Abstract
Background: Kidney yang deficiency syndrome (KYDS) is one of the most common syndromes treated with traditional Chinese medicine (TCM) among elderly patients. Shen Qi Wan (SQW) has been effectively used in treating various diseases associated with KYDS for hundreds of years. However, due to the complex composition of SQW, the mechanism of action remains unknown.
Purpose: To identify the mechanism of the SQW in the treatment of KYDS and determine the molecular targets of SQW.
Methods: The potential targets of active ingredients in SQW were predicted using PharmMapper. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were carried out using the Molecule Annotation System (MAS3.0). The protein–protein interaction (PPI) network of these potential targets and “components-targets-pathways” interaction networks were constructed using Cytoscape. We also established a KYDS rat model induced by adenine to investigate the therapeutic effects of SQW. Body weight, rectal temperature, holding power, water intake, urinary output, blood urea nitrogen (BUN), serum creatinine (Scr), adrenocorticotrophic hormone (ACTH), cortisol (CORT), urine total protein (U-TP), and 17-hydroxy-corticosteroid (17-OHCS) were measured. Additionally, the mRNA expression levels of candidates were detected by qPCR.
Results: KYDS-caused changes in body weight, rectal temperature, holding power, water intake, urinary output, BUN, Scr, ACTH, CORT, U-TP, and 17-OHCS were corrected to the baseline values after SQW treatment. We selected the top 10 targets of each component and obtained 79 potential targets, which were mainly enriched in the proteolysis, protein binding, transferase activity, T cell receptor signaling pathway, and focal adhesion. SRC, MAPK14, HRAS, HSP90AA1, F2, LCK, CDK2, and MMP9 were identified as targets of SQW in the treatment of KYDS. The administration of SQW significantly suppressed the expression of SRC, HSP90AA1, LCK, and CDK2 and markedly increased the expression of MAPK14, MMP9, and F2. However, HRAS levels remained unchanged.
Conclusion: These findings demonstrated that SQW corrected hypothalamic–pituitary–target gland axis disorder in rats caused by KYDS. SRC, MAPK14, HRAS, HSP90AA1, F2, LCK, CDK2, and MMP9 were determined to the therapeutic target for the further investigation of SQW to ameliorate KYDS.
Keywords: network pharmacology, gene ontology, potential targets, traditional Chinese medicine, phytotherapy, transcriptomics
Introduction
Kidney yang deficiency syndrome (KYDS) is a diagnostic pattern in traditional Chinese medicine (TCM) and was first documented in Huang Di Nei Jing, one of the four great classical textbooks of TCM (Nan et al., 2016). KYDS is characterized by warm dysfunction and a metabolic disorder of the body fluid, causing aversion to cold, cold limbs, cold of waist and back, soreness and weakness of waist and knee, tinnitus, fatigue, impairment of hearing, and looseness of teeth (Lu et al., 2011; Tan et al., 2014; Rong et al., 2016; Xiong et al., 2019). Modern studies have indicated that functional disorders with different degrees of hypothalamic–pituitary–target gland axis, including adrenal glands, thyroids, and gonads, are the crucial pathological mechanism leading to KYDS (Lu et al., 2011; Tan et al., 2014; Nan et al., 2016; Zhang et al., 2017; Tang et al., 2018). KYDS can be present in chronic diseases such as rheumatoid arthritis, hypertension, and diabetes, posing a considerable challenge to the medical system. A valid and classic rat model of KYDS has been developed via the administration of a high dose of adenine by oral gavage, which precipitates in renal tubules, leading chronic renal failure, and the animals exhibit the clinical characteristics of KYDS.
Shen Qi Wan (SQW) is a frequently used Chinese formula described by Zhang Zhongjing in Synopsis of Prescriptions of the Golden Chamber (also named Jin Kui Yao Lue in Mandarin). It can be traced back to nearly 2,000 years ago in ancient China (Xiong et al., 2015). The SQW formula is based on the combinatorial principle of “emperor-minister-adjuvant-courier” (jun-chen-zuo-shi in Chinese) to combine multiple herbs. The jun herb of SQW contains Cinnamomum cassia (L). J.Presl and Aconitum carmichaelii Debeaux to treat the main cause or primary symptoms of KYDS. The chen herb of SQW is Rehmannia glutinosa (Gaertn). DC., Cornus officinalis Siebold & Zucc., and Dioscorea oppositifolia L. assist the jun herb to enhance its therapeutic effects and relieve the accompanying symptoms. The zuo shi herb includes Poria cocos (Schw). Wolf, Alisma plantago-aquatica L., and Paeonia × suffruticosa Andrews to counteract the possible toxicity or side effects of other herbs and to ensure the absorption of the formula components and help deliver or guide them to the target organs (Qiu, 2007; Yao et al., 2013). For centuries, SQW has been effectively used in treating various diseases associated with KYDS. However, the therapeutic mechanism remains unknown, the complexity of multiple components, multiple targets, and multiple pathways involved in KYDS make it difficult to elucidate using classical pharmacological approaches.
Network pharmacology is a distinctive new approach based on advances in polypharmacology and network biology to shift away from the traditional “one drug, one target” strategy and move toward sub-network targets and systems, providing a more comprehensive understanding of the mechanism, targets, and pathways behind drug action (Tang and Aittokallio, 2014; Poornima et al., 2016). With the combination of the “medicines-targets” network and biological system network, network pharmacology is becoming more widely known and more frequently used in the field of drug research. PharmMapper (http://www.lilab-ecust.cn/pharmmapper/) is a freely accessed web server designed to identify potential target candidates for probe small molecules of interest using pharmacophore mapping approach (Liu et al., 2010). PharmMapper provides deeper insights and scientific evidence for TCM and helps identify potential targets of Chinese herbs and their underlying mechanisms.
In the present study, investigations based on the pharmacology database and previous studies were conducted to investigate the warm yang and the involvement of several compounds of interest. Potential targets of SQW were predicted by reverse docking to analyze the biological information of potential targets and associated pathways using the network pharmacology method. Moreover, we aimed to identify the potential therapeutic target genes and explore the effects of SQW on the mRNA expression levels of the candidate targets to preliminarily discuss the involvement of the candidate targets in KYDS.
Materials and Methods
Compound Preparation
To collect the compounds of SQW, we combined the Traditional Chinese Medicine Systems Pharmacology Database (TcmSP™, http://lsp.nwu.edu.cn), a unique system pharmacology platform designed for Chinese herbal medicines (Liu et al., 2016) and the review of previous studies (Wang et al., 2016). In addition, we used China National Knowledge Infrastructure (CNKI) and PubMed to obtain information on the modern pharmacology of the compounds in SQW. CAS No. comes from the Chemical Abstract Service (http://www.cas.org/). We finally selected several compounds of each herb in SQW, every compound we chose has various pharmacological effects such as vascular and tracheal relaxation effect; anti-thrombotic, anti-apoptotic, anti-oxidative effects; and anti-inflammatory and immunomodulatory effects.
Preparation of Mol2 Format Files
Using the software ChemBioDraw Ultra 14.0 (Version 14, PerkinElmer Inc), we transformed the structures of active components into the sdf structure format. Then, we transformed the sdf structure format into mol2 format files using ChemBio3D Ultra 14.0 (Version 14, PerkinElmer Inc) to obtain the corresponding three-dimensional molecular ball-and-stick model.
Prediction and Screening of Targets
To predict the potential target candidates, we imported the mol2 format files into the freely accessed web server of the target database of pharmacophore PharmaMapper website (http://www.lilab-ecust.cn/pharmmapper/) to perform reverse docking. Subsequently, we employed UniProtKB (http://www.uniprot.org/), which is the central hub for the collection of functional information on proteins, to correct the unstandardized drug target naming by converting the protein names with the species limited to “Homo sapiens” to its official symbol. We selected the top 10 targets of each active component for the subsequent study.
Construction of Protein–Protein Interaction Network for Potential Targets
By using the STRING (Version 10.0) database (http://version10.string-db.org//), we identified the direct physical interactions of proteins and their functional interactions (Wu et al., 2016). We uploaded the gene symbols of potential targets and drew a protein–protein interaction (PPI) network graph online to evaluate the interactions among the potential targets. Then, we imported the PPI data in text format into Cytoscape (http://www.cytoscape.org/) to visualize relationships and used its network analyzer plugin to calculate the degree of PPI network.
Investigation of Biological Information for Potential Targets of SQW
We imported the potential targets into the Bio database (http://bioinfo.capitalbio.com/mas3/ Version 3.37) to perform the analysis for GO and KEGG pathway enrichment and then screened for pathways with a cut-off p < 0.05 (Wu et al., 2016).
Construction of the Component-Target-Pathway Network
Based on the screening of pathways with their corresponding targets and components, we created a component-target-pathway illustration using Cytoscape, which not only applies to visualizing biological pathways and intermolecular interaction networks but also supplies a basic set of features for data integration, analysis, and visualization for complicated network analysis (Liu et al., 2016). In the network, the node stands for the constituents of SQW, chemical components, component targets, and component pathways. These constituents are connected by an edge when a target is a potential target of a compound. With this network, we studied the effects of multiple components, multiple targets, and multiple pathways of SQW, which ameliorates KYDS.
Animal Study and Sample Collection
The animal study was approved by the Ethics of Committee of Zhejiang Chinese Medical University. Thirty male Wistar rats (250 ± 30 g, animal license no. SCXK-2013-0033) were obtained from the Animal Center of Zhejiang Chinese Medicine University [Laboratory rearing room Permit No. SYXK (Zhejiang) 2013-0184]. All of the animals were housed at 22 ± 2°C with 50–60% relative humidity. A 12 h light/12 h dark cycle was set, and the animals had free access to standard diet and water. All animals were randomly divided into the control group (n = 10), KYDS model group (n = 10), and SQW group (n = 10). In the first 21 consecutive days, the control group was administered normal saline, the model group and the SQW group were administered adenine (Lot: 131203. Shanghai Bo’ao Biological Technology Co., Ltd) at 200 mg/kg per day. From the 22nd day, the control group was administered normal saline, the model group was administered adenine at 200 mg/kg and normal saline via gavage after 1 h per day in the next 21 consecutive days, and the SQW group was administered adenine at 200 mg/kg per day and SQW (Lot: 130904. Henan WanXi Pharmaceuticals Co., Ltd) at 3g/kg via gavage after 1 h per day for the next 21 consecutive days. The body weight, rectal temperature, and holding power were detected every 4 days. Water intake and urinary output were observed every 7 days. The urine was obtained from metabolism cages and was used to detect the urine total protein (U-TP) by an automatic biochemical analyzer; 17-hydroxy-corticosteroids (17-OHCSs) were measured using ELISA kits (CAS: 14020809, Biovol Technologies Co. Ltd. Shanghai) after adenine administration at day 21. Blood samples were collected from the heart after pentobarbital sodium (45 mg/kg, i.p.) anesthesia, and then the kidney tissues were rapidly excised, quickly frozen in liquid nitrogen, and stored at −80°C to perform the quantitative real-time PCR assays. Serum was separated by centrifugation at 3,000 rpm for 15 min at 4°C after standing for 30 min to detect blood urea nitrogen (BUN) and serum creatinine (Scr) with an automatic biochemical analyzer (Hitachi, Japan); adrenocorticotrophic hormone (ACTH) and cortisol (CORT) were measured using enzyme-linked immunosorbent assay (ELISA) kits (CAS: 140208, 14020807. Biovol Technologies Co. Ltd., Shanghai). Throughout the experimental period, no animals died before the experimental endpoint. Euthanasia was performed under sodium pentobarbital anesthesia followed by cardiac puncture/kidney removal for all animals.
Quantitative Real-Time PCR Analysis
Total RNA separation and extraction methods were performed according to the instructions of the TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa, Clontech). Spectrophotometric measurements at 260/280 nm (Thermo Scientific, USA) were used to determine the purities and concentrations of the total RNA samples. Reverse transcription reactions were performed using 300 ng of RNA with PrimerScript™ RT Master Mix (Perfect Real Time) for cDNA. Table 1 lists the primer sequences. SRC, MAPK14, HRAS, HSP90AA1, F2, LCK, CDK2, and MMP9 gene expression was investigated. The samples were exposed to pre-denaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s, and annealing at 60°C for 30 s. The dissolution curve conditions were 65°C for 0.05 s and 95°C for 0.5 s using 5 µL 5× SYBR Green qPCR Mix, 0.4 µL 20 µmol/L forward primer, 0.4 µL 20 µmol/L reverse primer, and 1 µL cDNA. Water was added to achieve a total volume of 10 µL. β-Actin was used as the internal control, and the data were analyzed using the 2-ΔΔCt method. The experiment was repeated three times.
Table 1.
Primers used for qPCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| SRC | AAGCTTAGGTCTGGCATGGT | ATGGGCTTACGGGGTTACAA |
| MAPK14 | GCTTACCGATGACCACGTTC | CGTGGCCTCGGAAATACAAG |
| HRAS | GTACTTGGGTGATGGTTACGTT | AGACTTGGCGCTTGTAAAGGA |
| HSP90AA1 | GGGGAAATGACAGGGAAGGA | AGCAATTCTCCTGTCAGCCT |
| F2 | CACGGCTACGGATGTGTTCTG | ACCCTCAGCACAGTTACCTTC |
| LCK | TTACCTACCCGCGCTCCTGTGTCCC | CTGGGAAGTCAGTGTCAAACCA |
| CDK2 | TACTTTGGGAGGCTGAGGTG | TTCCCCTCCCACTGATTTCC |
| MMP9 | CCCCGGTACCGAAGGCGAAATGCTTTGCCC | CCCCCTCGAGGGTGAGAACCGAAGCTTCTG |
| β-actin | GCTCTCTTCCAGCCTTCCTT | GGTCTTTACGGATGTCAACG |
SRC, proto-oncogene tyrosine-protein kinase SRC; MAPK14, mitogen-activated protein kinase 14; HRAS, GTPase HRas; HSP90AA1, heat shock protein HSP 90α; F2, prothrombin; LCK, proto-oncogene tyrosine-protein kinase LCK; CDK2, cell division protein kinase 2; MMP9, matrix metalloproteinase-9.
Standards and Chemicals
The standards of o-anisaldehyde (purity >96%), higenamine (purity >98%), and coryneine chloride (purity >98%) were purchased from Yuanye Biological Technology Co., Ltd (Shanghai, China). Salsolinol standard (purity >98%) was purchased from Tauto Biotech Co., Ltd (Shanghai, China). And cinnamic acid standard (purity >98%) was from Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). HPLC-grade acetonitrile and methanol were purchased from Tedia (Fairfield, USA). HPLC-grade phosphoric acid was supplied from Shanghai Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China). Distilled water was used throughout the study.
Preparation of Reference and Sample Solutions
A mixed standard solution was obtained by dissolving the five standards (o-anisaldehyde, higenamine, coryneine chloride, salsolinol, and cinnamic acid) in methanol. The final concentration is 2 mg/ml. SQW was ultrasonically extracted by 10-fold volume pure water twice for 30 min each time. The solution was concentrated to 40 mg/ml then filtered by a 0.22-μm Millipore filter. The injection volume was 10 μl in the same.
Liquid Chromatographic Analysis
Samples were analyzed using the ACQUITY UPLC system (Waters Corp., Milford, MA, USA), equipped with a quaternary pump and a variable wavelength ultraviolet (UV) detector. Elution of analytes was achieved on an Agilent EC-C18 column (100× 3.0 mm, 2.7 μm diameter) at a flow rate of 0.3 ml/min. The mobile phase was acetonitrile (A) and 0.1% phosphoric acid solution (B). The gradient program was as follow: 0–5 min, 0.5–2% A; 5–6 min, 2–30% A; 6–8 min, 30–50% A; 10–12 min, 50–70% A; 12–15 min, 70–0.5% A. The UV detection wavelength was at 208 nm. The column temperature was set at 25°C.
Statistical Analysis
The SPSS 22.0 statistical software package (SPSS, Chicago, IL, USA) was used for the analysis of variance followed by one-way ANOVA. Data were presented as the mean values ± standard deviation. Statistical significance was considered if p < 0.05 was observed.
Results
Compound Information
A search of the TcmSP™ identified 1,345 items, including 130 in A. carmichaelii Debeaux, 200 in C. cassia (L.) J.Presl, 151 in R. glutinosa (Gaertn.) DC., 452 in C. officinalis Siebold & Zucc., 142 in D. oppositifolia L., 68 in P. cocos (Schw). Wolf, 92 in A. plantago-aquatica L., and 110 in Paeonia × suffruticosa Andrews. Wang et al. (2016), in “an integrated chinmedomics strategy for discovery of effective constituents from traditional herbal medicine,” reported 84 compounds, among which 51 compounds in negative ion mode and 33 compounds in positive ion mode were identified from SQW. Moreover, 20 compounds absorbed into the blood, such as azelaic acid-O-glucuronide, jionoside D, azelaic acid, and poricoic acid, had a strong relationship with the therapeutic effect of SQW on KYDS. Database search and current studies were used to select the chemical components of SQW according to its pharmacological activities, such as a cardiac-stimulating effect, heightened adrenal cortex function, promoting diuresis and detumescence, invigorating spleen and dampness removal, as shown in Table 2 . Higenamine, coryneine chloride, salsolinol, o-anisaldehyde, cinnamic acid, catalpol, acteoside, loganin, diosgenin, morroniside, pachymic acid, acetophenone, paeoniflorin, alisol A, and alisol B were probably associated with warming yang attributes of SQW, which were used for further study.
Table 2.
Chemical information for the constituents of SQW.
| Medicinal herbs | Compounds | MW | Structure | Composition | CAS no. | Pharmacological activities | References |
|---|---|---|---|---|---|---|---|
|
Fuzi
(Aconitum carmichaelii Debeaux) |
Higenamine | 271.32 | 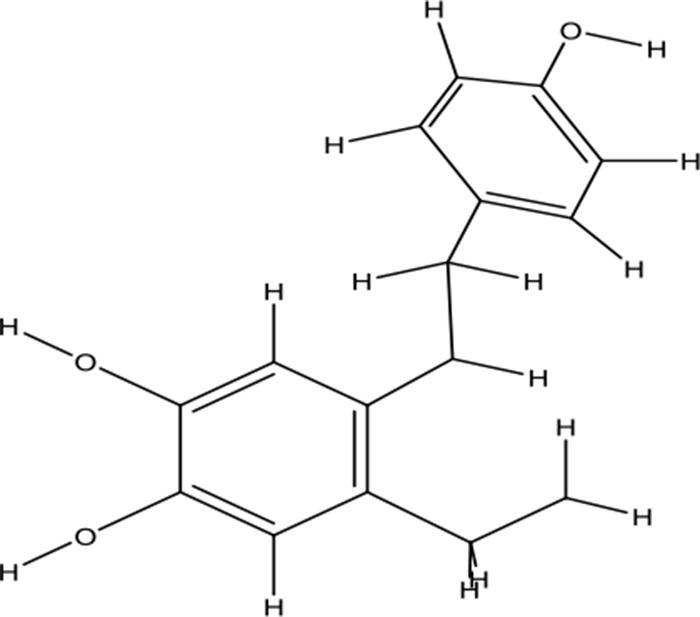 |
C16H17NO3 | 5843-65-2 | Anti-inflammatory; anti-oxidative; anti-apoptotic | Zhang et al., 2014; Liu et al., 2015; Wei et al., 2015 |
| Coryneine chloride | 203.67 | 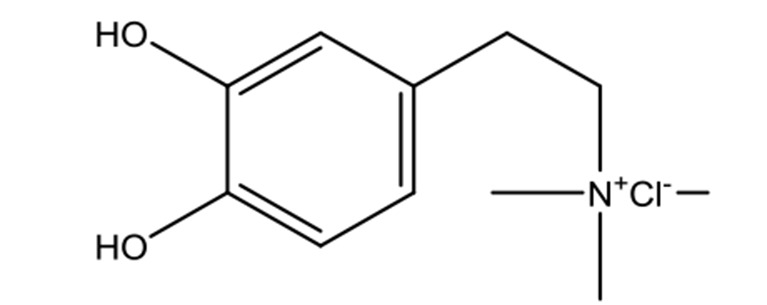 |
C9H14ClNO2 | 1477-68-5 | Cardiac-stimulating | Li et al., 2010 | |
| Salsolinol | 179.22 | 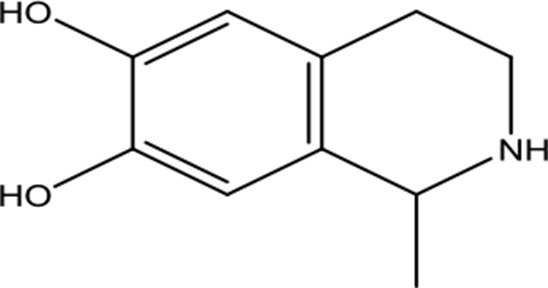 |
C10H13NO3 | 27740-96-1 | Cardiac-stimulating | Chen and Liang, 1982; Yang et al., 2014 |
|
|
RouGui
(Cinnamomum cassia (L.) J.Presl) |
o-Anisaldehyde | 162.19 | 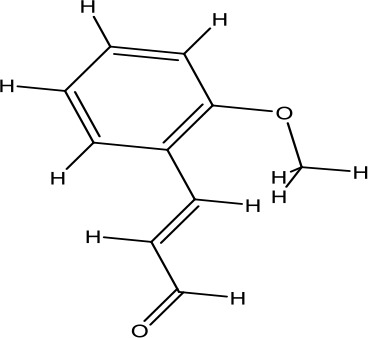 |
C10H10O2 | 1504-74-1 | Improve blood supply of cardiac muscle; anti-shock | Hasegawa et al., 2002; Chang et al., 2013; Ma et al., 2018 |
| Cinnamic acid | 148.16 | 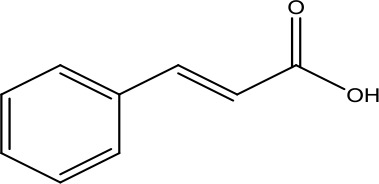 |
C9H8O2 | 621-82-9 | Protect myocardial function | Li et al., 2019 | |
|
Dihuang
(Rehmannia glutinosa (Gaertn.) DC.) |
Catalpol | 362.33 | 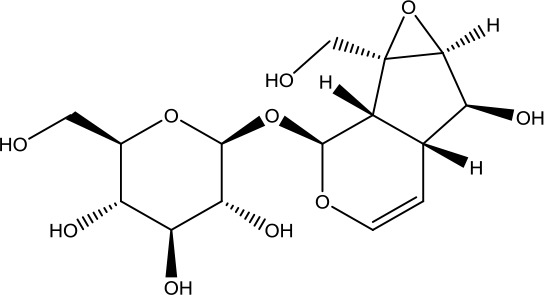 |
C15H22O10 | 2415-24-9 | Hypoglycemic effect; anti-tumor; anti-inflammatory |
Youn et al., 2018; Yuan et al., 2018 |
| Acteoside | 624.59 | 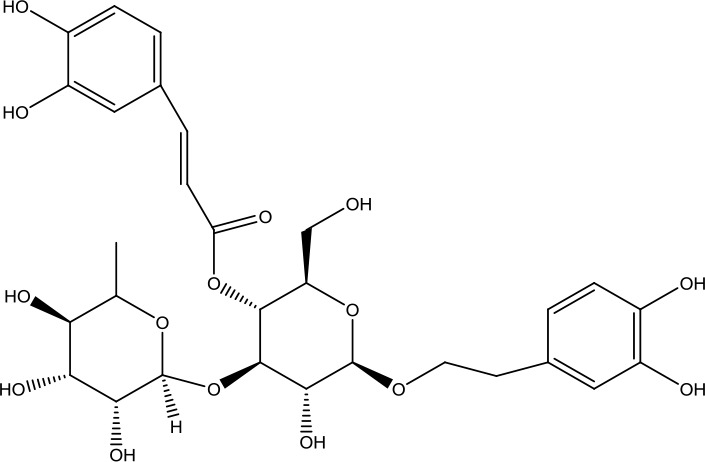 |
C29H36O15 | 61276-17-3 | Neuroprotective effect; antioxidant effect; immunomodulatory effect | Huang et al., 2012; Deng et al., 2016 | |
|
Shanzhuyu
(Cornus officinalis Siebold & Zucc.) |
Morroniside | 406.38 | 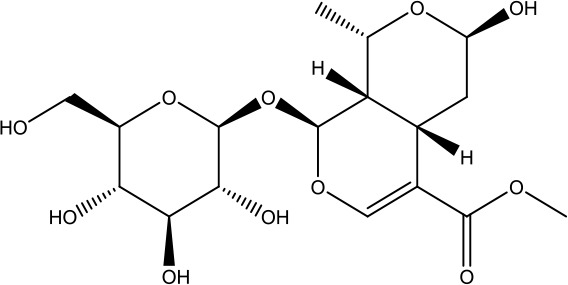 |
C17H26O11 | 25406-64-8 | Neuroprotective effect; antioxidant effect | Wang et al., 2010; Huang et al., 2018 |
| Loganin | 390.38 | 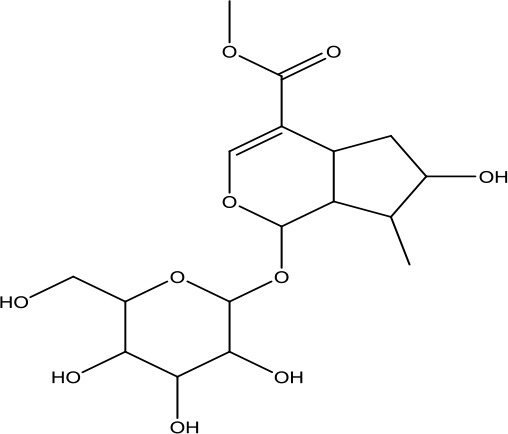 |
C17H26O10 | 18524-94-2 | Anti-inflammatory; immunomodulatory effect |
He et al., 2016; Wang et al., 2018 | |
|
Shanyao
(Dioscorea oppositifolia L.) |
Diosgenin | 414.63 | 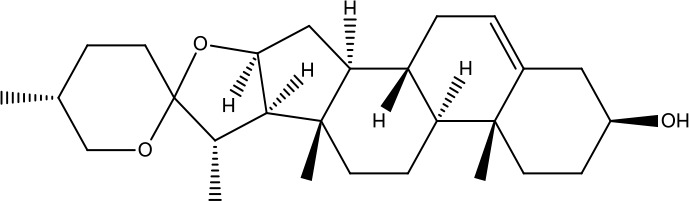 |
C27H42O3 | 512-04-9 | Anti-tumor; anti-inflammatory; antioxidant effect |
Yan et al., 2013; Guo et al., 2016 |
|
Fuling
(Poria cocos(Schw.) Wolf) |
Pachymic acid | 528.76 | 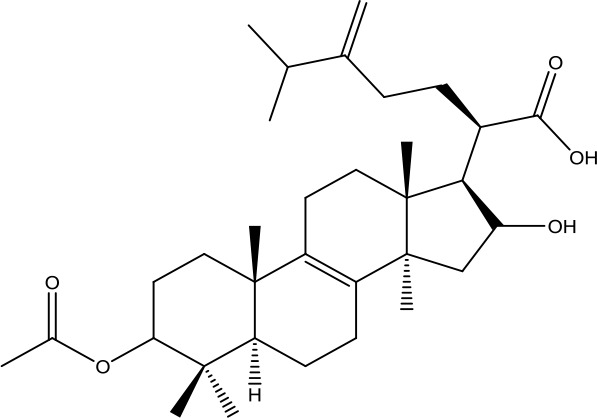 |
C33H52O5 | 29070-92-6 | Anti-tumor; anti-inflammatory; antioxidant effect; hypoglycemic effect |
Li et al., 2017; Qian et al., 2018 |
| Acetophenone | 166.18 | 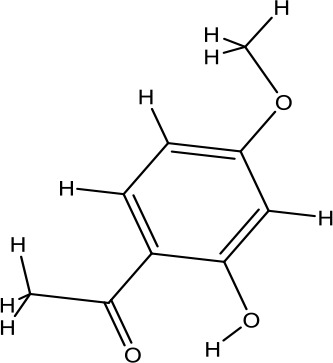 |
C9H10O3 | 552-41-0 | Anti-inflammatory; anti-allergy; neuroprotective effect; anti-tumor |
Sun et al., 2015; Tanaka et al., 2016 | |
|
Danpi
(Paeonia × suffruticosa Andrews) |
Paeoniflorin | 480.47 | 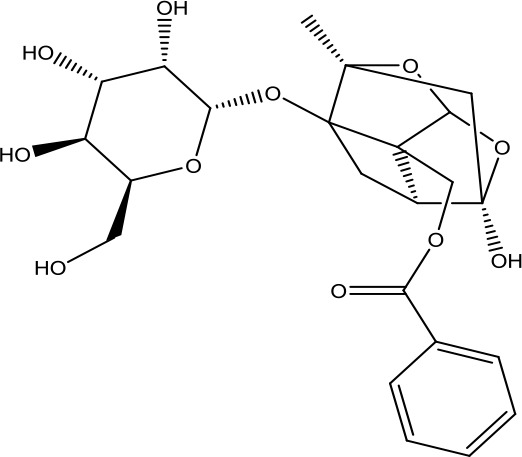 |
C23H28O11 | 23180-57-6 | Anti-inflammatory; antioxidant effect; antidepressant; enrich the blood |
Wu et al., 2009; Zhou et al., 2017 |
| Alisol A | 490.72 | 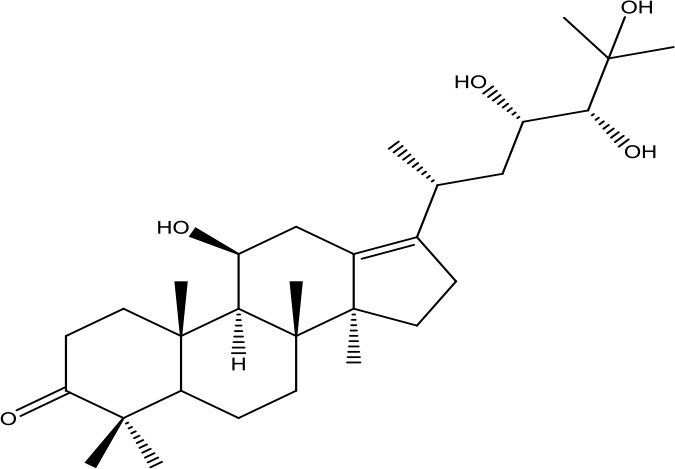 |
C30H50O5 | 19885-10-0 | Diuretic action; anti-atherosclerosis |
Yu et al., 2011; Zhang et al., 2015 | |
|
Zexie
(Alisma plantago-aquatica L.) |
Alisol B | 472.70 | 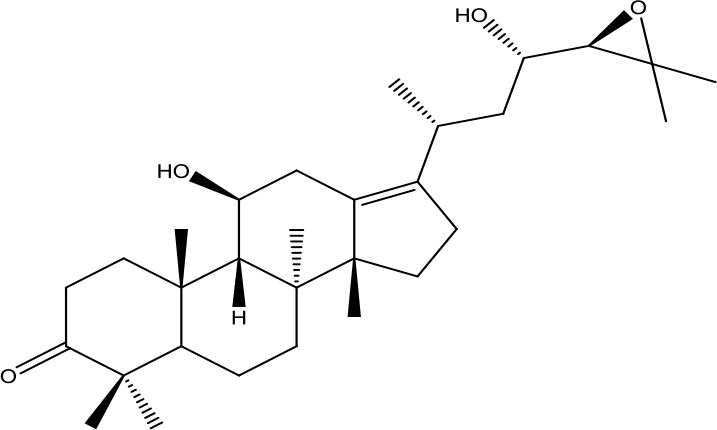 |
C30H48O4 | 18649-93-9 | Diuretic action; | Gu et al., 2015 |
Construction of the Interaction Network and Network Analysis
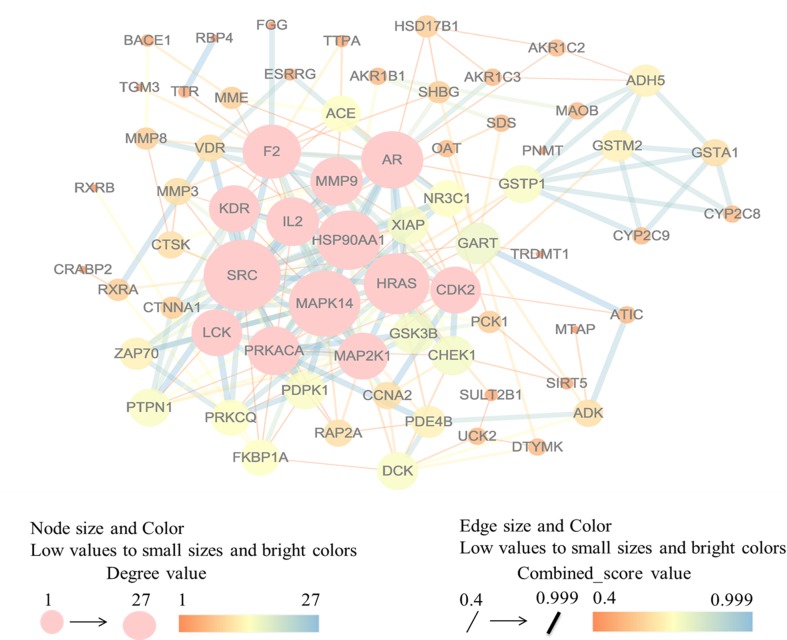
Ranked by fit score in descending order, three hundred potential targets were predicted by PharmMapper. We selected the top 10 targets of each component, if one gene symbol of a component with a different subunit remained. Subsequently, 79 potential targets were selected for further investigation. In the present study, the components of SQW could dock the same or different targets, implying that SQW had a therapeutic effect on the treatment of KYDS through a “multiple components-multiple targets” mechanism. We evaluated 79 potential targets by using the STRING version 10.0 database to identify the interactions between identified 68 proteins. Then, we constructed a PPI network ( Figure 1 ) by using Cytoscape. We deleted the isolated pairs of linked nodes, which were not meaningful. The resulting network was composed of 68 nodes and 229 edges, with 27 as the maximum degree of connectivity of a node and 1 as the minimum. We evaluated a node with a degree, which denotes the number of edges between a node and other nodes in a network. A high-degree node was the most influential node in the network, and a hub node was a component of a network with a high-degree node. The average degree of connectivity of the nodes in the network was 6.74, and the standard deviation was 5.84. In this study, we selected the hub nodes with a degree of connectivity set as ≥ the mean value + standard deviation. SRC (degree = 27), MAPK14 (degree = 24), HRAS (degree = 21), HSP90AA1 (degree = 20), AR (degree = 19), F2 (degree = 17), PRKACA (degree = 14), MMP9 (degree = 14), IL2 (degree = 14), MAP2K1 (degree = 13), LCK (degree = 13), KDR (degree = 13), and CDK2 (degree = 13). The hub nodes maintain the stability of the network and show the occurrence of KYDS, which involves multiple genes and multi-dimensional regulation.
Figure 1.
Candidate target genes identified in the protein-protein interaction network constructed using Cytoscape software.
GO Enrichment and Pathway Analysis for Potential Targets of SQW
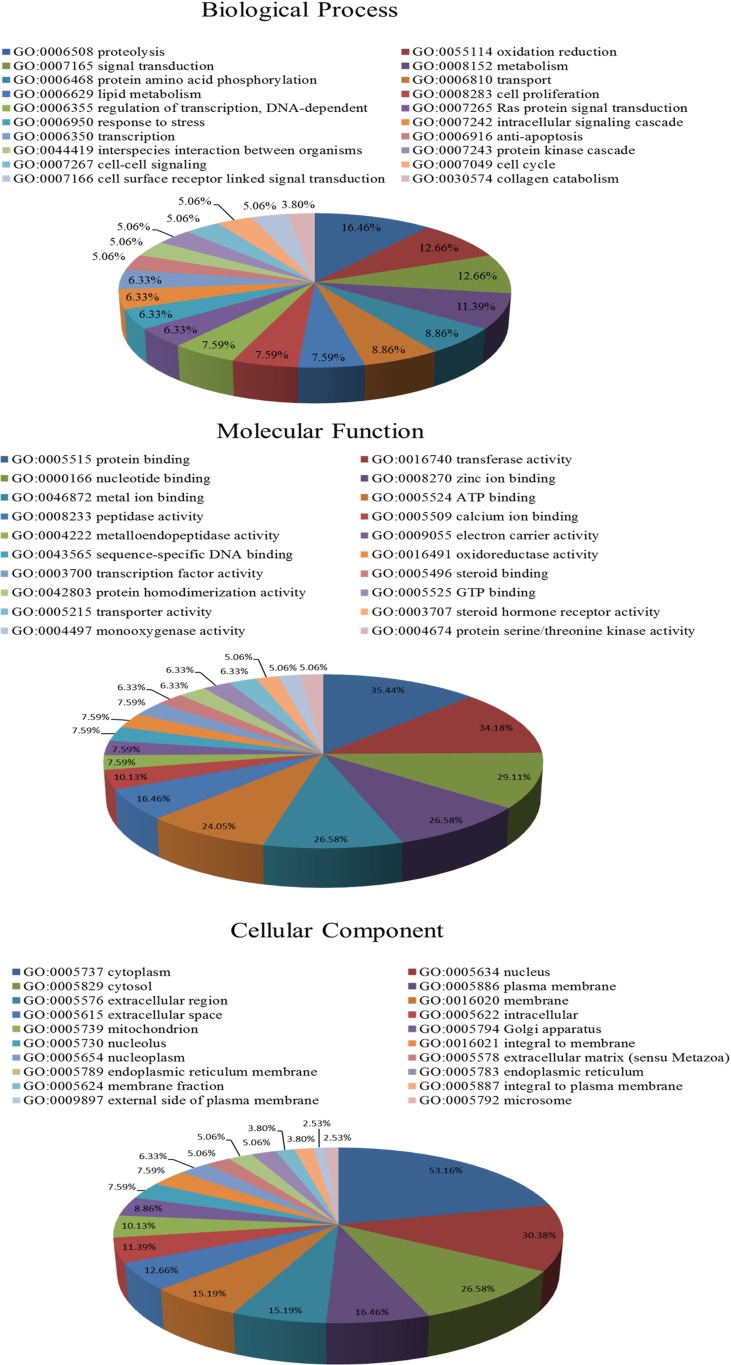
We imported the selected potential 79 target genes into the Molecule Annotation System for GO enrichment and pathway analysis. GO analysis results revealed that the functions of these potential targets are related to many biological processes that may be important for the occurrence and development of KYDS, such as proteolysis, oxidation reduction, signal transduction, and metabolism. Binding (protein, nucleotide, zinc ion, metal ion) and activity (transferase, peptidase) are closely related in molecular function and the cellular components, including cytoplasm, nucleus, and cytosol, these proteins were ranked highly as potential targets ( Figure 2 ).
Figure 2.
GO enrichment analysis of the potential target genes predicted in the PharmMapper database. (A) Biological process enrichment; (B) molecular function enrichment; (C) cell component enrichment.
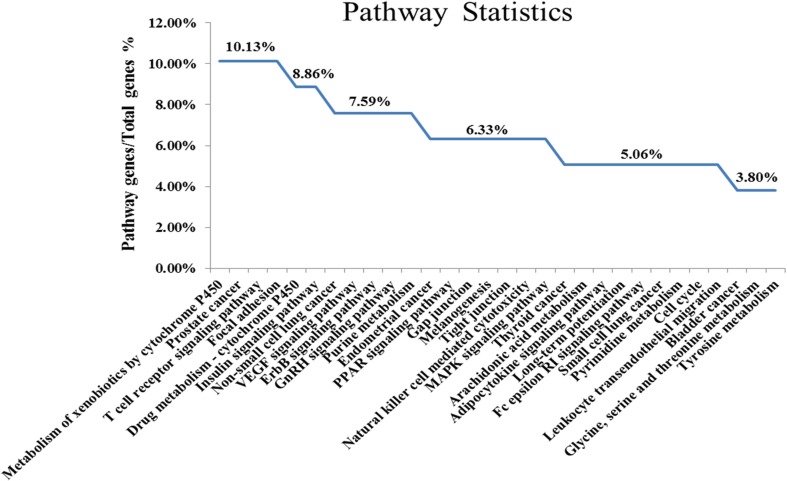
A total of 105 pathways were obtained by GO analysis, from which we selected the top 76 pathways that met the criterion of p < 0.05. Numerous pathways for potential target genes were identified. Our study found that the ErbB signaling pathway, VEGF signaling pathway, and MAPK signaling pathway are associated with signal transduction, the insulin signaling pathway, metabolism of xenobiotics by cytochrome P450, drug metabolism—cytochrome P450, and the PPAR signaling pathway. Androgen and estrogen metabolism are associated with the endocrine system. The focal adhesion and the T cell receptor signaling pathway are also closely related to immunological stress or inflammation. Moreover, we found some disease-related pathways such as prostate cancer, non-small cell lung cancer, endometrial cancer, and thyroid cancer, which indicate that SQW has a potential application in other diseases ( Figure 3 ). The results prompted that SQW ameliorated the imbalance of body by regulating the neurological, endocrine, and immune processes.
Figure 3.
KEGG pathway enrichment of the potential target genes in SQW (top 30).
Pharmacology Network of SQW
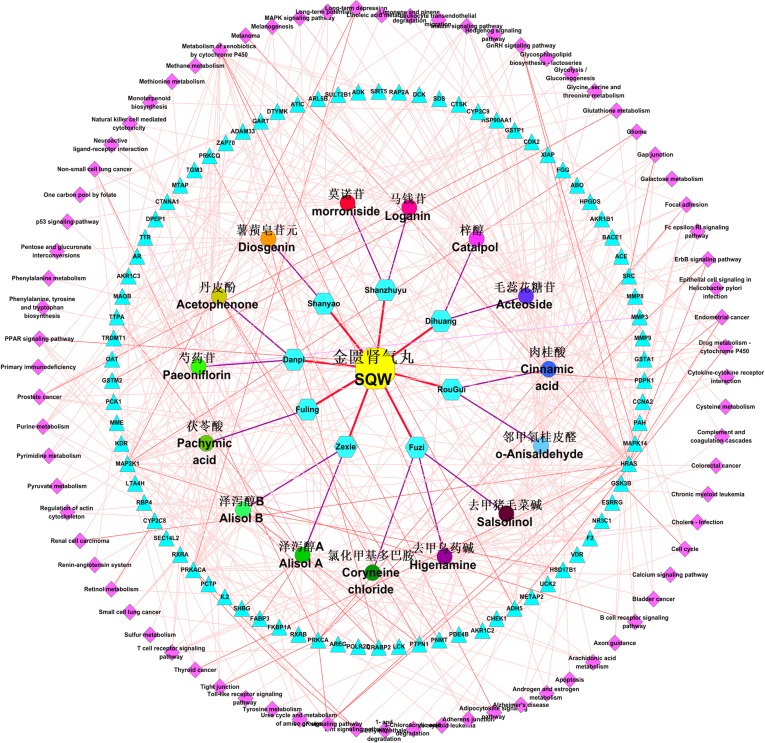
We constructed a pharmacology network of SQW ( Figure 4 ) using the Cytoscape software, which showed the relationships among the constituents, chemical components, and potential targets of SQW and the selected 76 pathways (p < 0.05). We obtained a preliminary understanding of the mechanism of SQW through this network. The potential targets of the effective components are distributed in different metabolic pathways to jointly affect the occurrence and development of KYDS.
Figure 4.
Pharmacology network of the “components-targets-pathways” regulated by SQW (pink. diamonds indicate pathways, cerulean triangles indicate targets, different colored circles indicate the chemical components, cerulean hexagons indicate the herbs in SQW, and the yellow octagon represents SQW. Red lines represent the relation of herbs with SQW; purple lines indicate the relation of herbs between its chemical components; and light pink lines indicate the relation of chemical components, component targets, and component pathways).
Adenine-Induced KYDS
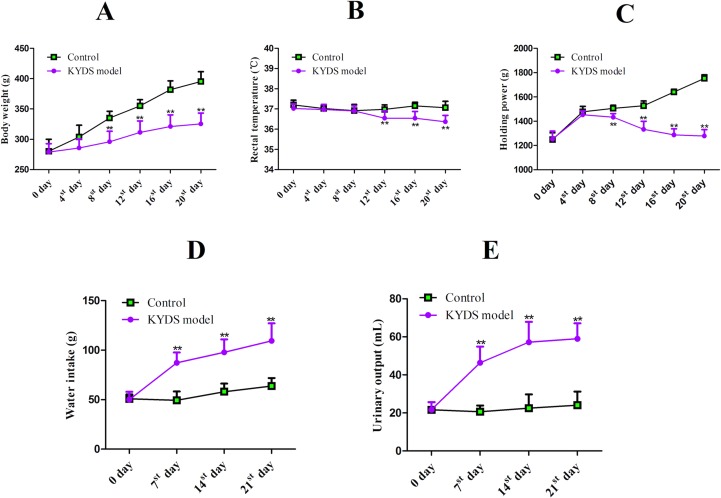
To validate the establishment of the animal model, the body weight, rectal temperature, and the holding power were measured on days 0, 4, 8, 12, 16, and 20. The results ( Figure 5A, B, and C ) demonstrated that the body weight, temperature, and the holding power values of the KYDS model rats gradually decreased as the time increased compared to those of the rats in the control groups (p < 0.01), whereas the water intake and urinary output of KYDS model rats were higher than those of the rats in the control groups (p < 0.01) on the 7th, 14th, and 20th days ( Figure 5D and E ). As shown in Table 3 , the contents of BUN, Scr, ACTH, and CORT were determined in rat serum on the 21st day. The BUN and Scr of the KYDS model were significantly increased (p < 0.01), whereas the ACTH and CORT of the KYDS model rats were significantly lower than those of the rats in the control group (p < 0.01). Moreover, the concentration of 17-OHCS in the KYDS rats was decreased compared with that in the control group rats (p < 0.01), but the U-TP in the urine of KYDS model rats was significantly increased (p < 0.01). These results indicated that the rats presented symptoms such as sluggishness, languorousness, and a crouched posture, which are the typical pathological features of KYDS. The biochemical results indicated that the KYDS model was successfully established for subsequent experiments.
Figure 5.
The effect of adenine on body weight (A), rectal temperature (B), holding power (C), water intake (D), and urinary output (E) in rats. **p < 0.01, the KYDS model group (n = 20) versus the control group (n = 10). Values are presented as mean values ± SD. The p-values were calculated using a one-way ANOVA.
Table 3.
Effects of adenine on BUN, Scr, ACTH, CORT, U-TP, and 17-OHCS in rats.
| Groups | BUN (mmol/l) | Scr (µmol/l) | ACTH (ng/ml) | CORT (ng/ml) | U-TP (mg/24 h) | 17-OHCS (nmol/l) |
|---|---|---|---|---|---|---|
| Control | 6.12 ± 0.95 | 58.70 ± 2.50 | 88.93 ± 6.43 | 64.89 ± 6.57 | 21.72 ± 5.90 | 29.20 ± 9.62 |
| KYDS model | 45.55 ± 13.18** | 223.00 ± 64.19** | 74.26 ± 16.80** | 41.87 ± 18.20** | 30.27 ± 3.53** | 22.27 ± 3.53** |
The values are presented as the means ± SD. **p < 0.01 KYDS model group (n = 20) compared with control group (n = 10).
Treatment of KYDS With SQW
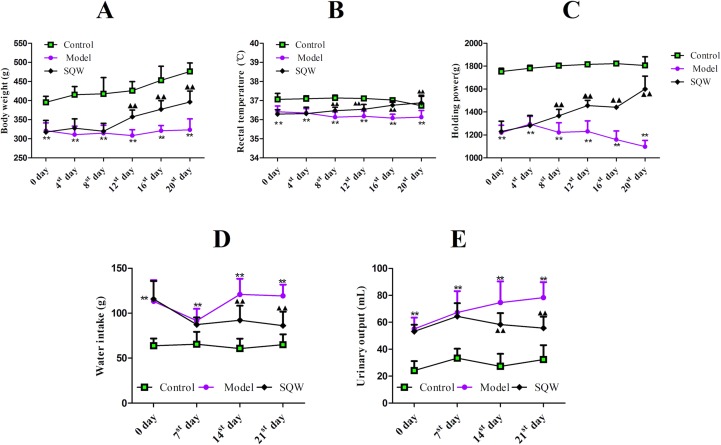
Treatment group rats recuperated after intra-gastric administration of SQW. The body weight improved significantly in the SQW-treated rats compared to that in model group rats (p < 0.01) on the 12th day of intra-gastric administration of SQW ( Figure 6A ). The rectal temperature and holding power of the SQW-treated rats were ameliorated compared with that in the model groups (p < 0.01) at the beginning of the 8th day of intra-gastric administration of SQW ( Figure 6B and C ). The levels of water intake and urinary output in the SQW-treated rats gradually returned to the baseline levels (p < 0.01) of the control group ( Figure 6D and E ). The body weight, rectal temperature, holding power, water intake, and urinary output of the model groups showed significant differences compared with those in the control groups (p < 0.01) throughout the treatment period. As shown in Table 4 , the SQW treatment obviously improved the numeral values of ACTH, CORT, and 17-OHCS (p < 0.01), while BUN, Scr, and U-TP showed a significant decrease compared with the model groups (p < 0.01), demonstrating that SQW could effectively ameliorate KYDS and had a therapeutic effect on the rat KYDS models.
Figure 6.
Effects of SQW on body weight (A), rectal temperature (B), holding power (C), water intake (D), and urinary output (E) in rats. **p < 0.01, the model group (n = 10) versus the control group (n = 10). ▲▲ p < 0.01, the SQW group (n = 10) versus the model group (n = 10). Values are presented as the means ± SD. The p-values were calculated using a one-way ANOVA.
Table 4.
Effects of SQW treatment on BUN, Scr, ACTH, CORT, U-TP, and 17-OHCS in rats.
| Groups | BUN (mmol/l) |
Scr (µmol/l) |
ACTH (ng/ml) |
CORT (ng/ml) |
U-TP (mg/24 h) |
17-OHCS (nmol/l) |
|---|---|---|---|---|---|---|
| Control | 5.69 ± 0.61 | 53.50 ± 4.18 | 101.11 ± 6.57 | 82.15 ± 8.47 | 28.73 ± 6.51 | 45.31 ± 3.62 |
| Model | 34.37 ± 12.99** | 179.79 ± 47.52** | 87.04 ± 5.07** | 38.75 ± 5.96** | 51.58 ± 8.59** | 32.01 ± 2.40** |
| SQW | 10.41 ± 3.02▲▲ | 67.21 ± 13.82▲▲ | 99.00 ± 3.56▲▲ | 55.51 ± 4.35▲▲ | 27.86 ± 5.81▲▲ | 41.19 ± 3.01▲▲ |
The values are presented as mean values ± SD. **p < 0.01 compared with the control group (n = 10); ▲▲ p < 0.01 SQW group (n = 10) compared with the model group (n = 10).
Results of qPCR for Candidate Target Genes
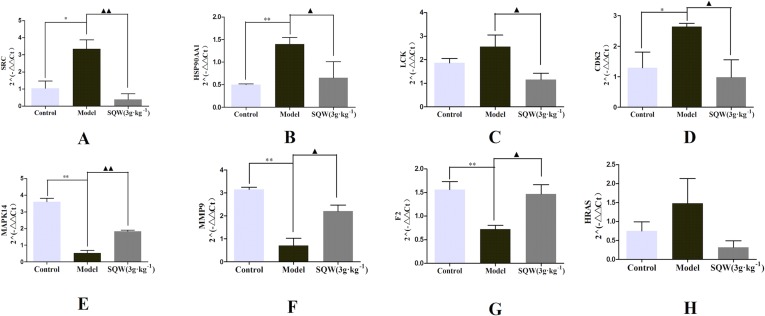
The hub genes were identified in the PPI network with high degree of connectivity. Among them, SRC, MAPK14, HRAS, HSP90AA1, F2, LCK, CDK2, and MMP9 were closely related to the emperor’s constituents. Then, we explored the effect of SQW on mRNA expression in the kidney using qPCR, as shown in Figure 7 . The mRNA expression levels of SRC, HSP90AA1, LCK, and CDK2 in the SQW-treated group were significantly decreased compared to those in the model group ( Figure 7A–D ), whereas MAPK14, MMP9, and F2 expression levels were significantly higher in the SQW-treated group than those in the model group ( Figure 7E–G ). Although the mRNA expression levels of HRAS showed no significant difference compared with the model group, there was a weak trend ( Figure 7H ). These results indicate that SQW treatment could effectively ameliorate KYDS via the synergy of multiple targets.
Figure 7.
The effects of the control, KYDS model, and SQW treatments (3 g/kg/day) on SRC (A), HSP90AA1 (B), LCK (C), CDK2 (D), MAPK14 (E), MMP9 (F), F2 (G), HRAS (H) mRNA expression in the kidney. Values are expressed as mean values ± SD. **p < 0.01 or *p < 0.05 compared with the control group; ▲▲ p < 0.01 or ▲ p < 0.05 compared with the model group.
Results of Ultra-Performance Liquid Chromatography
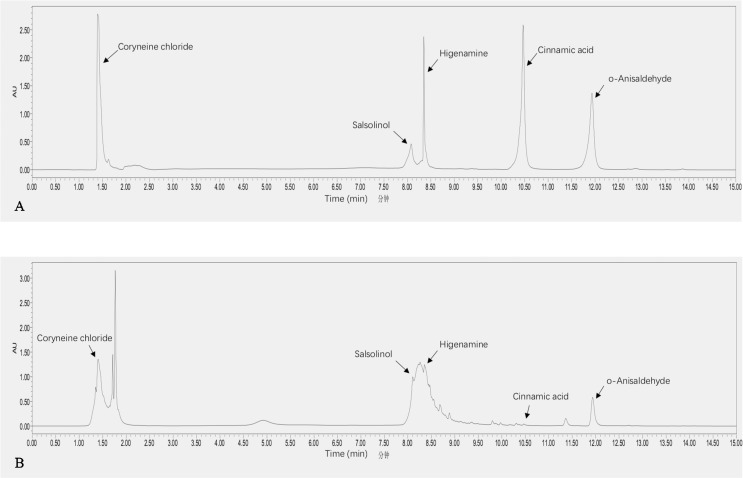
The main five components (higenamine, coryneine chloride, salsolinol, o-anisaldehyde, and cinnamic acid), which belong to the jun herbs Ramulus Cinnamomi and Radix aconiti lateralis preparata, were detected by UPLC in SQW ( Figure 8 ). The gradient program was as follow: 0–5 min, 0.5–2% A; 5–6 min, 2–30% A; 6–8 min, 30–50% A; 10–12 min, 50–70% A; 12–15 min, 70–0.5% A. The UV detection wavelength was at 208 nm. However, the peak of cinnamic acid seemed to be weak.
Figure 8.
The performances of higenamine, coryneine chloride, salsolinol, o-anisaldehyde, and cinnamic acid in SQW (40 mg/ml) (B) and in mixed standards (2 mg/ml) (A). The UV detection wavelength was at 208 nmOs.
Discussion
KYDS is most prevalent in older men and women and increases with age (Chen et al., 2010; Rong et al., 2016). TCM considers KYDS to be a complex kidney disorder, and “kidney yang” activates the power of human vitality (Lu et al., 2011; Huang et al., 2013; Zhao et al., 2013). The primary cause of KYDS is a decline in kidney-yang and transformative action, which is similar to a debilitating disease, such as chronic prostatitis, nephrotic syndrome, adrenocortical insufficiency, chronic nephritis, and diabetes mellitus in Western medicine. SQW is a typical TCM formula widely used for the treatment of chronic diseases associated with KYDS in China. Nevertheless, due to the complex pathological properties and multiple targets in KYDS, it is not easy to explore the mechanism of action of SQW using traditional methods. In this study, reverse pharmacophore docking and network pharmacology strategies were used to study the characteristics of “multiple components-multiple targets-multiple pathways” associated with SQW in the treatment of KYDS.
PharmMapper was designed to identify potential target candidates for given small molecules (drugs, natural products, or other newly discovered compounds with unidentified binding targets) by the mutual recognition of space and the ability to find the best mapping configurations (Liu et al., 2010). Ma et al. (2016) showed the “multiple components-multiple targets-multiple pathways” mechanism of Naoxintong capsule with the PharmMapper database and network pharmacology. Tao et al. (2016) used PharmMapper and the KEGG bioinformatics websites to predict the target proteins and related pathways of Chuanbei Pipa dropping pills to clarify the anti-inflammatory and cough-suppressing mechanisms. Using a network pharmacology method provides a basis for understanding the mechanism of action of SQW and is indispensable in the study of complex drugs.
We successfully predicted the drug targets of 15 compounds in SQW. The results of PPI network suggested 13 hub genes, which play important roles in the PPI. Among them, SRC, MAPK14, HRAS, HSP90AA1, F2, LCK, CDK2, and MMP9 were closely associated with higenamine, coryneine chloride, salsolinol, o-anisaldehyde, and cinnamic acid. These compounds were found in R. Cinnamomi and Radix aconiti lateralis preparata, the jun herb of SQW treating the main cause or primary symptoms of KYDS.
Proto-oncogene tyrosine-protein kinase Src (SRC) is a non-receptor tyrosine kinase. Once SRC is activated, the intracellular signal transduction cascades are triggered and subsequently multiple cellular functions such as cell proliferation, differentiation, and metabolism are modulated. Further investigations revealed that SRC activation is critically involved in the development of chronic kidney disease. Yan et al. (2016) observed that SRC kinase is activated in cultured kidney fibroblasts, and the inhibition of SRC by PP1, a selective small-molecule inhibitor of SRC kinase, appeared to disrupt TGFβ1/Smad3 and epidermal growth factor receptor (EGFR) signaling. Another study demonstrated that the inhibitor of SRC kinase effectively blocked the expression of α-SMA, which is associated with the progression of renal fibrogenesis (Hu et al., 2014). Even more, SRC can be activated by autophosphorylation of Tyr416, which is induced in response to a wide variety of cytokines/growth factors/transmembrane receptor proteins, including receptor tyrosine kinases, cytokine receptors, TGF-β1, and EGF (Yan et al., 2016; Zhou and Liu, 2016). Thus, SRC may be a potential therapeutic target for the treatment of chronic renal fibrosis with KYDS.
Mitogen-activated protein kinase 14 (MAPK14) encodes P38 mitogen-activated protein kinase and can be activated by various environmental stressors and pro-inflammatory cytokines (Han et al., 2015). MAPK14 regulates the activation of several transcription factors responses, including gene expression, growth, inflammation, metabolism, and apoptosis (Umasuthan et al., 2015). MAPK14 activity-deficient mice had less kidney dysfunction, inflammation, and apoptosis in acute folate nephropathy, while MAPK14 siRNA targeting decreased inflammation and cell death in cultured tubular cells (Ortiz et al., 2017). We conclude that MAPK14 promoted kidney injury through the promotion of inflammation and cell death and that it is a putative novel therapeutic target of SQW to ameliorate KYDS.
HRAS, a small GTPase from the Ras family, encodes the GTPase HRas, which is also known as the transforming protein p21 (Sugita et al., 2018). HRAS plays a role in regulating the growth, differentiation, and death of endothelial cells while enhancing the effects of the growth factor (Burgoyne et al., 2012). Moreover, HRAS participates in focal adhesion and the MAPK pathway by relieving inflammation (Tao et al., 2016).
Heat shock protein (HSP) is a highly conserved protein that is synthesized in response to physical, chemical, biological, and/or mental stimulation. Heat shock protein HSP 90α (HSP90AA1) belongs to the HSP90 protein superfamily, which is a molecular chaperone of numerous oncoproteins and a mediator of cellular homeostasis to maintain cell survival under stimulation (Trepel et al., 2010). Hsp 90 inhibition represses the TLR4-mediated NF-κB activity primarily through IKK to reduce renal ischemia-reperfusion acute injury (O’Neill et al., 2015). Moreover, inhibiting HSP90 activation prevents the development of renal fibrosis through the degradation of TβRII depending on Smurf2-mediation (Noh et al., 2012). These intriguing findings suggest that the kidney-protective functions of SQW may occur by regulating the expression of HSP90AA1.
Coagulation factor II (F2) encodes the prothrombin protein, which functions in blood homeostasis, inflammation, and wound healing. Qi deficiency and blood stasis are the key factors of KYDS, which is characterized by decreased gasification, a disorder of vital energy and blood, and cold limbs. We speculate that blood rheology abnormalities cause the deficiency of heat production and the ability of the kidney-yang to transfer body fluid into energy. However, the function of F2 in KYDS should be further studied.
Tubular epithelial cells (TECs) play an important role in renal diseases, especially in tubulointerstitial inflammation and fibrosis, which is a pathological process involved in a variety of cytokines and inflammatory mediators. Lymphocyte-specific protein-tyrosine kinase (LCK) and a SRC family protein-tyrosine kinase are located in the cytoplasm of TECs and form the key signal transduction molecule in the process of intracellular signal transduction (Singh et al., 2017). Li et al. have investigated the effect of the LCK pathway activation on the IL-12 signal transduction of TECs and found that LCK may regulate the LCK c-Jun signaling pathways in TEC, while the inflammation of TEC mediated by the activation of the LCK pathway is related to the expression of c-Jun promoted by IL-12 (Li et al., 2001).
Glomerular mesangial cell proliferation is a common pathological feature of many glomerular diseases (Lin et al., 2017). Cyclin-dependent kinase 2 (CDK2) is a serine/threonine-protein kinase involved in the control of the cell cycle. Yu et al. (2007) observed that the proliferation of mesangial cells is directly related to the high expression of CDK2, which indicates that SQW probably improves KYDS by depressing the expression of CDK2.
Renal fibrosis is a common disease with pathological characteristics of the accumulation of extracellular matrix (ECM) and also strongly associated with the progression of chronic kidney disease to end-stage renal disease. Matrix metalloproteinases (MMPs) are renal, physiological regulators of ECM degradation. Matrix metalloproteinase 9 (MMP9), a 92 kDa type IV collagenase, can specifically degrade type IV and V collagens and gelatin to maintain homeostasis of the ECM in the kidney (Lenz et al., 2000). ECM components accumulate due to an imbalance in ECM production and defective ECM degradation by proteolytic enzymes during renal fibrosis (Tsai et al., 2012). The results of GO enrichment showed that protein hydrolysis has an important role, which is consistent with the function of MMP9. The preliminary results in our research demonstrated that the medicated serum of 3.0 and 6.0 g/kg SQW significantly increased the expression of MMP9 protein in NRK-52E cells. Target prediction also showed that salsolinol is associated with MMP9, MMP3, and MMP8, suggesting an interaction relationship between SQW and the matrix metalloproteinases (MMPs) family. These findings suggest that SQW reduces the accumulation of EMC in renal epithelial cells via the metalloproteinases.
Moreover, the effect of SQW on AQPs, the aquaporin channel family, and on the relation between AQP1 and MMP9 showed a trend of enhancement to promote the migration of renal TECs for renal injury repair. Moreover, SQW has a therapeutic effect on water metabolism disorder by promoting the mRNA and protein expression levels of AQP2. Furthermore, SQW significantly increased the ACTH, while CORT regulated the hypothalamic-pituitary-adrenal axis to exploit the R. Cinnamomi and Radix aconiti lateralis preparata role in tonifying the kidney yang (Xu et al., 2014). These results are consistent with the therapeutic effect of SQW observed in the present paper.
Our study showed that SQW treatment dramatically improved the common physiological symptoms of KYDS and had protective effects on the hypothalamic-pituitary-adrenal axis in KYDS model rats. The potential targets of SQW were identified using PharmMapper, bioinformatics, and PPI network analysis. We found 79 potential target genes and identified SRC, MAPK14, HRAS, HSP90AA1, F2, LCK, CDK2, and MMP9 as the key potential therapeutic targets of SQW. The 79 target genes mainly related to the metabolism of xenobiotics by cytochrome P450, prostate cancer, and the T cell receptor signaling pathway. Wang et al. (2016) also reported 14 important potential targets associated with the aldosterone-regulated sodium reabsorption and adrenergic signaling pathways. However, further studies are required to confirm the results of this study. We explored the potential targets and pathways of SQW from a different perspective and using novel methods, and we conclude that multiple components, multiple targets, and multiple pathways of SQW led to a therapeutic effect on KYDS. This study shows that cell proliferation, differentiation, apoptosis, migration (SRC, HRAS, HSP90AA1, CDK2), and ameliorating chronic kidney disease (MAPK14, F2, LCK, MMP9) appear to play important roles in the therapeutic effect of SQW.
In this study, SQW ameliorated KYDS characteristics in rats presumably by eight target genes. Further studies are needed to analyze the protein levels of these targets. Moreover, other species should be considered for further verification.
Conclusion
In summary, SQW has a therapeutic effect on the treatment of KYDS through the “multiple components-multiple targets-multiple pathways” mechanism. We found that SRC, MAPK14, HRAS, HSP90AA1, F2, LCK, CDK2, and MMP9 genes were highly involved and may be potential targets in the treatment of KYDS.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
This study was carried out in accordance with the recommendations of the Ethics of Committee of Zhejiang Chinese Medical University (permit number: SYXK 2013-0115). The protocol was approved by the Ethics of Committee of Zhejiang Chinese Medical University (permit number: SYXK 2013-0115). All procedures were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Author Contributions
JZ, CH, YY and CL conceived and designed the experiments. JZ and CH performed the experiments. HC, XZ and YZ analyzed the data. JZ, HC, TE, YY and CL contributed reagents/materials/analysis tools. JZ and TE wrote and edited the paper. JZ and CH contributed equally to this work. TE, YY and CL contributed equally to this work.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81673839 and 81373507), the Project of National Great New Drug Research and Development (No. 2012ZX09503001-001), and Science and Technology Innovation Project of Zhejiang Province College Students (Grant No. 2017R410049) and Zhejiang Province Administration of Traditional Chinese Medicine (no. 2015ZA073). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
17-OHCS, 17-hydroxy-corticosteroid; ACTH, adrenocorticotrophic hormone; AQP, aquaporin; BUN, blood urea nitrogen; CAS, Chemical Abstract Service; CDK2, cell division protein kinase 2; CNKI, China National Knowledge Infrastructure; CORT, cortisol; ECM, extracellular matrix; F2, prothrombin; GO, Gene Ontology; HPLC, high performance liquid chromatography; HRAS, GTPase HRas; HSP90AA1, heat shock protein HSP 90α; KEGG, Kyoto Encyclopedia of Genes and Genomes; KYDS, kidney yang deficiency syndrome; LCK, Lymphocyte-specific protein-tyrosine kinase LCK; MAPK14, mitogen-activated protein kinase 14; MMP9, matrix metalloproteinase-9; PPI, protein–protein interaction; Scr, serum creatinine; SQW, Shen Qi Wan; SRC, proto-oncogene tyrosine-protein kinase SRC; STRING, (Search Tool for the Retrieval of Interacting Genes/Proteins; TCM, traditional Chinese medicine; TcmSP™, Traditional Chinese Medicine Systems Pharmacology Database; UPLC, ultra-performance liquid chromatography; U-TP, urine total protein.
References
- Burgoyne J. R., Haeussler D. J., Kumar V., Ji Y., Pimental D. R., Zee R. S., et al. (2012). Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. FASEB J. 26, 832–841. 10.1096/fj.11-189415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. T., Chang W. L., Hsu J. C., Shih Y., Chou S. T. (2013). Chemical composition and tyrosinase inhibitory activity of Cinnamomum cassia essential oil. Bot Stud. 54, 10. 10.1186/1999-3110-54-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. H., Liang X. T. (1982). Studies on the constituents of lateral root of Aconitum carmichaeli Debx. Yao Xue Xue Bao 17, 792–794. 10.16438/j.0513-4870.1982.10.011 [DOI] [PubMed] [Google Scholar]
- Chen R. Q., Wong C. M., Cao K. J., Lam T. H. (2010). An evidence-based validation of traditional Chinese medicine syndromes. Complement. Ther. Med. 18, 199–205. 10.1016/j.ctim.2010.05.036 [DOI] [PubMed] [Google Scholar]
- Deng Z. J., Liu R. X., Qi Y. Q., Wang J. J., Liu X. W., Guo J. W. (2016). Pharmacokinetic effect of acteoside in blood deficiency rats. Zhong Yao Cai 39, 395–397. 10.13863/j.issn1001-4454.2016.02.039 [DOI] [PubMed] [Google Scholar]
- Gu W., Geng C., Xue W., Wu Q., Chao J., Xu F., et al. (2015). Characterization and function of the 3-hydroxy-3-methylglutaryl-CoA reductase gene in Alisma orientale (Sam). Plant Physiol. Biochem. 97, 378–389. 10.1016/j.plaphy.2015.10.031 [DOI] [PubMed] [Google Scholar]
- Guo Y., Xing E., Liang X., Song H., Dong W. (2016). Effects of total saponins from Rhizoma Dioscoreae Nipponicae on expression of vascular endothelial growth factor and angiopoietin-2 and Tie-2 receptors in the synovium of rats with rheumatoid arthritis. J. Chin. Med. Assoc. 79, 264–271. 10.1016/j.jcma.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Han D., Scott E. L., Dong Y., Raz L., Wang R., Zhang Q. (2015). Attenuation of mitochondrial and nuclear p38alpha signaling: a novel mechanism of estrogen neuroprotection in cerebral ischemia. Mol. Cell. Endocrinol. 400, 21–31. 10.1016/j.mce.2014.11.010 [DOI] [PubMed] [Google Scholar]
- Hasegawa A., Yoshino M., Nakamura H., Ishii I., Watanabe T., Kiuchi M., et al. (2002). Identification of inhibitory component in cinnamon-O-methoxycinnamaldehyde inhibits CYP1A2 and CYP2E1-. Drug Metab. Pharmacokinet. 17, 229–236. 10.1007/s00228-006-0253-5 [DOI] [PubMed] [Google Scholar]
- He K., Song S., Zou Z., Feng M., Wang D., Wang Y., et al. (2016). The hypoglycemic and synergistic effect of loganin, morroniside, and ursolic acid isolated from the fruits of Cornus officinalis. Phytother. Res 30, 283–291. 10.1002/ptr.5529 [DOI] [PubMed] [Google Scholar]
- Hu M., Che P., Han X., Cai G. Q., Liu G., Antony V., et al. (2014). Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J. Pharmacol. Exp. Ther. 351, 87–95. 10.1124/jpet.114.216044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Yang J., Lu X., Deng Y., Xiong Z., Li F. (2013). An integrated plasma and urinary metabonomic study using UHPLC-MS: intervention effects of Epimedium koreanum on ‘Kidney-Yang Deficiency syndrome’ rats. J. Pharm. Biomed. Anal. 76, 200–206. 10.1016/j.jpba.2012.12.022 [DOI] [PubMed] [Google Scholar]
- Huang J., Zhang Y., Dong L., Gao Q., Yin L., Quan H., et al. (2018). Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. J. Ethnopharmacol. 213, 280–301. 10.1016/j.jep.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Huang L. T., Sun S. P., Zheng Y. (2012). Simultaneous determination and optimization of extraction process of catalpol and acteoside from rehmanniae radix. Zhong Yao Cai 35, 1318–1322. 10.13863/j.issn1001-4454.2012.08.042 [DOI] [PubMed] [Google Scholar]
- Lenz O., Elliot S. J., Stetler-Stevenson W. G. (2000). Matrix metalloproteinases in renal development and disease. J. Am. Soc. Nephrol. 11, 574–581. 10.1089/end.2000.14.225 [DOI] [PubMed] [Google Scholar]
- Li M., Wang S., Zhang Y., He L. (2010). An online coupled cell membrane chromatography with. LC/MS method for screening compounds from Aconitum carmichaeli Debx. acting on VEGFR-2. J. Pharm. Biomed. Anal. 53, 1063–1069. 10.1016/j.jpba.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Li Q., Li Z., Li Y. (2001). Investigation of lck on IL-12 signal transduction in tubular epithelial cells. Zhonghua Wei Sheng Wu Xue He Mian Yi Xue Za Zhi 21, 590–593. 10.3760/j:issn:0254-5101.2001.06.002 [DOI] [Google Scholar]
- Li S., Zhang J., Li S., Liu C., Liu S., Liu Z. (2017). Extraction and separation of lactate dehydrogenase inhibitors from Poria cocos (Schw). J. Sep. Sci. 40, 1773–1783. 10.1002/jssc.201700054 [DOI] [PubMed] [Google Scholar]
- Li Z., Wen R., Du Y., Zhao S., Zhao P., Jiang H., et al. (2019). Simultaneous quantification of fifteen compounds in rat plasma by LC-MS/MS and its application to a pharmacokinetic study of Chaihu-Guizhi decoction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1105, 15–25. 10.1016/j.jchromb.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Lin N., Ji Z., Huang C. (2017). Smad7 alleviates glomerular mesangial cell proliferation via the ROS-NF-κB pathway. Exp. Cell Res. 361, 210–216. 10.1016/j.yexcr.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Liu C., Zhu C., Wang G., Xu R., Zhu Y. (2015). Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal ischemia-reperfusion injury in mice. Inflamm. Res. 64, 395–403. 10.1007/s00011-015-0817-x [DOI] [PubMed] [Google Scholar]
- Liu H., Zeng L., Yang K., Zhang G. (2016). A network pharmacology approach to explore the pharmacological mechanism of xiaoyao powder on anovulatory infertility. Evid. Based Complement. Alternat. Med. 2016, 2960372. 10.1155/2016/2960372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ouyang S., Yu B., Liu Y., Huang K., Gong J., et al. (2010). PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 38, 609–614. 10.1093/nar/gkq300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Xiong Z., Li J., Zheng S., Huo T., Li F. (2011). Metabonomic study on ‘kidney-yang. Talanta 83, 700–708. 10.1016/j.talanta.2010.09.026 [DOI] [PubMed] [Google Scholar]
- Ma N., Ding Y., Zhang Y., Zhang T., Yi Y., Wang B. (2018). Chemical fingerprinting and quantification of Chinese Cinnamomi Cortex by ultra high-performance liquid chromatography coupled with chemometric methods. Molecules 23, E2214. 10.3390/molecules23092214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Lv B., Li P., Jiang X., Zhou Q., Wang X., et al. (2016). Identification of “multiple components-multiple targets-multiple pathways” associated with naoxintong capsule in the treatment of heart diseases using UPLC/Q-TOF-MS and network pharmacology. Evid. Based Complement. Alternat. Med. 2016, 9468087. 10.1155/2016/9468087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Y., Zhou X., Liu Q., Zhang A., Guan Y., Lin S., et al. (2016). Serum metabolomics strategy for understanding pharmacological effects of ShenQi pill acting on kidney yang deficiency syndrome. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1026, 217–226. 10.1016/j.jchromb.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Noh H., Kim H. J., Yu M. R., Kim W. Y., Kim J., Ryu J. H., et al. (2012). Heat shock protein 90 inhibitor attenuates renal fibrosis through degradation of transforming growth factor-β type II receptor. Lab. Invest. 92, 1583–1596. 10.1038/labinvest.2012.127 [DOI] [PubMed] [Google Scholar]
- O’Neill S., Humphries D., Tse G., Marson L. P., Dhaliwal K., Hughes J., et al. (2015). Heat shock protein 90 inhibition abrogates TLR4-mediated NF-κB activity and reduces renal ischemia-reperfusion injury. Sci. Rep. 5, 12958. 10.1038/srep12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A., Husi H., Gonzalez-Lafuente L., Valiño-Rivas L., Fresno M., Sanz A. B., et al. (2017). Mitogen-activated protein kinase 14 promotes AKI. J. Am. Soc. Nephrol. 28, 823–836. 10.1681/ASN.2015080898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poornima P., Kumar J. D., Zhao Q., Blunder M., Efferth T. (2016). Network pharmacology of cancer: from understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacol. Res. 111, 290–302. 10.1016/j.phrs.2016.06.018 [DOI] [PubMed] [Google Scholar]
- Qian Q., Zhou N., Qi P., Zhang Y., Mu X., Shi X., et al. (2018). A UHPLC-QTOF-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1102–1103, 34–44. 10.1016/j.jchromb.2018.10.011 [DOI] [PubMed]
- Qiu J. (2007). Traditional medicine: a culture in the balance. Nature 448, 126–128. 10.1038/448126a [DOI] [PubMed] [Google Scholar]
- Rong R., Li R. R., Hou Y. B., Li J., Ding J. X., Zhang C. B., et al. (2016). Mahuang-Xixin-Fuzi decoction reduces the infection of influenza A virus in kidney-yang deficiency syndrome mice. J. Ethnopharmacol. 192, 217–224. 10.1016/j.jep.2016.07.017 [DOI] [PubMed] [Google Scholar]
- Singh D. K., Deshmukh R. K., Narayanan P. K., Shivaji S., Siva A. B. (2017). SRC family kinases in hamster spermatozoa: evidence for the presence of LCK. Reproduction 153, 655–669. 10.1530/REP-16-0591 [DOI] [PubMed] [Google Scholar]
- Sugita S., Enokida H., Yoshino H., Miyamoto K., Yonemori M., Sakaguchi T., et al. (2018). HRAS as a potential therapeutic target of salirasib RAS inhibitor in bladder cancer. Int. J. Oncol. 53, 725–736. 10.3892/ijo.2018.4435 [DOI] [PubMed] [Google Scholar]
- Sun M., Huang L., Zhu J., Bu W., Sun J., Fang Z. (2015). Screening nephroprotective compounds from Cortex Moutan by mesangial cell extraction and UPLC. Arch. Pharm. Res. 38, 1044–1053. 10.1007/s12272-014-0469-3 [DOI] [PubMed] [Google Scholar]
- Tan Y., Liu X., Lu C., He X., Li J., Xiao C., et al. (2014). Metabolic profiling reveals therapeutic biomarkers of processed Aconitum carmichaeli Debx in treating hydrocortisone induced kidney-yang deficiency syndrome rats. J. Ethnopharmacol. 152, 585–593. 10.1016/j.jep.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Tanaka R., Shibata H., Sugimoto N., Akiyama H., Nagatsu A. (2016). Application of a quantitative (1)H-NMR method for the determination of paeonol in moutan cortex, hachimijiogan and keishibukuryogan.. J. Nat. Med. 70, 797–802. 10.1007/s11418-016-1003-3 [DOI] [PubMed] [Google Scholar]
- Tang J., Aittokallio T. (2014). Network pharmacology strategies toward multi-target anticancer. Curr. Pharm. Des. 20, 23–36. 10.2174/13816128113199990470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Liu L., Qiu H., Shi W., Mao D. (2018). Analysis of gene expression and functional changes of adrenal gland in a rat model of kidney yang deficiency syndrome treated with Sini decoction. Exp. Ther. Med. 16, 3107–3115. 10.3892/etm.2018.6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Hou Y., Ma X., Liu D., Tong Y., Zhou H., et al. (2016). An integrated global chemomics and system biology approach to analyze the mechanisms of the traditional Chinese medicinal preparation Eriobotrya japonica–Fritillaria usuriensis dropping pills for pulmonary diseases. BMC Complement. Altern. Med. 16, 4. 10.1186/s12906-015-0983-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel J., Mollapour M., Giaccone G., Neckers L. (2010). Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537–549. 10.1038/nrc2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J. P., Liou J. H., Kao W. T., Wang S. C., Lian J. D., Chang H. R. (2012). Increased expression of intranuclear matrix metalloproteinase 9 in atrophic renal tubules is associated with renal fibrosis. PLoS One 7, e48164. 10.1371/journal.pone.0048164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umasuthan N., Bathige S., Noh J. K., Lee J. (2015). Gene structure, molecular characterization and transcriptional expression of two p38 isoforms (MAPK11 and MAPK14) from rock bream (Oplegnathus fasciatus). Fish Shellfish Immunol. 47, 331–343. 10.1016/j.fsi.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Wang L., Chen H., Jiang Y., Liu Z., Wang Q., Zheng X. (2018). Simultaneous determination of 11. high-polarity components from Fructus Corni: a quantitative LC-MS/MS method for improved quality control. J. Chromatogr. Sci. 56, 56–64. 10.1093/chromsci/bmx083 [DOI] [PubMed] [Google Scholar]
- Wang W., Xu J., Li L., Wang P., Ji X., Ai H., et al. (2010). Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res. Bull. 83, 196–201. 10.1016/j.brainresbull.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang A., Zhou X., Liu Q., Nan Y., Guan Y., et al. (2016). An integrated chinmedomics strategy for discovery of effective constituents from traditional herbal medicine. Sci. Rep. 6, 18997. 10.1038/srep18997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Wu H., Yu W., Yan X., Zhang X. (2015). Shenfu decoction as adjuvant therapy for improving quality of life and hepatic dysfunction in patients with symptomatic chronic heart failure. J. Ethnopharmacol. 169, 347–355. 10.1016/j.jep.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Wu H., Zhu Z., Zhang G., Zhao L., Zhang H., Zhu D., et al. (2009). Comparative pharmacokinetic study of paeoniflorin after oral administration of pure paeoniflorin, extract of Cortex Moutan and Shuang-Dan prescription to rats. J. Ethnopharmacol. 125, 444–449. 10.1016/j.jep.2009.07.019 [DOI] [PubMed] [Google Scholar]
- Wu Y. S., Chen Y. T., Bao Y. T., Li Z. M., Zhou X. J., He J. N., et al. (2016). Identification and verification of potential therapeutic target genes in berberine-treated Zucker diabetic fatty rats through bioinformatics analysis. PLoS One 11, e0166378. 10.1371/journal.pone.0166378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R., Li Y., Zheng K., Zhang T., Gao M., Li Y., et al. (2019). Er Shen Wan extract alleviates polyuria and regulates AQP 2 and AVPR 2 in a rat model of spleen-kidney yang deficiency-induced diarrhea. Biomed. Pharmacother. 110, 302–311. 10.1016/j.biopha.2018.11.147 [DOI] [PubMed] [Google Scholar]
- Xiong X., Wang P., Li X., Zhang Y. (2015). Shenqi pill, a traditional Chinese herbal formula, for the. Complement. Ther. Med. 23, 484–493. 10.1016/j.ctim.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Xu W. D., Wang X., Wang C. (2014). Study on rougui and fuzi’s role in tonifying kidney yang by shenqiwan from hypothalamic-pituitary-adrenal axis. J. Zhejiang Univ. Tradit. Chin. Med. 38, 831–836. 10.16466/j.issn1005-5509.2014.07.025 [DOI] [Google Scholar]
- Yan W., Ji L., Hang S., Shun Y. (2013). New ionic liquid-based preparative method for diosgenin from Rhizoma dioscoreae nipponicae. Pharmacogn. Mag. 9, 250–254. 10.4103/0973-1296.113282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Ma L., Zhou X., Ponnusamy M., Tang J., Zhuang M. A., et al. (2016). SRC inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int. 89, 68–81. 10.1038/ki.2015.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. L., Huang Z. F., Zhang Y. H., Liu Y. H., Liu Y. H., Chen Y., et al. (2014). Effects of steaming and baking on content of alkaloids in Aconite Lateralis Radix (fuzi). Zhongguo Zhong Yao Za Zhi 39, 4798–4803. 10.4268/cjcmm20142420 [DOI] [PubMed] [Google Scholar]
- Yao Y., Zhang X., Wang Z., Zheng C., Li P., Huang C., et al. (2013). Deciphering the combination principles of Traditional Chinese Medicine from a systems pharmacology perspective based on ma-huang decoction. J. Ethnopharmacol. 150, 619–638. 10.1016/j.jep.2013.09.018 [DOI] [PubMed] [Google Scholar]
- Youn U. J., Gu B. S., Kim K. H., Ha C., Jung I. C. (2018). Variation of main components according to the. J. Pharmacopuncture 21, 112–119. 10.3831/KPI.2018.21.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Yang H., Zhao J. (2007). Effect of valsartan on CDK_2 expression in mesangial cells of Thy1 glomerulonephritis rats. Chin. J. Integr. Tradit. West. Nephrol. 2, 81–83. 10.3969/j.issn.1009-587X.2007.02.006 [DOI] [Google Scholar]
- Yu Y., Li Q., Bi K., Xie P., Yang G., Chen X. (2011). A sensitive liquid chromatography-mass spectrometry method for simultaneous determination of alisol A and alisol A 24-acetate from Alisma orientale (Sam). Anal. Bioanal. Chem. 399, 1363–1369. 10.1007/s00216-010-4426-9 [DOI] [PubMed] [Google Scholar]
- Yuan H., Yang M., Han X., Ni X. (2018). The therapeutic effect of the Chinese Herbal Medicine Rehmanniae Radix Preparata in attention deficit hyperactivity disorder via reversal of structural abnormalities in the cortex. Evid. Based Complement. Alternat. Med. 2018, 3052058. 10.1155/2018/3052058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. H., Xin J., Fan L. X., Yin H. (2017). Intervention effects of Zuoguiwan containing serum on osteoblast through ERK1/2 and Wnt/β-catenin signaling pathway in models with kidney-yang-deficiency, kidney-yin-deficiency osteoporosis syndromes. Zhongguo Zhong Yao Za Zhi 42, 3983–3989. 10.19540/j.cnki.cjcmm.20170907.002 [DOI] [PubMed] [Google Scholar]
- Zhang Y. W., Li Q., Lv C. X., Liu X. J., Chen X. H., Bi K. S. (2015). Simultaneous determination of four active components in Alisma orientale (Sam). J. Pharm. Anal. 5, 85–92. 10.1016/j.jpha.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Li M., Wang Y., Wu J., Li J. (2014). Higenamine promotes M2 macrophage activation and. Int. Immunopharmacol. 23, 681–687. 10.1016/j.intimp.2014.10.022 [DOI] [PubMed] [Google Scholar]
- Zhao L., Wu H., Qiu M., Sun W., Wei R., Zheng X., et al. (2013). Metabolic signatures of kidney yang deficiency syndrome and protective effects of two herbal extracts in rats Using GC/TOF MS. Evid. Based Complement. Alternat. Med. 2013, 540957. 10.1155/2013/540957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Liu Y. (2016). Therapy for kidney fibrosis: is the SRC kinase a potential target? Kidney Int. 89, 12–14. 10.1016/j.kint.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Liu B., Meng J. (2017). Quality evaluation of raw moutan cortex using the AHP and Gray correlation-TOPSIS method. Pharmacogn. Mag. 13, 528–533. 10.4103/0973-1296.211029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.