pain & inflammation | Anti- inflammatory, antispasmodic & anti-disquiet effects, involvement of μ-opioid receptors | [16] |
| Xylopia aethiopica/ ethanolic fruit extract | Vincristine-induced neuropathy in Sprague-Dawley rats | 30–300 mg/kg, PO | ↓Tactile & cold allodynia, intermediate & mechanical hyperalgesia |
Inhibition of p38 &/or ERK1 & ERK2 pathways, ↓NF-κB activation |
[17] |
| Synedrella nodiflora/ hydroethanolic extract | Vincristine-induced neuropathy in Sprague-Dawley rats | 100, 300 & 1000 mg/kg, PO | ↓Tactile & cold allodynia, mechanical & thermal hyperalgesia |
Inhibition of pain stimuli propagation in the degenerated unmyelinated & myelinated C-, Aδ, & Aβ-fibres, ↓LPO |
[18] |
| Synedrella nodiflora/ hydroethanolic extract | Paclitaxel-induced neuropathy in Sprague-Dawley rats | 100, 300 & 1000 mg/kg, PO | ↓Thermal hyperalgesia | Involvement of glutamatergic neurotransmission, NMDA receptor, & purinergic pathways | [19] |
| Plantago sp. or Achyranthes sp. / aqueous extract | Paclitaxel-induced mechanical allodynia in mice | 0.3, 0.1 & 0.03 g/kg, PO | ↓Mechanical allodynia by Plantago sp. but not by Achyranthes sp. | Antioxidant activity | [20] |
| Palisota hirsuta/ ethanolic extract | Vincristine-induced tactile allodynia in Sprague Dawley rats | 30–300 mg/kg, PO |
↓Tactile allodynia, thermal & mechanical hyperalgesia, No significant effect on cold allodynia |
↓Inflammatory nociceptive reactions in peripheral nerve region, inflammatory, spinal nociceptive processes in dorsal root ganglia & dorsal horn neurons of the spinal cord, antidepressant& anxiolytic effects | [21] |
| Lithospermum sp./ aqueous extract |
In vitro: NGF-stimulated rat pheochromocytoma PC12 cells In vivo: Oxaliplatin-induced peripheral neuropathy in C57BL/6 mice |
250 mg/kg, PO |
In vitro: ↓Neurotoxicity of oxaliplatin, In vivo: ↓mechanical hypersensitivity ↓spinal activation of microglia & astrocytes, ↓multinucleolated DRG neurons, ↑IENFs density |
Anti-inflammatory activity in neuronal immune cells | [22] |
| Astragalus sp./ aqueous & hydroalcoholic extracts | Oxaliplatin-induced neuropathy in Sprague-Dawley rat | 300 mg/kg/day, PO |
↓Sensitivity to noxious & non-noxious mechanical stimuli, thermal allodynia (only hydroalcoholic extract) improvement in motor coordination in rotarod test, ↑p-NF-H expression in sciatic nerve, Improvement in morphological changes & ↓ATF-3 expression in DRG, ↓Nrf2 mRNA in DRG, ↓spinal & cerebral density of microglia & astrocytes |
– | [23] |
| Maerua angolensis/ petroleum ether/ethyl acetate stem bark extract | Vincristine-induced neuropathy in Swiss albino mice | 3, 10, & 20 mg/kg, PO |
↓Tactile & cold allodynia, ↓intermediate & mechanical hyperalgesia |
↓Phosphorylation of extracellular signal-related kinases & p38 MAPK, ↓NF-κB, ↓NO, interleukins & TNF-α |
[24] |
| Aconitum sp./ powder | Oxaliplatin-induced peripheral neuropathy in Sprauge-Dawley rats | 300 mg/kg, PO |
↓Cold & mechanical allodynia ↓activation of astrocytes in the spinal dorsal horn, ↓TNF-α & IL-1β |
↓ERK 1/2-phosphorlation, Regulation of NMDA & AMPA receptors via modulation of pro-inflammatory cytokines |
[25] |
| Ocimum sanctum/ hydroalcoholic extract & the saponin rich fraction | Vincristine-induced peripheral neuropathic pain in Wistar albino rats |
100 & 200 mg/kg, PO |
↓Cold allodynia, mechanical & heat hyperalgesia, tail cold hyperalgesia ↓total calcium, LPO, superoxide anion |
↓Oxidative stress & calcium levels | [26] |
| Cinnamomum cassia/ aqueous extract |
Oxaliplatin-induced neuropathic cold allodynia in rats |
100, 200, & 400 mg/kg, PO |
↓Cold allodynia, ↓astrocytes & microglia activation, ↓IL-1β & TNF in the spinal cord |
– | [27] |
| Camellia sinensis/ extract | Oxaliplatin-induced peripheral neuropathy in Sprague-Dawley rats | 300 mg/kg, PO |
↑Sensory & thermal threshold values, No significant change in sensory nerve conduction & number of apoptotic cells in DRG |
Involvement of antioxidative properties | [28] |
| Agrimonia eupatoria/ hydroalcoholic extract | Cisplatin-induced neuropathic pain in Sprague-Dawley rats | 200 mg/kg, PO |
↓Mechanical & thermal hyperalgesia, ↓cold allodynia |
– | [29] |
| Acorus calamus/ hydroalcoholic extract | Vincristine-induced neuropathy in Wistar rats | 100 & 200 mg/kg, PO |
↓Thermal & mechanical hyperalgesia, mechanical allodynia, ↓total calcium, MPO, superoxide anion, ↓axonal degeneration |
Antioxidant, anti-inflammatory, neuroprotective, & calcium inhibitory activity | [30] |
| Acorus calamus/ hydroalcoholic extract | Vincristine-induced neuropathy in Wistar rats | 100 & 200 mg/kg, PO |
↓Thermal hyperalgesia & allodynia, mechanical hyperalgesia & allodynia, ↓sciatic functional index ↓total calcium, MPO, superoxide anion, TNF-α |
Antioxidant, anti-inflammatory, calcium inhibitory actions | [31] |
| Ginkgo biloba/ extract | Vincristine-induced neuropathy in Sprague-Dawley rats | 50, 100, & 150 mg/kg, PO | ↓Mechanical & cold hyperalgesia dose-dependently |
Antioxidant properties, involvement of MAPK & NF-ĸB pathways, ↓NO and TNF-α, ↑peripheral nerve function recovery |
[32] |
| Tithonia tubaeformis/ hydromethanolic extract |
Acetic acid-induced abdominal constriction, Tail immersion test, Vincristine-induced neuropathy in BALB/c mice |
100 & 200 mg/kg, PO |
↓pain in abdominal constriction antinociceptive test & tail immersion test, ↓allodynia & thermal hyperalgesia, |
Central analgesic activity | [33] |
PO per oral, IP intraperitoneal, DRG dorsal root ganglia, NGF nerve growth factor, NO nitric oxide, TNF tumor necrosis factor, PG prostaglandin, NF-ĸB nuclear factor kappa B, LPO lipid peroxidation, MAPK Mitogen-activated protein kinase, MPO myeloperoxidase
Table 2.
Animal studies regarding the effect of phytochemicals in chemotherapy-induced neuropathy
| Phytochemical & plant name | Animal model | Dosage | Outcome | Possible mechanisms | Reference |
|---|---|---|---|---|---|
| Curcumin from Curcumin longa | Cisplatin-induced neuropathy in Wistar rats | 200 mg/kg, PO |
↓Thermal hyperalgesia ↑MNCV in the 8thweek, but not in the 5th week No significant decrease in myelin thickness ↓neuron loss, nuclear & nucleolar atrophy |
↓Oxidative stress | [34] |
| Xylopic acid from Xylopia aethiopica | Vincristine-induced neuropathy in Sprague-Dawley rats | 10–100 mg/kg, PO |
↓Tactile & cold allodynia, ↓static mechanical hyperalgesia & intermediate hyperalgesia |
Inhibition of p38 &/or ERK1 & ERK2 pathways ↓NF-κB activation ↓pain stimuli propagation in the degenerated unmyelinated & myelinated C-, Aδ- & Aβ-fibers Ca2+ channel-blocking effect, Inhibition of NMDA, adrenergic (β & α) & protein kinase A/C pathways |
[17] |
| Aucubin from Plantago sp. | Paclitaxel-induced allodynia in C57BL/6NCr mice | 50 mg/kg, IP | ↓Mechanical allodynia | Antioxidant activity | [20] |
| Thioctic acid | Vincristine- induced neuropathy in Sprague–Dawley rats | 1, 5 & 10 mg/kg, IP | ↓Tactile & cold allodynia |
↓LPO, ↓IL-1β, TNF-α & NO |
[35] |
| Curcumin | Cisplatin & oxaliplatin neuropathy in Wistar rats | 10 mg/kg, PO |
↓Neurotensin, insignificant decrease in platinum concentration, ↓demyelination |
Anti-inflammatory, antiapoptotic & antioxidant | [36] |
| Euphol from Euphorbia tirucalli | PGE2-induced acute & persistent hypersensitivity in Swiss mice & Wistar Hanover rats, respectively | 30 mg/kg, PO |
↓Mechanical hypersensitivity ↓persistent hypersensitivity ↓hypersensitivity dose-dependently |
↓PKCε ↓NF-ҡB & CREB ↓COX-2 |
[37] |
| cAMP/PKA activation-induced mechanical hypersensitivity in Swiss mice | No inhibition | ||||
| PKCε activation-induced mechanical hypersensitivity in Wistar Hanover rats | ↓Hyperalgesia | ||||
| Paclitaxel-induced neuropathy in Swiss mice | ↓Mechanical hypersensitivity | ↓PKCε | |||
| Tumors-induced mechanical hypersensitivity in C57BL/6 mice | ↓Mechanical hypersensitivity | ||||
| Rutin, | Oxaliplatin-induced peripheral neuropathy in Swiss mice |
Both: 25, 50 & 100 mg/kg |
↓Mechanical & cold allodynia, |
↓MDA in spinal cord ↓Fos, nitrotyrosine & LPS-induced iNOS, |
[38] |
| Quercetin | Improvement of spinal morphological changes |
↓ROS & LPO (Quercetin) ↑GSH |
|||
| Matrine | Vincristine-induced neuropathy in mice | 15, 30 & 60 mg/kg, IP |
↓Mechanical & thermal hyperalgesia, ↓cold allodynia, |
↑SNAP & SNCV, ↓MDA, total Ca2+, MPO, TNF-α, IL-6, ↑TAOC, Gpx, SOD, IL-10 |
[39] |
| Matrine | Vincristine-induced neuropathy in mice | 15, 30 & 60 mg/kg, IP |
↓Mechanical hyperalgesia by repeated dose, ↓cold allodynia, no significant deference in thermal hyperalgesia |
Anti-inflammatory activity, ↓TNF-α, IL-1 & IL-6 |
[40] |
| Curcumin | Vincristine-induced neuropathy & formalin-induced nociception in Swiss albino mice | 15, 30 & 60 mg/kg, PO |
↑Pain threshold, ↓thermal allodynia, ↓Mechanical hyperalgesia, ↓SFI, ↓formalin-induced nociception in delayed phase, but not in acute phase ↓Total Ca ↑SOD, CAT, GPx, & GSH, ↓LPO, iNOS & NO |
↓Pro-inflammatory cytokines, Involvement of monoamine pathway |
[41] |
| Paeoniflorin | Paclitaxel-induced mechanical allodynia in C57BL/6NCr mice | 0.1 & 1%, topical |
↓Mechanical allodynia, time-dependently, ↓abnormal peripheral nerve activity, ↓demyelination & thinning, Involvement of A1 receptor, ↓CHOP |
↓Cytosolic Ca2+ ↓ER stress in Schwann cells |
[42] |
| Coumarin from Cinnamomum cassia | Oxaliplatin-induced neuropathy in Sprague-Dawley rats | 10 mg/kg, PO |
↑Mechanical threshold (insignificant), ↓cold allodynia |
↓TNF & IL-1β | [27] |
PO per oral, IP intraperitoneal, SFI sciatic functional index, LPO lipid peroxidation, MDA malondialdehyde, TNF tumor necrosis factor, NO nitric oxide, iNOS inducible nitric oxide synthase, IL interleukin, NF-ĸB nuclear factor ĸB, SOD superoxide dismutase, CAT catalase, GSH reduced glutathione, GPx glutathione peroxidase, ROS reactive oxygen species, COX cyclooxygenase, CHOP C/EBP homologous protein, ER endoplasmic reticulum, TAOC total antioxidant capacity, SNCV sensory nerve conduction velocity, SNAP sensory nerve action potential
Table 3.
Human studies regarding the effect of medicinal plants in chemotherapy-induced neuropathy
| Treatment | Design | Dosage | Duration | Results | Reference |
|---|---|---|---|---|---|
| α-lipoic acid | Randomized, double-blind, placebo-controlled trial in 70 subjects with chemotherapy-induced peripheral neuropathy | 600 mg | 24 w | No significant difference in any of the clinical outcomes | [43] |
| α-lipoic acid+ Boswellia serrata + MSM + bromelain | Prospective study in 25 subjects with chemotherapy-induced peripheral neuropathy | α-lipoic acid (240 mg) + B. serrata (40 mg) + MSM (200 mg) + bromelain (20 mg) | 12 w | ↓Pain (VAS), sensor and motor neuropathic impairment (NCI-CTC score), TNSc, mISS | [44] |
| Goshajinkigan | Randomized controlled trial in 29 patients with ovarian or endometrial cancer underwent TC therapy and developed peripheral neuropathy | 7.5 g | 6 w |
↓Frequency of abnormal CPT, No significant change in VAS, NCI-CTCAE neuropathy grade, FACT-Taxane & CPT ranges |
[45] |
| Nabiximols (oromucosal spray) | Double-blind, placebo-controlled, crossover pilot trial in 16 patients with chemotherapy-induced neuropathic pain | Maximum 12 puff per day | 24 w | ↓Pain (NRS-PI) | [46] |
| Goshajinkigan | Retrospective study in 73 colorectal cancer patients with oxaliplatin-induced peripheral neuropathy | 2.5 g, TDS | ≥4 w | ↓Deleterious effects in comparison to non-treated patients | [47] |
VAS visual analogue scale, MSM Methylsulfonylmethane, TNSC Total Neuropathy Score clinical version, mISS group sensory sum score, NCI-CTCAE National Cancer Institute–Common Toxicity Criteria for Adverse Event, NRSPI numeric rating scale for pain intensity, CPT current perception threshold, FACT-Taxane functional assessment of cancer therapy-Taxane
Chemotherapeutic agents with high risk of neuropathy
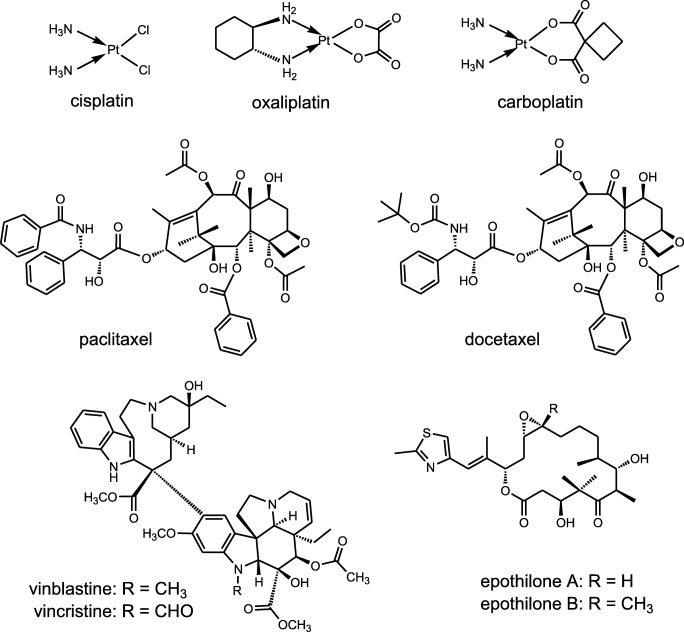
Chemotherapy agents inducing CIPN can be divided into subgroups based on their category and mechanisms of action. Platinum agents like cisplatin, oxaliplatin and carboplatin, vinca alkaloids like vincristine and vinblastine, taxanes like paclitaxel and docetaxel, epothilones like ixabepalone and newer agents such as bortezomib, thalidomide and lenalidamide are more prone to cause peripheral neuropathy (Fig. 2) [6, 7, 10].
Fig. 2.
Structures of some chemotherapeutic agents causing neurotoxicity
Platinum agents specifically produce sensory ganglionopathy through interstrand and intrastrand crosslinking of DNA or between DNA and proteins. This effect may occur for months after treatment. Amongst platinum agents, neuropathy caused by cisplatin is generally the most severe form, followed by oxaliplatin and carboplatin [48–51]. Vinca alkaloids cause neuropathy via disorientation of microtubules of mitotic spindle and disrupting the axonal transport [51, 52]. Neurotoxicity induced by taxanes is mostly sensory and the exact mechanism is still unknown. Based on a clinical study, disturbance of cell body and axonal transport as a result of formation of dysfunctional microtubules is suggested to be the main mechanism of neurotoxicity induced by taxanes [53]. Epothilones, including epothilone A, epothilone B, and epothilone D, seem to induce neurotoxicity with similar mechanism as taxanes by stabilizing the microtubules [51]. Bortezomib is classified as a proteasome inhibitor and has a multifactorial role in the pathogenesis of neuropathy. Mitochondrial injury, dysregulation of mitochondrial calcium homoeostasis, autoimmune factors, and blockade of neuronal survival are proposed to be the possible mechanisms of bortezomib-induced neuropathy [54]. Other causative chemotherapeutic agents such as thalidomide and lenalidamide are also considered to induce neuropathy. The induction of neuropathy in patients receiving chemotherapy also depends on genetic susceptibility; however, it is not yet completely demonstrated [55].
Underlying mechanisms in the pathogenesis of CIPN
Various possible mechanisms of action for plant extracts and/ or phytochemicals have been proposed as primary and/or secondary prevention of CIPN.
Inflammation is an immune response that occurs due to cell/tissue injury and initiate the secretion of proinflammatory cytokines such as interleukins (ILs) and prostaglandins to the injured site [56]. Inflammation and muscle cramps are amongst the common complications of CIPN which can be relieved with decrease in the level of prostaglandins, cytokines and antispasmodic agents [57].
Activation of μ-opioid receptors by some phytochemicals, such as salvinorin A, leads to antinociceptive effects in both peripheral and central nervous systems [58, 59].
Another important mechanism involved in the antihyperalgesic activity of phytochemicals in CIPN is the regulation of nitric oxide (NO) pathway [60, 61]. In the early stage of neurotoxicity, an increase in inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS) and intracellular calcium ion, as well as a decrease in reduced glutathione (GSH) is observed. Hence, inhibition of iNOS gene expression helps to prevent neuronal death and neuropathic pain [38].
One of the common pathways in the pathogenesis of nearly all types of inflammatory disorders, including CIPN, is the activation of nuclear factor- κB (NF-κB), p38 mitogen-activated protein kinase (MAPK), or extracellular signal-related kinase (ERK) 1 and ERK2 pathways. Moreover, the phosphorylation of ERK results in development of neuropathic pain through the activation of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) [62]. Additionally, TNF-α enhances activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor and IL-1β phosphorylates the N-methyl-D-aspartate (NMDA) receptor. This results in NO production and NMDA receptor activation which increases the influx of calcium ion which consequently develops neuropathic pain [25].
Protein kinase A, also known as cyclic adenosine monophosphate (cAMP)-dependent protein kinase, is a family of enzymes which have several cellular functions including lipid metabolism, modulating voltage-gated sodium currents, and regulating cAMP second messenger related cascades. Activation of protein kinase A mediates the hyperalgesia and downregulates μ-opioid receptors, which are related to pain regulation [17, 63]. Additionally, activation of protein kinase C (PKC), especially PKCɛ which is involved in controlling the other proteins function, is related to induce neuropathic pain through triggering pro-inflammatory pathways, sensitization of nociceptive neurons, exposing the hidden calcium channels, or change in the conductance of ion channels. PKCɛ also mediates the activation of a cellular transcription factor called cAMP response element-binding protein (CREB) and cyclooxygenase-2 (COX-2) upregulation in primary afferent neurons which are related to inflammatory processes [37, 64].
Some conventional antidepressant drugs including tricyclic antidepressants are beneficial for neuropathic pain; thus, considering their mechanism of action can be helpful in understanding the pathogenesis of neuropathy [65–67]. Decrease in monoamine contents especially noradrenaline and serotonin in spine causes nociceptive effects; therefore, inhibition of monoamine reuptake may have analgesic effects [41, 68, 69]. Adrenergic receptors (α and β) especially α2 subtype which are located on post-ganglionic sympathetic terminals are also involved in the induction of hyperalgesia [17, 70, 71].
Some studies proposed that the activation of the immune system is contributed to the induction of inflammation in the satellite cells of the dorsal root ganglion and can cause neuropathic pain [22, 72], so plant-derived natural agents with modulatory effects on immune system may prevent the pathological stimulation of immune system.
Mitochondrial dysfunction caused by chemotherapy agents as the trigger of neuronal apoptosis leads to hyperalgesia and phytochemicals with anti-apoptotic effect can be helpful to prevent this process [36, 73].
The same as several neurological disorders, reactive oxygen species (ROS) contribute to the development of neuropathy and neuropathic pain. Thus, plants with antioxidant activity, especially those preventing the lipid peroxidation, can improve the overall condition in CIPN [74]. The reaction between NO and superoxide forms peroxynitrite, a potent oxidant which leads to formation of reactive radicals that are responsible for neuronal death. Moreover, free radicals react mainly with lipids and tyrosine which is evident from the increase in nitrotyrosine and malondialdehyde (MDA) production.
Some chemotherapy agents can promote demyelination of neurons [10]. Myelin sheaths are formed by Schwann cells and are impaired through endoplasmic reticulum (ER) stress. ER is an essential cellular organelle for synthesis and folding of secretory proteins and storage of calcium ion. The perturbation of ER homeostasis such as increase in intracellular calcium ions leads to ER stress and the organelle provokes apoptotic signals.
CHOP gene expression as a marker of ER stress is upregulated during the ER stress-mediated apoptosis pathway. Inhibition of ER stress by the upregulation of CHOP can prevent neuropathic pain induced by this pathway [42, 75].
Medicinal plants used in the treatment of CIPN
Matricaria chamomilla L. (chamomile) hydroalcoholic extract mainly contains apigenin, bisabolol and chamazulene which have sedative, anti-inflammatory, anti-spasmodic and antioxidant effects. In an animal model of cisplatin-induced neuropathic pain, M. chamomilla extract demonstrated analgesic effects. The extract could also represent antinociceptive properties in both early and delayed phase of formalin test. It is worthy to mention that activation of C fiber and peripheral stimulation induces pain in the early phase of formalin test; while the inflammatory reactions are the main cause of delayed phase [15]. Chamomile extract has also exhibited neuroprotective properties via reduction of oxidative stress in several animal models [76, 77]. This effect can be partially mediated by apigenin, a flavonoid with an affinity to benzodiazepine receptors [15]. Also, neuroprotective effects of apigenin via antioxidant properties is demonstrated in several studies. This effect is evident from the reduction of biomarkers of oxidative damage such as ROS and lipid peroxidation (LPO), as well as proinflammatory cytokines [18].
Salvia officinalis L. (sage) extract has shown anti-inflammatory properties in the second phase of formalin test in a dose-dependent manner [16]. Sage hydroethanolic extract could also relieve vincristine-induced pain in a mice model of neuropathy [16]. As different species of the genus Salvia contain a diterpene called salvinorin A, an agonist of μ opioid receptors, these receptors may be involved in the antinociceptive effects of S. officinalis [78]. Rosmarinic acid of sage extract is also effective in the protection of neural cells against apoptosis via inhibition of caspase-3 overactivation and DNA damage [79].
Xylopia aethiopica (Dunal) A. Rich., commonly called as African pepper, was traditionally used for headache, neuralgia and colic pain. The ethanolic fruit extract was significantly effective for pain management in the animal model of vincristine-induced neuropathy. The plant contains diterpenes such as kaurenoic acid and xylopic acid which are responsible for antihyperalgesic and anti-inflammatory effects in neuropathic pain, possibly via inhibition of p38 and/ or ERK1 and ERK2 pathways that leads to deactivation of NF-κB, inhibition of NMDA, adrenergic and protein kinase A/C pathways [17]. The fruit extract has also demonstrated antioxidant activity via improvement of endogenous antioxidant defense mechanisms such as superoxide dismutase (SOD) and catalase (CAT) in an animal model of brain oxidative damage [80]. Xylopic acid from X. aethiopica ethanolic fruit extract has shown antihyperalgesic and anti-inflammatory effects in neuropathic pain with low toxicity [17].
Synedrella nodiflora (L.) Gaertn. (nodeweed) hydroethanolic extract has demonstrated to have antinociceptive, sedative, anticonvulsant and antioxidant effects in animal models. The extract has a low toxicity and contains several bioactive compounds such as glycosides, saponins, alkaloids and tannins which are involved in various biological activities. The extract could significantly improve neuropathic pain in vincristine- and paclitaxel-induced neuropathy in rats [19, 81]. The inhibition of painful stimuli in the degenerated unmyelinated and myelinated C-, Aδ-, and Aβ-fibers and antioxidant effects might be the probable mechanism of action against CIPN [81]. Also, since paclitaxel-induced neuropathy is involved with the activation of NMDA receptors and glutamatergic system, these pathways may be the topic of future studies to clarify the antinociceptive mechanisms of nodeweed [19].
Plantaginis Semen (seeds from Plantago sp.) is one of the ingredients of a traditional Japanese herbal formulation, goshajinkigan, that has been demonstrated to reduce the peripheral neuropathy. The plant extract could significantly improve neuropathy induced by paclitaxel in mice [20]. Aucubin, the main component of the aqueous extract, is an iridoid glycoside with strong antioxidant activity and neuroprotective effects via prevention of nuclear damage [82] which is demonstrated in several in vitro and in vivo models [83, 84]. Aucubin could attenuate the allodynia induced by paclitaxel; however, geniposide acid as its precursor and catalpol as its metabolite showed no preventive effect on neuropathic pain [20].
Palisota hirsuta (Thunb.) K.Schum. is an African herb with various traditional indications such as kidney pains, toothache and arthritis pain. Ethanolic extract of the leaves has shown significant anti-inflammatory, antioxidant and antinociceptive effects. It could attenuate the mechanical and thermal hyperalgesia and tactile allodynia in animal model of vincristine-induced neuropathic pain; while it has less effect on cold allodynia [21]. The extract has demonstrated antinociceptive activity which is partially mediated by opioid receptors via NO-cGMP-ATP-sensitive K+ channel [34]. Furthermore, it has anxiolytic and antidepressant effects in central nervous system which would be demanded for neuropathic pain [21].
Aqueous extract of Lithospermi Radix (roots of Lithospermum sp.) has a significant effect on relieving the oxaliplatin-induced neuropathy in vitro by suppressing the spinal activation of astrocytes and microglia cells and decreasing the mechanical hypersensitivity in oxaliplatin-induced neuropathy. Considerable loss of intraepidermal nerve fibers (IENFs) was observed in skin of foot pads of animals treated with oxaliplatin, a phenomenon causing numbness in clinical setting, which was also significantly improved by the plant extract. Additionally co-administration of the extract with chemotherapeutic agents did not diminish their antitumor activity in human colorectal (HCT116) and non-small cell lung carcinoma (A549) cells [22]; however, the possibility of pharmacokinetic interaction can not be ruled out unless with an in vivo study.
Astragalus is a plant genus with a wide variety of species all over the world. The hydroalcoholic and aqueous root extract of Astragalus sp. both could relieve neuropathic pain via central nervous system in oxaliplatin-induced neurotoxicity. The plant has several bioactive components such as isoflavonoids that are mostly found in hydroalcoholic extract, triterpenoid saponins, polysaccharides, and amino butyric acids. It could prevent axonal damage by downregulating the phosphorylated heavy neurofilament. Furthermore, 50% hydroalcoholic extract decreased the number of microglia and astrocytes in the dorsal horn of the spinal cord and brain and lead to decrease of pain hypersensitivity. It is found that 50% hydroalcoholic extract is the most effective preparation, possibly due to the presence of higher amount of bioactive components responsible to decrease pain hypersensitivity [23].
Maerua angolensis DC. is a medicinal plant from the family Capparaceae which is well studied regarding its pharmacological activities in nervous system such as anticonvulsant effects via GABAergic and NO-cGMP signaling [85], antidepressant and anxiolytic activities [86], as well as analgesic effects [87]. The plant showed antihyperalgesic effect in the second phase of formalin test using the petroleum ether/ethyl acetate stem bark extract. Moreover, it could effectively reduce vincristine-induced neuropathic pain via its antioxidant effects and blockade of calcium channels in sensory nerves [24].
Aconiti Tuber, commonly called Buja, is the corms of Aconitum sp. which is a component of Gyejigachulbu-tang (GBT) used as an herbal medicine in East Asia. Buja attenuates activation of astrocytes in the dorsal horn of spinal cord and downregulates the production of pro-inflammatory cytokines including TNF-α and IL-1β which results in decreased cold and mechanical allodynia in the animal model of oxaliplatin-induced neuropathy [25].
Ocimum sanctum L. (holy basil) is a plant mostly found in India and is used for several purposes in Ayurveda. The plant has demonstrated anti-tumorigenesis and nerve tonic effects in the experimental studies and has shown ameliorative effect on vincristine-induced neuropathy [26]. Also, animal studies in cerebral ischemia and in vitro evaluations on H2O2-induced neuronal cell damage suggest O. sanctum as an important neuroprotective medicinal plant which mostly acts via protection of cells from oxidative damage [88, 89]. Saponins such as ursolic acid and oleanolic acid are the main constituents of the hydroalcoholic extract of the plant proved to be responsible for attenuating vincristine-induced neuropathy via antioxidant effects and decrease in the calcium level [26].
Cinnamomum cassia (L.) D. Don (cinnamon) is a well-known medicinal plant to manage neurological problems due to the growing body of evidence supporting its neuroprotective effects. It is a well-discussed plant to improve memory [90, 91] and is recently attracted the attention of scientists in Parkinson’s disease [92]. The plant contains several compounds such as coumarins, cinnamic acid, as well as cinnamaldehyde, a major component in the essential oil which has demonstrated to regulate iNOS, COX-2 and NF-κB signaling during neuroinflammation [93]. Aqueous extract of cinnamon bark (Cinnamomi Cortex) and twig (Cinnamomi Ramulus) were both found to have analgesic effects. The oral administration of Cinnamomi Cortex extract to rats with CIPN reduced the spinal TNF level and deactivated the spinal astrocytes and microglia in a dose-dependent manner; whereas no significant effect was observed with Cinnamomi Ramulus [27]. Moreover, coumarin extracted from C. cassia is reported to have analgesic effects which can alleviate cold allodynia induced by chemotherapy agents [27].
Camellia sinensis (L.) Kuntze, commonly known as tea, is a rich source of polyphenolic compounds. According to the degree of fermentation, different types of tea are produced amongst which the most popular ones are black tea (produced under highest degree of fermentation), containing theaflavins as the main flavonoids, and green tea containing catechins such as gallocatechin, epigallocatechin, epicatechin, epigallocatechin-3-gallate (EGCG). The potent antioxidant properties of catechins prevent oxidative stress and decrease chemotherapy-related dose-limiting side effects in the early stage of therapy. Green tea could ameliorate oxaliplatin-induced allodynia in a rat model of CIPN. It also showed a synergistic anticancer effect via reducing the tumor size when co-administered with oxaliplatin; however, it could not prevent the morphometric or electrophysiological changes [28]. EGCG as one of the main components of green tea is widely studied in different models of neurological disturbances due to the antioxidant and anti-inflammatory properties [94, 95].
Agrimonia genus include several species of perennial herbs distributed in the North Temperate Zone. A. pilosa root extract contains tannins and aerial part extract contains flavonoids and triterpenoids with antioxidant effects which has demonstrated neuroprotective activities in vitro [96]. A. eupatoria could significantly alleviate cisplatin-induced neuropathic pain in rats. A. eupatoria and A. pilosa are species with nearly similar phytochemical composition like agrimony and agrimony lactone and both can alleviate CIPN [29].
Acorus calamus L. is a medicinal plant traditionally used for pain management. The aqueous and hydroalcoholic extracts contain glycosides, flavonoids, saponins, tannins, mucilage and volatile oil. Studies revealed that A. calamus exerted antioxidant, anti-inflammatory and neuroprotective effects in both central and peripheral nervous systems. The ethanolic extract showed antinociceptive properties through suppressing the voltage-gated calcium channels and inhibiting the production of the NO, IL-2 and TNF-α in animal model of CIPN [30, 31]. Saponins and β-asarone are suggested to be the major active ingredients of the plant which participate in neuroprotective properties of the plant via modulation of autophagy and anti-inflammatory properties [97, 98].
The leaf extract of Ginkgo biloba L., known as living fossil, contains flavone glycosides including quercetin, kaempferol, and isorhamnetin, as well as terpene lactones such as ginkgolides A, B, and C and bilobalide. This plant is commonly used as a supplement in different malfunctions of nervous system. It has demonstrated anti-inflammatory, antioxidant and analgesic effects in formalin-induced inflammation in rat. Ginkgo showed beneficial effects on the vincristine-induced neuropathy [32]. The plant was also effective in animal model of diabetic neuropathy which seems to be mediated via inhibition of oxidative- and nitrosative-induced neural damage [99]. Furthermore, ginkgo and its constituent, Ginkgolide B, could regulate the cellular pro-apoptotic pathways in an in vitro multicellular model of neural damage [100].
Tithonia tubaeformis (Jacq.) Cass. is a Mexican medicinal plant from Asteraceae family. Hydromethanolic extract of the plant showed significant analgesic effects in vincristine-induced animal model of neuropathy. Since the plant is not yet well investigated, future studies are necessary to clarify the main active ingredients, as well as the mechanisms of its antinociceptive activity [33].
Phytochemicals used in the treatment of CIPN
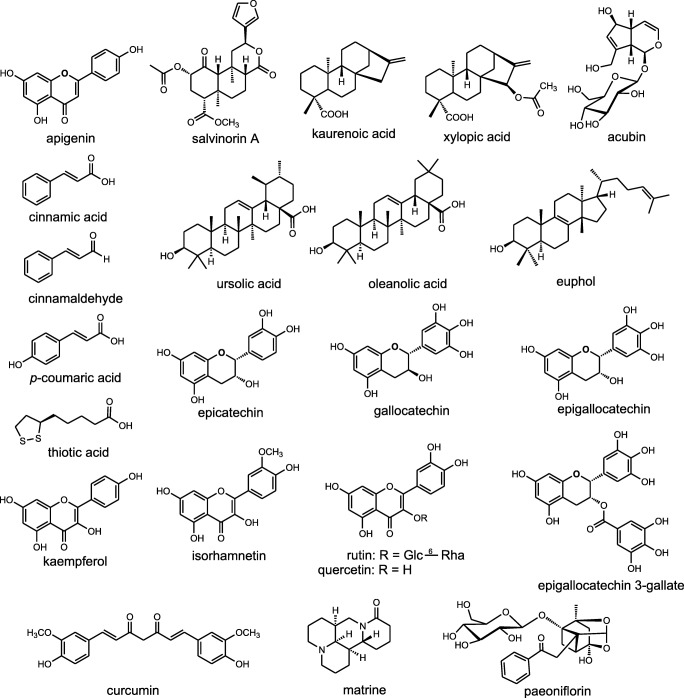
Fig. 3 shows the chemical structure of phytochemicals assessed in CIPN.
Fig. 3.
Chemical structure of phytochemicals assessed in chemotherapy-induced peripheral neuropathy
Curcumin, the main component of Curcuma longa L. (turmeric), is a polyphenol with antioxidant, antineoplastic and anti-inflammatory effects. Curcumin can improve myelin loss induced by chemotherapy agents through the reduction of oxidative stress and can alleviate both functional and structural defects observed in neuropathy. The co-administration of curcumin with chemotherapeutic drugs also increases the anti-proliferative activity of conventional anticancer agents in in vitro and in vivo models. Curcumin has analgesic effect only in the delayed phase of formalin test in animal model which shows its peripheral activity. In addition, it exhibited antinociceptive effects in a hot plate model, indicating its central activity [41]. Neuroprotective mechanisms of curcumin are studied in a growing body of literature which suggest this compound to have beneficial modulatory effects on NF-ĸB, COX-2, oxidative damage, and different types of neurotoxicity [101]. Curcumin is a relatively safe agent and can be tolerated at even high doses in human [36, 102]; however, preclinical studies showed pharmacokinetic interactions between curcumin and conventional drugs and thus, its concomitant administration with chemotherapeutic agents should be under close observation [103].
Paeoniflorin is a monoterpene glycoside extracted from Paeonia lactiflora Pall. root (Paeoniae Radix) and demonstrated to ameliorate mechanical allodynia induced by paclitaxel which seems to be mediated via adenosine A1 receptors [42]. In addition to its analgesic properties, it has neuroprotective activity via antioxidant and anti-apoptotic activity which is demonstrated in several in vitro and in vivo models of neurological disorders such as Alzheimer’s disease and Parkinson’s disease [104–106].
Rutin and Quercetin are flavonoids commonly found in numerous medical plants, as well as fruits and vegetables. Rutin is a water-soluble flavonoid that converts to quercetin in blood. Rutin and quercetin both have antioxidant, analgesic and anti-inflammatory effects. Antioxidant activity of quercetin has been found to be four times greater than Trolox (vitamin E analog) that leads to neural protection against oxidative stress [38]. Both flavonoid could significantly reduce mechanical and thermal hyperalgesia due to oxaliplatin-induced neuropathy which was, at least in part, due to the inhibitory effect on iNOS and oxidative stress [38]. Nrf2 and paraoxonase 2 (an endogenous antioxidant and anti-inflammatory enzyme) are also suggested as two main pathways being affected as a result of quercetin neuroprotective activity [107].
Matrine, a quinolizidine alkaloid, is a major bioactive compound of Sophora flavescens Aiton extract which showed anti-inflammatory, immunosuppressive and nociceptive properties. Repeated co-administration of matrine and anticancer drugs decreased mechanical allodynia in the animal model of vincristine-induced neuropathy with the lowest dose (15 mg/ kg) showing the best analgesic effect [39]. The effect was mediated via modulation of endogenous antioxidant defense mechanisms, as well as pro-inflammatory cytokines [39, 40].
Euphol, as main bioactive compound of Euphorbia tirucalli L., is a tetracyclic triterpene which has anticancer and anti-inflammatory properties [37]. Acute repeated administration of euphol produced dose-dependent antinociception and analgesic effects on peripheral neuropathy induced by paclitaxel. Several mechanisms are suggested for the analgesic properties of euphol including the involvement of PKCε, NF-ĸB, COX-2, and CREB [37], as well as a possible role for cannabinoid receptors [108].
Thioctic acid (α-lipoic acid) as a biological antioxidant has anti-inflammatory effect and reduces allodynia induced by vincristine via increase in nerve blood flow and conduction velocity as seen in in vivo test model [35]. There are also some clinical studies on this agent which are discussed below.
Clinical studies on the herbal medicines for CIPN
There are few number of clinical studies regarding the effect of herbal medicines in CIPN. Goshajinkigan is a polyherbal Kampo medicine from Japan containing Plantaginis Semen, Cinnamomi Cortex and eight other medicinal plants which is clinically assessed in several types of neuropathies. In a clinical study in 29 cancer patients underwent treatment with paclitaxel/carboplatin, Goshajinkigan was evaluated as a supplement for the management of CIPN. Six-week administration of Goshajinkigan, along with vitamin B12 showed analgesic effects in regard to visual analogue scale (VAS); however, determination of current perception thresholds (CPT) revealed no significant difference with the control group who only received vitamin B12 [45]. On the other hand, Yoshida et al. (2013) assessed the effect of the supplement in colorectal cancer patients treated with oxalipatin. This retrospective study compared the results of Goshajinkigan with those received no treatment for their CIPN. The analyses showed that Goshajinkigan-treated patients experienced fewer complications as the incidence of grade 3 CIPN was significantly lower compared with the non-treated patients [47]. This suggests that the negative results of the former study might be due to the administration of vitamin B12, a neuroprotective agent, in both test and control groups. Also, the type of cancer, as well as the chemotherapy was different between the two studies which may be involved in the controversial results observed in these two trials.
In a pilot study in 16 patients suffering from CIPN, an oromucosal spray containing cannabinoids (nabiximols) was evaluated for 24 weeks. Although the overall difference in pain was not significant between the two groups, a responder’s analysis showed lower incidence of pain in the nabiximols group [46]. Due to the small sample size of this study, future trials with larger sample sizes are essential to determine the overall efficacy of nabiximols in CIPN.
A multicomponent natural supplement containing 240 mg of α-lipoic acid, 40 mg of Boswellia serrata (frankincense), 200 mg of methylsulfonylmethane (MSM), and 20 mg of bromelain (a proteolytic enzyme naturally obtained from pineapple (Ananas comosus (L.) Merr.)) was clinically examined for its protective activity against CIPN. After 12 weeks of administration in 25 patients, the supplement could significantly decrease both sensory and motor impairments due to CIPN in comparison to the baseline values. Also, the supplement had no effect on the efficacy of the chemotherapeutic regimen; thus, the pharmacodynamics and pharmacokinetics of the anticancer agents seem to be unaltered [44]. It should be mentioned that α-lipoic acid was individually administered to patients with CIPN in another clinical trial with a dose of 600 mg per day; however, no significant clinical outcome was observed in comparison to placebo. High drop out rate of the latter trial may be a reason for this negative result which should be considered in future studies [43].
Discussion
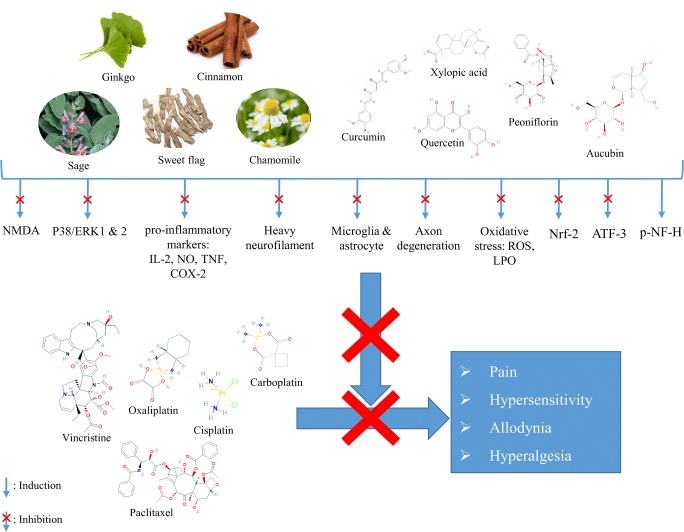
Chemotherapy, as one of the main treatment modalities of cancer, has several debilitating side effects amongst which one of the most important ones is neuropathy. Due to the failure of conventional therapies to totally control the condition, studies are seeking new approaches such as complementary and alternative medicine (CAM) to manage CIPN. Figure 4 shows a schematic view of the main mechanisms by which medicinal plants and their active components can prevent CIPN.
Fig. 4.
Schematic mechanisms of medicinal plants to prevent chemotherapy-induced neuropathy: NO: nitric oxide, TNF: tumor necrosis factor, PG: prostaglandin, NF-ĸB: nuclear factor kappa B, LPO: lipid peroxidation, ROS: reactive oxygen species, COX: cyclooxygenase, IL: interleukin, ERK: extracellular signal-related kinase, X: inhibition, ↓: induction
Several medicinal plants have shown beneficial effects in animal models of CIPN via different mechanisms such as anti-inflammatory, antinociceptive, and antioxidant properties. Such general mechanisms can help medicinal plants to be effective in most diseases whose pathologies are involved with inflammation and oxidative stress, such as CIPN. One of the gaps of the included studies is lack of detailed assessment of exact cellular and extracellular signaling pathways involved in the pharmacological activity of the evaluated plants. Only few number of studies straightly measured the level of inflammatory signaling mediators like Nrf2 or biomarkers of oxidative stress [23]. Moreover, most of the included studies suggested the “possible” mechanism of action based on the previously reported data on the pharmacological activities of the medicinal plants and/or their phytochemicals in pathological conditions other than CIPN. Thus, future studies are necessary to assess the detailed mechanisms of action in the specific animal models to better understand the molecular targets involved in CIPN treatment.
The most common categories of phytochemicals effective in CIPN are polyphenols such as curcumin and quercetin, as well as terpenoids like aucubin and xylopic acid which can give directions for the future researches. There are several polyphenol-rich and terpenoid-rich plants which can be the next candidates for the preclinical studies of CIPN.
Current review supports the beneficial effects of some medicinal plants in the animal models of CIPN; however, it should be considered that there is a long path from lab to clinic. There are only five clinical studies assessing natural agents in patients suffering from CIPN which provides a relatively low level of evidence. This can be due to the extremely complicated condition of the patients under chemotherapy. Chemotherapeutic agents are highly toxic and have narrow therapeutic indices, i.e. a serum concentration lower than the required level results in treatment failure and a higher level leads to severe adverse effects. This makes the oncologists to act cautious in accepting CAM suggestions in such patients. There is a growing body of evidence demonstrating the high risk of herb-drug interactions in concomitant use of medicinal plants with conventional drugs which, in some cases, lead to irreversible life-threatening events [103, 109]. In this regard, one of the ways to solve the problem is to look for local treatment options by which the risk of herb-drug interaction is ruled out. Also, sublingual administration of medicinal plants, such as in the form of nabiximoles oromucosal spray [46], can exclude the first pass metabolism of the phytochemicals; thus, lower total administered dose is needed which decreases the possibility of the drug interactions.
Plants such as chamomile, cinnamon, or tea are being used for thousands of years and are generally considered as safe agents with several clinical trials regarding their safety and efficacy in other indications. So, they can be evaluated in patients suffering from CIPN considering the previous dosing and safety data. Also, there are several medicinal plants investigated in other types of neuropathies like carpal tunnel syndrome or diabetic neuropathy which have shown beneficial effects in clinical trials; thus, may be considered as effective candidates in CIPN, as well. Additionally, as one of the main mechanisms of chemotherapeutic agents in the induction of neuropathy is oxidative damage to neural cells, antioxidant plants or phytochemicals with previously demonstrated antioxidant and neuroprotective activities can also be effective to manage this type of neuropathy, and thus should be consider for future CIPN studies.
Current review showed that there are several medicinal plants and phytochemicals which are able to decrease the symptoms of neuropathy in animal models of CIPN. Curcumin, rutin, quercetin, matrine, euphol, thioctic acid, rosmarinic acid and cannabinoids are the most relevant natural products with therapeutic effects in CIPN. Also, results obtained from present review revealed that medicinal plants including dunal (Xylopia aethiopica), nodeweed (Synedrella nodiflora), chamomile (Matricaria chamomilla L.), sage (Salvia officinalis L.), cinnamon (Cinnamomum cassia L.), and sweet flag (Acorus calamus L.) posssess protective or therapeutic activities in CIPN. Medicinal plant and their phytochmeicals perform their beneficial effects in CIPN through different pharmacological mechanisms including regulation of neural cell apoptosis, nitrergic and NF-ĸB pathways, inflammatory cytokines transduction signaling (TNF-α, ILs, COX-2), reduction of total calcium level, prevention of inflammatory response and axonal degeneration, as well as reinforcement of enzymatic antioxidant agents like SOD and CAT.
Conclusion
Overall, medicinal plants and phytochemicals are valuable sources of natural agents with beneficial effects in CIPN; however, current available evidences cannot fully support their application in human. Future mechanistic studies as well as well-designed clinical trials are essential to evaluate the safety and efficacy of medicinal plants and their isolated phytochemicals in patients with CIPN.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hughes RAC. Peripheral neuropathy. Br Med J. 2002;324(7335):466–469. doi: 10.1136/bmj.324.7335.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62(4):310–318. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller G. Diagnosing and managing mononeuropathies. Clin Med (Lond) 2004;4(2):113–117. doi: 10.7861/clinmedicine.4-2-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cascella M. Chemotherapy-induced peripheral neuropathy: limitations in current prophylactic strategies and directions for future research. Curr Med Res Opin. 2017;33(6):981–984. doi: 10.1080/03007995.2017.1284051. [DOI] [PubMed] [Google Scholar]
- 5.Malik B, Stillman M. Chemotherapy-induced peripheral neuropathy. Curr Neurol Neurosci Rep. 2008;8(1):56–65. doi: 10.1007/s11910-008-0010-5. [DOI] [PubMed] [Google Scholar]
- 6.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Brzeziński K. Chemotherapy-induced peripheral neuropathy. Part II. Prevention. Contemp Oncol (Pozn) 2012;16(3):258–261. doi: 10.5114/wo.2012.29296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Wong R, Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy–a case series. Acupunct Med. 2006;24(2):87–91. doi: 10.1136/aim.24.2.87. [DOI] [PubMed] [Google Scholar]
- 10.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 11.Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2014;(3):Cd005228. [DOI] [PubMed]
- 12.Bahramsoltani R, Farzaei MH, Farahani MS, Rahimi R. Phytochemical constituents as future antidepressants: a comprehensive review. Rev Neurosci. 2015;26(6):699–719. doi: 10.1515/revneuro-2015-0009. [DOI] [PubMed] [Google Scholar]
- 13.Farzaei MH, Shahpiri Z, Mehri MR, Bahramsoltani R, Rezaei M, Raeesdana A, Rahimi R. Medicinal plants in neurodegenerative diseases: perspective of traditional Persian medicine. Curr Drug Metab. 2018;19(5):429–442. doi: 10.2174/1389200219666180305150256. [DOI] [PubMed] [Google Scholar]
- 14.Shahpiri Z, Bahramsoltani R, Hosein Farzaei M, Farzaei F, Rahimi R. Phytochemicals as future drugs for Parkinson’s disease: a comprehensive review. Rev Neurosci. 2016;27(6):651–668. doi: 10.1515/revneuro-2016-0004. [DOI] [PubMed] [Google Scholar]
- 15.Abad ANA, Nouri MHK, Gharjanie A, Tavakoli F. Effect of Matricaria chamomilla Hydroalcoholic extract on cisplatin-induced neuropathy in mice. Chin J Nat Med. 2011;9(2):126–131. [Google Scholar]
- 16.Abad ANA, Nouri MHK, Tavakkoli F. Effect of Salvia officinalis hydroalcoholic extract on vincristine-induced neuropathy in mice. Chin J Nat Med. 2011;9(5):354–358. [Google Scholar]
- 17.Ameyaw EO, Woode E, Boakye-Gyasi E, Abotsi WKM, Kyekyeku JO, Adosraku RK. Anti-Allodynic and anti-hyperalgesic effects of an ethanolic extract and xylopic acid from the fruits of Xylopia aethiopica in murine models of neuropathic pain. Pharm Res. 2014;6(2):172–179. doi: 10.4103/0974-8490.129041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabavi SF, Khan H, D'onofrio G, Šamec D, Shirooie S, Dehpour AR, Argüelles S, Habtemariam S, Sobarzo-Sanchez E. Apigenin as neuroprotective agent: of mice and men. Pharmacol Res. 2018;128:359–365. doi: 10.1016/j.phrs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Amoateng P, Adjei S, Osei-Safo D, Kukuia KKE, Kretchy IA, Sarkodie JA, N'Guessan BB. Analgesic effects of a hydro-ethanolic whole plant extract of Synedrella nodiflora (L.) Gaertn in paclitaxel-induced neuropathic pain in rats. BMC Res Notes. 2017;10(1):226. doi: 10.1186/s13104-017-2551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andoh T, Kato M, Kitamura R, Mizoguchi S, Uta D, Toume K, Komatsu K, Kuraishi Y. Prophylactic administration of an extract from Plantaginis semen and its major component aucubin inhibits mechanical allodynia caused by paclitaxel in mice. J Tradit Complement Med. 2016;6(3):305–308. doi: 10.1016/j.jtcme.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boakye-Gyasi E, Kasanga EA, Biney RP, Abotsi WKM, Mensah KB, Woode E. Ameliorative effects of ethanolic leaf extract of Palisota hirsuta K. Schum (Commelinaceae) on vincristine-induced neuropathic pain in rats. J Applied Pharmaceut Sci. 2014;4(11):35–41. [Google Scholar]
- 22.Cho ES, Yi JM, Park JS, Lee YJ, Lim CJ, Bang OS, Kim NS. Aqueous extract of Lithospermi radix attenuates oxaliplatin-induced neurotoxicity in both in vitro and in vivo models. BMC Complement Altern Med. 2016;16(1):419. doi: 10.1186/s12906-016-1396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Cesare ML, Pacini A, Micheli L, Femia AP, Maresca M, Zanardelli M, et al. Astragali radix: could it be an adjuvant for oxaliplatin-induced neuropathy? Sci Rep. 2017;7:42021. doi: 10.1038/srep42021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Iliya HA, Abotsi WKM, Benneh C, Woode E. Maerua angolensis extract reduces allodynia and hyperalgesia in a mouse model of vincristine-induced peripheral neuropathy. J Applied Pharmaceut Sci. 2016;6(5):124–130. [Google Scholar]
- 25.Jung Y, Lee JH, Kim W, Yoon SH, Kim SK. Anti-allodynic effect of Buja in a rat model of oxaliplatin-induced peripheral neuropathy via spinal astrocytes and pro-inflammatory cytokines suppression. BMC Complement Altern Med. 2017;17(1). 10.1186/s12906-017-1556-z. [DOI] [PMC free article] [PubMed]
- 26.Kaur G, Jaggi AS, Singh N. Exploring the potential effect of Ocimum sanctum in vincristine-induced neuropathic pain in rats. J Brachial Plex Peripher Nerve Inj. 2010;5(1). [DOI] [PMC free article] [PubMed]
- 27.Kim C, Lee JH, Kim W, Li D, Kim Y, Lee K, et al. The Suppressive Effects of Cinnamomi Cortex and Its Phytocompound Coumarin on Oxaliplatin-Induced Neuropathic Cold Allodynia in Rats. Molecules. 2016;21(9). [DOI] [PMC free article] [PubMed]
- 28.Lee JS, Kim YT, Jeon EK, Won HS, Cho YS, Ko YH. Effect of green tea extracts on oxaliplatin-induced peripheral neuropathy in rats. BMC Complement Altern Med. 2012;12:124. doi: 10.1186/1472-6882-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KH, Rhee KH. Anti-nociceptive effect of agrimonia eupatoria extract on a cisplatin-induced neuropathic model. Afr J Tradit Complement Altern Med. 2016;13(5):139–144. doi: 10.21010/ajtcam.v13i5.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthuraman A, Singh N. Attenuating effect of hydroalcoholic extract of Acorus calamus in vincristine-induced painful neuropathy in rats. J Nat Med. 2011;65(3–4):480–487. doi: 10.1007/s11418-011-0525-y. [DOI] [PubMed] [Google Scholar]
- 31.Muthuraman A, Singh N, Jaggi AS. Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food Chem Toxicol. 2011;49(10):2557–2563. doi: 10.1016/j.fct.2011.06.069. [DOI] [PubMed] [Google Scholar]
- 32.Park HJ, Lee HG, Kim YS, Lee JY, Jeon JP, Park C, Moon DE. Ginkgo biloba extract attenuates hyperalgesia in a rat model of vincristine-induced peripheral neuropathy. Anesth Analg. 2012;115(5):1228–1233. doi: 10.1213/ANE.0b013e318262e170. [DOI] [PubMed] [Google Scholar]
- 33.Nawaz NUA, Saeed M, Rauf K, Usman M, Arif M, Ullah Z, Raziq N. Antinociceptive effectiveness of Tithonia tubaeformis in a vincristine model of chemotherapy-induced painful neuropathy in mice. Biomed Pharmacother. 2018;103:1043–1051. doi: 10.1016/j.biopha.2018.04.115. [DOI] [PubMed] [Google Scholar]
- 34.Woode E, Boakey-Giasi E, Ainooson GK, Ansah C, Duwiejua M. Anti-nociceptive effects and the mechanism of Palisota hirsuta K. Schum. Leaf extract in murine models. Int J Pharmacol. 2009;5(2):101–113. [Google Scholar]
- 35.Kahng J, Kim TK, Chung EY, Kim YS, Moon JY. The effect of thioctic acid on allodynia in a rat vincristine-induced neuropathy model. J Int Med Res. 2015;43(3):350–355. doi: 10.1177/0300060515569287. [DOI] [PubMed] [Google Scholar]
- 36.Al Moundhri MS, Al-Salam S, Al Mahrouqee A, Beegam S, Ali BH. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: some behavioral, biochemical, and histopathological studies. J Med Toxicol. 2013;9(1):25–33. doi: 10.1007/s13181-012-0239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutra RC, Bicca MA, Segat GC, Silva KABS, Motta EM, Pianowski LF, Costa R, Calixto JB. The antinociceptive effects of the tetracyclic triterpene euphol in inflammatory and neuropathic pain models: the potential role of PKCε. Neuroscience. 2015;303:126–137. doi: 10.1016/j.neuroscience.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 38.Azevedo MI, Pereira AF, Nogueira RB, Rolim FE, Brito GA, Wong DV, et al. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol Pain. 2013;9:53. doi: 10.1186/1744-8069-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong SS, Li YX, Zhang MT, Du J, Ma PS, Yao WX, et al. Neuroprotective effect of Matrine in mouse model of vincristine-induced neuropathic pain. Neurochem Res. 2016;41(11):3147–3159. doi: 10.1007/s11064-016-2040-8. [DOI] [PubMed] [Google Scholar]
- 40.Dun L, Li Y, Xu Y, Zhou R, Ma L, Jin S, et al. Antinociceptive effect of matrine on vincristine-induced neuropathic pain model in mice. Neurol Sci. 2014;35(6):815–821. doi: 10.1007/s10072-013-1603-6. [DOI] [PubMed] [Google Scholar]
- 41.Babu A, Prasanth KG, Balaji B. Effect of curcumin in mice model of vincristine-induced neuropathy. Pharm Biol. 2015;53(6):838–848. doi: 10.3109/13880209.2014.943247. [DOI] [PubMed] [Google Scholar]
- 42.Andoh T, Kobayashi N, Uta D, Kuraishi Y. Prophylactic topical paeoniflorin prevents mechanical allodynia caused by paclitaxel in mice through adenosine A1 receptors. Phytomedicine. 2017;25:1–7. doi: 10.1016/j.phymed.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Guo Y, Jones D, Palmer JL, Forman A, Dakhil SR, Velasco MR, Weiss M, Gilman P, Mills GM, Noga SJ, Eng C, Overman MJ, Fisch MJ. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: a randomized, double-blind, placebo-controlled trial. Support Care Cancer. 2014;22(5):1223–1231. doi: 10.1007/s00520-013-2075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desideri I, Francolini G, Becherini C, Terziani F, Delli Paoli C, Olmetto E, Loi M, Perna M, Meattini I, Scotti V, Greto D, Bonomo P, Sulprizio S, Livi L. Use of an alpha lipoic, methylsulfonylmethane and bromelain dietary supplement (opera((R))) for chemotherapy-induced peripheral neuropathy management, a prospective study. Med Oncol. 2017;34(3):46. doi: 10.1007/s12032-017-0907-4. [DOI] [PubMed] [Google Scholar]
- 45.Kaku H, Kumagai S, Onoue H, Takada A, Shoji T, Miura F, et al. Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: a multicenter collaborative study. Exp Ther Med. 2012;3(1):60–65. doi: 10.3892/etm.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manag. 2014;47(1):166–173. doi: 10.1016/j.jpainsymman.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida N, Hosokawa T, Ishikawa T, Yagi N, Kokura S, Naito Y, et al. Efficacy of goshajinkigan for oxaliplatin-induced peripheral neuropathy in colorectal cancer patients. J Oncol. 2013;2013:139740. doi: 10.1155/2013/139740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8(1):10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poklar N, Pilch DS, Lippard SJ, Redding EA, Dunham SU, Breslauer KJ. Influence of cisplatin intrastrand crosslinking on the conformation, thermal stability, and energetics of a 20-mer DNA duplex. Proc Natl Acad Sci U S A. 1996;93(15):7606–7611. doi: 10.1073/pnas.93.15.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudd GN, Hartley JA, Souhami RL. Persistence of cisplatin-induced DNA interstrand crosslinking in peripheral blood mononuclear cells from elderly and young individuals. Cancer Chemother Pharmacol. 1995;35(4):323–326. doi: 10.1007/BF00689452. [DOI] [PubMed] [Google Scholar]
- 51.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 52.Tanner KD, Levine JD, Topp KS. Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J Comp Neurol. 1998;395(4):481–492. [PubMed] [Google Scholar]
- 53.Hagiwara H, Sunada Y. Mechanism of taxane neurotoxicity. Breast Cancer. 2004;11(1):82–85. doi: 10.1007/BF02968008. [DOI] [PubMed] [Google Scholar]
- 54.Broyl A, Corthals SL, Jongen JL, van der Holt B, Kuiper R, de Knegt Y, et al. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol. 2010;11(11):1057–1065. doi: 10.1016/S1470-2045(10)70206-0. [DOI] [PubMed] [Google Scholar]
- 55.Chaudhry V, Cornblath D, Corse A, Freimer M, Simmons-O’Brien E, Vogelsang G. Thalidomide-induced neuropathy. Neurology. 2002;59(12):1872–1875. doi: 10.1212/01.wnl.0000037480.59194.85. [DOI] [PubMed] [Google Scholar]
- 56.Ferrero-Miliani L, Nielsen O, Andersen P, Girardin S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147(2):227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siegal T, Haim N. Cisplatin-induced peripheral neuropathy. Frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer. 1990;66(6):1117–1123. doi: 10.1002/1097-0142(19900915)66:6<1117::aid-cncr2820660607>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 58.Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of μ-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367(3):375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 59.Obara I, Makuch W, Spetea M, Schutz J, Schmidhammer H, Przewlocki R, et al. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur J Pharmacol. 2007;558(1–3):60–67. doi: 10.1016/j.ejphar.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 60.Bujalska M, Makulska-Nowak H. Bradykinin receptors antagonists and nitric oxide synthase inhibitors in vincristine and streptozotocin induced hyperalgesia in chemotherapy and diabetic neuropathy rat model. Neuro Endocrinol Lett. 2009;30(1):144–152. [PubMed] [Google Scholar]
- 61.Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93(3):584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- 62.Castrillo A, de Las HB, Hortelano S, Rodriguez B, Villar A, Bosca L. Inhibition of the nuclear factor kappa B (NF-kappa B) pathway by tetracyclic kaurene diterpenes in macrophages. Specific effects on NF-kappa B-inducing kinase activity and on the coordinate activation of ERK and p38 MAPK. J Biol Chem. 2001;276(19):15854–15860. doi: 10.1074/jbc.M100010200. [DOI] [PubMed] [Google Scholar]
- 63.Aley KO, Levine JD. Role of protein kinase a in the maintenance of inflammatory pain. J Neurosci. 1999;19(6):2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coderre TJ. Contribution of protein kinase C to central sensitization and persistent pain following tissue injury. Neurosci Lett. 1992;140(2):181–184. doi: 10.1016/0304-3940(92)90097-q. [DOI] [PubMed] [Google Scholar]
- 65.McQuay H, Tramer M, Nye B, Carroll D, Wiffen P, Moore R. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2–3):217–227. doi: 10.1016/s0304-3959(96)03140-5. [DOI] [PubMed] [Google Scholar]
- 66.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 67.Uhm JH, Yung WK. Neurologic complications of cancer therapy. Curr Treat Options Neurol. 1999;1(5):428–437. doi: 10.1007/s11940-996-0006-x. [DOI] [PubMed] [Google Scholar]
- 68.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 69.Zhao X, Xu Y, Zhao Q, Chen C-R, Liu A-M, Huang Z-L. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: descending monoamine system and opioid receptors are differentially involved. Neuropharmacology. 2012;62(2):843–854. doi: 10.1016/j.neuropharm.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 70.Tracey DJ, Cunningham JE, Romm MA. Peripheral hyperalgesia in experimental neuropathy: mediation by α2-adrenoreceptors on post-ganglionic sympathetic terminals. Pain. 1995;60(3):317–327. doi: 10.1016/0304-3959(94)00141-z. [DOI] [PubMed] [Google Scholar]
- 71.Woode E, Ameyaw E, Ainooson G, Abotsi W, Gyasi E, Kyekyeku J. Analgesic effects of an ethanol extract of the fruits of xylopia aethiopica and xylopic acid in murine models of pain: possible mechanism (s) Pharmacologia. 2013;4(4):285–300. [Google Scholar]
- 72.Warwick RA, Hanani M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur J Pain. 2013;17(4):571–580. doi: 10.1002/j.1532-2149.2012.00219.x. [DOI] [PubMed] [Google Scholar]
- 73.Cavaletti G, Alberti P, Frigeni B, Piatti M, Susani E. Chemotherapy-induced neuropathy. Curr Treat Options Neurol. 2011;13(2):180–190. doi: 10.1007/s11940-010-0108-3. [DOI] [PubMed] [Google Scholar]
- 74.Hassler SN, Johnson KM, Hulsebosch CE. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J Neurochem. 2014;131(4):413–417. doi: 10.1111/jnc.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bánhegyi G, Baumeister P, Benedetti A, Dong D, Fu Y, Lee AS, et al. Endoplasmic reticulum stress. Ann N Y Acad Sci. 2007;1113(1):58–71. doi: 10.1196/annals.1391.007. [DOI] [PubMed] [Google Scholar]
- 76.Ranpariya VL, Parmar SK, Sheth NR, Chandrashekhar VM. Neuroprotective activity of Matricaria recutita against fluoride-induced stress in rats. Pharm Biol. 2011;49(7):696–701. doi: 10.3109/13880209.2010.540249. [DOI] [PubMed] [Google Scholar]
- 77.Chandrashekhar VM, Ranpariya VL, Ganapaty S, Parashar A, Muchandi AA. Neuroprotective activity of Matricaria recutita Linn against global model of ischemia in rats. J Ethnopharmacol. 2010;127(3):645–651. doi: 10.1016/j.jep.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Grundmann O, Phipps SM, Zadezensky I, Butterweck V. Salvia divinorum and salvinorin a: an update on pharmacology and analytical methodology. Planta Med. 2007;73(10):1039–1046. doi: 10.1055/s-2007-981566. [DOI] [PubMed] [Google Scholar]
- 79.Iuvone T, De Filippis D, Esposito G, D'Amico A, Izzo AA. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J Pharmacol Exp Ther. 2006;317(3):1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- 80.Adaramoye OA, Popoola BO, Farombi EO. Effects of Xylopia aethiopica (Annonaceae) fruit methanol extract on gamma-radiation-induced oxidative stress in brain of adult male Wistar rats. Acta Biol Hung. 2010;61(3):250–261. doi: 10.1556/ABiol.61.2010.3.2. [DOI] [PubMed] [Google Scholar]
- 81.Amoateng P, Adjei S, Osei-Safo D, Ameyaw EO, Ahedor B, N'Guessan BB, et al. A hydro-ethanolic extract of Synedrella nodiflora (L.) Gaertn ameliorates hyperalgesia and allodynia in vincristine-induced neuropathic pain in rats. J Basic Clin Physiol Pharmacol. 2015;26(4):383–394. doi: 10.1515/jbcpp-2014-0084. [DOI] [PubMed] [Google Scholar]
- 82.Xue HY, Gao GZ, Lin QY, Jin LJ, Xu YP. Protective effects of aucubin on H2O2 -induced apoptosis in PC12 cells. Phytother Res. 2012;26(3):369–374. doi: 10.1002/ptr.3562. [DOI] [PubMed] [Google Scholar]
- 83.Xue HY, Lu YN, Fang XM, Xu YP, Gao GZ, Jin LJ. Neuroprotective properties of aucubin in diabetic rats and diabetic encephalopathy rats. Mol Biol Rep. 2012;39(10):9311–9318. doi: 10.1007/s11033-012-1730-9. [DOI] [PubMed] [Google Scholar]
- 84.Xue HY, Jin L, Jin LJ, Li XY, Zhang P, Ma YS, Lu YN, Xia YQ, Xu YP. Aucubin prevents loss of hippocampal neurons and regulates antioxidative activity in diabetic encephalopathy rats. Phytother Res. 2009;23(7):980–986. doi: 10.1002/ptr.2734. [DOI] [PubMed] [Google Scholar]
- 85.Benneh CK, Biney RP, Tandoh A, Ampadu FA, Adongo DW, Jato J, Woode E. Maerua angolensis DC. (Capparaceae) Stem Bark Extract Protects against Pentylenetetrazole-Induced Oxidative Stress and Seizures in Rats. Evid Based Complement Alternat Med. 2018;2018:9684138. doi: 10.1155/2018/9684138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benneh CK, Biney RP, Adongo DW, Mante PK, Ampadu FA, Tandoh A, et al. Anxiolytic and antidepressant effects of Maerua angolensis DC. Stem Bark Extract in Mice. Depress Res Treat. 2018;2018:1537371. doi: 10.1155/2018/1537371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azi IH, Boakye-Gyasi E, Donatus AW, Agyei AF, Woode E. Antinociceptive activity of various solvent extracts of Maerua angolensis DC stem bark in rodents. J Phytopharmacol. 2014;3(1):1–8. [Google Scholar]
- 88.Venuprasad MP, Hemanth Kumar K, Khanum F. Neuroprotective effects of hydroalcoholic extract of Ocimum sanctum against H2O2 induced neuronal cell damage in SH-SY5Y cells via its antioxidative defence mechanism. Neurochem Res. 2013;38(10):2190–2200. doi: 10.1007/s11064-013-1128-7. [DOI] [PubMed] [Google Scholar]
- 89.Yanpallewar SU, Rai S, Kumar M, Acharya SB. Evaluation of antioxidant and neuroprotective effect of Ocimum sanctum on transient cerebral ischemia and long-term cerebral hypoperfusion. Pharmacol Biochem Behav. 2004;79(1):155–164. doi: 10.1016/j.pbb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Momtaz S, Hassani S, Khan F, Ziaee M, Abdollahi M. Cinnamon, a promising prospect towards Alzheimer's disease. Pharmacol Res. 2018;130:241–258. doi: 10.1016/j.phrs.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Madhavadas S, Subramanian S. Cognition enhancing effect of the aqueous extract of Cinnamomum zeylanicum on non-transgenic Alzheimer's disease rat model: biochemical, histological, and behavioural studies. Nutr Neurosci. 2017;20(9):526–537. doi: 10.1080/1028415X.2016.1194593. [DOI] [PubMed] [Google Scholar]
- 92.Bae WY, Choi JS, Jeong JW. The Neuroprotective Effects of Cinnamic Aldehyde in an MPTP Mouse Model of Parkinson's Disease. Int J Mol Sci. 2018;19(2). [DOI] [PMC free article] [PubMed]
- 93.Chen YF, Wang YW, Huang WS, Lee MM, Wood WG, Leung YM, Tsai HY. Trans-Cinnamaldehyde, an essential oil in cinnamon powder, ameliorates cerebral ischemia-induced brain injury via inhibition of Neuroinflammation through attenuation of iNOS, COX-2 expression and NFκ-B signaling pathway. NeuroMolecular Med. 2016;18(3):322–333. doi: 10.1007/s12017-016-8395-9. [DOI] [PubMed] [Google Scholar]
- 94.Chen SQ, Wang ZS, Ma YX, Zhang W, Lu JL, Liang YR, et al. Neuroprotective Effects and Mechanisms of Tea Bioactive Components in Neurodegenerative Diseases. Molecules. 2018;23(3). [DOI] [PMC free article] [PubMed]
- 95.Kakuda T. Neuroprotective effects of the green tea components theanine and catechins. Biol Pharm Bull. 2002;25(12):1513–1518. doi: 10.1248/bpb.25.1513. [DOI] [PubMed] [Google Scholar]
- 96.Lee KY, Hwang L, Jeong EJ, Kim SH, Kim YC, Sung SH. Effect of neuroprotective flavonoids of Agrimonia eupatoria on glutamate-induced oxidative injury to HT22 hippocampal cells. Biosci Biotechnol Biochem. 2010;74(8):1704–1706. doi: 10.1271/bbb.100200. [DOI] [PubMed] [Google Scholar]
- 97.Muthuraman A, Singh N. Neuroprotective effect of saponin rich extract of Acorus calamus L. in rat model of chronic constriction injury (CCI) of sciatic nerve-induced neuropathic pain. J Ethnopharmacol. 2012;142(3):723–731. doi: 10.1016/j.jep.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 98.Mo ZT, Fang YQ, He YP, Zhang S. β-Asarone protects PC12 cells against OGD/R-induced injury via attenuating Beclin-1-dependent autophagy. Acta Pharmacol Sin. 2012;33(6):737–742. doi: 10.1038/aps.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang X, Zheng T, Hong H, Cai N, Zhou X, Sun C, Wu L, Liu S, Zhao Y, Zhu L, Fan M, Zhou X, Jin F. Neuroprotective effects of Ginkgo biloba extract and Ginkgolide B against oxygen-glucose deprivation/reoxygenation and glucose injury in a new in vitro multicellular network model. Front Med. 2018;12(3):307–318. doi: 10.1007/s11684-017-0547-2. [DOI] [PubMed] [Google Scholar]
- 100.Taliyan R, Sharma PL. Protective effect and potential mechanism of Ginkgo biloba extract EGb 761 on STZ-induced neuropathic pain in rats. Phytother Res. 2012;26(12):1823–1829. doi: 10.1002/ptr.4648. [DOI] [PubMed] [Google Scholar]
- 101.Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agthong S, Kaewsema A, Charoensub T. Curcumin ameliorates functional and structural abnormalities in cisplatin-induced neuropathy. Exp Neurobiol. 2015;24(2):139–145. doi: 10.5607/en.2015.24.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bahramsoltani R, Rahimi R, Farzaei MH. Pharmacokinetic interactions of curcuminoids with conventional drugs: a review. J Ethnopharmacol. 2017;209:1–12. doi: 10.1016/j.jep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 104.Zheng M, Liu C, Fan Y, Shi D, Zhang Y. Protective effects of Paeoniflorin against MPP(+)-induced neurotoxicity in PC12 cells. Neurochem Res. 2016;41(6):1323–1334. doi: 10.1007/s11064-016-1834-z. [DOI] [PubMed] [Google Scholar]
- 105.Gu X, Cai Z, Cai M, Liu K, Liu D, Zhang Q, et al. Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of Alzheimer's disease. Mol Med Rep. 2016;13(3):2247–2252. doi: 10.3892/mmr.2016.4805. [DOI] [PubMed] [Google Scholar]
- 106.Wang D, Wong HK, Feng YB, Zhang ZJ. Paeoniflorin, a natural neuroprotective agent, modulates multiple anti-apoptotic and pro-apoptotic pathways in differentiated PC12 cells. Cell Mol Neurobiol. 2013;33(4):521–529. doi: 10.1007/s10571-013-9914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Costa LG, Garrick JM, Roquè PJ, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxidative Med Cell Longev. 2016;2016:2986796. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dutra RC, Simão da Silva KA, Bento AF, Marcon R, Paszcuk AF, Meotti FC, et al. Euphol, a tetracyclic triterpene produces antinociceptive effects in inflammatory and neuropathic pain: the involvement of cannabinoid system. Neuropharmacology. 2012;63(4):593–605. doi: 10.1016/j.neuropharm.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 109.Soleymani S, Bahramsoltani R, Rahimi R, Abdollahi M. Clinical risks of St John's wort (Hypericum perforatum) co-administration. Expert Opin Drug Metab Toxicol. 2017;13(10):1047–1062. doi: 10.1080/17425255.2017.1378342. [DOI] [PubMed] [Google Scholar]