Summary
Objective
Physiological mechanical loading reduces inflammatory signalling in numerous cell types including articular chondrocytes however the mechanism responsible remains unclear. This study investigates the role of chondrocyte primary cilia and associated intraflagellar transport (IFT) in the mechanical regulation of interleukin-1β (IL-1β) signalling.
Design
Isolated chondrocytes and cartilage explants were subjected to cyclic mechanical loading in the presence and absence of the cytokine IL-1β. Nitric oxide (NO) and prostaglandin E2 (PGE2) release were used to monitor IL-1β signalling whilst Sulphated glycosaminoglycan (sGAG) release provided measurement of cartilage degradation. Measurements were made of HDAC6 activity and tubulin polymerisation and acetylation. Effects on primary cilia were monitored by confocal and super resolution microscopy. Involvement of IFT was analysed using ORPK cells with hypomorphic mutation of IFT88.
Results
Mechanical loading suppressed NO and PGE2 release and prevented cartilage degradation. Loading activated HDAC6 and disrupted tubulin acetylation and cilia elongation induced by IL-1β. HDAC6 inhibition with tubacin blocked the anti-inflammatory effects of loading and restored tubulin acetylation and cilia elongation. Hypomorphic mutation of IFT88 reduced IL-1β signalling and abolished the anti-inflammatory effects of loading indicating the mechanism is IFT-dependent. Loading reduced the pool of non-polymerised tubulin which was replicated by taxol which also mimicked the anti-inflammatory effects of mechanical loading and prevented cilia elongation.
Conclusions
This study reveals that mechanical loading suppresses inflammatory signalling, partially dependent on IFT, by activation of HDAC6 and post transcriptional modulation of tubulin.
Keywords: IFT, Primary cilia, Chondrocyte, IL-1β, HDAC6, Tubulin
Introduction
Primary cilia are slender organelles which regulate a variety of cell signalling pathways including hedgehog, wnt, growth factor and mechanosignalling [for review see1, 2, 3]. They consist of an axoneme, 2–5 μm in length and 0.25 μm in diameter, composed of tubulin, which is typically acetylated, forming a ring of nine stable microtubule doublets. The majority of proteins required for cilia structure and function are trafficked on and off the cilium by intraflagellar transport (IFT) as cilia lack protein synthesis machinery.
Among the various pathways regulated by primary cilia, recent studies by the authors have shown primary cilia and/or IFT, are required for pro-inflammatory signalling in response to the cytokine, interleukin-1β (IL-1β)4. Loss of cilia in chondrocytes with hypomorphic mutation of the IFT protein, IFT88, prevents the associated pro-inflammatory response. Interestingly, in endothelial cells, removal of cilia by complete deletion of IFT88 increased inflammatory gene expression in vivo5. Other recent studies further demonstrate an interplay between pro-inflammatory nuclear factor-kappa B (NF-kappa B) signalling and IFT6, 7.
Mechanical stimulation is anti-inflammatory in a variety of tissues8, 9. In cartilage cells, mechanical loading inhibits IL-1β-induced release of the pro-inflammatory mediators, nitric oxide (NO) and prostaglandin E2 (PGE2)10. Separate studies have shown mechanical loading regulates primary cilia structure via the tubulin de-acetylase, HDAC611, 12, 13. The resulting subtle changes in primary cilia structure may reflect or induce changes in ciliary IFT, and are associated with regulation of cilia and IFT-dependent signalling pathways including hedgehog11, wnt14 and mechanosignalling15. However no previous studies have investigated whether the anti-inflammatory effects of mechanical loading are mediated by regulation of IFT and primary cilia or the underpinning mechanisms involved. This study tests the hypothesis that mechanical loading suppresses the inflammatory response to IL-1β by HDAC6-dependent modulation of tubulin leading to changes in primary cilia elongation. We examined this behaviour in articular chondrocytes which are routinely exposed to physiological dynamic mechanical loading consisting of compressive, shear and tensile strains.
We show for the first time, that mechanical suppression of the inflammatory response to IL-1β in chondrocytes occurs in part via an IFT-dependent pathway regulated by HDAC6 activation and modulation of tubulin acetylation and polymerization.
Materials and methods
Cell and Tissue Culture
These studies used isolated bovine articular chondrocytes, bovine cartilage explants and a murine chondrocyte cell line with hypomorphic mutation of IFT88 (full details in SI).
Mechanical loading
Isolated chondrocytes were subjected to equibiaxial cyclic tensile strain (CTS) at 0.33 Hz, 0–10% strain using the Flexcell FX-5000T™ system (Flexcell Corp, PA). Cartilage explants were subjected to cyclic compressive strain (CCS) at 0.33 Hz, 0–10% peak strain using a well-established Bose loading system as previously described16. Explants were mounted in a standard 24 well plate with each explant centrally located within a washer. Loading was applied using hemispherical indenter pins with an initial tare load of 0.01N to guarantee contact. All mechanical loading was performed within a humidified incubator maintained at 37°C and 5% CO2.
Measurement of Sulphated glycosaminoglycan (sGAG), NO and PGE2 release in the culture media
sGAG release was quantified by Dimethylmethylene Blue (DMMB) assay. NO release was based on measurement of Nitrite (NO2), the stable product of NO detected, using the Griess assay. An immunoassay kit (KGE004B, R&D Systems, UK) was used to quantify PGE2 release.
Immunofluorescence
Chondrocytes were fixed with 4% paraformaldehyde for 10min, permeabilised with 0.5% triton-X/PBS for 5min and blocked with 5% goat serum for 1hr. Primary cilia were immuno-labelled using anti-acetylated α-tubulin (1:2000, T7451, Sigma Aldrich, Poole, UK) and Arl13b (1:2000, 17711-1-AP, Proteintech, Manchester UK) with overnight incubation at 4°C. Appropriate Alexa Fluor 488 and Alexa Fluor 633 conjugated second antibodies were used for fluorescent labelling and cells were counterstained with DAPI (1 μg/ml).
Confocal and super resolution microscopy
Primary cilia were visualised on a Leica TCS SP2 confocal microscope (x63/0.95NA objective). Confocal z-stack sections were made throughout the cell depth (0.5 μm step size, 2048 × 2048 form 0.116 μm pixel size). Super resolution structure illumination microscopy (sr-SIM) was performed on a Zeiss 710 ELYRA PS.1 microscope (x63/1.4 NA objective). Cilia length was quantified from maximum projection images using image J11. Cilia prevalence was measured from 10 random fields of view.
Western Blotting
Total protein was isolated using RIPA buffer (R0278, Sigma Aldrich) and quantified with Bicinchoninic acid (BCA) assay. To fractionate soluble and polymerized tubulin, soluble tubulin extraction buffer A (137 mM NaCl, 20 mM Tris-HCl, 1% Triton X-100, and 10% glycerol) was added to cells at 4°C for 3 min, plates were gently swirled. Buffer was removed and saved as soluble fraction. Immediately after, polymerized tubulin extraction buffer B (buffer A + 1% SDS) was added for 1 min, cells were scraped, and the polymerized fraction was incubated on ice for 30 min.
Proteins were separated by SDS–PAGE under reducing conditions, transferred to nitrocellulose membranes and blocked in odyssey blocking buffer (Li-Cor Cambridge, UK) before probing with specific primary antibodies and infrared secondary antibodies (Li-Cor). Proteins were visualized using the Li-Cor Odyssey and quantified using Li-Cor lite software.
HDAC6 activity measurement
HDAC6 activity was measured using a commercial HDAC6 fluorometric assay kit (K466-100, Biovision). This assay exploits the selectivity of tubacin for HDAC6 in combination with a fluorescent synthetic acetylated-peptide substrate to determine enzyme activity. Briefly, cultures were lysed and 10 μl mixed with acetylated substrate (sample) or with 2 μM tubacin and acetylated substrate (inhibitor control). Reaction mixtures were incubated for 30 min at 37°C. A fluorescent plate reader was used to measure deacetylase-dependent release of a 7-amino-4-trifluoromethylcoumarin fluorophore (excitation/emission at 350/490 nm). HDAC6 activity was calculated as [sample-inhibitor control].
Statistical analyses
Data analysis was conducted using GraphPad Prism 5 (GraphPad Software Inc, La Jolla CA). Parametric analyses were performed on normally distributed data (D'Agostino-Pearson normality test). Experiments were performed using cells isolated from at least two joints/animals each with three or more technical replicates. These sample sizes are considered appropriate for an exploratory study given the large effect sizes expected and minimal variation in measurements on chondrocytes isolated from separate animals. Throughout the figure legends, n denotes the total number of technical replicate and N the number of joints/animals from which cells/tissue was obtained. Data are expressed as mean ± 95% Confidence Interval (CI). Statistically significant differences are indicated at P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***).
Results
Mechanical loading inhibits chondrocyte IL-1β signalling and associated cartilage degradation
We examined the effect of mechanical loading on the response to IL-1β in primary chondrocytes. Incubation of isolated cells with IL-1β (10 ng/ml) resulted in accumulation of the inflammatory mediators, NO [P < 0.0001, Fig. 1(A)] and PGE2 [P < 0.0001, Fig. 1(B)] in the culture media. Simultaneous co-application of IL-1β with mechanical loading in the form of CTS, completely blocked the effect of IL-1β, causing no statistically significant differences between IL-1β treatment and untreated controls for both NO [P = 0.545, Fig. 1(A)] and PGE2 [P = 0.290, Fig. 1(B)].
Fig. 1.
Mechanical loading inhibits IL-1β signalling and cartilage degradation. (A) NO (nitrite) release and (B) PGE2 release were measured in the culture media (samples n = 6, from donors N = 2) for primary chondrocytes ± IL-1β (10 ng/ml) in the presence or absence of cyclic tensile strain (CTS, 0–10%, 0.33 Hz, 24 h). (C) NO release and (D) Sulphated glycosaminoglycan (sGAG) release were measured in the media (n = 6, N = 2) for full depth cartilage explants ± IL-1β (10 ng/ml) in the presence or absence of cyclic compressive strain (CCS, 0–10%, 0.33 Hz, 24 h). Statistical differences based on Two-way ANOVA with Bonferroni's post hoc test.
Cartilage explants treated with IL-1β (10 ng/ml) produced a similar response to that seen for isolated cells, with an increase in NO release which was completely blocked by mechanical loading in the form of CCS [P = 0.694, Fig. 1(C)]. IL-1β treatment increased sGAG release into the culture media, indicative of cartilage degradation. This catabolic response was completely inhibited by CCS [P = 0.533, Fig. 1(D)].
Together, these data confirm that mechanical loading inhibits inflammatory signalling in response to the pro-inflammatory cytokine, IL-1β, and reduces the associated downstream degradation of the cartilage extracellular matrix.
Mechanical loading blocks primary cilia elongation in response to IL-1β
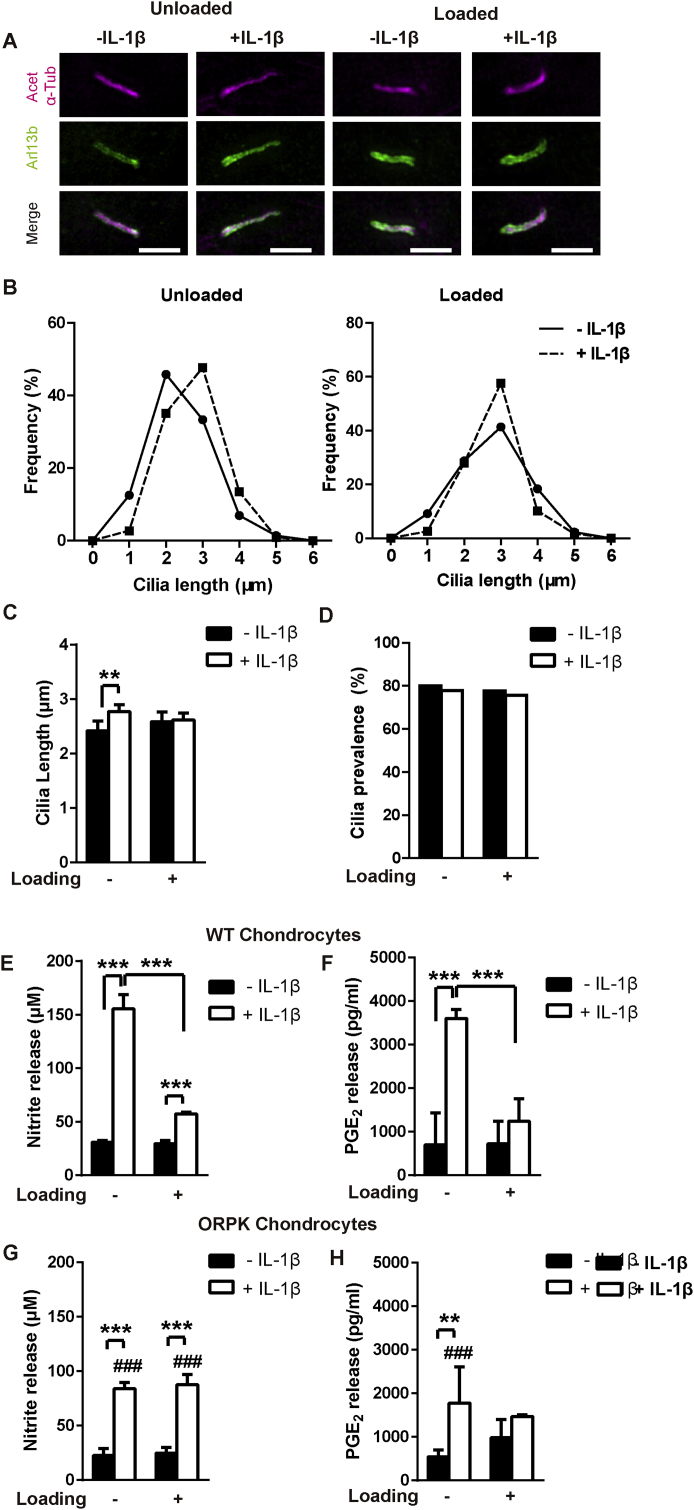
Previously we have shown that IL-1β causes primary cilia elongation4 while conversely cilia disassembly occurs in response to mechanical loading (CCS and CTS)11, 12. Therefore we examined whether CTS prevents cilia elongation in the presence of IL-1β. Isolated primary bovine chondrocytes were subjected to CTS prior to visualisation of cilia using immunofluorescence with confocal and sr-SIM [Fig. 2(A)]. Treatment with IL-1β at 1 ng/ml, produced a statistically significant increase in cilia length of 14% [P = 0.002, Fig. 2(B) and (C)]. However this effect was completely inhibited in mechanically loaded cells subjected to CTS [P = 0.913, Fig. 2(B) and (C)]. A similar effect was seen at 10 ng/ml IL-1β which produced a greater degree of cilia elongation in unloaded cells [43%, P < 0.001, Figs. S1(A)–(C)]. Treatment with IL-1β, at either 1 or 10 ng/ml, had no significant effect of cilia prevalence with or without CTS [Fig. 2(D) and S1(D)]. Thus mechanical loading, in the form of CTS, suppresses IL-1β-induced cilia elongation with no effect on cilia prevalence.
Fig. 2.
Mechanical loading blocks IL-1β induced elongation of chondrocyte primary cilia indicating disruption to intraflagellar transport (IFT) which is required for inflammatory signalling. (A) Representative SIM maximum intensity projection images of chondrocyte primary cilia ± IL-1β (1 ng/ml) in the presence or absence of mechanical loading in the form of cyclic tensile strain (CTS, 0–10%, 0.33 Hz, 24 h). Cilia were labelled for acetylated-α-tubulin (Acet-α-Tub, far-red) and arl13b (green). Scale bar represents 2 μm. (B) Frequency histograms of cilia length in unloaded and loaded cells and (C) corresponding mean cilia length (n ≈ 80–100 cilia) and (D) cilia prevalence (n ≈ 100 cells). Statistical analyses based on Mann–Whitney test for cilia length and Chi-square test for cilia prevalence. Changes in (E, G) nitrite and (F, H) PGE2 release for wild type (WT) (E, F) and ORPK (G, H) chondrocytes ± IL-1β (1 ng/ml) in the presence or absence of cyclic tensile strain (CTS, 0–10%, 0.33 Hz, 24 h, n = 4, N = 2). Statistical analyses based on two-way ANOVA with Bonferroni's post hoc test; # indicates significant difference between WT and ORPK.
Mechanical loading suppresses the inflammatory response through an IFT-dependent mechanism
To examine the role of primary cilia and associated IFT in mechanical regulation of inflammatory signalling we used an immortalised chondrocyte cell line generated from the Tg737ORPK mouse15. These chondrocytes exhibit hypomorphic mutation of IFT88 which causes cilia to either be absent, or severely stunted. This is illustrated by a significant reduction in expression of visible cilia from approximately 75% in wild type (WT) to 20% in ORPK cells [P < 0.001, Figs. S2(A) and (B)].
For WT cells, IL-1β (1 ng/ml) had no effect on cilia prevalence [P = 0.872, Fig. S2(B)] but increased cilia length [P < 0.001, Fig. S2(C)], as seen in primary bovine chondrocytes [Fig. 2(A)–(D)]. WT cells also demonstrated an inflammatory response to IL-1β, which was inhibited by mechanical loading in the form of CTS. Thus IL-1β significantly increased NO and PGE2 release [P < 0.001, Fig. 2(E) and (F)] while CTS inhibited both these responses, mimicking the behaviour observed in primary bovine chondrocytes.
For the ORPK cells that were seen to express detectable cilia, IL-1β (1 ng/ml) had no effect on prevalence [P = 0.615, Fig. S2(B)] but increased cilia length [P < 0.001, Fig. S2(C)]. As previously reported, ORPK cells exhibited an attenuated inflammatory response to IL-1β for both NO and PGE2 release, similar to the effect of CTS [P = 0.005, P < 0.001 respectively, Fig. 2(G) and (H)]. Importantly, mechanical loading (CTS) produced no further reduction in either NO or PGE2 release in ORPK cells in contrast to the behaviour in WT and primary cells.
These results suggest that the inflammatory response to IL-1β occurs via both IFT88-dependent and IFT88-independent pathways, in agreement with our previous studies4, Furthermore, the anti-inflammatory effects of mechanical loading inhibit the IFT-dependent pathway and hence also regulate ciliogenesis.
Mechanical loading activates HDAC6 to block IL-1β induced tubulin acetylation and polymerization
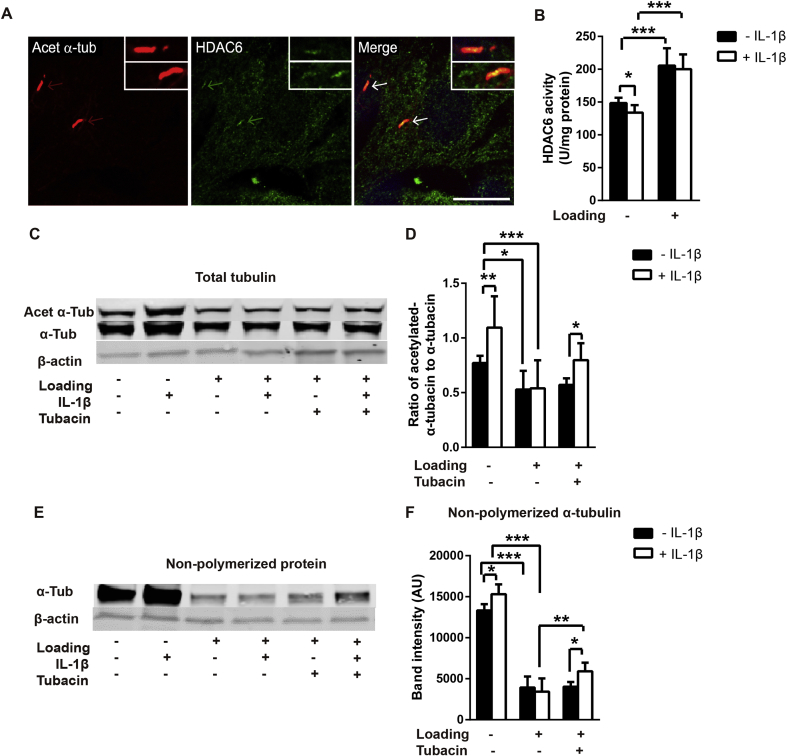
Previous studies suggest that HDAC6 activation and associated tubulin deacetylation, regulates changes in primary cilia length in response to mechanical loading11. We therefore sought to determine whether CTS modulated tubulin acetylation in the presence of IL-1β and if so, whether this was mediated via HDAC6. Confocal immunofluorescence revealed that HDAC6 was enriched within the cilia of primary articular chondrocytes [Fig. 3(A)]. HDAC6 activity was reduced by treatment with IL-1β in unloaded cells (P = 0.013) but was significantly up-regulated by CTS with and without IL-1β treatment [P < 0.001, Fig. 3(B)].
Fig. 3.
Mechanical loading blocks IL-1β induced tubulin acetylation and reduces non-polymerized tubulin expression via activation of HDAC6. (A) Representative confocal immunofluorescence for acetylated-α-tubulin (Acet-α-Tub, red), HDAC6 (green) in cultured chondrocytes showing co-localization on the cilia axoneme. (B) HDAC6 activity for cells ± IL-1β (1 ng/ml) in the presence or absence of cyclic tensile strain (CTS, 0–10%, 0.33 Hz, 24 h, n = 6, N = 2). Western blot analysis of tubulin based on (C–D) total protein levels and (E–F) non-polymerized protein for cells ± IL-1β (1 ng/ml) in the presence or absence of cyclic tensile strain (CTS) as above. (C and E) Representative blots and corresponding semi-quantitative analysis of (D) ratio of acetylated-α-tubulin to α-tubulin and (F) non-polymerized α-tubulin expression (n = 6). Statistical analyses based on two-way ANOVA with Bonferroni's post hoc test.
We measured the effects of CTS and IL-1β on the acetylation ratio of α-tubulin expression based on total protein levels. Treatment with IL-1β (1 ng/ml) increased acetylation of α-tubulin [P = 0.001, Fig. 3(C) and (D)]. A similarly response was also observed at 10 ng/ml IL-1β [P < 0.001, Fig. S3(C)]. CTS produced a significant reduction in α-tubulin acetylation ratio with and without IL-1β [P < 0.001 and P = 0.047, Fig. 3(D)], and completely blocked the acetylation response to IL-1β [P = 0.999, Fig. 3(D)].
To examine whether mechanical regulation of HDAC6 activity was responsible for changes in tubulin acetylation, cells were treated with the HDAC6 specific inhibitor, tubacin (SML0065, Sigma–Aldrich, Poole, UK) [Fig. 3(C)–(E)]. Consistent with its role as an inhibitor of deacetylation, tubacin (0.5 μM) increased total α-tubulin acetylation in unloaded cells [Fig. S3(A)]. Tubacin treatment significantly rescued the IL-1β induced increase in acetylation of α-tubulin in loaded cells [P = 0.039, Fig. 3(D)].
We also examined the effect of CTS on the pool of cytoplasmic, non-polymerized tubulin as this is necessary for incorporation into the cilia axoneme during cilia elongation18. Treatment with IL-1β significantly increased the presence of non-polymerized α-tubulin P = 0.028, Fig. 3(F)–(H)]. CTS induced a substantial reduction in non-polymerized tubulin expression (P < 0.001) and abolished the effect of IL-1β [P = 0.591, Fig. 3(F)–(H)]. In unloaded cells without IL-1β, tubacin increased the pool of non-polymerized α-tubulin [Fig. S3(B)]. Tubacin also significantly rescued the pool of non-polymerized α-tubulin in loaded cells treated with IL-1β [P = 0.003, Fig. 3(F)–(H)].
In the absence of IL-1β, tubacin did not restore the acetylation ratio of the amount of non-polymerized tubulin suggesting the reduction in tubulin acetylation and polymerization induced by loading alone may not be dependent on HDAC6. However, these studies suggest that mechanical loading activates HDAC6 which inhibits tubulin acetylation and polymerization induced by IL-1β.
Tubulin polymerization with taxol modulates IL-1β-induced cilia elongation and NO/PGE2 release
We next examined whether small molecule inhibition of polymerization would mimic the effects of CTS. We utilized taxol (T7191, Sigma–Aldrich, Poole, UK) to promote tubulin polymerisation, thus decreasing free cytoplasmic tubulin expression independent of HDAC6 activation. This effect on tubulin polymerisation was confirmed by confocal immunofluorescence [Fig. S4(A)] and western blot [Fig. S4(B)]. Taxol did not change cilia length in the absence of IL-1β but completely inhibited further cilia elongation caused by IL-1β [P < 0.001, Fig. 4(A)]. Taxol also produced a small but significant reduction in cilia prevalence, both with and without IL-1β [P < 0.001, Fig. 4(B)].
Fig. 4.
Reduction in non-polymerized tubulin by taxol treatment disrupts IFT-mediated cilia elongation and inhibits IL-1β signalling. Chondrocytes were cultured for 24 hrs with and without IL-1β (1 ng/ml) and taxol (15 μM). Corresponding measurement of (A) primary cilia length (n ≈ 100 cilia); and (B) prevalence (n ≈ 100 cells) and the release of the pro-inflammatory mediators, (C) NO and (D) PGE2 (n = 6). Statistical analyses based on Mann–Whitney test for cilia length, Chi-square test for cilia prevalence and two-way ANOVA with Bonferroni's post hoc test for NO and PGE2 release.
Taxol had no effect on IL-1β induced NO [Fig. 4(C)] and PGE2 release [Fig. 4(D)] in the absence of IL-1β, but significantly reduced the release of both pro-inflammatory mediators in the presence of IL-1β [P < 0.001 and P = 0.041 respectively]. Thus tubulin polymerization with taxol mimics the effect of CTS. This supports our hypothesis that the mechanically-induced reduction in non-polymerized α-tubulin is partially responsible for the anti-inflammatory effects of loading in the presence of IL-1β.
Inhibition of HDAC6 restores cilia elongation and blocks the anti-inflammatory effects of mechanical loading in the presence of IL-1β
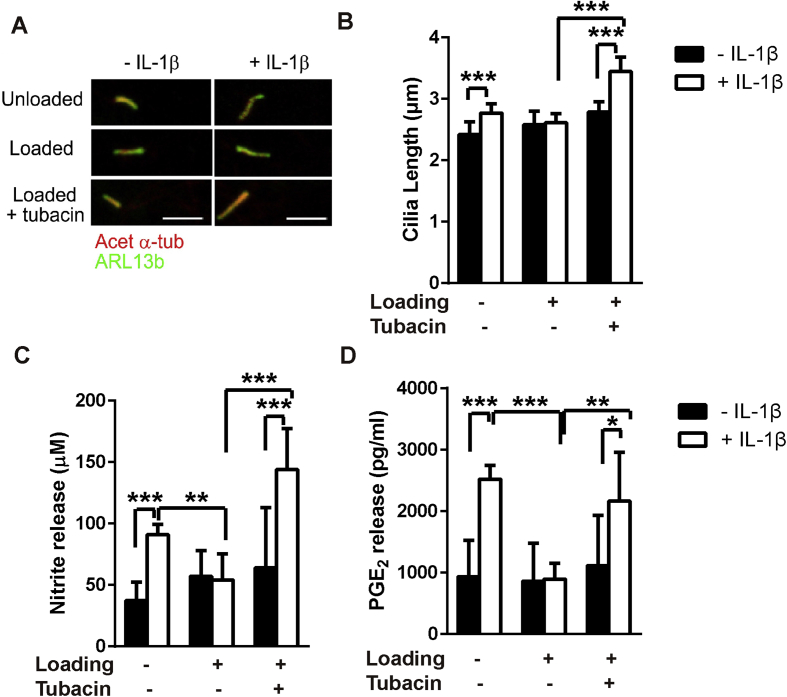
We next examined whether HDAC6 inhibition could block the anti-inflammatory effects of CTS on IL-1β signalling. Cells were subjected to CTS with and without IL-1β treatment in the presence/absence of tubacin. Immediately after mechanical loading cells were fixed and primary cilia visualised by confocal immunofluorescence [Fig. 5(A)]. In the absence of IL-1β, tubacin (0.5–2 μM) did not change cilia length or NO release [Figs. S5(A) and (B)]. However, tubacin (0.5 μM) blocked the effect of CTS such that cilia elongation in response to IL-1β (1 ng/ml) was fully restored in loaded chondrocytes [P < 0.001, Fig. 5(A) and (B)]. Similarly tubacin completely abolished the inhibitory effects of loading on NO [P < 0.001, Fig. 6(C)] and PGE2 release [P = 0.005, Fig. 5(D)] in response to IL-1β. Consequently, in loaded cells treated with tubacin, IL-1β significantly increased NO and PGE2, as seen in unloaded cells. A similar cilia elongation, NO and PGE2 response was also observed when cells were subjected to a higher concentration of 10 ng/ml IL-1β during CTS in the presence of tubacin [Fig. S6].
Fig. 5.
HDAC6 mediates the mechanical suppression of IL-1β-induced cilia elongation and inflammatory signalling. Chondrocytes were cultured on Flexcell membranes ± IL-1β (1 ng/ml), ± cyclic tensile strain (CTS, 0–10%, 0.33 Hz, 24 h) in the presence or absence of tubacin (0.5 μM). (A) Representative confocal immunofluorescence maximum intensity projection images of primary cilia labelled for acetylated-α-tubulin (Acet-α-Tub, red) and arl13b (green). Corresponding data for (B) cilia length (n ≈ 60–80 cilia), (C) NO release and (D) PGE2 release (n = 6, N = 2). Statistical analyses based on Kruskal Wallis test for cilia length and two-way ANOVA with Bonferroni's post hoc test for NO and PGE2 release.
Fig. 6.
In unloaded cells, tubacin prevents IL-1β-mediated tubulin acetylation, cilia elongation and nitrite release. (A) Cilia length (n ≈ 80–100 cilia) and (B) nitrite release (n = 6) for chondrocytes cultured in 0.2 μM, 0.5 μM, 1 μM or 2 μM tubacin ± IL-1β (1 ng/ml). Statistical analyses based on Mann–Whitney test for cilia length, one-way ANOVA with Bonferroni's post hoc test for NO release; * demonstrates statistical significance compared with untreated cells; # compared with cells treated with IL-1β in the absence of tubacin. (C–E) Western blot analysis of tubulin based on total protein levels for cells ± IL-1β (1 ng/ml) and ±tubacin (0.5 μM). (C) Representative blot and semi-quantitative analysis of (D) acetylated-α-tubulin and (E) α-tubulin expression and (F) the corresponding acetylation ratio (n = 3). Statistical analyses based on two-way ANOVA with Bonferroni's post hoc test.
These data suggest that the anti-inflammatory effect of mechanical loading is mediated by HDAC6 activation and post-transcriptional tubulin modification. This is associated with suppression of primary cilia elongation indicative of disrupted IFT.
In unloaded cells, pharmaceutical inhibition of IL-1β mediated tubulin acetylation suppresses inflammatory signalling and cilia elongation
Lastly we conducted a dose response analysis of the effects of tubacin (0.2, 0.5, 1 and 2 μM) on the response to IL-1β (0, 1, 5 and 10 ng/ml) in the absence of loading. In unloaded cells, treatment with tubacin at all concentrations blocked primary cilia elongation induced by IL-1β at 1 ng/ml [P < 0.001, Fig. 6(A)] as well as at 5 and 10 ng/ml IL-1β [Fig. S5(A)]. This inhibition of cilia elongation was accompanied by suppression of the associated NO release in the presence of 1 ng/ml IL-1β [P < 0.001, Fig. 6(B)]. There was a slight bell-shaped dose response with greatest inhibition of IL-1β signalling at 0.5 μM tubacin. The response was also seen at 5 ng/ml IL-1β but not at 10 ng/ml [Fig. S5(B)]. Consequently the anti-inflammatory efficacy of tubacin was greatest at lower concentrations of IL-1β, more similar to those seen in vivo [Fig. S5(C)].
Inhibition of cilia elongation and NO release by tubacin was associated with complete disruption of increased tubulin acetylation caused by IL-1β. Consequently, in the presence of tubacin, IL-1β had no effect on acetylated α-tubulin expression and acetylation ratio [P = 0.741 and P = 0.812 respectively, Fig. 6(D)–(F)]. Nevertheless, these results indicate that in unloaded cells with IL-1β, tubacin disrupts pro-inflammatory signalling associated with inhibition of IL-1β-mediated tubulin acetylation and cilia elongation. This therefore replicates the effect of mechanical loading.
In unloaded cells, in the absence of IL-1β, treatment with tubacin (0.5 μM) for 24 h increased expression of acetylated α-tubulin [P = 0.006, Fig. 6(D)], but had no effect on non-acetylated α-tubulin [P = 0.351, Fig. 6(E)] thereby increasing the acetylation ratio [P = 0.031, Fig. 6(F)]. A similar increase in acetylation ratio was also observed at 1 μM tubacin [P = 0.047, Fig. S3(A)]. This response is entirely consistent with the role of tubacin as an HDAC6 inhibitor. However, the normal effects of tubacin on acetylation of tubulin appear to be disrupted by the presence of IL-1β in unloaded cells in contrast to the behaviour in loaded cells. Treatment with IL-1β significantly increased acetylation [Fig. 6(C), (D) and 6(F)] as seen for unloaded cells on elastic membranes [Fig. 3(C)–(E)]. Interestingly, rather than further increasing acetylation, as would be expected for an HDAC6 inhibitor, and as seen in loaded cells, tubacin completely blocked the effect of IL-1β on acetylation [Fig. 6(C)–(F)]. The reason for this unexpected effect of tubacin in unloaded cells treated with IL-1β is unclear. Throughout these studies we demonstrate a consistent link between tubulin acetylation and IL-1β signalling which is disrupted by mechanical loading through an HDAC6 and IFT dependent mechanism regulating cilia elongation.
Discussion
In this study, we examine the mechanism through which mechanical stimulation suppresses the inflammatory response to IL-1β and the involvement of primary cilia and associated IFT. Within osteoarthritic synovial joints, IL-1β triggers inflammatory signalling leading to reduced synthesis of extracellular matrix proteins, collagen and aggrecan, and activation of metalloproteinases (MMPs) causing cartilage degradation. In agreement with previous studies10, 19, 20, we demonstrate that in isolated articular chondrocytes, mechanical loading in the form of CTS counteracts IL-1β induced production of NO and PGE2 which occur upstream of these events. CCS similarly blocked NO release in articular cartilage explants, as suggested previously21, and reduced downstream extracellular matrix degradation quantified by sGAG release. Throughout these studies we use 10% strain applied at 0.33 Hz, strains of this magnitude are within the typical range reported for articular cartilage subjected to normal physiological loading22, 23, 24, 30, 31 This regime reportedly activates membrane hyperpolarisation which is associated with an anabolic loading response25, 26, 27. Moreover, it has been effectively used in previous studies to modulate ciliary signalling pathways28, 29.
HDACs and histone acetyltransferases (HATs) cause post-translational modification of N-terminal tails of nuclear histone proteins as well as other non-histone proteins32. HDACs are a family of 18 enzymes that function to deacetylate histone proteins modulating chromosome structure thus contributing to regulation of gene transcription. Among HDACs, HDAC6 has unique characteristics because of its two catalytic deacetylase domains and one ubiquitin-binding domain, such that it inhibits α-tubulin acetylation without altering histone acetylation, gene expression or cell cycle progression17, 32. Consistent with this, mechanical activation of HDAC6 inhibited tubulin acetylation caused by IL-1β (Fig. 3). Treatment with the HDAC6 inhibitor, tubacin, restored tubulin acetylation and cilia elongation in loaded cells treated with IL-1β (Fig. 5).
Studies in osteoblasts suggest mechanical activation of HDAC6 may involve activation of TGF-β1 receptors33, 34. However other studies in lung epithelial cells report mechanical inhibition of HDAC6 activity35 suggesting the response may depend on cell type or loading regime. The enriched expression of HDAC6 on the chondrocyte primary cilium [Fig. 3(A)] suggests mechanical and pharmaceutical regulation of HDAC6 are closely associated with primary cilia and IFT. Indeed, previous studies report that HDAC6 mediates chondrocyte primary cilia disassembly in response to mechanical loading11, 34 and other physicochemical stimuli36, through an Aurora A kinase dependent pathway37. Changes in acetylation of ciliary tubulin likely alter the rate of ciliary disassembly, which will regulate cilia length as described by both the differential cargo-loading model of ciliogenesis38, and the original balance-point model39.
Cilia elongation requires a pool of free, non-polymerized cytosolic α-tubulin which can be trafficked to the cilia tip for incorporation into the growing axoneme18. The current study found mechanical loading reduced the amount of non-polymerized α-tubulin, partially dependent on HDAC6 activation [Fig. 3(F)–(H) S3B]. These findings agree with previous studies showing that HDAC6-mediated tubulin deacetylation decreases the pool of non-polymerized tubulin40. In the present study, induction of tubulin polymerization with taxol replicated the effect of loading on IL-1β-induced primary cilia elongation and NO release (Fig. 4). Furthermore, experiments using ORPK cells indicate that suppression of IL-1β-induced NO and PGE2 production by mechanical loading occurs through an IFT-dependent mechanism. Thus, ORPK cells with disrupted ciliogenesis, show a reduced inflammatory response and loss of anti-inflammatory mechanoregulation (Fig. 2). ORPK cells also show hyper-acetylation of tubulin41 which may further influence HDAC6 mediated mechanoregulation.
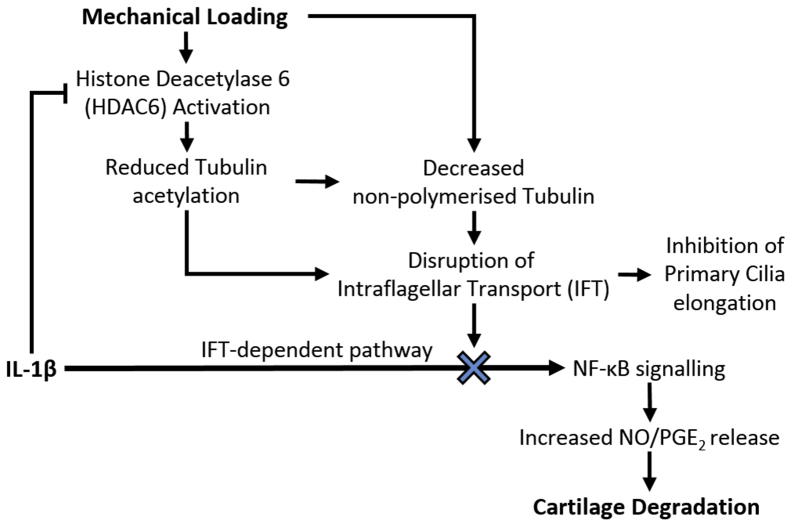
It is unclear whether mechanical loading regulates cilia length which then modulates IFT and associated signalling or whether loading regulates IFT directly resulting in changes in cilia length. In support of the former, previous studies demonstrate changes in cilia length inversely regulate the rate of IFT cargo injection and delivery of tubulin to the ciliary tip as an integral part of the differential cargo-loading and balance-point models controlling ciliogenesis38. However, although we have shown that IFT is required for IL-1β signalling, it is not clear whether this is mediated by primary cilia. Therefore we suggest that mechanical or pharmaceutical regulation of tubulin acetylation and polymerization, as shown here, may directly influence IFT, thereby controlling various IFT-dependent signalling pathways as well as primary cilia length. This is supported by previous studies showing that tubulin acetylation directly regulates IFT velocity in purified systems42. Indeed IFT proteins are also expressed in the cytoplasm where they may have non-canonical, non-ciliary functions43. Thus we suggest mechanical regulation of IL-1β-induced NO and PGE2 release occurs via HDAC6-mediated regulation of IFT. This in turn modulates cilia elongation in response to IL-1β (Fig. 7). Indeed the authors have previously reported regulation of IFT-dependent hedgehog signalling associated with mechanical, thermal and chemical modulation of HDAC6 with associated regulation of cilia length11, 36.
Fig. 7.
Proposed mechanism through which physiological mechanical loading suppresses IL-1β-induced NO/PGE2 production and downstream cartilage degradation. We suggest that the anti-inflammatory effects of mechanical loading are mediated by mechanosensitive HDAC6 activation resulting in disruption of tubulin acetylation and a reduction in the availability of non-polymerized tubulin, which disrupt IFT and associated primary cilia elongation. This mechanical modulation of IFT therefore suppresses IFT-dependent IL-1β signalling events in the form of NO and PGE2 release, thereby reducing cartilage degradation.
In loaded cells, HDAC6 inhibition with tubacin reversed the anti-inflammatory effects of mechanical loading, restoring an inflammatory response to IL-1β. By contrast in unloaded, cells tubacin blocked the inflammatory response. However in both cases, suppression of NO release was associated with inhibition of IL-1β-induced tubulin acetylation and cilia elongation. Thus the data support the overall hypothesis that mechanical modulation of tubulin acetylation regulates IFT to control IL-1β-induced NO and PGE2 release. Nevertheless, it remains unclear why in the presence of IL-1β, tubacin inhibits acetylation in unloaded cells. One possibility is that in unloaded cells treated with IL-1β, HDAC6 activity is reduced to such an extent that further suppression by tubacin causes a compensatory upregulation of the deacetylase, sirt-2 which blocks IL-1β-induced tubulin acetylation which has previously been reported in neurons44.
These findings in articular chondrocytes may also explain the anti-inflammatory effects of other forms of mechanical stimulation in other cell types. For example in endothelial cells, fluid shear forces are known to regulate cilia expression such that areas of the vascular system with steady blood flow have missing or stunted primary cilia. These same areas are less susceptible to inflammation and associated atherosclerotic plaque formation5, 45, further supporting our universal hypothesis that mechanical stimulation suppresses inflammatory signalling by regulating IFT and cilia elongation.
In conclusion, this study suggests a novel anti-inflammatory mechanism involving mechanoregulation of HDAC6, tubulin acetylation and polymerization which controls IFT thereby suppressing IL-1β-induced NO and PGE2 production and attenuating primary cilia elongation. Consequently, this study provides new mechanistic understanding of how mechanical forces may regulate the expanding range of IFT-dependent cell signalling pathways. The findings also support the possibility of novel therapeutic strategies using manipulation of tubulin acetylation and IFT to regulate inflammation and other IFT-dependent pathways important in diseases such as cancer, atherosclerosis and osteoarthritis.
Author contributions
All authors aided in revising this manuscript for intellectual content and approved the final version to be published.
Study design: Su Fu, Clare L Thompson, Martin M Knight.
Data acquisition: Su Fu, Clare L Thompson, Ahmed Ali.
Data analysis and interpretation: Su Fu, Clare L Thompson, Ahmed Ali, Wen Wang, Paul Chapple, Hannah M Mitchison, Phil L Beales, Angus K Wann, Martin M Knight.
Competing interests
The authors have no competing interests.
Role of the funding source
Su Fu is funded by the China Scholarship Council for his PhD studies at Queen Mary University of London. Dr Clare Thompson is supported by a project grant from the UK Medical Research Council (No:MR/L002876/1, PI: Knight). Beales is an NIHR Senior Investigator and is supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre (GOSH BRC). The super resolution microscope system was purchased through funding from the Institute of Bioengineering at Queen Mary University of London.
Acknowledgments
We thank Dr Sue McGlashan and Prof Courtney Haycraft for developing the ORPK chondrocyte cell line15.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joca.2019.03.003.
Contributor Information
S. Fu, Email: s.fu@qmul.ac.uk.
C.L. Thompson, Email: Clare.l.thompson@qmul.ac.uk.
A. Ali, Email: ahmed.ali1908@gmail.com.
W. Wang, Email: wen.wang@qmul.ac.uk.
J.P. Chapple, Email: j.p.chapple@qmul.ac.uk.
H.M. Mitchison, Email: h.mitchison@ucl.ac.uk.
P.L. Beales, Email: p.beales@ucl.ac.uk.
A.K.T. Wann, Email: angus.wann@kennedy.ox.ac.uk.
M.M. Knight, Email: m.m.knight@qmul.ac.uk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Goetz S.C., Anderson K.V. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muhammad H., Rais Y., Miosge N., Ornan E.M. The primary cilium as a dual sensor of mechanochemical signals in chondrocytes. Cell Mol Life Sci. 2012;69:2101–2107. doi: 10.1007/s00018-011-0911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singla V., Reiter J.F. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 4.Wann A., Knight M. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell Mol Life Sci. 2012;69:2967–2977. doi: 10.1007/s00018-012-0980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinsmore C., Reiter J.F. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep. 2016;17:156–166. doi: 10.15252/embr.201541019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wann A.K., Chapple J.P., Knight M.M. The primary cilium influences interleukin-1beta-induced NFkappaB signalling by regulating IKK activity. Cell Signal. 2014;26:1735–1742. doi: 10.1016/j.cellsig.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek H., Shin H.J., Kim J.-J., Shin N., Kim S., Yi M.-H. Primary cilia modulate TLR4-mediated inflammatory responses in hippocampal neurons. J Neuroinflammation. 2017;14:189. doi: 10.1186/s12974-017-0958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin T.M., Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Chatzizisis Y.S., Coskun A.U., Jonas M., Edelman E.R., Feldman C.L., Stone P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury T.T., Bader D.L., Lee D.A. Dynamic compression inhibits the synthesis of nitric oxide and PGE2 by IL-1β-stimulated chondrocytes cultured in agarose constructs. Biochem Biophys Res Commun. 2001;285:1168–1174. doi: 10.1006/bbrc.2001.5311. [DOI] [PubMed] [Google Scholar]
- 11.Thompson C., Chapple J., Knight M. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthritis Cartilage. 2014;22:490–498. doi: 10.1016/j.joca.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGlashan S.R., Knight M.M., Chowdhury T.T., Joshi P., Jensen C.G., Kennedy S. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int. 2010;34:441–446. doi: 10.1042/CBI20090094. [DOI] [PubMed] [Google Scholar]
- 13.Iomini C., Tejada K., Mo W., Vaananen H., Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray R., Wann A., Thompson C., Connelly J., Knight M. Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep. 2013;3:3545. doi: 10.1038/srep03545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wann A.K., Zuo N., Haycraft C.J., Jensen C.G., Poole C.A., McGlashan S.R. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2012;26:1663–1671. doi: 10.1096/fj.11-193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tilwani R., Vessillier S., Pingguan-Murphy B., Lee D., Bader D., Chowdhury T. Oxygen tension modulates the effects of TNFα in compressed chondrocytes. Inflamm Res. 2017;66:49–58. doi: 10.1007/s00011-016-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou H., Wu Y., Navre M., Sang B.C. Characterization of the two catalytic domains in histone deacetylase 6. Biochem Biophys Res Commun. 2006;341:45–50. doi: 10.1016/j.bbrc.2005.12.144. [DOI] [PubMed] [Google Scholar]
- 18.Sharma N., Kosan Z.A., Stallworth J.E., Berbari N.F., Yoder B.K. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell. 2011;22:806–816. doi: 10.1091/mbc.E10-03-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gassner R., Buckley M.J., Georgescu H., Studer R., Stefanovich-Racic M., Piesco N.P. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal S., Long P., Gassner R., Piesco N.P., Buckley M.J. Cyclic tensile strain suppresses catabolic effects of interleukin-1β in fibrochondrocytes from the temporomandibular joint. Arthritis Rheumatol. 2001;44:608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torzilli P.A., Bhargava M., Park S., Chen C.C. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage. 2010;18:97–105. doi: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guilak F., Ratcliffe A., Mow V.C. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 23.Madden R., Han S.K., Herzog W. Chondrocyte deformation under extreme tissue strain in two regions of the rabbit knee joint. J Biomech. 2013;46:554–560. doi: 10.1016/j.jbiomech.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Choi J.B., Youn I., Cao L., Leddy H.A., Gilchrist C.L., Setton L.A. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40:2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright M., Jobanputra P., Bavington C., Salter D.M., Nuki G. Effects of intermittent pressure-induced strain on the electrophysiology of cultured human chondrocytes: evidence for the presence of stretch-activated membrane ion channels. Clin Sci (Lond) 1996;90:61–71. doi: 10.1042/cs0900061. [DOI] [PubMed] [Google Scholar]
- 26.Wright M.O., Nishida K., Bavington C., Godolphin J.L., Dunne E., Walmsley S. Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: evidence of a role for alpha 5 beta 1 integrin as a chondrocyte mechanoreceptor. J Orthop Res. 1997;15:742–747. doi: 10.1002/jor.1100150517. [DOI] [PubMed] [Google Scholar]
- 27.Millward-Sadler S.J., Wright M.O., Lee H., Caldwell H., Nuki G., Salter D.M. Altered electrophysiological responses to mechanical stimulation and abnormal signalling through alpha5beta1 integrin in chondrocytes from osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:272–278. doi: 10.1053/joca.1999.0301. [DOI] [PubMed] [Google Scholar]
- 28.Thompson C.L., Chapple J.P., Knight M.M. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthritis Cartilage. 2014;22:490–498. doi: 10.1016/j.joca.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson C.L., Plant J.C., Wann A.K., Bishop C.L., Novak P., Mitchison H.M. Chondrocyte expansion is associated with loss of primary cilia and disrupted hedgehog signalling. Eur Cell Mater. 2017;34:128–141. doi: 10.22203/eCM.v034a09. [DOI] [PubMed] [Google Scholar]
- 30.Bleuel J., Zaucke F., Brüggemann G.-P., Niehoff A. Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review. PLoS One. 2015;10:e0119816. doi: 10.1371/journal.pone.0119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S., Hung C., Ateshian G. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage. 2004;12:65–73. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Yang X., Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 33.Lau K.-H.W., Kapur S., Kesavan C., Baylink D.J. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem. 2006;281:9576–9588. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- 34.Ehnert S., Sreekumar V., Aspera-Werz R.H., Sajadian S.O., Wintermeyer E., Sandmann G.H. TGF-β1 impairs mechanosensation of human osteoblasts via HDAC6-mediated shortening and distortion of primary cilia. J Mol Med. 2017;95:653–663. doi: 10.1007/s00109-017-1526-4. [DOI] [PubMed] [Google Scholar]
- 35.Geiger R.C., Kaufman C.D., Lam A.P., Budinger G.S., Dean D.A. Tubulin acetylation and histone deacetylase 6 activity in the lung under cyclic load. Am J Respir Cell Mol Biol. 2009;40:76–82. doi: 10.1165/rcmb.2007-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prodromou N.V., Thompson C.L., Osborn D.P., Cogger K.F., Ashworth R., Knight M.M. Heat shock induces rapid resorption of primary cilia. J Cell Sci. 2012;125:4297–4305. doi: 10.1242/jcs.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugacheva E.N., Jablonski S.A., Hartman T.R., Henske E.P., Golemis E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wren K.N., Craft J.M., Tritschler D., Schauer A., Patel D.K., Smith E.F. A differential cargo-loading model of ciliary length regulation by IFT. Curr Biol. 2013;23:2463–2471. doi: 10.1016/j.cub.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall W.F., Rosenbaum J.L. Intraflagellar transport balances continuous turnover of outer doublet microtubules. J Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuyama A., Shimazu T., Sumida Y., Saito A., Yoshimatsu Y., Seigneurin-Berny D. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berbari N.F., Sharma N., Malarkey E.B., Pieczynski J.N., Boddu R., Gaertig J. Microtubule modifications and stability are altered by cilia perturbation and in cystic kidney disease. Cytoskeleton. 2013;70:24–31. doi: 10.1002/cm.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed N.A., Cai D., Blasius T.L., Jih G.T., Meyhofer E., Gaertig J. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Taulet N., Vitre B., Anguille C., Douanier A., Rocancourt M., Taschner M. IFT proteins spatially control the geometry of cleavage furrow ingression and lumen positioning. Nat Commun. 2017;8:1928. doi: 10.1038/s41467-017-01479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maxwell M.M., Tomkinson E.M., Nobles J., Wizeman J.W., Amore A.M., Quinti L. The Sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS. Hum Mol Genet. 2011;20:3986–3996. doi: 10.1093/hmg/ddr326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Heiden K., Hierck B.P., Krams R., de Crom R., Cheng C., Baiker M. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–550. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.