Abstract
Species of Trichinella are a globally distributed assemblage of nematodes, often with distinct host ranges, which include people, domestic, and wild animals. Trichinella spp. are important in northern Canada, where dietary habits of people and methods of meat preparation (drying, smoking, fermenting as well as raw) increase the risk posed by these foodborne zoonotic parasites. Outbreaks in the arctic and subarctic regions of Canada and the United States are generally attributed to T. nativa (T2) or the T6 genotype, when genetic characterization is performed. We report the discovery of Trichinella pseudospiralis (T4), a non-encapsulated species, in a wolverine (Gulo gulo) from the Northwest Territories of Canada. This parasite has been previously reported elsewhere from both mammals and carnivorous birds, but our findings represent new host and geographic records for T. pseudospiralis. Multiplex PCR and sequencing of fragments of Cytochrome Oxidase Subunit I (COI) and D3 rDNA confirmed the identification. Phylogenetically, Canadian isolates linked with each other and others derived from Palearctic or Neotropical regions, but not elsewhere in the Nearctic (continental USA). We suggest that migratory birds might have played a role in the dispersal of this pathogen 1000's of km to northwestern Canada. Wolverines are not typically consumed by humans, and thus should not pose a direct food safety risk for trichinellosis. However, the current finding suggests that they may serve as an indicator of a broader distribution for T. pseudospiralis. Along with infection risk already recognized for T. nativa and Trichinella T6, our observations emphasize the need for further studies using molecular diagnostics and alternative methods to clarify if this is a solitary case, or if T. pseudospiralis and other freeze susceptible species of Trichinella (such as T. spiralis) circulate more broadly in wildlife in Canada, and elsewhere.
Keywords: Trichinella pseudospiralis, Wolverine, Canada, Palearctic, Neotropical
Graphical abstract
(Picture of wolverine: Wolverine picture: credit to Gustaf Samelius, Snow Leopard Trust, Seattle, Washington 98103, USA).

Highlights
-
•
First documentation of Trichinella pseudospiralis on the mainland of Canada.
-
•
Second molecular characterization of T. pseudospiralis from Canada.
-
•
Northernmost observation of T. pseudospiralis from North America.
-
•
Most closely related to isolates from Palearctic and Neotropics, not Nearctic.
1. Introduction
Among the 24 most significant foodborne parasitic diseases listed by the World Health Organization/United Nations Food and Agriculture Organization, Trichinella spiralis globally ranks seventh, with other Trichinella spp. ranked as 16th (FAO/WHO, 2014). In a systematic review, 65 818 human cases of trichinellosis were reported worldwide from 1986 to 2009 (Murrell and Pozio, 2011). From a public health perspective, species of Trichinella (largely T. nativa and Trichinella T6) were ranked third among nine zoonotic parasites in northern North America based on an evidence-based qualitative risk analysis (Jenkins et al., 2013).
People or other mammals and birds contract Trichinella spp. infection after consuming meat infected with larvae of these parasites (Gottstein et al., 2009; McIntyre et al., 2007; Serhir et al., 2001). Upon gastric digestion of infected meat in an exposed host, the first stage larvae are released, which penetrate intestinal mucosa, and undergo four molts before developing into adult worms within a few days of infection. Males and females copulate and after 5 days post infection, females start releasing newborn larvae, which travel via blood to predilection sites in the skeletal musculature. For encapsulated species of Trichinella such as T. spiralis, larvae encyst inside muscle cells, whereas in other species such as T. pseudospiralis, larvae remain unencapsulated. Clinical manifestations are rarely recognized in animals, but human patients may display symptoms including headache, fever, abdominal pain, diarrhea, myalgia, eyelid/facial edema, and even mortality due to cardiac manifestations, depending on infective dose and immune status (Gottstein et al., 2009). Trichinella pseudospiralis causes clinical manifestations similar to those caused by T. spiralis except with a more prolonged myopathy (Jongwutiwes et al., 1998). Diagnosing which species of Trichinella is responsible for human outbreaks is desirable, but rarely performed as it necessitates muscle biopsy and comparative molecular analyses. A broad understanding of the species/genotypes of Trichinella circulating in domestic and wild animals can aid understanding of transmission pathways, routes of exposure and developing possible management goals, because species differ in important characteristics, such as host affinities and tolerance to freezing.
All species and genotypes of Trichinella have been reported in mammals, whereas T. papuae and T. zimbabwensis also infect reptilian hosts. Trichinella pseudospiralis is the only species in the genus reported in both carnivorous birds and mammals, but the number of reports in mammals exceeds those in avian hosts (Pozio et al., 2009; Zamora et al., 2015). Worldwide, T. pseudospiralis has a cosmopolitan distribution and has been reported in 18 mammalian and eight avian species (Pozio, 2016). In Canada, T. spiralis has been eradicated from commercially raised pigs, and has only rarely been reported from wildlife (Gajadhar and Forbes, 2010). In contrast to the domestic cycle, a number of sylvatic species of Trichinella exist in Canadian wildlife, and include T. nativa (T2), T. murrelli (T5), Trichinella T6, and T. pseudospiralis (Jenkins et al., 2013). Trichinella nativa and Trichinella T6 are freeze-tolerant, and are the most common species found in wildlife hosts in the arctic and sub-arctic zones of Canada. Both species are commonly found in wolves (Canis lupus), wolverines (Gulo gulo), bears (Ursus spp.), arctic foxes (Vulpes lagopus), walruses (Odobenus rosmarus), and other carnivorous mammals (Gajadhar and Forbes, 2010; Jenkins et al., 2013; Sharma et al., 2018). Recent outbreaks of trichinellosis in Canada have been almost exclusively linked to consumption of game meat (black bear, grizzly bear, walrus) infected with T. nativa (Dalcin et al., 2017; Houze et al., 2009; McIntyre et al., 2007; Schellenberg et al., 2003; Serhir et al., 2001).
The public health importance of trichinellosis in the Canadian north necessitates continuing surveillance for species of Trichinella in a diverse assemblage of largely mammalian wildlife species. Wolverines are not commonly consumed, but are commonly harvested for fur, and have high prevalence (≥80%) of this parasite due to their high trophic position and scavenging lifestyle (Reichard et al., 2008; Sharma et al., 2018). Therefore, they make excellent sentinels for this and other food borne parasites. Here, we report the discovery of T. pseudospiralis in a wolverine from northern Canada, and discuss the epidemiological/phylogenetic associations of this isolate of T. pseudospiralis.
2. Material and methods
2.1. Sample collection and transport
Wolverine carcasses were submitted to the Department of Environment and Natural Resources, Government of the Northwest Territories as part of the fur harvest during 2005–2012 in Northwest Territories, and included 131 animals. Tongues and diaphragms were collected from wolverine carcasses and were stored at −20 OC before shipping to the Department of Veterinary Microbiology, Western College of Veterinary Medicine, Saskatoon, Saskatchewan, Canada.
2.2. Sample preparation, digestion and recovery of larvae
Fat and connective tissue were removed from the tongue (or diaphragm, if tongue was not available) of each wolverine, and muscle was cut into 0.5–1.0 cm cubes, mixed and a portion randomly selected to make up to 10 g. Muscle tissues were processed by the pepsin-HCl double separatory funnel digestion method (Forbes and Gajadhar, 1999). Trichinella first-stage larvae (L1) were identified based on morphology observed under the stereo-microscope, and counted. The burden of infection was estimated as larvae per gram (LPG). Larval motility was assessed by incubating a petri plate containing the larvae at 37OC for 30 min. Five individual larvae and a pool of ten larvae were collected in six 0.6 ml tubes containing 1X PCR buffer [10 mM Tris-HCl, pH 8.3, 50 mM HCl, 1.5 mM MgCl2, 0.01% (w/v) gelatin] and stored at −20 °C until used for molecular analysis. Compression of small tongue samples using a glass compressorium was also performed to determine if larvae in-situ were encapsulated.
2.3. Molecular identification and sequencing
Parasite genomic DNA was extracted from 5 individual larvae as well as a pool of 10 larvae using a Proteinase K extraction method (Scandrett et al., 2018). To identify species or genotype, primers amplifying internal transcribed spacer regions (ITS 1 and 2) as well as the expansion segment V (ESV) of the large subunit ribosomal DNA (Zarlenga et al., 1999) were used in a multiplex PCR assay. Positive controls of six recognized species of Trichinella (T. spiralis, T. nativa, T. britovi, T pseudospiralis, T murrelli and Trichinella T6), passaged in mice, were provided by the Centre for Food-borne and Animal Parasitology, Canadian Food Inspection Agency (CFIA), Saskatoon. The T. pseudospiralis isolate provided was originally recovered from a mountain lion (Puma concolor couguar) on Vancouver Island (British Columbia, Canada). This is the only other isolate of T. pseudospiralis reported from Canada and had not yet been characterized using the sequencing methods employed in this study. Samples were identified based on the banding patterns of amplified products on the 2.5% agarose gel stained with Red Safe (FroggaBio Inc, ON, Canada) and photographed using a Gel Doc system (Alpha Innotech AlphaImager digital imaging system).
To confirm and compare the sequence from the current T. pseudospiralis isolate with other isolates of different geographical origin (Eurasia, Australia, Canada and USA), the D3 domain of nuclear ribosomal DNA (D3 rDNA) and the mitochondrial cytochrome C oxidase subunit I (COI) gene were amplified by PCR using the primer pair 5-ACCCGTCTTGAAACACGGA-3 and 5-GATTAGTCTTTCGCCCCTA-3 and the primer pair 5-GTTTTTTGGGCATCCAGAAGTT-3 and 5-GAAGAAGGTCTAAGGAAGCATTTGA-3, respectively (Gasser et al., 2004; Krivokapich et al., 2015). Amplified segments of 400 bp of the D3 rDNA and 345 bp of the COI gene were purified using ExoSAP-IT as per manufacturer's instructions and sent to Macrogen Korea for sequencing (Macrogen Inc., Seoul, Korea). Consensus sequences for each locus were generated in Geneious 11.1.5 (Biomatters, Ltd., New Zealand) based on forward and reverse Sanger sequences. BLAST searches of the non-redundant nucleotide database at NCBI GenBank were used to confirm the Trichinella species diagnosis from the multiplex, and to obtain the nucleotide identity with other isolates. The nucleotide sequences of D3 rDNA and COI were deposited into the GenBank database under Accession Nos. MK333397, MK703809, MK333398 and MK713937. Multiple sequence alignments were carried out using Muscle 3.8.425 multi alignment program (Edgar, 2004) followed by manual optimization and comparison against the COI and D3 rDNA sequences of T. pseudospiralis from different geographical locations available in GenBank (Table 1). Phylogenetic analysis was performed in Geneious 11.1.5 using the neighbor-joining algorithm reconstructed from distances calculated using the HKY model of nucleotide substitution with 1000 bootstrap replicates. Maximum likelihood trees were generated in PhyML using the GTR + I + gamma substitution model (Guindon et al., 2010). Sequences from encapsulated Trichinella spiralis and Trichinella nelsoni were used to root the trees.
Table 1.
Comparison of percentage nucleotide identity of current T. pseudospiralis isolate with other isolates from different geographical origins.
| DNA sequence | Isolate Code | Host | Accession number | Geographic origin | Identity (%) | Reference |
|---|---|---|---|---|---|---|
| COI | Mountain Lion (Puma concolor couguar) | MK713937 | Canada (Vancouver Island) | 100 | Current study | |
| ISS13 | Raccoon dog (Nyctereutes procyonoides) | KM357408 | Russia (Krasnodar) | 100 | (Mohandas et al., 2014) | |
| ISS588 | Brown rat (Rattus norvegicus) | KM357409 | Russia (Kamchatka) | 100 | (Mohandas et al., 2014) | |
| ISS176 | Tawny eagle (Aquila rapax) | KM357410 | Kazakhstan | 100 | (Mohandas et al., 2014) | |
| Domestic pig (Sus scrofa) | KM063187 | South America (Argentina) | 100 | Krivokapich et al. (2015) | ||
| ISS141 | Tiger cat (Dasyurus maculatus) | EF601545 | Australia (Tasmania) | 97.6 | (Wu et al., 2007) | |
| ISS1132 | Wild pig (Sus scrofa) | EF601544 | USA (Texas) | 94.9 | (Wu et al., 2007) | |
| ISS470 | Black vulture (Coragypus atratus) | KM357411 | USA (Alabama) | 94.6 | (Mohandas et al., 2014) | |
| D3 rDNA | Mountain Lion (Puma concolor couguar) | MK703809 | Canada (Vancouver Island) | 100 | Current study | |
| ISS13 | Raccoon dog (Nyctereutes procyonoides) | AJ633056 | Russia (Krasnodar) | 100 | Gasser et al. (2004) | |
| Domestic pig (Sus scrofa) | KM063188 | South America (Argentina) | 100 | Krivokapich et al. (2015) | ||
| ISS470 | Black vulture (Coragypus atratus) | AJ633058 | USA (Alabama) | 99.3 | Gasser et al. (2004) | |
| ISS141 | Tiger cat (Dasyurus maculatus) | AJ633057 | Australia (Tasmania) | 97.5 | Gasser et al. (2004) |
As animals were harvested for purposes other than research, this animal use was considered a Level A Category of Invasiveness, as per the Canadian Council on Animal Care (CCAC) recommendations, and thus exempt from animal research ethics review at the University of Saskatchewan. We worked closely with the Government of the Northwest Territories for wildlife research and export permits.
3. Results
3.1. Microscopic examination
The positive animal was a one-year-old male, which had been killed by hunters or trappers in the South Slave Region (Latitude 60.83300 N, Longitude −117.20 W) in 2006. Digestion of tongue and diaphragm resulted in 12 (all comma-shaped, dead) and 44 (3 tightly coiled and 41 comma-shaped, dead) larvae, respectively. When repeated, digestion of tongue muscle resulted in 17 comma-shaped (dead) larvae. Mean LPG in diaphragm (4.4, 44 larvae/10 g) was more than that in tongue (1.45, 29 larvae/20 g). The overall mean larval burden was 2.4 LPG (tongue and diaphragm combined). None of the larvae were motile. We detected only two non-encapsulated Trichinella spp. larvae in 40 compressed tongue muscle samples (Fig. 1).
Fig. 1.
Photomicrograph of Trichinella pseudospiralis larva in compressed tongue muscle of a wolverine (Gulo gulo).
3.2. Multiplex PCR and DNA sequencing
Multiplex PCR revealed amplicons of approximately 310 bp, which corresponded to T. pseudospiralis. In order to confirm the species diagnosis and determine whether this isolate was closely related to any particular previously published isolates, identity among nucleotide sequences was considered and phylogenetic analyses were completed. High quality sequence for 339 base pairs of COI DNA and 400 base pairs of D3 rDNA was generated from the newly reported wolverine isolate. COI sequence showed 100% nucleotide identity with T. pseudospiralis isolates from Russia (Krasnodar and Kamchatka), Kazakhstan and Argentina, compared with 97.6% and 94.6–94.9% with T. pseudospiralis isolates from Australia and USA, respectively (Table 1). Similarly, the D3 rDNA sequence was identical to those of isolates from Russia, Argentina and Canada, and 99.3% and 97.5% with those of isolates from USA and Australia, respectively (Table 1). The mitochondrial and nuclear sequences from the described isolate shared no more than 89% identity with any other Trichinella species. Using T. spiralis (an encapsulated Trichinella species) as an outgroup, neighbor-joining and maximum likelihood phylogenetic trees for both mitochondrial and nuclear genes placed the new isolate within a strongly supported clade containing only T. pseudospiralis isolates (Fig. 2). Furthermore, the wolverine isolate clustered closely with the Canadian mountain lion isolate and with those of T. pseudospiralis previously documented from Russia and Argentina (Fig. 2) rather than isolates from the southern United States. Topologies and branch support were consistent between neighbor-joining and maximum likelihood estimated trees.
Fig. 2.
Neighbor-joining gene trees showing the relationship of the Trichinella pseudospiralis isolate reported here with previously described isolates. Sequence from (a.) the cytochrome oxidase 1 mitochondrial gene (COI) and (b.) the D3 domain of nuclear ribosomal DNA (D3 rDNA) placed the newly discovered isolate within a strongly supported clade containing isolates from Russia, Argentina, and Canada. This clade clearly delineated these isolates as belonging to T. pseudospiralis, but were notably distinct from conspecific isolates from Alabama and Texas in the continental United States. Tree topology was conserved between neighbor-joining, maximum likelihood, and Bayesian analyses with strong support for all interspecific nodes.
4. Discussion
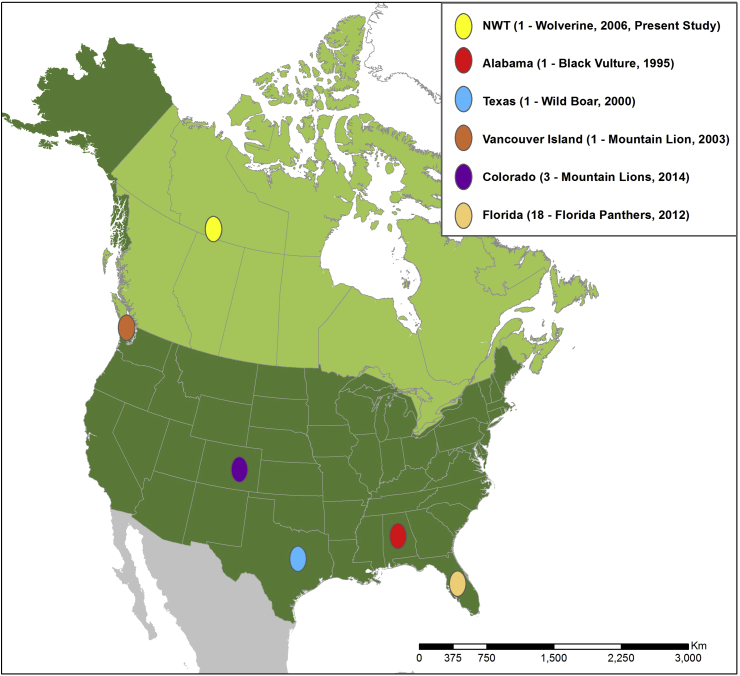
Globally, T. pseudospiralis has been reported in several wild animals, for example, raccoon dog (Nyctereutes procyonoides), lynx (Felis lynx), red fox (Vulpes vulpes), Florida panther (Puma concolor coryi), mountain lion etc (Airas et al., 2010; Gajadhar and Forbes, 2010; Reichard et al., 2015). We document the first report of T. pseudospiralis in wolverine and only the third report in any mustelid host; previous reports include badger (Meles meles) and American mink (Neovison vison) (Pozio, 2016). In North America, the first three reports were based on histology in a Coopers Hawk (Accipiter cooperi) from California (Wheeldon et al., 1982, Wheeldon et al., 1983) and muscle digests from a Great Horned Owl (Bubo virginianus) from Iowa, and a Pomarine Jaeger (Stercorarius pomarinus) from Alaska (Rausch et al., 1956; Zimmermann and Hubbard, 1969), but were identified prior to the advent of molecular diagnosis. The first verified North American isolate based on DNA hybridization using a species-specific probe was in a black vulture (Coragypus atratus) from Alabama (Lindsay et al., 1995). Based on the presence of unencapsulated, freeze susceptible larvae, the multiplex PCR results, and sequencing of both mitochondrial and nuclear DNA loci, we have confirmed that the isolate obtained from this wolverine is consistent with T. pseudospiralis. In addition to a new host record for T. pseudospiralis, this is the first documentation of the parasite on the mainland of Canada, the second report of T. pseudospiralis infecting a wild animal from Canada, and the northernmost observation of the parasite from North America (Table 2, Fig. 3). The only previous report of T. pseudospiralis in Canada was in a mountain lion from Vancouver Island in the southwestern region of the country in 2003 (Gajadhar and Forbes, 2010). Wolverines in North America have varied home ranges from 100 to 900 km2 but are known for long-range dispersal (Banci, 1994; Mulders, 2001). Assuming maximum dispersal of 1000 km of the animal under study, this wolverine likely originated within northwestern Canada.
Table 2.
North American and global reports of T. pseudospiralis in wild mammals and birds, respectively.
| North American reports | ||||
|---|---|---|---|---|
| Host | Geographic origin | Year | Number positive | Reference |
| Black vulture (Coragypus atratus) | Alabama, USA | 1995 | 1 | Lindsay et al. (1995) |
| Wild boar | Newcastle, Texas, USA | 2000 | 1 | Gamble et al. (2005) |
| Mountain lion (Puma concolor couguar) | Vancouver Island, Canada | 2003 | 1 | Gajadhar and Forbes (2010) |
| Florida panther (Puma concolor coryi) | Florida, USA | 2012 | 18 | Reichard et al. (2015) |
| Mountain lion (Puma concolor couguar) | Colorado, USA | 2014 | 3 | Reichard et al. (2017) |
| Wolverine (Gulo gulo) | Northwest Territories, Canada | 2006 | 1 | Current study |
| Global reports | ||||
| Rook (Corvus frugilegus) | Chimkent region, Kazakhstan | 1975 | 2 | Shaikenov (1980) |
| Tawny eagle (Aquila rapax) | Kazakhstan | 1980 | 1 | (Pozio et al., 1992) |
| Western marsh harrier (Circus aeroginosus) | Tasmania, Australia | 1990 | 1 | (Obendorf and Clarke, 1992) |
| Australian masked owl (Tyto novaehollandiae) | Tasmania, Australia | 1990 | 1 | (Obendorf and Clarke, 1992) |
| Black vulture (Coragypus atratus) | Alabama, USA | 1995 | 1 | Lindsay et al. (1995) |
| Tawny owl (Strix aluco) | Stockholm, Sweden | 1997 | 1 | (Hurníková et al., 2014) |
| Tawny owl (Strix aluco) | Uppsala, Sweden | 2011 | 1 | (Hurníková et al., 2014) |
| Tawny owl (Strix aluco) | Marche, Italy | 1998 | 1 | (Pozio et al., 1999) |
| Little owl (Athene noctua) | Marche, Italy | 1998 | 1 | (Pozio et al., 1999) |
| Common buzzard (Buteo buteo) | Krasnodar region, Russia | 2006 | 1 | Pozio (2016) |
Fig. 3.
Geographic locations of this and previously published reports of Trichinella pseudospiralis confirmed by multiplex PCR in North America (Lindsay et al., 1995., Gamble et al., 2005; Gajadhar and Forbes, 2010; Reichard et al., 2015, 2017).
We hypothesize that the arrival of T. pseudospiralis in this region could be mediated through migratory birds or mammals. Dietary habits of wolverines vary with season, availability and distribution of prey species and geographical locations. Wolverines are opportunistic foragers, primarily depending on carcasses of caribou (Rangifer tarandus), and other carrion and prey in the winter, shifting to vegetation and preying on small mammals, and birds in the summer (Pasitschniak-Arts and Larivière, 1995). Wild birds, especially raptors and birds such as jaegers that typically feed on small to medium sized mammals (e.g., rodents, shrews, lagomorphs), could spread T. pseudospiralis over great distances leading to the potential for establishment of new foci of infections in locations previously considered at no or low risk for this parasite (Zamora et al., 2015). Nucleotide sequence analysis placed the isolate discovered in wolverine among only other Canadian isolate previously documented from Canada (100% identity with isolate from a mountain lion from Vancouver Island), as well as those from distant and geographically disjunct localities in the Palearctic (Russia and Kazakhstan) and Neotropical regions (Argentina). In contrast, the wolverine-isolate was divergent from other geographically proximate locations in the Nearctic (continental USA). The closest match, sharing 100% nucleotide identity, was with Canadian, Russian, Kazakhstani and Argentinian isolates, raising the hypothesis of transmission pathways for this isolate linking Eurasia or the Neotropical region to Canada via migratory birds, especially birds of prey or via migratory carnivores within Canada.
Natural infections of T. pseudospiralis have been reported in eight species of birds, primarily raptors as well as corvids such as rook (Corvus frugilegus) from Russia and Kazakhstan (Table 2). The first natural infection in an avian species was reported among rooks, a large passerine (Corvidae- Corvus frugilegus), from the Chimkent region, Kazakhstan in 1975 (Shaikenov, 1980). Trichinella pseudospiralis has been reported in mammals more frequently than in birds, but there have been only a limited number of investigations in avian species (Pozio 2005). Additionally, the sensitivity of the digestion assay may be limited when applied to the generally smaller muscle samples (Gottstein et al., 2009) obtainable from birds. Of 23 previous reports, only one was from a bird in North America (Lindsay et al., 1995). Another possibility could be that this parasite was introduced to the region through a migratory terrestrial mammal covering the considerable distance required to reach this region. As the positive wolverine was located close to small human communities (Fort Providence, Fort Resolution, Hay River, and Enterprise), the wolverine may have acquired its infection by scavenging on discarded meat brought in by people from abroad; however, these are not major hubs for international travel or immigration. Based on this single finding of T. pseudospiralis, we cannot assume that it is actively circulating in northern Canadian wildlife; further sampling of wild mammals and birds from Northwest Territories and British Columbia and more broadly across North America seems warranted.
The higher burden of T. pseudospiralis larvae in this wolverine's diaphragm versus tongue is consistent with a report in foxes experimentally infected with other species of Trichinella (Kapel et al., 2005). In contrast, larvae of T. nativa and Trichinella T6 had a higher intensity of infection in tongue vs diaphragm in wolverine (Sharma et al., 2018). Although less sensitive than digestion (Forbes et al., 2003), the examination of tongue by compressorium was performed to attempt detection of in-situ larvae, particularly those with capsules. However, we did not detect any encapsulated larvae from this wolverine by this method, nor were any other species of Trichinella (such as T2 or T6) detected on multiplex PCR, but T. pseudospiralis.
In spite of the fact that we used a highly sensitive digestion method for recovery of Trichinella spp (Forbes et al., 2003), we found only one positive wolverine (of 131) with T. pseudospiralis, whereas prevalence of freeze-resistant species of Trichinella (T. nativa and Trichinella T6) was much higher (62%, 81/131) (Sharma et al., unpublished). The digestion assay is validated for the detection of live larvae in fresh samples; therefore using frozen samples (as in the situation here) may have reduced overall sensitivity especially for freeze susceptible species of Trichinella such as T. pseudospiralis. The tongue and diaphragm of wolverines tested were kept frozen at −20 °C prior to processing, and underwent two cycles of freeze-thaw, which might have killed larvae of T. pseudospiralis, and thus artificially decreased prevalence and intensity of T. pseudospiralis. Monitoring studies for Trichinella based on freshly harvested wildlife carcasses might reveal a higher prevalence of freeze-susceptible species such as T. pseudospiralis and T. spiralis than previously suspected. As well, a sequential sieving method could offer higher sensitivity than the standard artificial digestion method for larvae of freeze susceptible Trichinella species when sampling frozen tissue (Franssen et al., 2014), frequently the only option for wildlife.
Reports of T. pseudospiralis in harvested wild animals from North America indicate that this zoonotic species is circulating and poses a potential risk to public health. Human outbreaks in North America have not been attributed to T. pseudospiralis; however, definitive identification is rarely performed on human isolates (Dalcin et al., 2017; Gamble et al., 2005; Houze et al., 2009; McIntyre et al., 2007; Reichard et al., 2017; Serhir et al., 2001). Wolverines are not harvested for food but rather for fur; thus, the presence of T. pseudospiralis in this carnivore does not raise immediate public health concerns. As well, T. pseudospiralis is susceptible to freezing, lessening the likelihood of successful establishment in the Arctic, even if introduced with some regularity by migratory birds. Future regional surveillance efforts for Trichinella spp. could focus on wolverine (as a sentinel species), as well as other carnivorous or omnivorous wildlife, including game animals (such as bear and wild boar), rodents, and birds.
5. Conclusion
We report new host and geographic records for T. pseudospiralis in a wolverine, the first report of this parasite on the mainland of Canada. This isolate was most closely related to those from an island in western Canada, Russia and Argentina, but not to populations of parasites at geographically proximate localities from the continental USA. Distribution of T. pseudospiralis in subarctic Canada may emphasize the potential role of migratory birds in long distance dispersal and potential introduction of non-native pathogens into remote regions. Additional field sampling is needed to elucidate whether T. pseudospiralis is established in the Canadian North or has been introduced transiently to the area via carnivorous migratory birds or terrestrial mammals. Our discovery, linked to broader explorations of parasite diversity, emphasizes the importance of genetic identification of Trichinella using sequencing as well as the utility of monitoring for Trichinella spp. and other important food-borne parasites in wildlife, especially high trophic level carnivores and scavengers as an upstream measure of human risk.
Acknowledgement
We are thankful to the hunters/trappers and staff from the Government of the Northwest Territories Department of Environment and Natural Resources; without their involvement, this project would have not been possible. We thank Champika Fernando for technical help in the laboratory, and Bonnie Fournier (GIS and Wildlife Data Specialist, Government of NT) for preparation of maps. We also thank Kelly Konecsni for assembling the collection of reference isolates provided from CFIA. This study was financially supported by Discovery and Northern Research Supplement grants from the Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation Leaders Opportunity Fund, and the Western College of Veterinary Medicine Interprovincial and Wildlife Health Research Funds.
References
- Airas N., Saari S., Mikkonen T., Virtala A.M., Pellikka J., Oksanen A., Isomursu M., Kilpela S.S., Lim C.W., Sukura A. Sylvatic Trichinella spp. infection in Finland. J. Parasitol. 2010;96:67–76. doi: 10.1645/GE-2202.1. [DOI] [PubMed] [Google Scholar]
- Banci V. Wolverine. In: Ruggiero L.F., K.B.A, Buskirk S.W., Lyon J.L., Zielinski W.J., editors. The Scientific Basis for Conserving Forest Carnivores: American Marten, Fisher, lynx and Wolverine in the Western United States. US Department of Agriculture, Forest Service, Range Experiment Station; Fort Collins, Colorado: 1994. p. 184. General Technical Report RM-254. [Google Scholar]
- Dalcin D., Zarlenga D.S., Larter N.C., Hoberg E., Boucher D.A., Merrifield S., Lau R., Ralevski F., Cheema K., Schwartz K.L., Boggild A.K. Trichinella nativa outbreak with Rare Thrombotic Complications associated with meat from a black bear hunted in northern Ontario. Clin. Infect. Dis. 2017;64:1367–1373. doi: 10.1093/cid/cix165. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO . Report of a Joint FAO/WHO Expert Meeting, 3–7 September 2012. FAO Headquarters; Rome Italy: 2014. Multicriteria-based ranking for risk management of food-borne parasites.http://www.fao.org/3/a-i3649e.pdf Available from. [Google Scholar]
- Forbes L.B., Gajadhar A.A. A validated Trichinella digestion assay and an associated sampling and quality assurance system for use in testing pork and horse meat. J. Food Prot. 1999;62:1308–1313. doi: 10.4315/0362-028x-62.11.1308. [DOI] [PubMed] [Google Scholar]
- Forbes L.B., Parker S., Scandrett W.B. Comparison of a modified digestion assay with trichinoscopy for the detection of Trichinella larvae in pork. J. Food Prot. 2003;66:1043–1046. doi: 10.4315/0362-028x-66.6.1043. [DOI] [PubMed] [Google Scholar]
- Franssen F., Deksne G., Esite Z., Havelaar A., Swart A., van der Giessen J. Trend analysis of Trichinella in a red fox population from a low endemic area using a validated artificial digestion and sequential sieving technique. Vet Res. 2014;45:120. doi: 10.1186/s13567-014-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajadhar A.A., Forbes L.B. A 10-year wildlife survey of 15 species of Canadian carnivores identifies new hosts or geographic locations for Trichinella genotypes T2, T4, T5, and T6. Vet. Parasitol. 2010;168:78–83. doi: 10.1016/j.vetpar.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Gamble H.R., Pozio E., Lichtenfels J.R., Zarlenga D.S., Hill D.E. Trichinella pseudospiralis from a wild pig in Texas. Vet. Parasitol. 2005;132:147–150. doi: 10.1016/j.vetpar.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Gasser R.B., Hu M., Abs El-Osta Y.G., Zarlenga D.S., Pozio E. Nonisotopic single-strand conformation polymorphism analysis of sequence variability in ribosomal DNA expansion segments within the genus Trichinella (Nematoda: Adenophorea) Electrophoresis. 2004;25:3357–3364. doi: 10.1002/elps.200405985. [DOI] [PubMed] [Google Scholar]
- Gottstein B., Pozio E., Nockler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009;22:127–145. doi: 10.1128/CMR.00026-08. (Table of Contents) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Houze S., Ancelle T., Matra R., Boceno C., Carlier Y., Gajadhar A.A., Dupouy-Camet J. Trichinellosis acquired in Nunavut, Canada in September 2009: meat from grizzly bear suspected. Euro Surveill. 2009;14 [PubMed] [Google Scholar]
- Hurnikova Z., Hrckova G., Agren E., Komorova P., Forsman J., Chovancova B., Molnar L., Letkova V. First finding of Trichinella pseudospiralis in two tawny owls (Strix aluco) from Sweden. Helmintholgia. 2014;51:190–197. [Google Scholar]
- Jenkins E.J., Castrodale L.J., de Rosemond S.J., Dixon B.R., Elmore S.A., Gesy K.M., Hoberg E.P., Polley L., Schurer J.M., Simard M., Thompson R.C. Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv. Parasitol. 2013;82:33–204. doi: 10.1016/B978-0-12-407706-5.00002-2. [DOI] [PubMed] [Google Scholar]
- Jongwutiwes S., Chantachum N., Kraivichian P., Siriyasatien P., Putaporntip C., Tamburrini A., La Rosa G., Sreesunpasirikul C., Yingyourd P., Pozio E. First outbreak of human trichinellosis caused by Trichinella pseudospiralis. Clin. Infect. Dis. 1998;26:111–115. doi: 10.1086/516278. [DOI] [PubMed] [Google Scholar]
- Kapel C.M., Webster P., Gamble H.R. Muscle distribution of sylvatic and domestic Trichinella larvae in production animals and wildlife. Vet. Parasitol. 2005;132:101–105. doi: 10.1016/j.vetpar.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Krivokapich S.J., Prous C.L., Gatti G.M., Saldia L. First finding of Trichinella pseudospiralis in the Neotropical region. Vet. Parasitol. 2015;208:268–271. doi: 10.1016/j.vetpar.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Zarlenga D.S., Gamble H.R., al-Yaman F., Smith P.C., Blagburn B.L. Isolation and characterization of Trichinella pseudospiralis Garkavi, 1972 from a black vulture (Coragyps atratus) J. Parasitol. 1995;81:920–923. [PubMed] [Google Scholar]
- Mohandas N., Pozio E., La Rosa G., Korhonen P.K., Young N.D., Koehler A.V., Hall R.S., Sternberg P.W., Boag P.R., Jex A.R., Chang B.C., Gasser R.B. Mitochondrial genomes of Trichinella species and genotypes- a basis for diagnosis, and systematic and epidemiological explorations. Int. J. Parasitol. 2014;44:1073–1080. doi: 10.1016/j.ijpara.2014.08.010. [DOI] [PubMed] [Google Scholar]
- McIntyre L., Pollock S.L., Fyfe M., Gajadhar A., Isaac-Renton J., Fung J., Morshed M. Trichinellosis from consumption of wild game meat. CMAJ (Can. Med. Assoc. J.) 2007;176:449–451. doi: 10.1503/cmaj.061530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders R. 2001. Wolverine Ecology, Distribution, and Productivity in the Slave Geological Province-Final Report to the West Kitikmeot/Slave Study Society, Yellowknife, Northwest Territories; p. 92. Canada. [Google Scholar]
- Murrell K.D., Pozio E. Worldwide occurrence and impact of human trichinellosis, 1986-2009. Emerg. Infect. Dis. 2011;17:2194–2202. doi: 10.3201/eid1712.110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf D.L., Clarke K.P. Trichinella pseudospiralis infections in free-living Tasmanian birds. J. Helminthol. Soc.Washington. 1992;59:144–147. [Google Scholar]
- Pasitschniak-Arts M., Larivière S. Gulo gulo. Mamm. Species. 1995;499:1–10. [Google Scholar]
- Pozio E. The broad spectrum of Trichinella hosts: From cold- to ward-blooded animals. Vet. Parasitol. 2005;132:3–11. doi: 10.1016/j.vetpar.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Pozio E. Trichinella pseudospiralis an elusive nematode. Vet. Parasitol. 2016;231:97–101. doi: 10.1016/j.vetpar.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Pozio E., Goffredo M., Fico R., La Rosa G. Trichinella pseudospiralis in sedentary night-birds of prey from Central Italy. J. Parasitol. 1999;85:759–761. [PubMed] [Google Scholar]
- Pozio E., Hoberg E., La Rosa G., Zarlenga D.S. Molecular taxonomy, phylogeny and biogeography of nematodes belonging to the Trichinella genus. Infect. Genet. Evol. 2009;9:606–616. doi: 10.1016/j.meegid.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Pozio E., Shaikenov B., La Rosa G., Obendorf D.L. Allozymic and biological characters of Trichinella pseudospiralis isolates from free-ranging animals. J. Parasitol. 1992;78:1087–1090. [PubMed] [Google Scholar]
- Rausch R., Babero B.B., Rausch R.V., Schiller E.L. Studies on the helminth fauna of Alaska. XXVII. The occurrence of larvae of Trichinella spiralis in Alaskan mammals. J. Parasitol. 1956;42:259–271. [PubMed] [Google Scholar]
- Reichard M.V., Criffield M., Thomas J.E., Paritte J.M., Cunningham M., Onorato D., Logan K., Interisano M., Marucci G., Pozio E. High prevalence of Trichinella pseudospiralis in Florida panthers (Puma concolor coryi) Parasites Vectors. 2015;8:6. doi: 10.1186/s13071-015-0674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard M.V., Logan K., Criffield M., Thomas J.E., Paritte J.M., Messerly D.M., Interisano M., Marucci G., Pozio E. The occurrence of Trichinella species in the cougar Puma concolor couguar from the state of Colorado and other regions of North and South America. J. Helminthol. 2017;91:320–325. doi: 10.1017/S0022149X16000262. [DOI] [PubMed] [Google Scholar]
- Reichard M.V., Torretti L., Snider T.A., Garvon J.M., Marucci G., Pozio E. Trichinella T6 and Trichinella nativa in wolverines (Gulo gulo) from Nunavut, Canada. Parasitol. Res. 2008;103:657–661. doi: 10.1007/s00436-008-1028-y. [DOI] [PubMed] [Google Scholar]
- Scandrett B., Konecsni K., Lalonde L., Boireau P., Vallée I. Detection of natural Trichinella murrelli and Trichinella spiralis infections in horses by routine post-slaughter food safety testing. Food and Waterborne Parasitology. 2018;11:1–5. doi: 10.1016/j.fawpar.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg R.S., Tan B.J., Irvine J.D., Stockdale D.R., Gajadhar A.A., Serhir B., Botha J., Armstrong C.A., Woods S.A., Blondeau J.M., McNab T.L. An outbreak of trichinellosis due to consumption of bear meat infected with Trichinella nativa, in 2 northern Saskatchewan communities. J. Infect. Dis. 2003;188:835–843. doi: 10.1086/378094. [DOI] [PubMed] [Google Scholar]
- Serhir B., MacLean J.D., Healey S., Segal B., Forbes L. Outbreak of trichinellosis associated with arctic walruses in northern Canada, 1999. Can. Commun. Dis. Rep. 2001;27:31–36. [PubMed] [Google Scholar]
- Shaikenov B.S. Spontaneous infection of birds with Trichinella pseudospiralis Garkavi, 1972. Folia Parasitol. 1980;27:259–271. [Google Scholar]
- Sharma R., Harms N.J., Kukka P.M., Parker S.E., Gajadhar A.A., Jung T.S., Jenkins E.J. Tongue has higher larval burden of Trichinella spp. than diaphragm in wolverines (Gulo gulo) Vet. Parasitol. 2018;253:94–97. doi: 10.1016/j.vetpar.2018.02.032. [DOI] [PubMed] [Google Scholar]
- Wheeldon E.B., Dick T.A., Schulz T.A. First report of Trichinella spiralis var. pseudospiralis in North America. J. Parasitol. 1983;69:781–782. [PubMed] [Google Scholar]
- Wheeldon E.B., Kock M.D., Tucker R.L., Anderson M. Trichinellosis in a Cooper's hawk. J. Am. Vet. Med. Assoc. 1982;181:1385–1386. [PubMed] [Google Scholar]
- Wu Z., Snabel V., Pozio E., Hurnikova Z., Nareaho A., Nagano I., Takahashi Y. Genetic relationships among Trichinella pseudospiralis isolates from Australian, Nearctic, and Palearctic regions. Parasitol. Res. 2007;101:1567–1573. doi: 10.1007/s00436-007-0677-6. [DOI] [PubMed] [Google Scholar]
- Zamora M.J., Alvarez M., Olmedo J., Blanco M.C., Pozio E. Trichinella pseudospiralis in the Iberian peninsula. Vet. Parasitol. 2015;210:255–259. doi: 10.1016/j.vetpar.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Zarlenga D.S., Chute M.B., Martin A., Kapel C.M. A multiplex PCR for unequivocal differentiaition of all encapsulated and non-encapsulated genotypes of Trichinella. Int. J. Parasitol. 1999;29:1859–1867. doi: 10.1016/s0020-7519(99)00107-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann W.J., Hubbard E.D. Trichiniasis in wildlife of Iowa. Am. J. Epidemiol. 1969;90:84–92. doi: 10.1093/oxfordjournals.aje.a121053. [DOI] [PubMed] [Google Scholar]