Summary
The site-specific incorporation of deuterium (D) into small molecules is frequently used to access isotopically labeled compounds with broad utility in many research areas, such as drug development, mechanistic studies, and NMR analyses. Nevertheless, the deuteration of a stereocenter in an enantioselective manner, which could slow the metabolism and improve the bioavailability of bioactive molecules, remains challenging owing to the lack of established catalytic methods. Here, we report an asymmetric α-deuteration strategy for azaarenes with inexpensive D2O as the deuterium source. A cooperative visible light-driven photoredox and chiral Brønsted acid–catalyzed system using a Hantzsch ester as the terminal reductant has been developed, which enables racemic α-chloro-azaarenes and prochiral azaarene-substituted ketones to experience a single-electron reduction–enantioselective deuteration process. The transition metal-free method provides important chiral α-deuterated azaarenes in satisfactory yields with good to excellent enantioselectivities (up to 99% ee) and substantial deuterium incorporation.
Subject Areas: Catalysis, Organic Chemistry, Chemical Synthesis
Graphical Abstract

Highlights
-
•
Enantioselective deuteration enabled by photoredox asymmetric catalysis
-
•
D2O as the deuterium source
-
•
Azaarenes with a deuterated stereocenter
-
•
Transition-metal-free catalyst system
Catalysis; Organic Chemistry; Chemical Synthesis
Introduction
Deuterium (2H, D), an economical nonradioactive isotope, has been widely used in drug development, mechanistic studies, and NMR analyses (Elmore, 2009, Elmore and Bragg, 2015, Hall and Hanzlik, 1990, Nelson and Trager, 2003, MacDonald and Lu, 2002, Meanwell, 2011). A number of efficient deuterium-labeling techniques have been established through the increasing attention and contributions from various scientists (Alonso et al., 2002, Atzrodt et al., 2007, Loh et al., 2017, Zhang et al., 2019). The enantioselective incorporation of a deuterium atom on a stereocenter of a bioactive molecule could potentially slow its metabolism and improve its bioavailability (Maltais et al., 2009), but such processes remain underdeveloped (Hammadi et al., 1997, Lethu et al., 2018, Curran and Abraham, 1993, Curran and Ramamoorthy, 1993, Mohrig et al., 2011, Taglang et al., 2015, Hale and Szymczak, 2016, Palmer and Chirik, 2017, Zhao et al., 2012, Sakamoto et al., 2012, Sandoval et al., 2017). To date, few catalytic manifolds have been developed to accomplish this task (Taglang et al., 2015, Hale and Szymczak, 2016, Palmer and Chirik, 2017, Zhao et al., 2012, Sakamoto et al., 2012, Sandoval et al., 2017), and the existing systems of using the readily accessible racemic or prochiral feedstocks require prefabricated and expensive deuterium sources to attain excellent enantiofacial selectivity (Sakamoto et al., 2012, Sandoval et al., 2017). Azaarenes are ubiquitous in natural products and pharmaceuticals; however, to date, construction of their deuterated variants in enantioselective manner has not been reported.
Delivering a deuterium ion from inexpensive D2O to a prochiral tertiary carbanion in a chiral environment would be a promising strategy. Currently, H–D exchange reactions are among the main protocols for the preparation of deuterium-labeled compounds to meet the high demands (Atzrodt et al., 2007). The energy difference between de-protonation and de-deuteration that results in accumulation of deuterated species is subtle; therefore, achieving satisfactory enantioselectivity will be difficult using this strategy (Zhao et al., 2012). Recently, we reported the asymmetric construction of α-tertiary carbon stereocenters of electron-deficient azaarenes by developing a photoredox radical conjugate addition–enantioselective protonation reaction from vinylazaarenes (Yin et al., 2018). This study provided an important indication that single-electron reduction of the α-radical species is an irreversible strategy to generate α-carbanions of azaarenes. In photoredox catalysis (Yin et al., 2018, Prier et al., 2013, Shaw et al., 2016), the reductive dehalogenation of alkyl halides through a single-electron transfer (SET) fragmentation process enables the traceless formation of alkyl radicals (Hironaka et al., 1984, Staveness et al., 2016, Fukuzumi et al., 1990, Narayanam et al., 2009, Neumann et al., 2011, Tahara and Hisaeda, 2011, Maji et al., 2010, Li et al., 2018). Following our discovery, we explored the possibility of using this powerful strategy to generate α-radicals of azaarenes from α-halo-azaarenes. Notably, the ability of the subsequent process to cause protonation-like deuteration with D2O (Maji et al., 2010, Fan et al., 2018, Ju et al., 2018, Liao et al., 2018) but not hydrogen atom transfer (HAT) (Hironaka et al., 1984, Narayanam et al., 2009, Neumann et al., 2011) is crucial in the production of deuterated stereocenters. Meanwhile, to achieve high D incorporation, excess D2O might be utilized to compete with the H+ from the sacrificial reductant and reaction environment, but this excess reagent would pose a formidable challenge for chiral Brønsted acid catalysis, a promising platform for derivatizing azaarene-based substrates (Yin et al., 2018, Hepburn and Melchiorre, 2016, Proctor et al., 2018, Xu et al., 2018).
Herein, we demonstrate proof of this concept and report the first enantioselective reductive dechlorination–deuteration of α-chloro-azaarenes under the visible-light-driven cooperative photoredox asymmetric catalysis (Brimioulle et al., 2015, Skubi et al., 2016, Rono et al., 2013) of a dicyanopyrazine-derived chromophore (DPZ) as a photosensitizer and a 1,1′-spirobiindane-7,7′-diol (SPINOL)-based chiral phosphoric acid (CPA) with D2O as the deuterium source (Figure 1). A variety of valuable chiral α-deuterated azaarenes featuring various substituents on the stereocenters were obtained in high yields, with excellent D incorporation and with good to excellent enantioselectivities. More importantly, this catalytic system is very versatile showing compatibility with asymmetric reduction–deuteration of azaarene-substituted ketones, leading to important α-deuterated azaarene-based secondary alcohols with satisfactory results.
Figure 1.
Outline of This Work
Results and Discussion
Reaction Optimization
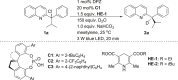
Recognizing the paucity in viable catalytic methods to access the enantioenriched derivatives of α-aryl-α-branched alkyl-substituted quinolines, which possess significant bioactive potential (Kaila et al., 2008, Huang et al., 2010), we began our investigation using 2-(1-chloro-2-methyl-1-phenylpropyl)quinoline (1a) as the model substrate. The reduction potential of 1a (Ered 1/2 = − 0.639 V vs. SCE in CH3CN) indicated that our developed metal-free DPZ (Ered 1/2 = −1.07 V vs SCE in CH3CN and −1.45 V vs SCE in CH2Cl2) will be able to reduce it through a reductive quenching cycle. Hence, we performed the initial study using 0.5 mol% DPZ, 1.5 equiv. of Hantzsch ester (HE) HE-1 as the terminal reductant and 10 equiv. of D2O as the deuterium source. Desired α-deuterated quinoline 2a was obtained in a 58% yield within 30 min (see entry 1, Table S1 in the Supplemental Information). The moderate D incorporation (53%) confirmed that the developed transformation might include a dechlorination-deuteration process. Subsequently, we evaluated a range of chiral H-bonding catalysts, reductants, and reaction parameters (see Tables S1, S2 and S3). The reaction performed in mesitylene at 25°C for 20 min in the presence of 1.0 mol% DPZ, 20 mol% SPINOL-CPA C1, 1.5 equiv. of HE-1, 1.0 equiv. of NaHCO3, and 150 equiv. of D2O afforded enantioenriched product 2a in a 75% yield with 93% ee and >95% D incorporation (entry 1, Table 1). Catalysts C2 and C3 provided 2a in 22% ee and 28% ee, respectively (entries 2–3), indicating that the substituents at the 6,6′-positions of SPINOL had a substantial influence on the enantioselectivity of the transformation. HE-2, with a tert-butyl ester, provided a slightly lower enantioselectivity (entry 4). When the reductant was changed from an HE to a tertiary amine, i.e., iPr2EtN, the reaction became sluggish, leading to 2a in 25% yield with 39% ee (entry 5). Other photoredox catalysts such as [Ir(ppy)2(dtbpy)]PF6 and 1,3-dicyano-2,4,5,6-tetrakis(N,N-diphenylamino)benzene (4DPAIPN) (Luo and Zhang, 2016) were tested, but no improvements in the yield and enantioselectivity were observed (entries 6–7). Using a stronger inorganic base, Na2CO3 instead of NaHCO3, as the acid-binding agent also deteriorated the reaction outcome (entry 8). Of note, the reaction conducted in the absence of catalyst C1 generated 2a in a similar yield and D incorporation, suggesting that a considerable competitive racemic background transformation is active (entry 9). The subsequent control experiments confirmed that DPZ, visible light, and the oxygen-free environment are indispensable to this deuteration reaction (entries 10–12).
Table 1.
Optimization of the Reaction Conditions
 | ||||
|---|---|---|---|---|
| Entry | Variation from Standard Conditions | Yield (%)a | ee (%)b | D Incorp. (%)c |
| 1 | None | 75 | 93 | >95 |
| 2 | C2 instead of C1 | 52 | 22 | >95 |
| 3 | C3 instead of C1 | 69 | 28 | >95 |
| 4 | HE-2 instead of HE-1 | 76 | 91 | 93 |
| 5 | iPr2EtN instead of HE-1 | 25 | 39 | 86 |
| 6 | Ir(III)d instead of DPZ | 52 | 76 | 88 |
| 7 | 4DPAIPN instead of DPZ | 43 | 73 | 90 |
| 8 | Na2CO3 instead of NaHCO3 | 51 | 61 | 89 |
| 9 | No C1 | 73 | NA | >95 |
| 10 | No DPZ | 0 | NA | NA |
| 11 | No light | 0 | NA | NA |
| 12 | Under air | 0 | NA | NA |
NA, not available; 4DPAIPN, 1,3-dicyano-2,4,5,6-tetrakis(N,N-diphenylamino)benzene.
The reaction was performed on a 0.05 mmol scale. The wavelength of the 3 W blue LED was 410–510 nm.
Yield of isolated product.
Determined by HPLC analysis on a chiral stationary phase.
Determined by 1H NMR spectroscopy.
Ir(III) = [Ir(ppy)2(dtbpy)]PF6.
Substrate Scope with Respect to α-Chloro Azaarenes
With the optimum reaction conditions in hand, the scope of this asymmetric α-deuteration of azaarenes was examined (Figure 2). A wide range of α-chloro-2-quinolines containing both aryl and alkyl groups on the sp3−C were evaluated. The transformations proceeded rapidly and smoothly, furnishing chiral products 2a-cc in 58%–89% yields with 80%–95% ee and high levels of D incorporation within 20–40 min. The introduction of distinct electron-withdrawing or electron-donating substituents at the para- and meta-positions (2b-f and 2i-l) of the α-aryl ring did not affect the excellent enantioselectivity. Methyl group at the ortho-position (2g-h) decreased the enantioselectivity slightly to 90% ee probably owing to steric hindrance. A similar steric effect was observed when bulkier fused aromatic rings, such as 1-naphthyl (2m), 2-naphthyl (2n), and 9-phenanthryl (2o), were in the α-position of the 2-quinolines. The catalytic system was also compatible with α-chloro-2-quinolines with diverse linear (2p-t), bulky branched (2u), and cyclic (2v-y) α-alkyl groups, based on the good to excellent enantioselectivities obtained with these substrates. With respect to the quinoline moiety, the installation of substituents on its 4-, 5-, and 6-positions resulted in the corresponding products 2z-cc with excellent enantioselectivities. Other such α-chloro-azaarenes were also tested. Chiral α-deuterated 1-isoquinoline (2dd), 6-phenanthridine (2ee), and 2-benzothiazole (2ff) were obtained in 71%–81% yields with 80%–83% ee and excellent D incorporation, which highlights the generality of this catalysis platform. As an exception, when the azaarene was 2-pyridine, the corresponding product 2gg was obtained with only moderate enantioselectivity. Noticeably, 4DPAIPN was used as the photoredox catalyst to furnish 2ee, 2ff, and 2gg as DPZ could not promote the transformation. To define the substrate scope, the phenyl group of 1a was changed as benzyl group; however, product 2hh was achieved in 65% yield with >95% D incorporation but only 38% ee, which thus still represents a challenging task. The reaction to access 2a on a 5.0 mmol scale gave a similar yield, enantioselectivity, and D incorporation (footnote a), suggesting the potential scalability of this method.
Figure 2.
Enantioselective Dehalogenative Deuteration (0.1 mmol scale)
Mechanistic Studies
To probe the mechanism, compound 3, i.e., non-deuterated racemic 2a, was evaluated under the standard reaction conditions (Figure 3A). After 1 h, the ee value of 3 remained 0% and no deuterated product 2a was observed. Similarly, chiral 2a with 93% ee was examined using 20 mol% diphenyl phosphate C21 instead of C1, and no changes in the enantiomeric purity or D incorporation were found. Consequently, the effects of H–D exchange for product 2 on the enantioselectivity and D incorporation could be excluded.
Figure 3.
Control Experiments and the Proposed Mechanism
(A) Control experiments for the H–D exchange of reduction product and the conversion of 1a with an excitation wavelength from 474 to 505 nm.
(B) The possible mechanism for the photoredox-catalyzed enantioselective α-deuteration of Azaarenes.
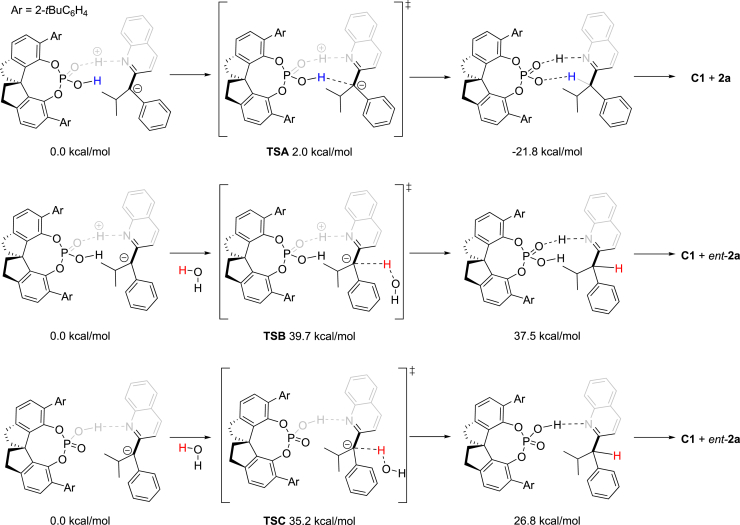
On the account of the excitation spectra of DPZ and HE-1, Stern-Volmer experiments were first conducted using an excitation wavelength of 415 nm (see Supplemental Information). Luminescence quenching of *DPZ (Et(S*/S⋅−) = +1.42 V vs. SCE in CH3CN and +0.91 V vs. SCE in CH2Cl2) by HE-1 (Ered 1/2 = +0.97 V vs. SCE in CH3CN) and *HE-1 (Et(S*/S⋅−) ≈ –2.28 V vs. SCE in CH3CN) (Jung et al., 2016) by 1a (Ered 1/2 = –0.639 V vs. SCE in CH3CN) was observed. Meanwhile, no measurement of *DPZ by 1a in the absence and presence of C1 was revealed. Since the excitation of HE-1 to *HE-1 is impossible at λ > 445 nm according to its excitation spectrum, such experiments were performed with an excitation wavelength of 448 nm, indicating no measurable luminescence quenching of *DPZ by HE-1 or 1a and *HE-1 by 1a. We also tested the transformation of 1a with a laser line filter (CWL = 488 ± 2 nm, FWHM = 10 ± 2 nm, emission wavelengths from 474 to 505 nm, Figure 3A), and no reaction was found. The results suggest the indispensable role of *HE-1 in the reaction. Therefore, the catalytic cycle likely begins from the SET of *DPZ with trace HE-1⋅ (Ered 1/2 ≈ –0.76 V vs. SCE), which is generated from *HE-1 and α-chloro-azaarenes 1 under irradiation with visible light (Figure 3B) (Cao et al., 2019). The success of 4DPAIPN (Ered 1/2 = –1.52 V vs. SCE in CH3CN) (Luo and Zhang, 2016) but failure of DPZ in the transformations of 6-phenanthridine-derived (1ee, Ered 1/2 = –0.963 V vs. SCE in CH3CN) and 2-benzothiazole-derived (1ff, Ered 1/2 = –1.227 V vs. SCE in CH3CN) substrates demonstrates that DPZ⋅− should engage in the SET reduction of 1 to furnish radical species I. It is also noteworthy that no transformation of 1a was observed in the absence of DPZ (entry 10, Table 1). Accordingly, a HAT (Lee et al., 2017) or a reduction–protonation/deuteration process between HE-1⋅ and radical I seems unfavorable. To provide HE-1⋅ to propagate the photocatalytic cycle, the reduction of I by *HE-1 with a stronger reductive ability than HE-1⋅ thus represents the most likely approach. Owing to the free proton from catalyst C1 and high concentration of D2O, deuterated C1, i.e., D-C1, would serve as a more stable hydrogen-bond donor to interact with the nitrogen atom in the azaarene, providing the enantiocontrolling environment for the deuteration of prochiral carbanion intermediate II to give chiral products 2 in high enantioselectivities. With respect to the enantioselective deuteration process, the intermediate II could directly abstract deuterium ion from the reaction system through the pathway a. However, the ability of P=O of CPA as Lewis base to grab a deuterium also leads to an alternative pathway b to deliver deuterium ion. To better understand the mechanism, DFT calculations were carried out (for computational details see Supplemental Information), and the calculated ΔG values are shown in Figure 4. The data suggest that the formation of (R)-2a through TSA only requires 2.0 kcal/mol, but affording (S)-2a through the proton from H2O (D2O) attacked by the α-anion of quinoline has to overcome high energy barriers (39.7 and 35.2 kcal/mol). This result is consistent with the experimental results. In this context, although chiral catalyst CPA cannot accelerate the transformations, it will act as a deuterium transfer interchange to enable the elusive enantioselective manifold with high enantioselectivity.
Figure 4.
Calculated ΔG Values of Possible Pathways for the Formation of 2a or ent-2a
Extension of the Method to Azaarene-Based Ketones
Azaarene-substituted secondary alcohols, as another kind of α-tertiary carbon-based azaarene derivative, are also important building blocks of numerous pharmaceuticals and biologically active compounds (Rennison et al., 2007). The chiral transition metal-catalyzed asymmetric transfer hydrogenation of aryl N-heteroaryl ketones with sodium formate as a hydrogen source has been developed to access the chiral variants (Liu et al., 2018). However, this strategy is only suitable for aryl N-heteroaryl ketones and is likely not compatible to introduce deuterium on the stereocenters with D2O as the deuterium reagent. Given that azaarene-substituted ketones are readily accessible and abundant, we anticipated evaluating the possibility of this double single-electron reduction–enantioselective deuteration method for these feedstocks, to directly provide the enantioenriched α-deuterated azaarene-substituted secondary alcohols. Literature investigation showed that azaarene rings are more liable to reduction than carbonyls in azaarene-substituted ketone derivatives in the presence of CPA catalyst and HE reductant (Rueping and Antonchick, 2007). Thus, the chemoselectivity with enantioselectivity and D incorporation would constitute formidable challenges in the desired transformations. Phenyl(quinolin-2-yl)methanone (4a, Ep1 = –0.905 V, Ep2 = –1.434 V vs. SCE in CH3CN) was first selected to be examined under the standard reaction conditions (see Table 1). Although the desired product 5a was obtained with moderate enantioselectivity (61% ee), the high yield (80%) and moderate D incorporation (72%) prompted us to explore a series of chiral CPAs and the reaction parameters (see Table S4). To our delight, when 0.5 mol% DPZ, 10 mol% CPA C2, 1.5 equiv. of HE-1, 200 equiv. of D2O, 0.2 equiv. of NaCl as an additive, and CMPE as the solvent were used, at −5°C, 5a was achieved in 96% yield with 91% ee and 91% D incorporation (Figure 5). A series of 2-quinoline-substituted ketones were next examined, leading to chiral products 5b-m in 65%–99% yields with 80%–99% ee and excellent D incorporation. Satisfactory results were achieved with fused aromatic (5g) and heteroaromatic (5h-i) rings as well as alkyls (5l-m) as the substituents of ketones underscore the generality of this catalytic system. Furthermore, ketones substituted with other azaarenes such as 6-phenanthridine (5n), N-Boc-2-benzimidazole (5o), and 2-benzothiazole (5p) were also compatible. It is worth mentioning that, although 2-(α-hydroxybenzyl)-benzimidazole (HBB) is a highly selective and potent inhibitor of picornavirus multiplication in cell culture, no catalytic method to access its optical pure isomer has been established (Tamm and Eggers, 1963, Kadin et al., 1964, Akihama et al., 1968, Tamm et al., 1969). The success in the synthesis of its chiral variant featuring a deuterium on the stereocenter, i.e., 5o, highlights the significance of this methodology.
Figure 5.
Enantioselective Reductive Deuteration (0.1 mmol scale)
Conclusion
In summary, we have developed the first catalytic asymmetric α-deuteration of azaarenes. Through cooperative, visible-light-driven photoredox and chiral H-bonding catalysis, both racemic α-chloro-azaarenes and prochiral azaarene-substituted ketones can undergo a double single-electron reduction–enantioselective deuteration process with a Hantzsch ester as the terminal reductant and inexpensive D2O as the deuterium source. The developed reaction can furnish a variety of important enantioenriched α-deuterated azaarenes in satisfactory yields with high D incorporation. Although excess D2O is used and a strong racemic background process occurs directly through the intrinsic redox catalytic cycle, good to excellent enantioselectivities were obtained, which confirms the efficacy of the catalytic system. Therefore, we anticipate that this work will inspire the pursuit of the D2O-enabled asymmetric construction of C–D bonds in various valuable molecules through this highly reactive radical-based dual catalysis platform.
Limitations of the Study
In both reaction systems, pyridine-based substrates were not applicable given the unsatisfactory enantioselectivity. With respect to the dehalogenative deuteration, poor enantioselectivity of mono-azaarene-substituted alkyl chlorides represents another important limitation.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Grants from the National Natural Science Foundation of China (21672052, 21603062), Young Elite Scientists Sponsorship Program by CAST (2017QNRC001), and Henan University are gratefully acknowledged. The calculations are supported by the High Performance Computing (HPC) Center of Henan Normal University.
Author Contributions
Z.J. conceived and designed the study and wrote the paper. T.S., Y.L., C.L., and B.Q. performed the experiments and analyzed the data. B.Q. prepared the Supplemental Information. N.M. conducted DFT calculations. G.C. collected HRMS data. X.Z. synthesized some of the substrates. All authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.06.007.
Contributor Information
Baokun Qiao, Email: qiaobaok@163.com.
Zhiyong Jiang, Email: chmjzy@henu.edu.cn.
Data and Software Availability
The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1893142 (8) and 1902192 (5n) and can be obtained free of charge from www.ccdc.cam.ac.uk/getstructures.
Supplemental Information
References
- Akihama S., Okude M., Sato K., Iwabuchi S. Inhibitory effect of 1,2-bis(2-benzimidazolyl)1,2-ethanediol derivatives on poliovirus. Nature. 1968;217:562–563. doi: 10.1038/217562a0. [DOI] [PubMed] [Google Scholar]; Akihama, S., Okude, M., Sato, K., and Iwabuchi, S.. (1968). Inhibitory effect of 1,2-bis(2-benzimidazolyl)1,2-ethanediol derivatives on poliovirus. Nature 217, 562−563. [DOI] [PubMed]
- Alonso F., Beletskaya I.P., Yus M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 2002;102:4009–4091. doi: 10.1021/cr0102967. [DOI] [PubMed] [Google Scholar]; Alonso, F., Beletskaya, I.P., and Yus, M.. (2002). Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 102, 4009−4091. [DOI] [PubMed]
- Atzrodt J., Derdau V., Fey T., Zimmermann J. The renaissance of H/D exchange. Angew. Chem. Int. Ed. 2007;46:7744–7765. doi: 10.1002/anie.200700039. [DOI] [PubMed] [Google Scholar]; Atzrodt, J., Derdau, V., Fey, T., and Zimmermann, J.. (2007). The renaissance of H/D exchange. Angew. Chem. Int. Ed. 46, 7744−7765. [DOI] [PubMed]
- Brimioulle R., Lenhart D., Maturi M.M., Bach T. Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed. 2015;54:3872–3890. doi: 10.1002/anie.201411409. [DOI] [PubMed] [Google Scholar]; Brimioulle, R., Lenhart, D., Maturi, M.M., and Bach, T.. (2015). Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed.. 54, 3872−3890. [DOI] [PubMed]
- Cao K., Tan S.M., Lee R., Yang S., Jia H., Zhao X., Qiao B., Jiang Z. Catalytic enantioselective addition of prochiral radicals to vinylpyridines. J. Am. Chem. Soc. 2019;141:5437–5443. doi: 10.1021/jacs.9b00286. [DOI] [PubMed] [Google Scholar]; Cao, K., Tan, S.M., Lee, R., Yang, S., Jia, H., Zhao, X., Qiao, B., and Jiang, Z.. (2019). Catalytic enantioselective addition of prochiral radicals to vinylpyridines. J. Am. Chem. Soc. 141, 5437−5443. [DOI] [PubMed]
- Curran D.P., Abraham A.C. 1,2-Asymmetric induction in radical reactions. Deuteration and allylation reactions of β-oxy-o-iodoanilides. Tetrahedron. 1993;49:4821–4840. [Google Scholar]; Curran, D.P., and Abraham, A.C.. (1993). 1,2-Asymmetric induction in radical reactions. Deuteration and allylation reactions of β-oxy-o-iodoanilides. Tetrahedron 49, 4821−4840.
- Curran D.P., Ramamoorthy P.S. 1,2-Asymmetric induction in radical reactions. Deuteration and allylation reactions of β-oxy-α-bromo esters. Tetrahedron. 1993;49:4841–4858. [Google Scholar]; Curran, D.P., and Ramamoorthy, P.S.. (1993). 1,2-Asymmetric induction in radical reactions. Deuteration and allylation reactions of β-oxy-α-bromo esters. Tetrahedron 49, 4841−4858.
- Elmore C.S. The use of isotopically labeled compounds in drug discovery. Annu. Rep. Med. Chem. 2009;44:515–534. [Google Scholar]; Elmore, C.S.. (2009). The use of isotopically labeled compounds in drug discovery. Annu. Rep. Med. Chem. 44, 515−534.
- Elmore C.S., Bragg R.A. Isotope chemistry; a useful tool in the drug discovery arsenal. Bioorg. Med. Chem. Lett. 2015;25:167–171. doi: 10.1016/j.bmcl.2014.11.051. [DOI] [PubMed] [Google Scholar]; Elmore, C.S., and Bragg, R.A.. (2015). Isotope chemistry; a useful tool in the drug discovery arsenal. Bioorg. Med. Chem. Lett. 25, 167−171. [DOI] [PubMed]
- Fan X., Gong X., Ma M., Wang R., Walsh P.J. Visible light-promoted CO2 fixation with imines to synthesize diaryl α-amino acids. Nat. Commun. 2018;9:4936. doi: 10.1038/s41467-018-07351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fan, X., Gong, X., Ma, M., Wang, R., and Walsh, P.J.. (2018). Visible light-promoted CO2 fixation with imines to synthesize diaryl α-amino acids. Nat. Commun.. 9, 4936. [DOI] [PMC free article] [PubMed]
- Fukuzumi S., Mochizuki S., Tanaka T. Photocatalytic reduction of phenacyl halides by 9,10-dihydro-10-methylacridine: control between the reductive and oxidative quenching pathways of tris(bipyridine) ruthenium complex utilizing an acid catalysis. J. Phys. Chem. 1990;94:722–726. [Google Scholar]; Fukuzumi, S., Mochizuki, S., and Tanaka, T.. (1990). Photocatalytic reduction of phenacyl halides by 9,10-dihydro-10-methylacridine: control between the reductive and oxidative quenching pathways of tris(bipyridine) ruthenium complex utilizing an acid catalysis. J. Phys. Chem. 94, 722−726.
- Hale L.V.A., Szymczak N.K. Stereoretentive deuteration of α-chiral amines with D2O. J. Am. Chem. Soc. 2016;138:13489–13492. doi: 10.1021/jacs.6b07879. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hale, L.V.A., and Szymczak, N.K.. (2016). Stereoretentive deuteration of α-chiral amines with D2O. J. Am. Chem. Soc. 138, 13489−13492. [DOI] [PMC free article] [PubMed]
- Hall L.R., Hanzlik R.P. Kinetic deuterium isotope effects on the N-demethylation of tertiary amides by cytochrome P-450. J. Biol. Chem. 1990;265:12349–12355. [PubMed] [Google Scholar]; Hall, L.R., and Hanzlik, R.P.. (1990). Kinetic deuterium isotope effects on the N-demethylation of tertiary amides by cytochrome P-450. J. Biol. Chem. 265, 12349−12355. [PubMed]
- Hammadi A., Ménez A., Genet R. Asymmetric deuteration of N-acetyl-(Z)-α,β-dehydrotryptophan-(L)-phenylalanine methyl ester produced by (L)-tryptophan 2’,3’-oxidase from chromobacterium vioaceum. A new route for stereospecific labelling of peptides. Tetrahedron. 1997;53:16115–16122. [Google Scholar]; Hammadi, A., Menez, A., and Genet, R.. (1997). Asymmetric deuteration of N-acetyl-(Z)-α,β-dehydrotryptophan-(L)-phenylalanine methyl ester produced by (L)-tryptophan 2’,3’-oxidase from chromobacterium vioaceum. A new route for stereospecific labelling of peptides. Tetrahedron 53, 16115−16122.
- Hepburn H.B., Melchiorre P. Brønsted acid-catalysed conjugate addition of photochemically generated α-amino radicals to alkenylpyridines. Chem. Commun. (Camb.) 2016;52:3520–3523. doi: 10.1039/c5cc10401g. [DOI] [PubMed] [Google Scholar]; Hepburn, H.B., and Melchiorre, P.. (2016). Bronsted acid-catalysed conjugate addition of photochemically generated α-amino radicals to alkenylpyridines. Chem. Commun. (Camb.). 52, 3520−3523. [DOI] [PubMed]
- Hironaka K., Fukuzumi S., Tanaka T. Tris(bipyridyl) ruthenium (II)-photosensitized reaction of 1-benzyl-1,4-dihydronicotinamide with benzyl bromide. J. Chem. Soc. Perkin Trans. 1984;2:1705–1709. [Google Scholar]; Hironaka, K., Fukuzumi, S., and Tanaka, T.. (1984). Tris(bipyridyl) ruthenium (II)-photosensitized reaction of 1-benzyl-1,4-dihydronicotinamide with benzyl bromide. J. Chem. Soc. Perkin Trans. 2 1705−1709.
- Huang A., Moretto A., Janz K., Lowe M., Bedard P.W., Tam S., Di L., Clerin V., Sushkova N., Tchernychev B. Discovery of 2-[1-(4-chlorophenyl)cyclopropyl]-3-hydroxy-8-(trifluoromethyl)quinoline-4-carboxylic acid (PSI-421), a P-selectin inhibitor with improved pharmacokinetic properties and oral efficacy in models of vascular injury. J. Med. Chem. 2010;53:6003–6017. doi: 10.1021/jm9013696. [DOI] [PubMed] [Google Scholar]; Huang, A., Moretto, A., Janz, K., Lowe, M., Bedard, P.W., Tam, S., Di, L., Clerin, V., Sushkova, N., Tchernychev, B., et al. (2010). Discovery of 2-[1-(4-chlorophenyl)cyclopropyl]-3-hydroxy-8-(trifluoromethyl)quinoline-4-carboxylic acid (PSI-421), a P-selectin inhibitor with improved pharmacokinetic properties and oral efficacy in models of vascular injury. J. Med. Chem. 53, 6003−6017. [DOI] [PubMed]
- Ju T., Fu Q., Ye J.H., Zhang Z., Liao L.L., Yan S.S., Tian X.Y., Luo S.P., Li J., Yu D.G. Selective and catalytic hydrocarboxylation of enamides and imines with CO2 to generate α,α-disubstituted α-amino acids. Angew. Chem. Int. Ed. 2018;57:13897–13901. doi: 10.1002/anie.201806874. [DOI] [PubMed] [Google Scholar]; Ju, T., Fu, Q., Ye, J.H., Zhang, Z., Liao, L.L., Yan, S.S., Tian, X.Y., Luo, S.P., Li, J., and Yu, D.G.. (2018). Selective and catalytic hydrocarboxylation of enamides and imines with CO2 to generate α,α-disubstituted α-amino acids. Angew. Chem. Int. Ed.. 57, 13897−13901. [DOI] [PubMed]
- Jung J., Kim J., Park G., You Y., Cho E.J. Selective debromination and α-hydroxylation of α-bromo ketones using Hantzsch esters as photoreductants. Adv. Synth. Catal. 2016;358:74–80. [Google Scholar]; Jung, J., Kim, J., Park, G., You, Y., and Cho, E.J.. (2016). Selective debromination and α-hydroxylation of α-bromo ketones using Hantzsch esters as photoreductants. Adv. Synth. Catal.. 358, 74−80.
- Kadin S.B., Eggers H.J., Tamm I. Synthesis and virus-inhibitory activity of D- and L-isomers of 2-(α-hydroxybenzyl)-benzimidazole. Nature. 1964;201:639–640. doi: 10.1038/201639a0. [DOI] [PubMed] [Google Scholar]; Kadin, S.B., Eggers, H.J., and Tamm, I.. (1964). Synthesis and virus-inhibitory activity of D- and L-isomers of 2-(α-hydroxybenzyl)-benzimidazole. Nature 201, 639−640. [DOI] [PubMed]
- Kaila N., Janz K.M., Huang A., Moretto A.F., Bedard P.W. Preparation of quinoline compounds as selectin inhibitors for disease treatment. PCT Int. Appl. 2008 121817 A3. [Google Scholar]; Kaila, N., Janz, K.M., Huang, A., Moretto, A.F., and Bedard, P.W.. (2008). Preparation of quinoline compounds as selectin inhibitors for disease treatment. PCT Int. Appl., WO 2008121817 A2 20081009.
- Lee K.N., Lei Z., Ngai M.-Y. β-Selective reductive coupling of alkenylpyridines with aldehydes and imines via synergistic Lewis acid/photoredox catalysis. J. Am. Chem. Soc. 2017;139:5003–5006. doi: 10.1021/jacs.7b01373. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, K.N., Lei, Z., and Ngai, M.-Y.. (2017). β-Selective reductive coupling of alkenylpyridines with aldehydes and imines via synergistic Lewis acid/photoredox catalysis. J. Am. Chem. Soc. 139, 5003−5006. [DOI] [PMC free article] [PubMed]
- Lethu S., Ano H., Murata M., Matsuoka S. Enantioselective deuteration of β-substituted α,β-unsaturated esters by rhodium-1,2-bis(2,5-diphenylphospholano)ethane. Eur. J. Org. Chem. 2018;2018:235–239. [Google Scholar]; Lethu, S., Ano, H., Murata, M., and Matsuoka, S.. (2018). Enantioselective deuteration of β-substituted α,β-unsaturated esters by rhodium-1,2-bis(2,5-diphenylphospholano)ethane. Eur. J. Org. Chem. 235−239.
- Li J., Kong M., Qiao B., Lee R., Zhao X., Jiang Z. Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat. Commun. 2018;9:2445. doi: 10.1038/s41467-018-04885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li, J., Kong, M., Qiao, B., Lee, R., Zhao, X., and Jiang, Z.. (2018). Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat. Commun.. 9, 2445. [DOI] [PMC free article] [PubMed]
- Liao L.-L., Cao G.-M., Ye J.-H., Sun G.-Q., Zhou W.-J., Gui Y.-Y., Yan S.-S., Shen G., Yu D.-G. Visible-light-driven external-reductant-free cross-electrophile couplings of tetraalkyl ammonium salts. J. Am. Chem. Soc. 2018;140:17338–17342. doi: 10.1021/jacs.8b08792. [DOI] [PubMed] [Google Scholar]; Liao, L.-L., Cao, G.-M., Ye, J.-H., Sun, G.-Q., Zhou, W.-J., Gui, Y.-Y., Yan, S.-S., Shen, G., and Yu, D.-G.. (2018). Visible-light-driven external-reductant-free cross-electrophile couplings of tetraalkyl ammonium salts. J. Am. Chem. Soc. 140, 17338−17342. [DOI] [PubMed]
- Liu Q., Wang C., Zhou H., Wang B., Lv J., Cao L., Fu Y. Iridium-catalyzed highly enantioselective transfer hydrogenation of aryl N-heteroaryl ketones with N-oxide as a removable ortho-substituent. Org. Lett. 2018;20:971–974. doi: 10.1021/acs.orglett.7b03878. [DOI] [PubMed] [Google Scholar]; Liu, Q., Wang, C., Zhou, H., Wang, B., Lv, J., Cao, L., and Fu, Y.. (2018). Iridium-catalyzed highly enantioselective transfer hydrogenation of aryl N-heteroaryl ketones with N-oxide as a removable ortho-substituent. Org. Lett. 20, 971−974. [DOI] [PubMed]
- Loh Y.Y., Nagao K., Hoover A.J., Hesk D., Rivera N.R., Colletti S.L., Davies I.W., MacMillan D.W.C. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science. 2017;358:1182–1187. doi: 10.1126/science.aap9674. [DOI] [PMC free article] [PubMed] [Google Scholar]; Loh, Y.Y., Nagao, K., Hoover, A.J., Hesk, D., Rivera, N.R., Colletti, S.L., Davies, I.W., and MacMillan, D.W.C.. (2017). Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182−1187. [DOI] [PMC free article] [PubMed]
- Luo J., Zhang J. Donor−acceptor fluorophores for visible−light-promoted organic synthesis: photoredox/Ni dual catalytic C(sp3)−C(sp2) cross-coupling. ACS Catal. 2016;6:873–877. [Google Scholar]; Luo, J., and Zhang, J.. (2016). Donor−acceptor fluorophores for visible−light-promoted organic synthesis: photoredox/Ni dual catalytic C(sp3)−C(sp2) cross-coupling. ACS Catal.. 6, 873−877.
- MacDonald D., Lu P. Determination of DNA structure in solution: enzymatic deuteration of the ribose 2’carbon. J. Am. Chem. Soc. 2002;124:9722–9723. doi: 10.1021/ja026678r. [DOI] [PubMed] [Google Scholar]; MacDonald, D., and Lu, P.. (2002). Determination of DNA structure in solution: enzymatic deuteration of the ribose 2’carbon. J. Am. Chem. Soc. 124, 9722−9723. [DOI] [PubMed]
- Maji T., Karmakar A., Reiser O. Visible-light photoredox catalysis: dehalogenation of vicinal dibromo-, α-halo-, and α,α-dibromocarbonyl compounds. J. Org. Chem. 2010;76:736–739. doi: 10.1021/jo102239x. [DOI] [PubMed] [Google Scholar]; Maji, T., Karmakar, A., and Reiser, O.. (2010). Visible-light photoredox catalysis: dehalogenation of vicinal dibromo-, α-halo-, and α,α-dibromocarbonyl compounds. J. Org. Chem. 76, 736−739. [DOI] [PubMed]
- Maltais F., Jung Y.C., Chen M., Tanoury J., Perni R.B., Mani N., Laitinen L., Huang H., Liao S., Gao H. In vitro and in vivo isotope effects with hepatitis C protease inhibitors: enhanced plasma exposure of deuterated telaprevir versus telaprevir in rats. J. Med. Chem. 2009;52:7993–8001. doi: 10.1021/jm901023f. [DOI] [PubMed] [Google Scholar]; Maltais, F., Jung, Y.C., Chen, M., Tanoury, J., Perni, R.B., Mani, N., Laitinen, L., Huang, H., Liao, S., Gao, H., et al. (2009). In vitro and in vivo isotope effects with hepatitis C protease inhibitors: enhanced plasma exposure of deuterated telaprevir versus telaprevir in rats. J. Med. Chem. 52, 7993−8001. [DOI] [PubMed]
- Meanwell N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011;54:2529–2591. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]; Meanwell, N.A.. (2011). Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529−2591. [DOI] [PubMed]
- Mohrig J.R., Reiter N.J., Kirk R., Zawadski M.R., Lamarre-Vincent N. Effect of buffer general acid−base catalysis on the stereoselectivity of ester and thioester H/D exchange in D2O. J. Am. Chem. Soc. 2011;133:5124–5128. doi: 10.1021/ja200014c. [DOI] [PubMed] [Google Scholar]; Mohrig, J.R., Reiter, N.J., Kirk, R., Zawadski, M.R., and Lamarre-Vincent, N.. (2011). Effect of buffer general acid−base catalysis on the stereoselectivity of ester and thioester H/D exchange in D2O. J. Am. Chem. Soc. 133, 5124−5128. [DOI] [PubMed]
- Narayanam J.M.R., Tucker J.W., Stephenson C.R.J. Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 2009;131:8756–8757. doi: 10.1021/ja9033582. [DOI] [PubMed] [Google Scholar]; Narayanam, J.M.R., Tucker, J.W., and Stephenson, C.R.J.. (2009). Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 131, 8756−8757. [DOI] [PubMed]
- Nelson S.D., Trager W.F. The use of deuterium isotope effects to probe the active site properties, mechanism of cytochrome P450-catalyzed reactions, and mechanisms of metabolically dependent toxicity. Drug Metab. Dispos. 2003;31:1481–1489. doi: 10.1124/dmd.31.12.1481. [DOI] [PubMed] [Google Scholar]; Nelson, S.D., and Trager, W.F.. (2003). The use of deuterium isotope effects to probe the active site properties, mechanism of cytochrome P450-catalyzed reactions, and mechanisms of metabolically dependent toxicity. Drug Metab. Dispos.. 31, 1481−1489. [DOI] [PubMed]
- Neumann M., Füldner S., König B., Zeitler K. Metal−free, cooperative asymmetric organophotoredox catalysis with visible light. Angew. Chem. Int. Ed. 2011;50:951–954. doi: 10.1002/anie.201002992. [DOI] [PubMed] [Google Scholar]; Neumann, M., Fuldner, S., Konig, B., and Zeitler, K.. (2011). Metal−free, cooperative asymmetric organophotoredox catalysis with visible light. Angew. Chem. Int. Ed.. 50, 951−954. [DOI] [PubMed]
- Palmer W.N., Chirik P.J. Cobalt-catalyzed stereoretentive hydrogen isotope exchange of C(sp3)−H bonds. ACS Catal. 2017;7:5674–5678. doi: 10.1021/acscatal.7b02051. [DOI] [PMC free article] [PubMed] [Google Scholar]; Palmer, W.N., and Chirik, P.J.. (2017). Cobalt-catalyzed stereoretentive hydrogen isotope exchange of C(sp3)−H bonds. ACS Catal. 7, 5674−5678. [DOI] [PMC free article] [PubMed]
- Prier C.K., Rankic D.A., MacMillan D.W.C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prier, C.K., Rankic, D.A., and MacMillan, D.W.C.. (2013). Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322−5363. [DOI] [PMC free article] [PubMed]
- Proctor R.S.J., Davis H.J., Phipps R.J. Catalytic enantioselective Minisci-type addition to heteroarenes. Science. 2018;360:419–422. doi: 10.1126/science.aar6376. [DOI] [PubMed] [Google Scholar]; Proctor, R.S.J., Davis, H.J., and Phipps, R.J.. (2018). Catalytic enantioselective Minisci-type addition to heteroarenes. Science 360, 419−422. [DOI] [PubMed]
- Rennison D., Bova S., Cavalli M., Ricchelli F., Zulian A., Hopkins B., Brimble M.A. Synthesis and activity studies of analogues of the rat selective toxicant norbormide. Bioorg. Med. Chem. 2007;15:2963–2974. doi: 10.1016/j.bmc.2007.02.012. [DOI] [PubMed] [Google Scholar]; Rennison, D., Bova, S., Cavalli, M., Ricchelli, F., Zulian, A., Hopkins, B., and Brimble, M.A.. (2007). Synthesis and activity studies of analogues of the rat selective toxicant norbormide. Bioorg. Med. Chem. 15, 2963−2974. [DOI] [PubMed]
- Rono L.J., Yayla H.G., Wang D.Y., Armstrong M.F., Knowles R.R. Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric aza-Pinacol cyclization. J. Am. Chem. Soc. 2013;135:17735–17738. doi: 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]; Rono, L.J., Yayla, H.G., Wang, D.Y., Armstrong, M.F., and Knowles, R.R.. (2013). Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric aza-Pinacol cyclization. J. Am. Chem. Soc. 135, 17735−17738. [DOI] [PubMed]
- Rueping M., Antonchick A.P. Organocatalytic enantioselective reduction of pyridines. Angew. Chem. Int. Ed. 2007;46:4562–4565. doi: 10.1002/anie.200701158. [DOI] [PubMed] [Google Scholar]; Rueping, M., and Antonchick, A.P.. (2007). Organocatalytic enantioselective reduction of pyridines. Angew. Chem. Int. Ed.. 46, 4562−4565. [DOI] [PubMed]
- Sakamoto T., Mori K., Akiyama T. Chiral phosphoric acid catalyzed enantioselective transfer deuteration of ketimines by use of benzothiazoline as a deuterium donor: synthesis of optically active deuterated amines. Org. Lett. 2012;14:3312–3315. doi: 10.1021/ol3012869. [DOI] [PubMed] [Google Scholar]; Sakamoto, T., Mori, K., and Akiyama, T.. (2012). Chiral phosphoric acid catalyzed enantioselective transfer deuteration of ketimines by use of benzothiazoline as a deuterium donor: synthesis of optically active deuterated amines. Org. Lett. 14, 3312−3315. [DOI] [PubMed]
- Sandoval B.A., Meichan A.J., Hyster T.K. Enantioselective hydrogen atom transfer: discovery of catalytic promiscuity in flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 2017;139:11313–11316. doi: 10.1021/jacs.7b05468. [DOI] [PubMed] [Google Scholar]; Sandoval, B.A., Meichan, A.J., and Hyster, T.K.. (2017). Enantioselective hydrogen atom transfer: discovery of catalytic promiscuity in flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 139, 11313−11316. [DOI] [PubMed]
- Shaw M.H., Twilton J., MacMillan D.W.C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shaw, M.H., Twilton, J., and MacMillan, D.W.C.. (2016). Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898−6926. [DOI] [PMC free article] [PubMed]
- Skubi K.L., Blum T.R., Yoon T.P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 2016;116:10035–10074. doi: 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Skubi, K.L., Blum, T.R., and Yoon, T.P.. (2016). Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035−10074. [DOI] [PMC free article] [PubMed]
- Staveness D., Bosque I., Stephenson C.R.J. Free radical chemistry enabled by visible light-induced electron transfer. Acc. Chem. Res. 2016;49:2295–2306. doi: 10.1021/acs.accounts.6b00270. [DOI] [PMC free article] [PubMed] [Google Scholar]; Staveness, D., Bosque, I., and Stephenson, C.R.J.. (2016). Free radical chemistry enabled by visible light-induced electron transfer. Acc. Chem. Res. 49, 2295−2306. [DOI] [PMC free article] [PubMed]
- Taglang C., Martίnez-Prieto L.M., del Rosal I., Maron L., Poteau R., Philippot K., Chaudret B., Perato S., Lone S.A., Puente C. Enantiospecific C−H activation using ruthenium nanocatalysts. Angew. Chem. Int. Ed. 2015;54:10474–10477. doi: 10.1002/anie.201504554. [DOI] [PubMed] [Google Scholar]; Taglang, C., Martίnez-Prieto, L.M., del Rosal, I., Maron, L., Poteau, R., Philippot, K., Chaudret, B., Perato, S., Lone, S.A., Puente, C., et al. (2015). Enantiospecific C−H activation using ruthenium nanocatalysts. Angew. Chem. Int. Ed. 54, 10474−10477. [DOI] [PubMed]
- Tahara K., Hisaeda Y. Eco-friendly molecular transformations catalyzed by a vitamin B12 derivative with a visible-light-driven system. Green Chem. 2011;13:558–561. [Google Scholar]; Tahara, K., and Hisaeda, Y.. (2011). Eco-friendly molecular transformations catalyzed by a vitamin B12 derivative with a visible-light-driven system. Green Chem. 13, 558−561.
- Tamm I., Eggers H.J. Specific Inhibition of replication of animal viruses. Science. 1963;142:24–33. doi: 10.1126/science.142.3588.24. [DOI] [PubMed] [Google Scholar]; Tamm, I., and Eggers, H.J.. (1963). Specific Inhibition of replication of animal viruses. Science 142, 24−33. [DOI] [PubMed]
- Tamm I., Eggers H.J., Bablanian R. Structural requirements of selective inhibition of enteroviruses by 2-(α-hydroxybenzyl)-benzimidazole and related compounds. Nature. 1969;223:785–788. doi: 10.1038/223785a0. [DOI] [PubMed] [Google Scholar]; Tamm, I., Eggers, H.J., and Bablanian, R.. (1969). Structural requirements of selective inhibition of enteroviruses by 2-(α-hydroxybenzyl)-benzimidazole and related compounds. Nature 223, 785−788. [DOI] [PubMed]
- Xu C., Muir C.W., Leach A.G., Kennedy A.R., Watson A.J.B. Catalytic enantioselective synthesis of α-chiral azaheteroaryl ethylamines by asymmetric protonation. Angew. Chem. Int. Ed. 2018;57:11374–11377. doi: 10.1002/anie.201806956. [DOI] [PubMed] [Google Scholar]; Xu, C., Muir, C.W., Leach, A.G., Kennedy, A.R., and Watson, A.J.B.. (2018). Catalytic enantioselective synthesis of α-chiral azaheteroaryl ethylamines by asymmetric protonation. Angew. Chem. Int. Ed. 57, 11374−11377. [DOI] [PubMed]
- Yin Y., Dai Y., Jia H., Li J., Bu L., Qiao B., Zhao X., Jiang Z. Conjugate addition−enantioselective protonation of N-aryl glycines to α-branched 2-vinylazaarenes via cooperative photoredox and asymmetric catalysis. J. Am. Chem. Soc. 2018;140:6083–6087. doi: 10.1021/jacs.8b01575. [DOI] [PubMed] [Google Scholar]; Yin, Y., Dai, Y., Jia, H., Li, J., Bu, L., Qiao, B., Zhao, X., and Jiang, Z.. (2018). Conjugate addition−enantioselective protonation of N-aryl glycines to α-branched 2-vinylazaarenes via cooperative photoredox and asymmetric catalysis. J. Am. Chem. Soc. 140, 6083−6087. [DOI] [PubMed]
- Zhang M., Yuan X.-A., Zhu C., Xie J. Deoxygenative deuteration of carboxylic acids with D2O. Angew. Chem. Int. Ed. 2019;58:312–316. doi: 10.1002/anie.201811522. [DOI] [PubMed] [Google Scholar]; Zhang, M., Yuan, X.-A., Zhu, C., and Xie, J.. (2019). Deoxygenative deuteration of carboxylic acids with D2O. Angew. Chem. Int. Ed. 58, 312−316. [DOI] [PubMed]
- Zhao Y., Lim X., Pan Y., Zong L., Feng W., Tan C.-H., Huang K.-W. Asymmetric H−D exchange reactions of fluorinated aromatic ketones. Chem. Commun. (Camb.) 2012;48:5479–5481. doi: 10.1039/c2cc31443f. [DOI] [PubMed] [Google Scholar]; Zhao, Y., Lim, X., Pan, Y., Zong, L., Feng, W.., and Tan, C.-H.; Huang, K.-W.. (2012). Asymmetric H−D exchange reactions of fluorinated aromatic ketones. Chem. Commun. (Camb.). 48, 5479−5481. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.