Abstract
Objectives
There are many studies about Iranian clinical pharmacists’ interventions and their impacts on medication safety and cost. The aim of this study is to collect data and critically evaluate the clinical and economic effects of Iranian clinical pharmacist interventions and activities. To our best of knowledge, this research is the first review of publications about Iranian clinical pharmacists’ interventions and activities.
Evidence acquisition.
Six online databases, including PubMed, Scopus, Medline, Cochrane Central Register of Controlled Trials, Cochrane Database of Systemic Reviews, and Google Scholar were searched using the terms ‘“Iranian”, “clinical pharmacist”, ‘adverse drug reactions”, “medication errors”, “drug interaction”, “drug utilization evaluation”, “cost”, and “interventions” for English studies conducted in Iran and described clinical pharmacist-initiated interventions, published before December 2018. The search and extraction process followed PRISMA guidelines. Observational or retrospective studies, clinical trials, congress abstracts, and case reports or case series were excluded. The search strategy after full-text review identified 39 articles matching the eligibility criteria.
Results
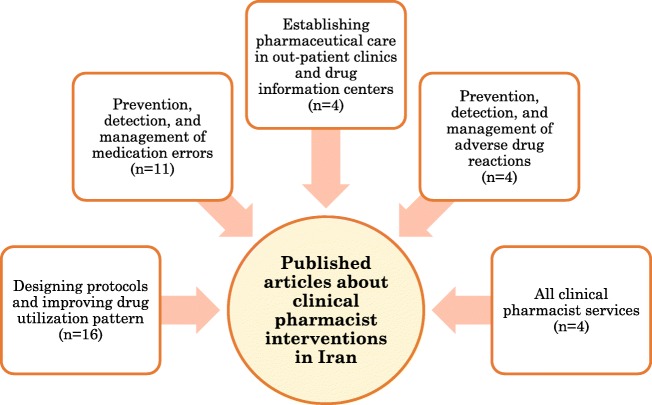
Thirty-nine articles were recruited. They included establishing pharmaceutical care in out-patient clinics and drug information centers (n = 4); prevention, detection, and management of adverse drug reactions(n = 4), designing protocols and improving drug utilization pattern(n = 16), prevention, detection, and management of medication errors (n = 11), and all clinical pharmacist services(n = 4). Most clinical pharmacist interventions and activities were regarding designing protocols, improving drug utilization pattern, as well as detection, prevention, and management of medication errors. About three-fourth (74.35%) of included studies were from either ambulatory care or in-patient settings in Tehran. The median (interquartile range) duration of intervention as well as follow-up phases was 9 (5) months.
Conclusion
Data of our review support the beneficial role of clinical pharmacists in the improvement of quality, safety, and efficiency of patients’ pharmaceutical care in Iran.
Graphical abstract.
Clinical pharmacists’ interventions in Iran
Keywords: Clinical pharmacy, Interventions, Clinical and economic impacts, Iran
Introduction
Clinical pharmacists are practitioners with advanced education and training, providing direct patient care and comprehensive drug management to patients and health-care providers, such as physicians and nurses [1]. Most clinical pharmacists work in hospital wards and they have attracted a significant attention worldwide as an important member of the patient care team for both ambulatory and in-patient care settings. Their practices aim to optimize the use of medications and improve patient outcomes by selecting appropriate drug, dosage form and route of administration, as well as monitoring and identification of adverse effects of drugs and their economic efficiency [2, 3].
It has been shown that pharmacists can improve medication adherence, knowledge and appropriateness of prescribed drugs, and at the same time, they can reduce the hospital stay [3]. Despite capability of pharmacists to assist with many of the challenges that currently the healthcare system is facing, it has been shown that pharmacists are considered as the most underutilized health care professionals [4]. Nowadays, the increased pressure on healthcare resources is threatening the pharmacists and the danger of erosion of pharmacists’ previous gains is being felt [5]. Therefore, it is necessary to provide sufficient information regarding clinical benefits, as well as cost effectiveness data on pharmacy services to justify the pharmacists’ capabilities and their important roles in health care systems [6].
Clinical pharmacy residency program was started in Iran in 1994. Now, after nearly 20 years, there are more than 100 graduated clinical pharmacy specialists in our country. Most of them are faculty members of national universities and they also work in various wards of teaching hospitals [7]. There are many studies about Iranian clinical pharmacists’ interventions and their effects on ADR, ME and medication cost but to our best of knowledge, there has been no comprehensive systematic assessment of the overall impact of Iranian clinical pharmacists’ services on patient outcomes and treatment costs. Therefore, the aim of this study is to collect data and critically evaluate the clinical and economic effects of Iranian clinical pharmacists’ interventions and activities. To our best of knowledge, this research is the first review of publications regarding Iranian clinical pharmacists’ interventions and activities. The underpinning research question for this systematic review was ‘How do the professional activities of a clinical pharmacist in Iran impact on the patient outcomes, as well as cost of drug therapy?
The goal in identifying the impact of clinical pharmacists’ interventions is to provide evidence tosupport their continued integration in the health care teams.
Methods
This systematic review follows the recommendations of the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement [8]. In order to conduct the literature research and subsequently guide a screening process for relevant articles, a research question was generated, being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS).
Aliterature review was conducted using PubMed, Scopus, Medline, Cochrane Central Register of Controlled Trials, Cochrane Database of Systemic Reviews, and Google Scholar as online databases Searched keywords were as follows: “Iranian”, “clinical pharmacist”, “adverse drug reactions”, “medication errors”, “drug interaction”, “drug utilization evaluation”, “cost”, and “interventions”. Searches were performed by two authors to confirm consistency and accuracy of results. Search results from multiple databases were transferred to a reference manager, End Note X7. The inclusion criteria for this systematic review were as follows: Studies published in English language, studies published as peer reviewed full-text articles, studies assessed an intervention performed by a clinical pharmacist or team of clinical pharmacists in either out-patient or inpatient settings, as at least a main surrogate end point. Non-peer reviewed literature, including government documents, technical reports, newspaper articles, letters to the editor, media releases, as well as non-interventionalobservational or retrospective studies, clinical trials, congress abstracts, systematic reviews, meta-analyses and case reports or case series were not eligible for inclusion.
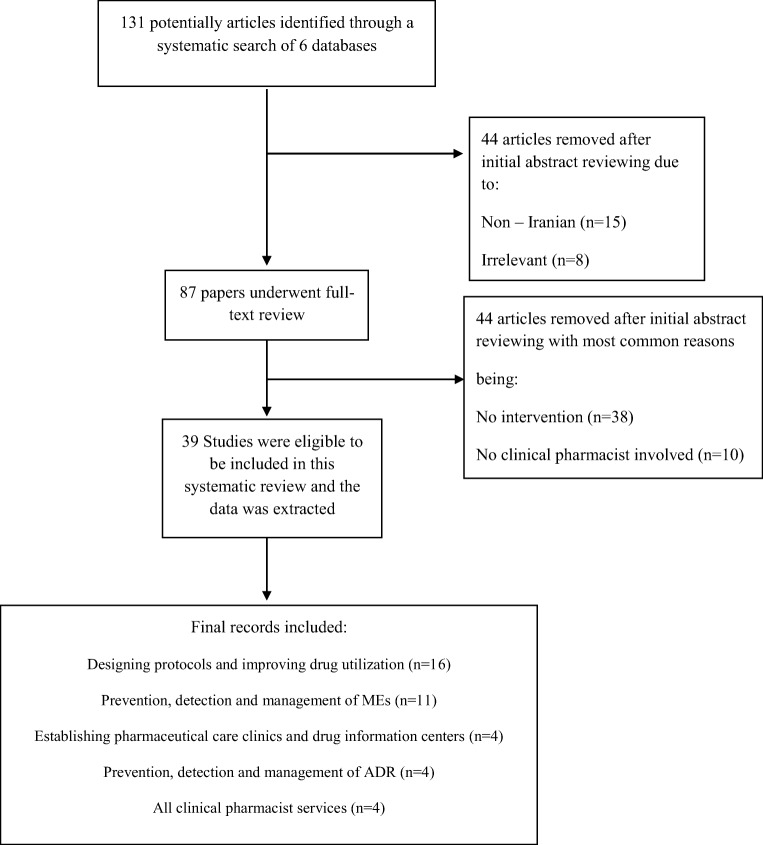
Figure 1 shows a flow chart of the reviewing and screening process. At first stage, titles were evaluated to identify potentially relevant articles and remove non-pertinent studies. At second stage, the abstracts were reviewed. If the inclusion criteria were not met, study was excluded. The full text of studies that had not been excluded, were reviewed at stage 3. The studies included in the systematic review were reviewed by all the authors to ensure that they met inclusion criteria. Any discrepancies were discussed by the authors to reach a final decision. At fourth stage, the articles were classified into five primary groups based on the type of intervention conducted by the clinical pharmacists: Establishing pharmaceutical care in out-patient clinics and drug information centers; prevention, detection, and management of ADR; designing protocols and improving drug utilization pattern; prevention, detection, and management of MEs; and all clinical pharmacist services. It should be mentioned that several studies could fall into multiple categories, so the categorization was performed based on the main type of intervention conducted by clinical pharmacists and these studies were not repeated in different categories. Then, the data was extracted from recruited articles, including author details, year published, study setting, participant number, study period, study design, type of intervention, and primary as well as secondary outcomes.
Fig. 1.
The flow diagram of the study
Results
131 articles were initially identified by the search method, based on their titles which was decreased to 87 articles after abstract screening, with full-text reviewing leaving 39articles matching the inclusion criteria. Common reasons for excluding articles were being non-Iranian or irrelevant, lack of clinical pharmacists in the study, being case report, and not discussing any intervention,
The details of 39 eligible studies are shown in Table 1, all of which were Iranian studies conducted and published before October 2018 and evaluated economical and clinical outcomes of clinical pharmacists’ interventions. Topics of recruited studies were as follows: Establishing pharmaceutical care in out-patient clinics and drug information centers(n = 4);prevention, detection, and management of ADR(n = 4); designing protocols and improving drug utilization pattern(n = 16), prevention, detection, and management of MEs(n = 11),and all clinical pharmacist services(n = 4).
Table 1.
Details of recruited studies in the systematic review including City, Study setting, Population/Service, Study duration, studied parameter(s), Intervention/Tools, Validation, and Main outcome(s)
| Reference | City | Study setting | Population/Service | Study duration | Studied parameter(s) | Intervention/Tools | Validation | Main outcome(s) |
|---|---|---|---|---|---|---|---|---|
| Baniasadi et al. [13] | Tehran | All wards of a teaching hospital | 6840 patients | 12 months | Adverse drug reactions |
- ADR reporting yellow form - Publishing monthly ADR bulletin - Providing training lectures |
-WHO definition of ADR - WHO dentition of serious ADRs - Naranjo’s algorithm and Schumock scale |
- Anti-infectives and ceftriaxone were as the most common medication class and agent related to ADRs, respectively - Incidence of ADRs reported by physicians and nurses was somewhat low |
| Dashti-Khavidaki et al. [43] | Tehran | Infectious diseases and nephrology wards of a teaching hospital | 1105 patients | 12 months | Number and type of clinical pharmacy services | Pharmacotherapy monitoring form | Guideline of Society of Hospital Pharmacy of Australia | - More than two-fifth of clinical pharmacy services consisted of discontinuation of unnecessary drugs and changing in dose or frequency of prescribed drugs. |
| Vessal [33] | Shiraz | Nephrology ward of a university hospital in Shiraz | 76 patients | 4 months |
- Number and types of prescribing errors -Level of Harm - Number of interventions |
- Medical chart review - Identifying errors and making interventions after agreement of the attending physician. |
American Society of Health-System pharmacist (ASHP) |
- Types of prescribing errors and their frequencies were as follows: wrong frequency, wrong drug selection, and overdose were the most common prescribing errors. - The attending physician agreed to 96.5% of the prescription errors detected, and interventions were made. |
| Fahimi et al. [44] | Tehran | All wards of a teaching hospital | 345 patients | 12 months | Number and type of clinical pharmacy interventions |
- Medication counseling/advice - Drug information forms |
Not defined |
- Drug information, dose adjustment, and therapeutic reduction/addition as the most common interventions - Percentage of interventions per patient-medication exposure equals to 24.28% |
| Khalili et al. [17] | Tehran | Infectious diseases ward of a teaching hospital |
- Pre-intervention phase: 186 patients - Post-intervention phase: 154 patients |
- Pre-intervention phase: 4 months - Post-intervention phase: 4 months |
Appropriateness of deep vein thrombosis prophylaxis |
- Internal guideline - Oral recommendations during medical rounds - Pharmacotherapy notes |
Guidelines for DVT prophylaxis (not specially defined) | - Significant decrease in the number of patients who should receive DVT prophylaxis but anticoagulants were not administered |
| Khalili et al. [18] | Tehran | Infectious diseases ward of a teaching hospital |
- Pre-intervention phase: 212 patients - Post-intervention phase: 113 patients |
- Pre-intervention phase: 4 months - Post intervention phase: 4 months |
Acid suppression therapy for stress ulcer prophylaxis | - Guideline development | American Society of Health-System Pharmacists (ASHP) guidelines | Significant reduction in the use of acid suppression therapy overall and also in patients without an absolute indication for stress ulcer prophylaxis |
| Abbasi-Nazari et al. [34] | Tehran | 4 wards of a teaching hospital | 460 patients | 6 months | Drug-food interactions |
- Teaching classes -Information pamphlets |
International references of drug-food interactions | Significant reduction in the rate of incorrect drug administration and absorption drug-food interactions |
| Fahimi et al. [9] | Tehran | Anticoagulation clinic of a teaching hospital | 76 patients | 14 months |
- Therapeutic International Normalized Ratio (INR) - Warfarin indication and ADR |
- Regular patient visits and follow up - Patient education package - INR logbook for patients |
7th American College of Chest Physician guideline |
- About half of patients reached the target INR on follow-up visits. - Increasing warfarin dose was the most common clinical pharmacist intervention |
| Khalili et al. [35] | Tehran | Infectious diseases ward of a teaching hospital | 861 patients | 1 year | Medication errors | Medical chart review | Medication errors classification based on Pharmaceutical Care Network Europe Foundation |
- 0.13 medication errors per patient were detected. - Physicians and nurses were responsible for most of medication errors |
| Khalili et al. [14] | Tehran | Teaching hospital | 100 healthcare workers |
- Pre-implementation phase: 1 month - Post-implementation phase: 3 months |
Knowledge, attitude and perception about ADR |
- A validated questionnaire - Workshops, meetings, and presentation |
WHO definition of ADR | - All participants knew Iranian Pharmacovigilance Center after the intervention phase |
| Dashti-Khavidaki et al. [36] | Tehran | 4 ICUs of two hospitals |
- Control group: 36 patients - Case group: 31 patients |
Implementation period: 1 month | Nurses’ knowledge and practice regarding medications delivery via enteral feeding tube |
- Preparing a questionnaire - Preparing and educating a booklet - Preparing a detailed working instruction |
Not defined | - Significant increase in the mean scores of knowledge and practice questions about medication administration through enteral feeding tubes |
| Dashti-Khavidaki et al. [20] | Tehran | Hemodialysis ward of a teaching hospital | 86 patients | 6 months | Relevant laboratory data (bone metabolism parameters, anemia parameters, and serum lipid profile) | Regular medical visits | NKF-K/DOQI guidelines | - Improvement in the management of complications in CKD patients such as Ca × P product, iPTH as well as hemoglobin concentrations, serum ferritin, total cholesterol, LDL cholesterol, and triglyceride |
| Abbasi-Nazari et al. [37] | Tehran | ICU and surgery wards of a teaching hospital | 46 patients | 9 months | Errors in the preparation and administration of IV drugs |
- Observation - Education via installation of wall posters and giving informative pamphlets |
Two well-known references | A significant decrease in the rate of errors regarding preparation and administration of IV drugs |
| Khalili et al. [38] | Tehran | Infectious diseases ward of a teaching hospital |
- Pre-intervention group: 1040patients - Post-intervention group: 956 patients |
12 months |
- Number and type of medication errors - Type, frequency, clinical significance, and economic significance of clinical pharmacy interventions - Nursing staff satisfaction with clinical pharmacy interventions |
- A validated questionnaire - Medical chart review |
Medication error: Pharmaceutical Care Network Europe Foundation Clinical significance of clinical pharmacy interventions: Guideline of Society of Hospital Pharmacy of Australia |
- Incorrect dose, omission, and medication were the most frequent medication error types. - Direct medication cost per patient was decreased about 3.8% following clinical pharmacist’s interventions. |
| Dashti-Khavidaki et al. [21] | Tehran | Hemodialysis ward of a teaching hospital |
- Case group: 26 patients - Control group: 34 patients |
6 months | Health-related quality of life (HRQOL) of hemodialysis patients |
- Weekly and monthly patients’ visits - Publishing booklets - Patient education |
Medical Outcome Study 36-Item Short-Form Health Survey (SF-36) questionnaire | Pharmaceutical care significantly improved HRQOL especially in the role-emotional, mental health, social functioning, and general health dimension |
| Salehifar et al. [15] | Mazandaran province | All hospitals of Mazandaran province | 793 yellow cards | 6 years | Incidence, pattern, and seriousness of ADRs |
- Developing pharmacovigilance committees - ADR yellow cards |
Not defined | - Majority of reported ADRs were related to time after the involvement of clinical pharmacy |
| Allameh et al. [45] | Tehran | All wards of a teaching hospital | 2227 interventions and 925 patients’ visits | 28 months | Frequency, severity, procedures, and accuracy of clinical pharmacists’ interventions | Collecting all clinical pharmacists’ interventions and visits | Modified version of Safety Assessment Code (SAC)-score | - Most clinical pharmacist interventions were recommended to physicians and considered as appropriate and relevant |
| Mousavi et al. [19] | Tehran | Nephrology ward of a teaching hospital |
- Pre-intervention: 387 patients - Post-intervention: 242 patients |
Pre-intervention: 6 months Post-intervention: 6 months |
Appropriateness and direct cost of stress ulcer prophylaxis |
- Guideline implementation - Educational classes for medical doctors - Ward rounds along with physicians |
American Society of Health-System Pharmacists (ASHP) guidelines | - Significant relative reduction in the inappropriate stress ulcer prophylaxis prescribing rate and related cost |
| Tavakoli-Ardakani et al. [22] | Tehran | BMT center of a teaching hospital |
- Control group: 30 patients - Interventional group: 30 patients |
14 months | Clinical, nutritional, and paraclinical factors | Individualized parenteral nutrition | Harris-Benedict equation | Significant improvement in clinical outcome and hematologic responses |
| Sistanizad et al. [30] | Tehran | 2 ICUs of a teaching hospital | Not defined |
Pre-restriction: 6 months Post-restriction: 9 months |
- Bacterial susceptibilities - Defined daily doses |
- Carbapenems restriction program - Daily rounds with the intensivist |
WHO collaborating center for drug statistics methodology |
- Significant increase in the sensitivity of P. aeroginosa to imipenem - Significant decrease in the carbapenems’ defined daily dose |
| Mousavi et al. [23] | Tehran | BMT wards of a teaching hospital |
- Intervention group: 29 patients - Control group:30 patients |
12 months | Nutritional status and clinical as well as safety outcome indexes | All the procedures of PN including ordering, preparation, monitoring, and discontinuation |
Standard guideline of nutrition support (not specially defined) |
- Significantly improved nutritional status and clinical outcomes but more episodes of hyperglycemia |
| Baniasadi et al. [16] | Tehran | 8 wards of a teaching hospital | 8559 patients | 12 months | Incidence, pattern, seriousness, and preventability of ADRs | ADR-reporting yellow cards |
- WHO definition of ADR - WHO dentition of serious ADRs - Naranjo’s algorithm and Schumock scale |
- Anti-infective agents accounted for the most frequently reported ADRs - Fifty-four and eighteen ADRs were classified as serious and preventable, respectively |
| Farsaei et al. [24] | Tehran | Infectious diseases ward of a teaching hospital |
Pre-intervention: 66 patients Post-intervention: 139 patients |
Pre-intervention: 16 months Post-intervention: 16 months |
Glycemic control |
Different interventions such as: - Holding oral anti-diabetic medications - Correction of daily insulin dose - Supplemental insulin dose |
ADA guideline | - Significant increase in the percentage of controlled random blood sugars |
| Entezari-Maleki et al. [11] | Tehran | Drug and poison information call center affiliated to a teaching pharmacy | 110,310 phone calls | 24 months |
- Frequency and type of calls - Demographic features and profession of clients |
- Answering healthcare professionals’ inquiries - Consulting staffs - Evaluating the accuracy and quality of staff answers |
Drug information references | 585 and 420 potential cases of ADRs and major DDIs were detected |
| Gharekhani et al. [40] | Tehran | Nephrology ward of a teaching hospital | 406 patients | 18 months | Medication errors |
- Regular pharmacotherapy rounds - Medical chart review |
- Medication errors: Pharmaceutical Care Network Europe Foundation - Clinical significance: National Council for Medication Error Reporting and Prevention |
Clinical pharmacists’ interventions decreased direct-related costs of medication errors by 4.3% |
| Namazi et al. [39] | Shiraz | Neurology wards of two teaching hospitals | 589 patients | 7 months | Incidence and risk factors of potential drug-drug interaction |
- Medical chart review - Unofficially administrative and prescription interventions |
Lexi-Comp | - Near three-fourth of unofficially administrative and prescription interventions were accepted by both physicians and nurses |
| Tavakoli-Ardakani et al. [31] | Tehran | ICU and hematology-oncology wards of a teaching hospital |
- Before intervention: 77 patients - Post-intervention: 82 patients |
12 months | Vancomycin use parameters |
- Medical chart review - Discussions with physician |
Center of Disease Control and prevention (CDC) and Infectious Diseases Society of America (IDSA) guidelines | - Significant improvement in appropriate initiation of vancomycin (but not dosing regimen and duration of therapy) |
| Hassani et al. [41] | Tehran | Infectious diseases ward of a teaching hospital | 419 patients | 8 months | Frequency and type of clinical pharmacists’ interventions and medication errors |
- Medical chart review - Pharmacotherapy monitoring forms |
- DVT: Caprini risk assessment model - SUP: American Society of Health-System Pharmacists (ASHP) guideline |
Monitoring vancomycin level, changing the frequency, duration or dose of drugs were the most common clinical pharmacists’ interventions |
| Mahmoudi et al. [25] | Shiraz | All wards of a teaching hospital |
- Control group: 6470 patients - Interventional group: 6210 patients |
- Control group: 4 months - Interventional group: 4 months |
- Total number of unit drug used and direct cost of albumin, enoxaparin, and IV pantoprazole - Clinical outcome indexes |
Guideline development and implementation | Updated international consensus guidelines (not specially defined) | Significant reduction in the total number of unit drug used and direct cost of studied medications without changing clinical outcome |
| Haghbin et al. [42] | Shiraz | Pediatric Intensive Care Unit of a teaching hospital | 41 patients | 6 months | Medication errors |
- A special, local safety guideline - Instruction about correct administration, prescription and transcription |
Not defined |
- 42 interventions were implemented - 80% of interventions were accepted and led to the correction of medication errors |
| Foroughinia et al. [7] | Shiraz | Neurology ward of a teaching hospital | 123 patients | 5 months | Drug-related problems | Pharmacotherapy consult | Pharmaceutical Care Network Europe classification |
- Prescriber informed was the most frequent intervention - The rate of clinical pharmacist’s intervention acceptance by physician was 41.91%. |
| Jahangard-Rafsanjani et al. [10] | Tehran | A teaching pharmacy | 287 patients | 4 months |
- Follow up with physician, - Physician plan for further work-up and treatment -Life style modifications |
Giving a clinical summary sheet to patients and encouraging them to follow up with their physician | Not defined | -Adherence to the follow up recommendation was 46% among high risk patients for cardiovascular diseases |
| Zolfagharian et al. [26] | Mashhad | 4 wards of a teaching hospital |
Before intervention: 50 patients After intervention: 50 patients |
Pre-implementation phase: 6 months Post-implementation phase: 6 months |
Pattern of use (indication, vial numbers, dose, and time duration) and direct cost of albumin |
- Developing a standard protocol - Medical chart review |
American Hospital Formulary Service (AHFS) and American Society of Health-System Pharmacists (ASHP) |
- Non-significant reduction of improper albumin use -Significant reduction in inappropriate dose, duration of therapy, the number of consumed vial, and the average cost of albumin for each patient |
| Laki et al. [27] | Tehran |
tertiary referral university-affiliated hospital. |
Phase 1: 100 patients Phase 2: 84 patients Phase 3: 66 patients |
Phase 1: 45 days Phase 2: 45 days Phase 3: 45 days |
Pattern, indication, dose and treatment duration of Albumin |
Developing albumin use guideline and presenting it to medical team - Evaluation of albumin use via reviewing order sheets |
Locally developed evidence-based guideline |
- Albumin orders with appropriate indication increased significantly - The frequency of inappropriate orders reduced significantly |
| Vazin et al. [32] | Shiraz | 4 ICUs of a teaching hospital | 100 patients | 11 months | Indication and dose of colistin |
- Medical chart review -Pharmacotherapy notes |
Standard guideline (not specially defined) | - Colistin was discontinued in all patients in whom empirical therapy was continued incorrectly |
| Karimzadeh et al. [12] | Shiraz | Drug and poison information center affiliated to a teaching hospital | 485 phone calls | 12 months |
- Frequency and type of calls - Demographic features and profession of clients |
- Answering healthcare professionals’ inquiries - Consulting staff and residents - Evaluating the accuracy and quality of staff answers |
Drug information references |
-Major questions were asked from the health-care team (the nursing group). - Drug indication, ADR, storage, the method of preparation and administration were the |
| Mahmoodpoor et al. [46] | Tabriz | Trauma ICU of a University hospital | 242 patients and 832 interventions | 9 months | Number, type, and clinical significance of clinical pharmacy interventions | Collecting all clinical pharmacists’ interventions and visits |
- The Australian guideline - standard of practice for clinical pharmacy practice |
- Most interventionsconcerned adding a new medication to a drug regimen or switching to a needed new medication. |
| Dastan et al. [28] | Tehran | National Research Institute of Tuberculosis and Lung Disease | Pre-implementation phase: 90 patients Post-implementation phase: 45 patients |
Pre-implementation phase: 66 days Post-implementation phase: 66 days |
-Appropriate and inappropriate indications of Albumin - Quantity of albumin administered - Inappropriate cost of albumin use |
- The introduction of evidence-base guideline for albumin via a pharmacist-led audit and feedback intervention |
Studies literally available in hand - Guidelines derived from expert consensus |
Inappropriate use of albumin was decreased significantly by 79.3% leading to 38,800 USD reduction in inappropriate costs of albumin. |
| Vazin et al. [29] | Shiraz | clinical wards of a teaching hospital |
Pre-implementation phase: 4946 patients Post-implementation phase: 4895 patients |
Pre-implementation phase: 6 months Post-implementation phase: 6 months |
- Clinical outcomes (hospital length of stay and all-cause in-hospital mortality) - Prescription number ofalbumin, IVIG, and IV pantoprazole, direct cost of each studied medication and alternative medication costs |
Guideline development and implementation through a pre-designed indication checklist for albumin, IVIG, and IV pantoprazole |
Online UptoDate, Micromedex, American Society of Health System Pharmacists guidelines and Applied Therapeutics: The Clinical Use of Drugs by Koda-Kimble and Young |
- The total number of administered medications significantly decreased by 50.76%. - The direct cost of albumin and IV pantoprazole significantly decreased (55.8% and 83.92%, respectively). - The monthly direct cost of all three medications decreased by $77,720 (55.88%). |
Establishing pharmaceutical care in out-patient clinics and drug information centers
Fahimi et al. in 2011 evaluated the adequacy of anticoagulation and the effect of consultation services by clinical pharmacists in the first pharmacist-managed anticoagulation clinic in Iran. During a 14-month period, all patients on warfarin therapy were regularly monitored and consulted based on the 7th ACCP guideline. Education package and International Normalized Ratio (INR) logbook were also given to each patient. Among different clinical pharmacists’ interventions, increasing the dose (31.6%) was the most common one. About half (47.7%) of patients reached the target INR on follow-up visits. In this outpatient setting, apart from INR monitoring, clinical pharmacists also educated patients about warfarin drug-drug and drug-food interactions, as well as their nutrition and disease [9].
Another study was conducted in Pharmacotherapy Consultation Clinic within 13-Aban pharmacy area by Jahangard-Rafsanjani and her colleagues in 2017, in which the outcomes and feasibility of a pharmacy-based cardiovascular screening program were assessed. The patients were evaluated regarding their demographic and clinical profile, major cardiovascular risk factors, exercise habits, medical conditions, medications, and family history; then Framingham risk score was calculated. Recommendations regarding diet, weight management, physical activity, and tobacco cessation, as well as printed education materials were provided to all patients. Additionally, a clinical summary sheet signed by a clinical pharmacist was given to all high-risk. Participants were contacted one month later and they were asked if they had followed given recommendation. The results showed that adherence to the follow up recommendation was 46% among high risk patients for cardiovascular diseases [10].
Drug and poison information call centers (DPIC) are another area in which Iranian clinical pharmacists have been involved. Entezari-Maleki et al. in 2014 assessed all call services delivered to the DPIC of 13-Aban pharmacy, operated by the department of clinical pharmacy during 2 years. Of the total 110,310 calls services delivered to this center, 585 and 420 potential cases of ADRs and major DDIs were detected, respectively. The clinical pharmacists educated patients about the correct time, dose, administration route and also drug-drug as well as drug-food interactions. They also consulted health-care professionals about stability, storage, compatibility, and all other drug information issues. They concluded that DPIC can offer drug consultation, as well as detecting and preventing ADRs and DDIs, to patients’ health promotion and improvement in their pharmacotherapy [11].
Karimzadeh et al. in 2017 reported the annual contacts to DPIC of Shiraz Namazi hospital, the largest referral university hospital in the southwest of Iran, that were under the scientific supervision of clinical pharmacists. Four hundred and eighty-five contacts were registered. The most common types of questions were drug indication (13.3%), ADRs (13.3%), storage (11.8%), and the method of preparation as well as administration (11.7%). The majority of questions were asked by the health-care team working in Namazi hospital and mostly from the nursing group. They concluded that DPICs in the hospitals are suitable services for reducing the rate of ADRs and MEs, as well as improve the pattern of medication use which can result in cost saving [12].
Prevention, detection and management of ADR
The roles of clinical pharmacists in ADR reporting were assessed by 4 studies. Baniasadi et al. in 2008 developed an ADR system at MasihDaneshvari hospital affiliated to Shahid Beheshti University of Medical Sciences. During the period of 12 months, healthcare professionals were educated and encouraged to report all suspected ADRs through yellow forms via publishing monthly ADR bulletins and providing training lectures. One hundred twelve spontaneous reports were received from 7 wards. The most common medication class and agent reported to induce ADRs were anti-infectives and ceftriaxone, respectively. The most frequently affected system was the skin and appendages system. 19and 25 ADRs were identified to be serious and preventable, respectively. Authors believed that the rate of ADRs reported by healthcare professionals (physicians and nurses) in their hospital was somewhat low [13].
Khalili et al. in 2012published an article regarding knowledge, attitude and perceptions/practices of healthcare workers about ADR in a single-center interventional study on 100 healthcare workers of Imam Khomeini Hospital. They designed a validate questionnaire based on WHO definition about ADR. In the first phase of the study, they invited medical students, nurses, physicians and pharmacists to participate in the study and fill the questionnaire. Then the educational phase including workshops, meetings and presentation was done by clinical pharmacists. During this one-month period, the participants learned how to fill a yellow card and clinical pharmacists emphasized on the importance, seriousness, preventability, necessity and advantages of reporting ADR. After 3 months, participants filled the same questionnaire. The results showed that 91.5% of the healthcare workers of hospital never reported any ADR.49% of participants were not even aware of Iranian Pharmacovigilance Center before the intervention phase. However, after that, all of them knew this center [14].
Salehifar et al. in 2013 collected and published all of the reported ADRs during 2004–2010 in the Mazandaran province, north of Iran and evaluated the role of clinical pharmacists in the improvement of a pharmacovigilance system. Since 2007, clinical pharmacists from Mazandran University have involved in ADR committees. From 2004 to 2010, 793 yellow cards were completed by health care providers from all hospitals. The majority of ADRs (95.2%) were related to the time after the involvement of clinical pharmacists. The most common drugs associated with the ADRs were ceftriaxone, diclofenac, and vancomycin. Forty-one ADRs were reported as serious [15].
Baniasadi et al. in 2014 evaluated the role of clinical pharmacy residents in reporting ADRs within the MasihDaneshvari teaching hospital. Clinical pharmacy residents were trained to report all suspected ADRs through ADR-reporting yellow cards. During one-year period of study, 202 ADRs from 8 wards were reported. Anti-infective agents were accounted for the most frequently reported ADRs and among anti-infective agents, rifampin was accounted for the highest number of reported ADRs. The most frequently affected system was gastro-intestinal. 44 and 18 ADRs were classified as serious and preventable, respectively [16].
Designing protocols, improving drug utilization, and cost saving
Khalili et al. in 2010 evaluated the appropriateness of deep vein thrombosis (DVT) prophylaxis before and after pharmacist intervention in the infectious ward of Imam Khomeini hospital in Tehran. During the pre-intervention phase of study, they assessed the patient’s risk factors for DVT during hospitalization period and physician’s approaches to DVT prophylaxis. Based on the available literature and guidelines, clinical pharmacists prepared an internal guideline for DVT prophylaxis. In the post-intervention phase, clinical pharmacist assessed DVT risk and gave recommendation on the appropriateness of prescribing anticoagulants as DVT prophylaxis for physicians. Before clinical pharmacist’s intervention, 69.9% of patients had appropriate indication. After the intervention, 88.4% of patients were prescribed anticoagulants appropriately. A statistically significant decrease was also observed in the number of patients who had the indication for prophylactic treatment of DVT but did not receive any anticoagulants [17].
Inappropriate use of acid suppression therapy (AST) for stress ulcer prophylaxis (SUP) has become increasingly common in recent years. Thus, Iranian clinical pharmacists performed two interventional studies in this field. Khalili et al. in 2010 conducted a prospective, pre-post intervention study to determine the effects of a clinical pharmacist intervention including AST prescribing and adherence to guideline for use of SUP in the infectious ward of Imam Khomeini hospital. In the pre-intervention phase (4-months), baseline SUP prescribing patterns were assessed. Then, he clinical pharmacists prepared an internal guideline in accordance with American Society of Health-System Pharmacists (ASHP) guideline for use of SUP and educated the physicians who monitored and visited the hospitalized patients during a 4-month period. The results implicated that the clinical pharmacists’ intervention was associated with significant reduction in the use of acid suppression therapy overall and also in patients without an absolute indication for SUP [18].
A similar study was done by Mousavi et al. in 2013 in the nephrology ward of the same hospital. In comparison to the pre-intervention phase, significant relative reduction in the inappropriate SUP prescription rate and related cost (by about 44% and 67%, respectively) was observed after the intervention [19].
Considering the fact that end-stage renal disease (ESRD) patients have different complications and they take several medications, two studies specifically evaluated clinical pharmacist’s interventions in hemodialysis (HD) patients in Iran. Dashti-Khavidaki et al. in 2012 designed a six-month prospective study in which clinical pharmacists conducted medical visits and adjusted the patients’ medications based on their laboratory data and NKF-K/DOQI guidelines in the hemodialysis ward of Imam Khomeini hospital in Tehran. According to their study, hemoglobin concentration increased and serum ferritin reached target value in anemic patients. Serum calcium concentration was increased and decreased in hypo calcemic and hypercalcemic patients, respectively. An improvement in Ca × P product as well as intact parathyroid hormone concentrations were also observed. Triglycerides, total cholesterol and low-density lipoprotein cholesterol decreased to near-optimal values [20].
The same study group in 2013 assessed the use of pharmaceutical care to improve health-related quality of life (HRQOL) in HD patients of Imam Khomeini hospital in Tehran. In comparison to the control group that received only standard care of the ward, case group received clinical pharmacist-led pharmaceutical care in addition to the standard care. During weekly visits, clinical pharmacists assessed different aspects of patients’ pharmacotherapy and gave required advices as well as educations. Two published booklets regarding correct drug administration and nutrition by clinical pharmacists were also given to patients. It was observed at the end of the study period (6 months) that median of HRQOL improved in case group especially in the role-emotional, mental health, social functioning, and general health dimensions in compare to control group [21].
Comparison of clinical pharmacist-based parenteral nutrition (PN) with conventional PN in Iran was studied in two articles in the bone marrow transplantation (BMT) settings. The first one was designed by Tavakoli et al. in 2013 to evaluate the effect of individualized PN based on Harris-Benedict equation in patients undergoing autologous hematopoietic stem cell transplantation at Taleghani BMT Centre in Tehran. They compared different clinical (duration of hospital stays, fever, infection and mortality), nutritional (bodyweight and days of PN) and paraclinical factors (serum proteins, white blood cell (WBC), platelet engraftment, packed cell and platelet transfusions) between patients received individualized PN and those received conventional PN. This study demonstrated that patients received individualized PN had less weight loss, lower incidence of infection, more rapid platelet engraftment, shorter duration of hospital stay, and less units of packed-cell transfusions [22].
At the same year, Mousavi et al. published the second study about clinical pharmacist-based PN service for BMT patients at Shariati hospital in Tehran. They compared nutritional status, clinical outcome indexes (length of hospital stay, rate of graft versus host disease, time to engraftment, and mortality rate) and safety profile (hyperglycemia, hepatic dysfunction, catheter infection) between the intervention and control groups. Patients in the control group received a routine nutrition support protocol. In this group, PN was started on the first day after transplantation regardless of tolerance of oral feeding and was continued until the catheter was removed at discharge. In contrast, PN was started in only certain conditions like oral intake ≤50%, inability to use enteral feeding, and serum albumin level < 3 g/dl in the intervention group. In this group, patients received PN based on the standard guidelines of nutrition support. All the procedures of PN (order, preparation, monitoring, and discontinuation) were carried out by the clinical pharmacy team. The intervention was associated with fewer days of PN, more improved nutritional status, shorter length of hospital stay, and more episodes of hyperglycemia in comparison to the control group [23].
Regarding blood glucose monitoring, Farsaei et al. in 2014evaluated the impact of clinical pharmacist interventions on the glycemic control in the infectious disease ward of Imam Khomeini Hospital in Tehran. Different interventions were implemented by clinical pharmacists in this regard, such as holding oral anti-diabetic medications (if necessary, based on blood glucose and illness status), correction of daily insulin dose (based on blood sugar monitoring), and supplemental insulin dose (if necessary). They also educated both nurses and patients about correct technique for insulin administration and blood glucose measurement by glucometer. The percentage of controlled random blood sugar was significantly higher in the post-intervention group than that in pre-intervention group (22.3% versus 13.8%, respectively). Similar, but not statistically significant, increasing trend was identified about percentage of controlled fasting blood sugars after the intervention [24].
Mahmoudi et al. in 2015 designed an 8-month study to evaluate both clinical and economic impacts of pharmaceutical practice guideline implementation for three costly medications, including albumin, enoxaparin, and pantoprazole in a tertiary hospital in Shiraz. They prepared a practice guideline for usage of these three costly medications based on the consensus guidelines. The results showed that in the pre-intervention period, 51.2%, 26%, and 67% of albumin, enoxaparin, and pantoprazole administrations were inappropriate, respectively. Guideline implementation resulted in significant reduction in the total value of costly administered drugs by 56%per month which accounted for85,625USD. Regarding clinical outcomes, the incidence of venous thromboembolic as well as gastrointestinal bleeding events, length of hospital stay, and mortality rate were comparable between pre- and post-intervention phases [25].
Three studies specifically assessed the interventions of clinical pharmacists in optimizing the utilization of albumin, as one of the costliest medications in hospitals, and its possible economic impacts. In 2017, Zolfagharian et al. evaluated and compared the appropriateness of albumin usage before and after implementation of a local guideline in4wards with the highest albumin consumption statistics at a teaching hospital in Mashhad. During the preparation phase, clinical pharmacists designed a standard protocol on albumin indications based on guidelines, such as American Hospital Formulary Service (AHFS) and ASHP. During the next 6 months (post-implementation phase), medical records of 50 patients receiving albumin were reviewed. The results showed a non-significant reduction of improper albumin use rate (from 62% to 57.5%). However, the average number of consumed albumin vials (from 8.80 to 4.15 vials), dose (from 52.63to 13.04 g) as well as duration of inappropriate albumin therapy (from 52.63 to 13.04 days) and the cost (from 317.78 to 149.81USD) for each patient were significantly decreased [26].
Another investigation was done by Laki et al. in 2017 aimed to evaluate the effects of hospital-wide interventions to optimize albumin use in a teaching clinical setting in Tehran. This study was comprised of three phases: In the first phase the pattern of baseline albumin use was determined. In the second phase, the designed guideline was presented to the physicians. In the third stage, the physicians were asked to fill the order sheet and send it to the hospital pharmacy. The interventions significantly increased albumin orders with appropriate indication (from 42% to 62%) and reduced the frequency of inappropriate orders (from 58% to 27%) over the three phases of the study [27].
Finally, Dastan et al. conducted a two-phase study, comprising an observational drug utilization evaluation and a pharmacist-led audit and feedback interventional study in a tertiary referral hospital in Tehran, in 2018. During the interventional phase, physicians were asked to fill out the albumin request form and send the form to the pharmaceutical care department. Then, the form was reviewed by a clinical pharmacist. The results showed inappropriate prescription of 78.4% and 38.4% of albumin vials during the observational and interventional phases, respectively. The intervention caused a significant decrease in the unjustified use of albumin (79.3%), leading to38,800 USD reduction in inappropriate costs of albumin [28].
Also, Vazin et al. conducted a six-month prospective study in clinical wards of Namazi hospital in Shiraz to evaluate the impact of an intervention by the pharmaceutical care unit on the use pattern of three high-cost medications, including albumin, IVIG, and IV pantoprazole and their direct costs. The physicians were asked to complete indications checklists designed by a clinical pharmacist for this purpose when ordering these drugs. Then trained general pharmacists examined these checklists and were authorized to either approve or disapprove the indication forms under the supervision of clinical pharmacists. The result showed that the total number of administered medications decreased significantly by 50.76% during the intervention period. Additionally, the direct cost of albumin and IVpantoprazole significantly decreased (55.8% and 83.92%, respectively) [29].
There are also three articles about Iranian clinical pharmacists’ interventions on antibiotic use and stewardship including colistin, carbapenems, and vancomycin at teaching hospitals.
Sistanizad et al. in 2013 studied the effect of an antibiotic stewardship program (ASP) by carbapenems restriction on gram-negative antimicrobial resistance in 2 ICUs of a teaching hospital in Tehran. Carbapenem (imipenem and meropenem) uses were restricted to only culture proven multi-drug resistance bacteria with the absence of sensitivity to other antimicrobial agents, under close supervision of infectious disease specialist and the clinical pharmacist in one of the studied ICUs. Compared to the pre-intervention period, the sensitivity of P. aeruginosa (rather than Klebsiella and Acinetobacter) to imipenem, and carbapenems’ defined daily dose was significantly increased and decreased, respectively [30].
Tavakoli-Ardakani et al. in 2015 designed a pre-post intervention study to determine the accuracy of vancomycin use before and after clinical pharmacist intervention in patients admitted to the ICU and hematology-oncology wards of Taleghani hospital in Tehran. Pharmacist interventions were categorized into three main sets: appropriate initiation, duration, and dosing regimen of vancomycin therapy. After pharmacist intervention, a significant improvement was observed in the appropriate initiation of vancomycin (but not dosing regimen and duration of therapy). Pharmacist discussions with the physician resulted in vancomycin discontinuation and dosage adjustment in 50% and 30.77% of cases, respectively [31].
Beside drug use evaluation, Vazin et al. in 2017 also reported interventions of a clinical pharmacist in detecting and correcting inappropriate dose and indication of colistin in 100 patients in 4 ICUs of a referral hospital in Shiraz. Clinical pharmacist’s suggestions regarding correcting colistin dose, based on patients clinical and paraclinical conditions were accepted by physicians in89% of cases. The acceptance rate of clinical pharmacist’s suggestions was 100% in the case of incorrect continuation of empirical therapy [32].
Detection, prevention, and management of medication errors
Clinical pharmacists’ practices in the detection, prevention, and management of medication errors (MEs) have been the subject of 11interventional studies in Iran.
In 2009, a 4-month study was conducted by Vessal to determine the role of a clinical pharmacist on detection and prevention of prescription errors at the nephrology ward of a referral hospital in southern of Iran. Eighty-six prescribing errors including wrong frequency, wrong drug selection, overdose, failure to discontinue, failure to order, under- dose, wrong time, monitoring, wrong route, and drug interactions occurred in 60.5% of hospital admissions. 96.5% of the prescription errors were accepted by the attending physician and interventions were made. The most common errors were related to immunosuppressive and anti-infective medications [33].
In 2011, Abbasi-Nazari et al. assessed the role of clinical pharmacists in educating nurses to reduce drug-food interactions (absorption phase) in 4 wards of a teaching hospital. This study was designed in 3 phases. In the first phase, only drug-food interactions were recorded by a trained pharmacy student. During the second phase, clinical pharmacists prepared information pamphlets and trained nurses. In the third phase, nursing practices were observed again one month later. Nurse training was associated with significant reduction in the rate of incorrect drug administration (from 44.6% to 31.5%) and also absorption drug-food interactions [34].
Khalili et al. in 2011assessedthe role of clinical pharmacist’s interventions within 1 year in the detection and prevention of MEs in the infectious disease ward of Imam Khomeini hospital in Tehran. They detected 112 MEs among 861patients,of which drug dosing, choice, use and, interactions were the most common ones. Clinical pharmacists made recommendations about all detected MEs and health care provider team accepted them [35].
In 2012, Dashti-Khavidaki et al designed a case-control interventional study about the role of clinical pharmacist in improving medication administration through enteral feeding tubes by nurses. In the pre-interventional phase, they assessed baseline knowledge of nurses by different questions regarding medication preparation, tube flushing, recognizing drug-drug/drug-food interactions, and recognizing dosage forms. In the interventional phase, they educated nurses in the case group by preparing evidence-based booklet and classes. After 3 months, nurses in both case and control groups were evaluated again. Findings indicated a significant increase in the mean scores of knowledge and practice questions in the case group but no change or even reduction in the control group [36].
Abbasi-Nazari et al. in 2012 published another study about the effects of nurse’s education on reduction of errors in intravenous (IV) drugs preparation and administration in intensive care unit (ICU) and surgical ward of a teaching hospital in Tehran. In the pre-intervention phase, IV drug preparation and administration by nurses were monitored. In the interventional phase, nurses were educated via installation of wall posters and giving informative pamphlets. Finally, IV drug preparation and administration by nurses was observed again after the educational phase. This study showed a significant difference between the rate of ME before and after interventions in either ICU or surgery and the total two wards [37].
Khalili et al. in 2012conducted another study at an infectious disease ward to determine the frequency and type of MEs, the type of clinical pharmacy interventions, acceptance of pharmacist interventions by health-care provider team, nursing staff satisfaction with clinical pharmacy services, and the probable economic impact of clinical pharmacy interventions. Among different types of MEs, the most common ones were incorrect dose (35.5%), omission error (24.3%), and incorrect medication (14.3%).The mean number of clinical pharmacist interventions per patient was 3.2.More than half of (59.8%) clinical pharmacy interventions were associated with adding a drug to treatment regimen, drug discontinuation, and changing the frequency, duration or dose of drugs.39% of clinical pharmacists’ interventions had moderate to major financial benefits. Although not reaching the level of statistical significance, the direct medication cost per patient was decreased about 3.8% (from 153.9 to 148.1 USD) following clinical pharmacist’s interventions. The acceptance rate of clinical pharmacist interventions by healthcare provider team, including nurses and physicians was 80% [38].
Namazi et al. in 2014 studied potential drug-drug interaction (DDIs) in neurology wards of Namazee and Faghihi hospitals in Shiraz. During the study period, 4539 DDIs were detected by the Lexi-Comp software. The most common type C, D, and X DDIs were between Heparin-Aspirin (23.40%), Warfarin-Aspirin (16.30%), and Omeprazole-Clopidogrel (0.60%), respectively. They found 484 ADEs, of which the most common were platelet and clotting disorders. Among all 421 clinical pharmacist’s administrative and prescription interventions, 74.24% were accepted by both physicians and nurses [39].
Gharekhani et al. in 2014evaluated the frequency, types, direct-related costs of MEs, as well as clinical pharmacist interventions during 18 months at the nephrology ward of Imam Khomeini hospital. During this study, MEs were detected, managed, and recorded by the clinical pharmacists. Among406 studied patients and 7762 ordered medications, the rate of ME was 3.5 errors per patient and 0.18 errors per ordered medication. More than 95% of MEs occurred at the prescription stage. Preventing MEs by clinical pharmacists’ interventions decreased direct medication costs by 4.3% [40].
In an eight-month prospective study in 2015, Hassani et al. determined the frequency and type of clinical pharmacist’s interventions and MEs in the infectious disease ward of Loghman hospital, affiliated to Shahid Beheshti University of Medical Sciences in Tehran. A clinical pharmacist collected all patients’ data admitted to the infectious disease, completed pharmacotherapy monitoring forms and extracted MEs. They used Caprini risk assessment model for DVT prophylaxis and ASHP guideline and articles for SUP. DVT prophylaxis, SUP prophylaxis, and vancomycin monitoring were the most common MEs. The most common clinical pharmacist intervention was requesting vancomycin level assays in the patients followed by adding a drug to the treatment regimen, and changing the frequency, duration or dose of drugs [41].
Foroughinia et al. in 2016 evaluated the clinical pharmacists’ interventions in patients admitted to the neurology ward of Faghihi hospital in Shiraz. Apart from 168 ME detected by a general pharmacist, a total of 346 interventions were done by the clinical pharmacist. Prescriber informed was the most frequent intervention (28.6%). The acceptance rate of clinical pharmacist’s intervention by physician was 41.91% [7].
Haghbin et al. in 2016 evaluated the incidence, types and outcomes of MEs in patients admitted to pediatric ICUs (PICU) during 6 months at Namazi hospital in Shiraz. A trained pharmacist under the supervision of a clinical pharmacist evaluated the patients’ medications for MEs by direct observational method. The pharmacist intervened via instructing about correct administration, prescription and transcription, only in situations that ME could cause substantial harm to patient (level 2–6). The most frequent MEs were administration errors. Of 42 pharmacist interventions, 80% of them were accepted and led to the correction of MEs [42].
All clinical pharmacists’ interventions
Four articles described all clinical pharmacist services and interventions at 3teaching hospitals in Tehran and Tabriz. In 2009, Dashti-Khavidaki et al. collected the data regarding all clinical pharmacist services in the nephrology and infectious disease wards of Imam Khomeini hospital within 1 year in Tehran. They defined clinical pharmacist services as any recommendation by a clinical pharmacist relevant to patient pharmacotherapy whether or not it resulted in any changes by physicians. A total number of 1386 services for 1105 patients were identified. More than two-fifth (45.4%) of clinical pharmacy services consisted of discontinuation of unnecessary drugs and changing in dose or frequency of prescribed drugs. Regarding clinical significance, about half of clinical pharmacist services (45.2%) had moderate-to life saving clinical significance. More than one-third (32%) of clinical pharmacist services were aimed to reduce drug costs. The acceptance rate of clinical pharmacist services by the physicians was 94.5%.
Fahimi et al. in 2010 reported the results of one-year (from January 2006 to January 2007) experience of clinical pharmacy establishment program at Masih Daneshvari hospital in Tehran. Among 772 interventions, the highest rate related to drug information (22.30%) followed by dose adjustment (13.57%) and therapeutic reduction/addition (12.88%). The calculated percentage of interventions per patient-medication exposure was24.28% [44].
At the same clinical setting, Allameh et al. published an article in 2012in which all clinical pharmacists’ interventions during 28 months from January 2008 to June 2011 (except for the period from September 2008 to February 2009 and October 2009 to March 2011) were reported. Of the total 3152 records, 2227 were recognized as interventions. The most common intervention was improper medication use (36.2%). 75.4% of all interventions were classified as grade 1 (minor potential inconvenience). Most interventions (97.6%)were considered to be appropriate and relevant [45].
Finally, in 2018, Mahmoodpoor et al. reported clinical pharmacy services during 9 months in the ICU of Shohada hospital in Tabriz. During the study period, a total of 832 interventions on 242 patients were performed by the clinical pharmacist. The intensivists accepted approximately 93.6% of the interventions. The most common intervention was adding a new medication to a drug regimen or switching to a needed new one. Also, the clinical pharmacist provided drug information to employees and medical staff in 13% of interventions [46].
Discussion
This systematic review evaluated studies that investigated clinical pharmacists’ interventions and their impacts on clinical and economical outcomes in Iran. To our best of knowledge, this study is the first review of publications regarding Iranian clinical pharmacists’ interventions and their related benefits.
Regarding improvement in the pattern of medication use (as one of the most frequent interventions of Iranian clinical pharmacists), most studies have been dedicated to antibiotics, anticoagulants, and albumin. Today, global concerns about antibiotic resistance highlight the importance of antibiotic stewardship programs. Clinical pharmacists, as the key and central member of antibiotic stewardship team, has been reported to have substantial impacts on reducing MEs, antibiotic resistance rates, length of hospital stay, and also the cost in Western countries [47–49]. Similarly, at least one study in Iran has clearly demonstrated the beneficial interventions of clinical pharmacists in decreasing carbapenem defined daily dose and burden of resistant pathogens in the ICU [30].
Among anticoagulants with narrow therapeutic window, warfarin has many inconveniences, such as needing regular monitoring, variability of the patient’s response, risk of bleeding and drug-drug as well as drug-food interactions. Several studies have demonstrated that anticoagulation management services or clinics can lead to a significant improvement in anticoagulation management in the outpatient settings. Schilling et al. conducted a cluster randomized trial in which the impact of an inpatient Pharmacist-Directed Anticoagulation Service (PDAS) on transition of care and safety of patients receiving warfarin anticoagulation was assessed. In the PDAS group, warfarin dosing, monitoring, patient education, and transition of care was managed by a specialized team of clinical pharmacists that cooperated with physicians and outpatient anticoagulation clinic staff. An improvement was observed in the safety and efficiency of the care provided by this new service in certain subsets of more complex patients [50]. According to Entezari-Maleki et al. systematic review, pharmacist-led services were superior to usual medical care in achieving INR within normal range, reducing the probable bleedings and its interactions [51]. This issue was also observed in the first official pharmacist-based warfarin-monitoring service in Iran [9].
In terms of ADR and MEs, several studies in Iran clearly implicated the undeniable and considerable role of clinical pharmacists in early detection, management, and prevention of these undesirable and potentially harmful events. The annual number of ADR reporting in Iran (about 4300 reports in the year 2008) is much lower than WHO standards (200 reports per1,000,000 inhabitants per year) [52]. Therefore, as implicated by Baniasadi et al., pharmaceutical care team including hospital pharmacists, and clinical pharmacist specialists as well as residents could improve the ADR reporting system in Iran [16].
Beside ADR and MEs, cost is the other item that has been considered in several studies included in this review. Medication cost reduction by clinical pharmacist interventions ranges from 3.8% to 67%. Health Revolution Program (since May 2013) has been highlighted the issue of medication cost saving in our country. The prominent study that specifically focused on this issue was performed by Mahmoudi et al. in a teaching hospital in Shiraz, in which clinical pharmacists interventions were associated with considerable cost-saving [25]. Similar findings (55.88% that equals to 77,720 USD cost reduction per month) have been observed for albumin, intravenous pantoprazole, and intravenous immune globulin in another referral hospital in Shiraz [29].
Most clinical pharmacist interventions have been reported from in-patient settings in Iran, mostly Tehran. Iranian clinical pharmacists actively attend or give services in various hospital wards such as emergency department, cardiovascular disease, nephrology, infection disease, hematology-oncology, kidney transplantation, liver transplantation, BMT, geriatric, pediatric, pulmonary, endocrinology, neurology, gastroenterology, and ICU. Regarding outpatient and ambulatory care settings in Iran, only 4studies have been published from the anticoagulation clinic, pharmacotherapy consultation clinic within a community pharmacy, and DPICs. In these centers, clinical pharmacists review patient’ spharmacotherapy, assess possible MEs as well as ADRs, and educate patients. For example, Fahimi et al. in their warfarin clinic informed patients regarding benefits of warfarin therapy, bleeding and thrombosis symptoms, interactions, and management of missed dose(s). [9] Various types of pharmacist-managed clinics (PMCs) have been well documented in the literature including pharmacist-managed asthma clinics and immunization services, as well as anticoagulation, hyperlipidemia, Helicobacter pylori infection, diabetes, hypertension, latent tuberculosis infection, pain, smoking cessation, and cancer chemotherapy. PMCs have beneficial impacts in terms of patients’ adherence to treatment and their knowledge about pharmacotherapy, cost-effectiveness, and the treatment outcomes [53, 54]. There are few published reports about the implementation of PMCs and their clinical impact in Iran. Although other unpublished activities in the ambulatory care are currently being conducted in our country, this field is somewhat overlooked by both clinical pharmacists and health policy-makers and planners in our country and needs more attention.
Few studies reported the acceptance rate of clinical pharmacist interventions by healthcare provider team (nurses and physicians) in Iran. This ranged from 41.91% to 94.5%. This wide variation in the acceptance rate of clinical pharmacist interventions can be partially explained by the time duration that the wards have been received services from clinical pharmacists. For example, this rate was 94.5%in the nephrology and infectious disease wards of Imam Khomeini hospital in Tehran, capital of Iran, in which clinical pharmacist services have been provided for more than 2 decades. In this regard, about three-fourth(74.35%) of included studies were from either ambulatory care or in-patient settings in Tehran [43]. In contrast, acceptance rate was reported to be 41.91% in a neurology ward of a teaching hospital in Shiraz where clinical pharmacy services are relatively new [7]. The acceptance rate range reported from European and American studies is 73–89% and 85–99%, respectively [38].
There are several limitations in our study which should be considered when interpreting the results of this systematic review: First, many studies had small sample sizes, and most of them were single-center,. Therefore, their findings may be biased and not be reproducible and generalizable. Second, providing several definitions for outcomes, such as ADEs, ADRs, and MEs are confusing. Third, some studies exploited direct observational methods that may underestimate the rate of MEs. At the same time, the role of patients in ME was not considered. In addition, severity and clinical significance of MEs were not considered in most investigations. Fourth, relevant clinical outcome indexes (e.g., thromboembolic events in the case of DVT prophylaxis or GI bleeding in the case of SUP) were not considered and duration of intervention as well as follow-up phases were relatively short in most studies [median (interquartile range),9 (5) months]. Fifth, cost reported in the included studies were direct medication cost. Until other relevant costs, such as labor costs of pharmacists as well as labor cost of nurses costs for storing, dispensing, preparing, and administering the medication have not been determined, the real economic impact of clinical pharmacy interventions should be interpreted with caution. A final and potential limitation of this study is that synthesizing results across studies was impossible, due to heterogeneity of study methodologies, types of interventions, outcomes assessment and the settings where the studies were taken place. Thus, results were not synthesized and an assessment of bias risk was not performed.
Conclusion
Data of our review support the beneficial role of clinical pharmacists in the improvement of quality, safety, and efficiency of patients’ pharmaceutical care in Iran. Clinical pharmacists’ interventions have been associated with improved health outcomes, decreased health care resources used, and subsequently reduction in treatment costs. Most clinical pharmacist interventions and activities are about designing protocols, improving drug utilization pattern, as well as detection, prevention, and management of MEs Health care teams would benefit from the involvement of a clinical pharmacist in their team to ensure the accurate and prompt provision of information related to different medications to prescribers, as well as patients. However, the extent of the effect of clinical pharmacists’ interventions and the expected funds required to run clinical pharmacy services is unknown. Therefore, further studies are required to allow a more accurate assessment of benefits provided by clinical pharmacists and the cost-effectiveness of their interventions.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Afsaneh Noormandi, Email: yalda.nor@gmail.com.
Iman Karimzadeh, Email: karimzadehiman@yahoo.com.
Mahtabalsadat Mirjalili, Email: mahtab.mirjalili@gmail.com.
Hossein Khalili, Phone: +98-21-66954709, Email: khalilih@tums.ac.ir, Email: Khalilih@sina.tums.ac.ir.
References
- 1.Chamoun NR, Zeenny R, Mansour H. Impact of clinical pharmacy interventions on medication error nodes. Int J Clin Pharm. 2016;38(6):1436–1444. doi: 10.1007/s11096-016-0384-4. [DOI] [PubMed] [Google Scholar]
- 2.Jacobi J. Clinical pharmacists: practitioners who are essential members of your clinical care team. Revista Médica Clínica Las Condes. 2016;27(5):571–577. doi: 10.1016/j.rmclc.2016.09.002. [DOI] [Google Scholar]
- 3.Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–964. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 4.Niznik JD, He H, Kane-Gill SL. Impact of clinical pharmacist services delivered via telemedicine in the outpatient or ambulatory care setting: a systematic review. Res Social Adm Pharm. 2018;14(8):707–717. doi: 10.1016/j.sapharm.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Zellmer WA. Pharmacy's future: transformation, diffusion, and imagination. Am J Health Syst Pharm. 2010;67(14):1199–1204. doi: 10.2146/ajhp090539. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher J, McCarthy S, Byrne S. Economic evaluations of clinical pharmacist interventions on hospital inpatients: a systematic review of recent literature. Int J Clin Pharm. 2014;36(6):1101–1114. doi: 10.1007/s11096-014-0008-9. [DOI] [PubMed] [Google Scholar]
- 7.Foroughinia F, Tazarehie SR, Petramfar P. Detecting and managing drug-related problems in the neurology ward of a tertiary care teaching hospital in Iran: a clinical pharmacist's intervention. J Res Pharm Pract. 2016;5(4):285–289. doi: 10.4103/2279-042X.192455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahimi F, Sharif-Kashani B, Hossein-Ahmadi Z, Salamzadeh J, Namdar R, Mousavi S, et al. The first pharmacist-based warfarin-monitoring service in Iran. Journal of Pharmaceutical Health Services Research. 2011;2(1):59–62. doi: 10.1111/j.1759-8893.2010.00021.x. [DOI] [Google Scholar]
- 10.Jahangard-Rafsanjani Z, Hakimzadeh N, Sarayani A, Najafi S, Heidari K, Javadi MR, et al. A community pharmacy-based cardiovascular risk screening service implemented in Iran. Pharm Pract (Granada) 2017;15(2):919. doi: 10.18549/PharmPract.2017.02.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Entezari-Maleki T, Taraz M, Javadi MR, Hajimiri MH, Eslami K, Karimzadeh I, et al. A two-year utilization of the pharmacist-operated drug information center in Iran. J Res Pharm Pract. 2014;3(4):117–122. doi: 10.4103/2279-042X.145368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimzadeh I, Vazin A, Talebnia N, Hatami-Mazinani N, Mahi-Birjand M. Performance of drug and poison information center within a Referral University hospital in southwest of Iran. Journal of Pharmaceutical Care. 2018;5(3–4):49–55. [Google Scholar]
- 13.Baniasadi S, Fahimi F, Shalviri G. Developing an adverse drug reaction reporting system at a teaching hospital. Basic Clin Pharmacol Toxicol. 2008;102(4):408–411. doi: 10.1111/j.1742-7843.2008.00217.x. [DOI] [PubMed] [Google Scholar]
- 14.Khalili H, Mohebbi N, Hendoiee N, Keshtkar AA, Dashti-Khavidaki S. Improvement of knowledge, attitude and perception of healthcare workers about ADR, a pre- and post-clinical pharmacists' interventional study. BMJ Open. 2012;2:e000367. doi: 10.1136/bmjopen-2011-000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salehifar E, Ala S, Amini M, Azhdari E, Mir-Shafa F. The role of clinical pharmacists in the improvement of a pharmacovigilance system: a review of the reported adverse drug reactions during 2004-2010 in Mazandaran Province of Iran. Journal of Pharmaceutical Care. 2015;1(1):8–12. [Google Scholar]
- 16.Baniasadi S, Habibi M, Haghgoo R, Karimi Gamishan M, Dabaghzadeh F, Farasatinasab M, Farsaei S, Gharekhani A, Kafi H, Karimzadeh I, Kharazmkia A, Najmeddin F, Nikvarz N, Oghazian MB, Rezaee H, Sadeghi K, Tafazzoli A, Shahsavari N, Fahimi F. Increasing the number of adverse drug reactions reporting: the role of clinical pharmacy residents. Iran J Pharm Res. 2014;13(1):291–297. [PMC free article] [PubMed] [Google Scholar]
- 17.Khalili H, Dashti-Khavidaki S, Talasaz AH, Mahmoudi L, Eslami K, Tabeefar H. Is deep vein thrombosis prophylaxis appropriate in the medical wards? A clinical pharmacists' intervention study. Pharm World Sci. 2010;32(5):594–600. doi: 10.1007/s11096-010-9412-y. [DOI] [PubMed] [Google Scholar]
- 18.Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N, et al. J Manag Care Pharm. 2010;16(2):114–121. doi: 10.18553/jmcp.2010.16.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mousavi M, Dashti-Khavidaki S, Khalili H, Farshchi A, Gatmiri M. Impact of clinical pharmacy services on stress ulcer prophylaxis prescribing and related cost in patients with renal insufficiency. Int J Pharm Pract. 2013;21(4):263–269. doi: 10.1111/ijpp.12005. [DOI] [PubMed] [Google Scholar]
- 20.Dashti-Khavidaki S, Khalili H, Shahverdi S, Abbasi MR, Lessan-Pezeshki M. The role of clinical pharmacy services in achieving treatment targets in Iranian haemodialysis patients. Singap Med J. 2012;53(9):599–603. [PubMed] [Google Scholar]
- 21.Dashti-Khavidaki S, Sharif Z, Khalili H, Badri S, Alimadadi A, Ahmadi F, Gatmiri M, Rahimzadeh S. The use of pharmaceutical care to improve health-related quality of life in hemodialysis patients in Iran. Int J Clin Pharm. 2013;35(2):260–267. doi: 10.1007/s11096-012-9748-6. [DOI] [PubMed] [Google Scholar]
- 22.Tavakoli-Ardakani M, Neman B, Mehdizadeh M, Hajifathali A, Salamzadeh J, Tabarraee M. Clinical effect of individualized parenteral nutrition vs conventional method in patients undergoing autologous hematopoietic SCT. Bone Marrow Transplant. 2013;48(7):958–962. doi: 10.1038/bmt.2012.280. [DOI] [PubMed] [Google Scholar]
- 23.Mousavi M, Hayatshahi A, Sarayani A, Hadjibabaie M, Javadi M, Torkamandi H, Gholami K, Ghavamzadeh A. Impact of clinical pharmacist-based parenteral nutrition service for bone marrow transplantation patients: a randomized clinical trial. Support Care Cancer. 2013;21(12):3441–3448. doi: 10.1007/s00520-013-1920-6. [DOI] [PubMed] [Google Scholar]
- 24.Farsaei S, Karimzadeh I, Elyasi S, Hatamkhani S, Khalili H. Glycemic control in the infectious diseases ward; role of clinical pharmacist interventions. J Infect Dev Ctries. 2014;8(4):480–489. doi: 10.3855/jidc.3357. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoudi L, Karamikhah R, Mahdavinia A, Samiei H, Petramfar P, Niknam R. Implementation of pharmaceutical practice guidelines by a project model based: clinical and economic impact. Medicine (Baltimore) 2015;94(42):e1744. doi: 10.1097/MD.0000000000001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zolfagharian F, Ghazanfari S, Elyasi S, Iraji P, Saberi MR, Vahdati-Mashhadian N, Mohammadpour AH. Drug utilization evaluation of albumin in a teaching hospital of Mashhad, Iran: an interventional pre-post design study. Int J Clin Pharm. 2017;39(4):704–711. doi: 10.1007/s11096-017-0458-y. [DOI] [PubMed] [Google Scholar]
- 27.Laki B, Taghizadeh-Ghehi M, Assarian M, Heidari K, Torkamandi H, Javadi MR, Gholami K. Effect of hospital-wide interventions to optimize albumin use in a tertiary hospital. J Clin Pharm Ther. 2017;42(6):704–709. doi: 10.1111/jcpt.12566. [DOI] [PubMed] [Google Scholar]
- 28.Dastan F, Jamaati H, Emami H, Haghgoo R, Eskandari R, Hashemifard SS, Khoddami F, Mirshafiei Langari Z. Reducing inappropriate utilization of albumin: the value of pharmacist-led intervention model. Iran J Pharm Res. 2018;17(3):1125–1129. [PMC free article] [PubMed] [Google Scholar]
- 29.Vazin A, Karimzadeh I, Karamikhah R, Oveisi Z, Mohseni S, Keykhaee M, Roshanfard F, Sabet E, Zargari-Samadnejad A. Clinical and economical impacts of guideline implementation by the pharmaceutical care unit for high cost medications in a referral teaching hospital. BMC Health Serv Res. 2018;18(1):815. doi: 10.1186/s12913-018-3627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sistanizad M, Kouchek M, Miri M, Goharani R, Solouki M, Ayazkhoo L, et al. Carbapenem restriction and its effect on bacterial resistance in an intensive care unit of a teaching hospital. Iran J Pharm Res. 2013;12(3):503–509. [PMC free article] [PubMed] [Google Scholar]
- 31.Tavakoli-Ardakani M, Ghassemi S, Alizadeh AM, Salamzadeh J, Ghadiani M, Ghassemi S. Effects of pharmacist intervention on the utilization of vancomycin in a teaching hospital. Iran J Pharm Res. 2015;14(4):1281–1288. [PMC free article] [PubMed] [Google Scholar]
- 32.Vazin A, Karimzadeh I, Zand A, Hatami-Mazinani N, Firouzabadi D. Evaluating Adherence of Health-Care Team to Standard Guideline of Colistin Use at Intensive Care Units of a Referral Hospital in Shiraz, Southwest of Iran. Adv Pharm Bull. 2017;7(3):391–397. doi: 10.15171/apb.2017.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vessal G. Detection of prescription errors by a unit-based clinical pharmacist in a nephrology ward. Pharm World Sci. 2010;32(1):59–65. doi: 10.1007/s11096-009-9341-9. [DOI] [PubMed] [Google Scholar]
- 34.Abbasi Nazari M, Salamzadeh J, Hajebi G, Gilbert B. The role of clinical pharmacists in educating nurses to reduce drug-food interactions (absorption phase) in hospitalized patients. Iran J Pharm Res. 2011;10(1):173–177. [PMC free article] [PubMed] [Google Scholar]
- 35.Khalili H, Farsaei S, Rezaee H, Dashti-Khavidaki S. Role of clinical pharmacists' interventions in detection and prevention of medication errors in a medical ward. Int J Clin Pharm. 2011;33(2):281–284. doi: 10.1007/s11096-011-9494-1. [DOI] [PubMed] [Google Scholar]
- 36.Dashti-Khavidaki S, Badri S, Eftekharzadeh SZ, Keshtkar A, Khalili H. The role of clinical pharmacist to improve medication administration through enteral feeding tubes by nurses. Int J Clin Pharm. 2012;34(5):757–764. doi: 10.1007/s11096-012-9673-8. [DOI] [PubMed] [Google Scholar]
- 37.Abbasinazari M, Zareh-Toranposhti S, Hassani A, Sistanizad M, Azizian H, Panahi Y. The effect of information provision on reduction of errors in intravenous drug preparation and administration by nurses in ICU and surgical wards. Acta Medica Iranica. 2012;50(11):771–777. [PubMed] [Google Scholar]
- 38.Khalili H, Karimzadeh I, Mirzabeigi P, Dashti-Khavidaki S. Evaluation of clinical pharmacist's interventions in an infectious diseases ward and impact on patient's direct medication cost. Eur J Intern Med. 2013;24(3):227–233. doi: 10.1016/j.ejim.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Namazi S, Pourhatami S, Borhani-Haghighi A, Roosta S. Incidence of potential drug-drug interaction and related factors in hospitalized neurological patients in two Iranian teaching hospitals. Iran J Med Sci. 2014;39(6):515–521. [PMC free article] [PubMed] [Google Scholar]
- 40.Gharekhani A, Kanani N, Khalili H, Dashti-Khavidaki S. Frequency, types, and direct related costs of medication errors in an academic nephrology ward in Iran. Ren Fail. 2014;36(8):1268–1272. doi: 10.3109/0886022X.2014.934650. [DOI] [PubMed] [Google Scholar]
- 41.Hassani S, Eshraghi A, Hematian F, Taheri M, Sahraei Z. Role of clinical pharmacists in early detection, reporting and prevention of medication errors in a medical Ward. Journal of Pharmaceutical Care. 2017;3(3–4):54–60. [Google Scholar]
- 42.Haghbin S, Shahsavari S, Vazin A. Medication errors in Pediatric Intensive Care Unit: incidence, types and outcome. Trends in Pharmaceutical Sciences. 2016;2(2).
- 43.Dashti-Khavidaki S, Khalili H, Hamishekar H, Shahverdi S. Clinical pharmacy services in an Iranian teaching hospital: a descriptive study. Pharm World Sci. 2009;31(6):696–700. doi: 10.1007/s11096-009-9336-6. [DOI] [PubMed] [Google Scholar]
- 44.Fahimi F. Implementation of a clinical pharmacy education program in a teaching hospital: resident oriented documentation and intervention. Iran J Pharm Res. 2010;9(3):297–302. [PMC free article] [PubMed] [Google Scholar]
- 45.Allameh Z, Mehrpooya M, Baniasadi S, Fahimi F. Clinical pharmacists’ interventions in a teaching hospital: types, severity, procedures, and accuracy. Research in Pharmaceutical Sciences. 2012;7(5):954. [Google Scholar]
- 46.Mahmoodpoor A, Kalami A, Shadvar K, Entezari-Maleki T, Hamishehkar H. Evaluation of clinical pharmacy Services in the Intensive Care Unit of a Tertiary University hospital in the northwest of Iran. J Res Pharm Pract. 2018;7(1):30–35. doi: 10.4103/jrpp.JRPP_17_82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasquale TR, Komorny KM, Letting-Mangira D, Peshek S. A pharmacist-physician antibiotic support team. P AND T. 2004;29(1):33–40. [Google Scholar]
- 48.Tonna AP, Stewart D, West B, Gould I, McCaig D. Antimicrobial optimisation in secondary care: the pharmacist as part of a multidisciplinary antimicrobial programme--a literature review. Int J Antimicrob Agents. 2008;31(6):511–517. doi: 10.1016/j.ijantimicag.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Saizy-Callaert S, Causse R, Furhman C, Le Paih MF, Thebault A, Chouaid C. Impact of a multidisciplinary approach to the control of antibiotic prescription in a general hospital. J Hosp Infect. 2003;53(3):177–182. doi: 10.1053/jhin.2002.1307. [DOI] [PubMed] [Google Scholar]
- 50.Schillig J, Kaatz S, Hudson M, Krol GD, Szandzik EG, Kalus JS. Clinical and safety impact of an inpatient pharmacist-directed anticoagulation service. J Hosp Med. 2011;6(6):322–328. doi: 10.1002/jhm.910. [DOI] [PubMed] [Google Scholar]
- 51.Entezari-Maleki T, Dousti S, Hamishehkar H, Gholami K. A systematic review on comparing 2 common models for management of warfarin therapy; pharmacist-led service versus usual medical care. J Clin Pharmacol. 2016;56(1):24–38. doi: 10.1002/jcph.576. [DOI] [PubMed] [Google Scholar]
- 52.Vessal G, Mardani Z, Mollai M. Knowledge, attitudes, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Pharm World Sci. 2009;31(2):183–187. doi: 10.1007/s11096-008-9276-6. [DOI] [PubMed] [Google Scholar]
- 53.Snella KA, Sachdev GP. A primer for developing pharmacist-managed clinics in the outpatient setting. Pharmacotherapy. 2003;23(9):1153–1166. doi: 10.1592/phco.23.10.1153.32758. [DOI] [PubMed] [Google Scholar]
- 54.Yamada K, Nabeshima T. Pharmacist-managed clinics for patient education and counseling in Japan: current status and future perspectives. J Pharm Health Care Sci. 2015;1:2. doi: 10.1186/s40780-014-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]