Summary
N-functionalization of amines with CO2 and H2 is one of the most important processes to make use of CO2. Although noble metal-based catalysts with remarkable performance have been widely used in this process, developing efficient non-noble-metal-based catalysts remains a grand challenge. Herein, we report In2O3 nanocrystals with high density of grain boundaries (HGB-In2O3), which show excellent activity toward methylation of amines. Impressively, HGB-In2O3 achieved the optimal yield of 82.7% for N,N-dimethylaniline with a mass activity of 21.2 mmol·g−1h−1 in methylation of N-methylaniline, comparable to noble-metal-based catalysts. As a bonus, HGB-In2O3 held noticeable stability, remarkable selectivity, and comprehensive applicability. Further mechanistic studies revealed that the presence of high density of grain boundaries not only facilitated the adsorption and activation of CO2 to generate CH3OH as the intermediate but also enhanced the activation of N-H bond in amines, contributing to the attractive activity of HGB-In2O3 toward methylation of amines.
Subject Areas: Catalysis, Materials Characterization Techniques, Nanomaterials
Graphical Abstract

Highlights
-
•
We prepared In2O3 nanocrystals with high density of grain boundaries (HGB-In2O3)
-
•
HGB-In2O3 gained 82% yield of N,N-dimethylaniline in N-methylaniline methylation
-
•
The grain boundaries in In2O3 facilitated the adsorption and activation of CO2
-
•
The grain boundaries in In2O3 enhanced the activation of N-H bond in amines
Catalysis; Materials Characterization Techniques; Nanomaterials
Introduction
Owing to the superfluous consumption of fossil fuels, anthropogenic emissions of CO2 to the atmosphere are rapidly increasing, which gives rise to global warming (Bhanja et al., 2018, Molla et al., 2017, Khan et al., 2016, Li et al., 2018). In this case, reduction of CO2 into fuels utilizing electric (Fernández-Alvarez and Oro, 2018, Goeppert et al., 2014, Liu et al., 2017, Zhang et al., 2019) or solar (Sun et al., 2018, Wang et al., 2018, Hou et al., 2019) energy was considered as an efficient approach to mitigate the environmental problem. Besides, converting CO2 into high value-added fine chemicals by organic reaction is also an effective way to recycle CO2, which has attracted intensive attention around the world. In particular, N-functionalization of amines with CO2 and H2 is one of the most important processes to make use of CO2 efficiently (Liu et al., 2015, Liu et al., 2018, Nguyen et al., 2015, Sorribes et al., 2015, Li et al., 2013a, Li et al., 2013b, Du et al., 2015, Yuan and Lin, 2015, Zhang et al., 2015, Zhang et al., 2017, Toyao et al., 2017, Ju et al., 2017, Choi and Hong, 2018, Beydoun et al., 2014, Li et al., 2013a, Li et al., 2013b, Beydoun et al., 2013, Kon et al., 2014, Cui et al., 2014a, Cui et al., 2014b, Yang et al., 2015, Elangovan et al., 2016, Natte et al., 2017, Cui et al., 2014a, Cui et al., 2014b). Thanks to the efforts from a number of research groups, noble metal-based catalysts including both homogeneous and heterogeneous ones, which have been proved to achieve high activity and selectivity, are mainly used in this process. Typical noble metal-based catalysts for the reaction include Re, Pt, Pd, Ru, Rh, Au, and their complexes (Liu et al., 2015, Nguyen et al., 2015, Sorribes et al., 2015, Li et al., 2013a, Li et al., 2013b, Du et al., 2015, Yuan and Lin, 2015, Zhang et al., 2015, Zhang et al., 2017, Toyao et al., 2017, Ju et al., 2017, Choi and Hong, 2018, Beydoun et al., 2014, Li et al., 2013a, Li et al., 2013b, Beydoun et al., 2013, Kon et al., 2014, Cui et al., 2014a, Cui et al., 2014b). For instance, a homogeneous well-defined [Ru(triphos)(tmm)] catalyst was reported by Beydoun and coworkers. In the reductive methylation of amines by using CO2 and H2, the desired product was isolated with 83% yield (Beydoun et al., 2014). Another notable example is the ruthenium-pincer-type complexes, which were found to attain remarkable turnover numbers of up to 1,940,000 (Zhang et al., 2015). Cui et al. described an efficient procedure for the reductive amination of CO2 using Pd/CuZrOx catalyst, which can be realized with up to 97% yield under relatively mild reaction conditions (Cui et al., 2014a, Cui et al., 2014b). As for non-noble-metal-based catalysts, active and high-cost reactants, such as methanol and phenylsilane instead of CO2 and H2, were generally required in N-functionalization of amines to achieve the desired catalytic activity (Yang et al., 2015, Elangovan et al., 2016, Natte et al., 2017, Liu et al., 2018, Cui et al., 2014a, Cui et al., 2014b). Therefore developing efficient non-noble-metal-based catalysts for N-functionalization of amines with CO2 and H2 remains a grand challenge.
Herein, we report a rational design of In2O3 nanocrystals with high density of grain boundaries (HGB-In2O3), which shows remarkable catalytic performance toward methylation of amines using CO2 and H2. During the methylation of N-methylaniline, HGB-In2O3 achieved an optimal yield of 82.7% for N,N-dimethylaniline, which is not inferior to noble metal-based catalysts. In addition, 84% of the original reaction activity for HGB-In2O3 was preserved after five rounds of reaction. Besides, HGB-In2O3 exhibited excellent applicability in the methylation of amines. Further mechanistic studies revealed that the presence of high density of grain boundaries not only facilitated the adsorption and activation of CO2 to generate CH3OH as the intermediate, but also enhanced the activation of N-H bond in amines, which led to the attractive catalytic activity of HGB-In2O3 toward methylation of amines.
Results
Synthesis and Structural Characterizations of In2O3 Nanocrystals
To begin with, the metal-organic frameworks containing indium ions (In-MOFs) were synthesized in a Teflon-lined autoclave at 150°C for 20 h. Figure S1 shows representative scanning electron microscopic (SEM) images of the as-obtained In-MOFs, indicating the formation of stacked structure with single layer having thickness of ∼1 μm. In-MOFs were then calcined into powder in a muffle furnace at 350°C and kept for 3 h. Figure 1A shows a high-angle annular dark-field scanning transmission electron microscopic (HAADF-STEM) image of the as-obtained powder. Most of the nanocrystals take a square morphology with an average size of about 14.4 nm (Table S1). The composition and crystalline structure of the as-synthesized powder were further analyzed by X-ray diffraction (XRD). The XRD profile shown in Figure 1D of the powder can be indexed to a highly crystalline In2O3 phase with a body-centered cubic structure (JCPDS#89-4595). Further observation of the high-resolution HAADF-STEM of In2O3 displays an individual nanocrystal, in which two sets of fringes with interplanar spacing of 2.41 Å and 2.83 Å are observed, relating to the {411} and {222} planes, respectively. Obviously, the individual nanocrystal is determined to be multiple grains instead of a single grain. As a result, there remains abundant grain boundaries highlighted by yellow dotted lines in Figures 1B and S2. This sample with high density of grain boundaries was named as HGB-In2O3. For analysis of the chemical compositions of the nanostructure, the STEM energy-dispersive X-ray elemental mapping images of nanocrystal are shown in Figure 1C, demonstrating the homogeneous distribution of both In and O throughout the nanocrystal. When conducting the synthetic procedure similar to that of the HGB-In2O3 except for changing the calcined temperature from 350 to 600°C, nanocrystals with an average size of about 22.2 nm containing grain boundaries were formed (Figure S3 and Table S1). Owing to lower density of grain boundaries compared with HGB-In2O3, this sample was named as LGB-In2O3. For comparison, In2O3 nanocrystals without grain boundaries (NGB-In2O3) were also prepared according to a previously reported method (Gao et al., 2017, Albani et al., 2017). These NGB-In2O3 nanocrystals have an average size of about 20.2 nm (Figure S4 and Table S1). Besides, the Brunauer-Emmett-Teller surface areas of all samples were also measured (Table S1), where no obvious difference was observed for HGB-In2O3, LGB-In2O3, and NGB-In2O3. In addition, we estimated the density of grain boundary (DGB) of the samples based on the Formulas 1 and 2 in Transparent Methods. Accordingly, the DGB for HGB-In2O3 and LGB-In2O3 were estimated to be 180,000 and 53,000 m/mg, respectively (Table S1). To characterize the electronic properties of the obtained samples, we conducted X-ray photoelectron spectroscopic (XPS) measurements of In2O3. The binding energies of In 3d5/2 and In 3d3/2 in HGB-In2O3 are 444.3 and 451.9 eV, which are same as that in LGB-In2O3 and 0.2 eV higher relative to that in NGB-In2O3, respectively (Figure 1E) (Gu et al., 2015, Xu et al., 2007). The O 1s XPS spectra of In2O3 nanocrystals exhibited three distinct peaks. The prominent peak at 529.7 eV was assigned to O species in internal In2O3 nanocrystals. Another binding energy of O 1s was 531.3 eV, corresponding to that of the species (such as O2 and CO2) adsorbed on the surface of samples. Besides, for HGB-In2O3 and LGB-In2O3, there existed peak at 530.4 eV, which was assigned to O species in In2O3 at grain boundaries (Figure 1F) (Ding et al., 2015).
Figure 1.
Structural Characterizations of In2O3 Nanocrystals
(A) HAADF-STEM image of the HGB-In2O3 nanocrystals.
(B) High-resolution HAADF-STEM image of an individual HGB-In2O3 nanocrystal.
(C) STEM-energy-dispersive X-ray elemental mapping of an individual HGB-In2O3 nanocrystal.
(D) XRD profiles of HGB-In2O3, LGB-In2O3, and NGB-In2O3.
(E) In 3d XPS spectra for HGB-In2O3, LGB-In2O3, and NGB-In2O3 nanocrystals.
(F) O 1s XPS spectra for HGB-In2O3, LGB-In2O3, and NGB-In2O3 nanocrystals.
Catalytic Properties of HGB-In2O3 in CO2 Fixation
The catalytic properties of the as-obtained In2O3 were evaluated in methylation of amines using CO2 and H2. The model substrate was N-methylaniline leading to N,N-dimethylaniline. Each reaction was performed under 70 bar of mixed gas (CO2/H2 = 1:3) at 180°C by using tetrahydrofuran as the solvent. A blank test was conducted without any catalyst, in which no product was observed. Figure 2A illustrates the product yields of adding 25 mg of In2O3 catalysts with different density of grain boundaries. When the reaction was catalyzed by HGB-In2O3, N,N-dimethylaniline was produced attaining an yield of 82.7% after 9 h. In comparison, the yields decreased to only 21.7% and 12.6% catalyzed by LGB-In2O3 and NGB-In2O3 under the same reaction condition, respectively. Thus HGB-In2O3 catalyst exhibits remarkable activity and selectivity, which was even comparable to the noble metal-based catalysts (Li et al., 2013a, Li et al., 2013b, Beydoun et al., 2013, Kon et al., 2014, Cui et al., 2014a, Cui et al., 2014b). To further investigate the diversity of catalytic property, we calculated the mass activity for In2O3 catalysts at 180°C. As shown in Figure 2B, the mass activity of HGB-In2O3 is 21.2 mmol·g−1 h−1, which is almost 4 and 7 times as high as that of LGB-In2O3 and NGB-In2O3, respectively. We then plotted the profile of the mass activity versus DGB over different catalysts, where an almost linear correlation was observed (Figure S5). This indicated that grain boundaries played the dominated role in catalytic performance. In addition, the stability of HGB-In2O3 was also studied by performing successive rounds of reaction. As revealed in Figures 2C and S6, almost 84% of the original reaction activity was preserved after five rounds with product of N,N-dimethylaniline. In addition, the XRD patterns of the recovered HGB-In2O3 were also indexed to a highly crystalline In2O3 phase (JCPDS#89-4595) (Figure S7A). Although the morphology of the recovered HGB-In2O3 had a slight change, grain boundaries in HGB-In2O3 were still preserved after five cycles based on the high-resolution HAADF-STEM image (Figure S7B). Both XRD and HAADF-STEM results proved the high stability of HGB-In2O3, which is extremely important for potential applications in industrial processes by reducing the cost and pollution efficiently. Furthermore, hot filtration tests showed that the reaction was a heterogeneous catalysis (Figure S8). In the filtrate, only 0.29% of In element relative to HGB-In2O3 was leached determined by inductively coupled plasma atomic emission spectrometry. Figure 2D shows a systematic comparison of the methylation of various substituted anilines using HGB-In2O3 as catalyst under 70 bar mixed gas (CO2/H2 = 1:3) at 180°C for 24 h. When 4-chloroaniline was used as reactant, 4-chloro-N,N-dimethylaniline was obtained in high yield of 94.2%. On the contrary, in the transformations of aniline and 2-fluoroaniline, the yields of N,N-dimethylaniline and N,N-dimethyl-2-fluoroaniline slightly dropped to 90.9% and 89.2%, respectively. In addition, 74.3% and 53.2% of indole and 2,4,6-trimethylaniline converted into N-methylindole and N,N,2,4,6-pentamethylaniline, respectively. Notably, when using octylamine as the substrate, 26.3% of conversion was achieved with 99% selectivity of N-methyloctan-1-amine. As a result, HGB-In2O3 exhibited excellent applicability in the methylation of amines.
Figure 2.
Catalytic Performance of HGB-In2O3, LGB-In2O3, and NGB-In2O3 in CO2 Fixation
(A) Yield of N,N-dimethylaniline catalyzed by HGB-In2O3, LGB-In2O3, and NGB-In2O3 in methylation of N-methylaniline at 180°C for 9 h.
(B) Comparison of mass activity with HGB-In2O3, LGB-In2O3, and NGB-In2O3 as catalysts in methylation of N-methylaniline at 180°C for 9 h.
(C) Yield of N,N-dimethylaniline catalyzed by HGB-In2O3 over the course of five rounds of successive reaction at 180°C for 9 h.
(D) Yields of 4-chloro-N,N-dimethylaniline, N,N-dimethylaniline, N,N-dimethyl-2-fluoroaniline, N-methylindole, and N,N,2,4,6-pentamethylaniline catalyzed by HGB-In2O3 in methylation of 4-chloroaniline, aniline, 2-fluoroaniline, indole, and 2,4,6-trimethylaniline at 180°C for 24 h, respectively.
Mechanistic Studies of Remarkable Catalytic Activity for HGB-In2O3 in CO2 Fixation
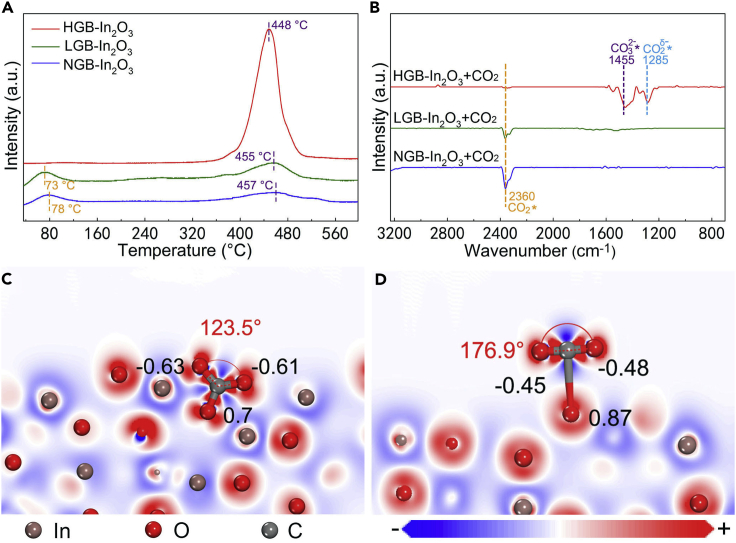
To elucidate the role of grain boundary in catalytic reaction, we investigated the interaction between CO2 and In2O3 catalysts. CO2 temperature-programmed desorption (CO2-TPD) measurements of In2O3 nanocrystals were implemented. Figure 3A illustrates CO2-TPD spectra of In2O3 catalysts with different densities of grain boundaries. In the presence of HGB-In2O3, a prominent peak of desorption appeared at 448°C, which corresponds to the chemisorbed CO2. For LGB-In2O3 and NGB-In2O3, two obscure desorption peaks emerging at 455°C and 457°C were in line with chemisorbed CO2 and the other two weak peaks at around 70°C–80°C conformed to physisorbed CO2. The adsorption capability was compared on the basis of the peak area lying on the premise of setting the equivalent mass of each sample to 30 mg. The area of chemisorption peak of HGB-In2O3 was almost 8 and 11 times larger than that of LGB-In2O3 and NGB-In2O3, respectively. To further gain insight into the interaction between CO2 and In2O3 catalysts, we carried out in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopic measurements. After treatment with CO2 at 180°C for 30 min, the in situ DRIFT spectrum of HGB-In2O3 exhibited a peak at 1,455 cm−1 assigned to CO32−* species and another one at 1,285 cm−1 assigned to CO2δ−* (Graciani et al., 2014). With regard to LGB-In2O3 and NGB-In2O3 after exposure to CO2 at 180°C for 30 min, peaks at 2,360 cm−1 appeared, corresponding to the frequency of physisorbed CO2* species (Figure 3B) (Wang et al., 2009). As a result, grain boundaries are beneficial to the chemisorption of CO2. To rationalize the remarkable function of grain boundary in activation of CO2, we carried out density functional theory (DFT) calculations. One atomic model for single-crystal In2O3 was established along the (111) facet, which was named as In2O3(111). Another model consisted of two grains formed along (110) and (111) facets, respectively (Figure S9). This model involving a grain boundary was named as In2O3(GB). First-principle simulations were performed to calculate the adsorption energies of CO2 on In2O3(111) and In2O3(GB). The adsorption energy of CO2 on In2O3(GB) was −1.66 eV, much higher than that on In2O3(111) with the value as low as −0.1 eV. Figures 3C and 3D reveal the adsorption geometries of CO2 on In2O3(GB) and In2O3(111). For In2O3(GB), there exist higher negative charge density of surface O atoms and lower positive charge density of C atoms on the surface than that for In2O3(111). Thus, CO2 obtained more negative electrons when absorbed in In2O3(GB), promoting the process of activation. Notably, the interatomic bond angles of CO2 decreased from 180° to 176.9° and 123.5° on In2O3(111) and In2O3(GB), respectively. The greater reduction of the bond angle led by grain boundaries increases the internal energy of CO2 molecule, further making it unstable and prone to reaction. Thus, grain boundaries encourage the adsorption and activation of CO2. We also performed in situ DRIFT measurement to study the activation of H2. Before recording the DRIFT spectrum, HGB-In2O3 was exposed to H2 (1 bar) at 180°C for 1 h. As shown in DRIFT spectrum, the set of frequencies at 3,660–3,551 cm−1 corresponding to the stretching vibration of O-H were observed (Figure S10). It was thus speculated that H2 was dissociated after adsorbing on the oxygen atoms of In2O3.
Figure 3.
Mechanistic Studies of the Role of Grain Boundary in CO2 Activation
(A) CO2-TPD profiles of HGB-In2O3, LGB-In2O3, and NGB-In2O3 nanocrystals.
(B) In situ DRIFT spectra of HGB-In2O3, LGB-In2O3, and NGB-In2O3 nanocrystals after treatment with CO2 at 180°C for 30 min.
(C) The geometrical configurations and electron density difference of CO2 adsorbed on In2O3(GB).
(D) The geometrical configurations and electron density difference of CO2 adsorbed on In2O3(111).
Furthermore, the interaction between amines and In2O3 catalysts was also explored. Figure 4A shows the in situ DRIFT spectra of HGB-In2O3, LGB-In2O3, and NGB-In2O3 after treatment with N-methylaniline at 180°C for 30 min. With regard to HGB-In2O3, peaks at 692–750, 1,262, 1,506–1,603, 2,904–3,049, and 3,416 cm−1 appeared, corresponding to the bending vibration of C-H, the stretching vibrations C-N, the vibration of benzene skeleton, the stretching vibrations of C-H, and the stretching vibration of N-H, respectively. Considering that the fracture of N-H plays a pivotal role in the methylation of amines, we focused on the distinction of peaks for the stretching vibration of N-H among In2O3 catalysts. In the spectra of LGB-In2O3 and NGB-In2O3, the peaks for the stretching vibration of N-H shifted to 3,422 and 3,443 cm−1, respectively. Thus, HGB-In2O3 was found to illustrate the lowest wave number of the peaks among the three samples, indicating the largest length of N-H bond. Therefore, grain boundary benefits the activation of N-H bond in amines. DFT calculations were conducted on In2O3(GB) and In2O3(111) to further verify the function of grain boundary in activating N-H bond. The adsorption energy of N-methylaniline on In2O3(GB) was −0.48 eV, implying exothermic adsorption on In2O3(GB). In comparison, the adsorption energy of 0.33 eV for In2O3(111) demonstrates that the adsorption of N-methylaniline on In2O3(111) was endothermic. As shown in Figures 4B and 4C, the length of N-H bond was 1.029 Å when absorbed on In2O3(GB), whereas for In2O3(111), the length of N-H bond decreased to 1.023 Å. The elongation of bond length was able to induce red-shift of stretching vibration frequency for adsorbed N-methylaniline on In2O3(GB), well consistent with the observation in in situ DRIFT measurements.
Figure 4.
Mechanistic studies of remarkable catalytic activity for HGB-In2O3
(A) In situ DRIFT spectra of HGB-In2O3, LGB-In2O3, and NGB-In2O3 nanocrystals after treatment with N-methylaniline at 180°C for 30 min.
(B) The geometrical configurations and electron density difference of N-methylaniline adsorbed on In2O3(GB).
(C) The geometrical configurations and electron density difference of N-methylaniline adsorbed on In2O3(111).
(D) Products of CO2 hydrogenation over HGB-In2O3, LGB-In2O3, and NGB-In2O3 nanocrystals at 180°C after 8 h.
(E) Yield of N,N-dimethylaniline when CH3OH and N-methylaniline react at 180°C catalyzed by HGB-In2O3, LGB-In2O3 and NGB-In2O3 for 4 h.
For methylation of amines with CO2 and H2, the selectivity for product varies with the intermediate of the reaction. For instance, amide is generated when CO serves as the intermediate, whereas aniline is synthesized by forming CH3OH intermediate (Li et al., 2013a, Li et al., 2013b, Cui et al., 2014a, Cui et al., 2014b, Tlili et al., 2014, Dang et al., 2015, Tsarev et al., 2015, Ogata et al., 2018, Fernández-Alvarez and Oro, 2018, Goeppert et al., 2014, Liu et al., 2017, Zhang et al., 2019). To determine the intermediate for methylation of amines, 70 bars of CO2/H2 mixed gas (CO2:H2 = 1:3) was allowed to react at 180°C for 8 h with In2O3 catalysts. After completion of the reaction, CH3OH was detected as the main product except a spot of CO (Figure 4D). Therefore, CH3OH is considered to be the intermediate, conducive to methylation of amines. In addition, HGB-In2O3 was endowed with the highest yield of CH3OH with a value of 17.7 mmol; owing to this, grain boundary promoted the activation of CO2 (Figure 4D). In situ DRIFT measurement was also employed to further explore the reaction intermediate. After exposing HGB-In2O3 to the mixed gas (CO2:H2 = 1:3, 1 bar) at 180°C for 1 h, the DRIFT spectrum was recorded. As shown in Figure S10, two sets of frequencies were observed. One set of frequency at 2,360 cm−1 corresponded to the physisorbed CO2* species. The other set of frequencies at 3,645–3,619, 2,968–2,863, 1,580, and 1,050 cm−1 corresponded to the stretching vibration of O-H, the stretching vibration of C-H, the bending vibration of C-H, and the stretching vibration of C-O in CH3OH* species, respectively. This result confirmed that methanol was the intermediate during the catalytic reaction, which was consistent with the previous results (Gao et al., 2017, Ye et al., 2013). Moreover, to rationalize the function of activating amines in the methylation reaction, we applied CH3OH and N-methylaniline to react at 180°C catalyzed by In2O3 catalysts. As shown in Figure 4E, when catalyzed by HGB-In2O3, N,N-dimethylaniline was produced attaining the yield of 79.8% after 4 h, 1.2 and 2.8 times higher than those of LGB-In2O3 and NGB-In2O3, respectively. Thus the activation of amines can indeed control the activity of methylation reaction, which can be facilitated by grain boundaries. Taking the discussion above into account, we proposed the reaction pathway of methylation of amines as illustrated in Figure S11. Collectively, grain boundaries not only facilitated the adsorption and activation of CO2 to generate CH3OH as the intermediate (Equation 1, Figure S11) but also enhanced the activation of N-H bond in amines (Equation 3, Figure S11), which led to the attractive catalytic activity of HGB-In2O3 toward methylation of amines.
Discussion
In conclusion, given the high cost of noble metal-based catalysts, we reported a rational design of HGB-In2O3 nanocrystals, which achieved remarkable catalytic performance toward methylation of amines with CO2 and H2. We designed a series of In2O3 nanocrystals with different density of grain boundaries, i.e., HGB-In2O3, LGB-In2O3, and NGB-In2O3. During the methylation of N-methylaniline, HGB-In2O3 achieved an optimal yield of 82.7% for N,N-dimethylaniline, 3.8 and 6.6 times as high as those of LGB-In2O3 and NGB-In2O3, respectively. Further mechanistic studies revealed that the presence of high density of grain boundaries not only facilitated the adsorption and activation of CO2 to generate CH3OH as the intermediate but also enhanced the activation of N-H bond in amines, which led to the attractive catalytic activity of HGB-In2O3 toward methylation of amines. This work not only develops a catalyst with high density of grain boundaries to achieve the methylation of amines but also opens up new possibilities for designing efficient non-noble-metal-based catalysts.
Limitations of the Study
Although HGB-In2O3 exhibited excellent catalytic performance in the methylation of aromatic amines, the activity was still unsatisfactory when using fatty amines as substrates.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant no. 51801235, 11875258, 11505187), the Start-up Funding of Central South University (No. 502045005), the Fundamental Research Funds for the Central Universities of Central South University (No. 2018zzts402), and the Fundamental Research Funds for the Central Universities (No. WK2310000066, WK2060190081).
Author Contributions
Lirong Wang and J.C. equally contributed to this work. Lirong Wang, S.L., and Liangbing Wang designed the studies and wrote the paper. Lirong Wang and L.X. synthesized catalysts. Lirong Wang and S.Y. performed catalytic tests. X.Z., J.G., L.X., and Lirong Wang conducted XRD, TPD, and in situ DRIFT measurements. J.Z., X.Z., J.Y. and L.Z. conducted XPS measurements. J.C. conducted DFT calculations. All authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.06.005.
Contributor Information
Xusheng Zheng, Email: zxs@ustc.edu.cn.
Shuquan Liang, Email: lsq@csu.edu.cn.
Liangbing Wang, Email: wanglb@csu.edu.cn.
Supplemental Information
References
- Albani D., Capdevila-Cortada M., Vile G., Mitchell S., Martin O., Lopez N., Perez-Ramirez J. Semihydrogenation of acetylene on indium oxide: proposed single-ensemble catalysis. Angew. Chem. Int. Ed. 2017;56:10755–10760. doi: 10.1002/anie.201704999. [DOI] [PubMed] [Google Scholar]; Albani, D., Capdevila-Cortada, M., Vile, G., Mitchell, S., Martin, O., Lopez, N. and Perez-Ramirez, J. (2017). Semihydrogenation of Acetylene on Indium Oxide: Proposed Single-Ensemble Catalysis. Angew. Chem. Int. Ed. 56, 10755-10760. [DOI] [PubMed]
- Beydoun K., vom Stein T., Klankermayer J., Leitner W. Ruthenium-catalyzed direct methylation of primary and secondary aromatic amines using carbon dioxide and molecular hydrogen. Angew. Chem. Int. Ed. 2013;52:9554–9557. doi: 10.1002/anie.201304656. [DOI] [PubMed] [Google Scholar]; Beydoun, K., vom Stein, T., Klankermayer, J. and Leitner, W. (2013). Ruthenium-catalyzed direct methylation of primary and secondary aromatic amines using carbon dioxide and molecular hydrogen. Angew. Chem. Int. Ed. 52, 9554-9557. [DOI] [PubMed]
- Beydoun K., Ghattas G., Thenert K., Klankermayer J., Leitner W. Ruthenium-catalyzed reductive methylation of imines using carbon dioxide and molecular hydrogen. Angew. Chem. Int. Ed. 2014;53:11010–11014. doi: 10.1002/anie.201403711. [DOI] [PubMed] [Google Scholar]; Beydoun, K., Ghattas, G., Thenert, K., Klankermayer, J. and Leitner, W. (2014). Ruthenium-catalyzed reductive methylation of imines using carbon dioxide and molecular hydrogen. Angew. Chem. Int. Ed. 53, 11010-11014. [DOI] [PubMed]
- Bhanja P., Modak A., Bhaumik A. Supported porous nanomaterials as efficient heterogeneous catalysts for CO2 fixation reactions. Chemistry. 2018;24:7278–7297. doi: 10.1002/chem.201800075. [DOI] [PubMed] [Google Scholar]; Bhanja, P., Modak, A., and Bhaumik, A. (2018). Supported porous nanomaterials as efficient heterogeneous catalysts for CO2 fixation reactions. Chemistry 24, 7278-7297. [DOI] [PubMed]
- Choi G., Hong S.H. Selective monomethylation of amines with methanol as the C1 source. Angew. Chem. Int. Ed. 2018;57:6166–6170. doi: 10.1002/anie.201801524. [DOI] [PubMed] [Google Scholar]; Choi, G. and Hong, S. H. (2018). Selective monomethylation of amines with methanol as the C1 source. Angew. Chem. Int. Ed. 57, 6166-6170. [DOI] [PubMed]
- Cui X., Zhang Y., Deng Y., Shi F. N-Methylation of amine and nitro compounds with CO2/H2 catalyzed by Pd/CuZrOx under mild reaction conditions. Chem. Commun. (Camb.) 2014;50:13521–13524. doi: 10.1039/c4cc05119j. [DOI] [PubMed] [Google Scholar]; Cui, X., Zhang, Y., Deng, Y. and Shi, F. (2014). N-Methylation of amine and nitro compounds with CO2/H2 catalyzed by Pd/CuZrOx under mild reaction conditions. Chem. Commun. (Camb.) 50, 13521-13524. [DOI] [PubMed]
- Cui X., Dai X., Zhang Y., Deng Y., Shi F. Methylation of amines, nitrobenzenes and aromatic nitriles with carbon dioxide and molecular hydrogen. Chem. Sci. 2014;5:649–655. [Google Scholar]; Cui, X., Dai, X., Zhang, Y., Deng, Y. and Shi, F. (2014). Methylation of amines, nitrobenzenes and aromatic nitriles with carbon dioxide and molecular hydrogen. Chem. Sci. 5, 649-655.
- Dang T.T., Ramalingam B., Seayad A.M. Efficient ruthenium-catalyzed N-methylation of amines using methanol. ACS Catal. 2015;5:4082–4088. [Google Scholar]; Dang, T. T., Ramalingam, B. and Seayad, A. M. (2015). Efficient ruthenium-catalyzed N-methylation of amines using methanol. ACS Catal. 5, 4082-4088
- Ding M., Meng D., Tang Y., Liu C., Luo S. One-dimensional porous Ag/AgBr/TiO2 nanofibres with enhanced visible light photocatalytic activity. Chem. Pap. 2015;69:1411–1420. [Google Scholar]; Ding, M., Meng, D., Tang, Y., Liu, C. and Luo, S. (2015). One-dimensional porous Ag/AgBr/TiO2 nanofibres with enhanced visible light photocatalytic activity. Chem. Pap. 69 , 1411-1420.
- Du X.L., Tang G., Bao H.L., Jiang Z., Zhong X.H., Su D.S., Wang J.Q. Direct methylation of amines with carbon dioxide and molecular hydrogen using supported gold catalysts. ChemSusChem. 2015;8:3489–3496. doi: 10.1002/cssc.201500486. [DOI] [PubMed] [Google Scholar]; Du, X. L., Tang, G., Bao, H. L., Jiang, Z., Zhong, X. H., Su, D. S. and Wang, J. Q. (2015). Direct methylation of amines with carbon dioxide and molecular hydrogen using supported gold catalysts. ChemSusChem 8, 3489-3496. [DOI] [PubMed]
- Elangovan S., Neumann J., Sortais J.B., Junge K., Darcel C., Beller M. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 2016;7:12641–12648. doi: 10.1038/ncomms12641. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elangovan, S., Neumann, J., Sortais, J. B., Junge, K., Darcel, C. and Beller, M. (2016). Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 7, 12641-12648. [DOI] [PMC free article] [PubMed]
- Fernández-Alvarez F.J., Oro L.A. Homogeneous catalytic reduction of CO2 with silicon-hydrides, state of the art. ChemCatChem. 2018;10:4783–4796. [Google Scholar]; Fernandez-Alvarez, F.J., and Oro, L.A. (2018). Homogeneous catalytic reduction of CO2 with Silicon-Hydrides, State of the Art. ChemCatChem 10, 4783-4796.
- Gao P., Li S., Bu X., Dang S., Liu Z., Wang H., Zhong L., Qiu M., Yang C., Cai J. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017;9:1019–1024. doi: 10.1038/nchem.2794. [DOI] [PubMed] [Google Scholar]; Gao, P., Li, S., Bu, X., Dang, S., Liu, Z., Wang, H., Zhong, L., Qiu, M., Yang, C., Cai, J.et al. (2017). Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019-1024. [DOI] [PubMed]
- Goeppert A., Czaun M., Jones J.P., Surya Prakash G.K., Olah G.A. Recycling of carbon dioxide to methanol and derived products - closing the loop. Chem. Soc. Rev. 2014;43:7995–8048. doi: 10.1039/c4cs00122b. [DOI] [PubMed] [Google Scholar]; Goeppert, A., Czaun, M., Jones, J.P., Surya Prakash, G.K., and Olah, G.A. (2014). Recycling of carbon dioxide to methanol and derived products - closing the loop. Chem. Soc. Rev. 43, 7995-8048. [DOI] [PubMed]
- Graciani J., Mudiyanselage K., Xu F., Baber A.E., Evans J., Senanayake S.D., Stacchiola D.J., Liu P., Hrbek J., Sanz J.F., Rodriguze J.A. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science. 2014;345:546–550. doi: 10.1126/science.1253057. [DOI] [PubMed] [Google Scholar]; Graciani, J., Mudiyanselage, K., Xu, F., Baber, A.E., Evans, J., Senanayake, S.D., Stacchiola, D.J., Liu, P., Hrbek, J., Sanz, J.F., and Rodriguze, J.A. (2014). Highly Active Copper-Ceria and Copper-Ceria-Titania Catalysts for Methanol Synthesis from CO2. Science 345, 546-550. [DOI] [PubMed]
- Gu F., Nie R., Han D., Wang Z. In2O3–graphene nanocomposite based gas sensor for selective detection of NO2 at room temperature. Sens. Actuator. B Chem. 2015;219:94–99. [Google Scholar]; Gu, F., Nie, R., Han, D. and Wang, Z. (2015). In2O3-graphene nanocomposite based gas sensor for selective detection of NO2 at room temperature. Sens. Actuator B-Chem. 219, 94-99.
- Hou T., Luo N., Cui Y.-T., Lu J., Li L., MacArthur K.E., Heggen M., Chen R., Fan F., Tian W. Selective reduction of CO2 to CO under visible light by controlling coordination structures of CeOx-S/ZnIn2S4 hybrid catalysts. Appl. Catal. B. 2019;245:262–270. [Google Scholar]; Hou, T., Luo, N., Cui, Y.-T., Lu, J., Li, L., MacArthur, K.E., Heggen, M., Chen, R., Fan, F., Tian, W., et al. (2019). Selective reduction of CO2 to CO under visible light by controlling coordination structures of CeOx-S/ZnIn2S4 hybrid catalysts. Appl. Catal. B 245, 262-270.
- Ju P., Chen J., Chen A., Chen L., Yu Y. N-formylation of amines with CO2 and H2 using Pd–Au bimetallic catalysts supported on polyaniline-functionalized carbon nanotubes. ACS Sustain. Chem. Eng. 2017;5:2516–2528. [Google Scholar]; Ju, P., Chen, J., Chen, A., Chen, L. and Yu, Y. (2017). N-formylation of amines with CO2 and H2 using Pd-Au bimetallic catalysts supported on polyaniline-functionalized carbon nanotubes. ACS Sustain. Chem. Eng. 5, 2516-2528.
- Khan M.U., Wang L., Liu Z., Gao Z., Wang S., Li H., Zhang W., Wang M., Wang Z., Ma C. Pt3Co octapods as superior catalysts of CO2 hydrogenation. Angew. Chem. Int. Ed. 2016;55:9548–9552. doi: 10.1002/anie.201602512. [DOI] [PubMed] [Google Scholar]; Khan, M.U., Wang, L., Liu, Z., Gao, Z., Wang, S., Li, H., Zhang, W., Wang, M., Wang, Z., Ma, C., et al. (2016). Pt3Co octapods as superior catalysts of CO2 hydrogenation. Angew. Chem., Int. Ed. 55, 9548-9552. [DOI] [PubMed]
- Kon K., Siddiki S.M., Onodera W., Shimizu K. Sustainable heterogeneous platinum catalyst for direct methylation of secondary amines by carbon dioxide and hydrogen. Chem. Eur. J. 2014;20:6264–6267. doi: 10.1002/chem.201400332. [DOI] [PubMed] [Google Scholar]; Kon, K., Siddiki, S. M., Onodera, W. and Shimizu, K. (2014). Sustainable heterogeneous platinum catalyst for direct methylation of secondary amines by carbon dioxide and hydrogen. Chem. Eur. J. 20, 6264-6267. [DOI] [PubMed]
- Li Y., Fang X., Junge K., Beller M. A general catalytic methylation of amines using carbon dioxide. Angew. Chem. Int. Ed. 2013;52:9568–9571. doi: 10.1002/anie.201301349. [DOI] [PubMed] [Google Scholar]; Li, Y., Fang, X., Junge, K. and Beller, M. (2013). A general catalytic methylation of amines using carbon dioxide. Angew. Chem. Int. Ed. 52, 9568-9571. [DOI] [PubMed]
- Li Y., Sorribes I., Yan T., Junge K., Beller M. Selective methylation of amines with carbon dioxide and H2. Angew. Chem. Int. Ed. 2013;52:12156–12160. doi: 10.1002/anie.201306850. [DOI] [PubMed] [Google Scholar]; Li, Y., Sorribes, I., Yan, T., Junge, K. and Beller, M. (2013). Selective methylation of amines with carbon dioxide and H2. Angew. Chem. Int. Ed. 52, 12156-12160. [DOI] [PubMed]
- Li H., Wang L., Dai Y., Pu Z., Lao Z., Chen Y., Wang M., Zheng X., Zhu J., Zhang W. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 2018;13:411–417. doi: 10.1038/s41565-018-0089-z. [DOI] [PubMed] [Google Scholar]; Li, H., Wang, L., Dai, Y., Pu, Z., Lao, Z., Chen, Y., Wang, M., Zheng, X., Zhu , J., Zhang, W., et al. (2018). Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411-417. [DOI] [PubMed]
- Liu Q., Wu L., Jackstell R., Beller M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015;6:5933–5947. doi: 10.1038/ncomms6933. [DOI] [PubMed] [Google Scholar]; Liu, Q., Wu, L., Jackstell, R. and Beller, M. (2015). Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 6, 5933-5947. [DOI] [PubMed]
- Liu X.F., Li X.Y., Qiao C., Fu H.C., He L.N. Betaine catalysis for hierarchical reduction of CO2 with amines and hydrosilane to form formamides, aminals, and methylamines. Angew. Chem. Int. Ed. 2017;56:7425–7429. doi: 10.1002/anie.201702734. [DOI] [PubMed] [Google Scholar]; Liu, X.F., Li, X.Y., Qiao, C., Fu, H.C., and He, L.N. (2017). Betaine catalysis for hierarchical reduction of CO2 with amines and hydrosilane to form formamides, aminals, and methylamines. Angew. Chem. Int. Ed. 56, 7425-7429. [DOI] [PubMed]
- Liu W., Sahoo B., Spannenberg A., Junge K., Beller M. Tailored cobalt-catalysts for reductive alkylation of anilines with carboxylic acids under mild conditions. Angew. Chem. Int. Ed. 2018;57:11673–11677. doi: 10.1002/anie.201806132. [DOI] [PubMed] [Google Scholar]; Liu, W., Sahoo, B., Spannenberg, A., Junge, K. and Beller, M. (2018). Tailored cobalt-catalysts for reductive alkylation of anilines with carboxylic acids under mild conditions. Angew. Chem. Int. Ed. 57, 11673-11677. [DOI] [PubMed]
- Molla R.A., Bhanja P., Ghosh K., Islam S.S., Bhaumik A., Islam S.M. Pd nanoparticles decorated on hypercrosslinked microporous polymer: a highly efficient catalyst for the formylation of amines through carbon dioxide fixation. ChemCatChem. 2017;9:1939–1946. [Google Scholar]; Molla, R.A., Bhanja, P., Ghosh, K., Islam, S.S., Bhaumik, A., and Islam, S.M. (2017). Pd nanoparticles decorated on hypercrosslinked microporous polymer: A highly efficient catalyst for the formylation of amines through carbon dioxide fixation. ChemCatChem 9, 1939-1946.
- Natte K., Neumann H., Jagadeesh R.V., Beller M. Convenient iron-catalyzed reductive aminations without hydrogen for selective synthesis of N-methylamines. Nat. Commun. 2017;8:1344–1352. doi: 10.1038/s41467-017-01428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Natte, K., Neumann, H., Jagadeesh, R. V. and Beller, M. (2017). Convenient iron-catalyzed reductive aminations without hydrogen for selective synthesis of N-methylamines. Nat. Commun. 8, 1344-1352. [DOI] [PMC free article] [PubMed]
- Nguyen T.V., Yoo W.J., Kobayashi S. Effective formylation of amines with carbon dioxide and diphenylsilane catalyzed by chelating bis(tzNHC) rhodium complexes. Angew. Chem. Int. Ed. 2015;54:9209–9212. doi: 10.1002/anie.201504072. [DOI] [PubMed] [Google Scholar]; Nguyen, T. V., Yoo, W. J. and Kobayashi, S. (2015). Effective Formylation of Amines with Carbon Dioxide and Diphenylsilane Catalyzed by Chelating bis(tzNHC) Rhodium Complexes. Angew. Chem. Int. Ed. 54, 9209-9212. [DOI] [PubMed]
- Ogata O., Nara H., Fujiwhara M., Matsumura K., Kayaki Y. N-monomethylation of aromatic amines with methanol via PN(H)P-pincer Ru catalysts. Org. Lett. 2018;20:3866–3870. doi: 10.1021/acs.orglett.8b01449. [DOI] [PubMed] [Google Scholar]; Ogata, O., Nara, H., Fujiwhara, M., Matsumura, K. and Kayaki, Y. (2018). N-Monomethylation of Aromatic Amines with Methanol via PN(H)P-Pincer Ru Catalysts. Org. Lett. 20, 3866-3870 [DOI] [PubMed]
- Sorribes I., Cabrero-Antonino J.R., Vicent C., Junge K., Beller M. Catalytic N-alkylation of amines using carboxylic acids and molecular hydrogen. J. Am. Chem. Soc. 2015;137:13580–13587. doi: 10.1021/jacs.5b07994. [DOI] [PubMed] [Google Scholar]; Sorribes, I., Cabrero-Antonino, J. R., Vicent, C., Junge, K. and Beller, M. (2015). Catalytic N-Alkylation of Amines Using Carboxylic Acids and Molecular Hydrogen. J. Am. Chem. Soc. 137, 13580-13587. [DOI] [PubMed]
- Sun S., Watanabe M., Wu J., An Q., Ishihara T. Ultrathin WO3·0.33H2O nanotubes for CO2 photoreduction to acetate with high selectivity. J. Am. Chem. Soc. 2018;140:6474–6482. doi: 10.1021/jacs.8b03316. [DOI] [PubMed] [Google Scholar]; Sun, S., Watanabe, M., Wu, J., An, Q., and Ishihara, T. (2018). Ultrathin WO3·0.33H2O Nanotubes for CO2 Photoreduction to Acetate with High Selectivity. J. Am. Chem. Soc. 140, 6474-6482. [DOI] [PubMed]
- Tlili A., Frogneux X., Blondiaux E., Cantat T. Creating added value with a waste: methylation of amines with CO2 and H2. Angew. Chem. Int. Ed. 2014;53:2543–2545. doi: 10.1002/anie.201310337. [DOI] [PubMed] [Google Scholar]; Tlili, A., Frogneux, X., Blondiaux, E. and Cantat, T. (2014). Creating added value with a waste: methylation of amines with CO2 and H2. Angew. Chem. Int. Ed. 53, 2543-2545 [DOI] [PubMed]
- Toyao T., Siddiki S., Morita Y., Kamachi T., Touchy A.S., Onodera W., Kon K., Furukawa S., Ariga H., Asakura K. Rhenium-loaded TiO2: a highly versatile and chemoselective catalyst for the hydrogenation of carboxylic acid derivatives and the N-methylation of amines using H2 and CO2. Chem. Eur. J. 2017;23:14848–14859. doi: 10.1002/chem.201702801. [DOI] [PubMed] [Google Scholar]; Toyao, T., Siddiki, S., Morita, Y., Kamachi, T., Touchy, A. S., Onodera, W., Kon, K., Furukawa, S., Ariga, H., Asakura, K., et al. (2017). Rhenium-loaded TiO2: a highly versatile and chemoselective catalyst for the hydrogenation of carboxylic acid derivatives and the N-methylation of amines using H2 and CO2. Chem. Eur. J. 23, 14848-14859. [DOI] [PubMed]
- Tsarev V.N., Morioka Y., Caner J., Wang Q., Ushimaru R., Kudo A., Naka H., Saito S. N-methylation of amines with methanol at room temperature. Org. Lett. 2015;17:2530–2533. doi: 10.1021/acs.orglett.5b01063. [DOI] [PubMed] [Google Scholar]; Tsarev, V. N., Morioka, Y., Caner, J., Wang, Q., Ushimaru, R., Kudo, A., Naka, H. and Saito, S. (2015). N-methylation of amines with methanol at room temperature. Org. Lett. 17, 2530-2533 [DOI] [PubMed]
- Wang X., Schwartz V., Clark J.C., Ma X., Overbury S.H., Xu X., Song C. Infrared study of CO2 sorption over “molecular basket” sorbent consisting of polyethylenimine-modified mesoporous molecular sieve. J. Phys. Chem. C. 2009;113:7260–7268. [Google Scholar]; Wang, X., Schwartz, V., Clark, J. C., Ma, X., Overbury, S. H., Xu, X., and Song, C. (2009). Infrared study of CO2 sorption over “molecular basket” sorbent consisting of polyethylenimine-modified mesoporous molecular sieve. J Phys Chem. C, 113, 7260-7268.
- Wang S., Guan B.Y., Lou X.W.D. Construction of ZnIn2S4-In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. J. Am. Chem. Soc. 2018;140:5037–5040. doi: 10.1021/jacs.8b02200. [DOI] [PubMed] [Google Scholar]; Wang, S., Guan, B.Y., and Lou, X.W.D. (2018). Construction of ZnIn2S4-In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. J. Am. Chem. Soc. 140, 5037-5040. [DOI] [PubMed]
- Xu J.Q., Chen Y.P., Pan Q.Y., Xiang Q., Cheng Z.X., Dong X.W. A new route for preparing corundum-type In2O3 nanorods used as gas-sensing materials. Nanotechnology. 2007;18:115615–115621. [Google Scholar]; Xu, J. Q., Chen, Y. P., Pan, Q. Y., Xiang, Q., Cheng, Z. X. and Dong, X. W. (2007). A new route for preparing corundum-type In2O3 nanorods used as gas-sensing materials. Nanotechnology 18, 115615-115621.
- Yang Z., Yu B., Zhang H., Zhao Y., Ji G., Liu Z. Fluoro-functionalized polymeric N-heterocyclic carbene-zinc complexes: efficient catalyst for formylation and methylation of amines with CO2 as a C1-building block. RSC Adv. 2015;5:19613–19619. [Google Scholar]; Yang, Z., Yu, B., Zhang, H., Zhao, Y., Ji, G. and Liu, Z. (2015). Fluoro-functionalized polymeric N-heterocyclic carbene-zinc complexes: efficient catalyst for formylation and methylation of amines with CO2 as a C1-building block. RSC Adv. 5, 19613-19619.
- Ye J., Liu C., Mei D., Ge Q. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): a DFT study. ACS Catal. 2013;3:1296–1306. [Google Scholar]; Ye, J., Liu, C., Mei, D., and Ge, Q. (2013). Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): A DFT study. ACS Catal. 3, 1296-1306.
- Yuan R., Lin Z. Mechanistic insight into the gold-catalyzed carboxylative cyclization of propargylamines. ACS Catal. 2015;5:2866–2872. [Google Scholar]; Yuan, R. and Lin, Z. (2015). Mechanistic insight into the gold-catalyzed carboxylative cyclization of propargylamines. ACS Catal. 5, 2866-2872.
- Zhang L., Han Z., Zhao X., Wang Z., Ding K. Highly efficient ruthenium-catalyzed N-formylation of amines with H2 and CO2. Angew. Chem. Int. Ed. 2015;54:6186–6189. doi: 10.1002/anie.201500939. [DOI] [PubMed] [Google Scholar]; Zhang, L., Han, Z., Zhao, X., Wang, Z. and Ding, K. (2015). Highly Efficient Ruthenium-Catalyzed N-Formylation of Amines with H2 and CO2. Angew. Chem. Int. Ed. 54, 6186-6189. [DOI] [PubMed]
- Zhang Y., Wang H., Yuan H., Shi F. Hydroxyl group-regulated active nano-Pd/C catalyst generation via in situ reduction of Pd(NH3)xCly/C for N-formylation of amines with CO2/H2. ACS Sustain. Chem. Eng. 2017;5:5758–5765. [Google Scholar]; Zhang, Y., Wang, H., Yuan, H. and Shi, F. (2017). Hydroxyl Group-Regulated Active Nano-Pd/C Catalyst Generation via in Situ Reduction of Pd(NH3)xCly/C for N-Formylation of Amines with CO2/H2. ACS Sustain. Chem. Eng. 5, 5758-5765.
- Zhang J., Qian Q., Wang Y., Asare Bediako B.B., Cui M., Yang G., Han B. Synthesis of acetamides using CO2, methanol, H2 and amines. Green. Chem. 2019;21:233–237. [Google Scholar]; Zhang, J., Qian, Q., Wang, Y., Asare Bediako, B.B., Cui, M., Yang, G., and Han, B. (2019). Synthesis of acetamides using CO2, methanol, H2 and amines. Green Chemistry 21, 233-237.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.