Abstract
Background
The major adverse effect associated with systemic administration of Fluconazole (FLZ), is hepatic toxicity. FLZ is most commonly used antifungal drug in treatment of invasive fungal infections.
Methods
FLZ toxicity was challenged by individual and in combination of three vitamins (B1, B2 B3). Animals were divided nine groups with six animals in each group. FLZ, at a dose of 50 mg/kg b.w, was orally administered for 90 days in experimental animals. Vitamins as individual or in combination was administered concomitantly to challenge or alleviate the toxicity of FLZ. They were sacrificed at the end of protocol for biochemical and histopathology analysis. Focus was made to observe the role of these micro nutrient’s (vitamins) on liver for alteration in of pathological and physiological effects by FLZ in the Wistar albino rats.
Results
Combination of vitamin B1 + B2 + B3 in FLZ induced toxicity was able to restore the level of alkaline phosphatase (ALP) near to normal but with high level of ALP in B1 Control group. Aspartate aminotransferase (AST) was restored to normal in FLZ + B1, FLZ + B2 and FLZ + B1 + B2 + B3 groups and vice versa in FLZ + B3 group animals. Further the level of alanine aminotransferase (ALT) was restored to normal in FLZ + B3 animals. There were no significant changes found in total bilirubin (TBI), and direct bilirubin (DBI) as compare to normal control. Histopathological studies on animals’ studies validated the serological results in normalizing the cellular architecture of liver.
Conclusions
Restoration of altered biochemical parameters and cellular architecture of hepatocytes by different combination of these vitamins proves the chemo preventive potential of these micro nutrients’ in FLZ toxicity.
Graphical abstract.
Vitamin B combination attenuates fluconazole toxicity.
Keywords: Fluconazole, Toxicity, Thiamine, Riboflavin, Niacin
Introduction
Immunosuppressant therapy in the management of organ transplantation, malignancy, and trauma makes patient susceptible to invasive fungal infections (IFIs) [1, 2]. Fluconazole (FLZ), is widely used for the treatment of various fungal infections including many IFIs [3, 4]. The antifungal potential of FLZ is attributed by inhibition of cytochrome P450 dependent C14 lanosterol demethylase, involved in biosynthesis of ergosterol in it [5, 6]. It has been reported in previous studies that the therapeutic regimen of FLZ generally intended to ameliorate the condition of immunocompromised patients and is major key factor for chronic hepatotoxicity, nevertheless, its mechanism is not completely deciphered and yet to be established [7, 8].
It is well established and proved that vitamins play a vital role in alleviating severe and chronic illnesses associated with adverse effect of many modern drug therapy. Vitamins are classed into fat-soluble and water-soluble, water-soluble vitamins include vitamin B and vitamin C (ascorbic acid). Furthermore, there are different forms of like Thiamine, Riboflavin and Niacin along with Biotin, Pyridoxal and cyanocobalamin etc. Thiamine, Riboflavin and Niacin also known as vitamin B1, vitamin B2, and vitamin B3 respectively (hereafter referred as B1, B2, and B3) are the prominent types and plays crucial role in many pathological and physiological functions of human body. B1 plays a crucial role in the generation of adenosine triphosphates (ATPs) via carbohydrate catabolism and is also involved in nerve functioning as well as in the synthesis of DNA and RNA [9]. Similarly, B2 is involved in several metabolic redox reactions and is essentially required in citric acid cycle and electron transport chain [10]. B2 has a key role in the catabolism of glucose and fatty acids (beta-oxidation) which helps is production of ATPs needed for the survival of cells. Analogously, B3 with its two co-enzymes viz., nicotinamide adenine dinucleotide phosphate (NADP) and nicotinamide adenine dinucleotide (NAD) plays an important role in energy transfer reactions which are in turn associated with the metabolism of alcohol, fat and glucose [11]. NADP is a coenzyme required for the synthesis of nucleic acids and lipids in the cell [12]. The combination of nicotinamide, B2 and ascorbic acid has potential antioxidant activity to appease the hepatotoxicity induced by thioacetamide [13]. Additionally, the hepatorenal and hematological toxicity induced by antitubercular drugs is remarkably ameliorated by the combination of methionine and vitamin B-complex [14]. Studies have established the potential benefits in the treatment of peg-asparaginase induced hyperbilirubinemia by the combination of L-carnitine and vitamin B complex [15]. Thus, it is observed that vitamins B complex plays significant role in the treatment of various diseased conditions and this made us to explore and exploit their role in treatment of toxicity induced by FLZ.
FLZ is often used in treatment of various conditions of fungal infection, including invasive candidiasis, esophageal and oropharyngeal candidiasis, and cryptococcosis [16]. The limitation of its use in fungal infection is very much associated with adverse effect on liver tissue and must be impeded to ensure better patient compliance and improved treatment [17, 18]. The inference of existing scientific literature and previous studies related to vitamin B, we tried to curb the FLZ induced hepatotoxicity by individual or in combination of Vitamin B1, B2 and B3.
Methods
Animals
Wistar strain albino male/female rats (100–120 g) were randomly segregated in nine groups with six animals in each group. All the animals were housed under standard laboratory condition (temperature of 22 ± 2 °C, relative humidity 55 ± 5% and 12 h light/dark cycle) at the Department of Biochemistry; Faculty of Science, King Abdulaziz University. The animals were given free access to standard pellet chow and purified RO water ad libitum. The experimental protocol was approved by Animal Ethical Committee of Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia with Ethical Approval number 171060176. All the national and institutional guidelines for the use and care of laboratory animals were followed with utmost care.
Drugs and chemicals
FLZ was procured from Ontop Pharmaceuticals Pvt. Ltd. (Trade name: AF-150 dispersible tablets). Pure samples of vitamin B1, B2, and B3 were received as gift samples from Jamjoom Pharmaceuticals Co., Jeddah, Saudi Arabia.
Experimental design
Animals were acclimatization for 1 week before commencement of experimental study, 54 rats were randomly segregated into nine groups with 6 animals in each group. FLZ (50 mg/kg) was administered daily by oral gavage. All the drugs (vitamins and FLZ) were administered to the specific groups for a period of 90 days.
Group I: Served as normal control, rats in this group were fed only normal diet. Group II: Served as disease control and were treated with a dose of FLZ at 50 mg/kg body weight (FLZ Control group). Group III: Treated withFLZ as in group II followed by daily administration of vitamin B1 ad libitum in drinking water (50 mg/100 ml) for 90 days (FLZ + Vitamin B1). Group IV: Treated withFLZ as in group II and was subsequently administered vitamin B2 ad libitum in drinking water (50 mg/100 ml) for 90 days (FLZ + Vitamin B2). Group V: Treated withFLZ as in group II and was administered vitamin B3 ad libitum in drinking water (50 mg/100 ml) for 90 days (FLZ + Vitamin B3). Group VI: (Vitamin B1 control group), Group VII: (Vitamin B2 control group), and Group VIII: (Vitamin B3 control group), animals were given free access to drinking water containing vitamin B1, B2 and B3 (50 mg of each in 100 ml/day) for 90 days respectively. Group IX: Received FLZ treatment as in group II and was administered with vitamin B1, B2, and B3 in drinking water (50 mg of each in 100 ml water everyday) for the remaining period of study (FLZ + Vit B1 + Vit B2 + Vit B3). The animals were kept on overnight fasting before they were sacrificed; blood serum was collected for the estimation of different biochemical parameters.
Determination of biochemical parameters
On the 90th day of study, all the animals were fasted overnight and on the next day under mild anesthesia, the blood samples (10 ml approx.) were withdrawn from all the rats by either retro-orbital puncture or cardiac puncture technique. The blood samples were collected in an EDTA anticoagulant tubes. Serum from collected blood was separated by centrifugation (15,000 rpm for 10 min) and stored at 2–4 °C for further tests and experimentation [19, 20]. Biochemical parameters like aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) total bilirubin (TBI), and direct bilirubin (DBI) were determined by regular biochemical kits purchased from Nicholas India Private Limited using semi-auto analyzer (Nicholas India Private Limited).
Histopathology
After the withdrawal of blood, all the animals were euthanized using diethyl ether and ketamine and the liver was isolated from each animal. Liver tissue samples were extracted and immediately fixed in formalin (10%) and were subsequently dehydrated by passing through a graded series of alcohol and paraffin infiltration. The semi-automated microtome was used to prepare 5 μm sections then the samples were dried in an oven at 37 °C overnight. Hematoxylin and eosin were used for staining and images were captured by light microscopy.
Statistical analysis
Statistical analysis of experimental data was performed using one-way analysis of variance (ANOVA), followed by Tukey’s test (Prism 8.0 Graph pad prism software). The results were expressed as the mean ± SEM; P values (t-test) of different biochemical parameters were considered increasingly significant in the following order <0.05(*), <0.01(**), <0.001(***) and <0.0001(****).
Results
Liver function parameters
Table 1 Enumerate the treatment with vitamins on hepatic parameters, ALP, AST, ALT, and DBI. FLZ elucidated hepatotoxicity in group II, III, IV, V and IX. The parameters have shown significant elevation in FLZ control group while they were near to normal in groups of animals on treatment of B1, B2 and B3. Group IX showed the maximum efficacy in lowering the hepatotoxicity.
Table 1.
Liver function biochemical parameters of different groups treated with fluconazole and Vitamins B1, B2 & B3
| SN | Group | ALP | ALT | AST | TBI | DBI |
|---|---|---|---|---|---|---|
| 1 | Normal Control | 102 ± 12 | 64 ± 2.5 | 105 ± 7.5 | 0.15 ± 0.0076 | 0.06 ± 0.0037 |
| 2 | FLZ Control | 145 ± 8.3*/a | 74 ± 5 6 ns/a | 130 ± 13 ns/a | 0.10 ± 0.011 **/a | 0.033 ± 0.0033 **/a |
| 3 | FLZ + B1 | 130 ± 13ns/b | 81 ± 1 3 ns/b | 115 ± 9 2 ns/b | 0.13 ± 0.00 5 ns/b | 0.023 ± 0.00 4 ns/b |
| 4 | FLZ + B2 | 120 ± 3.5 */b | 71 ± 2 9 ns/b | 102 ± 11 ns/b | 0.12 ± 0.00 3 ns/b | 0.03 ± 0.00 3 ns/b |
| 5 | FLZ + B3 | 141 ± 12ns/b | 66 ± 4 1 ns/b | 127 ± 5 5 ns/b | 0.12 ± 0.0 2 ns/b | 0.053 ± 0.0056 */b |
| 6 | B1 Control | 247 ± 12 ****/b | 79 ± 4 5 ns/b | 123 ± 0. 7 ns/b | 0.13 ± 0.0022 **/b | 0.045 ± 0.0022 */b |
| 7 | B2 Control | 115 ± 13ns/b | 75 ± 2 5 ns/b | 117 ± 5 4 ns/b | 0.11 ± 0.00 5 ns/b | 0.045 ± 0.0 05ns/b |
| 8 | B3 Control | 132 ± 17ns/b | 59 ± 7 5 ns/b | 95 ± 5.1 */b | 0.13 ± 0.00 2 ns/b | 0.05 ± 0.0 */b |
| 9 | FLZ + B1 + B2 + B3 | 115 ± 1.9 **/b | 74 ± 7 3 ns/b | 116 ± 2 5 ns/b | 0.11 ± 0.00 7 ns/b | 0.047 ± 0.0033 */b |
All values are expressed as mean ± S.E.M for each group (n = 6). Where: ns –not significant; aNormal Vs disease control group; bDisease Control Vs Drug Control/ Therapeutic group; * P < 0.05; **P < 0.01; ***P < 0.001; **** P < 0.0001
Alp
FLZ treated groups exhibited significant (p < 0.05) elevated levels of serum ALP, in the FLZ control group. ALP level was remarkably (p < 0.0001) increased to 1.5 times (145 ± 8.3 IU/L) as compared to the normal control group (102 ± 12 IU/L). ALP level in FLZ + B1 + B2 + B3 group was significantly (p < 0.01) controlled and decreased (115 ± 1.9 IU/L) in this group of treatment (Table 1).
Alt
An insignificant rise in the level of ALT was observed in the FLZ control group (74 ± 5.6 IU/L) as compared to normal control (64 ± 2.5 IU/L). FLZ + B3 group (66 ± 4.1 IU/L) showed near normal levels of ALT whereas, high variation was observed in rest of the animals from various groups (Table 1).
AST
Serum AST level variation in FLZ administered groups was significant but in those without the administration of FLZ the changes in AST levels were statistically insignificant, evident from the Table 1. The administration of vitamin B combination led to the normalization of serum AST levels in groups administered with mono therapy of vitamin B1, B2 or B3, but maximum alleviation of hepatotoxicity was observed in group IX, a combination of B1, B2 and B3 therapeutic group (Table 1).
TBI
A significant reduction (P < 0.01) in the levels of total bilirubin in FLZ group (0.10 ± 0.011 IU/L) as compared to normal control (0.15 ± 0.003 IU/L). Among all the treatment groups i.e. FLZ + B1, B1 and B3 Control groups showed similar efficacy as their mean value of TBI was very much close to normal control group (NC 0.15 ± 0.0076 IU/L; FLZ + B1 0.13 ± 0.0015 IU/L) (Table 1). FLZ + B2 and FLZ + B3 followed with 0.12 ± 0.00 IU/L and 0.12 ± 0.00 IU/L respectively.
DBI
The level of DBI was significantly (P < 0.01) decreased (50% low) in the FLZ group (0.033 ± 0.0033 IU/L) as compared to normal control (0.06 ± 0.003 IU/L). Two therapeutic groups viz.FLZ + B3 and FLZ + B1 + B2 + B3 retained the serum levels of DBI and were analogous to that of the normal control (P < 0.05) (Table 1).
Histopathology
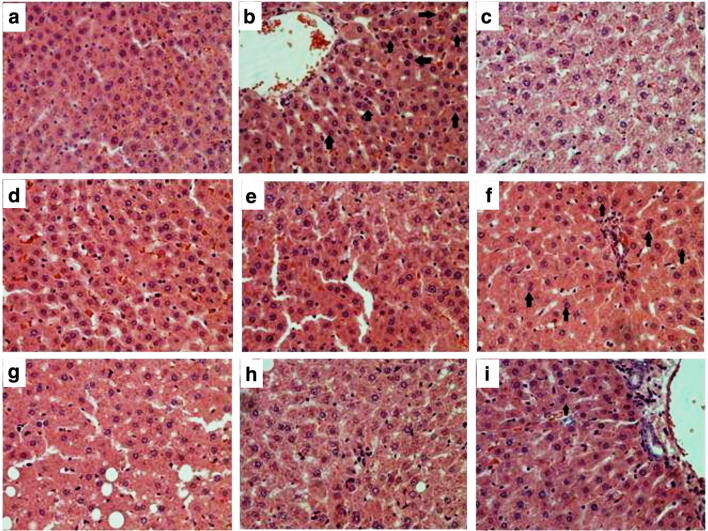
The histopathological analysis showed the presence of normal hepatocytes in normal control group; the cellular structure was intact with distinct central vein, regular sinusoids, and radiating hepatocytes arrangement (Fig. 1a). FLZ control group revealed distorted and binucleated cellular architecture (Fig. 1b black arrows), the central vein area was also affected by the infiltration of abnormal hepatocytes. The drug control groups i.e., B1 control (Fig. 1c), B2 control (Fig. 1d) and B3 control (Fig. 1e) showed no significant differences in comparison to the normal control. Improvement in the hepatic structure was noted with diminished congestion and reduced numbers of binucleated hepatocytes in the treatment groups (Fig. 1f, g, h and i), especially in the combination therapy group (FLZ + B1 + B2 + B3) where the results revealed indifferent hepatic architecture to normal group.
Fig. 1.
Effect of vitamins (B1, B2 and B3) in FLZ administered hepatotoxicity in different groups of rats: a Normal control; b FLZ control; c B1 control; d B2 control; e B3 control; f FLZ + B1 group; g FLZ + B2; h FLZ + B3; i FLZ + B1 + B2 + B3. The above pictures for each group were chosen randomly. Black arrows depict bi-nucleated cellular architecture. The slides were H&E stained and the original magnification was 40×
Discussion
FLZ based antifungal therapy is well accepted and established for patients with different types of invasive fungal infection particularly invasive candidiasis in which the immunogenic response is disrupted. The major complications of FLZ regimen in treatment of various fungal infection is chronic hepatotoxicity. Present study is an attempt to alter the serum levels of biochemical parameters in liver incurred by the administration of FLZ. Existing literature and studies imply that much of the toxicities associated with FLZ is due to the alteration of sterol synthesis, butmore concrete and mechanistic approach is needed to affirm this notion [7]. FLZ is a powerful inhibitor of CYP 3A4 and hence can induce severe toxicity from drugs that are normally metabolized by CYP3A4. Consequently, it leads to significant increase in plasma levels of biomarkers due to the absence of oxidizing enzymes which are indispensable for carrying out normal metabolic processes [21]. Earlier dose-dependent studies have also proved the toxicity of FLZ injuring liver [22, 23], which was evident in the presented study and hence our study was in line with modern concept research.
ALP is basically zinc based enzymewith a serine at the active center; they release inorganic phosphate and are normal constituents of all tissues. In liver, ALP is found near the surface of sinusoidal hepatocytes and in the bile canaliculi [24]. Abnormal level of ALP corresponds to liver malfunction and/or its inflammation. In FLZ control group, serum ALP was significantly altered, which indicates hepatic injury, whereas these levels were successfully restored to normal range by the combination of FLZ + B1 + B2 + B3 indicating its hepatoprotective nature of FLZ induce toxicity. Other mono therapy by vitamin B1, B2 or B3 were also able to restored ALP level elevated by FLZ, but the combination of all three in FLZ + B1 + B2 + B3 group was most significant. This was further supported by histopathology of liver in different treatment groups.
Most sensitive hepatic marker enzymes used for determination of liver function are the aminotransferases and are considered as the standard biomarkers of hepatic necrosis, injury or toxicity. ALT and AST catalyze the transfer of α-amino acids of alanine and aspartate respectively to the α-keto group of ketoglutaric acid. Alteration in the level of ALT and AST indicates hepatic damage and liver dysfunction. In present work, FLZ induced liver injury was confirmed by the alteration of serum levels of AST and ALT. The liver cells contain high concentration of AST and ALT in their cytoplasm, AST is more predominant in mitochondria [25]. Liver cells damage leads to the leakage of liver marker enzymes in plasma, resulting in an increased level of AST and ALT. Hence, an increased AST and ALT level indicate the hepatocellular damage and loss of its regular functional capacity [17]. After the treatment of FLZ induced toxicity with different vitamin combination, the altered AST and ALT levels were restored, with much significant in FLZ + B2 group animals. AST level in FLZ + B1 + B2 + B3 group animals were also restored to normal level as compared to FLZ control. This indicates that these dose combinations of vitamins are effective in recovering FLZ induced hepatocyte damage. The histopathological recovery further substantiates the alleviation of hepatotoxicity in FLZ + B1 + B2 + B3 group.
Bilirubin is an endogenous substance produced from the breakdown of RBC and their elevation in serum level asserts hepatic dysfunction [26]. It is excreted in urine and bile, and its elevated in certain hepatic diseases or malfunction [27]. In this study, we measured bilirubin in TBI and DBI form. Level of TBI and DBI remained unchanged in disease control as well as in the treatment control groups hence indicating that single and multiple administration of vitamin B does not affect extraction and metabolism of bilirubin. However, its level was decreased in disease control as well as treatment groups, which is generally not a concern and does not give any idea about hepatic dysfunction.
The histopathological reports revealed that micronutrients treatment has potential for reducing the hepatotoxicity caused by the administration of FLZ. It was evident from the absence of cellular deformities and binucleated hepatocytes in the liver section of rats treated with the vitamin B1, B2 and B3. Moreover, it was confirmed by our study that the combination therapy (B1 + B2 + B3) can reverse the histopathological changes caused by the toxic effect of FLZ. Therefore, with these significant results we can conclude that the liver toxicity caused by FLZ therapy can be remarkably alleviated by vitamins.
Furthermore, it is established from previous literature that Vitamin B1, B2 and B3 protects the liver. Thiamine (Vitamin B1) prevents oxidative damage of liver cells by acting as an antioxidant [28] and its mechanism is still unexplored. Additionally, Riboflavin (Vitamin B2) like thiamine shows antioxidant effects and protects hepatocytes against injury. The mechanism of Riboflavin hepatoprotection is due to its intrinsic antioxidant potential, reduction in iNOS/eNOS hepatic expression and decrease in NO levels [29]. Nicotinamide (Vitamin B3) also protects liver cells by cAMP/PKA/CREB pathway-dependent upregulation of Sirt1 [30]. Our research gives idea about how combinations of vitamins with FLZ (FLZ + B1 + B2 + B3, FLZ + B1 and FLZ + B3) is effective in restoring abnormal function of liver. This beneficial effect may be due to additive or synergistic effect of the combination of all three vitamin; B1, B2 and B3.
Hence, we can conclude from our research study that the hepatotoxicity caused by the administration of FLZ can be alleviated using the combination of vitamin B1, B2 and B3 and with different combinations. The possible mechanism underlying this treatment is the additive effect of individual antioxidant activity of these vitamins. Further study required to establish exact mechanism of action of these combinations.
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under the grant no. G-389.130-38. The authors, therefore, acknowledge with thanks DSR for their technical and financial support.
The authors thank Kaleemuddin Mohammed, Ph.D. research scholar at our department for his assistance in performing experiments and drafting this manuscript.
Compliance with ethical standards
Conflict of interest
The Authors declare that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fahad A. Al-Abbasi, Phone: +966 559399935, Email: alabassif@hotmail.com
Firoz Anwar, Phone: +966 505132306, Email: firoz_anwar2000@yahoo.com.
References
- 1.Cavayas YA, Yusuff H, Porter R. Fungal infections in adult patients on extracorporeal life support. Crit Care. 2018;22(1):98. doi: 10.1186/s13054-018-2023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Leary RA, Einav S, Leone M, Madách K, Martin C, Martin-Loeches I. Management of invasive candidiasis and candidaemia in critically ill adults: expert opinion of the European Society of Anaesthesia Intensive Care Scientific Subcommittee. J Hosp Infect. 2018;98(4):382–390. doi: 10.1016/j.jhin.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Cho SY, Lee DG, Choi JK, Lee HJ, Kim SH, Park SH, Choi SM, Choi JH, Yoo JH, Kim YJ, Kim HJ, Min WS, Back H, Kang S, Lee EK. Cost-benefit analysis of Posaconazole versus FLZ or Itraconazole as a primary antifungal prophylaxis in high-risk hematologic patients: a propensity score-matched analysis. Clin Ther. 2015;37(9):2019–2027. doi: 10.1016/j.clinthera.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 4.van Prehn J, Menke-van der Houven van Oordt CW, de Rooij ML, Meijer E, Bomers MK, van Dijk K. Hepatosplenic Candidiasis Without Prior Documented Candidemia: An Underrecognized Diagnosis? Oncologist. 2017;22(8):989–994. doi: 10.1634/theoncologist.2017-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhi V, Colburn D, Williams SJ, Hop CE, Dresser MJ, Chandra P, Graham RA. A clinical drug-drug interaction study to evaluate the effect of a proton-pump inhibitor, a combined P-glycoprotein/cytochrome 450 enzyme (CYP)3A4 inhibitor, and a CYP2C9 inhibitor on the pharmacokinetics of vismodegib. Cancer Chemother Pharmacol. 2016;78(1):41–49. doi: 10.1007/s00280-016-3020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanini I, Rizzetto L, Rivero D, Carbonell S, Gut M, Heath S, Gut IG, Trabocchi A, Guarna A, Ben Ghazzi N, Bowyer P, Kapushesky M, Cavalieri D. Deciphering the mechanism of action of 089, a compound impairing the fungal cell cycle. Sci Rep. 2018;8(1):5964. doi: 10.1038/s41598-018-24341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gearhart MO. Worsening of liver function with FLZ and review of azole antifungal hepatotoxicity. Ann Pharmacother. 1994;28(10):1177–1181. doi: 10.1177/106002809402801009. [DOI] [PubMed] [Google Scholar]
- 8.Srichatrapimuk S, Sungkanuparph S. Integrated therapy for HIV and cryptococcosis. AIDS Res Ther. 2016;13(1):42. doi: 10.1186/s12981-016-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lonsdale D. Thiamin. Adv Food Nutr Res. 2018;83:1–56. doi: 10.1016/bs.afnr.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Saedisomeolia A, Ashoori M. Riboflavin in human health: a review of current evidences. Adv Food Nutr Res. 2018;83:57–81. doi: 10.1016/bs.afnr.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Oka S, Hsu CP, Sadoshima J. Regulation of cell survival and death by pyridine nucleotides. Circ Res. 2012;111(5):611–627. doi: 10.1161/CIRCRESAHA.111.247932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen B, Brown AD, Kuhlberg J, Millar L, Nichols M, Economos C, Allender S. Understanding a successful obesity prevention initiative in children under 5 from a systems perspective. PLoS One. 2018;13(3):e0195141. doi: 10.1371/journal.pone.0195141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashandy SAE, Ebaid H, Abdelmottaleb Moussa SA, Alhazza IM, Hassan I, Alaamer A, al Tamimi J. Potential effects of the combination of nicotinamide, vitamin B2 and vitamin C on oxidative-mediated hepatotoxicity induced by thioacetamide. Lipids Health Dis. 2018;17(1):29. doi: 10.1186/s12944-018-0674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amagon KI, Awodele O, Akindele AJ. Methionine and vitamin B-complex ameliorate antitubercular drugs-induced toxicity in exposed patients. Pharmacol Res Perspect. 2017;5(5):e00360. doi: 10.1002/prp2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rausch CR, Paul S, Marx KR, Jabbour E, Pemmaraju N, Ferrajoli A, Kantarjian H. L-carnitine and vitamin B complex for the treatment of Pegasparaginase-induced hyperbilirubinemia. Clin Lymphoma Myeloma Leuk. 2018;18(5):e191–e195. doi: 10.1016/j.clml.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Pound MW, Townsend ML, Dimondi V, Wilson D, Drew RH. Overview of treatment options for invasive fungal infections. Med Mycol. 2011;49(6):561–580. doi: 10.3109/13693786.2011.560197. [DOI] [PubMed] [Google Scholar]
- 17.Björnsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50(3):511–517. doi: 10.1016/j.jhep.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Gayam V, Khalid M, Dahal S, Garlapati P, Gill A. Hyperacute liver injury following intravenous FLZ: a rare case of dose-independent hepatotoxicity. J Fam Med Prim Care. 2018;7(2):451–454. doi: 10.4103/jfmpc.jfmpc_330_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afzal M, Khan R, Kazmi I, Anwar F. Hepatoprotective potential of new steroid against carbon tetrachloride-induced hepatic injury. Mol Cell Biochem. 2013;378(1–2):275–281. doi: 10.1007/s11010-013-1618-6. [DOI] [PubMed] [Google Scholar]
- 20.Fernández I, Peña A, Del Teso N, Pérez V, Rodríguez-Cuesta J. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J Am Assoc Lab Anim Sci. 2010;49(2):202–206. [PMC free article] [PubMed] [Google Scholar]
- 21.Rezaei-Matehkolaei A, Khodavaisy S, Alshahni MM, Tamura T, Satoh K, Abastabar M, et al. In Vitro antifungal activity of novel Triazole Efinaconazole and five comparators against dermatophyte isolates. Antimicrob Agents Chemother. 2018;62(5). [DOI] [PMC free article] [PubMed]
- 22.Khoza S, Moyo I, Ncube D. Comparative hepatotoxicity of FLZ, ketoconazole, Itraconazole, terbinafine, and Griseofulvin in rats. J Toxicol. 2017;2017(6746989):1–9. doi: 10.1155/2017/6746989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyriakidis I, Tragiannidis A, Munchen S, Groll AH. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf. 2017;16(2):149–165. doi: 10.1080/14740338.2017.1270264. [DOI] [PubMed] [Google Scholar]
- 24.Rosalki SB, Mcintyre N. Biochemical investigations in the management of liver disease. Oxford textbook of clinical hepatology. 2. New York: Oxford University Press; 1999. pp. 503–521. [Google Scholar]
- 25.Drotman RB, Lawhorn GT. Serum enzymes are indicators of chemical induced liver damage. Drug Chem Toxicol. 1978;1:163–171. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 26.Santiago RP, Vieira C, Adanho CSA, Santana SS, Guarda CC, Figueiredo CVB, Fiuza LM, Pitanga TN, Ferreira JRD, Aleluia MM, Oliveira RM, Zanette DL, Lyra IM, Goncalves MS. Laboratory and genetic biomarkers associated with cerebral blood flow velocity in hemoglobin SC disease. Dis Markers. 2017;2017:6359871. doi: 10.1155/2017/6359871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bulmer AC, Coombes JS, Blanchfield JT, Toth I, Fassett RG, Taylor SM. Bile pigment pharmacokinetics and absorption in the rat: therapeutic potential for enteral administration. Br J Pharmacol. 2011;164(7):1857–1870. doi: 10.1111/j.1476-5381.2011.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uysal HB, Dağlı B, Yılmaz M, Kahyaoğlu F, Gökçimen A, Ömürlü İK, Demirci B. Biochemical and histological effects of thiamine pyrophosphate against acetaminophen-induced hepatotoxicity. Basic Clin Pharmacol Toxicol. 2016;118(1):70–76. doi: 10.1111/bcpt.12496. [DOI] [PubMed] [Google Scholar]
- 29.Sanches SC, Ramalho LN, Mendes-Braz M, Terra VA, Cecchini R, Augusto MJ, Ramalho FS. Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem Toxicol. 2014;67:65–71. doi: 10.1016/j.fct.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Dou X, Li S, Zhang X, Zeng Y, Song Z. Nicotinamide ameliorates palmitate-induced ER stress in hepatocytes via cAMP/PKA/CREB pathway-dependent Sirt1 upregulation. Biochim Biophys Acta. 2015;1853(11):2929–2936. doi: 10.1016/j.bbamcr.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]