Abstract
Background
Antibiotic resistant strains of Pseudomonas aeruginosa are the cause of Gram negative nosocomial infections especially among the immunosuppressed patients. The bacteria contains las I and las R genes that play very important roles in the pathogenesis and mechanisms of aggression. These genes can be influenced by the quorum sensing (QS) system and such mechanism is becoming clinically important worldwide. This study aimed to investigate the preventive effects of green coffee extract (GCE) on the expression of pathogenesis-related genes, las I and las R in P. aeruginosa.
Methods
A total of fifty four P. aeruginosa strains were isolated out of 100 clinical samples collected from the infectious wards in different hospitals (Tehran province) using conventional microscopic and biochemical methods. Susceptibility of the isolates to different antibiotics, GCE and chlorogenic acid were elucidated. Multiplex polymerase chain reaction (PCR) and real-time PCR were performed to detect and quantify the expression levels of las I and las R genes. The presence of chlorogenic acid in GCE was confirmed by HPLC.
Results
Antibiotic susceptibility tests revealed multidrug resistance among the clinical isolates of those 40 strains were resistant to ciprofloxacin (74.07%), 43 to ceftazidime (79.26%), 29 to amikacin (53.7%), 42 to ampicillin (77.77%), 17 to colistin (31.48%), 40 to gentamicin (74.77%), and 50 to piperacillin (92.59%). PCR outcomes exhibited that the frequency of las I and las R genes were 100% in resistant and sensitive strains isolated from clinical and standard strains of P. aeruginosa (ATCC 15449). Real-time PCR analyses revealed that GCE significantly prevented the expression of las I and las R genes in P. aeruginosa. GCE at concentration level as low as 2.5 mg/mL could prevent the expression of lasI and lasR genes in P. aeruginosa clinical isolates.
Conclusion
The presence and expression levels of las I and las R genes in P. aeruginosa isolates were investigated when the bacteria was exposed to GCE. Our results tend to suggest that genes involved in pathogenesis of:Pseudomonas aeruginosa are down regulated by quorum sensing effect of chlorogenic acid and therefore GCE could be useful as an adjuvant in combating multidrug resistance strains of Pseudomonas aeruginosa.
Keywords: Pseudomonas aeruginosa, Chlorogenic acid, Green coffee extract, Quorum sensing
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen which causes extremely severe infections in immunocompromised patients, especially in those who suffer from cancer and AIDS or individuals with high-degree burns or cystic fibrosis. Studies have shown that pathogenicity of P. aeruginosa depends on virulence factors such as elastase, alkaline protease, phospholipase C, exotoxin A, exoenzyme S, pyocyanine, pyoverdine, hydrogen cyanide, ramnolipid, and several other factors. The ability P. aeruginosa to form biofilm, is one of its major properties that contributes to its antibiotic resistance and pathogenicity. Most of virulence-associated genes in P. aeruginosa such as las I and las R are express and regulate by quorum sensing system [1, 2].
Quorum Sensing (QS) is a cell-to-cell communication pathway using small molecules (SMs) of single-cell organisms that act through certain communication molecules releasing into the medium and then their binding to the relevant receptors. Therefore, the cells are able to exchange information, directly or indirectly, which affects their genes translation and transcription. QS is important to control the virulence factors expressions, resistance to antimicrobial agents and biofilm formation. Biofilms with high antibiotic resistance are considered to be a dynamic community of bacteria and a major problem in treating infectious diseases. Both las I and las R play important roles in QS and biofilm formation; hence, prevention of their expression could be a great approach in controlling P. aeruginosa infection [3, 4].
P. aeruginosa contains a QS system consisting of two sets of genes, las I-las R and rhl I-rhl R. While las I and rhl I direct the synthesis of acyl-homoserine lactones (acyl-HSL), the las R and rhl R genes produce transcriptional regulators that stimulate the target virulence genes. This phenomenon has been detected and described in clinical isolates using molecular techniques [5–7].
As resistance to current antimicrobial drugs are emerging and new medicines are becoming scarce to combat the resistant bugs, QS could open a window to assist the human being to use quorum quenching compounds as adjuvants in antimicrobial therapy [8, 9].
Although plants are free of any organized immune system to fight off aggressive pathogens, unlike humans and mammals, but they have the ability to produce special secondary metabolites called “quorum quenching compounds” to combat external pathogens. Natural and biological compounds have inspired pharmaceutical industries to design new antibiotics for treatment of various infections. Anti-quorum sensing compounds can be obtained from various natural sources such as plants and mushrooms. Since co-existence of bacteria with plants and fungi goes back millions of years ago, it is likely that different mechanisms have been developed during the co-evolution to control QS [10–12].
Green coffee is a good source of chlorogenic acid, a phenolic compound from hydroxy-cinnamic acid family. Studies on green coffee extract (GCE) showed that chlorogenic acid has antioxidant, anti-inflammatory, anti-cancer, anti- type 2 diabetic, and anti-hypertension activities. Chlorogenic acid also has a potent cytotoxicity on microorganisms including bacteria, fungi, molds, viruses, and bacterial biofilms including P. aeruginosa biofilms [13, 14]. In addition to green coffee, other plants such as apples, artichoke, betel, burdock, carrots, eggplants, eucommia, grapes, honeysuckle, kiwi fruit, pears, plums, potatoes, tea, tobacco leaves, tomato and wormwood also contain variable amounts of chlorogenic acid [14–20]. As P. aeruginosa is a model of pathogenic bacterium with clinical importance and possess several virulence factors, this study intended to investigate the preventive effects of GCE on the expression of pathogenesis-related genes, las I and las R in P. aeruginosa.
Materials and methods
Bacterial isolation
A total of 100 patients with suspected pseudomonas infection who were referred or hospitalized at Tehran medical centers (e.g. Imam Khomeini Hospital), were selected randomly. Samples were collected and transferred to the Quality Control laboratories of the Quality Assurance Research Center of Tehran University of Medical Sciences. The samples were cultured on MacConkey agar(Merck, Germany) and cetrimide agar(Merck, Germany). The cultures were incubated at 37 °C for 24 h until colonies were appeared. To obtain pure culture, each colony was sub-cultured on the growth media using the conventional streaking method. Gram-staining was performed on single colonies and Gram-negative bacteria were subjected to biochemical assays including Oxidative/Fermentative (O/F), oxidase, urease, indole, methyl red, voges proskauer (VP), simmon citrate, acid production of sugars (mannitol, galactose, fructose and glucose), and growth at 42 °C tests [21].
Coffee extraction
Green coffee beans powder (Coffee arabica L.) were purchased from Bonyan Salamat Kasra Co., Iran. Green coffee powder (8.0 g) was dissolved in 40 mL boiling distilled water. The mixture was kept in a boiling water bath for 3–5 min. The brewed coffee was filtered using Whatman No. 1 filter paper and was collected in a sterile container [22].
Determination of chlorogenic acid of GCE by HPLC
Chromatography
A stainless steel column L1 (150 × 4.6 mm i.d.) particle size 5 μm, was used and the column temperature was maintained at 25 °C. The mobile phase was 0.5% formic acid in water and methanol (80:20) at a flow rate of 1.2 mL/min. The injection volume was 20 μl and the wavelength of detector was set at 278 nm.
Standards and samples
To get the standard curve of chlorogenic acid (Sigma), five different calibration solutions were prepared in acetonitrile. The optimum ranges of concentrations were calculated between 5 to 60 ppm. Linear calibration curves were acquired by drawing the peak areas of the different individual compounds [23].
Antibiotic susceptibility profile of isolated P. aeruginosa strains
The antibiotic susceptibility assay was performed using standard antibiotic disks (Padtan Teb, Tehran, Iran) to determine the antibiotic resistance profile of the isolated P. aeruginosa strains based on Kirby-Bauer method and CLSI (Clinical and Laboratory Standards Institute) guidelines. The experimental antibiotics used included ciprofloxacin, ceftazidime, amikacin, imipenem, colistin, piperacillin, and gentamicin [24].
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of GCE
The MIC and MBC levels of lyophilized powder of green coffee for P. aeruginosa isolates were determined by micro-broth dilution method using a 96-well microplate. GCE was prepared in doubling dilution manner at concentrations ranging from 0.31–80 mg/mL in test tubes. In the vertical section of the plate with 8 wells labeled after Latin letters A to H and aliquots of 100 μL of different concentrations of GCE were deposited by sterile samplers. Similar procedure was performed for 9 other columns of the plate. As a result, the MICs of 10 microbial strains were examined by using just single plate. Wells containing only Mueller Hinton broth (MHB) Medium (Merck, Germany) and those loaded with 100 μL of medium plus 100 μL of bacteria were assumed as controls. Culture of each P. aeruginosa isolate with concentration of approximately equal to standard 0.5 McFarland (1.5 × 108 CFU/mL) was freshly prepared in the physiologic serum. Aliquots of 0.1 mL of each microbial suspension were added into the 9.9 mL of MHB medium in test tubes and were stirred to produce a concentration of 1.5 × 106 CFU/mL. Contents (100 μL) of each tubes were added to the A to H wells (rows 1 to 10). Then 100 μL of the culture medium containing 100 μL of bacteria was added to the chlorogenic acid (already present in the wells) and the microplates were stored at 37 °C for 18–24 h. To determine the MBC, a loop of the wells containing GCE and the experimental bacteria was removed without opacity and cultured on a plate containing a Mueller-Hinton Agar (Merck, Germany), kept at 37 °C for 18–24 h. The concentration of the antimicrobial agent that killed the microorganisms or stopped their growth was determined and recorded as the amount of MBC of GCE [24]. Ciprofloxacin was used as positive control in side-by-side comparison with GCE.
Investigation of P. aeruginosa las I and las R genes with multiplex PCR
The presence of las I and las R genes in the resistant and sensitive isolate and also that of the standard strain of P. aeruginosa were checked out using multiplex PCR (Bio Rad, USA) using specific primers.
The bacterial strains were cultured in a Soybean Casein Digest Broth Medium, and then the DNA was extracted using a commercial kit (Sina Clone kit PR881613C, Iran) according to the manufacturer’s instruction. The DNA concentration was measured by spectrophotometer at 260 nm and 280 nm. Absorption ratio of <1.7 (260 nm to 280 nm) indicates sample contamination with protein and an absorption ratio of >2 indicates the presence of residual RNA. Genomic DNA was stored at −20 °C until use.
Multiplex PCR was designed in a final volume of 20 μL containing 10 μL PCR mastermix, 1 μL of each primer (10 pmol), 4 μL DNA (250 ng) and 2 μL distilled water. Sequences of primers and the lengths of the amplification products are listed in Table 1 (24). Multiplex PCR was performed at initial temperature of 95 °C for 5 min, 35 cycles at 95 °C for 30 s, annealing at 55 °C for 45 s, and extension at 72 °C for 1 min. At the end, the final extension step was carried out at 72 °C for 10 min [24, 25]. The PCR product was electrophoresed in 1.2% agaros (Fermentase USA) and after staining with ethidium bromide and visualized by UV light illumination (Bio Rad-USA).
Table 1.
Primers used for multiplex PCR
| Primer | Sequence (5′ to 3′) | Product size |
|---|---|---|
| Las R (F) | GCTTCACACGAGAGAACAC | 243 bp |
| Las R (R) | TCTGATCTTGCCTCTCAGG | |
| Las I (F) | AGGTGTTCTTCAGCATGTAGG | 121 bp |
| Las I (R) | GATGGTTATGACGCACTCAGT |
Determination of las I and las R gene expression in the presence of GCE using real-time PCR
Regarding the MIC value of green coffee (2.5 mg/mL), prior to PCR, 0.625 mg of GCE was added to 10 mL BHI broth, which was equivalent to the half-McFarland. After 12 h, RNA was extracted using the following method.
RNA extraction
RNX plus Micro kit (Cinagene RTPL12, Iran) was utilized to extract RNA from bacterial suspensions (Las I and Las R positive bacteria), in the logarithmic growth phase (OD600 = 0.4–0.6). A DNase kit (I) (Cinagene RTPL12,Iran) was exploited to remove genomic DNA.
Quantitative and qualitative RNA evaluation
The extracted RNA was evaluated quantitatively and qualitatively in order to ensure the accuracy of the experimental samples is as precise as possible. In the quantitative method, the RNA absorbance was detected at 260/280 nm. Briefly, 2 μL of the extracted RNA was placed in nanotubes and the absorbance wad recorded at wavelengths of 260 and 280 nm. The ratio between these two wavelengths, and the ratio of 260/230 nm, was also investigated.
Synthesis of cDNA
In a new test tube, 1 μg of RNA, 1 μL of the enzyme buffer, 1 μL of DNase and 5 μL of RNase inhibitor were added accordingly. The mixture was diluted to 10 μL using RNase-free water and placed at 37 °C for 30 min. Next, 1 μL of 0.5 M Ethylenediaminetetraacetic acid (EDTA) was added and the tube was kept at 65 °C for 10 min for DNase inactivation. After 10 min, the process continued by addition of Random Hexamer (0.5 μL) and Oligo dT (0.5 μL), heated at 65 °C for 5 min. Afterwards, the samples were kept on ice. 13 μL of the above mixture was added to each tube and was stored at 25 °C for 5 min, 42 °C for 60 min, and 70 °C for 10 min. The single-stranded cDNA was stored at −20 °C.
Real-time PCR
PCR was performed in a total volume of 20 μL as follows: 10 μL master mix cyber amplicon (2x), 6 μL diethyl pyrocarbonate (DEPC) water, 1 μL (10 pmol) of each primer (Table 1), and 2 μL (500 ng) cDNA. Duplication was induced in the ABI Step one system (USA) with the initial denaturation at 95 °C for 1 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 40 s, and the extension at 72 °C for 60 s. The housekeeping gyrase gene (16S) was used as the internal control [24, 25]. Specific software was used to calculate the gene expression level and to plot the corresponding graphs. The expression level was interpreted by comparing the relative measurement of mRNA expression in comparison with the standard strain, P. aeruginosa ATCC 15449.
Statistical analysis
Data were the mean of at least three replications and were analyzed by t-test method in Excel 2010.
Results
Bacteria isolation
Out of 100 clinical samples, 54 strains turned to be P. aeruginosa using microscopic and biochemical methods.
Antibiotic susceptibility profile of P. aeruginosa strains
Table 2 depicts the antibiotic susceptibility pattern of the isolated P. aeruginosa strains against ciprofloxacin (5 μg), ceftazidime (30 μg), amikacin (30 μg), imipenem (10 μg), colistin (10 μg), gentamycin (10 μg) and piperacillin (100 μg). The isolates showed high degrees of resistance to various antimicrobial agents. Most of the clinical isolates of P. aeruginosa (92.59%) were resistant to piperacillin, while only 32.48% was resistant to clindamycin.
Table 2.
The percentage of antibiotic resistance of the clinical isolates of Pseudomonas aeruginosa
| Ciprofloxacin (CIP) 5 μg/disc |
Ceftazidime (CAZ) 30 μg/disc |
Amikacin (AM) 30 μg/disc |
Imipenem (IPM) 10 μg/disc |
Colistin (CL) 10 μg/disc |
Gentamycin (GM) 10 μg/disc |
Piperacillin (PIP) 100 μg/disc |
|---|---|---|---|---|---|---|
| %74.07 | %79.62 | %53.7 | %77.77 | %31.48 | %74.07 | %62.59 |
HPLC
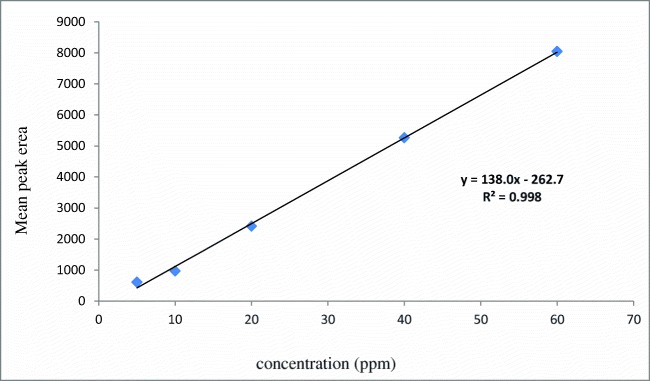
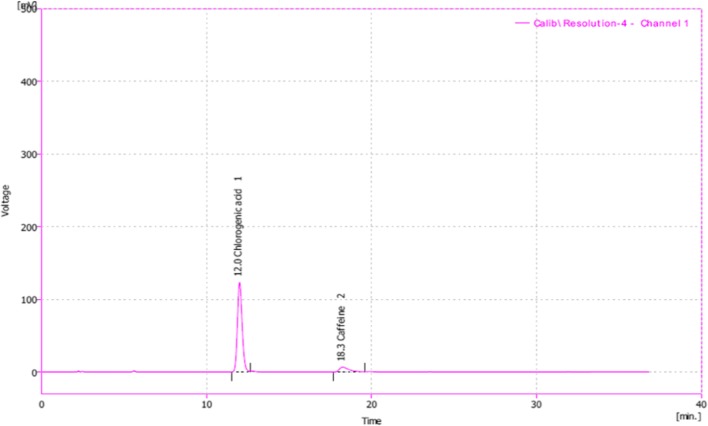
The calibration curve of different concentrations of chlorogenic acid are shown in Fig. 1. The calibration plot of the analyte was excellent and linear, over the concentrations ranging from 5 to 60 ppm, also the correlation coefficient value was calculated to be 0.998. The Limit of detection (S/N = 3) and the Limit of quantification (S/N = 9) were 2.5 and 7.6518 ppm, respectively. The retention times and areas under the curve were approximately similar in all injections. The standard samples of chlorogenic acid were detected at concentrations of 5 to 60 ppm. The chromatograms of chlorogenic acid in the standard solutions (60 ppm), and for Green coffee beans powder extracts (detected after 12 min of chlorogenic acid administration) are illustrated in Figs. 2 and 3.
Fig. 1.
Calibration curve of standard chlorogenic acid
Fig. 2.
Typical chromatogram of Chlorogenic acid and Caffeine standard
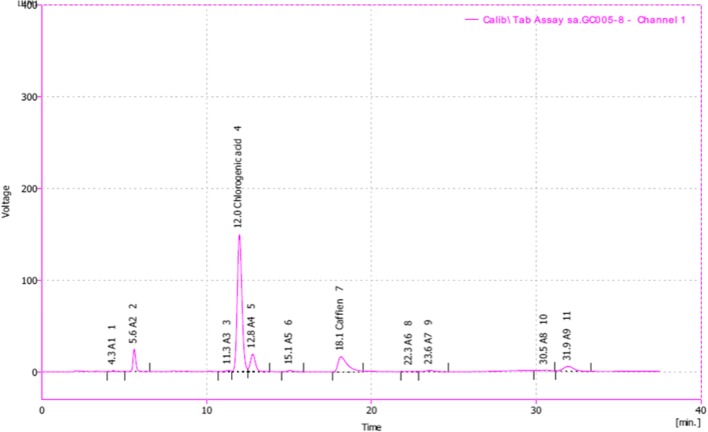
Fig. 3.
Typical chromatogram of Green coffee beans powder extracts. As HPLC analysis showed, GCE contained 63.24% chlorogenic acid and 13.95% Caffeine
MIC and MBC of GCE
The opacity of the sample wells were compared with the negative control which contained only the culture media. Both the MIC and MBC of the GCE against P. aeruginosa were found to be 2.5 mg/mL. Ciprofloxacin was used as positive control with the MIC value of 0.04 μg/mL against P. aeruginosa (ATCC 15449).
Investigation of P. aeruginosa las I and las R genes with multiplex PCR
The frequency of las I and las R genes in resistant, sensitive and standard strains was 100% (Table 3 and Fig. 4).
Table 3.
Frequency of las I and las R genes in Pseudomonas aeruginosa clinical isolates
| Number (percent|) | Tested strains | Gene |
|---|---|---|
| 0(0) | Resistant | Las R |
| 0 (0) | Las I | |
| 6 (100) | Las I and Las R | |
| 0 (0) | Sensitive | Las R |
| 0 (0) | Las I | |
| 5 (100) | Las I and Las R | |
| 0 (0) | Standard (P. aeruginosa ATCC 15449). | Las R |
| 0 (0) | Las I | |
| 1 (100) | Las I and Las R |
Fig. 4.
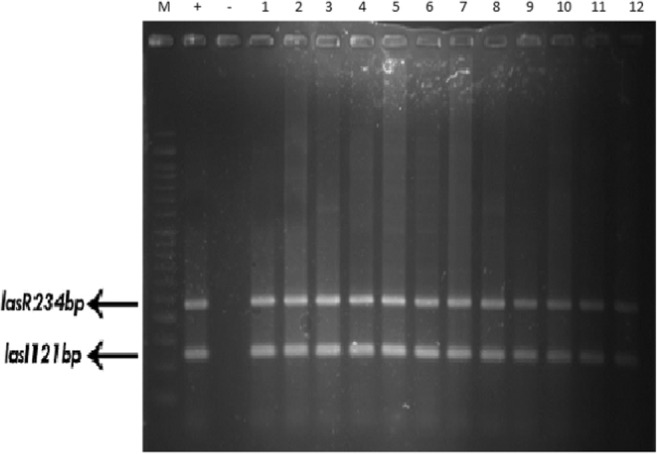
Detection of las I and las R chromosomal genes by multiplex PCR. wells 1–12, clinically isolated P. aeruginosa; well +, positive control (P. aeruginosa ATCC 15449) with las I and las R genes; well -, negative control (distilled water)
Determination of las I and las R gene expression in the presence of GCE using real-time PCR
The data which were obtained from real time PCR were analyzed by 2–ΔΔCt formula. The las I and las R gene expression in P. aeruginosa were decreased 0.49 and 0.75 fold after treatment by GCE in comparison with not-treated P. aeruginosa samples as control (P < 0.05). It is proved that the GCE is able to decrease the P. aeruginosa biofilm formation by decrease in las I and las R expression.
Discussion
Clinical isolates of P. aeruginosa are pathogenic microorganisms with substantial interest among hospitalized patients. Most of the virulence-associated genes of P. aeruginosa are expressed and regulated by a system called QS system. It was reported that the pathogenesis and aggression of P. aeruginosa is highly contributed to the presence of las I and las R genes. The main point is that these genes can be influenced by the QS system and such mechanism is becoming clinically important worldwide. Antibiotics are defined as chemical compounds to treat infections. Overuse or inaccurate prescription of antibiotics can lead to resistance in bacteria. Natural products such as coffee and tea are known to possess antimicrobial effects and can be potential antimicrobial agents in treatment of infectious diseases [26, 27].
In a study conducted on the anti-QS fluorescence of Florida plant extracts against P. aeruginosa, the plant extracts modified the expression level of different genes, including las R and las I in bacteria, which have been affected by the QS system. Sepahi et al. showed that the essential oil of Ferula and Dorema at a concentration of 25 μg/mL could inhibit QS system [28].
Real-time PCR assays were performed to detect the expression levels of las R and las I genes in P. aeruginosa. The presence of these genes in the present study was observed in the entire isolated strains. These results were consistent with the report examining the existence of las R and las I in the urinary tract infection isolates of P. aeruginosa [29].
However, this is the first report regarding the expression statues of the desired genes in the presence of plant extracts or herbal essential oils. We found that GCE at concentration of 2.5 mg/mL has the ability to exhibit antimicrobial effect and can eliminate the most resistant bacteria P. aeruginosa. The amount of chlorogenic acid of the coffee extract was also measured by HPLC. HPLC quantification revealed that the amount of chlorogenic acid in GCE was 63/24% w/w. Chlorogenic acid is a phenolic compound with significant benefits towards preventing certain diseases including cardiovascular disease, type 2 diabetes, alzheimer’s, also demonstrated to have antibacterial and anti-inflammatory activities. Chlorogenic acid regulates insulin function in humans’ body as can reduce calorie intake from fat deposits and prevents 45% of fat absorption, leading to release of G6P, an enzyme responsible for the blood sugar balance. GCE is one of the main natural sources of chlorogenic acid which contains about 12 to 5 g of this compound per gram [30–32].
In a study by Craig et al. the chlorogenic acid content of five different GCE was quantified with HPLC-UV method, showing that the GCE had 32.24% to 52.65% w/w chlorogenic acid [33].
At concentrations of 16–8 μg, Chlorogenic acid was found to have anti-bacterial effect, besides at concentrations 16–32 μg possessed antifungal properties on trimethoprim/sulfamethoxazole resistant Stenotrophomonas maltophilia. It has also been reported that chlorogenic acid has an excellent anti-biofilm activity at different concentrations in vitro condition [34].
According to several independent studies, chlorogenic acid in GTE affected the cell membrane and the enzymatic reactions, besides showed significant antimicrobial effect and eliminated heavy metals [35].
The level of resistance of the individual clinical isolates to current antibiotics were high. More than 70 % of the isolates were resistant to most of the antibiotics except the end stage antibiotic, colistin which showed a lower resistance of about 30%.
There has been trend to substitute the failed antibiotics with those conferring sensitive or less resistance when combating an infectious disease and less attention has been paid to the intrinsic mechanisms behind the bacterial resistance.
Chlorogenic acid is an antimicrobial agent with quorum sensing activity with the ability of down regulating the virulence genes of P. aeruginosa and therefore could be used as an adjuvant along with synthetic antimicrobial drugs to combat the resistant nosocomial infections.
Authors’ contributions
This study was PhD thesis of HJ who did all the practical experiments. HJ contributed in the conception, design of the study, data collation and manuscript preparation. NS contributed in the conception and the design of the study. JN contributed in the design of the study. MD supervised the gene analysis experiments. MRF contributed in the conception, the design of the study and gave revisions of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by Deputy of Research, Tehran University of Medical Sciences, (grant No. 31002).
Compliance with ethical standards
This study was approved by the Ethics Committee of the Tehran University of Medical Sciences, Tehran, Iran (ethics committee reference number: IR. TUMS. REC. 1394.2108).
Competing interests
The authors declare that they have no competing interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brooks G, Carroll KC, Butel J, Morse S. Jawetz Melnick & Adelbergs Medical Microbiology 26/E: McGraw Hill Professional; 2012.
- 2.Karatuna O, Yagci A. Analysis of quorum sensing-dependent virulence factor production and its relationship with antimicrobial susceptibility in Pseudomonas aeruginosa respiratory isolates. Clin Microbiol Infect. 2010;16(12):1770–1775. doi: 10.1111/j.1469-0691.2010.03177.x. [DOI] [PubMed] [Google Scholar]
- 3.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280(5361):295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 4.Annapoorani A, Umamageswaran V, Parameswari R, Pandian SK, Ravi AV. Computational discovery of putative quorum sensing inhibitors against LasR and RhlR receptor proteins of Pseudomonas aeruginosa. J Comput Aided Mol Des. 2012;26(9):1067–1077. doi: 10.1007/s10822-012-9599-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Conibear TC, Bandara R, Aliwarga Y, Stapleton F, Willcox MD. Type III secretion system–associated toxins, proteases, serotypes, and antibiotic resistance of Pseudomonas aeruginosa isolates associated with keratitis. Curr Eye Res. 2006;31(4):297–306. doi: 10.1080/02713680500536746. [DOI] [PubMed] [Google Scholar]
- 6.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 7.Wagner VE, Frelinger JG, Barth RK, Iglewski BH. Quorum sensing: dynamic response of Pseudomonas aeruginosa to external signals. Trends Microbiol. 2006;14(2):55–58. doi: 10.1016/j.tim.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Reading NC, Sperandio V. Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett. 2005;254(1):1–11. doi: 10.1111/j.1574-6968.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- 9.Raina S, Vizio DD, Odell M, Clements M, Vanhulle S, Keshavarz T. Microbial quorum sensing: a tool or a target for antimicrobial therapy? Biotechnol Appl Biochem. 2009;54(2):65–84. doi: 10.1042/BA20090072. [DOI] [PubMed] [Google Scholar]
- 10.Adonizio A, Kong K-F, Mathee K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008;52(1):198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fratianni F, Pepe R, Nazzaro F. Polyphenol composition, antioxidant, antimicrobial and quorum quenching activity of the “Carciofo di Montoro”(Cynara cardunculus var. scolymus) global artichoke of the Campania region, southern Italy. Food Nutr Sci. 2014;5(21):2053–2062. [Google Scholar]
- 12.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31(2):224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22(3):358. doi: 10.3390/molecules22030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozuma K, Tsuchiya S, Kohori J, Hase T, Tokimitsu I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens Res. 2005;28(9):711–718. doi: 10.1291/hypres.28.711. [DOI] [PubMed] [Google Scholar]
- 15.Ochiai R, Jokura H, Suzuki A, Tokimitsu I, Ohishi M, Komai N, et al. Green coffee bean extract improves human vasoreactivity. Hypertens Res. 2004;27(10):731–737. doi: 10.1291/hypres.27.731. [DOI] [PubMed] [Google Scholar]
- 16.Shimoda H, Seki E, Aitani M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement Altern Med. 2006;6(1):9. doi: 10.1186/1472-6882-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki A, Kagawa D, Ochiai R, Tokimitsu I, Saito I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens Res. 2002;25(1):99–107. doi: 10.1291/hypres.25.99. [DOI] [PubMed] [Google Scholar]
- 18.Blum J, Lemaire B, Lafay S. Effect of a green decaffeinated coffee extract on glycaemia. NutraFoods. 2007;6:13–17. [Google Scholar]
- 19.Farah A, Donangelo CM. Phenolic compounds in coffee. Braz J Plant Physiol. 2006;18(1):23–36. doi: 10.1590/S1677-04202006000100003. [DOI] [Google Scholar]
- 20.Suárez-Quiroz ML, Taillefer W, López Méndez EM, González-Ríos O, Villeneuve P, Figueroa-Espinoza M-C. Antibacterial activity and antifungal and anti-Mycotoxigenic activities against aspergillus flavus and A. ochraceus of green coffee Chlorogenic acids and dodecyl Chlorogenates. J Food Saf. 2013;33(3):360–368. doi: 10.1111/jfs.12060. [DOI] [Google Scholar]
- 21.Garrity GM. Bergey's manual of systematic bacteriology: volume one: the archaea and the deeply branching and phototrophic Bacteria: Springer Science & Business Media; 2012.
- 22.Almeida AAP, Farah A, Silva DA, Nunan EA, MBA G. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J Agric Food Chem. 2006;54(23):8738–8743. doi: 10.1021/jf0617317. [DOI] [PubMed] [Google Scholar]
- 23.Ayelign A, Sabally K. Determination of chlorogenic acids (CGA) in coffee beans using HPLC. Am J Res Commun. 2013;1(2):78–91. [Google Scholar]
- 24.Kadhim D, Ali MR. Prevalence study of quorum sensing groups among clinical isolates of Pseudomonas aeruginosa. Int J Curr Microbiol App Sci. 2014;3(11):204–215. [Google Scholar]
- 25.Thuptimdang P, Limpiyakorn T, McEvoy J, Prüß BM, Khan E. Effect of silver nanoparticles on Pseudomonas putida biofilms at different stages of maturity. J Hazard Mater. 2015;290:127–133. doi: 10.1016/j.jhazmat.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 26.Hoque MM, Ahmad M, Khisa S, Uddin MN, Jesmine R. Antibiotic resistance pattern in Pseudomonas aeruginosa isolated from different clinical specimens. JAFMC. 2016;11(1):45–49. [Google Scholar]
- 27.Singh Arora D, Jeet Kaur G, Kaur H. Antibacterial activity of tea and coffee: their extracts and preparations. Int J Food Prop. 2009;12(2):286–294. doi: 10.1080/10942910701675928. [DOI] [Google Scholar]
- 28.Sepahi E, Tarighi S, Ahmadi FS, Bagheri A. Inhibition of quorum sensing in Pseudomonas aeruginosa by two herbal essential oils from Apiaceae family. J Microbiol. 2015;53(2):176–180. doi: 10.1007/s12275-015-4203-8. [DOI] [PubMed] [Google Scholar]
- 29.Senturk S, Ulusoy S, Bosgelmez-Tinaz G, Yagci A. Quorum sensing and virulence of Pseudomonas aeruginosa during urinary tract infections. The Journal of Infection in Developing Countries. 2012;6(06):501–507. doi: 10.3855/jidc.2543. [DOI] [PubMed] [Google Scholar]
- 30.Farah A, Monteiro M, Donangelo CM, Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr. 2008;138(12):2309–2315. doi: 10.3945/jn.108.095554. [DOI] [PubMed] [Google Scholar]
- 31.Ranheim T, Halvorsen B. Coffee consumption and human health–beneficial or detrimental?–mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol Nutr Food Res. 2005;49(3):274–284. doi: 10.1002/mnfr.200400109. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 33.Craig AP, Fields C, Liang N, Kitts D, Erickson A. Performance review of a fast HPLC-UV method for the quantification of chlorogenic acids in green coffee bean extracts. Talanta. 2016;154:481–485. doi: 10.1016/j.talanta.2016.03.101. [DOI] [PubMed] [Google Scholar]
- 34.Karunanidhi A, Thomas R, Van Belkum A, Neela V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. Biomed Res Int. 2012;2013. [DOI] [PMC free article] [PubMed]
- 35.Kabir F, Katayama S, Tanji N, Nakamura S. Antimicrobial effects of chlorogenic acid and related compounds. J Korean Soc Appl Biol Chem. 2014;57(3):359–365. doi: 10.1007/s13765-014-4056-6. [DOI] [Google Scholar]