Abstract
Introduction
Isolated dissection of the superior mesenteric artery (SMA) is rare and remains the most common reason for aneurysmal degeneration of the vessel. The treatment is challenging and not standardised. The purpose of this report is to demonstrate that coiling of the false lumen is a good alternative for dissecting SMA aneurysms.
Report
A 50 year old male presented with a 3.3 cm dissecting aneurysm of the SMA and epigastric pain of moderate severity. More than 50% of the ileal arteries arose from the collapsed true lumen. Via transfemoral access the true lumen was catheterised. An open cell balloon expandable stent was deployed at the proximal and a closed cell self expandable stent at the distal end of the dissection flap. Through the cells of the first stent a microcatheter was advanced into the false lumen and 33 coils were deployed into the aneurysm sac. A stent graft was deployed within the first stent leading to the total exclusion of the aneurysm. Follow up at three months was uneventful and the patency was assessed by contrast enhanced ultrasound.
Discussion
Coiling of the false aneurysm is a good alternative for dissecting SMA aneurysms, where no other open surgical or endovascular options are applicable.
Keywords: Coiling, Dissecting aneurysm, Dissection, Endovascular, Mesentery artery
Highlights
-
•
Spontaneous isolated SMA dissection is the most frequent type of visceral artery dissection.
-
•
Spontaneous isolated SMA dissection is the most frequent cause of SMA dissecting aneurysms.
-
•
Treatment of SMA dissecting aneurysms remains controversial.
-
•
Coiling of the false lumen and stenting of the true lumen is an effective method of treatment.
Introduction
Spontaneous isolated superior mesenteric artery (SMA) dissection represents the most frequent type of visceral artery dissection with a mostly asymptomatic course. As a result, dissection remains the most common cause of SMA aneurysm.1 Although the current literature reports an increasing incidence of isolated SMA dissection,1, 2 a post-dissection SMA aneurysm remains rather a rare finding. It is related to significant morbidity and mortality rates and the treatment can be challenging.
Herein, a case is presented of treatment by stenting of the true lumen and coiling of the false lumen. This is one of a few reported cases of post-dissection SMA aneurysm treated with a similarly complex technique.
Case report
A 50 year old male presented with a chronic epigastric pain of moderate severity for the previous two months. No other symptoms were present. Physical examination was unremarkable. Past medical history consisted of arterial hypertension for many years and the patient was on antihypertensive medication. In the surgical history, the patient reported a cholecystectomy three years before.
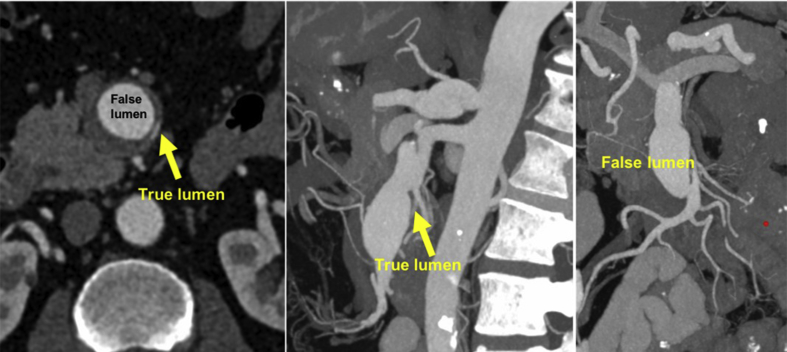
A computed tomography angiogram (CTA) revealed a solitary and complex 3.3 cm dissecting aneurysm of the SMA (Fig. 1) and post-dissection mild dilatation (1.8 cm) of the coeliac trunk. The dissecting aneurysm was classified as type V according to Li's classification or type I according to Yun's.2 The true lumen was collapsed. The median colic artery and the first four ileal arteries arose from the true lumen along the aneurysm sac. No signs of bowel ischaemia were present. The very proximal part of the SMA was healthy without dissection with a maximum diameter of 9 mm and the distal dissection free segment of the vessel revealed a diameter of 7.6 mm. Complete blood count and basic biochemistry were within normal values.
Figure 1.
Post-dissection aneurysm of the superior mesenteric artery with collapse of the true lumen and involvement of the ileal arteries in the aneurysm sac.
Technique
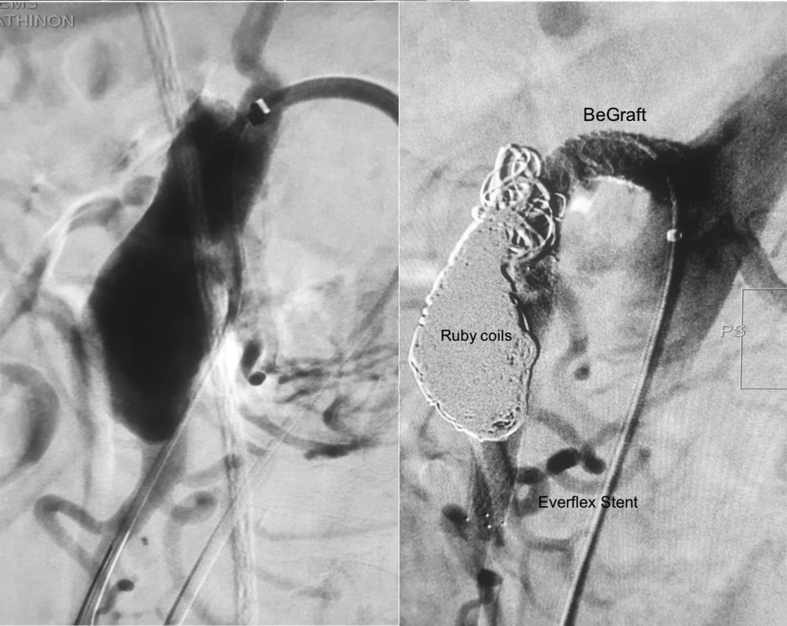
A 5F sheath was introduced through a femoral access. After an exchange of the starting wire with a 0.035″ stiff Terumo wire, the sheath was replaced with a 6F 55 cm catheter (Cook Medical Inc., Bloomington, IN, USA). The true lumen of the SMA was successfully catheterised using a Berenstein catheter (Merit Medical OEM, South Jordan, UT, USA) and a 0.014″ Choice PT floppy guidewire (Boston Scientific Corp., Malborough, MA, USA). The catheter was advanced into the true lumen over the 0.014″ guidewire. The guidewire was removed and after confirmation that the catheter was within the true lumen, a Rosen wire (Cook Medical, USA) was advanced into the distal main trunk of the SMA. A balloon expandable 8 × 57 mm Dynamic stent (Biotronik GmbH & Co, Berlin, Germany) was delivered expanding the true lumen. A self expandable 8 × 60 mm Everflex stent (Medtronic Inc., Minneapolis, MN, USA) was deployed further into the true lumen, expanding the latter along the whole length of the dissection to healthy segments of the SMA. The angiogram confirmed that the true lumen and the ileal branches remained patent. A 5F microcatheter (Merit Medical, USA) was inserted through the cells of the Dynamic stent and the proximal tear of the dissection into the aneurysm false lumen. Thirty-three Ruby coils (Penumbra Inc., Alameda, CA, USA) of various diameters were deployed into the aneurysm sac fully excluding the aneurysm from the blood flow. As the tips of some coils protruded through the proximal tear into the cells of the stent, an 8 × 57 mm covered stent, BeGraft (Bentley Innomed GmbH, Hechingen, Germany) was deployed from the orifice of the SMA to the orifice of the first ileal branch. Proximal flaring of the covered stent was performed using a 10 × 40 mm balloon in order to adapt to the diameter of the native vessel. Final angiography confirmed the technical success of the procedure (Fig. 2). The access point was sealed with an 8F Angio-Seal device (Terumo Medical Corp., Somerset, NJ, USA).
Figure 2.
Aneurysm exclusion with coiling of the false lumen and recanalisation of the true lumen with a proximal stent graft and a distal bare metal stent.
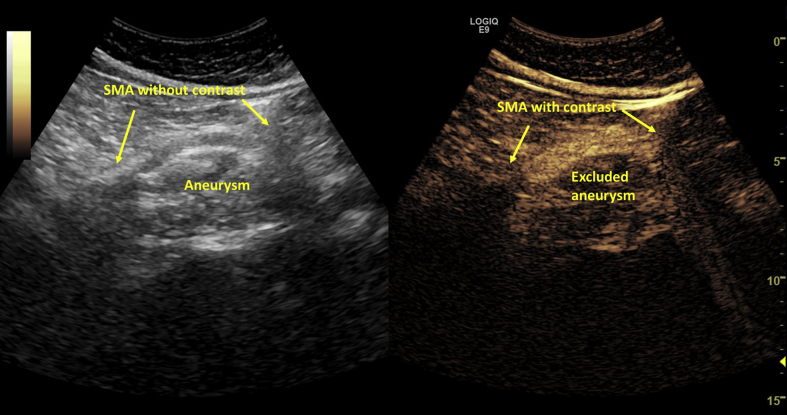
The patient recovered fully from the general anaesthesia and the post-operative course was uneventful. The patient was discharged on the second post-operative day and a contrast enhanced ultrasound scan (SonoVue, Bracco Imaging, Amsterdam, The Netherlands) confirmed the successful exclusion of the aneurysm at discharge and at three month follow up (Fig. 3). The patient has remained free of symptoms since the procedure and CTA is planned at six months.
Figure 3.
Assessment of stent patency and the exclusion of the aneurysm sac with contrast enhanced ultrasound. SMA = superior mesenteric artery.
Discussion
Spontaneous isolated SMA dissection represents 8% of all visceral artery dissections and has an overall prevalence of 0.09% in cadaveric studies.3 Isolated SMA dissection is the most common cause of SMA dissecting aneurysm, although other causes exist.4, 5, 6, 7 Despite the increase in the reported incidence of SMA dissecting aneurysms because of the widespread application of CTA, these are still considered rather rare findings.
The dissection process can occupy various lengths of the SMA. Untreated SMA dissection is related to high morbidity and mortality, caused by progressive ischaemia of the bowel or aneurysm rupture.8, 9 Some authors suggest that medical treatment is acceptable and report low morbidity and mortality rates, but their conclusions are often based on a limited number of cases or smaller dissecting aneurysms.1 Asymptomatic post-dissection SMA aneurysms are rarely described in the literature. Clinical manifestations vary widely depending on the size of the dissecting aneurysm, the degree of the compromise of the true lumen, and on the number of collateral arteries.10 In the present case the dissection involved a good length of the main trunk of the SMA and the orifices of the first five SMA branches. The patient reported epigastric pain; an expected symptom, given the significant size of the aneurysm. Laboratory tests did not demonstrate elevated serum amylase or other pathological findings.
Open repair of this SMA dissecting aneurysm would have been technically feasible, but it would also be related to significant technical difficulties and post-operative morbidity. The main challenge consists of the complete peri-pancreatic replacement of the aneurysm with inclusion of all ileal branches and the median colic artery on the graft. The alternative option of ligation and bypassing would lead to bowel ischaemia due to the ileal branches arising directly from the affected SMA segment. On the other hand, the endovascular approach that the team preferred led to the complete and safe exclusion of the aneurysmal sac, expansion of the true lumen, and the preservation of blood flow. A recent publication by Jia et al.11 concluded that results of using either covered or bare stents are similar. In the current case, the use of a covered stent for the whole length was not feasible due to the coverage of relevant SMA branches. Only one covered stent was used at the entry point in order to minimise the risk of the protruding coil edges resulting in migration and distal embolisation. Furthermore, Jia's finding might not be applicable to the current case as the cases did not appear to have a re-entry point and there is no mention of the type and branching of those cases.
A potential alternative endovascular approach could also be the implantation of flow diverter but the proximal and distal diameter of the vessel made this approach not applicable.
Based on this case, coiling of a post-dissection SMA aneurysm is both technically feasible and of benefit to the patient, although meticulous pre-operative planning and extensive knowledge of the characteristics of the endovascular materials involved are necessary. In that case, the exclusion of the aneurysm was assessed with contrast enhanced ultrasound sonography, which avoids any artefacts of the coils on patency checks compared with CTA.
Conclusion
Although further study is necessary, coiling of the false aneurysm using a microcatheter and stenting of the true lumen appears to be a safe and effective treatment option for dissecting SMA aneurysms where no other open surgical or endovascular options are applicable.
Conflict of interest
None.
Funding
None.
References
- 1.Wang J., He Y., Zhao J., Yuan D., Xu H., Ma Y. Systematic review and meta-analysis of current evidence in spontaneous isolated celiac and superior mesenteric artery dissection. J Vasc Surg. 2018;68 doi: 10.1016/j.jvs.2018.05.014. 1228-1240 e1229. [DOI] [PubMed] [Google Scholar]
- 2.Li D.L., He Y.Y., Alkalei A.M., Chen X.D., Jin W., Li M. Management strategy for spontaneous isolated dissection of the superior mesenteric artery based on morphologic classification. J Vasc Surg. 2014;59:165–172. doi: 10.1016/j.jvs.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Karaolanis G., Antonopoulos C., Tsilimigras D.I., Moris D., Moulakakis K. Spontaneous isolated superior mesenteric artery dissection. Systematic review and meta-analysis. Vascular. 2019;27:324–337. doi: 10.1177/1708538118818625. [DOI] [PubMed] [Google Scholar]
- 4.Garrett H.E., Jr. Options for treatment of spontaneous mesenteric artery dissection. J Vasc Surg. 2014;59 doi: 10.1016/j.jvs.2014.01.040. 1433-1439 e1431-1432. [DOI] [PubMed] [Google Scholar]
- 5.Park Y.J., Park K.B., Kim D.I., Do Y.S., Kim D.K., Kim Y.W. Natural history of spontaneous isolated superior mesenteric artery dissection derived from follow-up after conservative treatment. J Vasc Surg. 2011;54:1727–1733. doi: 10.1016/j.jvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Cormier F., Ferry J., Artru B., Wechsler B., Cormier J.M. Dissecting aneurysms of the main trunk of the superior mesenteric artery. J Vasc Surg. 1992;15:424–430. [PubMed] [Google Scholar]
- 7.Javerliat I., Becquemin J.P., d'Audiffret A. Spontaneous isolated dissection of the superior mesenteric artery. Eur J Vasc Endovasc Surg. 2003;25:180–184. doi: 10.1053/ejvs.2002.1785. [DOI] [PubMed] [Google Scholar]
- 8.Sidawy A.N., Perler B.A. 9th ed. Elsevier; Philadelphia, PA: 2019. Rutherford's vascular surgery and endovascular therapy. [Google Scholar]
- 9.Jeong M.J., Kwon H., Kim A., Ko G.Y., Han Y., Kwon T.W. Clinical outcomes of conservative treatment in patients with symptomatic isolated spontaneous renal artery dissection and comparison with superior mesenteric artery dissection. Eur J Vasc Endovasc Surg. 2018;56:291–297. doi: 10.1016/j.ejvs.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y., Yoshimuta T., Kimura K., Iino K., Tamura Y., Sakata K. Clinical characteristics of spontaneous isolated visceral artery dissection. J Vasc Surg. 2018;67:1127–1133. doi: 10.1016/j.jvs.2017.08.054. [DOI] [PubMed] [Google Scholar]
- 11.Jia Z., Su H., Chen W., Ni G., Qi C., Gu J. Endovascular treatment of patients with isolated mesenteric artery dissection aneurysm: bare stents alone versus stent assisted coiling. Eur J Vasc Endovasc Surg. 2019;57:400–406. doi: 10.1016/j.ejvs.2018.08.057. [DOI] [PubMed] [Google Scholar]