Abstract
Aim
To examine the feasibility of an individual, supervised, structured moderate-to-high intensity cycle ergometer exercise training immediately before radiotherapy in patients undergoing concomitant chemoradiotherapy for locally advanced non-small cell lung cancer (NSCLC).
Background
Lung cancer is the most common form of cancer. Despite significant advancements in therapy and supportive care it is still the leading cause of cancer-related death worldwide.
Materials and methods
Randomized controlled study design; patients with NSCLC receiving concomitant chemoradiotherapy were recruited and randomly assigned to either the exercise (EXE) or the control (CON) group. Exercise training consisted of 20 min moderate-to-high intensity aerobic interval training 5 times per week (Mon–Fri) prior to radiotherapy. Secondary outcomes were assessed at baseline and after 7 weeks: peak oxygen consumption (VO2peak), functional capacity (6MWD), pulmonary function (FEV1), psychosocial parameters (quality of life (FACT-L), anxiety and depression (HADS)) and cancer-related side effects (reported daily).
Results
Fifteen patients were included. All patients completed a baseline test, while 13 patients were eligible for a posttest. The recruiting rate was 44.1% and the overall attendance rate to exercise was 90.0% with an adherence rate to full exercise participation of 88.1%. No adverse events or any unexpected reactions were observed during the exercise sessions. No significant differences were observed within or between groups from baseline to post intervention in any of the secondary outcomes.
Conclusion
This study demonstrated ‘proof of principle’ that daily moderate-to-high intensity cycle ergometer exercise was feasible, safe and well tolerated among newly diagnosed patients with locally advanced NSCLC undergoing concomitant chemoradiotherapy. Larger randomized controlled trials are warranted.
Keywords: Chemoradiotherapy, Non-small cell lung cancer, Cancer-related-fatigue, VO2peak, 6MWD, Quality of Life
1. Background
Lung cancer is the most common form of cancer and the leading cause of cancer-related mortality worldwide.1, 2 Non-small cell lung Cancer (NSCLC) represents approximately 80–85% of all lung cancer cases and about 75% of these patients are inoperable.2, 3 Patients with locally advanced NSCLC (stage III) are treated with chemoradiotherapy (CRT) with a curative intent. Despite significant advancements in irradiation techniques, many patients experience local or metastatic progression and the 5-year survival rate in the most promising of studies does not exceed 40%.4 On top of this, patients with NSCLC are found to have a significant increase of treatment related toxic side effects during CRT compared to chemotherapy or radiotherapy alone.5
Exercise intervention studies have shown that physical activity can safely improve physical and psychological conditions as well as reduce cancer-related side effects in cancer patients with NSCLC.6, 7, 8, 9, 10, 11 However, most of these studies include patients with earlier stage of disease (NSCLC I–III) with resectable lung cancer or patients only undergoing chemotherapy. Inactivity is a significant issue in lung cancer and development of new strategies to enhance physical activity in clinical practice is necessary. Radiotherapy is delivered in daily fractions (5 fractions per week) for 6–7 weeks. Patients report that “lack of time” and “medical appointments” is a barrier to enhance physical activity during treatment.12 No study has yet investigated the implementation of aerobic exercise training prior to radiotherapy in patients with NSCLC receiving concomitant chemoradiotherapy and it has been questioned whether it was feasible.
Accordingly, we conducted a randomized, controlled feasibility study. This study examined the feasibility of 20 min daily, individual, supervised, structured moderate-to-high intensity cycle ergometer exercise immediately before radiotherapy in patients undergoing concomitant chemoradiotherapy for locally advanced NSCLC.
2. Aim
The primary aim was to check feasibility and the secondary aim was to examine the effect of intervention on cardiopulmonary endpoints, quality of life (QoL), anxiety, depression and cancer-related side effects.
3. Materials and methods
From March 2017 to January 2018 we enrolled patients with locally advanced NSCLC referred to concomitant chemoradiotherapy. The study design was a randomized controlled feasibility study, randomizing to standard care or standard care and exercise. Inclusion criteria were referral for concomitant chemoradiotherapy of 66 Gy in 33 fractions, age >18 years, WHO performance status 0–1 and signed informed consent. Exclusion criteria were: patients with any symptoms or circumstances that advise against physical activity; symptomatic heart disease, e.g. arrhythmia or myocardial infarction within the last three months; and patients who did not read or speak Danish. Patients were randomized after baseline testing in a 1:1 manner to either the exercise group (EXE) or the control group (CON). Randomization was stratified by gender in blocks of eight and the randomization sequence was generated by a computer program and put into sealed opaque envelopes made by an outside party. The study was approved by the Danish Data Protection Agency (Case no: 2012-58-0004) and by the Regional Ethics Committee for the Capital Region (Case no: H-16048479). The study is registered at clinicaltrails.gov (NCT03066271) and all data is stored in research electronic data capture (RedCap).
At baseline, information about demographic and health history was recorded from the patient's medical records and a socioeconomic questionnaire. Information about depression and anxiety (HADS), QoL (FACT-L) and activity level (IPAQ-L) was collected by questionnaires. Baseline testing consisted of measurements of aerobic capacity , functional capacity (6-min walk distance (6-MWD) test) and lung function (FEV1). Before each physical testing, vital parameters were measured and if one of the following criteria was met, the patient was prohibited from testing that day. The criteria were diastolic blood pressure <45 or >95, heart rate (HR) at rest >115/min, temperature >38 °C, respiratory rate at rest >30/min, infection requiring treatment.
3.1. Intervention group – standard care and exercise
The exercise intervention was carried out as daily (Mon–Fri), supervised, individual, structured exercise training, performed on an ergometer cycle (Monark LC4) before the patient's radiotherapy fraction. The intervention period equaled the patients’ individual number of radiotherapy sessions over a 7-week period and the exercise training was carried out just next to the accelerator. Each session lasted 20 min and was supervised by an exercise physiologist or a physiotherapist. The exercise training comprised a 5-min warm-up phase followed by three 5-min exercise phases. Warm-up consisted of light stationary cycling, individually adjusted to 50–60% of the patient's peak power output determined at the incremental cycle test (iPPO). The first and the third exercise phase comprised of interval training consisting of 5 × 30-s intervals at 80–95% of iPPO, with each interval separated by a 30-s pause. The second exercise phase consisted of continuous cycling at an intensity equaling 80% of the patient's iPPO. Over the 7 weeks, the intensities were increased progressively from 50%, 80%, 70% and 80% of iPPO to 60%, 95%, 80% and 95% of iPPO according to the four phases. All patients wore HR monitors during the exercise sessions. HR, Watt (W) and revolutions per minute (RPM) were monitored using Monark Test Software. At each session all patients rated their motivation to exercise, perceived exertion following exercise and dyspnea at a visual analogue scale (VAS). After the exercise session patients rated their self-perceived exertion perception (RPE) using the modified Borg scale. In addition, the patients were equipped with a Garmin® vívosmart® HR activity tracker every day, 24 h, during the course of radiotherapy and were asked to rate their daily degree of side effects on a record sheet handed out weekly.
3.2. Control group – standard care
Patients who were randomized to CON received no exercise training. The patients wore a Garmin® vívosmart® HR activity tracker every day and rated their daily degree of side effects on a record sheet handed out weekly as did the patients in EXE. Data from the activity tracker and the filled-out record sheets were collected every week to ensure participation in CON.
3.3. Aerobic capacity
To determine , an incremental stationary ergometer cycle test was performed. All tests were performed on a Monarch 839 E ergometer cycle and gas analyses were performed by breath-by-breath system and calculated as an average over 15 s using the Oxygen Pro, Jaeger measurement system. The test started with a 4-min warm-up with a burden of 20–80 Watt (W), after which the workload was increased with 5–15 W/min until volitional exhaustion or until a symptom-limitation was achieved. During the test, HR (FT1 polar) was continuously monitored. After each test, maximal HR, length of the test (minutes and seconds) was noted to calculate iPPO. In the final seconds of the test, RPE was evaluated using the modified Borg Scale. The testing protocol was conducted in accordance with protocols from previous studies and guidelines from the American Thoracic Society and the American College of Chest Physicians.13, 14
3.4. Physiological assessments
Functional capacity was measured by a 6-min walk distance test and the test was performed according to the protocol of the American Thoracic Society.15 To determine lung function forced expiratory volume in 1 s (FEV1) was measured in a standing position using spirometry of the Oxygen Pro, Jaeger Measurements System according to the ATS and ERS guidelines.16 To assess well-being, the Hospital Anxiety and Depression Scale (HADS) was administered. QoL was assessed using the Functional Assessment of Cancer Therapy-Lung (FACT-L), which contains 36 items grouped into five different categories. Questionnaires were coded according to questionnaire manuals.17, 18, 19, 20
3.5. Activity and side effects
During the course of CRT, daily activity and side effects were monitored. Activity data (steps, distance, and intensity minutes) was monitored by a Garmin® vívosmart® HR activity tracker and collected by the software Garmin connect. Steps were counted by a three-axis accelerometer and distance was calculated by a standardized step length (0.7 m) multiplied by steps. The activity tracker calculates intensity minutes by comparing HR data to the average resting HR. Side effects (appetite, nausea, vomit, diarrhea, constipation, arthralgia, myalgia, other pains, physical fatigue, mental fatigue, and treatment-induced fatigue) were rated on a 0–4 scale and noted daily on a paper handed out weekly during the course of radiotherapy.
3.6. Statistical analysis
All statistical analyses were performed using the statistical program R. Differences in physiological and psychosocial outcomes between EXE and CON were evaluated using analysis of covariance (ANCOVA) of posttest measurements with baseline values as covariate including data from only the patients who completed both the baseline- and posttests. Differences in side effects and activity level were tested using an unpaired t-test. All statistical tests used a significance level of 5% (p value <0.05). Due to the perspective of this study all statistical analyses were exploratory. All values are expressed as mean ± standard deviation (SD) unless otherwise mentioned.
3.7. Sample size
The sample size calculation was based on an expected change in (L O2/min) and was based on earlier data, where 30 patients who participated in a 20-week exercise intervention achieved an increase in of 0.380 L O2/min, assuming a SD of 0.49 L O2/min.6 The sample size calculation was based on an unpaired t-test with following assumptions: Type-I error: 0.05; type-II error: 0.20 which gave a power of 80%; MIREDIF: 0.380 L O2/min; SD: 0.49 L O2/min. The calculation gave a sample size of 28 in each group. We expected a dropout rate of 40% during the intervention; therefore, 80 patients (40 in each group (=1.40 × 28)) was aimed to obtain the required number of patients.
3.8. Feasibility
Feasibility was measured by the recruiting rate, retention rate, attendance, adherence and safety. The study was defined as successful if the patients completed the prescribed exercise for at least two-thirds of the time19 and if there were no differences in harms between CON and EXE. Attendance is calculated as a percentage and is equal to the total number of exercise sessions attended divided by the total number of exercise sessions prescribed.21 Adherence to prescribed exercise session is equal to exercise sessions performed by a full exercise program divided by session attended and is calculated as percentage.
4. Results
4.1. Patient flow
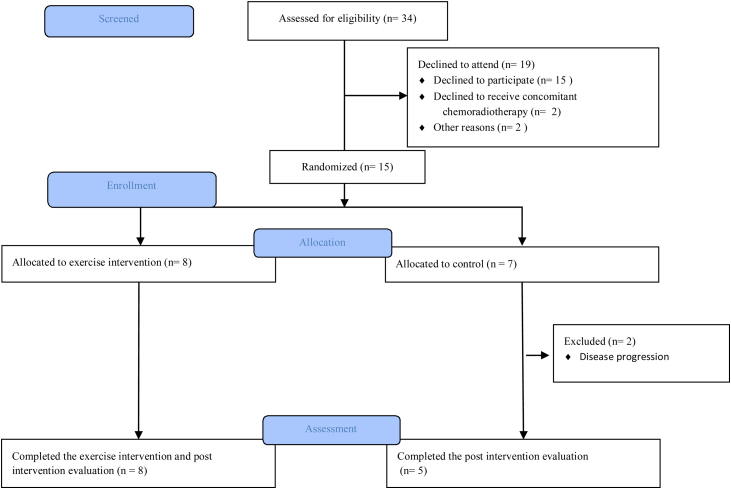
From March 2017 to January 2018, 34 patients with locally advanced NSCLC were screened for eligibility (Fig. 1). Of these, 15 (44.1%) patients signed informed consent and were included (ten females, five males). All patients completed baseline test and 8 patients were randomized to EXE and 7 patients to CON. Baseline testing was carried out 21 days before to five days after the first fraction of radiotherapy. Two patients were excluded after baseline testing but before the intervention due to disease progression, both were randomized to CON. There were no dropouts during the intervention. Thirteen patients were eligible for posttest. All 13 patients completed posttest which was carried out zero to nine days after the last radiation fraction. Slow inclusion rate was due to a reduced flow of patients and for time reasons: the inclusion period terminated before the intended number of patients was included. Baseline characteristics for all included patients are shown in Table 1.
Fig. 1.
Study flow shown. Overview of eligibility, randomization and follow-up in the study.
Table 1.
Characteristics of included patients at baseline (n = 15).
| Variables | Exercise (n = 8) | Control (n = 7) |
|---|---|---|
| Age, years | 64 ± 5.8 | 65 ± 4.7 |
| Female sex, n | 5 (62.5%) | 5 (71.4%) |
| Body mass index, kg/m2 | 24.1 ± 4.4 | 24.2 ± 1.9 |
| Smocking history | ||
| Never | 1 (12.5%) | 0 (0.0%) |
| Former | 5 (62.5%) | 4 (57.1%) |
| Current | 2 (25.0%) | 3 (42.9%) |
| Pack-years | 35 ± 19.9 | 38 ± 13.8 |
| Level of education | ||
| 10th grade or less | 2 (25.0%) | 2 (28.6%) |
| Vocational education | 2 (25.0%) | 2 (28.6%) |
| Further education (<3 years) | 2 (25.0%) | 0 (0.0%) |
| Higher education (3–4 years) | 2 (25.0%) | 1 (14.3%) |
| Master degree (5 years or more) | 0 (0.0%) | 2 (28.6%) |
| Stage | ||
| IIIa | 3 (37.5%) | 2 (28.6%) |
| IIIb | 4 (50.0%) | 4 (57.1%) |
| IV | 1 (12.5%) | 1 (14.3%) |
| Pathology | ||
| Adenocarcinoma | 5 (62.5%) | 4 (57.1%) |
| Squamous carcinoma | 3 (37.5%) | 3 (42.9%) |
| Cytostatica | ||
| Cisplatin + vinorelbin | 6 (75.0%) | 6 (85.7%) |
| Carboplatin + vinorelbin | 2 (25.0%) | |
| Pulmonary function | ||
| Predicted FEV1, L | 2.2 ± 0.74 | 2.3 ± 0.65 |
| Predicted FEV1, % | 86.4 ± 26.8 | 98.6 ± 23.8 |
| Physiological measurements | ||
| , ml O2/min | 1.328 ± 0.458 | 1.460 ± 0.431 |
| , ml O2/kg/min | 20 ± 3.6 | 22 ± 6.2 |
| iPPO, W | 90 ± 29 | 101 ± 57 |
| 6MWD, m | 437 ± 126 | 515 ± 124 |
| Systolic blood pressure, mmHg | 138 ± 21 | 131 ± 16 |
| Diastolic blood pressure, mmHg | 85 ± 16 | 80 ± 9 |
| HRmax, beats/min | 153 ± 18 | 144 ± 22 |
| HRrest, beats/min | 91 ± 17 | 72 ± 9 |
Data are presented as mean ± SD or n (%). Only 7 patients are included in HRmax for EXE. Six patients are included in cytostatica for CON. FEV1: forced expired volume in 1 s; : maximal oxygen uptake; iPPO: incremental peak power output; 6MWD: 6 min walk distance; HR: heart rate.
4.2. Feasibility
The exercise intervention comprised 31 (range: 26–33 sessions) prescribed exercise sessions over the 7-week period. The overall attendance rate to exercise was 90.0% (range: 53.8–100.0%). Of the 90.0% attendance, the adherence rate to full exercise participation was 88.1% (range: 70.0–100.0%) and 11.9% (range: 0.0–30.0%) was performed by a modified program due to early exhaustion, pause during the exercise session or practical reasons as earlier start of radiotherapy on a given day. The exercise intensity was 52.5 ± 4.7%, 77.6 ± 6.7%, 64.5 ± 7.3% and 77.3 ± 7.3% of iPPO during the four phases of the exercise sessions, corresponding to 74.7 ± 10.6%, 84.3 ± 7.4%, 87.0 ± 7.6% and 90.7 ± 6.2% of HRmax with a mean RPE of 14 ± 1.5.
4.3. Safety
Two patients were hospitalized during the course due to chemotherapy adverse events. No adverse events or any unexpected reactions were observed during the exercise sessions.
4.4. Physiological endpoints
Changes in cardiopulmonary endpoints are shown in Table 2. The explorative statistical analyses included 8 patients in EXE, except the analysis for iPPO (n = 7). Five patients were included for analyses in CON. No significant differences were observed within or between groups from baseline to post intervention in any cardiopulmonary endpoints.
Table 2.
Between-group differences between baseline and after intervention for physiological outcomes.
| Variable | Exercise |
Control |
Covariance analysis difference between groups |
|||
|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | Mean (95%-CI) | P value | |
| Pulmonary function | ||||||
| FEV1, L | 2.2 ± 0.7 | 2.0 ± 0.6 | 2.6 ± 0.7 | 2.6 ± 0.6 | 0.31 (−0.07 to 0.70) | 0.101 |
| FEV1, % of Predicted | 86.4 ± 26.8 | 80.1 ± 25.0 | 97.1 ± 24.2 | 100.0 ± 0.6 | 11.59 (−3.13 to 26.32) | 0.110 |
| Physiological measurements | ||||||
| , ml O2/min | 1.33 ± 0.46 | 1.25 ± 0.30 | 1.68 ± 0.27 | 1.65 ± 0.27 | 0.21 (−0.08 to 0.51) | 0.142 |
| , ml O2/kg/min | 19.5 ± 3.6 | 18.7 ± 2.8 | 24.5 ± 5.2 | 23.8 ± 6.6 | 1.26 (−3.68 to 6.20) | 0.583 |
| iPPO, W | 90 ± 29 | 93 ± 21 | 130 ± 35 | 132 ± 36 | 7.10 (−14.62 to 28.83) | 0.478 |
| 6MWD, m | 437 ± 126 | 470 ± 82 | 574 ± 89 | 577 ± 67 | 18.41 (−15.51 to 52.34) | 2.543 |
| Systolic BP, mmHg | 138 ± 21 | 134 ± 28 | 130 ± 18 | 127 ± 17 | −0.84 (−23.48 to 21.80) | 0.936 |
| Diastolic BP, mmHg | 85 ± 16 | 83 ± 9 | 83 ± 6.6 | 80 ± 6 | −2.61 (−11.84 to 6.62) | 0.543 |
| HRmax, beats/min | 153 ± 18 | 154 ± 20 | 153 ± 18 | 157 ± 12 | 2.52 (−15.49 to 20.52) | 0.759 |
| HRrest, beats/min | 91 ± 17 | 90 ± 20 | 72 ± 10 | 74 ± 15 | 0.65 (−12.30 to 18.96) | 0.646 |
Data are presented as mean ± SD. In data for HRmax only 7 patients are included in EXE. FEV1: forced expired volume in 1 s; : maximal oxygen uptake; iPPO: incremental peak power output; 6MWD: 6 min walk distance; HR: heart rate; CI: confidence interval.
4.5. Patients reported outcome
Analyses for QoL were made with 7 patients in EXE and 5 patients in CON. The QoL results are shown in Table 3. There were no significant changes from baseline to post in EXE or CON. Furthermore, there were no significant differences between groups for any of the parameters in FACT-L. Results from the HADS Scale showed no significant within or between group differences from baseline to post (Table 3). Numbers of patients included for analyses were as described for QoL.
Table 3.
Between-group differences between baseline and after intervention for FACT-L and HADS.
| Variable | Baseline |
Post |
Covariance analysis difference between groups |
|||
|---|---|---|---|---|---|---|
| Exercise | Control | Exercise | Control | Mean (95%-CI) | P value | |
| FACT-L | ||||||
| Physical well-being | 21.4 ± 6.4 | 19.2 ± 8.3 | 20.9 ± 5.6 | 22.0 ± 4.3 | 2.20 (−3.21 to 7.61) | 0.382 |
| Social well-being | 22.4 ± 6.4 | 25.3 ± 2.3 | 23.3 ± 5.8 | 24.2 ± 3.8 | −1.50 (−5.07 to 2.08) | 0.369 |
| Emotional well-being | 17.7 ± 4.5 | 18.4 ± 3.1 | 18.9 ± 3.8 | 20.2 ± 3.1 | 1.09 (−3.38 to 5.55) | 0.596 |
| Functional well-being | 17.6 ± 5.4 | 17.4 ± 7.1 | 18.0 ± 5.5 | 17.4 ± 4.7 | −0.52 (−6.85 to 5.79) | 0.854 |
| Lung cancer subscale | 17.4 ± 4.5 | 19.4 ± 3.4 | 18.0 ± 6.7 | 22.8 ± 2.3 | 4.62 8 (−3.14 to 12.38) | 0.211 |
| Trial outcome index | 56.4 ± 12.9 | 56.0 ± 17.2 | 56.9 ± 14.7 | 62.2 ± 10.4 | 5.49 (−11.47 to 22.46) | 0.483 |
| FACT-General | 79.1 ± 12.7 | 80.2 ± 16.4 | 81.0 ± 12.5 | 83.8 ± 12.9 | 2.98 (−12.61 to 17.20) | 0.735 |
| FACT-Lung | 96.6 ± 14.8 | 99.6 ± 19.0 | 99.0 ± 16.2 | 106.6 ± 14.3 | 6.69 (−13.83 to 27.20) | 0.480 |
| HADS | ||||||
| Anxiety | 6.5 ± 4.7 | 5.6 ± 1.5 | 4.1 ± 3.1 | 4.4 ± 3.4 | 0.33 (−2.99 to 3.64) | 0.829 |
| Depression | 4.6 ± 5.4 | 5.4 ± 4.9 | 3.0 ± 1.4 | 4.8 ± 3.4 | 0.64 (−2.62 to 3.91) | 0.667 |
Data are presented as mean ± SD. Data only included for 7 patients in EXE. Higher scores in FACT-L indicate higher QoL. In HADS higher scores indicate a great extent of anxiety and depression. FACT-L: Functional Assessment of Cancer Therapy-Lung; HADS: Hospital Anxiety and Depression Scale; CI: confidence interval.
4.6. Activity and side effects during the intervention
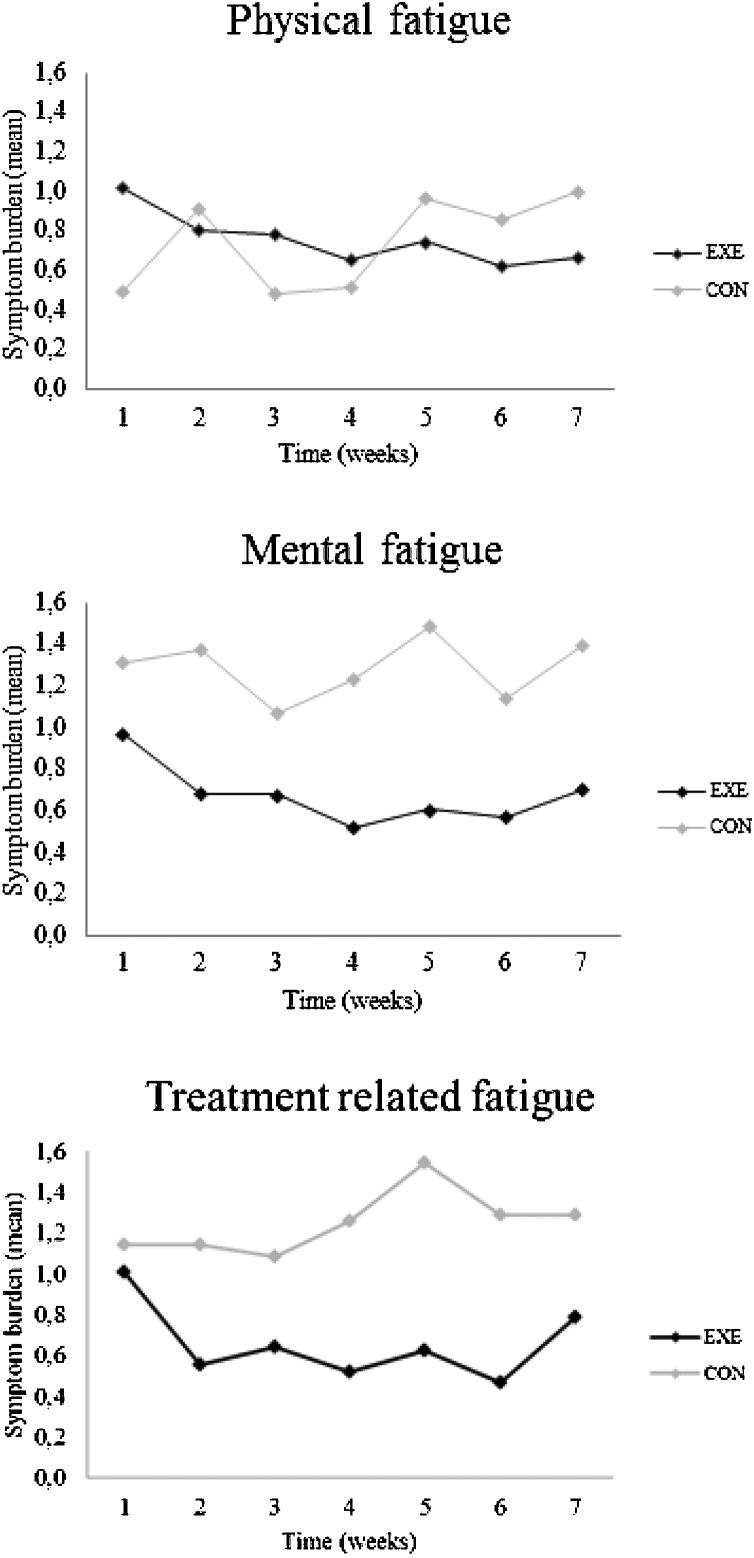
Activity data was collected during a period of 39–51 days. Data derived from the Garmin® activity tracker showed no significant differences between EXE and CON in steps, distance or intensity minutes (Table 4). Statistical analyses were made with 8 patients in EXE and five in CON. Data was included for all 13 patients who completed the intervention. Fig. 2 shows a tendency to lower mental and treatment-related fatigue burden in EXE compared to CON but the results did not reach statistical significance. Data is only shown for fatigue.
Table 4.
Between-group differences in activity data collected during the intervention.
| Variable | Exercise | Control | Mean diff. (95%-CI) | P value |
|---|---|---|---|---|
| Steps (day) | 6254 ± 2337 | 7572 ± 2445 | 1318 (−4458 to 1821) | 0.3632 |
| Distance (day) | 4.71 ± 1.83 | 6.06 ± 3.33 | 1.35 (−4.241 to 1.546) | 0.3081 |
| Intensity minutes (day) | 28 ± 27 | 19 ± 17 | −9.28 (−17.522 to 36.072) | 0.462 |
Data are represented as mean ± SD. Data collected during the intervention period.
Fig. 2.
Changes in patient reported side effects during the intervention period: (A) physical fatigue, (B) mental fatigue and (C) treatment related fatigue.
5. Discussion
This is the first study to test whether daily individual, supervised, structured exercise training is feasible and safe for patients with locally advanced NSCLC while undergoing concomitant chemoradiotherapy. The principal finding of this study is that 7 weeks of 20-min daily moderate-to-high intensity cycle ergometer exercise is feasible, safe and well tolerated during concomitant chemoradiotherapy in patients with locally advanced NSCLC.
The recruiting rate in this study (44.1%) was similar to earlier studies including patients with NSCLC. Kuehr et al.22 had a recruiting rate of 49.4% (including stages IIa–IV), and Jones et al.8 reported a recruiting rate of 50.0% (including stages I–IIIB). In contrast to these studies, Quist et al.11 had a 25.9% rate (including III–IV NSCLC and SCLC-ED). In the present study all patients completed posttest (retention rate: 100%), which was excellent compared to the above-mentioned exercise studies.8, 11 All 8 patients randomized to EXE could perform daily exercise concurrent to receiving concomitant chemoradiotherapy. Contrary to this Edvardsen et al.6 showed that patients with resectable NSCLC treated with chemotherapy were forced to postpone their training until they had completed the last course of treatment. As the exercise training was carried out next to the accelerator patients only had to appear 30 min before planned radiotherapy session. The attendance was high (90.0%) which was similar compared to previous studies.8, 22 Kuehr et al.22 showed a 95% adherence rate in a hospital-based exercise program and 77% adherence rate in a home-based program, while Quist et al.11 had a rate of 73.3% and 8.7% in supervised group training and home-based training, respectively. Thus, indicating that meeting patients in their current setting is favorable. No patients missed their radiotherapy session due to participating in the study and we observed no adverse event during the exercise sessions or during the baseline- and posttest.
The study showed a high attendance (90.0%) and adherence (88.1%), but due to the included number of patients we demonstrated no statistical significant differences in any of the secondary outcome for any of the groups following 7 weeks of exercise training. This might be due to the small sample size included. Even though there were no significant findings in the current study data showed a tendency toward a lower mental and treatment related fatigue burden in EXE compared with CON. Similar findings have been observed in another study with a significant effect in fatigue after 6 weeks of exercise.23 Additionally, in a qualitative study Adamsen et al.24 showed that treatment related fatigue could be transformed to a more positively perceived exercise induced fatigue as a result of participating in exercise.
The strength of this study is that the patients were met in their current situation with the exercise intervention carried out next to the accelerator. In this way, the design was tested in a real life situation, necessary in order to test the perspective of increasing oxygen availability prior to radiotherapy. Furthermore, the exercise was tailored and adjusted individually and this exercise-dose (intensity, frequency, duration) has been implemented before in NSCLC patients.9 Jones et al.9 reported that 2 patients experienced an abnormal decline in systolic blood pressure >20 mm Hg (hypotension) which normalized after exercise discontinuation. Other studies, including the same patient group experienced no exercise-related adverse events.7, 8, 11, 22 The fact that daily side effect, activity level, motivation to exercise, perceived exertion following exercise, dyspnea and RPE were strictly, individually monitored over a 7-week period strengthens this study. These data revealed if the exercise training was well tolerated and made it possible to adjust the intensity during the intervention period. The achieved exercise intensities with corresponding HR levels emphasize that the prescribed exercise intervention was successful and well tolerated. All the above-mentioned circumstances have enhanced the safety of this study. The training components (intensity, frequency, duration) in the intervention can easily be reproduced and thus raise the external validity.
The present setting was feasible for the included patients. The perspective of exercise before radiotherapy is that aerobic exercise may provide a novel means to overcome tumor microenvironment-associated therapy resistance in human solid tumors by increased oxygen availability. Due to structural and functional abnormalities in tumor blood vessels oxygen supply can be heterogeneous and result in intratumoral regions of hypoxia.25 Preclinical studies have shown that tumors with hypoxic regions are more resistant to radiotherapy than tumors without hypoxia.26, 27 Little is known, however, about the acute effect of aerobic exercise on blood flow to human tumor tissue. This key focus is emerging in the literature, and for now still at a hypothesis-generating level.28, 29, 30, 31
There are several limitations to the current study. The number of eligible patients, which relies on the fluctuations in newly diagnosed NSCLC patients receiving concomitant chemoradiotherapy, was lower than expected. Therefore, the inclusion period terminated before the calculated number of patients was achieved. Our findings may therefore be limited for generalization because of the small sample size. We only included locally advanced patients with NSCLC, and only a subset of the eligible patients were willing to participate in the study, which may have resulted in a selective, more strongly motivated group. Owing to the hypothesis-generated, prospective and explorative design of this study, it has not been possible to confirm the described outcome measures. To examine the secondary outcomes, the intervention should be investigated in a bigger randomized controlled study including the prescribed number of patients from the sample size calculation.
In conclusion, this study demonstrated ‘proof of principle’ that daily moderate-to-high intensity cycle ergometer exercise was feasible, safe and well tolerated during concomitant chemoradiotherapy in patients with locally advanced NSCLC. Large prospective randomized controlled trials are warranted to definitively address this question.
Conflict of interests
None declared.
Financial disclosure
The study was supported by grants from the Toyota-Foundation.
Acknowledgement
The authors acknowledge the support and expertise from the Department of Oncology, Section of Radiotherapy, University Hospital Copenhagen, Rigshospitalet.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Detterbeck F.C., Boffa D.J., Kim A.W., Tanoue L.T. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Walraven I., van den Heuvel M., van Diessen J. Long-term follow-up of patients with locally advanced non-small cell lung cancer receiving concurrent hypofractionated chemoradiotherapy with or without cetuximab. Radiother Oncol. 2016;118(3):442–446. doi: 10.1016/j.radonc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Kale M.S., Mhango G., Gomez J.E. Treatment toxicity in elderly patients with advanced non-small cell lung cancer. Am J Clin Oncol. 2017;40(5):470–476. doi: 10.1097/COC.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edvardsen E., Skjonsberg O.H., Holme I., Nordsletten L., Borchsenius F., Anderssen S.A. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax. 2015;70(3):244–250. doi: 10.1136/thoraxjnl-2014-205944. [DOI] [PubMed] [Google Scholar]
- 7.Hwang C.L., Yu C.J., Shih J.Y., Yang P.C., Wu Y.T. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer. 2012;20(12):3169–3177. doi: 10.1007/s00520-012-1452-5. [DOI] [PubMed] [Google Scholar]
- 8.Jones L.W., Eves N.D., Peterson B.L. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients: a pilot study. Cancer. 2008;113(12):3430–3439. doi: 10.1002/cncr.23967. [DOI] [PubMed] [Google Scholar]
- 9.Jones L.W., Peddle C.J., Eves N.D. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110(3):590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 10.Quist M., Adamsen L., Roerth M., Laursen J.H., Christensen K.B., Langer S.W. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety and depression in patients with advanced-stage lung cancer undergoing chemotherapy. Integr Cancer Ther. 2015 doi: 10.1177/1534735415572887. [DOI] [PubMed] [Google Scholar]
- 11.Quist M., Rorth M., Langer S. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer. 2012;75(2):203–208. doi: 10.1016/j.lungcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Adamsen L., Stage M., Laursen J., Rorth M., Quist M. Exercise and relaxation intervention for patients with advanced lung cancer: a qualitative feasibility study. Scand J Med Sci Sports. 2012;22(6):804–815. doi: 10.1111/j.1600-0838.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- 13.Moller T., Lillelund C., Andersen C. The challenge of preserving cardiorespiratory fitness in physically inactive patients with colon or breast cancer during adjuvant chemotherapy: a randomised feasibility study. BMJ Open Sport Exerc Med. 2015;1(1):e000021. doi: 10.1136/bmjsem-2015-000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Thoracic Society, American College of Chest Physicians ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 15.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 16.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Resp J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Cella D.F., Tulsky D.S., Gray G. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 18.Craig C.L., Marshall A.L., Sjostrom M. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Holle V., De Bourdeaudhuij I., Deforche B., Van Cauwenberg J., Van Dyck D. Assessment of physical activity in older Belgian adults: validity and reliability of an adapted interview version of the long International Physical Activity Questionnaire (IPAQ-L) BMC Public Health. 2015;15:433. doi: 10.1186/s12889-015-1785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawley-Hague H., Horne M., Skelton D.A., Todd C. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open. 2016;6(6):e011560. doi: 10.1136/bmjopen-2016-011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuehr L., Wiskemann J., Abel U., Ulrich C.M., Hummler S., Thomas M. Exercise in patients with non-small cell lung cancer. Med Sci Sports Exerc. 2014;46(4):656–663. doi: 10.1249/MSS.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 23.Adamsen L., Quist M., Andersen C. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamsen L., Midtgaard J., Andersen C., Quist M., Moeller T., Roerth M. Transforming the nature of fatigue through exercise: qualitative findings from a multidimensional exercise programme in cancer patients undergoing chemotherapy. Eur J Cancer Care (Engl) 2004;13(4):362–370. doi: 10.1111/j.1365-2354.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 25.Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 26.Gulliksrud K., Ovrebo K.M., Mathiesen B., Rofstad E.K. Differentiation between hypoxic and non-hypoxic experimental tumors by dynamic contrast-enhanced magnetic resonance imaging. Radiother Oncol. 2011;98(3):360–364. doi: 10.1016/j.radonc.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Ovrebo K.M., Gulliksrud K., Mathiesen B., Rofstad E.K. Assessment of tumor radioresponsiveness and metastatic potential by dynamic contrast-enhanced magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2011;81(1):255–261. doi: 10.1016/j.ijrobp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Siemann D.W., Horsman M.R. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther. 2015;153:107–124. doi: 10.1016/j.pharmthera.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiggins J.M., Opoku-Acheampong A.B., Baumfalk D.R., Siemann D.W., Behnke B.J. Exercise and the tumor microenvironment: potential therapeutic implications. Exerc Sport Sci Rev. 2018;46(1):56–64. doi: 10.1249/JES.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 30.Colliez F., Gallez B., Jordan B.F. Assessing tumor oxygenation for predicting outcome in radiation oncology: a review of studies correlating tumor hypoxic status and outcome in the preclinical and clinical settings. Front Oncol. 2017;7:10. doi: 10.3389/fonc.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hojman P., Gehl J., Christensen J.F., Pedersen B.K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27(1):10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]