Summary
Chiral homoallylic amines not only are found in pharmaceutically relevant compounds but also serve as versatile building blocks for chemical synthesis. However, catalytic allylation of ketimines with allylboronates, an attractive approach to synthesize chiral homoallylic amine scaffolds remain scarce. Herein, we develop a highly enantioselective allylation of isatin-derived ketimines with boron allylation reagents catalyzed by a Bi(OAc)3-chiral phosphoric acid catalyst system. The reactions are remarkably efficient and mild, most of which were completed in less than an hour at room temperature with only 1/2 mol% (Bi(OAc)3/CPA) catalyst loading. A wide range of chiral 3-allyl 3-aminooxindoles were obtained in excellent yields and enantioselectivities. The synthetic utility was demonstrated by efficient formal synthesis of (+)-AG-041R and (−)-psychotriasine. Preliminary mechanism was studied by control experiments and theoretical calculations.
Subject Areas: Catalysis, Organic Synthesis, Organic Reaction
Graphical Abstract

Highlights
-
•
Asymmetric allylation of ketimines
-
•
Bi(OAc)3-chiral phosphoric acid catalyst
-
•
Downstream synthetic transformations
Catalysis; Organic Synthesis; Organic Reaction
Introduction
Chiral homoallylic amines not only are widely found in natural products and pharmaceutically relevant compounds (Guan et al., 2003, Ghosh et al., 2006) but also serve as versatile building blocks for chemical synthesis (Scheme 1A) (Sirasani and Andrade, 2011, Lathrop et al., 2016). Therefore, the asymmetric synthesis of chiral homoallylic amine scaffolds is of great interest in the organic chemistry community (Kumar et al., 2016, Wan et al., 2017). In this context, the asymmetric addition reaction of allylboronates to imines has been recognized as one of the most efficient methods for the construction of chiral homoallylic amines (Kennedy and Hall, 2003, Yus et al., 2011, Huo et al., 2014). Compared with the additions of allylboronates to aldimines (Lou et al., 2007, Lou and Schaus, 2008, Silverio et al., 2013, Wu et al., 2014, Jiang et al., 2017a, Jiang et al., 2017b, Jiang and Schaus, 2017), the corresponding asymmetric allylation of ketimines remains scarce, probably owing to the low reactivity of ketimines. Pioneering enantioselective allylation of acyclic ketimines with allylboronates by using DuPHOS-CuF catalyst has been demonstrated in 2006 by Shibasaki group (Scheme 1B) (Wada et al., 2006). In addition, Rh (and Co)-catalyzed enantioselective additions of potassium allyltrifluoroborates to cyclic N-sulfonyl α-ketiminoesters were also reported (Scheme 1C) (Luo et al., 2012, Hepburn et al., 2013, Hepburn and Lam, 2014, Wu et al., 2018). Very recently, Hoveyda reported NHC-CuCl complex-catalyzed highly stereoselective additions of versatile allyl groups to N-H ketimines (Scheme 1D) (Jang et al., 2017). Other methods involve using enantiomerically pure boron allylation reagent (Scheme 1E) (Chen and Aggarwal, 2014) or chiral inducing amine alcohol reagent (Scheme 1F) (Tan et al., 2017). Despite the mentioned achievements, several limitations, including high catalyst loading, long reaction time, harsh reaction conditions, and limited substrates, remain vast challenges to this field. Furthermore, such endeavors have been relying on either the utilization of canonical transition metal catalysis or stoichiometric chiral reagent. In consequence, the discovery of an efficient catalyst system that could enable the allylation of ketimines by allylboronate reagents in a more efficient and stereoselective fashion would provide access to chiral homoallylic amines in a sustainable manner.
Scheme 1.
Construction of Chiral Homoallylic Amines through Addition of Allylboronates to Ketimines
(A) Examples of biologically active homoallylamines.
(B) Cu-catalyzed addition of allylboronates to ketimines.
(C) Rh- or Co-catalyzed addition of allylboronates to ketimines.
(D) Cu-catalyzed three-component reaction of N–H ketimines.
(E) Addition of chiral allylboronates to ketimine.
(F) Addition of allylboronates to ketimines controlled by chiral reagent.
(G) Bi-catalyzed addition of allylboronates to ketimines.
Over the past few decades, chiral Lewis acid catalysis, a significant approach to obtain optically active compounds, had been well developed (Yamamoto, 2000, Yamamoto and Futatsugi, 2005, Yamamoto and Ishihara, 2008, Liu et al., 2011, Liu et al., 2014, Lv and Luo, 2013, Mlynarski, 2017). Although rare-earth metals, the first-row transition metals, and boron-type compounds are the most popular Lewis acid catalysts, the chiral alkaline-earth metal-based catalysts have also attracted ever-growing interest for meeting the needs of green sustainable chemical synthesis (Hatano et al., 2010, Zhang et al., 2011, Zheng et al., 2011, Li et al., 2013). Bismuth compounds, due to their low toxicity and non-corrosiveness, have always been considered as suitable for designing environmentally benign catalysts (Bothwell et al., 2011, Salvador et al., 2012, Ollevier, 2013, Ondet et al., 2017). However, the asymmetric bismuth catalysis remains a relatively unexplored field (Wada et al., 1997, Kobayashi et al., 2005, Kobayashi and Ogawa, 2006, Koch and Peters, 2007, Koch and Peters, 2011, Lassauque et al., 2009, Mahajan et al., 2011, Li et al., 2012, Kitanosono et al., 2013, Isomura et al., 2019). Thus, the development of efficient catalytic transformations using chiral bismuth system is highly meaningful and desirable.
Chiral 3-amino-2-oxindole is an important structural motif in medicinally relevant compounds (Zhou et al., 2010, Singh and Desta, 2012, Cao et al., 2018). Especially, the homoallylic aminooxindole derivatives not only act directly as an inhibitor of HIV-1 protease (Scheme 1A) but also can be converted into aminooxindole frameworks presented in alkaloids (Scheme 3B and 3C). In 2013, Nakamura demonstrated the enantioselective allylation of isatin-derived ketimines catalyzed by Pd-pincer-complexes and AgF under strict reaction conditions (−30°C) (Nakamura et al., 2013). In 2016, Cai group reported an enantioselective In(OTf)3-catalyzed allylation of ketimines derived from isatins with highly toxic allyltributyltin; however, this method is not suitable for the substrates with electron-withdrawing groups (Chen and Cai, 2016). Herein, we report a Bi(III)-catalyzed asymmetric allylation of isatin-derived ketimines with allylboronates under rather mild reaction conditions (Scheme 1G). A wide range of chiral 3-allyl 3-aminooxindoles were smoothly obtained in excellent yields with exceptional stereocontrol to forge the quaternary stereogenic carbon centers (Scheme 1G).
Scheme 3.
Large-Scale Reaction and Transformations of the Products
(A) The gram-scale reaction.
(B) The versatile transforms of 3a.
(C) The formal synthesis of (+)-AG-041R.
Reagents and conditions: (a) KMnO4, NaIO4, H2O, room temperature, 2 d; (b) Et3N, ClCO2Et, THF, −10°C, 1 h; (c) NaBH4, H2O, 0°C to room temperature, 4 h; (d) DMP, NaHCO3, DCM, room temperature, 1 h; (e) CH3NH2⋅HCl, Et3N, MgSO4, MeOH, room temperature, overnight, then NaBH4, 0°C; (f) 9-BBN, THF, 0°C to room temperature, 24 h; (g) AcONa, H2O2 (30% aq.), 0°C to room temperature, 5 h; (h) Ph3P, DEAD, DCM, 0°C to room temperature, overnight; (i) NaH, DMF, Allyl bromide, room temperature, 30 min; (j) Grubbs second, toluene, 60°C, 20 min; (k) MeI, Cs2CO3, CH3CN, room temperature, 8 h; (l) TFA, DCM, room temperature, 3 h; (m) 2 M NaOH (aq.), MeOH, 2 h; (n) MsCl, NaHCO3, CH3CN, 80°C, 18 h.
Results and Discussion
Optimization of the Reaction Conditions
Binaphthols have been proved to be efficient catalysts for the reactions of boronate with ketones (Lou et al., 2006, Barnett et al., 2009, Alam et al., 2015) and aldimines (Lou et al., 2007, Lou and Schaus, 2008, Jiang et al., 2017a, Jiang et al., 2017b, Jang et al., 2017); we initially attempted the reaction of the isatin-derived N-Boc-protected ketimine 1a and allylboronic acid pinacol ester 2a with binaphthol 4, yet catalyst 4 could not promote this reaction (Table 1, entry 1). Then we turned our attention to chiral phosphoric acids, which have also been considered as good catalysts to realize the allylboration of aldehydes (Jain and Antilla, 2010). Although chiral phosphoric acid (S)-5a indeed catalyzed the reaction to give product 3a with 85.9: 14.1 er, only 17% yield was obtained after 48h (Table 1, entry 2). The reactivity is obviously unsatisfactory. We suspected that the Brønsted acidity of chiral phosphoric acid is not strong enough to simultaneously activate ketimine 1a and allylboronate 2a. Inspired by Luo's asymmetric binary acid catalysis (Lv et al., 2011, Lv et al., 2013, Hashimoto et al., 2013, Hatano et al., 2015, Wang et al., 2017, Zhang et al., 2017a) and the bismuth catalyzed allylation of para-quinone with allylboronate 2a developed by our group (Zhang et al., 2017b), we proposed that this transformation was likely to be promoted by the BiX3-chiral phosphoric acid catalyst system and the use of chiral phosphoric acid could ensure the stereochemistry of this process. Gratifyingly, in the presence of (S)-5a and Bi(OAc)3, the model reaction gave product 3a in quantitative yield with 87.9: 12.1 er (Table 1, entry 3). We then examined other (S)-BINOL chiral phosphoric acid with Bi(OAc)3, but no better results were achieved (Table 1, entries 4–8). Screening of solvents (Table 1, entries 9–15) revealed that the reaction was favored in Et2O (Table 1, entry 15). When catalyst loading was lowered to 1 mol% Bi(OAc)3 and 2 mol% (S)-5a, the yield (96%) and enantioselectivity (99.1: 0.9 er) essentially remained the same in comparison with those with high catalyst loading (Table 1, entry 16). The counter anions of Bi(III) and different Lewis acids were also investigated in the model reaction. The use of other bismuth salts resulted in either low reactivities or poor stereoselectivities (Table 1, entries 17–22). Exploring other metal acetates, including Sc(III), In(III), and Y(III), almost all showed poor catalytic activities (Table 1, entries 23–28). Thus, the optimal reaction conditions were finally determined to be 1 mol% Bi(OAc)3 and 2 mol% (S)-5a in Et2O (0.2 M) at room temperature (Table 1, entry 16).
Table 1.
Reaction Optimization
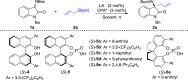 | ||||||
|---|---|---|---|---|---|---|
| Entry | LA | CPA | Solvent | Time | Yielda/% | erb |
| 1c | – | 4 | DCM | 64 h | n.r. | – |
| 2c | – | 5a | DCM | 48 h | 17 | 85.9:14.1 |
| 3 | Bi(OAc)3 | 5a | CHCl3 | 20 min | 99 | 87.9:12.1 |
| 4 | Bi(OAc)3 | 5b | CHCl3 | 25 min | 88 | 61.7:38.3 |
| 5 | Bi(OAc)3 | 5c | CHCl3 | 40 min | 98 | 75.3:24.7 |
| 6 | Bi(OAc)3 | 5d | CHCl3 | 25 min | 95 | 55.1:44.9 |
| 7 | Bi(OAc)3 | 5e | CHCl3 | 25 min | 92 | 50.4:49.6 |
| 8 | Bi(OAc)3 | 6a | CHCl3 | 80 min | 99 | 17.0:83.0 |
| 9 | Bi(OAc)3 | 5a | DCM | 20 min | 89 | 86.0:14.0 |
| 10 | Bi(OAc)3 | 5a | Toluene | 15 min | 98 | 97.7:2.3 |
| 11 | Bi(OAc)3 | 5a | EA | 15 min | 96 | 98.3:1.7 |
| 12 | Bi(OAc)3 | 5a | CH3CN | 50 min | 99 | 88.2:11.8 |
| 13 | Bi(OAc)3 | 5a | THF | 75 min | 93 | 97.0:3.0 |
| 14 | Bi(OAc)3 | 5a | Dioxane | 45 min | 99 | 96.6:3.4 |
| 15 | Bi(OAc)3 | 5a | Et2O | 20 min | 99 | 99.2:0.8 |
| 16d | Bi(OAc)3 | 5a | Et2O | 35 min | 96 | 99.1:0.9 |
| 17d | Bi(OTf)3 | 5a | Et2O | 24 h | 30 | 57.0:43.0 |
| 18d | BiCl3 | 5a | Et2O | 24 h | 27 | 53.9:46.1 |
| 19d | BiBr3 | 5a | Et2O | 24 h | 81 | 52.6:47.4 |
| 20d | BiI3 | 5a | Et2O | 24 h | 92 | 65.5:34.5 |
| 21d | Bi(OH)3 | 5a | Et2O | 24 h | 20 | 94.8:5.2 |
| 22d | Bi(OiPr)3 | 5a | Et2O | 60 h | 94 | 94.9:5.1 |
| 23d | Sc(OAc)3 | 5a | Et2O | 72 h | <5 | – |
| 24d | In(OAc)3 | 5a | Et2O | 72 h | 31 | 55.4:44.6 |
| 25d | Cu(OAc)2 | 5a | Et2O | 52 h | trace | – |
| 26d | AgOAc | 5a | Et2O | 25 h | 12 | 55.9:44.1 |
| 27d | Y(OAc)3 | 5a | Et2O | 25 h | trace | – |
| 28d | La(OAc)3 | 5a | Et2O | 48 h | 12 | 69.0:31.0 |
The reactions were carried out with 1a (0.1 mmol), 2a (0.12 mmol), Bi(OAc)3 (2 mol%), and CPA (3 mol%) in 0.5 mL solvent at room temperature.
Yield of isolated products.
Determined by HPLC analysis.
The reactions were carried out with 1a (0.1 mmol), 2a (0.12 mmol), 10 mol% catalyst in 0.5 mL DCM at room temperature.
The reactions were carried out with 1a (0.2 mmol), 2a (0.24 mmol), Bi(OAc)3 (1 mol%), and (S)-5a (2 mol%) in 1.0 mL Et2O at room temperature.
Substrate Scope
We then explored the substrate scope of the allylation of isatin-derived ketimines under the optimal reaction conditions. We first investigated the substituents on the phenyl ring of the isatin. As shown in Figure 1, this protocol is amenable to most of N-Boc-protected ketimines derived from N-benzylisatins bearing electron-donating or electron-withdrawing substituents and halogen atoms on the phenyl ring, leading to chiral 3-allyl 3-aminooxindole products (Figures 1, 3a-3p) in high yields (73%–99%) with good to excellent enantioselectivities (91.7: 8.3–99.3: 0.7 er). However, electron-withdrawing substituents on the C5 and C7 of the phenyl ring led to reduced stereoselectivities (Figure 1, 3g and 3p).
Figure 1.
Scope of Substituents on the Phenyl Ring
The reactions were carried out with 1 (0.2 mmol), 2a (0.24 mmol), Bi(OAc)3 (1 mol%), and (S)-5a (2 mol%) in 1.0 mL Et2O at room temperature. The absolute configuration of the product was determined by X-ray analysis of 10. Isolated yields. The er values were determined by HPLC analysis.
Subsequently, the effect of the protecting group at the N1-position were examined (Figure 2). To our delight, the expected product 3q was afforded from ketimine 1q without protecting group on the N1-atom in 99% yield and 85.1: 14.9 er. An elevated 98.8: 1.2 er was obtained after one single recrystallization from ethyl acetate/n-pentane. In addition, isatin-derived ketimines with phenyl, acetyl, alkyl (R’ = Me, allyl, methoxymethyl or CH2CH(OEt)2) at the N1-position, were also efficiently transformed into the corresponding allylic products (3r-3v) with good to excellent enantioselectivities (92.4: 7.6–99.5: 0.5 er). When the substituents at the N1-position of ketimines 1 were substituted benzyl groups, we found that the electron effect or the steric hindrance had almost no effect on the reaction results (Figure 2, 3x-3z and 3aa–3af).
Figure 2.
Scope of Protecting Group at the N1-Position and Other N-Substituted Ketimines
The reactions were carried out with 1 (0.2 mmol), 2a (0.24 mmol), Bi(OAc)3 (1 mol%), and (S)-5a (2 mol%) in 1.0 mL Et2O at room temperature. Isolated yields. The er values were determined by HPLC analysis.
The er in bracket was afforded after recrystallization from ethyl acetate/n-pentane.
Furthermore, other N-alkoxycarbonyl ketimines can also react with 2a and give the products (3ag and 3ah) in good yields and excellent enantioselectivities (Figure 2). And N-phenyl ketimine 1ai could also be transformed into the corresponding allylation product 3ai under the optimal conditions in excellent yield (98%) with good enantioselectivity (94.1:5.9 er).
To expand the scope of this Bi(OAc)3/CPA catalyzed asymmetric allylation method, some other ketimines were also investigated (Scheme 2). To our delight, not only the cyclic N-sulfonyl α-ketiminoester 1aj but also the N-Boc ketimine 1ak derived from pyrazolin-5-one could work smoothly under the optimal conditions and give the desired products 3aj (Wu et al., 2018) and 3ak in excellent yields with good enantioselectivities. In addition, this catalytic system was also proved to be suitable for the asymmetric allylation of isatin (Scheme 2) (Itoh et al., 2009).
Scheme 2.
Other Ketimine Skeletons and Scope of Allyl Boron Reagent
Condition A: 1 (0.2 mmol), 2a (0.24 mmol), Bi(OAc)3 (1 mol%), and (S)-5a (2 mol%) in 1.0 mL Et2O at room temperature; Condition B: 1al (0.2 mmol), 2a (0.24 mmol), Bi(OAc)3 (1 mol%), and (S)-6a (2 mol%) in 1.0 mL cyclohexane at room temperature. Isolated yields. The er values were determined by high-performance liquid chromatography (HPLC) analysis.
Further exploration of the substrate scope was focused on the allyl boron reagent (Scheme 2). When potassium allyltrifluoroborate and allyl boric acid were used, the corresponding product 3a was obtained with 92.4:7.6 er and 93.1:6.9 er, respectively. It should be noted that this Bi(OAc)3/CPA catalytic system is applicable to a variety of boron allylation reagent, whereas previous reports are often limited to the particular one. The α-addition product 3am (91% yield, 5.8:1 d.r., and 96.6:3.4 er) resulted in the reaction of 1a and 2d. When β-methyl branch allylboronic acid pinacol ester reacted with 1a under the optimal conditions, the desired product 3an was obtained in good yield (84%) with depressed enantioselectivity (79.4:20.6 er). Moreover, the reaction of pinacolyl isoprenylboronate and ketimine 1a could also give the desired product 3ao in good yield (69%) and moderate enantioselectivity (84.4:15.6 er).
Large-Scale Reaction and Synthetic Applications
To probe the efficiency of current asymmetric allylation strategy in preparative synthesis, a gram-scale reaction of 1a and 2a was investigated under optimal reaction conditions. To our delight, the corresponding product 3a was obtained without any loss of the enantioselectivity (Scheme 3A). To illustrate the applicability of our method in organic synthesis, the product was applied to synthesize some pharmaceuticals and N-containing heterocyclic oxindole compounds. Firstly, as shown in Scheme 3B, the allylation product 3a underwent complete oxidation and reduction to give the compound 7. Compound 7 can be oxidized to an aldehyde intermediate and provided key compound 8 by reductive amination, which can be converted to (−)-psychotriasine (Dai et al., 2017). Compound 3a underwent hydroboration-oxidation followed by an intramolecular Mitsunobu reaction to afford spirocyclic amine 10 (98.7:1.3 er). The N-allylation of 3a can also offer product 11 in high yield, and its ring-closing metathesis gave spirocyclic amine 12 in high yield with maintained er value by using Grubbs second catalyst. In addition, the β-amino ester 13 was afforded by oxidation of 3a followed by esterification. Boc removal followed by cyclization led to spiro-β-lactam 15 in 67% yield and 98.8:1.2 er. Thereafter, oxidation of 3v followed by an esterification afforded compound 16 without any loss of enantioselectivity (99.6:0.4 er). And the compound 16 could be transformed into (+)-AG-041R, which is a potent gastrin/CCK-B receptor antagonist (Scheme 3C) (Sato et al., 2009).
Mechanistic Considerations
We performed control experiments to investigate whether bismuth acetate and chiral phosphoric acid work in a synergic manner on the activity and enantioselectivity of the asymmetric allylation (Table 2). The reaction proceeded smoothly in the presence of Bi(OAc)3 and gave a racemic product (Table 2, entry 1). When only chiral phosphoric acid (S)-5a existed, 14% yield and 62.4: 37.6 er could be achieved in 48 h (Table 2, entry 2). Considering that the hydrolysis of Bi(OAc)3 produces acetic acid, we performed the reaction under the condition of only 2 mol% AcOH, and 12% racemic product could be given (Table 2, entry 3). If adding 3 mol% (S)-5a on the basis of condition C, we could afford the product in 17% yield with 71.6: 28.4 er in 48 h (Table 2, entry 4). Therefore, the effect of Lewis acid's hydrolysis on the reaction results could be excluded. These experimental results demonstrated that the reactivity and stereoselectivity should be controlled by Bi(OAc)3 and chiral phosphoric acid together.
Table 2.
Control Experiments
 | ||
|---|---|---|
| Entry | Conditions | Results |
| 1 | Only 2 mol% Bi(OAc)3 | 1.5 h, 99% yield, rac |
| 2 | Only 3 mol% (S)-5a | 48 h, 14% yield, 62.4:37.6 er |
| 3 | Only 2 mol% AcOH | 48 h, 12% yield, rac |
| 4 | 2 mol% AcOH +3 mol% (S)-5a | 48 h, 17% yield, 71.6:28.4 er |
Preliminary experiments were conducted to illustrate the mechanism of the Bi(OAc)3/CPA catalytic system. ESI-MS experiment (cationic mode) gave two peaks m/z 1027.27 and 1728.45 corresponding to 5a⋅Bi(OAc)2 and (5a)2⋅Bi(OAc)2 (for details, see Supplemental Information). A positive nonlinear effect between the catalyst's er value and product's er value was observed under optimal reaction conditions (Figure 3) (Liu et al., 2011, Wang et al., 2017), which indicates that more than one molecule of the chiral acid (S)-5a is likely to be involved in the transition state of the enantio-differentiating step. The α-selectivity was observed with 1-methylallylboronic acid pinacol ester (Scheme 2, substrate scope part); thus, we speculated that the reaction should occur through a B-to-Bi transmetalation process (Chakrabarti et al., 2010).
Figure 3.
Nonlinear Effect Experiment
For the major diastereomer, determined by HPLC analysis on a chiral stationary phase, averaged over two runs (see also Table S3).
The mechanism of the catalytic system has been further investigated by theoretical calculations (for computational details, see Supplemental Information). Two mechanistic possibilities that differ by the coordination number were considered. A single CPA ligand is present in M1, whereas two chiral ligands are present in M2 (Figure 4). Mechanism M1 can be discarded based on the large energy barrier (at least 6.1 kcal/mol unfavorable) in which the single CPA served as a typical anionic ligand. Two CPA ligands perform different roles in M2, in which one serves as a typical anionic ligand and the other performs as a neutral ligand and acid catalyst simultaneously. We have examined different relative orientations of substrate 1q and Bi-allyl species (details in the Supplemental Information), and the most stable TSs corresponding to the structure was shown (M2 in Figure 4). On examination of TS-2p-(R), we found that the C=O group of the ketimine is coordinated with the Bi and the C=N group is activated by the proton of phosphoric acid simultaneously. In the most stable TS-2P-(R), substrate 1q is oriented with the bulky Boc group into an open quadrant of the catalyst and TS-2P-(S) with the bulky Boc group toward the catalyst lying 1.1 kcal/mol above the most stable TS. Calculations predict 86.5: 13.5 er for the (R)-product, which is consistent well with experimental 85.1: 14.9 er.
Figure 4.
Transition State Structures and Relative Free Energies (in kcal/mol)
See also Figures S167–S170; and Tables S3 and S4–S10.
Limitations of Study
The reaction only gave poor yield (30%) and poor enantioselectivity (57.0:43.0 er) with the widely used Bi(OTf)3 instead of Bi(OAc)3 (Table 1, entry 17).
Conclusion
In summary, we have developed a highly efficient and enantioselective asymmetric allylation of isatin-derived ketimines with allylboronates promoted by a binary acid system containing bismuth acetate and chiral phosphoric acid. As far as we know, this is the first successful application of the catalyst system of Bi(III) Lewis acid and chiral phosphoric acid in asymmetric catalysis. This is an unreported catalytic system in asymmetric allylation of ketimines. As a result, a series of chiral 3-allyl 3-aminooxindoles were obtained in excellent yields (up to 99%) and enantioselectivities (up to 99.5: 0.5 er). The synthetic utility was demonstrated not only by formal synthesis of (+)-AG-041R and (−)-psychotriasine but also by the transformation of the allylation products into valuable chiral 3-spirocyclic oxindoles. Preliminary mechanism study by control experiments and theoretical calculations shows that two chiral phosphoric acids, in which one serves as an anionic ligand and the other performs as a neutral ligand and acid catalyst simultaneously, have participated in this allylation strategy. We anticipate that this work will provide a broad prospect for the future application of bismuth in asymmetric catalysis.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The project was supported by NSFC (21390400). We thank Prof. Jian Lv for helpful discussion. We also thank Mrs Zhi-Yan Li in ICCAS for MS analysis.
Author Contributions
J.W. developed the asymmetric catalytic reaction. J.W. and Q.Z. expanded the substrate scope, performed the synthetic applications, and characterized all the products. B.Z. and C.Y. performed the theoretical calculations. X.L. directed the investigations. J.W., X.L., and J.-P.C. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.06.006.
Data and Software Availability
The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1849656 (10) and can be obtained free of charge from www.ccdc.cam.ac.uk/getstructures.
Supplemental Information
References
- Alam R., Vollgraff T., Eriksson L., Szabó K.J. Synthesis of adjacent quaternary stereocenters by catalytic asymmetric allylboration. J. Am. Chem. Soc. 2015;137:11262–11265. doi: 10.1021/jacs.5b07498. [DOI] [PubMed] [Google Scholar]; Alam, R., Vollgraff, T., Eriksson, L. and Szabo, K.J.. (2015). Synthesis of adjacent quaternary stereocenters by catalytic asymmetric allylboration. J. Am. Chem. Soc. 137, 11262-11265. [DOI] [PubMed]
- Barnett D.S., Moquist P.N., Schaus S.E. The mechanism and an improved asymmetric allylboration of ketones catalyzed by chiral biphenols. Angew. Chem. Int. Ed. 2009;48:8679–8682. doi: 10.1002/anie.200904715. [DOI] [PMC free article] [PubMed] [Google Scholar]; Barnett, D.S., Moquist, P.N. and Schaus, S.E.. (2009). The mechanism and an improved asymmetric allylboration of ketones catalyzed by chiral biphenols. Angew. Chem. Int. Ed. 48, 8679-8682. [DOI] [PMC free article] [PubMed]
- Bothwell J.M., Krabbez S.W., Mohan R.S. Applications of bismuth(III) compounds in organic synthesis. Chem. Soc. Rev. 2011;40:4649–4707. doi: 10.1039/c0cs00206b. [DOI] [PubMed] [Google Scholar]; Bothwell, J.M., Krabbez, S.W. and Mohan, R.S.. (2011). Applications of bismuth(III) compounds in organic synthesis. Chem. Soc. Rev. 40, 4649-4707. [DOI] [PubMed]
- Cao Z.-Y., Zhou F., Zhou J. Development of synthetic methodologies via catalytic enantioselective synthesis of 3,3-disubstituted oxindoles. Acc. Chem. Res. 2018;51:1443–1454. doi: 10.1021/acs.accounts.8b00097. [DOI] [PubMed] [Google Scholar]; Cao, Z.-Y., Zhou, F. and Zhou, J.. (2018). Development of synthetic methodologies via catalytic enantioselective synthesis of 3,3-disubstituted oxindoles. Acc. Chem. Res. 51, 1443-1454. [DOI] [PubMed]
- Chakrabarti A., Konishi H., Yamaguchi M., Schneider U., Kobayashi S. Indium(I)-catalyzed asymmetric allylation, crotylation, and α-chloroallylation of hydrazones with rare constitutional and high configurational selectivities. Angew. Chem. Int. Ed. 2010;49:1838–1841. doi: 10.1002/anie.200906308. [DOI] [PubMed] [Google Scholar]; Chakrabarti, A., Konishi, H., Yamaguchi, M., Schneider, U. and Kobayashi, S.. (2010). Indium(I)-catalyzed asymmetric allylation, crotylation, and α-chloroallylation of hydrazones with rare constitutional and high configurational selectivities. Angew. Chem. Int. Ed. 49, 1838-1841. [DOI] [PubMed]
- Chen J.L.-Y., Aggarwal V.K. Highly diastereoselective and enantiospecific allylation of ketones and imines using borinic esters: contiguous quaternary stereogenic centers. Angew. Chem. Int. Ed. 2014;53:10992–10996. doi: 10.1002/anie.201407127. [DOI] [PubMed] [Google Scholar]; Chen, J.L.-Y. and Aggarwal, V.K.. (2014). Highly diastereoselective and enantiospecific allylation of ketones and imines using borinic esters: contiguous quaternary stereogenic centers. Angew. Chem. Int. Ed. 53, 10992-10996. [DOI] [PubMed]
- Chen T., Cai C. Imidazolylpyridine-In(OTf)3 catalyzed enantioselective allylation of ketimines derived from isatins. Org. Biomol. Chem. 2016;14:5019–5022. doi: 10.1039/c6ob00551a. [DOI] [PubMed] [Google Scholar]; Chen, T. and Cai, C.. (2016). Imidazolylpyridine-In(OTf)3 catalyzed enantioselective allylation of ketimines derived from isatins. Org. Biomol. Chem. 14, 5019-5022. [DOI] [PubMed]
- Dai J., Xiong D., Yuan T., Liu J., Chen T., Shao Z. Chiral primary amine catalysis for asymmetric Mannich reactions of aldehydes with ketimines: stereoselectivity and reactivity. Angew. Chem. Int. Ed. 2017;56:12697–12701. doi: 10.1002/anie.201706304. [DOI] [PubMed] [Google Scholar]; Dai, J., Xiong, D., Yuan, T., Liu, J., Chen, T. and Shao, Z.. (2017). Chiral primary amine catalysis for asymmetric Mannich reactions of aldehydes with ketimines: stereoselectivity and reactivity. Angew. Chem. Int. Ed. 56, 12697-12701. [DOI] [PubMed]
- Ghosh A.K., Schiltz G., Perali R.S., Leshchenko S., Kay S., Walters D.E., Koh Y., Maeda K., Mitsuya H. Design and synthesis of novel HIV-1 protease inhibitors incorporating oxyindoles as the P’2-ligands. Bioorg. Med. Chem. Lett. 2006;16:1869–1873. doi: 10.1016/j.bmcl.2006.01.011. [DOI] [PubMed] [Google Scholar]; Ghosh, A.K., Schiltz, G., Perali, R.S., Leshchenko, S., Kay, S., Walters, D.E., Koh, Y., Maeda, K. and Mitsuya, H.. (2006). Design and synthesis of novel HIV-1 protease inhibitors incorporating oxyindoles as the P’2-ligands. Bioorg. Med. Chem. Lett. 16, 1869-1873. [DOI] [PubMed]
- Guan J., Mathai S., Harris P., Wen J.-Y., Zhang R., Brimble M., Gluckman P. Peripheral administration of a novel diketopiperazine, NNZ 2591, prevents brain injury and improves somatosensory-motor function following hypoxia-ischemia in adult rats. Neuropharmacology. 2003;53:749–762. doi: 10.1016/j.neuropharm.2007.08.010. [DOI] [PubMed] [Google Scholar]; Guan, J., Mathai, S., Harris, P., Wen, J.-Y., Zhang, R., Brimble, M. and Gluckman, P.. (2003). Peripheral administration of a novel diketopiperazine, NNZ 2591, prevents brain injury and improves somatosensory-motor function following hypoxia-ischemia in adult rats. Neuropharmacology. 53, 749-762. [DOI] [PubMed]
- Hashimoto T., Galvez A.O., Maruoka K. In situ assembled boronate ester assisted chiral carboxylic acid catalyzed asymmetric trans- aziridinations. J. Am. Chem. Soc. 2013;135:17667–17670. doi: 10.1021/ja407764u. [DOI] [PubMed] [Google Scholar]; Hashimoto, T., Galvez, A.O. and Maruoka, K.. (2013). In situ assembled boronate ester assisted chiral carboxylic acid catalyzed asymmetric trans- aziridinations. J. Am. Chem. Soc. 135, 17667-17670. [DOI] [PubMed]
- Hatano M., Moriyama K., Maki T., Ishihara K. Which is the actual catalyst: chiral phosphoric acid or chiral calcium phosphate. Angew. Chem. Int. Ed. 2010;49:3823–3826. doi: 10.1002/anie.201000824. [DOI] [PubMed] [Google Scholar]; Hatano, M., Moriyama, K., Maki, T. and Ishihara, K.. (2010). Which is the actual catalyst: chiral phosphoric acid or chiral calcium phosphate. Angew. Chem. Int. Ed. 49, 3823 -3826. [DOI] [PubMed]
- Hatano M., Goto Y., Izumiseki A., Akakura M., Ishihara K. Boron tribromide-assisted chiral phosphoric acid catalyst for a highly enantioselective Diels-Alder reaction of 1, 2- dihydropyridines. J. Am. Chem. Soc. 2015;137:13472–13475. doi: 10.1021/jacs.5b08693. [DOI] [PubMed] [Google Scholar]; Hatano, M., Goto, Y., Izumiseki, A., Akakura, M. and Ishihara, K.. (2015). Boron tribromide-assisted chiral phosphoric acid catalyst for a highly enantioselective Diels-Alder reaction of 1, 2- dihydropyridines. J. Am. Chem. Soc. 137, 13472-13475. [DOI] [PubMed]
- Hepburn H.B., Lam H.W. The isomerization of allylrhodium intermediates in the rhodium-catalyzed nucleophilic allylation of cyclic imines. Angew. Chem. Int. Ed. 2014;53:11605–11610. doi: 10.1002/anie.201407233. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hepburn, H.B. and Lam, H.W.. (2014). The isomerization of allylrhodium intermediates in the rhodium-catalyzed nucleophilic allylation of cyclic imines. Angew. Chem. Int. Ed. 53, 11605-11610. [DOI] [PMC free article] [PubMed]
- Hepburn H.B., Chotsaeng N., Luo Y., Lam H.W. Enantioselective rhodium-catalyzed allylation of cyclic imines with potassium allyltrifluoroborates. Synthesis. 2013;45:2649–2661. [Google Scholar]; Hepburn, H.B., Chotsaeng, N., Luo, Y. and Lam, H.W.. (2013). Enantioselective rhodium-catalyzed allylation of cyclic imines with potassium allyltrifluoroborates. Synthesis. 45, 2649-2661.
- Huo H.X., Duvall J.R., Huang M.Y., Hong R. Catalytic asymmetric allylation of carbonyl compounds and imines with allylic boronates. Org. Chem. Front. 2014;1:303–320. [Google Scholar]; Huo, H.X., Duvall, J.R., Huang, M.Y. and Hong, R.. (2014). Catalytic asymmetric allylation of carbonyl compounds and imines with allylic boronates. Org. Chem. Front. 1, 303-320.
- Isomura M., Petrone D.A., Carreira E.M. Coordination-induced stereocontrol over carbocations: asymmetric reductive deoxygenation of racemic tertiary alcohols. J. Am. Chem. Soc. 2019;141:4738–4748. doi: 10.1021/jacs.9b00862. [DOI] [PubMed] [Google Scholar]; Isomura, M., Petrone, D.A. and Carreira, E.M.. (2019). Coordination-induced stereocontrol over carbocations: asymmetric reductive deoxygenation of racemic tertiary alcohols. J. Am. Chem. Soc. 141, 4738−4748. [DOI] [PubMed]
- Itoh J., Han S.B., Krische M.J. Enantioselective allylation, crotylation, and reverse prenylation of substituted isatins: iridium-catalyzed C-C bond- forming transfer hydrogenation. Angew. Chem. Int. Ed. 2009;48:6313–6316. doi: 10.1002/anie.200902328. The absolute configuration of the product is determined by comparison with the data in the literature. [DOI] [PMC free article] [PubMed] [Google Scholar]; The absolute configuration of the product is determined by comparison with the data in the literature: Itoh, J., Han, S.B. andKrische, M.J. (2009). Enantioselective allylation, crotylation, and reverse prenylation of substituted isatins: iridium-catalyzed C-C bond- forming transfer hydrogenation. Angew. Chem. Int. Ed. 48, 6313-6316. [DOI] [PMC free article] [PubMed]
- Jain P., Antilla J.C. Chiral Brønsted acid-catalyzed allylboration of aldehydes. J. Am. Chem. Soc. 2010;132:11884–11886. doi: 10.1021/ja104956s. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jain, P. and Antilla, J.C.. (2010). Chiral Bronsted acid-catalyzed allylboration of aldehydes. J. Am. Chem. Soc. 132, 11884-11886. [DOI] [PMC free article] [PubMed]
- Jang H., Romiti F., Torker S., Hoveyda A.H. Catalytic diastereo- and enantioselective additions of versatile allyl groups to N-H ketimines. Nat. Chem. 2017;9:1269–1275. doi: 10.1038/nchem.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jang, H., Romiti, F., Torker, S. and Hoveyda, A.H.. (2017). Catalytic diastereo- and enantioselective additions of versatile allyl groups to N-H ketimines. Nat. Chem. 9, 1269-1275. [DOI] [PMC free article] [PubMed]
- Jiang Y., Schaus S.E. Asymmetric petasis borono-mannich allylation reactions catalyzed by chiral biphenols. Angew. Chem. Int. Ed. 2017;56:1544–1548. doi: 10.1002/anie.201611332. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jiang, Y. and Schaus, S.E.. (2017). Asymmetric petasis borono-mannich allylation reactions catalyzed by chiral biphenols. Angew. Chem. Int. Ed. 56, 1544-1548. [DOI] [PMC free article] [PubMed]
- Jiang Y., Diagne A.B., Thomson R.J., Schaus S.E. Enantioselective synthesis of allenes by catalytic traceless petasis reactions. J. Am. Chem. Soc. 2017;139:1998–2005. doi: 10.1021/jacs.6b11937. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jiang, Y., Diagne, A.B., Thomson, R.J. and Schaus, S.E.. (2017a). Enantioselective synthesis of allenes by catalytic traceless petasis reactions. J. Am. Chem. Soc. 139, 1998-2005. [DOI] [PMC free article] [PubMed]
- Jiang Y., Thomson R.J., Schaus S.E. Asymmetric traceless petasis borono-mannich reactions of enals: reductive transposition of allylic diazenes. Angew. Chem. Int. Ed. 2017;56:16631–16635. doi: 10.1002/anie.201708784. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jiang, Y., Thomson, R.J. and Schaus, S.E.. (2017b). Asymmetric traceless petasis borono-mannich reactions of enals: reductive transposition of allylic diazenes. Angew. Chem. Int. Ed. 56, 16631-16635. [DOI] [PMC free article] [PubMed]
- Kennedy J.W.J., Hall D.G. Recent advances in the activation of boron and silicon reagents for stereocontrolled allylation reactions. Angew. Chem. Int. Ed. 2003;42:4732–4739. doi: 10.1002/anie.200301632. [DOI] [PubMed] [Google Scholar]; Kennedy, J.W.J. and Hall, D.G.. (2003). Recent advances in the activation of boron and silicon reagents for stereocontrolled allylation reactions. Angew. Chem. Int. Ed. 42, 4732-4739. [DOI] [PubMed]
- Kitanosono T., Ollevier T., Kobayashi S. Iron- and bismuth-catalyzed asymmetric Mukaiyama aldol reactions in aqueous media. Chem. Asian J. 2013;8:3051–3062. doi: 10.1002/asia.201301149. [DOI] [PubMed] [Google Scholar]; Kitanosono, T., Ollevier, T. and Kobayashi, S.. (2013). Iron- and bismuth-catalyzed asymmetric Mukaiyama aldol reactions in aqueous media. Chem. Asian J. 8, 3051-3062. [DOI] [PubMed]
- Kobayashi S., Ogawa C. New entries to water-compatible Lewis acids. Chem. Eur. J. 2006;12:5954–5960. doi: 10.1002/chem.200600385. [DOI] [PubMed] [Google Scholar]; Kobayashi, S. and Ogawa, C.. (2006). New entries to water-compatible Lewis acids. Chem. Eur. J. 12, 5954-5960. [DOI] [PubMed]
- Kobayashi S., Ogino T., Shimizu H., Ishikawa S., Hamada T., Manabe K. Bismuth triflate-chiral bipyridine complexes as water-compatible chiral Lewis acids. Org. Lett. 2005;7:4729–4731. doi: 10.1021/ol051965w. [DOI] [PubMed] [Google Scholar]; Kobayashi, S., Ogino, T., Shimizu, H., Ishikawa, S., Hamada, T. and Manabe, K.. (2005). Bismuth triflate-chiral bipyridine complexes as water-compatible chiral Lewis acids. Org. Lett. 7, 4729-4731. [DOI] [PubMed]
- Koch F.M., Peters R. Catalytic enantio- and diastereoselective formation of β-sultones: ring-strained precursors for enantioenriched β-hydroxysulfonyl derivatives. Angew. Chem. Int. Ed. 2007;46:2685–2689. doi: 10.1002/anie.200604796. [DOI] [PubMed] [Google Scholar]; Koch, F.M. and Peters, R.. (2007). Catalytic enantio- and diastereoselective formation of β-sultones: ring-strained precursors for enantioenriched β-hydroxysulfonyl derivatives. Angew. Chem. Int. Ed. 46, 2685-2689. [DOI] [PubMed]
- Koch F.M., Peters R. Lewis acid/base catalyzed [2+2]-cycloaddition of sulfenes and aldehydes: a versatile entry to chiral sulfonyl and sulfinyl derivatives. Chem. Eur. J. 2011;17:3679–3692. doi: 10.1002/chem.201003542. [DOI] [PubMed] [Google Scholar]; Koch, F.M. and Peters, R.. (2011). Lewis acid/base catalyzed [2+2]-cycloaddition of sulfenes and aldehydes: a versatile entry to chiral sulfonyl and sulfinyl derivatives. Chem. Eur. J. 17, 3679-3692. [DOI] [PubMed]
- Kumar D., Vemula S.R., Balasubramanian N., Cook G.R. Indium-mediated stereoselective allylation. Acc. Chem. Res. 2016;49:2169–2178. doi: 10.1021/acs.accounts.6b00362. [DOI] [PubMed] [Google Scholar]; Kumar, D., Vemula, S.R., Balasubramanian, N. and Cook, G.R.. (2016). Indium-mediated stereoselective allylation. Acc. Chem. Res. 49, 2169-2178. [DOI] [PubMed]
- Lassauque N., Franciò G., Leitner W. Nickel-catalyzed asymmetric hydrovinylation using Lewis acid activation. Eur. J. Org. Chem. 2009;2009:3199–3202. [Google Scholar]; Lassauque, N., Francio, G. and Leitner, W.. (2009). Nickel-catalyzed asymmetric hydrovinylation using Lewis acid activation. Eur. J. Org. Chem. 2009, 3199-3202.
- Lathrop S.P., Pompeo M., Chang W.T., Movassaghi M. Convergent and biomimetic enantioselective total synthesis of (−) - Communesin F. J. Am. Chem. Soc. 2016;138:7763–7769. doi: 10.1021/jacs.6b04072. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lathrop, S.P., Pompeo, M., Chang, W.T. and Movassaghi, M.. (2016). Convergent and biomimetic enantioselective total synthesis of (−) - Communesin F. J. Am. Chem. Soc. 138, 7763-7769. [DOI] [PMC free article] [PubMed]
- Li Z., Plancq B., Ollevier T. Bismuth triflate-catalyzed asymmetric allylation of aromatic aldehydes. Chem. Eur. J. 2012;18:3144–3147. doi: 10.1002/chem.201103646. [DOI] [PubMed] [Google Scholar]; Li, Z., Plancq, B. and Ollevier, T.. (2012). Bismuth triflate-catalyzed asymmetric allylation of aromatic aldehydes. Chem. Eur. J. 18, 3144-3147. [DOI] [PubMed]
- Li G., Liang T., Wojtas L., Antilla J.C. An asymmetric Diels–Alder reaction catalyzed by chiral phosphate magnesium complexes: highly enantioselective synthesis of chiral spirooxindoles. Angew. Chem. Int. Ed. 2013;52:4628–4632. doi: 10.1002/anie.201209295. [DOI] [PubMed] [Google Scholar]; Li, G., Liang, T., Wojtas, L. and Antilla, J.C.. (2013). An asymmetric Diels-Alder reaction catalyzed by chiral phosphate magnesium complexes: highly enantioselective synthesis of chiral spirooxindoles. Angew. Chem. Int. Ed. 52, 4628 -4632. [DOI] [PubMed]
- Liu X., Lin L., Feng X. Chiral N, N’-dioxides: new ligands and organocatalysts for catalytic asymmetric reactions. Acc. Chem. Res. 2011;44:574–587. doi: 10.1021/ar200015s. [DOI] [PubMed] [Google Scholar]; Liu, X., Lin, L. and Feng, X.. (2011). Chiral N, N’-dioxides: new ligands and organocatalysts for catalytic asymmetric reactions. Acc. Chem. Res. 44, 574-587. [DOI] [PubMed]
- Liu X., Lin L., Feng X. Chiral N, N’-dioxide ligands: synthesis, coordination chemistry and asymmetric catalysis. Org. Chem. Front. 2014;1:298–302. [Google Scholar]; Liu, X., Lin, L. and Feng, X.. (2014). Chiral N, N’-dioxide ligands: synthesis, coordination chemistry and asymmetric catalysis. Org. Chem. Front. 1, 298-302.
- Lou S., Schaus S.E. Asymmetric petasis reactions catalyzed by chiral biphenols. J. Am. Chem. Soc. 2008;130:6922–6923. doi: 10.1021/ja8018934. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lou, S. and Schaus, S.E.. (2008). Asymmetric petasis reactions catalyzed by chiral biphenols. J. Am. Chem. Soc. 130, 6922-6923. [DOI] [PMC free article] [PubMed]
- Lou S., Moquist P.N., Schaus S.E. Asymmetric allylboration of ketones catalyzed by chiral diols. J. Am. Chem. Soc. 2006;128:12660–12661. doi: 10.1021/ja0651308. [DOI] [PubMed] [Google Scholar]; Lou, S., Moquist, P.N. and Schaus, S.E.. (2006). Asymmetric allylboration of ketones catalyzed by chiral diols. J. Am. Chem. Soc.. 128, 12660-12661. [DOI] [PubMed]
- Lou S., Moquist P.N., Schaus S.E. Asymmetric allylboration of acyl imines catalyzed by chiral diols. J. Am. Chem. Soc. 2007;129:15398–15404. doi: 10.1021/ja075204v. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lou, S., Moquist, P.N. and Schaus, S.E.. (2007). Asymmetric allylboration of acyl imines catalyzed by chiral diols. J. Am. Chem. Soc. 129, 15398-15404. [DOI] [PMC free article] [PubMed]
- Luo Y., Hepburn H.B., Chotsaeng N., Lam H.W. Enantioselective rhodium-catalyzed nucleophilic allylation of cyclic imines with allylboron reagents. Angew. Chem. Int. Ed. 2012;51:8309–8313. doi: 10.1002/anie.201204004. [DOI] [PubMed] [Google Scholar]; Luo, Y., Hepburn, H.B., Chotsaeng, N. and Lam, H.W.. (2012). Enantioselective rhodium-catalyzed nucleophilic allylation of cyclic imines with allylboron reagents. Angew. Chem. Int. Ed. 51, 8309-8313. [DOI] [PubMed]
- Lv J., Luo S. Asymmetric binary acid catalysis: chiral phosphoric acid as dual ligand and acid. Chem. Commun. 2013;49:847–858. doi: 10.1039/c2cc34288j. [DOI] [PubMed] [Google Scholar]; Lv, J. and Luo, S.. (2013). Asymmetric binary acid catalysis: chiral phosphoric acid as dual ligand and acid. Chem. Commun.. 49, 847-858. [DOI] [PubMed]
- Lv J., Zhang L., Zhou Y., Nie Z., Luo S., Cheng J.-P. Asymmetric binary acid catalysis: a regioselectivity switch between enantioselective 1, 2- and 1, 4- addition through different counteranions of InIII. Angew. Chem. Int. Ed. 2011;50:6610–6614. doi: 10.1002/anie.201101254. [DOI] [PubMed] [Google Scholar]; Lv, J., Zhang, L., Zhou, Y., Nie, Z., Luo, S. and Cheng, J.-P.. (2011). Asymmetric binary acid catalysis: a regioselectivity switch between enantioselective 1, 2- and 1, 4- addition through different counteranions of InIII. Angew. Chem. Int. Ed. 50, 6610-6614. [DOI] [PubMed]
- Lv J., Zhang L., Luo S., Cheng J.-P. Switchable diastereoselectivity in enantioselective [4+2] cycloadditions with simple olefins by asymmetric binary acid catalysis. Angew. Chem. Int. Ed. 2013;52:9786–9790. doi: 10.1002/anie.201304561. [DOI] [PubMed] [Google Scholar]; Lv, J., Zhang, L., Luo, S. and Cheng, J.-P.. (2013). Switchable diastereoselectivity in enantioselective [4+2] cycloadditions with simple olefins by asymmetric binary acid catalysis. Angew. Chem. Int. Ed. 52, 9786-9790. [DOI] [PubMed]
- Mahajan N., Koul S., Razdan T.K. Bismuth triflate- L(-)- proline catalyzed synthesis of chiral 2, 5- diaryl-2, 3- dihydropyrano[2, 3-b]quinolin- 4- ones. J. Heterocyclic Chem. 2011;48:1302–1307. [Google Scholar]; Mahajan, N., Koul, S. and Razdan, T.K.. (2011). Bismuth triflate- L(-)- proline catalyzed synthesis of chiral 2, 5- diaryl-2, 3- dihydropyrano[2, 3-b]quinolin- 4- ones. J. Heterocyclic Chem. 48, 1302-1307.
- Mlynarski J. Wiley-VCH; 2017. Chiral Lewis Acids in Organic Synthesis. [Google Scholar]; Mlynarski, J.. (2017). Chiral Lewis Acids in Organic Synthesis (Wiley-VCH, Weinheim, Germany).
- Nakamura S., Hyodo K., Nakamura M., Nakane D., Masuda H. Catalytic enantioselective allylation of ketimines by using palladium pincer complexes with chiral bis(imidazoline)s. Chem. Eur. J. 2013;19:7304–7309. doi: 10.1002/chem.201300685. [DOI] [PubMed] [Google Scholar]; Nakamura, S., Hyodo, K., Nakamura, M., Nakane, D. and Masuda, H.. (2013). Catalytic enantioselective allylation of ketimines by using palladium pincer complexes with chiral bis(imidazoline)s. Chem. Eur. J. 19, 7304-7309. [DOI] [PubMed]
- Ollevier T. New trends in bismuth-catalyzed synthetic transformations. Org. Biomol. Chem. 2013;11:2740–2755. doi: 10.1039/c3ob26537d. [DOI] [PubMed] [Google Scholar]; Ollevier, T.. (2013). New trends in bismuth-catalyzed synthetic transformations. Org. Biomol. Chem. 11, 2740-2755. [DOI] [PubMed]
- Ondet P., Lemière G., Duñach E. Cyclisations catalysed by bismuth(III) triflate. Eur. J. Org. Chem. 2017;2017:761–780. [Google Scholar]; Ondet, P., Lemiere, G. and Duñach, E.. (2017). Cyclisations catalysed by bismuth(III) triflate. Eur. J. Org. Chem. 2017 761-780.
- Salvador J.A.R., Figueiredo S.A.C., Pinto R.M.A., Silvestre S.M. Bismuth compounds in medicinal chemistry. Future Med. Chem. 2012;4:1495–1523. doi: 10.4155/fmc.12.95. [DOI] [PubMed] [Google Scholar]; Salvador, J.A.R., Figueiredo, S.A.C., Pinto, R.M.A. and Silvestre, S.M.. (2012). Bismuth compounds in medicinal chemistry. Future Med. Chem. 4, 1495-1523. [DOI] [PubMed]
- Sato S., Shibuya M., Kanoh N., Iwabuchi Y. An expedient route to a potent gastrin/CCK-B receptor antagonist (+)-AG-041R. J. Org. Chem. 2009;74:7522–7524. doi: 10.1021/jo901352u. [DOI] [PubMed] [Google Scholar]; Sato, S., Shibuya, M., Kanoh, N. and Iwabuchi, Y.. (2009). An expedient route to a potent gastrin/CCK-B receptor antagonist (+)-AG-041R. J. Org. Chem. 74, 7522-7524. [DOI] [PubMed]
- Silverio D.L., Torker S., Pilyugina T., Vieira E.M., Snapper M.L., Haeffner F., Hoveyda A.H. Simple organic molecules as catalysts for enantioselective synthesis of amines and alcohols. Nature. 2013;494:216–221. doi: 10.1038/nature11844. [DOI] [PMC free article] [PubMed] [Google Scholar]; Silverio, D.L., Torker, S., Pilyugina, T., Vieira, E.M., Snapper, M.L., Haeffner, F. and Hoveyda, A.H.. (2013). Simple organic molecules as catalysts for enantioselective synthesis of amines and alcohols. Nature. 494, 216-221. [DOI] [PMC free article] [PubMed]
- Singh G.S., Desta Z.Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012;112:6104–6155. doi: 10.1021/cr300135y. [DOI] [PubMed] [Google Scholar]; Singh, G.S. and Desta, Z.Y.. (2012). Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 112, 6104 -6155. [DOI] [PubMed]
- Sirasani G., Andrade R.B. Total synthesis of (−) - leuconicine a and B. Org. Lett. 2011;13:4736–4737. doi: 10.1021/ol202056w. [DOI] [PubMed] [Google Scholar]; Sirasani, G. and Andrade, R.B.. (2011). Total synthesis of (−) - leuconicine a and B. Org. Lett. 13, 4736-4737. [DOI] [PubMed]
- Tan Q., Wang X., Xiong Y., Zhao Z., Li L., Tang P., Zhang M. Chiral amino alcohol accelerated and stereocontrolled allylboration of iminoisatins: highly efficient construction of adjacent quaternary stereogenic centers. Angew. Chem. Int. Ed. 2017;56:4829–4833. doi: 10.1002/anie.201700581. [DOI] [PubMed] [Google Scholar]; Tan, Q., Wang, X., Xiong, Y., Zhao, Z., Li, L., Tang, P. and Zhang, M.. (2017). Chiral amino alcohol accelerated and stereocontrolled allylboration of iminoisatins: highly efficient construction of adjacent quaternary stereogenic centers. Angew. Chem. Int. Ed. 56, 4829-4833. [DOI] [PubMed]
- Wada M., Takahashi T., Domae T., Fukuma T., Miyoshi N., Smith K. Asymmetric trimethylsilylcyanation of aldehydes utilizing chiral bismuth compounds. A frontier in bismuth mediated synthetic reactions. Tetrahedron Asymmetry. 1997;8:3939–3946. [Google Scholar]; Wada, M., Takahashi, T., Domae, T., Fukuma, T., Miyoshi, N. and Smith, K.. (1997). Asymmetric trimethylsilylcyanation of aldehydes utilizing chiral bismuth compounds. A frontier in bismuth mediated synthetic reactions. Tetrahedron Asymmetry. 8, 3939-3946.
- Wada R., Shibuguchi T., Makino S., Oisaki K., Kanai M., Shibasaki M. Catalytic enantioselective allylation of ketoimines. J. Am. Chem. Soc. 2006;128:7687–7691. doi: 10.1021/ja061510h. [DOI] [PubMed] [Google Scholar]; Wada, R., Shibuguchi, T., Makino, S., Oisaki, K., Kanai, M. and Shibasaki, M.. (2006). Catalytic enantioselective allylation of ketoimines. J. Am. Chem. Soc. 128, 7687-7691. [DOI] [PubMed]
- Wan L., Tian L., Liu J., Niu D. Iridium-catalyzed asymmetric umpolung allylation of N-fluorenyl imines to prepare 1, 4-disubstituted homoallylic amines. Synlett. 2017;28:2051–2056. [Google Scholar]; Wan, L., Tian, L., Liu, J. and Niu, D.. (2017). Iridium-catalyzed asymmetric umpolung allylation of N-fluorenyl imines to prepare 1, 4-disubstituted homoallylic amines. Synlett. 28, 2051-2056.
- Wang L., Lv L., Zhang J., Luo S. Catalytic regio- and enantioselective [4+2] annulation reactions of non-activated allenes by a chiral cationic indium complex. Angew. Chem. Int. Ed. 2017;56:10867–10871. doi: 10.1002/anie.201704020. [DOI] [PubMed] [Google Scholar]; Wang, L., Lv, L., Zhang, J. and Luo, S.. (2017). Catalytic regio- and enantioselective [4+2] annulation reactions of non-activated allenes by a chiral cationic indium complex. Angew. Chem. Int. Ed. 56, 10867-10871. [DOI] [PubMed]
- Wu H., Haeffner F., Hoveyda A.H. An efficient, practical, and enantioselective method for synthesis of homoallenylamides catalyzed by an aminoalcohol-derived, boron-based catalyst. J. Am. Chem. Soc. 2014;136:3780–3783. doi: 10.1021/ja500374p. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wu, H., Haeffner, F. and Hoveyda, A.H.. (2014). An efficient, practical, and enantioselective method for synthesis of homoallenylamides catalyzed by an aminoalcohol-derived, boron-based catalyst. J. Am. Chem. Soc. 136, 3780-3783. [DOI] [PMC free article] [PubMed]
- Wu L., Shao Q., Yang G., Zhang W. Cobalt-catalyzed asymmetric allylation of cyclic ketimines. Chem. Eur. J. 2018;24:1241–1245. doi: 10.1002/chem.201704760. [DOI] [PubMed] [Google Scholar]; Wu, L., Shao, Q., Yang, G. and Zhang, W.. (2018). Cobalt-catalyzed asymmetric allylation of cyclic ketimines. Chem. Eur. J. 24, 1241-1245. [DOI] [PubMed]
- Yamamoto H. Wiley-VCH; 2000. Lewis Acids in Organic Synthesis. [Google Scholar]; Yamamoto, H.. (2000). Lewis Acids in Organic Synthesis (Wiley-VCH, Weinheim, Germany).
- Yamamoto H., Futatsugi K. “Designer acids”: combined acid catalysis for asymmetric synthesis. Angew. Chem. Int. Ed. 2005;44:1924–1942. doi: 10.1002/anie.200460394. [DOI] [PubMed] [Google Scholar]; Yamamoto, H. and Futatsugi, K.. (2005). “Designer acids”: combined acid catalysis for asymmetric synthesis. Angew. Chem. Int. Ed. 44, 1924-1942. [DOI] [PubMed]
- Yamamoto H., Ishihara K. Wiley-VCH; 2008. Acid Catalysis in Modern Organic Synthesis. [Google Scholar]; Yamamoto, H. and Ishihara, K.. (2008). Acid Catalysis in Modern Organic Synthesis (Wiley-VCH, Weinheim, Germany).
- Yus M., González-Gómez J.C., Foubelo F. Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 2011;111:7774–7854. doi: 10.1021/cr1004474. [DOI] [PubMed] [Google Scholar]; Yus, M., Gonzalez-Gomez, J.C. and Foubelo, F.. (2011). Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 111, 7774-7854. [DOI] [PubMed]
- Zhang Z., Zheng W., Antilla J.C. Highly enantioselective catalytic benzoyloxylation of 3-aryloxindoles using chiral VAPOL calcium phosphate. Angew. Chem. Int. Ed. 2011;50:1135–1138. doi: 10.1002/anie.201006595. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang, Z., Zheng, W. and Antilla, J.C.. (2011). Highly enantioselective catalytic benzoyloxylation of 3-aryloxindoles using chiral VAPOL calcium phosphate. Angew. Chem. Int. Ed. 50, 1135 -1138. [DOI] [PMC free article] [PubMed]
- Zhang L., Zhang J., Ma J., Cheng D.-J., Tan B. Highly atroposelective synthesis of arylpyrroles by catalytic asymmetric Paal-Knorr reaction. J. Am. Chem. Soc. 2017;139:1714–1717. doi: 10.1021/jacs.6b09634. [DOI] [PubMed] [Google Scholar]; Zhang, L., Zhang, J., Ma, J., Cheng, D.-J. and Tan, B.. (2017a). Highly atroposelective synthesis of arylpyrroles by catalytic asymmetric Paal-Knorr reaction. J. Am. Chem. Soc. 139, 1714-1717. [DOI] [PubMed]
- Zhang Z.-P., Dong N., Li X. Bismuth-catalyzed allylation of para-quinone methides. Chem. Commun. (Camb.) 2017;53:1301–1304. doi: 10.1039/c6cc06605d. [DOI] [PubMed] [Google Scholar]; Zhang, Z.-P., Dong, N. and Li, X.. (2017b). Bismuth-catalyzed allylation of para-quinone methides. Chem. Commun. (Camb.) 53, 1301-1304. [DOI] [PubMed]
- Zheng W., Zhang Z., Kaplan M.J., Antilla J.C. Chiral calcium VAPOL phosphate mediated asymmetric chlorination and Michael reactions of 3-substituted oxindoles. J. Am. Chem. Soc. 2011;133:3339–3341. doi: 10.1021/ja109824x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zheng, W., Zhang, Z., Kaplan, M.J. and Antilla, J.C.. (2011). Chiral calcium VAPOL phosphate mediated asymmetric chlorination and Michael reactions of 3-substituted oxindoles. J. Am. Chem. Soc. 133, 3339-3341. [DOI] [PMC free article] [PubMed]
- Zhou F., Liu Y.-L., Zhou J. Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal. 2010;352:1381–1407. [Google Scholar]; Zhou, F., Liu, Y.-L. and Zhou, J.. (2010). Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal. 352, 1381 -1407.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









