Abstract
Background
Nanosuspensions, liquid dispersions with nanometer size distribution, are becoming trendy in pharmaceutical practice to formulate poorly water-soluble drugs and to enhance their bioavailability. Generally, nanosuspensions are produced in two main approaches; top-down or bottom-up. The former is based on size-reduction of large particles via milling or high pressure homogenization. The latter is focused on the mechanisms of nucleation and particle growth.
Methods
In this review, the critical factors influencing the kinetics or dynamics of nucleation and growth are discussed. Subsequently, the mechanisms of nanosuspension instability as well as strategies for stabilization are elaborated. Furthermore, the effects of stabilizers on key parameters of instability as well as the process of choosing an appropriate stabilizer is discussed.
Results
Steric and electrostatic stabilizations or combination of them is essential for nanosuspensions formulation to prevent coagulation. Accordingly, some characteristics of stabilizers play critical role on stability and optimization of nanosuspensions; i.e., HLB and concentration. Nevertheless, after reviewing various articles, it is ascertained that each formulation requires individual selection of surfactants according to the parameters of the particle surface and the medium.
Conclusions
Based on the results, application of excipients such as stabilizers requires proper optimization of type and concentration. This implies that each formulation requires its own optimization process.
Graphical Abstract.
ᅟ
Keywords: Nanosuspensions, Bottom-up, Nucleation, Particle growth, Electrostatic stabilization, Steric hindrance
Introduction
Bioavailability is one of the limiting factors in drug development with chemicals having poor water solubility [1–5]. An array of solubilizing agents as well as various techniques have been employed to increase the solubility of such drugs, including surfactants, co-solvents, pH adjusted solutions, emulsions, liposomes, cyclodextrins and solid dispersions [6, 7]. However, the majority of these techniques require additional excipients, which themselves can be a safety challenge [2]. In addition, these techniques offer little or no help in formulations where the parent molecules are poorly soluble in both aqueous and non-aqueous media [8].
The tendency of solute dissolution in specific solvent is determined by similar or dissimilar molecular interactions between the solute and solvent. In this way, environmental condition such as pressure and temperature influence the cohesion and adhesion force and eventually the intermolecular interactions. Of particular note, any further addition of solute above the solvent capacity leads to precipitation. All together Noyes–Whitney equation (Eq. 1), is very practical to get the valuable information about the process of dissolution.
| 1 |
Nanosuspensions, liquid dispersions with nanometer size distribution, help enhance the bioavailability of poorly soluble chemicals. In this regard, particle size as well as size distribution significantly influence the dissolution rate; thereby are among key determinants of the bioavailability. In particular, fine particles with high specific surface areas exhibit faster dissolution rate, which is demonstrated by Noyes–Whitney equation (Eq. 1) [7, 9–11].
According to this equation (Eq. 1), dissolution rate of a particle (dc/dt) is inversely related to diffusion distance (h), and directly to the particle surface area (A) and saturation solubility [12]. Also, solubility is correlated to the rate of dissolution, albeit in some cases the case is more complex. Solubility is thermodynamically defined as the maximum amount of solute that can hold the pure solvent. So, solubility is affected by environmental conditions such as pH, temperature, ionic strength of solvent and solute. However, the rate of dissolution is a kinetic process. This implies that the extent of solubility of one solute can be poor or good independent of the rate of dissolution.
However, according to Ostwald–Freundlich equation (Eq. 2), in apposition to microparticles, the saturation solubility and equilibrium solubility of spherical particles with size of smaller than 1000 nm can influence the particle size.
| 2 |
That Cs is solubility, C∞ is solubility of the solid which consist of large particles, σ is interfacial tension substance and ρ is the solid density [13]. Also, according to Prandtl equation (Eq. 3) hydrodynamic boundary layer thickness will decrease and surface specific dissolution rate will increase by reduction of particle size [14].
| 3 |
Where L is length of the surface in the direction of flow, k is denotes a constant, V is relative velocity of the flowing liquid against a flat surface and hH is the hydrodynamic boundary layer thickness.
Also, the formation of nanosuspensions not only increases the surface area but also enhances the saturation solubility of the solute in medium, resulting in better bioavailability [12, 15–18]. Moreover, the increase of mucoadhesivity and attachment to surfaces/cell membranes are other characteristics of nanosuspensions aiding in efficacious drug delivery [7, 9, 10]. Furthermore, nanosuspensions require no co-solvents, and allow higher drug loading compared with other formulations [19]. Better antitumor activity is also reported with nanosuspensions due to higher extravasation and remaining of particles at the vicinity of tumor [20].
Formulation of pharmaceuticals as nanosuspension was introduced in 1990, and the first product of this form appeared in the market in 2000 [10]. Ever since, a variety of micro- and/or nano- particles with proper size distribution have been widely used for drug delivery of poorly soluble chemicals [21]. These included of Rapamune® (sirolimus), Emend® (aprepitant), Megace®ES (Megestrole), Triglide® and Tricor® (fenofibrate), [8, 22–24]. In the most cases, particles are stabilized using appropriate polymers and/or surfactants in nanosuspensions [8, 25].
Generally, nanosuspensions are produced via either top-down or bottom-up processes [1, 2]. While the former mainly includes size reduction via milling, the latter involves precipitation and supersaturation. The main factors determining an effective top-down formulation process are elaborated elsewhere [26]. Here, we review the mechanisms of nanosuspension formulation in bottom-up approach and, also, we elaborate the parameters influencing the quality and stability of these formulations.
Mechanism of nanoparticle formation
Top-down
Top-down approaches are based on the size-reduction and breaking down of large materials into particles with nanometer dimensions via milling, high pressure homogenization and pulsed laser fragmentation [27, 28]. Milling is performed using a rotating instrument in which particles are mixed with milling pearls with constant rotation, resulting in crystals or amorphous particles with reduced size [2, 26, 29, 30]. However, high speed rotation may generate lots of heat, causing degradation of thermal-sensitive agents [2]. Also, milling can cause surface activation of drug particles, influencing several physiochemical properties of them such as their flow ability [31]. High-pressure homogenization (HPH) is also applied for nanocrystal production. In particular, the piston-gap homogenizer and microfluidizer are two main types of homogenizers frequently used for particle size reduction [29]. In this regard, several parameters are critical in HPH including pressure, cycle number, stabilizer type, temperature of process and stabilizing concentration [32, 33]. Pulsed Laser Ablation (PLA) and Pulsed Laser Deposition (PLD) are advanced techniques based on the absorption of the energy by the material and transformation to thermal and/or chemical energy to break (inter) molecular bonds of the bulk material. This method usually leads to smaller particles with a wide size distribution which can be considered a disadvantages [28].
The Gibbs free energy will change during the size reduction in top-down process due to formation of new surfaces. This will result in thermodynamic instability of nanosuspension. Therefore, proper stabilizers are required to reduce the particle free energy [34]. The process of top-down nano formulation is discussed elsewhere [26] and is beyond the scope of this review.
Bottom-up
The bottom-up approach is based on precipitation of supersaturated solutions [35]. It is frequently employed for the production of nanosuspensions both in bulk solutions or in single droplets [36]. This method is used in a number of pharmaceutical processes such as solvent–anti-solvent technique, supercritical fluid processing, spray drying, and emulsion–solvent evaporation [8, 37]. Of particular note, nanoparticles are obtained after several steps including supersaturation, nucleation, diffusion of the solute molecules and nanoparticle growth [38].
The bottom-up approach offers various advantages such as production of monodisperse particles, i.e., narrow range of size distribution, application of low energy and low processing temperature for thermolabile drugs, and no need for advanced equipment and generally an economically procedure. Recently, some pharmaceutical products have been produced and marketed in this method, such as Griseofulvin (Gris-PEG®) and Nabilone (Cesamet®) [39]. In addition, an investigation has proved the nanosuspension of Danazol (gonadotropin inhibitor) has more improvement in comparison with micro-suspension of marketed Danazol [40, 41]. Interestingly, fenoibrate [42] and Nitrendipine nanosuspensions were manufactured with the aid of combination methods [43].
However, generation of different unstable polymorphs, hydrates and solvates such as needle shaped particles caused by rapid growth can be undesirable. Also, application of non-solvents or anti-solvents are not favorable, given that even a few amount of residual medium can lead to physicochemical instability. Therefore, non-solvents are removed either via evaporative precipitation into aqueous solution (EPAS) or supercritical fluid (SCF) which need high pressure pump, temperature and fine nozzle designing.
Combination method
It is, however, noteworthy that some studies suggest a combination of top-down and bottom-up approaches as an optimal method for the particle preparation with size and stability. In this way, NANOEDGE™, H 69, CT (combination technology), H 96 and H 42 technology have been introduced to combine bottom-up and top down technology to reach effective reduction of particle size, e.g., preparation of atorvastatin nanoparticles via combination of anti-solvent precipitation after probe sonication method [44]. When these two methods are implemented simultaneously, a number of distinct nuclei with a certain size are created and inhibit the formation of nuclei with larger size. In addition, the combination of these methods leads to reach a narrow polydispersity index (PDI) and the range of particle size lies within a certain range that decreases the particle growth [2, 24, 35, 44, 45].
Nucleation and particle growth
Particle formation in bottom-up approach is initiated by nucleation followed by particle growth, which is, indeed, considered as the most important step in nanosuspension formulation [46]. As described by Reiss [47] and laMer and Dinegar [48], particle formation begins with short nucleation bursts and consequent molecule-by-molecule diffusion leading to particle growth. However, nucleation may result in either crystals or amorphous nanoparticles.
The first step of nucleation is monomer aggregation in supersaturated systems. So, the likelihood of addition of one monomer to the nucleus is directly proportional to its concentration in the bulk solution as well as its ability to diffuse to the nucleus surface [46]. In this regard, when the primary nucleus is spherical, the rate of diffusion of monomer (Q) follows the Fick’s law (Eq. 4); where R is the primary nucleus radius, D is the diffusion coefficient of monomers, Cb is the concentration of monomer in the bulk solution, and S0 is the solubility [46, 49].
| 4 |
The free energy per monomer in the solution is higher than that in crystalline phase. Therefore, monomeric molecules tend to aggregate to maintain a low-energy state. Likewise, a small nucleus has higher surface area compared to large ones, resulting in higher interface interaction with solute molecules and higher level of energy. Hence, small nuclei combine together to reduce the system’s free energy, ultimately leading to the particle growth [46].
It is noteworthy that coagulation of nanoparticle are also determined by other factors, described in Navier-Stokes (NS) equations such as mass, momentum and energy conservation of Newtonian-fluids. NS equations are applicable for incompressible or compressible, low or high speed, inviscid or viscous flows. The compact form of Navier-Stokes equations for Newtonian fluid is (Eq. 5):
| 5 |
Where ρ is density, is change of velocity with time, (V. ∇)V is convective term, ∇P is pressure term (fluid flows in the direction of largest change in pressure), ρg is bode force term (external forces acting on the fluid, such as gravity and electromagnetic) and μ∇2V is viscosity controlled velocity diffusion. This equation (Eq. 5) represents how all Newtonian fluids move [50].
Kinetics of nucleation and particle growth
The rate of formation and growth of the nuclei is determined by Arrhenius model (Eq. 6). Here, K is the nucleation constant, Ea is the activation energy, and A0 is the pre-exponential factor. These parameters are, in turn, influenced by the number and orientation of particles, surface tension, medium viscosity, temperature, pressure and external driving forces [51, 52]. In particular, Ea diminishes at higher supersaturation, leading to higher nucleation probability and speed [53].
| 6 |
The effect of temperature
It is expected for particle growth to decrease at higher temperatures because of particle re-dissolution. However, the Brownian motions will also increase with temperature, leading to improvement of particle orientation and the force of intermolecular interactions, which are in favor of particle growth. Furthermore, temperature increases the particles hydrodynamic diameter (diameter of particle that is coated with hydrated counter ion), resulting in lower particle repulsion, ultimately leading to particle adhesion and nanoparticles growth. In fact, the effect of temperature on nucleation depends on the degree of supersaturation of solution [51, 52].
The effect of surface tension
The rate of nucleation is a critical determinant of particle size and size distribution. In this regard, Arrhenius equation (Eq. 7) describes the effects of different variables on nucleation rate [54]. Of particular note, nucleation rate constant () depends on the concentration of stabilizers. In fact, increase of stabilizer concentration in the solution decreases surface tension, thereby inhibits nucleation rate and increases crystallization time [53]. In addition, surfactants alter γ, the surface free energy, thereby modulate the nucleation speed [54].
| 7 |
The effect of supersaturation
The ratio of anti-solvent to solvent increases at high degree of supersaturation, resulting in a greater nucleation rate, which eventually enhances the number but decreases the size of particles. In fact, higher degree of supersaturation (S in Eq. 7) enhances nucleation rate, and eventually results in smaller particle size [45]. In this regard, small changes in S dramatically alters nucleation rate; Kwon and Hyeon showed that an increase of S by two folds (from 2 to 4) lead to a significant increase of nucleation rate up to 1070 times [54]. It is noteworthy that particle growth is directly proportional to both particles count and their size. Thus, a shrink in particle size appears to neutralize the effects of supersaturation on particle number. Nevertheless, the overall outcome of an increase in supersaturation favors particle growth [45].
The effect of molecular diffusion
The nanoparticles diameter is determined according to LSW (Lifshitz–Slyozov–Wagner) model [51, 55–57]; where n depends on the formation and growth of the particles.
| 8 |
When n is 2, particle growth is mainly controlled by ions diffusion in the matrix; i.e., diffusion of ions in solution. Meanwhile, when n is 3, the particle growth depends on volumetric diffusion in the matrix; i.e., diffusion of ions on the particle surface. Accordingly, n = 4 implies that dissolution kinetics at both particles surface and the matrix ought to be counted in particle growth, demonstrating dissolving-precipitation phenomena at the solid-liquid interface [51, 56]. It is noteworthy that n can never be 1 and the 1/n exponent would always be less than 1, because particle size does not follow first order kinetics model. In fact, a decrease in the surface/volume ratio due to particle growth tends to negatively influence the ripening rate (k) due to reducing the available surface for deposition.
In this regard, k is correlated to diffusion coefficient of solute molecules in the medium, bulk solublity of dispersed phase (C∞), the solid-liquid interfacial tension (γ) and the molar volume of dispersed phase (Vm) [55]. As demonstrated in Eq. 9, k is almost constant in specified isothermal systems.
| 9 |
The surface nucleation, a critical component of particle growth, varies in different systems and depends on the dimensions of length and time, τ and k1 respectively (Eq. 10) [58, 59].
| 10 |
The crystal growth depends, also, on the concentration of bulk solution as well as its related concentration to crystal surface. In particular, the rate of crystal growth (RG) is determined by Eq. 11; where kG is the coefficient of growth rate, Cb is the solute concentration in bulk solution, C∗ is the solute concentration in equilibrium after nanosuspension formation, and g is the total growth condition [60].
| 11 |
The crystal growth is composed of two sequential steps. The first step involves solute transfer to the interfacial solid-liquid surface; i.e., the diffusion of monomers to the surface. In the next step, the solute integrates with the solution and separates from the crystal lattice.
Thermodynamics of nucleation and particle growth
The effect of free energy
The bottom-up approach in nanosuspension formulation is based on the supersaturation of the solution followed by precipitation of the nanoparticles. Assessment of the thermodynamics of homogenous nucleation has revealed that the Gibbs free energy of nanoparticles is determined by the sum of surface free energy of nanoparticles and bulk free energy (Eq. 12). Accordingly, the bulk free energy (∆Gv) is calculated by Eq. 13. In particular, ∆Gv is directly proportional to temperature (T), Boltzmann constant (kB), degree of supersaturation (S), and inversely to the molar volume (v) [46, 54, 61].
| 12 |
| 13 |
The rate of particle formation, stabilization, and size, depend on the sum of free energy (Eqs. 14 and 15). In fact, the higher the total free energy, the lower the rate of particle formation. It should be noted that the surface free energy of particles in solution is more than in crystal form. Thus particles tend to form large agglomerates or precipitate in the form of crystals. Of particular note, the radius of critical nuclei (rcrit), nuclei with maximal free energy per monomer without further re-dissolution in medium, is directly proportional to their free energy (∆Gcrit) [46, 54, 61].
| 14 |
| 15 |
The effect of supersaturation
The precipitation of nanoparticles correlates with the degree of supersaturation. Indeed, the lower the degree of supersaturation, the slower the rate of nucleation. In this regard, “metastable zone” is a concentration range at which nucleation does not occur regardless of time. During the nanosuspension generation process, the concentration of solute molecules rise to form “clusters” which are prerequisites for nanoparticle formation [46].
According to the Eq. 7, the rate of clustering of molecules as well as its thermodynamics depends on the degree of supersaturation and the molar volume of the nucleus. In fact, solute molecule continues to cluster until ∆Gcrit becomes negative. In this regard, ∆Gcrit, Gibbs free energy to form the critical nucleus, is calculated by Eq. 16; where γsl is the surface tension between liquid and solid surface [46].
| 16 |
As demonstrated in Eq. 16, the degree of supersaturation is inversely related to ∆Gcrit. Thus, solutions become thermodynamically unstable and tend to crystallize when the degree of supersaturation increases [53].
The steady state concentration in which clusters are stable (Cn∗) is close to the equilibrium concentration, and is calculated using Eq. 17. Here, ∆Gcritrepresents the free energy of forming a critical cluster from free monomers, and Ctot is the total concentration of substance in the system, which is approximately equal to the free monomer concentration [46].
| 17 |
For the assessment of ∆Gcrit, the capillarity approximation has been used. In other words, the free energy of a cluster is established on the free energy of a macroscopic solid and the cluster–water interfacial tension [62]. The formation of critical clusters per unit of time represents the transport of monomers to the clusters, and is calculated by nucleation rate (J) in unit of bulk volume in steady state concentration (Eq. 18); where Z is Zeldovich factor, and ψ∗ = rcrit/(λ + rcrit) [46].
| 18 |
The effect of molecular diffusion
The nucleation rate is related to diffusion and λ (effective length) as well as the interfacial tension (γsl) and Cb/S0, which is dependent to degree of supersaturation. It is noteworthy that when a change in λ is compensated by simultaneous change in γsl, the nucleation rate maintains a constant value [46].
Some polymers with surface adsorption can enhance nucleation rate. In fact, these polymers ought to modulate one or more of the parameters discussed above. When, a specific polymer produces distinct effects on each parameter, the geometric sum of effects determines the final outcome [53].
Thermodynamics of surfactant adsorption and nucleation
Generally, nanosuspension is assuming a dispersion of hydrophobic nanoparticles in water as a hydrophilic medium. The enormous increase of surface area due to reduction of particle size results in increase of particles’ free energy while The interfacial tension remains constant, and is considered the main reason of thermodynamic instability of nanosuspensions [8]. Accordingly, to reduce the total surface energy of the system, nanoparticles tend to accumulate and reduce the total surface area with flocculation aggregation or crystal growth. Thus, crystallization as the main step in bottom-up approach can itself result in instability of nanosuspension, if is not appropriately controlled. In this regard, stabilizers are added to reduce the free energy of the system by decreasing the interfacial tension. This is fulfilled through electrostatic or steric stabilization or both [55].
The effect of surfactant lipophilicity and concentration
The adsorption of surfactants and polymers on the surface of nanoparticles play a pivotal role in the formation and stabilization of nanosuspensions. Furthermore, not only the surfactant adsorption to the solid surface but also its orientation influences the energy in the case of ∆G, ∆H and ∆S. In this regard, the hydrophilic: lipophilic balance (HLB) of surfactants is a critical parameter [63]. In fact, a change in the energy levels of the adsorbate species, the interfacial plate, and free surfactant in the bulk solution ultimately leads to the selective partitioning of the adsorbate into the interfacial region [63].
The mixture of surfactant/polymer and drug crystallites determines the melting point depression, and depends on their concentration, though their relationship is not necessarily linear [64]. The concentration of surfactant at the interface (Cinterface)is in balance with its’ bulk concentration (Cbulk), and is calculate by Eq. 19. In this regard, the negative value of Gibbs free energy indicates the favorable conditions for absorption of surfactant from the bulk medium to the interface.
| 19 |
The effect of dispersion medium
The absorption of surfactants in the solid-liquid interface depends on several factors in the solid plate, in the surfactant, and in the medium. The latter includes the nature of solvent, pH, temperature, ionic strength, lateral interaction between the adsorbed molecules, and presence of other dissolved materials, i.e., electrolytes or short chain additives such as alcohol an urea [63, 65].
An array of interactions is demonstrated at the interface such as electrostatic, covalent, hydrogen or non-polar bonds [64, 65], among which hydrogen bonds strongly influence the nucleation process [53]. Albeit, a specific type of interaction is dominant for each polymer. In this regard, polymers like PVP, with only one hydrogen bonding functional group per monomer, are not strongly adsorbed onto the crystal faces, and are less likely to induce habit modification [53]. For example, Lindfors L. et al., found that the growth rate significantly decreased in the presence of 0.01% (w/w) PVP, whereas nucleation rate was not affected. This is explained by similar values of D, λ, γsl, and Cb/S0 with and without PVP, small critical radius, and the fact that there is no significant polymer adsorption on these small particles [46].
Although hydrogen bonds play the main role in surface adsorption, the sum of all additive forces determines the level of free energy. For instance, Celecoxib is more likely to produce hydrogen bonds with the hydroxyl group of hydroxypropyl cellulose (HPC) than with the ether group of Pluronic F-127 (PLU), but the latter produce stronger effects on crystallization. This indicates that the final effect on crystallization is determined by the sum of interactions [64]. Thus, ∆Gads is considered when analyzing the surface energy (Eq. 20). In fact, the level of energy is a combination of electric attraction force between solid plate and the surfactant (∆Gelec), covalent bonding (∆Gchem), cohesive chain-chain interaction among adsorbed long chain surfactant species (∆Gc − c) and similar interactions between the hydrocarbon chains and hydrophobic sites on the solid plate (∆Gc − s), hydrogen bonding (∆GH), and the solvation or de-solvation energy () [65].
| 20 |
This implies that several processes at the interface including ion exchange (the exchange of surfactants with opposite charges), ion pairing (the absorption of surfactants in non-occupied positions), acid-base interaction (Lewis acid and base interaction with hydrogen bonds), π electron polarization (the force between side chains with positive charge), dispersion force (such as London and Van Der Waals forces), hydrophobic interactions (interaction between the non-polar side chains of surfactant and hydrophobic surface of nanoparticles) [63].
The effect of interfacial layer thickness
The thickness of interfacial layer (τ) is of notable importance. In this regard, the level of energy at the interface depends on the concentration of surfactant as well as the interfacial thickness (Гi) (Eq. 21) [65].
| 21 |
The interface is a very dynamic region. In fact, diffusion, convection, adsorption, and desorption of stabilizer on the new surface of nanoparticle can occur simultaneously [66–68]. The physicochemical property of new particle surfaces, medium and surfactant is critical to minimize the Gibbs free energy. Thus, stabilization of a specific nanosuspension demands selection of a stabilizer with appropriate properties [69]. Moreover, the mixture ought to be incubated for a specific time span to ensure proper coverage of the surface; the length of which depends on physicochemical properties of the surfactant such as molecular weight and hydration value as well as the viscosity of medium [70].
The effect of surface polarity
Surface polarity influences the surface interaction of not only ionic but also non-ionic surfactants. In fact, the absorption of nonionic surfactant on the articles depends on the chemical potential of bulk solution, and is defined by Flory-Huggins Eq. (Eq. 22); where, the s represents surface, and q is the number of solute segments in contact with the surface. The m is the number of segment-surface nearest neighbors divided by the lattice coordination number, and r is the molecular size ratio of solute to solvent. The net interaction between components 1 (water as solvent) and 2 (surfactant as solvate) is expressed as χ1, 2. In this regard, χ1, s − χ2, s indicates the change of surface contact from solvent to solute, and represents the differential strength of solute-solvent interaction in the bulk solution and in the surface phase [71].
| 22 |
This model is applied for the absorption of nonionic surfactant to the non-polar surface such as polystyrene. In this regard, approximately 20% of free energy of absorption of nonionic surfactants on latex surface is due to the replacement of surface-water contacts with surface-surfactant contacts, i.e., (χ1, s − χ2, s). The remaining 80% is related to the orientation of surfactant molecules at the surface, expressed as .
At the interface, the hydrophilic parts of surfactant molecule are in contact with water, whereas hydrophobic parts orient toward particle surface [71]. This indicates lower possibility of unfavorable contacts of hydrocarbon chain to the aqueous solution. Therefore, the water–surfactant interaction at the surface will be weaker than that in bulk solution (χs < χ).
The χ parameter is the sum of fractional effects of interactions between water-hydrophilic moiety of surfactant, water-hydrophobic moiety of surfactant, water-water, and hydrophilic-hydrophobic moieties. Hence, χ is related to the HLB of surfactant and the HLB will be HLB = 20ωhydrophilic in which ωhydrophilic is the weight fraction of hydrophilic part of surfactant [71].
Nanosuspension instability
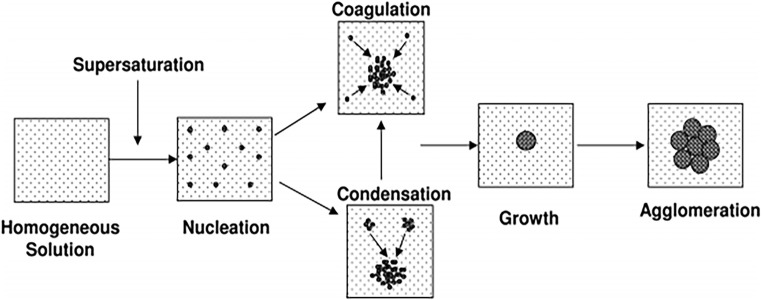
The process of particle formation in nanosuspensions generally follows 2 steps, nucleation and particle growth. In this regard, uncontrolled growth can cause instability of the suspension (Fig. 1) [60, 61]. Instability at some, but not all, levels is reversible with appropriate intervention. Thus, proper control of the processing time is critical to get nanoparticles with desirable size without instability.
Fig. 1.
Schematic of particle precipitation process. Reprinted with permission from [159]. Copyright (2009) American Chemical Society
Particle growth in nanosuspensions is explained by various mechanisms, including aggregation [55, 56], coalescence [56, 61, 72], crystal growth [55, 60, 73], Ostwald ripening [55, 74, 75], orientated attachment [56, 76], self-assembly, and mesoscopic transformation of nanoparticles via a restructuring process [77–79]. Depending on the system, one or several mechanisms may be involved in nucleation and growth of particle size [56].
Particle agglomeration is the outcome of attractive forces between the particles in the absence of considerable energy barrier; the latter is proven to be more important [80]. In this regard, high levels of hydrophobicity of particles decrease the wettability of their surface, resulting in aggregation [81]. Accordingly, hydrophobic particles tend to agglomerate to reduce the system free energy, as shown in the case of naproxen particles [82].
The lyophobic nanosuspension is produced by dispersion of hydrophobic materials in hydrophilic aqueous medium. In this system, increase of the surface area, because of nano-formulation, enhances the contacts between particles and outer medium, ultimately resulting in high surface energy. This can cause thermodynamic instability [8] and aggregation of nanoparticles [81].
Mechanisms of nanosuspension instability/stabilization
As discussed above, the mechanisms of nanoparticle growth and instability are similar. In fact, inappropriate growth can cause instability of the system. Hence, both formation and instability of nanoparticles can be determined by similar variables and principles.
Nanoparticle growth in lamer mechanism
The nucleation and particles growth is divided into 3 steps according to lamer mechanism. At first, increase in monomer concentration in supersaturated solution causes nucleation bursts in the medium. This results in a decrease of monomer concentration, and if continued, halts the process of nucleation. If monomer concentration is high enough to prevent nucleation arrest, the nuclei will grow due to monomer diffusion of supersaturated solutions [54, 83]. Thus, monomer concentration as well as factors influencing its diffusion plays pivotal roles not only in nucleation and growth but also in system stability. In this regard, storage at low temperatures, removal of residual solvents, applications of steric barrier around the particles, higher viscosity and lower diffusion coefficients of the medium ought to reduce monomer diffusion to the surface and inhibit particle growth (Eqs. 9, 10 and 11).
Ostwald ripening
Ostwald ripening was introduced in 1900 by Gibbs–Thomson law, and is based on the changes in the solubility of nanoparticles according to their size. It can cause significant instability in nanosuspensions [2], especially in long term storage [55]. In fact, higher solubility of nanoparticles causes re-dissolving and elimination of small particles. In the meantime, large particles tend to grow inappropriately because of low level of surface energy [84, 85].
Owing to application of surfactants in nanosuspension formulations and because of their adsorption at particles surface, Ostwald ripening is illustrated by Lifshitz–Slyozov–Wagner (LSW) theory (Eq. 23). Here, r is the radius of spherical nanocrystal, t is the time of radius changing, and KD is the diffusion coefficient. The ω is monomer flux coefficient which demonstrates the ease of material movements in the surfactant layer [74].
| 23 |
In LSW theory, the term is specific for each system. For instance, in the case of bare-nanocrystals it equals to 9/4. However, if surfactants are utilized, one ought to consider the thickness of surfactant layer on the nanoparticle surface (δ) (Eq. 24) [74].
| 24 |
The kinetics of Ostwald ripening is determined by two basic parameters; the ability of solute molecules to diffuse to the particle surface, and the rate of crystal growth/dissolution by attachment-detachment of the solute. In this regard, the ripening is controlled by the slower step. That is, Ostwald ripening becomes diffusion controlled, if crystal growth/dissolution is rapid; and it becomes interface controlled, when solute diffusion is faster than its incorporation/elimination to/from the particles [55].
The effects of surfactants
According to Eq. 24, surfactants influence diffusion coefficient as well as solubility and surface tension. It is also noteworthy that growth depends on t, though not linearly. Thereby, surfactants have an enormous effect on the process of ripening. In fact, they decrease growth by reducing Ostwald ripening [74]. In contrast, according to the LSW theory, the concentration of the dispersed phase directly influence the rate of Ostwald ripening [2]. Therefore, surfactants with higher solubilizing property, causing a rise in particle concentration in bulk solution, increase the rate of Oswald ripening [2].
The effects of size distribution
Size distribution is another determinant of particle growth. In this regard, Ostwald ripening requires poly disperse particles with some solubility in medium [55]. Because large particles tend to grow and small particles tend to fade, a narrow size distribution is essential to prevent particle growth due to Ostwald ripening and to maintain the stability of nanosuspension [86].
Digestive ripening
Digestive ripening is another mechanism describing the effects of particle size on the stability of nanosuspensions. In contrast to Ostwald ripening, digestive ripening argues that it is possible to reconstruct particles in order to increase the stability of system. In fact, the ultimate goal is to enhance dissolving of large particles and growth of small particles [87].
Finke-Watzky two step mechanism
Finke-Watzky mechanism illustrates nucleation and growth as consequent events. In this mechanism, the particle growth is autocatalytic with the formed nucleus, and is defined by Eqs. 25 and 26 [88]. The formation of nucleus is a chemical process, and is beyond the scope of this review. However, a fraction of substance A is primarily converted to substance B. Then A and B bind together, and two moles of B are ultimately formed. Consequently, particles grow in size during this process.
| 25 |
| 26 |
Coalescence and orientated attachment
Coalescence and orientated attachment describe particle growth in a similar manner. The former defines particle attachment with no orientation, yet the latter demands crystallographic alignment of particles for their attachment, including continuous crystallographic planes [76].
Equation 27 shows the relationship between the rate constant (K2) of orientated attachment reaction and particle radius (r); the NA is the Avogadro constant. In particular, the rate of particle growth is directly proportional to particle radius and diffusion coefficient (DA) [56].
| 27 |
Equation 28 represent relationship between rate constant (K2) of orientated attachment reaction and viscosity. On the other hand, the correlation between DA in Eq. (27) and viscosity is illustrated with Stokes-Einstein model (Eq. 31). Also, Eq. 28 argues that particle growth is directly related with temperature (T) and is inversely associated with viscosity (η). Furthermore, viscosity of the solvent as well as the solid volumetric fraction (ϕ) determine the total viscosity of system (Eq. 29), thereby influencing the kinetics of particle growth [56].
| 28 |
| 29 |
It is noteworthy that viscosity depends also on temperature, according to Arrhenius model described in Eq. 30 [56]. Thus, the effects of viscosity on growth rate ought to be adjusted to temperature. In fact, increase of viscosity at low temperatures (around the melting point of solvent) significantly inhibits particle growth. In contrast, viscosity tends to remain moderately constant at high temperatures, conferring a linear relationship between growth rate (K2) and temperature (Fig. 2) [56].
| 30 |
The Influence of solids concentration in dispersion medium
Fig. 2.

Temperature dependence of the reaction rate constant. Reprinted with permission from [56]. Copyright (2005) John Wiley and Sons
Solid content of the system is another determinant of viscosity in Einstein equation (Eq. 29). In fact, the solid volumetric fraction (ϕ), assuming particles as highly dispersed rigid spheres, increases viscosity and, thereby, inhibits particle growth in colloidal nanosuspensions [56]. It is, therefore, suggested that dilution of nanosuspension, by reducing the total viscosity, promotes particle growth and increase particle size. For instance, Daebis et al., showed that reducing the viscosity of Itraconazol nanosuspension through serial dilution resulted in particle growth from 682.3 nm ± 55 nm (PDI 0.38 ± 0.05) to 828 nm ± 86.3 μm (PDI 0.48 ± 0.104) [89].
The effect of stabilizers on system viscosity
According to Stokes-Einstein model (Eq. 31), the diffusion of a molecule is inversely proportional to its diameter (d). Also, temperature (T) influences the diffusion through alteration of systems viscosity [90]. For instance, HPMC enhances the viscosity much more than do Poloxamer 407 and PVA (Fig. 3a). Hence, the former more effectively inhibits the diffusion of the solute molecules and particle growth [90]. In contrast, Poloxamer 407, with the lowest viscosity, results in higher growth rate (Eq. 28). In this regard, temperature more significantly influences the viscosity of solutions containing HPMC (Fig. 3b) [90].
| 31 |
Intra particle re-shaping
Fig. 3.
Dynamic viscosity of crystallization medium containing different polymers (a), and at different temperatures (b) (n = 3). Reprinted with permission from [90]. Copyright (2012) Springer Nature
Intraparticle ripening explains the change of particle shape at the nanomaterial surface over time. It requires specific conditions in which the energy of monomers in bulk solution is lower or equal compared to that in crystal surface. This implies absence of monomer diffusion/influx to the particle surface. However, the irregularity of particle shape can cause subtle differences in surface energy on an individual particle, leading to dissolution of some regions (high energy surfaces) and growth of others (low energy surfaces) within a particle. Thus, an individual particle is constantly eroded at one side and formed at another side [91, 92].
The role of particle morphology on stability
Although needle shape crystal structures are more common in bottom-up approach, an array of particle morphologies is possible such as amorphous, crystal or metastable [46, 93, 94]. For instance, preparation of nanoparticles with anti-solvent method produce amorphous, polycrystalline, single crystals or metastable forms [90, 95–98]. Furthermore, localized amorphous particles are seen in some cases, which are the result of inhibition of crystal growth. This can lead to instability of the nanosuspensions, especially in long term storage [90].
Generally, application of inorganic materials such as calcium carbonate [99, 100], cadmium sulfide [101], and zinc oxide [77] results in crystal formation with broad range of crystal habits. In contrast, organic materials in the formulation give amorphous products or crystals with varied shapes [90, 95].
The morphology of particles influence their stability through several mechanisms such as Ostwald ripening [102]. In particular, the mobility of drug molecules in crystal structure is less than that in amorphous form [34]. Also, the relatively high energy of amorphous structures makes them unstable, thus they tend to transform to more stable crystal form [102]. The surface energy of crystals will rise, if the localized amorphous regions are generated on the surface [103]. This increases the chance of re-ordering or re-crystallization, and compromises physicochemical stability during formulation or long term storage [2]. Therefore, crystal particles confer better stability to the system [69]. However, this may interfere with the dissolution rate of nanosuspension [1, 23, 81, 104].
The primary nanoparticles formed during the process are either amorphous or metastable [95]. The other particles are resulted from the aggregation of primary particles, which explains their varied forms as amorphous, polycrystalline, or single crystals [90, 95–98]. However, recrystallization after aggregation may change the particles structure [96–98].
The effect of surfactants
Surfactants or polymers in the formulation can influence the crystal state as well as crystallization rate. For examples, the type and concentration of surfactant has an important impact on crystallinity and size of amylase [105] and celecoxib particles [106]. Accordingly, PVP, but not PEG, distinctly improves the in vitro drug release behavior, and causes significant melting point depression and polymorphic change in celecoxib nanocrystals [64]. In addition, PVP and/or HPMC changed the morphology, size and crystallization rate of bicalutamide and griseofulvin nanoparticles [46, 107]. Of particular note, PVP (0.01% w/w) inhibits crystal formation and alters the crystal structure to amorphous particles (Fig. 4) [46]. Surfactants, also, alter the crystal structure of buparvaquone [70] and isradipine [108]. In contrast, neither nanosizing nor surfactants influence the crystallinity or morphology of nifedipine particles [109].
The effect of dispersion medium
Fig. 4.
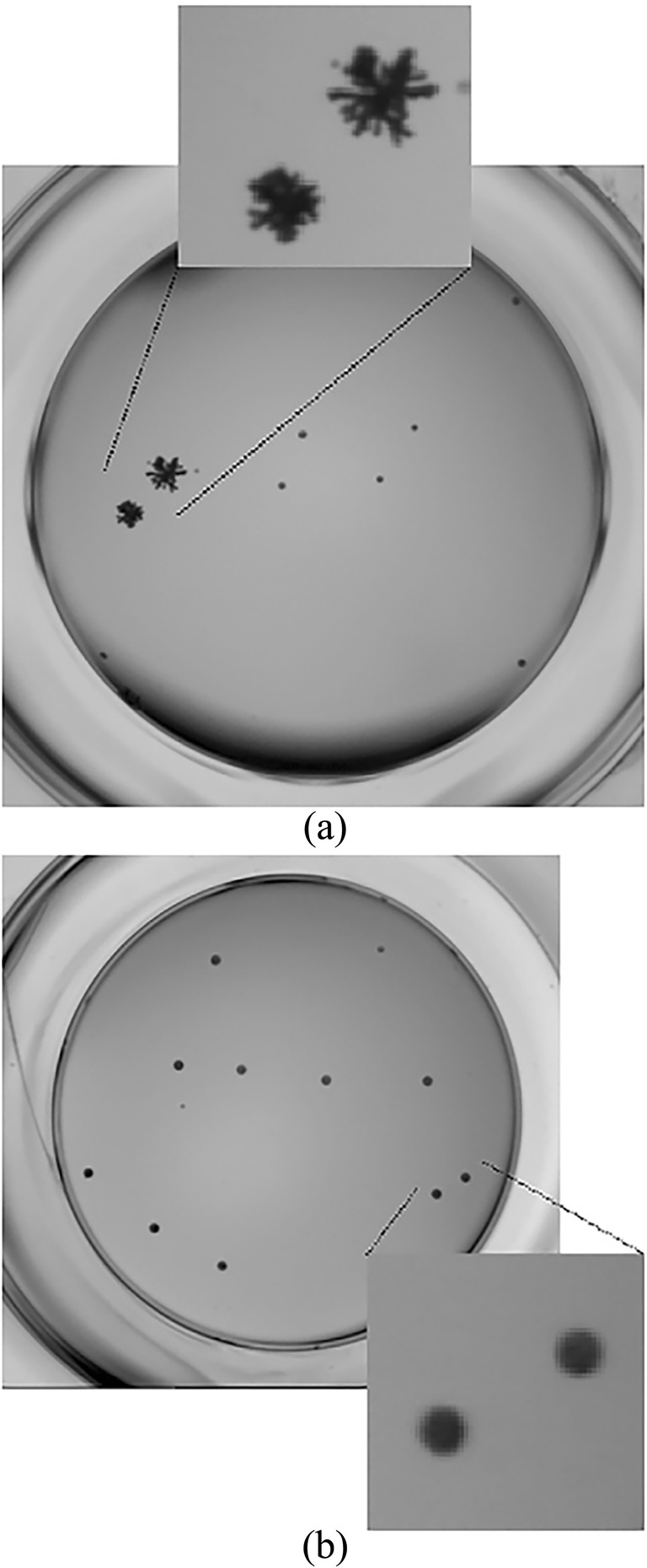
Images from nucleation experiments carried out at an initial concentration drug concentration of 440 μM, corresponding to a supersaturation of 30: a polymer free system; b at the presence of PVP (0.01% w/w). The diameter of the wells is 5 mm. Reprinted with permission from [46]. Copyright (2008) Elsevier
An array of solvents or dispersion media are employed for the preparation of nanosuspensions, including methanol, acetone, ethanol, acetonitrile, liquid paraffin, isopropylmyristate, propylene glycol dicaprylate, caprylic/capric triglyceride, oleic acid, oleyl alcohol, triacetin, and propylene carbonate [108, 110, 111]. In this regard, the choice of an appropriate organic solvent or dispersion medium is critical to control the size and stability of drug nanocrystals during precipitation [110]. In fact, the crystal form as well as its behavior is determined by some parameters such as the composition of nanoparticles, the crystallization temperature, and presence of solvents/co-solvents [107].
Particle growth as well as morphology and behavior are influenced by residual solvents or impurities in the system. Of particular note, solvent polarity decreases the nanoparticle size and growth [107] In fact, impurities can interact physically or chemically with the surface [53] or can be incorporated in crystal lattice [46]. In addition, the hydrate, solvate or anhydrous forms of materials may be produced after nanosuspension formation [90]. It is noteworthy that the residual solvent or impurities in the particle can cause safety issues [2, 8].
The effect of external energies
The external energies such as sonication and homogenization are also applied to reduce particle size in bottom-up process. In the meantime, they can alter nanoparticle morphology or behavior [108]. In particular, homogenization can reduce the crystal size or even transform them into cubic or round structures. For instance, homogenization induces formation of amorphous layers around the crystals in buparvaquone nanosuspension [70]. In addition, simultaneous application of surfactants and homogenization may produce distinct effects on particle coating and morphology [70].
The role of surface tension and intermolecular forces on stability
Interfacial tension is a key factor in nucleation and growth [46]. As described previously in Eq. 7, surface tension (γ) is inversely related to nucleation rate (J). Thus, reduction of surface tension increases nucleation rate, eventually decreasing the particle size and growth (crystallization) [46, 112–114].
Surface tension in a system is defined as the interactions between different surfaces (Young–Laplace model, Eq. 32) [46, 71, 115]. Here, γsl, γsv, and γlv represent the surface tension between solid-liquid, solid-vapor, and liquid-vapor interfaces, respectively. In addition, the interaction between particles and medium as well as crystal growth rate decrease with lower contact angel of solid-liquid interface (θ) [46].
| 32 |
According to Bragg–Williams theory (Eq. 33), the interfacial tension is directly related to the sum of intermolecular attractions (χ), including hydrogen, hydrophobic, dipole and Van Der Waals interactions [46, 71, 115]. In addition, the fraction of nearest neighbors in a plane below or above the interface (m) as well as the cross-sectional area per molecule (a) are critical [46, 71, 115]. This implies that the wider the nanoparticle surface, the higher the possibility of Van Der Waals interactions, thus, the higher the surface tension.
| 33 |
Types of interparticulate forces
The forces between particles influence nanosuspension stability. In fact, interparticle forces alter the rate of adhesion/repulsion and consequently aggregation/disaggregation of nanoparticles in liquid medium. This include repulsive forces such as Van Der Waals, electrostatic, and hard sphere as well as attractive forces such as steric [116]. The DLVO theory (named after Derjaguin, Landau, Verwey and Overbeek) represents the stability of colloidal nanoparticles according to interparticle forces [117]. In this regard, interparticle force is the sum of adhesion and cohesion forces (Eq. 34) [63].
| 34 |
Van Der Waals force
The Van Der Waals force is calculated by Eq. 35; where A is Hamaker constant, a is the radius of similar spherical particles, and H represents the minimum distance between two particles [118]. The Hamaker constant (A) in dispersed medium is replaced by Aeff, which depends on the nature of dispersed particles and the medium (Eq. 36). The similar polarity of particles and medium results in lower Aeff. According to Eq. 35 smaller values of Aeff would lead to less attraction between the particles.
This decrease Van Der Waals repulsions, resulting in particle aggregation and instability. In this condition the potential energy will decrease [63].
| 35 |
| 36 |
Electrostatic force
Electrostatic force with repulsive nature between two semi-charged particles is calculated by Eq. 37; where εr represents the dielectric constant of the dispersion medium, and the a is hydrodynamic radial. In contrast to Van Der Waals force, the electrical force is always positive. Electrostatic force is greater when the distance between particles (h) is minimum. Furthermore, it highly depends on zeta potential (ψ0), electrolyte content of the medium, and the ratio of particle size to electrical double layer, a/(1/κ) = κa, [63, 119].
The effective electrical double layer is called Debye length (1/κ), and represents the thickness of stern layer (Eq. 38). Here, F is the Faraday constant, Ci is the molar concentration of any ion in the solution phase, and Zi is the valence of the ions [63].
Of particular note, addition of electrolytes, via increase of κ and decrease of VR, can cause coagulation. In contrast, increasing the zeta potential ψ0 as well as surfactants enhance the system VR and decrease particle coagulation.
| 37 |
| 38 |
Zeta potential is the net charge around the particles after neutralization with opposite ions in the medium [120]. Indeed, the first absorbed monolayer, the inner Helmholtz layer, is consist of ions with opposite charge to that of nanoparticle surface, and are fixed and dehydrated ions. The second monolayer, the outer Helmholtz layer, is composed of fixed but hydrated ions with similar charge to dispersion medium. Helmholtz layers together are called the Stern layer.
Adjustment of surface tension and intermolecular forces
Intramolecular forces are controlled via two main mechanisms; electrostatic stabilization or steric hindrance. The former is based on the electrostatic interaction between the particle surface and ionic surfactants or charged polymers [121]. The later, however, describes adsorption of stabilizers onto the surface of drug nanoparticle via non-covalent forces [80].
Electrostatic stabilization
As a general rule, nanosuspensions are stabilized by electrostatic repulsion with zeta potentials of at least 30 mv [9, 70, 86, 122–124]. Furthermore, increase of zeta potential to 60 mv confers more stability to the formulation, whereas vast aggregation occurs in zeta potentials lower than 5 mv [125, 126]. However, to stabilize nanosuspensions containing both electrostatic and steric stabilizers, an absolute zeta potential of ±20 mv appear to be sufficient [9, 70, 86, 122, 123].
The effect of ionic strength of dispersion medium
The ionic strength of the medium is a key factor that influences the inter-particles electrostatic repulsive forces and inhibits particle aggregation [127]. Electrostatic stabilization is a simple and low cost method for nanosuspension stabilization [128]. According to Eqs. 37 and 38, increase of the ionic strength reduces the thickness of double layer, leading to a fall in the repulsion force between particles [127]. The change in ionic strength could be due to ingredients in the formulation or nanosuspension contact with body fluids. In this regard, body fluids exhibit a broad range of pH and electrolyte content [125]. Also, processing conditions such as heat sterilization [129] or changing the medium pH [130] can alter the electrostatic strength. Thus, alternative methods of sterilization such as filtration or buffering systems are recommended to prevent instability of the formulation [131].
The effect of surfactants
Surfactants/polymers decrease electrostatic force and change the dielectric constant of medium. Therefore, they diminish repulsion between nanoparticles [132]. Zeta potential is influence by the type as well as the concentration of employed stabilizers or electrolytes in the medium [70]. In this regard, steric stabilizers, such as HPMC and MC, alter the zeta potential through surface coverage [55]. As such, negatively or positively charged surfactant/polymers influence the total zeta potential of nanoparticles. For instance, negatively charged ionic surfactants, such as SLS, improves the Helmholts internal layer as well as the negative zeta potential in a concentration dependent manner [55]. Accordingly, increase of the concentration of low molecular weight chitosan enhances the positive charge at the surface of atorvastatin nanocrystal [44]. Although, high zeta potential inhibits the nanosuspension aggregation, it may impair interactions between nanoparticles and surfactants with similar charge, resulting in inefficient coverage. In the same manner, electrolytes in the medium can diminish the charged layer around the particles, resulting in a decrease in zeta potential.
Electrical charges of the particles and surfactant are key players in surfactant adsorption to the surface. However, pH, temperature, light, chemical nature and composition of the particle surface and medium influence electrical charge [126, 133–136]. For instance, change in the medium pH alters zeta potential, and causes repulsion between ibuprofen nanoparticles and HPMC [2].
Steric hindrance
A substantial body of evidence indicates that polymers efficiently prevent nanoparticles from aggregation and agglomeration during preparation and/or incubation [1]. In this regard, the affinity of nanoparticle for the polymer is a critical determinant of the efficacy of stabilization [111].
Formation of steric barrier around the particles is one of the main mechanisms of stabilizer-induced inhibition of aggregation and agglomeration [1]. In fact, steric stabilizers decrease particle contacts with the medium as well as attraction forces between particles (Eq. 35). Accordingly, polymer-coated drug nanoparticles exhibit lower Brownian motions, which are another mechanism for inhibition of particle attraction and aggregation [70, 137]. In addition, polymer adsorption prevents attachment/detachment of molecules of particle surface, thereby inhibiting Ostwald ripening [53, 138–140]. Also, polymers and nonionic surfactants tend to increase the solution viscosity, which in turn provides a barrier against aggregation (Eq. 28).
Several variables of the particle, the medium and the stabilizer influence the efficacy of steric barrier. In this regard, polymers appear to provide a superior steric barrier compared with surfactant shaving small molecular structure such as sodium lauryl sulfate [69, 141]. This is explained by more effective inhibition of steric repulsion due to fast/tight adsorption and effective surface coverage [89, 141, 142]. Of particular note, the thickness of polymer layer ought to be about 10 nm in order to provide efficient steric stabilization [70, 143].
Adsorption of polymers onto hydrophobic surfaces is due to the hydrophobicity of polymer chain. On the other hand, the hydrophilic moiety of polymer interacts with medium. So, the chain morphology plays a pivotal role in polymer adsorption and steric stabilization [69, 86]. In particular, long swinging hydrophilic chains appear to provide better steric stability and protect more efficiently against aggregation [144]. Furthermore, a range of particles (340–440 nm) were obtained in formulations which differed only on the molecular weight of the stabilizer, chitosan. Indeed, low molecular weight chitosan yielded smaller nanocrystals compared to moderate or high molecular weight counterparts [44]. However, polymers with higher molecular weight tend to reduce the rate of surface adsorption, which is also an essential determinant of stability [34].
Application of block copolymers is shown to provide better control/reduction of the particle shape and size [145]. In this regard, poloxamer is a block copolymer of polyethylene oxide (PEO), as hydrophilic segment, and polypropylene oxide (PPO), as hydrophobic segment. The latter moiety helps surface adsorption, while the former provides steric hindrance surrounding the particles, thus protecting against aggregation [89].
The efficacy of polymers in particle adhesion depends on temperature. For example, chitosan fails to produce steric stabilization to the atorvastatin nanocrystals at high temperatures (40 °C), resulting in agglomeration [45]. As such, the protective effects of HPMC against aggregation disappear, when temperature rises to 45 °C due to dehydration of polymer chains or Ostwald ripening [55].
Steric stabilization has several advantages over electrostatic stabilization. 1: the particles stabilized by steric hindrance are re-dispersible [146, 147]; 2: dispersion medium elimination is possible even in high concentration of nano-crystals [7, 148]; 3: addition of electrolytes during ‘salting out’ process does not influence the stability of formulation [149, 150]; and 4: it can be adopted for systems with multiple phases [151].
Choosing the appropriate stabilizer
Stabilizers are extensively used in nanosuspension formulations to adjust thermodynamic and/or mechanical parameters in order to prevent or delay agglomeration [86, 152]. Surfactants as well as polymers or a mixture of both are employed as stabilizers. In particular, an appropriate stabilizer with proper quantity confer particle stability via steric or electrical barriers, leading to balance of the surface energy [68, 69, 153, 154].
Surfactants and polymers tend to decrease Van Der Waals forces, resulting in a decrease of Boltzmann constant (KB), interfacial tension, and crystallization rate [155]. However, the effect of surfactants/polymers is not limited to the surface tension. Rather, they can increase the viscosity of system [53, 112], which interferes with mass diffusion (D) from solution to solid–liquid surface, and decreases the growth rate (Eq. 31) [56, 156–159]. In this regard, particle growth due to higher HPMCs ratio in formulation is ascribed to the increase of viscosity of solution [160].
An array of steric stabilizers are employed in the formulation of nanosuspensions (Table 1), including hydroxypropyl methyl cellulose (HPMC) [34, 109, 161], hydroxypropyl cellulose (HPC), polyvinyl pyrrolidone (PVP K-30), Poloxamer (188 and 407), Vitamin E TPGS [34, 162], serum proteins (e.g. albumin) [45] and polyvinyl alcohol (PVA) [6, 7]. Accordingly, several electrostatic stabilizers are also used to provide stability to the formulation, including polysorbates (mainly Tween®80), sodium lauryl sulfate (SLS) [34, 124], amphoteric surfactant (e.g. lecithin) [70], lipoid S75 [120], and eudragit® [163]. Serum proteins, such as albumin, were also extensively employed as emulsifier to help stabilize oil-in-water emulsions [45, 164]. Hypothetically, serum proteins can also provide stability to nanocrystals because of their ability to interact with hydrophobic surfaces. Therefore, they confer steric barriers to nanocrystals aggregation and growth, especially during consecutive centrifugation and drying [165, 166].
Table 1.
Stabilizers that used in various nanosuspension formulations
| Article title | Drug name | Stabilizer name | The best stabilizer | Additives |
|---|---|---|---|---|
|
Clarithromycin Dissolution Enhancement by Preparation of Aqueous Nanosuspensions Using Sonoprecipitation Technique [1] |
Clarithromycin | HPMC E5, HPMC E15, HPMC E50, Tween®80, Poloxamer 188, NaCMC, PVA | HPMC E5, HPMC E15, HPMC E50 | Orthophosphoric acid |
| A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions [2] | Ibuprofen USP | SLS, kollidon 30 (PVP K-30), Poloxamer 188, Poloxamer 407, Tween®80, HPMCs = K3, E3, E5, E15, A15 premium LV grades | HPMCs = K3, E3, E5, E15, A15 premium LV grades | |
| Chitosan based atorvastatin nanocrystals: effect of cationic charge on particle size, formulation stability, and in-vivo efficacy [44] | Atorvastatin (ATR) | Chitosan of different molecular weights (CSL-40, CSM-480, CSH-850 kDa); viscosities (20, 200, and 800 cps), Poloxamer 407, Labrasol® | Labrasol®-low molecular chitosan | |
| Effects of surfactants on size and structure of amylose nanoparticles prepared by precipitation [105] | Amylose with content of 99.5% | Tween80, Span80 | Tween 80-Span 80 | DMSO (solvent) |
| Formulation and Characterization of Itraconazole Oral Nanosuspension: Methyl Cellulose as Promising Stabilizer [89] | Itraconazole(ITZ) | SLS, PVP K25, HPMC E15, Poloxamer188 (P.188), Poloxamer 407 (P.407), methylcellulose (MC) |
1-Poloxamer 407 (P.407) 2-Poloxamer188 (P.188) 3-SLS |
|
| Investigation of Effect of Different Stabilizers on Formulation of ZaltoprofenNanosuspension [86] | Zaltoprofen | Pluronic F68 (Poloaxamer 188), Pluronic F127 (Poloxamer 407), Sodium lauryl sulphate (SLS) | Pluronic F68-SLS | |
| Nanosuspension Based In Situ Gelling Nasal Spray of Carvedilol: Development, In Vitro and In Vivo Characterization [110] | Carvedilol (CRV) | Poloxamer 407 (Lutrol F127), Poloxamer 188 (Lutrol F68), SLS, Tween®80, Solutol, PVP K-30, Stearic acid, Oleic acid, Span 40 | Tween80 |
Methanol Ethanol Acetone |
| Method for screening of solid dispersion formulations of low-solubility compounds—Miniaturization and automation of solvent casting and dissolution testing [163] | JNJ-25894934 | hydroxypropyl cellulose (HPC-SL), hydroxypropyl methyl cellulose acetate phthalate (HPMCP 50), Kollidon VA-64 (BASF, USA), Plasdone K29–32 (ISP, USA), acrylate-based (Eudragit® L100 and Eudragit® RS100), Vitamin E TPGS, SLS, Pluronic F127 NF (Poloxamer 407,BASF, USA), Tween®80, Crodesta F-160, Crodesta F-110, Cremophor EL, Volpo 10 | Plasdone K29–32 (ISP, USA)&Cremophor EL | solvents used (acetone, ethanol,n-propanol) |
|
Evaluation of Tadalafil Nanosuspensions and Their PEG Solid Dispersion Matrices for Enhancing Its Dissolution Properties [177] |
Tadalafil | PVP K30, Methocel E5 LV, Pluronic F-68, SLS, Na docusate, Triton X-100, Cremophor EL, Brij®58, Tween®80, Span20, Span60, Span80 | Tween®80-Span80 1:1 |
Acetone (solvent) Water (anti-solvent) PEG 4000 (Solid Dispersion Matrices) |
Stabilizers are not equal efficacious for all formulations. For example, PVP is effectively used in nanosuspension formulations of phenytoin [167], probucol [168], hydrocortisone acetate [53], celecoxib [64], ibuprofen [141] and griseofulvin [107]. Conversely, it fails to inhibit aggregation in itraconazole nanosuspensions because of low affinity to the particle surface. In this regard, more viscose polymers such as HPMC and MC have successfully been employed for the stabilization of itraconazole nanocrystals [169].
Surfactants can supply better wetting of the nanoparticles than do macromolecular hydrocolloids (PVP, HPMC and MC). This indicates better dispersion with the former [86, 89]. In addition, high molecular weight polymers (Poloxamer 407) provide higher degree of steric hindrance, more size reduction, and better dispersion compared to their counterparts with short side chains (Poloxamer 188) [89]. However, Lee et al., reported no correlation between the molecular weight and steric repulsion [69].
Some nonionic surfactants like Tween®80, Cremophor EL, Pluronic F68, and TPGS are reported to inhibit the activity of P-glycoprotein efflux pumps, which are responsible for backward pumping of the drug molecules and decrease of their intracellular concentration. Also, surfactants enhance drug penetration and increase drug absorption via paracellular route. These mechanisms are additional to the effects of surfactants on particle behavior, and can explain better disposition of the drug molecules in target cells [170]. However, it should be noted that perturbation of the integrity of cell membrane can cause cytotoxicity.
Effect of surfactant HLB
Hydrophilic Lipophilic Balance (HLB) is a key feature in surfactants and influences the outcome of their application in formulations [2]. Actually, it demonstrates the magnitude of water to lipid attraction of a specific surfactant; i.e., those with lower HLB are more lipophilic and vice versa. Thus, HLB values of surfactants can assist in selecting the proper stabilizer. Albeit, there are some studies which highlight that HLB is not an appropriate criterion for stabilizer selection. The HLB values of some stabilizers are summarized in the Table 2.
Table 2.
The HLB value of some stabilizer which is used for nanosuspensions stability
| Stabilizer name | HLB value |
|---|---|
| HPMCS | 10–12 |
| Tween®80 | 15 |
| Poloxamer 188 (Pluronic F-68) | 29 |
| PVA | 18 |
| SLS | ≈40 |
| Poloxamer 407 (Pluronic F-127) | 22 |
| Labrasol® | 12 |
| Span 80 | 4.3 |
| Solutol | ~15 |
| Span 40 | 6.7 |
| Vitamin E TPGS | ≈13.2 |
| Crodesta F-160 | 14.5 |
| Crodesta F-110 | 12 |
| Cremophor EL | 12–14 |
| Volpo 10 | Volpo N10 = 12.4 |
| Span 20 | 8.6 |
| Span 60 | 4.7 |
| Brij® 58 | 15.7 |
Generally, nanosuspensions are hydrophobic drugs dispersed in hydrophilic media. Therefore, hydrophobic drug molecules tend to have more interactions with hydrophobic surfactants (low HLB), resulting in more reduction of surface energy, better dissolution, and smaller particles. For instance, there is a positive correlation between the size of ibuprofen nanoparticles, as lyophobic materials, and the HLB value of non-ionic stabilizer in bottom-up approach (Fig. 5). Conversely, larger particles are obtained when polymer with higher HLB are implemented [2].
Fig. 5.
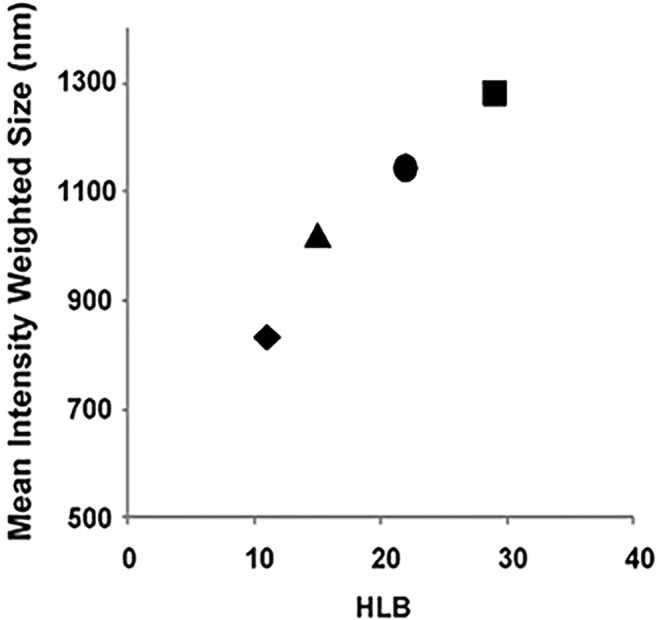
Particle size of ibuprofen suspensions as a function of HLB value of the stabilizer: precipitation. (♦) HPMCs, (▲) Tween®80, (●) Pluronic F-127 and (■) Pluronic F-68. Reprinted with permission from [2]. Copyright (2009) Elsevier
The favorable range of stabilizer HLB to get the minimum size and maximum stability of nanosuspension is 10–15 (Tables 1 and 2) [1, 2]. For example, among SLS (HLB 40), Span40 (HLB 6.7) [171] and Tween®80 (HLB 15), the latter was the best particle size reducer [172]. In this regard, surfactants with high HLB impair the ability of ionic stabilizers for size reduction. This is explained by inter-surfactant repulsion because of increase of particle charge density [44]. This phenomenon was observed in the preparation of atorvastatin nanocrystals; where labrasol® (HLB 12) was the best stabilizer compared with PVP, Tween®40 and Tween®80 with higher HLB [44]. However, each formulation requires individual selection of surfactants according to the parameters of the particle surface and the medium. It is noteworthy that surfactants with high HLB >14 may have harmful effects such as cytotoxicity, immunogenicity, or poor biodegradability [44, 173].
Effect of surfactant concentration
Application of surfactant with high concentration may adversely influence the nanosuspension formation and stabilization. In this regard, the surfactant solubility limit may cause precipitation at high concentrations. In addition, formation of a third layer around the particles can cause repulsion [105]. Accordingly, high concentrations of hydrophilic surfactants can promote nanoparticle dissolution through micelle formation, and cause instability via Ostwald ripening [69, 141, 174]. For instance, ibuprofen nanoparticles were more soluble in the presence of SLS, Tween®80 and Pluronic F-127, leading to erosion of small molecules and growth of large ones, i.e., instability of the formulation [2].
The concentration of stabilizer is a key factor in nanosuspension stability, and requires optimization for each formulation. At low concentrations, the efficacy of stabilizer diminishes because of low potency of surface coverage to form steric hindrance. For instance, the suitable concentration of MC for optimal size reduction and stabilization of itraconazole nanoparticles was 40% [89]. Moreover dilution of nanosuspension, by decreasing the efficacy of steric stabilizer for surface coverage, induces aggregation [89, 175]. In this regards, itraconazole particles grow in size due to dilution of formulation and reduction of HPMC concentration [175]. Accordingly, insufficient amount of stabilizer is reported to be the cause of agglomeration in zaltoprofen nanosuspension, whereas excess amounts of it induce Ostwald ripening [86].
Thus, too little or too much surfactant/stabilizer can yield unfavorable particles. In this regard, absence of surfactant in amylose nanosuspension resulted in large particles, whereas small particles of interest were obtained with surfactant at the concentration of 0.5% (w/v) [105]. Conversely, high concentrations of surfactant resulted in large agglomerate [105]. In the same manner, the optimal concentration of low molecular weight chitosan for the stabilization of atorvastatin was 0.3% (w/w), and increasing its concentration to 0.4 and 0.5% (w/w) led to particle agglomeration [44]. The paradoxical agglomeration because of high concentrations of stabilizer is explained by Ostwald ripening as well as increase of osmotic pressure. While the former enhances the solubility and causes erosion of small and growth of large particles, the latter induce attraction between colloidal particles [176].
Combination of surfactants
Combination of different surfactants/polymers is implemented when the optimal condition is not conceivable with a single stabilizer. The absorption of surfactant depends on the nature of particle surface. For instance, lyophobic nanoparticles such as tadalafil [177] and amylose [105] exhibit hydrophobic surface. So, surfactants with lower HLB tend to bind better on these surfaces. However, hydrophilic surfactants (high HLB) have shown higher steric stabilization. Thus, surfactants with low HLB may not be suitable choices in all cases. In fact, a combination of hydrophilic and hydrophobic surfactants seems to offer a better choice. In this regard, combination of tween®80 (HLB 15) with span80 (HLB 4.3) produced optimal amylose or tadalafil nanoparticles (Table 3) [105, 177]. However, these formulations required different ratios of tween®80 to span80.
Table 3.
Effect of surfactant HLB on mean size and PDI of amylase nanoparticles. Reprinted with permission from [105]. Copyright (2016) Indian Academy of Sciences
| Tween80/Span80 (w/w) | HLB | Mean size (nm) | PDI |
|---|---|---|---|
| 0/100 | 4.30 | 174.8 ±17.7 | 0.380 ± 0.063 |
| 25/75 | 6.98 | 183.1 ± 3.7 | 0.402 ± 0.032 |
| 50/50 | 6.65 | 173.1 ± 2.2 | 0.422 ± 0.017 |
| 75/25 | 12.33 | 155.2 ± 2.8 | 0.340 ± 0.032 |
| 100/0 | 15.0 | 157.4 ± 3.8 | 0.394 ± 0.020 |
Combination of polymers is shown to reduce particle size more efficiently than either of them alone, demonstrating synergistic effects [163]. In addition, simultaneous application of steric and electrostatic stabilizers, known as electrosteric stabilization, provides better protection against agglomeration [178]. For instance, charged polymers (cationic or anionic) such as chitosan confer both electrostatic repulsion and steric stabilization, thus prevent the adhesion of nanoparticles more efficiently [44]. Chitosan, also, enhances bioavailability due to mucoadhesion [179]. Accordingly, combination of high-Tg polymers (HPMCP 50 or Eudragit® L100) with surfactants enhances the dissolution rate, stability, and bioavailability of nanoparticulate drugs [163].
The synergistic effect of mixed surfactants on nanosuspension stability is explained by better coverage of the particle surface. In this regard, low amounts of Span80 penetrates into the gaps between Tween®80 molecules (Fig. 6), resulting in better coating of the nanoparticle surface. In fact, Span80 as inhibits the interactions between polar groups of Tween®80 produces a monolayer around the nanoparticle. This allows maximum coating with minimum concentration of the surfactant, and prevents the perturbation of other parameters of stability due to high concentrations of surfactant [177]. Alternatively, Tween®80 can be absorbed as the second layer over the internal layer of Span80 coating the particle. This indicates a surfactant bilayer, but requires high concentrations of the surfactants [177].
Fig. 6.

Interaction models of surfactants (white triangles and circles) Span80 and (black triangles and circles) Tween®80. Reprinted with permission from [177]. Copyright (2014) Springer Nature
Although application of two stabilizers appears to provide better protection, it adds another level of complexity for the optimization of formulation. Particularly, the ratio of polymers ought to be optimized to get the desired level of stability. In this regard, combination of PVP and SLS confer better size reduction. Meanwhile, increase of PVP ratios hampers the stability of formulation [180]. In fact, PVP seems to inhibit the surface adsorption of SLS at high concentrations. This is explained by the fact that free PVP molecules can interact with SLS molecules due to opposite charges [167, 180].
According to the DLVO theory, the effect of mixing sequence has no influence which may be as a result of formation of right energy barrier between the particles and hence have good physical properties [117].
Drying for particle stability
Dry powder formulations to be reconstituted as nanosuspension are becoming trendy to enhance the stability of system during long term storage. Also, nanosuspensions in dry state are harnessed for the preparation of solid dosage form such as tablets, capsules, and pellets. In this regard, spray drying, freeze drying, spray freeze drying, pelletization and granulation have been successfully employed to transform the liquid nanosuspensions into dry powder [106, 181]. In fact, drying appears to suppress the electrostatic activity due to lower effects of electrolytes in dry state [128].
Various additives such as polymers, surfactants, cryoprotectants and lyoprotectants are used as stabilizer or cryo/thermal-protectant in formulation or drying process. These excipients can alter particle behavior because of surface interaction or change in medium properties [106, 182–184]. In addition, the selection of carriers should not also perturb the maintenance of physicochemical properties of the nanoparticle after re-dispersion [185]. In this regard, sugar base cryoprotectants such as sucrose decrease the surface zeta potential of itraconazole loaded poly (ε-caprolactone) nanoparticles by masking the OH group on the surface [186]. Accordingly, mannitol appears to result in lower polydispersity, more size reduction, and better cryopreservation compared to sucrose [89, 117].
Conclusion
Taken together, nanosuspensions are very useful for the formulation of poorly water soluble drug molecules, and can increase their bioavailability, thereby, therapeutic efficacy. The bottom-up approach involves precipitation of supersaturated solutions for naonoparticle formation. Several parameters in the particle, dispersion medium and formulation/storage condition are critical in the stability of nanosuspension. Nucleation and particle growth share similar mechanisms with instability, i.e., the same principles that cause nucleation and particle formation can cause inappropriate growth an agglomeration. Thus, application of excipients such as stabilizers requires proper optimization of type and concentration. This implies that each formulation requires its own optimization process, and if a drying process is employed, one ought to consider the effects additional variables to ensure the desired size and behavior of particles as well as the stability and therapeutic efficacy of formulation.
Acknowledgements
The authors gratefully acknowledge the financial support from Shahid Sadoughi University of Medical Sciences.
Abbreviations
- HPMC
Hydroxypropyl methyl cellulose
- MC
Methylcellulose
- HPC-SL
Hydroxypropyl cellulose
- HPMCP 50
Hydroxypropyl methyl cellulose acetate phthalate
- Tween®80
Polysorbate 80
- Poloxamers
Polyoxyethylene–polyoxypropylene block copolymer
- NaCMC
Sodium carboxy methyl cellulose
- PVA
Polyvinylalcohol
- SLS
Sodium lauryl sulfate
- PVP
Polyvinylpyrrolidone
- Labrasol®
CaprylocaproylPolyoxylglycerides
- Span 80
Sorbitanmonooleate
- Solutol
2-Hydroxyethyl-12-hydroxyoctadecanoate
- Span 40
(Sorbitanmonopalmitate)
- Plasdone (PVP/VA copolymer)
Polyvinylpyrrolidone-vinyl acetate copolymer
- Eudragit®
Polymethacrylates
- Vitamin E TPGS
Tocopherol polyethylene glycol succinate
- Span 20
Sorbitanmonolaurate
- Span 60
Sorbitanmonostearate
- Brij® 58
Polyoxyl 20 cetyl ether
- Cremophor EL
Polyoxyl 35 castor oil
- Volpo 10
Polyoxyl 10 oleyl ether
- Crodesta F-160
Sucrose stearate
- Crodesta F-110
Sucrose stearate (and) sucrose distearate
- Triton X-100
Polyethylene glycol tert-octylphenyl ether
Nomenclature
- A
Crystal surface area
- D
Diffusion coefficient
- d
Nanoparticles diameter
- d0
Nanoparticles diameter in the initial time
- ∆G
Gibbs free energy of a nanoparticle
- kB
Boltzmann constant
- R
Gas molar constant
- RG
Rate of crystal growth
- r
Particle radius
- S
Degree of supersaturation
- T
Absolute temperature
- v
Molar volume
- η
Viscosity
- γ
Surface tension
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Esfandi E, Ramezani V, Vatanara A, Najafabadi AR, Moghaddam SPH. Clarithromycin dissolution enhancement by preparation of aqueous nanosuspensions using sonoprecipitation technique. Iran J Pharm Res. 2014;13(3):809–818. [PMC free article] [PubMed] [Google Scholar]
- 2.Verma S, Gokhale R. Burgess DJ. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm. 2009;380(1–2):216–222. doi: 10.1016/j.ijpharm.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Bolourchian N, Mahboobian MM, Dadashzadeh S. The effect of PEG molecular weights on dissolution behavior of simvastatin in solid dispersions. Iran J Pharm Res. 2013;12(Suppl):11–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Saeedi M, Akbari J, Morteza-Semnani K, Enayati-Fard R, Sar-Reshteh-dar S, Soleymani A. Enhancement of dissolution rate of indomethacin using liquisolid compacts. Iran J Pharm Res. 2010:25–33. [PMC free article] [PubMed]
- 5.Lipinski C. Poor aqueous solubility—an industry wide problem in drug discovery. Am Pharm Rev. 2002;5(3):82–85. [Google Scholar]
- 6.Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56(7):827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 7.Kesisoglou F, Panmai S, Wu YH. Nanosizing - Oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–644. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs C, Muller RH. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002;19(2):189–194. doi: 10.1023/a:1014276917363. [DOI] [PubMed] [Google Scholar]
- 10.Muller RH, Gohla S, Keck CM. State of the art of nanocrystals - special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;78(1):1–9. doi: 10.1016/j.ejpb.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Mauludin R, Muller RH, Keck CM. Kinetic solubility and dissolution velocity of rutin nanocrystals. Eur J Pharm Sci. 2009;36(4–5):502–510. doi: 10.1016/j.ejps.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Müller RH, Akkar A. Drug nanocrystals of poorly soluble drugs. Encyclopedia of nanoscience and nanotechnology. 2. American Scientific Publishers; 2004. p. 627–38.
- 13.Sun J, Wang F, Sui Y, She Z, Zhai W, Wang C, et al. Effect of particle size on solubility, dissolution rate, and oral bioavailability: evaluation using coenzyme Q10 as naked nanocrystals. Int J Nanomedicine. 2012;7:5733. doi: 10.2147/IJN.S34365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller RH, Peters K. Nanosuspensions for the formulation of poorly soluble drugs: I. preparation by a size-reduction technique. Int J Pharm. 1998;160(2):229–237. [Google Scholar]
- 15.Müller RH, Benita S, Böhm BH. Emulsions and nanosuspensions for the formulation of poorly soluble drugs. Boca Raton: CRC Press; 1998. [Google Scholar]
- 16.Müller R, Jacobs C, Kayser O. Nanosuspensions for the formulation of poorly soluble drugs. Pharmaceutical emulsions and suspensions: Second Edition, REVISED AND EXPANDED. Boca Raton: CRC Press; 2000. pp. 383–407. [Google Scholar]
- 17.Muller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47(1):3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 18.Müller RH, Jacobs C, Kayser O. DissoCubes‚ a novel formulation for poorly soluble and poorly bioavailable drugs. modified-release drug delivery technology. Informa Healthcare; 2002. p. 135–49.
- 19.Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18(2):113–120. doi: 10.1016/s0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 20.Peters K, Leitzke S, Diederichs JE, Borner K, Hahn H, Muller RH, et al. Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine Mycobacterium avium infection. J Antimicrob Chemother. 2000;45(1):77–83. doi: 10.1093/jac/45.1.77. [DOI] [PubMed] [Google Scholar]
- 21.Samei M, Vatanara A, Fatemi S, Najafabadi AR. Process variables in the formation of nanoparticles of megestrol acetate through rapid expansion of supercritical CO 2. J Supercrit Fluids. 2012;70:1–7. [Google Scholar]
- 22.Sofie V, Jan V, Ludo F, Patrick A. Microcrystalline cellulose, a useful alternative for sucrose as a matrix former during freeze-drying of drug nanosuspensions–a case study with itraconazole. Eur J Pharm Biopharm. 2008;70(2):590–596. doi: 10.1016/j.ejpb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Lai F, Pireddu R, Corrias F, Fadda AM, Valenti D, Pini E, et al. Nanosuspension improves tretinoin photostability and delivery to the skin. Int J Pharm. 2013;458(1):104–109. doi: 10.1016/j.ijpharm.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Möschwitzer JP. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453(1):142–156. doi: 10.1016/j.ijpharm.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Moschwitzer J, Achleitner G, Pomper H, Muller RH. Development of an intravenously injectable chemically stable aqueous omeprazole formulation using nanosuspension technology. Eur J Pharm Biopharm. 2004;58(3):615–619. doi: 10.1016/j.ejpb.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Azad M, Dave R, Bilgili E. Nanomilling of drugs for bioavailability enhancement: a holistic formulation-process perspective. Pharmaceutics. 2016;8(2):17. doi: 10.3390/pharmaceutics8020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagener P, Lau M, Breitung-Faes S, Kwade A, Barcikowski S. Physical fabrication of colloidal ZnO nanoparticles combining wet-grinding and laser fragmentation. Applied Physics A. 2012;108(4):793–799. [Google Scholar]
- 28.Habiba K, Makarov VI, Weiner BR, Morell G. Fabrication of nanomaterials by pulsed laser synthesis. Manufacturing Nanostructures. Manchester, UK: One Central Press; 2014. [Google Scholar]
- 29.Liu P. Nanocrystal formulation for poorly soluble. Drugs. 2013.
- 30.Loh ZH, Samanta AK, Heng PWS. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian Journal of Pharmaceutical Sciences. 2015;10(4):255–274. [Google Scholar]
- 31.Huttenrauch R, Fricke S, Zielke P. Mechanical activation of pharmaceutical systems. Pharm Res. 1985;2(6):302–306. doi: 10.1023/A:1016397719020. [DOI] [PubMed] [Google Scholar]
- 32.Verma S, Lan Y, Gokhale R, Burgess DJ. Quality by design approach to understand the process of nanosuspension preparation. Int J Pharm. 2009;377(1–2):185–198. doi: 10.1016/j.ijpharm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Xiong R, Lu W, Li J, Wang P, Xu R, Chen T. Preparation and characterization of intravenously injectable nimodipine nanosuspension. Int J Pharm. 2008;350(1–2):338–343. doi: 10.1016/j.ijpharm.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh I, Bose S, Vippagunta R, Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm. 2011;409(1–2):260–268. doi: 10.1016/j.ijpharm.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Sinha B, Muller RH, Moschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453(1):126–141. doi: 10.1016/j.ijpharm.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Chan HK, Kwok PCL. Production methods for nanodrug particles using the bottom-up approach. Adv Drug Deliv Rev. 2011;63(6):406–416. doi: 10.1016/j.addr.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Date AA, Patravale VB. Current strategies for engineering drug nanoparticles. Curr Opin Colloid Interface Sci. 2004;9(3–4):222–235. [Google Scholar]
- 38.Dirksen JA, Ring TA. Fundamentals of crystallization - kinetic effects on particle-size distributions and morphology. Chem Eng Sci. 1991;46(10):2389–2427. [Google Scholar]
- 39.Chen A, Shi Y, Yan Z, Hao H, Zhang Y, Zhong J, et al. Dosage form developments of nanosuspension drug delivery system for oral administration route. Curr Pharm Des. 2015;21(29):4355–4365. doi: 10.2174/1381612821666150901105026. [DOI] [PubMed] [Google Scholar]
- 40.Du J, Li X, Zhao H, Zhou Y, Wang L, Tian S, et al. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm. 2015;495(2):738–749. doi: 10.1016/j.ijpharm.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Shegokar R, Müller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399(1–2):129–139. doi: 10.1016/j.ijpharm.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 42.Hu J, Ng WK, Dong Y, Shen S, Tan RB. Continuous and scalable process for water-redispersible nanoformulation of poorly aqueous soluble APIs by antisolvent precipitation and spray-drying. Int J Pharm. 2011;404(1–2):198–204. doi: 10.1016/j.ijpharm.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 43.Quan P, Xia D, Piao H, Piao H, Shi K, Jia Y, et al. Nitrendipine nanocrystals: its preparation, characterization, and in vitro–in vivo evaluation. AAPS PharmSciTech. 2011;12(4):1136–1143. doi: 10.1208/s12249-011-9682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurakula M, El-Helw AM, Sobahi TR, Abdelaal MY. Chitosan based atorvastatin nanocrystals: effect of cationic charge on particle size, formulation stability, and in-vivo efficacy. Int J Nanomedicine. 2015;10:321–334. doi: 10.2147/IJN.S77731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y, Wang ZH, Li T, McNally H, Park K, Sturek M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J Control Release. 2014;176:76–85. doi: 10.1016/j.jconrel.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindfors L, Forssen S, Westergren J, Olsson U. Nucleation and crystal growth in supersaturated solutions of a model drug. J Colloid Interface Sci. 2008;325(2):404–413. doi: 10.1016/j.jcis.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 47.Reiss H. The growth of uniform colloidal dispersions. J Chem Phys. 1951;19(4):482–487. [Google Scholar]
- 48.LaMer VK, Dinegar RH. Theory, production and mechanism of formation of monodispersed hydrosols. J Am Chem Soc. 1950;72(11):4847–4854. [Google Scholar]
- 49.Lindfors L, Skantze P, Skantze U, Westergren J, Olsson U. Amorphous drug nanosuspensions. 3. Particle dissolution and crystal growth. Langmuir. 2007;23(19):9866–9874. doi: 10.1021/la700811b. [DOI] [PubMed] [Google Scholar]
- 50.Garrick S, Lehtinen K, Zachariah M. Nanoparticle coagulation via a Navier–stokes/nodal methodology: evolution of the particle field. J Aerosol Sci. 2006;37(5):555–576. [Google Scholar]
- 51.Huang F, Zhang HZ, Banfield JF. Two-stage crystal-growth kinetics observed during hydrothermal coarsening of nanocrystalline ZnS. Nano Lett. 2003;3(3):373–378. [Google Scholar]
- 52.Bilecka I, Elser P, Niederberger M. Kinetic and thermodynamic aspects in the microwave-assisted synthesis of ZnO nanoparticles in benzyl alcohol. ACS Nano. 2009;3(2):467–477. doi: 10.1021/nn800842b. [DOI] [PubMed] [Google Scholar]
- 53.Raghavan SL, Trividic A, Davis AF, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 2001;212(2):213–221. doi: 10.1016/s0378-5173(00)00610-4. [DOI] [PubMed] [Google Scholar]
- 54.Kwon SG, Hyeon T. Formation mechanisms of uniform nanocrystals via hot-injection and heat-up methods. Small. 2011;7(19):2685–2702. doi: 10.1002/smll.201002022. [DOI] [PubMed] [Google Scholar]
- 55.Verma S, Kumar S, Gokhale R, Burgess DJ. Physical stability of nanosuspensions: investigation of the role of stabilizers on Ostwald ripening. Int J Pharm. 2011;406(1–2):145–152. doi: 10.1016/j.ijpharm.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 56.Ribeiro C, Lee EJ, Longo E. Leite ER. A kinetic model to describe nanocrystal growth by the oriented attachment mechanism. ChemPhysChem. 2005;6(4):690–696. doi: 10.1002/cphc.200400505. [DOI] [PubMed] [Google Scholar]
- 57.Viswanatha R, Santra PK, Dasgupta C, Sarma DD. Growth mechanism of nanocrystals in solution: ZnO, a case study. Phys Rev Lett. 2007;98(25):255501. doi: 10.1103/PhysRevLett.98.255501. [DOI] [PubMed] [Google Scholar]
- 58.Cabane H, Laporte D, Provost A. An experimental study of Ostwald ripening of olivine and plagioclase in silicate melts: implications for the growth and size of crystals in magmas. Contrib Mineral Petrol. 2005;150(1):37–53. [Google Scholar]
- 59.Solomatov VS, Stevenson DJ. Kinetics of crystal-growth in a terrestrial Magma Ocean. Journal of Geophysical Research-Planets. 1993;98(E3):5407–5418. [Google Scholar]
- 60.Schram CJ, Smyth RJ, Taylor LS, Beaudoin SP. Understanding crystal Growth kinetics in the absence and presence of a polymer using a rotating disk apparatus. Cryst Growth Des. 2016;16(5):2640–2645. [Google Scholar]
- 61.Thanh NT, Maclean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev. 2014;114(15):7610–7630. doi: 10.1021/cr400544s. [DOI] [PubMed] [Google Scholar]
- 62.Kashchiev D. Nucleation: basic theory with applications. Boston: Butterworth Heinemann; 2000. [Google Scholar]
- 63.Rosen MJ, Kunjappu JT. Surfactants and interfacial phenomena: John Wiley & Sons; 2012.
- 64.Lee H, Lee J. Dissolution enhancement of celecoxib via polymer-induced crystallization. J Cryst Growth. 2013;374:37–42. [Google Scholar]
- 65.Somasundaran P, Huang L. Adsorption/aggregation of surfactants and their mixtures at solid-liquid interfaces. Adv Colloid Interf Sci. 2000;88(1–2):179–208. doi: 10.1016/s0001-8686(00)00044-0. [DOI] [PubMed] [Google Scholar]
- 66.Grau MJ, Kayser O, Muller RH. Nanosuspensions of poorly soluble drugs - reproducibility of small scale production. Int J Pharm. 2000;196(2):155–159. doi: 10.1016/s0378-5173(99)00411-1. [DOI] [PubMed] [Google Scholar]
- 67.Lee J. Drug nano-and microparticles processed into solid dosage forms: physical properties. J Pharm Sci. 2003;92(10):2057–2068. doi: 10.1002/jps.10471. [DOI] [PubMed] [Google Scholar]
- 68.Berglund KD, Przybycien TM, Tilton RD. Coadsorption of sodium dodecyl sulfate with hydrophobically modified nonionic cellulose polymers. 1. Role of polymer hydrophobic modification. Langmuir. 2003;19(7):2705–2713. [Google Scholar]
- 69.Lee J, Lee SJ, Choi JY, Yoo JY, Ahn CH. Amphiphilic amino acid copolymers as stabilizers for the preparation of nanocrystal dispersion. Eur J Pharm Sci. 2005;24(5):441–449. doi: 10.1016/j.ejps.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 70.Muller RH, Jacobs C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 2002;237(1–2):151–161. doi: 10.1016/s0378-5173(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 71.Kronberg B, Stenius P. The effect of surface polarity on the adsorption of nonionic surfactants. I Thermodynamic considerations. J Colloid Interface Sci. 1984;102(2):410–417. [Google Scholar]
- 72.Zheng H, Smith RK, Jun YW, Kisielowski C, Dahmen U, Alivisatos AP. Observation of single colloidal platinum nanocrystal growth trajectories. Science. 2009;324(5932):1309–1312. doi: 10.1126/science.1172104. [DOI] [PubMed] [Google Scholar]
- 73.Harris MT, Byers CH. Effect of solvent on the homogeneous precipitation of Titania by titanium Ethoxide hydrolysis. J Non-Cryst Solids. 1988;103(1):49–64. [Google Scholar]
- 74.Clark MD. Growth laws for surfactant-coated nanocrystals: Ostwald ripening and size focusing. J Nanopart Res. 2014;16(2):2264. [Google Scholar]
- 75.Burlakov V. Ostwald ripening on nanoscale. arXiv preprint arXiv:07105224. 2007.
- 76.Niederberger M, Colfen H. Oriented attachment and mesocrystals: non-classical crystallization mechanisms based on nanoparticle assembly. Phys Chem Chem Phys. 2006;8(28):3271–3287. doi: 10.1039/b604589h. [DOI] [PubMed] [Google Scholar]
- 77.Liu Z, Wen XD, Wu XL, Gao YJ, Chen HT, Zhu J, et al. Intrinsic dipole-field-driven mesoscale crystallization of core-shell ZnO mesocrystal microspheres. J Am Chem Soc. 2009;131(26):9405–9412. doi: 10.1021/ja9039136. [DOI] [PubMed] [Google Scholar]
- 78.Privman VV, Goia DV, Park J, Matijevi cacute E. Mechanism of formation of monodispersed colloids by aggregation of nanosize precursors. J Colloid Interface Sci. 1999;213(1):36–45. doi: 10.1006/jcis.1999.6106. [DOI] [PubMed] [Google Scholar]
- 79.Colfen H, Mann S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew Chem Int Ed Engl. 2003;42(21):2350–2365. doi: 10.1002/anie.200200562. [DOI] [PubMed] [Google Scholar]
- 80.Ain-Ai A, Gupta PK. Effect of arginine hydrochloride and hydroxypropyl cellulose as stabilizers on the physical stability of high drug loading nanosuspensions of a poorly soluble compound. Int J Pharm. 2008;351(1–2):282–288. doi: 10.1016/j.ijpharm.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 81.Dharmalingam SR, Chidambaram K, Ramamurthy S, Nadaraju S. Effects of nanosuspension and inclusion complex techniques on the in vitro protease inhibitory activity of naproxen. Braz J Pharm Sci. 2014;50(1):165–171. [Google Scholar]
- 82.Mura P, Maestrelli F, Cirri M. Ternary systems of naproxen with hydroxypropyl-β-cyclodextrin and aminoacids. Int J Pharm. 2003;260(2):293–302. doi: 10.1016/s0378-5173(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 83.Sugimoto T, Shiba F, Sekiguchi T, Itoh H. Spontaneous nucleation of monodisperse silver halide particles from homogeneous gelatin solution I: silver chloride. Colloids Surf A Physicochem Eng Asp. 2000;164(2–3):183–203. [Google Scholar]
- 84.Lifshitz IM, Slyozov VV. The kinetics of precipitation from supersaturated solid solutions. J Phys Chem Solids. 1961;19(1–2):35–50. [Google Scholar]
- 85.Wagner C. Theorie der alterung von niederschlägen durch umlösen (Ostwald-reifung) Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für physikalische Chemie. 1961;65(7–8):581–591. [Google Scholar]
- 86.Papdiwal AP, Pande VV, Aher S. Investigation of effect of different stabilizers on formulation of zaltoprofen nanosuspension. Int J Pharm Sci Rev Res. 2014;27(2):244–249. [Google Scholar]
- 87.Lee W-R, Kim MG, Choi J-R, Park J-I, Ko SJ, Oh SJ, et al. Redox− transmetalation process as a generalized synthetic strategy for core− shell magnetic nanoparticles. J Am Chem Soc. 2005;127(46):16090–16097. doi: 10.1021/ja053659j. [DOI] [PubMed] [Google Scholar]
- 88.Watzky MA, Finke RG. Transition metal nanocluster formation kinetic and mechanistic studies. A new mechanism when hydrogen is the reductant: slow, continuous nucleation and fast autocatalytic surface growth. J Am Chem Soc. 1997;119(43):10382–10400. [Google Scholar]
- 89.Daebis NAO, El-Massik M, Abdelkader H. Formulation and characterization of Itraconazole Oral Nanosuspension: methyl cellulose as promising stabilizer. Ely J Pharm Res. 2015;1(1):102. [Google Scholar]
- 90.Xia D, Ouyang M, Wu JX, Jiang Y, Piao H, Sun S, et al. Polymer-mediated anti-solvent crystallization of nitrendipine: monodispersed spherical crystals and growth mechanism. Pharm Res. 2012;29(1):158–169. doi: 10.1007/s11095-011-0522-6. [DOI] [PubMed] [Google Scholar]
- 91.Peng X, Manna L, Yang W, Wickham J, Scher E, Kadavanich A, et al. Shape control of CdSe nanocrystals. Nature. 2000;404(6773):59–61. doi: 10.1038/35003535. [DOI] [PubMed] [Google Scholar]
- 92.Peng ZA, Peng XG. Nearly monodisperse and shape-controlled CdSe nanocrystals via alternative routes: nucleation and growth. J Am Chem Soc. 2002;124(13):3343–3353. doi: 10.1021/ja0173167. [DOI] [PubMed] [Google Scholar]
- 93.Lindfors L, Skantze P, Skantze U, Rasmusson M, Zackrisson A, Olsson U. Amorphous drug nanosuspensions. 1 Inhibition of Ostwald ripening. Langmuir. 2006;22(3):906–910. doi: 10.1021/la0523661. [DOI] [PubMed] [Google Scholar]
- 94.Lindfors L, Forssen S, Skantze P, Skantze U, Zackrisson A, Olsson U. Amorphous drug nanosuspensions. 2. Experimental determination of bulk monomer concentrations. Langmuir. 2006;22(3):911–916. doi: 10.1021/la052367t. [DOI] [PubMed] [Google Scholar]
- 95.Mullin JW. Crystallization: Butterworth-Heinemann. 2001. [Google Scholar]
- 96.Penn RL. Kinetics of oriented aggregation. J Phys Chem B. 2004;108(34):12707–12712. [Google Scholar]
- 97.Penn RL, Oskam G, Strathmann TJ, Searson PC, Stone AT, Veblen DR. Epitaxial assembly in aged colloids. J Phys Chem B. 2001;105(11):2177–2182. [Google Scholar]
- 98.Kulak AN, Iddon P, Li Y, Armes SP, Colfen H, Paris O, et al. Continuous structural evolution of calcium carbonate particles: a unifying model of copolymer-mediated crystallization. J Am Chem Soc. 2007;129(12):3729–3736. doi: 10.1021/ja067422e. [DOI] [PubMed] [Google Scholar]
- 99.Andreassen JP. Formation mechanism and morphology in precipitation of vaterite - nano aggregation or crystal growth? J Cryst Growth. 2005;274(1–2):256–264. [Google Scholar]
- 100.Shen Q, Wei H, Wang L, Zhou Y, Zhao Y, Zhang Z, et al. Crystallization and aggregation behaviors of calcium carbonate in the presence of poly(vinylpyrrolidone) and sodium dodecyl sulfate. J Phys Chem B. 2005;109(39):18342–18347. doi: 10.1021/jp052094a. [DOI] [PubMed] [Google Scholar]
- 101.Sugimoto T, Dirige GE, Muramatsu A. Formation mechanism of monodisperse CdS particles from concentrated solutions of Cd-EDTA complexes. J Colloid Interface Sci. 1996;182(2):444–456. doi: 10.1006/jcis.1996.0660. [DOI] [PubMed] [Google Scholar]
- 102.Ali HSM, York P, Blagden N. Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int J Pharm. 2009;375(1–2):107–113. doi: 10.1016/j.ijpharm.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 103.Heng JY, Thielmann F, Williams DR. The effects of milling on the surface properties of form I paracetamol crystals. Pharm Res. 2006;23(8):1918–1927. doi: 10.1007/s11095-006-9042-1. [DOI] [PubMed] [Google Scholar]
- 104.Lai F, Sinico C, Ennas G, Marongiu F, Marongiu G, Fadda AM. Diclofenac nanosuspensions: influence of preparation procedure and crystal form on drug dissolution behaviour. Int J Pharm. 2009;373(1–2):124–132. doi: 10.1016/j.ijpharm.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 105.Dong Y, Chang YJ, Wang Q, Tong J, Zhou J. Effects of surfactants on size and structure of amylose nanoparticles prepared by precipitation. Bull Mater Sci. 2016;39(1):35–39. [Google Scholar]
- 106.Dolenc A, Kristl J, Baumgartner S, Planinsek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm. 2009;376(1–2):204–212. doi: 10.1016/j.ijpharm.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 107.Kumar R, Siril PF. Controlling the size and morphology of griseofulvin nanoparticles using polymeric stabilizers by evaporation-assisted solvent-antisolvent interaction method. J Nanopart Res. 2015;17(6):256. [Google Scholar]
- 108.Tran TT, Tran PH, Nguyen MN, Tran KT, Pham MN, Tran PC, et al. Amorphous isradipine nanosuspension by the sonoprecipitation method. Int J Pharm. 2014;474(1–2):146–150. doi: 10.1016/j.ijpharm.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 109.Hecq J, Deleers M, Fanara D, Vranckx H, Amighi K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm. 2005;299(1–2):167–177. doi: 10.1016/j.ijpharm.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 110.Saindane NS, Pagar KP, Vavia PR. Nanosuspension based in situ gelling nasal spray of carvedilol: development, in vitro and in vivo characterization. AAPS PharmSciTech. 2013;14(1):189–199. doi: 10.1208/s12249-012-9896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato T, Takeuchi H, Sakurai T, Tanaka K, Matsuki K, Higashi K, et al. Characterization of a riboflavin non-aqueous nanosuspension prepared by bead milling for cutaneous application. Chem Pharm Bull (Tokyo) 2015;63(2):88–94. doi: 10.1248/cpb.c14-00641. [DOI] [PubMed] [Google Scholar]
- 112.Chari K, Antalek B, Kowalczyk J, Eachus RS, Chen T. Polymer− surfactant interaction and stability of amorphous colloidal particles. J Phys Chem B. 1999;103(45):9867–9872. [Google Scholar]
- 113.Matteucci ME, Hotze MA, Johnston KP, Williams RO., III Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir. 2006;22(21):8951–8959. doi: 10.1021/la061122t. [DOI] [PubMed] [Google Scholar]
- 114.Jongen N, Bowen P, Lemaitre J, Valmalette JC, Hofmann H. Precipitation of self-organized copper oxalate polycrystalline particles in the presence of hydroxypropylmethylcellulose (HPMC): control of morphology. J Colloid Interface Sci. 2000;226(2):189–198. [Google Scholar]
- 115.Kronberg B, Lindman B. Surfactants and polymers in aqueous solution. Chichester: John Wiley & Sons Ltd.; 2003. [Google Scholar]
- 116.Tadros TF. Control of the properties of suspensions. Colloids Surf. 1986;18(2–4):137–173. [Google Scholar]
- 117.Kumar MP, Rao YM, Apte S. Formulation of nanosuspensions of albendazole for oral administration. Curr Nanosci. 2008;4(1):53–58. [Google Scholar]
- 118.Ruiz-Cabello FJ, Trefalt G, Csendes Z, Sinha P, Oncsik T, Szilagyi I, et al. Predicting aggregation rates of colloidal particles from direct force measurements. J Phys Chem B. 2013;117(39):11853–11862. doi: 10.1021/jp406061f. [DOI] [PubMed] [Google Scholar]
- 119.Lyklema J. Principles of the stability of lyophobic colloidal dispersions in non-aqueous media. Adv Colloid Interf Sci. 1968;2(2):67–114. [Google Scholar]
- 120.Zhao YX, Hua HY, Chang M, Liu WJ, Zhao Y, Liu HM. Preparation and cytotoxic activity of hydroxycamptothecin nanosuspensions. Int J Pharm. 2010;392(1–2):64–71. doi: 10.1016/j.ijpharm.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 121.Van Eerdenbrugh B, Vermant J, Martens JA, Froyen L, Van Humbeeck J, Augustijns P, et al. A screening study of surface stabilization during the production of drug nanocrystals. J Pharm Sci. 2009;98(6):2091–2103. doi: 10.1002/jps.21563. [DOI] [PubMed] [Google Scholar]
- 122.Singare DS, Giriraj TK, Gowthamrajan K. Nanosuspension solidification technique: evaluation of high shear granulation as per industrial perspective.
- 123.Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364(1):64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 124.Jacobs C, Kayser O, Muller RH. Nanosuspensions as a new approach for the formulation for the poorly soluble drug tarazepide. Int J Pharm. 2000;196(2):161–164. doi: 10.1016/s0378-5173(99)00412-3. [DOI] [PubMed] [Google Scholar]
- 125.Müller RH, Hildebrand GE. Zetapotential und Partikelladung in der Laborpraxis(Einführung in die Theorie praktische Messdurchführung Dateninterpretation). Paperback APV; 1996.
- 126.Freitas C, Muller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN (TM)) dispersions. Int J Pharm. 1998;168(2):221–229. [Google Scholar]
- 127.Manciu M, Ruckenstein E. Role of the hydration force in the stability of colloids at high ionic strengths. Langmuir. 2001;17(22):7061–7070. [Google Scholar]
- 128.Van der Hoeven PC, Lyklema J. Electrostatic stabilization in non-aqueous media. Adv Colloid Interf Sci. 1992;42:205–277. [Google Scholar]
- 129.Tuomela A, Hirvonen J, Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics. 2016;8(2):16. doi: 10.3390/pharmaceutics8020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.George M, Ghosh I. Identifying the correlation between drug/stabilizer properties and critical quality attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur J Pharm Sci. 2013;48(1–2):142–152. doi: 10.1016/j.ejps.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62(11):1569–1579. doi: 10.1111/j.2042-7158.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- 132.Zhang H, Hollis CP, Zhang Q, Li T. Preparation and antitumor study of camptothecin nanocrystals. Int J Pharm. 2011;415(1–2):293–300. doi: 10.1016/j.ijpharm.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 133.Attard P, Antelmi D, Larson I. Comparison of the zeta potential with the diffuse layer potential from charge titration. Langmuir. 2000;16(4):1542–1552. [Google Scholar]
- 134.Bourikas K, Stylidi M, Kondarides DI, Verykios XE. Adsorption of acid orange 7 on the surface of titanium dioxide. Langmuir. 2005;21(20):9222–9230. doi: 10.1021/la051434g. [DOI] [PubMed] [Google Scholar]
- 135.Kim TH, Choi SM, Kline SR. Polymerized rodlike nanoparticles with controlled surface charge density. Langmuir. 2006;22(6):2844–2850. doi: 10.1021/la052949a. [DOI] [PubMed] [Google Scholar]
- 136.Liao D, Wu G, Liao B. Zeta potential of shape-controlled TiO 2 nanoparticles with surfactants. Colloids Surf A Physicochem Eng Asp. 2009;348(1):270–275. [Google Scholar]
- 137.Tanaka Y, Inkyo M, Yumoto R, Nagai J, Takano M, Nagata S. Nanoparticulation of poorly water soluble drugs using a wet-mill process and physicochemical properties of the nanopowders. Chem Pharm Bull. 2009;57(10):1050–1057. doi: 10.1248/cpb.57.1050. [DOI] [PubMed] [Google Scholar]
- 138.Raghavan SL, Schuessel K, Davis A, Hadgraft J. Formation and stabilisation of triclosan colloidal suspensions using supersaturated systems. Int J Pharm. 2003;261(1–2):153–158. doi: 10.1016/s0378-5173(03)00299-0. [DOI] [PubMed] [Google Scholar]
- 139.Ziller KH, Rupprecht HH. Control of crystal-Growth in drug suspensions .2. Influence of polymers on dissolution and crystallization during temperature cycling. Pharmazeutische Industrie. 1990;52(8):1017–1022. [Google Scholar]
- 140.Teeranachaideekul V, Junyaprasert VB, Souto EB, Muller RH. Development of ascorbyl palmitate nanocrystals applying the nanosuspension technology. Int J Pharm. 2008;354(1–2):227–234. doi: 10.1016/j.ijpharm.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 141.Choi JY, Yoo JY, Kwak HS, Nam BU, Lee J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr Appl Phys. 2005;5(5):472–474. [Google Scholar]
- 142.Walstra P. Formation of emulsions. 1983.
- 143.Müller RH. Colloidal carriers for controlled drug delivery and targeting: modification, characterization and in vivo distribution. London: Taylor & Francis; 1991. [Google Scholar]
- 144.Liu P, Rong X, Laru J, van Veen B, Kiesvaara J, Hirvonen J, et al. Nanosuspensions of poorly soluble drugs: preparation and development by wet milling. Int J Pharm. 2011;411(1–2):215–222. doi: 10.1016/j.ijpharm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 145.Wegner G, Baum P, Müller M, Norwig J, Landfester K (eds). Polymers designed to control nucleation and growth of inorganic crystals from aqueous media. Macromolecular Symposia. Wiley Online Library; 2001.
- 146.Shenoy DB, Sukhorukov GB. Engineered microcrystals for direct surface modification with layer-by-layer technique for optimized dissolution. Eur J Pharm Biopharm. 2004;58(3):521–527. doi: 10.1016/j.ejpb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 147.Giermanska-Kahn J, Schmitt V, Binks BP. Leal-Calderon F. A new method to prepare monodisperse Pickering emulsions. Langmuir. 2002;18(7):2515–2518. [Google Scholar]
- 148.Keck CM, Muller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62(1):3–16. doi: 10.1016/j.ejpb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 149.Zimmermann E, Muller RH. Electrolyte- and pH-stabilities of aqueous solid lipid nanoparticle (SLN (TM)) dispersions in artificial gastrointestinal media. Eur J Pharm Biopharm. 2001;52(2):203–210. doi: 10.1016/s0939-6411(01)00167-9. [DOI] [PubMed] [Google Scholar]
- 150.Einarson MB, Berg JC. Electrosteric stabilization of colloidal latex dispersions. J Colloid Interface Sci. 1993;155(1):165–172. [Google Scholar]
- 151.Shete G, Jain H, Punj D, Prajapat H, Akotiya P, Bansal AK. Stabilizers used in nanocrystal based drug delivery systems. Journal of Excipients and Food Chemicals. 2014;5(4):184–209. [Google Scholar]
- 152.Verma S, Huey BD, Burgess DJ. Scanning probe microscopy method for nanosuspension stabilizer selection. Langmuir. 2009;25(21):12481–12487. doi: 10.1021/la9016432. [DOI] [PubMed] [Google Scholar]
- 153.Jiang T, Han N, Zhao B, Xie Y, Wang S. Enhanced dissolution rate and oral bioavailability of simvastatin nanocrystal prepared by sonoprecipitation. Drug Dev Ind Pharm. 2012;38(10):1230–1239. doi: 10.3109/03639045.2011.645830. [DOI] [PubMed] [Google Scholar]
- 154.Berglund KD, Przybycien TM, Tilton RD. Coadsorption of sodium dodecyl sulfate with hydrophobically modified nonionic cellulose polymers. 2. Role of surface selectivity in adsorption hysteresis. Langmuir. 2003;19(7):2714–2721. [Google Scholar]
- 155.Tanvir S, Qiao L. Surface tension of Nanofluid-type fuels containing suspended nanomaterials. Nanoscale Res Lett. 2012;7(1):226. doi: 10.1186/1556-276X-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jackson KA. Kinetic Processes: Crystal Growth, Diffusion, and phase transformations in materials. Hoboken: Wiley; 2006. [Google Scholar]
- 157.Wong EM, Bonevich JE, Searson PC. Growth kinetics of nanocrystalline ZnO particles from colloidal suspensions. J Phys Chem B. 1998;102(40):7770–7775. [Google Scholar]
- 158.Oskam G, Nellore A, Penn RL, Searson PC. The growth kinetics of TiO2 nanoparticles from titanium(IV) alkoxide at high water/titanium ratio. J Phys Chem B. 2003;107(8):1734–1738. [Google Scholar]
- 159.Dalvi SV, Dave RN. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind Eng Chem Res. 2009;48(16):7581–7593. [Google Scholar]
- 160.Monnier H, Wilhelm AM, Delmas H. Influence of ultrasound on mixing on the molecular scale for water and viscous liquids. Ultrason Sonochem. 1999;6(1–2):67–74. doi: 10.1016/s1350-4177(98)00034-0. [DOI] [PubMed] [Google Scholar]
- 161.O'Connor SM, Gehrke SH. Synthesis and characterization of thermally-responsive hydroxypropyl methylcellulose gel beads. J Appl Polym Sci. 1997;66(7):1279–1290. [Google Scholar]
- 162.Zhang ZP, Tan SW, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 163.Shanbhag A, Rabel S, Nauka E, Casadevall G, Shivanand P, Eichenbaum G, et al. Method for screening of solid dispersion formulations of low-solubility compounds - miniaturization and automation of solvent casting and dissolution testing. Int J Pharm. 2008;351(1–2):209–218. doi: 10.1016/j.ijpharm.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 164.McClements DJ. Protein-stabilized emulsions. Curr Opin Colloid Interface Sci. 2004;9(5):305–313. [Google Scholar]
- 165.Jeyachandran YL, Mielczarski E, Rai B, Mielczarski JA. Quantitative and qualitative evaluation of adsorption/desorption of bovine serum albumin on hydrophilic and hydrophobic surfaces. Langmuir. 2009;25(19):11614–11620. doi: 10.1021/la901453a. [DOI] [PubMed] [Google Scholar]
- 166.Seo JH, Dembereldorj U, Park J, Kim M, Kim S, Joo SW. Facile internalization of paclitaxel on titania nanoparticles in human lung carcinoma cells after adsorption of serum proteins. J Nanopart Res. 2012;14(10):1–8. [Google Scholar]
- 167.Niwa T, Danjo K. Design of self-dispersible dry nanosuspension through wet milling and spray freeze-drying for poorly water-soluble drugs. Eur J Pharm Sci. 2013;50(3–4):272–281. doi: 10.1016/j.ejps.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 168.Pongpeerapat A, Wanawongthai C, Tozuka Y, Moribe K, Yamamoto K. Formation mechanism of colloidal nanoparticles obtained from probucol/PVP/SDS ternary ground mixture. Int J Pharm. 2008;352(1–2):309–316. doi: 10.1016/j.ijpharm.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 169.Mou D, Chen H, Wan J, Xu H, Yang X. Potent dried drug nanosuspensions for oral bioavailability enhancement of poorly soluble drugs with pH-dependent solubility. Int J Pharm. 2011;413(1–2):237–244. doi: 10.1016/j.ijpharm.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 170.Gao L, Liu G, Ma J, Wang X, Wang F, Wang H, et al. Paclitaxel nanosuspension coated with P-gp inhibitory surfactants: II. Ability to reverse the drug-resistance of H460 human lung cancer cells. Colloids Surf B: Biointerfaces. 2014;117:122–127. doi: 10.1016/j.colsurfb.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 171.Rowe RC, Sheskey PJ, Weller PJ. Handbook of pharmaceutical excipients. London: Pharmaceutical press; 2006. [Google Scholar]
- 172.Rajebahadur M, Zia H, Nues A, Lee C. Mechanistic study of solubility enhancement of nifedipine using vitamin E TPGS or solutol HS-15. Drug Deliv. 2006;13(3):201–206. doi: 10.1080/10717540500316094. [DOI] [PubMed] [Google Scholar]
- 173.Delongeas JL, de Conchard GV, Beamonte A, Bertheux H, Spire C, Maisonneuve C, et al. Assessment of Labrasol (R)/Labrafil (R)/Transcutol (R) (4/4/2, v/v/v) as a non-clinical vehicle for poorly water-soluble compounds after 4-week oral toxicity study in Wistar rats. Regul Toxicol Pharmacol. 2010;57(2–3):284–290. doi: 10.1016/j.yrtph.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 174.Adamson AW, Gast AP. Physical chemistry of surfaces. 1967.
- 175.Ghosh I, Schenck D, Bose S, Ruegger C. Optimization of formulation and process parameters for the production of nanosuspension by wet media milling technique: effect of vitamin E TPGS and nanocrystal particle size on oral absorption. Eur J Pharm Sci. 2012;47(4):718–728. doi: 10.1016/j.ejps.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 176.Dalvi SV, Dave RN. Analysis of nucleation kinetics of poorly water-soluble drugs in presence of ultrasound and hydroxypropyl methyl cellulose during antisolvent precipitation. Int J Pharm. 2010;387(1–2):172–179. doi: 10.1016/j.ijpharm.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 177.Obeidat WM, Sallam A-SA. Evaluation of tadalafil nanosuspensions and their PEG solid dispersion matrices for enhancing its dissolution properties. AAPS PharmSciTech. 2014;15(2):364–374. doi: 10.1208/s12249-013-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Romero-Cano MS, Martin-Rodriguez A, de las Nieves FJ. Electrosteric stabilization of polymer colloids with different functionality. Langmuir. 2001;17(11):3505–11. [Google Scholar]
- 179.Don TM, Hsu SC, Chiu WY. Structures and thermal properties of chitosan-modified poly (methyl methacrylate) J Polym Sci A Polym Chem. 2001;39(10):1646–1655. [Google Scholar]
- 180.Niwa T, Miura S, Danjo K. Universal wet-milling technique to prepare oral nanosuspension focused on discovery and preclinical animal studies - development of particle design method. Int J Pharm. 2011;405(1–2):218–227. doi: 10.1016/j.ijpharm.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 181.Detroja C, Chavhan S, Sawant K. Enhanced antihypertensive activity of candesartan cilexetil nanosuspension: formulation, characterization and pharmacodynamic study. Sci Pharm. 2011;79(3):635–651. doi: 10.3797/scipharm.1103-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen XX, Young TJ, Sarkari M, Williams RO, Johnston KP. Preparation of cyclosporine A nanoparticles by evaporative precipitation into aqueous solution. Int J Pharm. 2002;242(1–2):3–14. doi: 10.1016/s0378-5173(02)00147-3. [DOI] [PubMed] [Google Scholar]
- 183.Sarkari M, Brown J, Chen XX, Swinnea S, Williams RO, Johnston KP. Enhanced drug dissolution using evaporative precipitation into aqueous solution. Int J Pharm. 2002;243(1–2):17–31. doi: 10.1016/s0378-5173(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 184.Abdelwahed W, Degobert G, Fessi H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur J Pharm Biopharm. 2006;63(2):87–94. doi: 10.1016/j.ejpb.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 185.Elham G, Mahsa P, Vatanara A, Vahid R. Spray drying of nanoparticles to form fast dissolving glipizide. Asian Journal of Pharmaceutics. 2015;9(3):213–218. [Google Scholar]
- 186.Chasteigner Sd CG, Fessi H, Devissaguet J-P, Puisieux F. Freeze-drying of itraconazole-loaded nanosphere suspensions: a feasibility study. Drug Dev Res. 1996;38(2):116–124. [Google Scholar]