Graphical abstract
Keywords: Vaborbactam, Serine- and metallo-β-lactamase, Transition state analogue, Boronate inhibitor, β-Lactamase induction, Antibiotic resistance
Abstract
β-Lactams are the most successful antibacterials, yet their use is threatened by resistance, importantly as caused by β-lactamases. β-Lactamases fall into two mechanistic groups: the serine β-lactamases that utilise a covalent acyl-enzyme mechanism and the metallo β-lactamases that utilise a zinc-bound water nucleophile. Achieving simultaneous inhibition of both β-lactamase classes remains a challenge in the field. Vaborbactam is a boronate-based inhibitor that reacts with serine-β-lactamases to form covalent complexes that mimic tetrahedral intermediates in catalysis. Vaborbactam has recently been approved for clinical use in combination with the carbapenem meropenem. Here we show that vaborbactam moderately inhibits metallo-β-lactamases from all 3 subclasses (B1, B2 and B3), with a potency of around 20–100 fold below that by which it inhibits its current clinical targets, the Class A serine β-lactamases. This result contrasts with recent investigations of bicyclic boronate inhibitors, which potently inhibit subclass B1 MBLs but which presently lack activity against B2 and B3 enzymes. These findings indicate that cyclic boronate scaffolds have the potential to inhibit the full range of β-lactamases and justify further work on the development of boronates as broad-spectrum β-lactamase inhibitors.
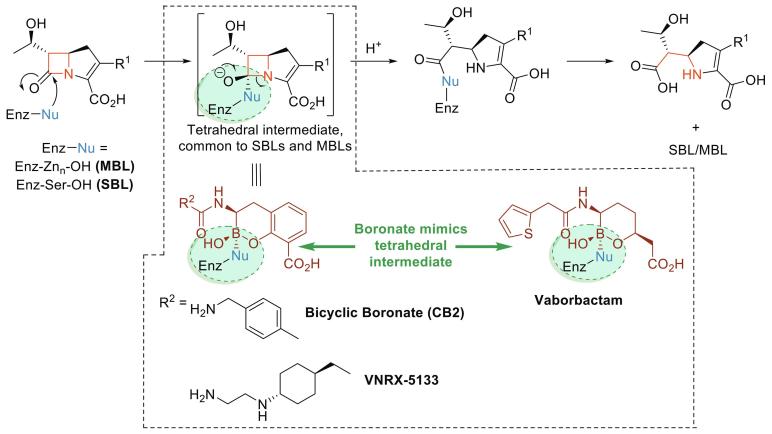
The β-lactams are amongst the most important antibacterials;1 their continued widespread use is challenged by resistance, most importantly due to β-lactamases.2 There are two mechanistically distinct types of β-lactamases: the serine–β-lactamases (SBLs; Ambler classes A, C and D)3 and the metallo–β-lactamases (MBLs; class B)4 (Fig. 1). SBL inhibitors (clavulanate, sulbactam and tazobactam) are established for clinical use when combined with a penicillin/cephalosporin.5 In combination with a cephalosporin, the non β-lactam SBL inhibitor Avibactam has been introduced as a broader–spectrum SBL inhibitor (active against classes A and C, with limited activity against class D).6, 7, 8 None of the clinically used SBL inhibitors inhibit MBLs. The β-lactams of the established SBL inhibitors are also increasingly subject to hydrolysis by MBLs/SBLs7, 8 and even the cyclic urea core of avibactam is susceptible to low-level hydrolysis by some MBLs.9 There is thus increasing interest in developing non-hydrolytically labile β-lactamase inhibitors.10 In this regard, boronic acids have long attracted attention since they can mimic the tetrahedral intermediates common to SBL and MBL catalysis (Fig. 1).11
Figure 1.
Outline mechanism of β-lactamase catalysis exemplified for a carbapenem. Note that the product can be produced in different tautomeric forms. The tetrahedral intermediate, common to both SBLs and MBLs, is mimicked by cyclic boronates.
Vaborbactam (formerly RPX7009) was developed to target SBLs of classes A and C12 and has been recently approved for clinical use in combination with meropenem (Vabomere).13, 14 In an initial study,12 vaborbactam was described as a sub-micromolar inhibitor of clinically relevant SBLs, with Ki values (using nitrocefin assays) for SBLs, including extended spectrum-β-lactamases (ESBLs), in the 10–100 nM range (CTX-M15 Ki 44 nM; SHV-12 Ki 29 nM; TEM-10 Ki 110 nM; KPC-2 carbapenemase Ki = 69 nM (all class A); Enterobacter cloacae cephalosporinase P99 Ki = 53 nM; Klebsiella pneumonia15 CMY-2 Ki 99 nM (class C)).12 A subsequent study reported that vaborbactam inhibition manifests fast-on-fast-off behaviour, a feature proposed to underlie lack of potent inhibition of the SHV-12 SBLs (and the TEM-42 ESBL).13 Co-administration of vaborbactam with a β-lactam antibiotic (primarily meropenem) manifests activity against bacterial strains harbouring genes encoding diverse class A enzymes (TEM-116; CTX-M, SHV, and TEM ESBLs; the KPC, FRI-1 and SME-2 carbapenemases) and the narrow spectrum oxacillinases OXA-2 and OXA-30.12 By contrast, vaborbactam combinations were not active against strains harbouring OXA-48 like class D SBLs, those hyperexpressing chromosomally encoded AmpC SBLs, and/or producing MBLs (i.e. the NDM, IMP or VIM carbapenemases).12, 13, 16, 17 Boronates with a ‘bicyclic’ scaffold such as cyclic boronate CB211, 18 (Fig. 1) can inhibit all four Ambler classes, with one such compound, VNRX-5133, in clinical trials11, 15, 18 (Fig. 1). By contrast, vaborbactam, which is principally ‘monocyclic’ in solution (Fig. 1), is reported not to inhibit MBLs.12, 13, 14
Here we report studies profiling the interactions of vaborbactam with representative enzymes of the three MBL subclasses (B1, B2, B3). The results reveal that vaborbactam shows weak inhibition activity of all three MBLs subclasses, including the clinically relevant B1 MBLs Verona Integron-encoded MBL (VIM)-1 and VIM-2, the New Delhi MBL (NDM)-1 and Imipenemase (IMP)-1; the B2 MBL Aeromonas hydrophila CphA (CphA) and the B3 MBL L1 from Stenotrophomonas maltophilia.
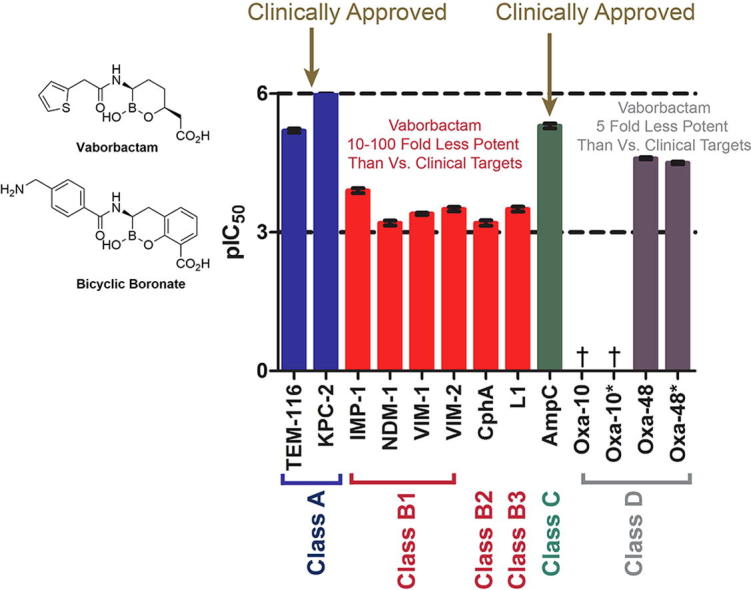
As anticipated, based on prior reports,12, 13, 17 vaborbactam inhibits representative SBLs from classes A and C, i.e. the class A narrow spectrum β-lactamase TEM-116 (IC50 = 6 μM), the Class A carbapenemase KPC-2 (IC50 = 90 nM), and the class C cephalosporinase AmpC from Pseudomonas aeruginosa (IC50 = 5 μM) (Table 1). Against the tested class D enzymes, moderate inhibition of the OXA-48 carbapenemase was observed (IC50 = 25 μM and IC50 = 32 μM in the presence of 100 mM NaHCO3), whilst only very low-level inhibition (<50%) of the narrow spectrum oxacillinase OXA-10 was observed using 400 μM vaborbactam (Table 1).
Table 1.
IC50 values and reported Ki values for vaborbactam against β-lactamases, compared to the reported values for vaborbactam and a bicyclic boronate.18†Weak inhibition (<50%) was observed for OXA-10 at the highest tested concentration (400 μM).
| Class | Enzyme | Vaborbactam IC50 [μM] | Vaborbactam Ki [nM] | Cyclic Boronate (CB2)11, 18 IC50 [μM] |
|---|---|---|---|---|
| A | TEM-116 | 6 μM | Not available | 0.003 μM18 |
| A | CTX-M15 | Not available | 44 nM12 | 0.013 μM11 |
| A | SHV-12 | Not available | 29 nM12 | Not available |
| A | TEM-10 | Not available | 110 nM12 | Not available |
| A | KPC-2 | 0.09 μM | 69 nM12 | 0.03 μM |
| B1 | IMP-1 | 126 μM | Not available | 1 μM18 |
| NDM-1 | 631 μM | Not available | 0.029 μM18 | |
| VIM-1 | 398 μM | Not available | 0.085 μM11 | |
| VIM-2 | 316 μM | Not available | 0.003 μM18 | |
| B2 | CphA | 631 μM | Not available | > 100 μM18 |
| B3 | L1 | 336 μM | Not available | Not inhibited19 |
| C | AmpC | 5 μM | Not available | 0.12 μM11 |
| C | P99 | Not available | 53 nM12 | Not available |
| C | CMY-2 | Not available | 99 nM12 | Not available |
| D | OXA-10 | > 400 μM | Not available | Not available |
| OXA-10 | > 400 μM | Not available | 5.1 μM11 | |
| (100 mM NaHCO3) | ||||
| OXA-48 | 25 μM | Not available | Not available | |
| OXA-48 | 32 μM | Not available | 2.6 μM11 | |
| (100 mM NaHCO3) |
Vaborbactam was then tested against a panel of MBLs (subclass B1: IMP-1, VIM-1, VIM-2, NDM-1; subclass B2: CphA and subclass B3: L1) comprising representatives of the three MBL subclasses (which differ in their active site architectures and Zn(II) requirements).18, 20, 21 Vaborbactam weakly inhibits all four of the tested B1 MBLs, VIM–1 (IC50 = 398 μM), VIM-2 (IC50 = 316 μM), NDM-1 (IC50 = 631 μM) and IMP-1 (IC50 = 126 μM), but at a much lower levels than observed for the SBLs. Similar low–level inhibition of the MBL subclass B2 CphA (IC50 = 631 μM) and the subclass B3 L1 (IC50 = 336 μM) was also observed (Table 1).
We investigated the antimicrobial activity of vaborbactam at a fixed concentration of 8 µg/mL (27 µM), in combination with meropenem against three E. coli and K. pneumoniae clinical isolates all co-expressing NDM-1, which is weakly inhibited by vaborbactam (IC50 = 631 μM). In accord with the literature data12, 13 and its relatively weak potency versus NDM-1 vaborbactam did not improve the MIC of meropenem against these strains (Supporting Information- Table 1.).
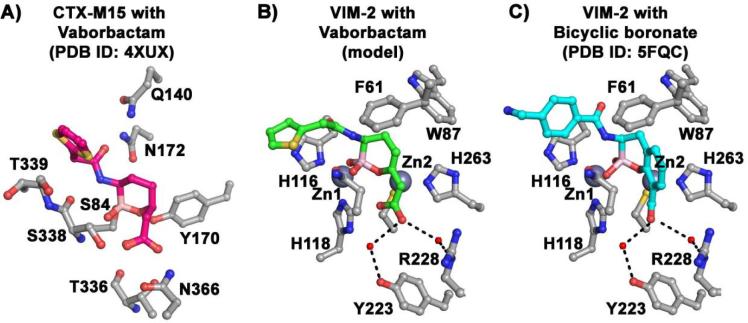
Although there are multiple crystal structures of boronates complexed to both SBLs22 and the related penicillin binding proteins,23 there are few with MBLs.11, 18 To investigate the possible structural basis of vaborbactam interaction with the MBLs, a model of vaborbactam bound to the B1 MBL VIM-2, based upon the binding mode of a bicyclic boronate (Fig. 1, PDB ID: 5FQC),18 was constructed (Fig. 2C).
Figure 2.
Model of vaborbactam binding to VIM-2 (B). Residues within 3.5 Å of vaborbactam are indicated. The model is presented alongside a view from a crystal structure of (A) vaborbactam bound to CTX-M15 (PDB ID: 4XUX) and (C) a bicyclic boronate bound to VIM-2 (PDB ID: 5FQC).18
The model implies that vaborbactam might bind in a similar manner to the bicyclic boronates (Fig. 2B and C),11, 24 with its ‘endocyclic’ boronate oxygen positioned to bind to the Zn(2) ion in the Cys-His-Asp site of the di-Zn(II) active site and the other two boronate oxygens positioned to bind to Zn(1) in the tri-His site. Since VIM-1 and VIM-2 employ different binding modes for the substrate carboxylate (VIM-2: Y224, R228 and VIM-1: H224,S228),24 the observation that VIM-1 and VIM-2 are inhibited to a similar degree by vaborbactam is notable. The modelled VIM-2 complex features water-mediated contacts between the vaborbactam carboxylate and Y224 and R228, as observed in our previous crystallographic characterisations of bicyclic boronate binding to MBLs18 (Fig. 2B and C).
The overall results reveal that, from comparison of IC50 values, vaborbactam manifests inhibition of SBLs (TEM-116, KPC-2 and AmpC, from classes A and C, respectively) that is 20 to 7000–fold more potent than that for the class B MBLs (IMP-1, VIM-1, VIM-2, NDM-1 and L1) and 5-fold more potent than reported for the class D SBL, OXA-48 (Table 1). With the class D enzymes (OXA-10 and OXA-48) vaborbactam manifests weak activity against the carbapenem hydrolysing class D (CHDL) SBL OXA-48, but no activity against the narrow spectrum oxacillinase OXA-10 (Fig. 3). These observations correlate with microbiological studies, wherein vaborbactam shows no activity against OXA-10/OXA–4817 and as reported here, NDM-1 producing strains. Although of weak potency against MBLs, vaborbactam exhibits greater activity against the MBLs than avibactam, which we have demonstrated to interact with some MBLs9 but which does not show any inhibition across the same range of inhibitor concentrations. Notably, vaborbactam shows some activity towards the (mono-Zn(II)) B2 MBL CphA and the B3 MBL L1 (Table 1). For the class B1 MBLs, vaborbactam was most potent against IMP-1 (126 µM), and less potent against VIM-1 and VIM-2 (398 and 316 µM, respectively) with the lowest activity observed against NDM-1 (631 µM).
Figure 3.
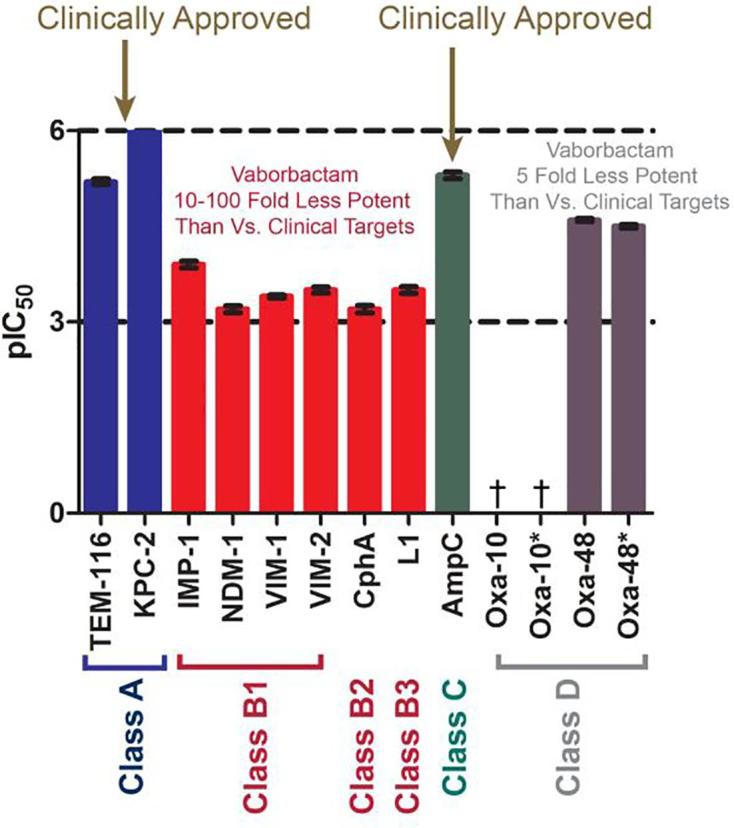
IC50 values for vaborbactam against the shown β-lactamases.
The results imply that whilst vaborbactam itself is very likely not useful against most, if not all, MBL-, and many SBL- (especially class D), producing strains, there is considerable potential for further optimisation of cyclic boronate based β -lactamase inhibitors. Boronates are being pursued as SBL/MBL/penicillin-binding protein (PBP) inhibitors, in part because of their ability to mimic potentially common tetrahedral intermediates in catalysis.12, 18 However, while such efforts are currently limited by the lack of useful (broad spectrum and potent) PBP inhibition by the boronates so far investigated, structure-activity relationship (SAR) information is emerging for SBL/MBL inhibition by different types of boronates. By contrast to the results for the monocyclic compound vaborbactam reported here, bicyclic boronates are capable of potent (nM) inhibition of MBLs of subclass B1 in addition to their activity against SBLs. However, the currently tested bicyclic boronates e.g. CB2, Table 1,18, 19 do not exhibit inhibitory activity against the B2 CphA (mono Zn(II)) or B3 L1 MBLs. It is notable that vaborbactam shows weak but detectable (μM) inhibition of both CphA and L1, raising the possibility that monocyclic boronates are potentially capable of supporting broader spectrum inhibitory activity against MBLs than their current bicyclic counterparts. Together with previous studies, including those with PBPs,23 these observations may reflect the increased conformational flexibility of monocyclic versus bicyclic boronates and, maybe, the increased propensity of the former to exist in an acyclic form. Further SAR on both mono- and bi-cyclic boronate based β-lactamase inhibitors is required.
We also observed substantial variations in vaborbactam potency within, as well as between, different MBL subclasses (B1-B3). The differences in vaborbactam activity against B1 MBLs (IMP-1 > VIM-1/VIM-2 > NDM-1), might relate to the active site of IMP-1 being more compact (on the basis of reported crystallographic studies) than that of NDM-1;25 bicyclic boronates inhibit IMP-1 less potently than VIM-1/-2 and NDM-1.11 For the class D enzymes, which require active site lysine carbamylation for activity;26 vaborbactam inhibition was unaffected by addition of NaHCO3 to the assay buffer, although this increased catalytic activity. This observation is consistent with reported studies on bicyclic boronates,18 but contrasts with results for avibactam.26 The molecular reasons for these variations in SAR for the different classes of boronate based inhibitors are presently unclear, but merit further detailed investigation given the desirability of developing very broad spectrum β–lactamase inhibitors, especially those active against carbapenemases, e.g. the VIM, IMP, NDM and OXA-48 enzymes, for which current inhibitors are largely ineffective.
Overall, our results identify vaborbactam as a low level pan β-lactamase inhibitor able to inhibit SBLs and MBLs of all classes. Together with recently reported studies on the structural bases of (bi)cyclic boronate inhibition of all classes of β-lactamases and PBPs, these data support the proposal that cyclic boronates constitute inhibitor templates of interest for development as β -lactamase inhibitors with wider spectra of activity than currently available agents.
Acknowledgements
We thank the Wellcome Trust, Cancer Research UK, the Medical Research Council, the SWON alliance (MR/N002679/1), the Biotechnology and Biological Research Council (BB/S50676X/1)BB/S50676X/1, and the Innovative Medicines Initiative (European Lead factory and ENABLE components), for funding our work on antibiotics, MBL fold enzymes, and β-lactamase inhibitors.
Footnotes
Supplementary data (Material and Methods) to this article can be found online at https://doi.org/10.1016/j.bmcl.2019.05.031.
Contributor Information
Christopher J. Schofield, Email: christopher.schofield@chem.ox.ac.uk.
Jürgen Brem, Email: jurgen.brem@chem.ox.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem, 2014;6: 25-64. [DOI] [PMC free article] [PubMed]
- 2.Versporten A., Bolokhovets G., Ghazaryan L. Antibiotic use in eastern Europe: a cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect Dis. 2014;14(5):381–387. doi: 10.1016/S1473-3099(14)70071-4. [DOI] [PubMed] [Google Scholar]; Versporten A, Bolokhovets G, Ghazaryan L, et al. Antibiotic use in eastern Europe: a cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect Dis, 2014;14(5): 381-387. [DOI] [PubMed]
- 3.Frere J.M., Joris B., Granier B., Matagne A., Jacob F., Bourguignon-Bellefroid C. Diversity of the mechanisms of resistance to beta-lactam antibiotics. Res Microbiol. 1991;142(6):705–710. doi: 10.1016/0923-2508(91)90084-n. [DOI] [PubMed] [Google Scholar]; Frere JM, Joris B, Granier B, Matagne A, Jacob F, Bourguignon-Bellefroid C. Diversity of the mechanisms of resistance to beta-lactam antibiotics. Res Microbiol, 1991;142(6): 705-710. [DOI] [PubMed]
- 4.Walsh T.R., Toleman M.A., Poirel L., Nordmann P. Metallo-β-lactamases: the quiet before the storm? Clini Microbiol Rev. 2005;18(2):306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: the quiet before the storm? Clini Microbiol Rev, 2005;18(2): 306-325. [DOI] [PMC free article] [PubMed]
- 5.Chellat M.F., Raguz L., Riedl R. Targeting Antibiotic Resistance. Angew Chem Int Ed. 2016;55(23):6600–6626. doi: 10.1002/anie.201506818. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chellat MF, Raguz L, Riedl R. Targeting Antibiotic Resistance. Angew Chem Int Ed, 2016;55(23): 6600-6626. [DOI] [PMC free article] [PubMed]
- 6.Docquier J.D., Mangani S. An update on β-lactamase inhibitor discovery and development. Drug Resist Updat. 2018;36:13–29. doi: 10.1016/j.drup.2017.11.002. [DOI] [PubMed] [Google Scholar]; Docquier JD, Mangani S. An update on β-lactamase inhibitor discovery and development. Drug Resist Updat, 2018;36: 13-29. [DOI] [PubMed]
- 7.Wang D.Y., Abboud M.I., Markoulides M.S., Brem J., Schofield C.J. The road to avibactam: the first clinically useful non-β-lactam working somewhat like a β-lactam. Future Med Chem. 2016;8(10):1063–1084. doi: 10.4155/fmc-2016-0078. [DOI] [PubMed] [Google Scholar]; Wang DY, Abboud MI, Markoulides MS, Brem J, Schofield CJ. The road to avibactam: the first clinically useful non-β-lactam working somewhat like a β-lactam. Future Med Chem, 2016;8(10): 1063-1084. [DOI] [PubMed]
- 8.Shields R.K., Chen L., Cheng S. Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob Agents Chemother. 2017;61(3). doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shields RK, Chen L, Cheng S, et al. Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob Agents Chemother, 2017;61(3). [DOI] [PMC free article] [PubMed]
- 9.Abboud M.I., Damblon C., Brem J. Interaction of Avibactam with Class B Metallo-β-Lactamases. Antimicrob Agents Chemother. 2016;60(10):5655–5662. doi: 10.1128/AAC.00897-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; Abboud MI, Damblon C, Brem J, et al. Interaction of Avibactam with Class B Metallo-β-Lactamases. Antimicrob Agents Chemother, 2016;60(10): 5655-5662. [DOI] [PMC free article] [PubMed]
- 10.Drawz S.M., Papp-Wallace K.M., Bonomo R.A. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58(4):1835–1846. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Drawz SM, Papp-Wallace KM, Bonomo RA. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother, 2014;58(4): 1835-1846. [DOI] [PMC free article] [PubMed]
- 11.Cahill S.T., Cain R., Wang D.Y. Cyclic Boronates Inhibit All Classes of β-Lactamases. Antimicrob Agents Chemother. 2017;61(4). doi: 10.1128/AAC.02260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cahill ST, Cain R, Wang DY, et al. Cyclic Boronates Inhibit All Classes of β-Lactamases. Antimicrob Agents Chemother, 2017;61(4). [DOI] [PMC free article] [PubMed]
- 12.Hecker S.J., Reddy K.R., Totrov M. Discovery of a Cyclic Boronic Acid β-Lactamase Inhibitor (RPX7009) with Utility vs Class A Serine Carbapenemases. J Med Chem. 2015;58(9):3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]; Hecker SJ, Reddy KR, Totrov M, et al. Discovery of a Cyclic Boronic Acid β-Lactamase Inhibitor (RPX7009) with Utility vs Class A Serine Carbapenemases. J Med Chem, 2015;58(9): 3682-3692. [DOI] [PubMed]
- 13.Lomovskaya O., Sun D., Rubio-Aparicio D. Vaborbactam : Spectrum of β-Lactamase Inhibition and Impact of Resistance Mechanisms on Activity in Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(11). doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: Spectrum of β-Lactamase Inhibition and Impact of Resistance Mechanisms on Activity in Enterobacteriaceae. Antimicrob Agents Chemother, 2017;61(11). [DOI] [PMC free article] [PubMed]
- 14.Lapuebla A., Abdallah M., Olafisoye O. Activity of Meropenem Combined with RPX7009, a Novel β-Lactamase Inhibitor, against Gram-Negative Clinical Isolates in New York City. Antimicrob Agents Chemother. 2015;59(8):4856–4860. doi: 10.1128/AAC.00843-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of Meropenem Combined with RPX7009, a Novel β-Lactamase Inhibitor, against Gram-Negative Clinical Isolates in New York City. Antimicrob Agents Chemother, 2015;59(8): 4856-4860. [DOI] [PMC free article] [PubMed]
- 15.Cahill S.T., Tyrrell J.M., Navratilova I.H. Studies on the inhibition of AmpC and other β-lactamases by cyclic boronates. Biochim Biophys Acta Gen Subj. 2019;1863(4):742–748. doi: 10.1016/j.bbagen.2019.02.004. [DOI] [PubMed] [Google Scholar]; Cahill ST, Tyrrell JM, Navratilova IH, et al. Studies on the inhibition of AmpC and other β-lactamases by cyclic boronates. Biochim Biophys Acta Gen Subj,. 2019;1863(4): 742-748. [DOI] [PubMed]
- 16.Livermore D.M., Mushtaq S. Activity of biapenem (RPX2003) combined with the boronate β-lactamase inhibitor RPX7009 against carbapenem-resistant Enterobacteriaceae. J Antimicrob Chemother. 2013;68(8):1825–1831. doi: 10.1093/jac/dkt118. [DOI] [PubMed] [Google Scholar]; Livermore DM, Mushtaq S. Activity of biapenem (RPX2003) combined with the boronate β-lactamase inhibitor RPX7009 against carbapenem-resistant Enterobacteriaceae. J Antimicrob Chemother, 2013;68(8): 1825-1831. [DOI] [PubMed]
- 17.Jorgensen S.C.J., Rybak M.J. Meropenem and Vaborbactam: Stepping up the Battle against Carbapenem-resistant Enterobacteriaceae. Pharmacotherapy. 2018;38(4):444–461. doi: 10.1002/phar.2092. [DOI] [PubMed] [Google Scholar]; Jorgensen SCJ, Rybak MJ. Meropenem and Vaborbactam: Stepping up the Battle against Carbapenem-resistant Enterobacteriaceae. Pharmacotherapy. 2018;38(4): 444-461. [DOI] [PubMed]
- 18.Brem J., Cain R., Cahill S. Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat Commun. 2016;7:12406. doi: 10.1038/ncomms12406. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brem J, Cain R, Cahill S, et al. Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat Commun, 2016;7: 12406. [DOI] [PMC free article] [PubMed]
- 19.Calvopina K., Hinchliffe P., Brem J. Structural/mechanistic insights into the efficacy of nonclassical β-lactamase inhibitors against extensively drug resistant Stenotrophomonas maltophilia clinical isolates. Mol Microbiol. 2017;106(3):492–504. doi: 10.1111/mmi.13831. [DOI] [PubMed] [Google Scholar]; Calvopina K, Hinchliffe P, Brem J, et al. Structural/mechanistic insights into the efficacy of nonclassical β-lactamase inhibitors against extensively drug resistant Stenotrophomonas maltophilia clinical isolates. Mol microbiol, 2017;106(3): 492-504. [DOI] [PubMed]
- 20.Abboud M.I., Hinchliffe P., Brem J. 19 F-NMR Reveals the Role of Mobile Loops in Product and Inhibitor Binding by the Sao Paulo Metallo-β-Lactamase. Angew Chem Int Ed. 2017;56(14):3862–3866. doi: 10.1002/anie.201612185. [DOI] [PMC free article] [PubMed] [Google Scholar]; Abboud MI, Hinchliffe P, Brem J, et al. 19 F-NMR Reveals the Role of Mobile Loops in Product and Inhibitor Binding by the Sao Paulo Metallo-β-Lactamase. Angew Chem Int Ed, 2017;56(14): 3862-3866. [DOI] [PMC free article] [PubMed]
- 21.Brem J., van Berkel S.S., Zollman D. Structural Basis of Metallo-β-Lactamase Inhibition by Captopril Stereoisomers. Antimicrob Agents Chemother. 2015;60(1):142–150. doi: 10.1128/AAC.01335-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brem J, van Berkel SS, Zollman D, et al. Structural Basis of Metallo-β-Lactamase Inhibition by Captopril Stereoisomers. Antimicrob Agents Chemother, 2015;60(1): 142-150. [DOI] [PMC free article] [PubMed]
- 22.Diaz D.B., Yudin A.K. The versatility of boron in biological target engagement. Nat Chem. 2017;9(8):731–742. doi: 10.1038/nchem.2814. [DOI] [PubMed] [Google Scholar]; Diaz DB, Yudin AK. The versatility of boron in biological target engagement. Nat Chem, 2017;9(8): 731-742. [DOI] [PubMed]
- 23.Contreras-Martel C., Amoroso A., Woon E.C. Structure-guided design of cell wall biosynthesis inhibitors that overcome β-lactam resistance in Staphylococcus aureus (MRSA) ACS Chem Biol. 2011;6(9):943–951. doi: 10.1021/cb2001846. [DOI] [PubMed] [Google Scholar]; Contreras-Martel C, Amoroso A, Woon EC, et al. Structure-guided design of cell wall biosynthesis inhibitors that overcome β-lactam resistance in Staphylococcus aureus (MRSA). ACS Chem Biol, 2011;6(9): 943-951. [DOI] [PubMed]
- 24.Salimraj R., Hinchliffe P., Kosmopoulou M. Crystal structures of VIM-1 complexes explain active site heterogeneity in VIM-class metallo-β-lactamases. FEBS J. 2019;286(1):169–183. doi: 10.1111/febs.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]; Salimraj R, Hinchliffe P, Kosmopoulou M, et al. Crystal structures of VIM-1 complexes explain active site heterogeneity in VIM-class metallo-β-lactamases. FEBS J,. 2019;286(1): 169-183. [DOI] [PMC free article] [PubMed]
- 25.Moali C., Anne C., Lamotte-Brasseur J. Analysis of the importance of the metallo-β-lactamase active site loop in substrate binding and catalysis. Chem Biol. 2003;10(4):319–329. doi: 10.1016/s1074-5521(03)00070-x. [DOI] [PubMed] [Google Scholar]; Moali C, Anne C, Lamotte-Brasseur J, et al. Analysis of the importance of the metallo-β-lactamase active site loop in substrate binding and catalysis. Chem Biol, 2003;10(4): 319-329. [DOI] [PubMed]
- 26.Lohans C.T., Wang D.Y., Jorgensen C. (13)C-Carbamylation as a mechanistic probe for the inhibition of class D β-lactamases by avibactam and halide ions. Org Biomol Chem. 2017;15(28):6024–6032. doi: 10.1039/c7ob01514c. [DOI] [PubMed] [Google Scholar]; Lohans CT, Wang DY, Jorgensen C, et al. (13)C-Carbamylation as a mechanistic probe for the inhibition of class D β-lactamases by avibactam and halide ions. Org Biomol Chem, 2017;15(28): 6024-6032. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.