Abstract
The overall survival rate of patients with osteosarcoma has remained stagnant at 15–30% for several decades. Although immunotherapy has revolutionized the oncology field, largely attributed to the success of immune-checkpoint blockade, the durability and efficacy of anti-PD1 (programmed cell death protein 1) mAb vary across different malignancies. Among the major reasons for tumor resistance to this immune checkpoint therapy is the absence of tumor-infiltrating cytotoxic T lymphocytes. However, the presence of intratumor exhausted PD1hi T cells also contributes to insensitivity to anti-PD1 treatment. In this study, we established the osteosarcoma mouse tumor model resistant to anti-PD1 mAb that harbored PD1hi T cells. Furthermore, flow cytometry analysis of tumor infiltrating leukocytes after treatment was used as a screening platform to identify agents that could re-sensitize T cells to anti-PD1 mAb. Results showed that anti-CD40 mAb treatment converted PD1hi T cells to PD1lo T cells, reversing phenotypic T cell exhaustion and sensitizing anti-PD1 refractory tumors to respond to anti-PD1 mAb. Results also showed that intratumor Treg presented with a less activated and attenuated suppressive phenotype after anti-CD40 mAb treatment. Our study provides proof of concept to systematically identify immune conditioning agents, which are able to convert PD1hi T cells to PD1lo T cells, with clinical implications in the treatment against refractory osteosarcoma to anti-PD1 mAb.
Keywords: Anti-CD40, PD1 blockade, Osteosarcoma, Immunotherapy
1. Introduction
As a highly malignant and the second most common bone-associated tumor in children and young adults, osteosarcoma is involving accumulation of multiple genetic and epigenetic abnormalities. The current clinical treatment still largely depends on the drugs initiated in the early 1980s with a dismal five-year survival rate of less than 20% [1], [2], [3]. Therefore, more efforts have to be made not only by discovering new agents with advanced specificity, but also by developing combined treatment approaches with enhanced efficacy [4].
Immune checkpoint inhibitors targeting cytotoxic T lymphocyte-associated protein 4 (CTLA4) and programmed cell death protein 1 (PD1) demonstrated durable clinical responses in various cancers including non-small cell lung cancer and melanoma, through disinhibiting the immune system. However, the durability and efficacy of anti-PD1 mAbs (nivolumab or pembrolizumab) varies [5], [6], [7], correlating with presence of neoantigens or levels of mutational burden in cancer [8], [9], [10]. The lack of tumor-infiltrating CD8+ T cells has been considered as one of the major reasons to lead to tumor resistance to anti-PD1 treatment [11]. Therefore, huge effort is needed to increase intratumor CD8+ T cells with different approaches to enhance the therapeutic efficacy of anti-PD1 treatment.
In tumor models infiltrated by PD1loCD8+ T cells, anti-PD1 mAb showed good therapeutic effects. In contrast, PD1hi CD8+ T cells is considered as a resistance biomarker to anti-PD1 treatment [12]. Interestingly, exhaustion of intratumor PD1hiCD8+ T cells was reversed to a PD1lo phenotype when regulatory T cells (Treg) were depleted conditionally [12], [13]. Unfortunately, although complete depletion of Treg can be performed by genetically modification in mouse models, it is limited in the clinic. Therefore, alternate strategies were explored to modulate levels of PD1 expression on T cells and then reverse the exhaustion state.
In this study, the osteosarcoma tumor model harboring PD1hi intratumor T cells was used and an agonistic anti-CD40 mAb was found to rapidly decrease PD1 expression on T cells. The reversal of T cell exhaustion was companied with significant increases in proliferative capacities of T cells and cytokine-production, as well as reactivated T cell phenotype, rendering resistant tumor cells sensitive to anti-PD1 treatment.
2. Materials and methods
2.1. Tumor cell line
The murine osteosarcoma K7M2 cell line was purchased from American Type Culture Collection. A master cell bank was expanded upon arrival. Cells were cultured in RPM-I1640 supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin in a humidified incubator at 37 °C with 5% CO2.
2.2. Mice
Female 6-week-old Balb/c mice were purchased from Vital River Ltd. (Beijing, China) and housed under specific pathogen-free condition. All animal studies were conducted according to Chinese animal care guidelines under an approved Institutional Animal Care and Use Committee protocol from Jilin University.
2.3. Reagents
Anti-CTLA4 mAb, anti-PD1 mAb, anti-mouse CD40 mAb and control IgG were purchased from BioXcell. All antibodies were used intraperitoneally at the dose as indicated.
2.4. Mouse tumor model and treatment
Tumor model was established by subcutaneously injecting 4 × 105 K7M2 cells into the right flank of Balb/c mice. Tumor volume was measured using a digital caliper. Once tumor volume reached 50–100 mm3, tumor-bearing mice were divided for different treatment (5 mice per group). Study would be terminated when tumor volume reached 1500 mm3. For flow cytometry analysis of immune cells in tumor tissues, tumor-bearing mice (400–600 mm3) were treated and tumor tissues were harvested 48 to 72 hrs post treatment.
2.5. Flow cytometric analysis of immune populations
Tumor tissue was removed and cut into small fragments. Single tumor cell suspension was obtained by digestion with tumor disassociation kit (Miltenyi Biotec, USA) at 37 °C and then filtering through 70 µm cell strainers. Mononuclear cells were enriched by a two-level percoll gradient. Following a wash with PBS, cells were stained with Aqua Live/Dead stain and fluoro-conjugated antibodies specific to cell surface markers (BioLegend). After fixation-permeabilization, cells were stained with fluorochrome labeled antibodies specific to intracellular markers (BioLegend) or with the isotype controls. BD LSRFortessa was sued for cell acquisition and data analysis was carried out using the FlowJo software.
2.6. Statistical analysis
Data were analyzed with SPSS 12.0 software (SPSS Inc., Chicago, IL, USA). Measurement data among groups were compared using one-way analysis of variance followed by Dunnett's t-test. A two-tailed p < 0.05 was considered as statistically significant.
3. Results
3.1. Anti-CD40 mAb treatment regulated PD1 expression on T cells
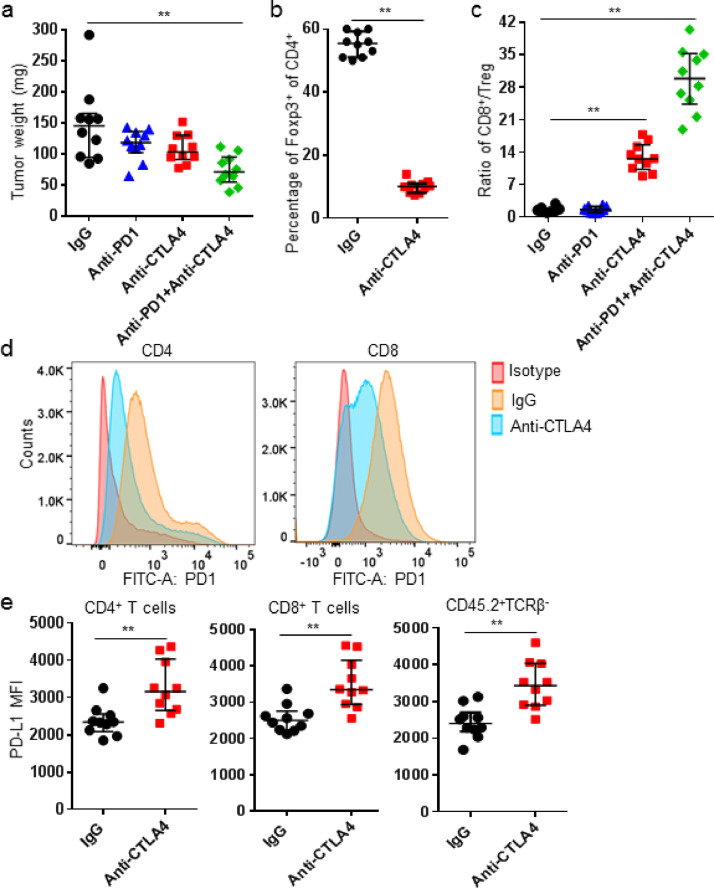
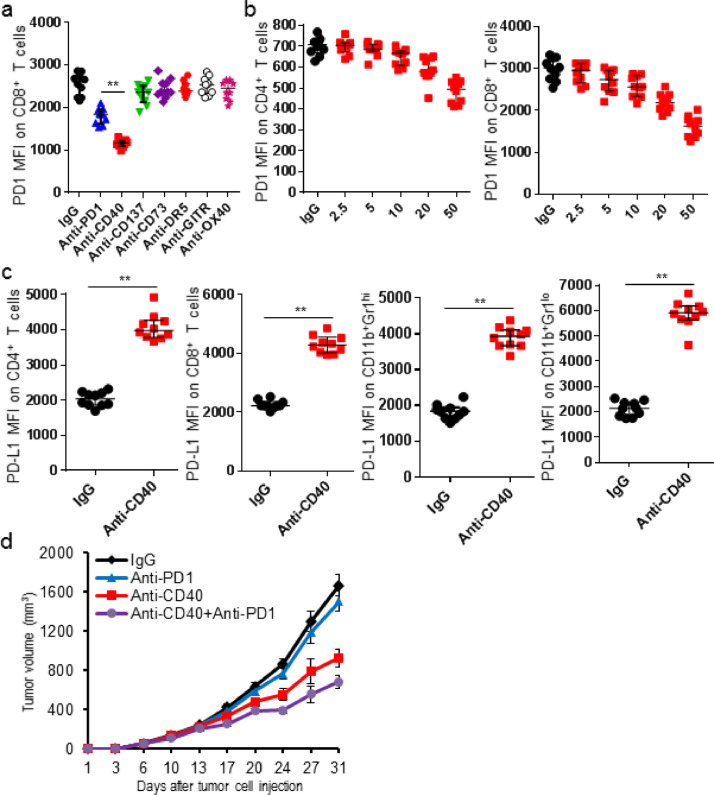
Systemic deletion of Treg rescued exhausted CD8+ T cells and increased the therapeutic efficacy of checkpoint blockade in cancer settings [12], [14]. In this study, we also found anti-CTLA4 treatment exerted Treg-depleting activity and furthermore down-regulated PD1 expression on T cells, resulting in an anti-PD1 resistant PD1hi phenotype to a sensitive PD1lo phenotype, and resultantly improved tumor control. Changes of PD1 expression on T cells caused a further increase in the ratio of intratumor CD8/Treg in tumor-bearing mice treated with anti-PD1 and anti-CTLA4 mAb. Different to the decreased PD1 expression on T cells, expression of PD-L1 increased significantly on myeloid cells and T cells in tumor tissues (Fig. 1). Therefore, other immune-based targets were then identified to sensitize tumor with PD1hi T cells to anti-PD1 treatment. Our data showed that the agonistic anti-CD40 mAb effectively lowered the expression of PD1 on T cells in tumor tissues in a dose-dependent manner. Not surprisingly, expression of PD-L1 increased on myeloid cells and intratumor T cells. Tumor-bearing mice were treated by anti-CD40 mAb with a single dose before the anti-PD1 treatment to confirm its immune conditioning effect. Results showed single dose of anti-CD40 mAb significantly suppressed tumor growth and enhanced the therapeutic efficacy of anti-PD1 treatment (Fig. 2).
Fig. 1.
Anti-CTLA4 treatment decreased PD1 expression on intratumor T cells. K7M2 tumor-bearing mice were treated with IgG (250 µg), anti-CTLA4 (200 µg), anti-PD1 (250 µg), or a combination of anti-CTLA4+anti-PD1 mAbs on days 10, 14, and 18. (a) On day 21, mice were sacrificed and tumor weights were recorded; (b) Frequencies of Foxp3+ of CD4+ T cells between control and anti-CTLA4 mAb treatment; (c) Intratumor CD8/Treg ratio was shown; (D) Representative overlaid PD1 histogram plots of CD4+ and CD8+ T cells; (e) PD-L1 MFI of indicated cell subsets was shown. Data were expressed as median ± interquartile (n = 10). *p < 0.05; **p < 0.01.
Fig. 2.
Anti-CD40 mAb reduced PD1 expression on intratumor T cell. K7M2 tumor-bearing mice were treated with the indicated antibodies or inhibitors, and tumors were harvested 3 days after treatments for flow cytometric analyses. (a) PD1 MFI of CD8+ T cells was shown; (b) Anti-CD40 mAb lowered PD1 expression on intratumor T cells in a dose-dependent manner; (c) PD-L1 MFI of indicated cell subsets was shown; (d) K7M2 tumor-bearing mice were treated with 100 µg of IgG or anti-CD40 mAb (Day 7), two days before the commencement of 250 µg of IgG or anti-PD1 mAb. IgG or anti-PD1 mAb was administered every four days for a total of 4 doses (Days 9, 13, 17, and 21). Tumor size was presented as mean ± SD. Data were expressed as median ± interquartile (n = 10) for scatter plot. *p < 0.05; **p < 0.01.
3.2. Anti-CD40 mAb treatment changed intratumor T cell phenotype
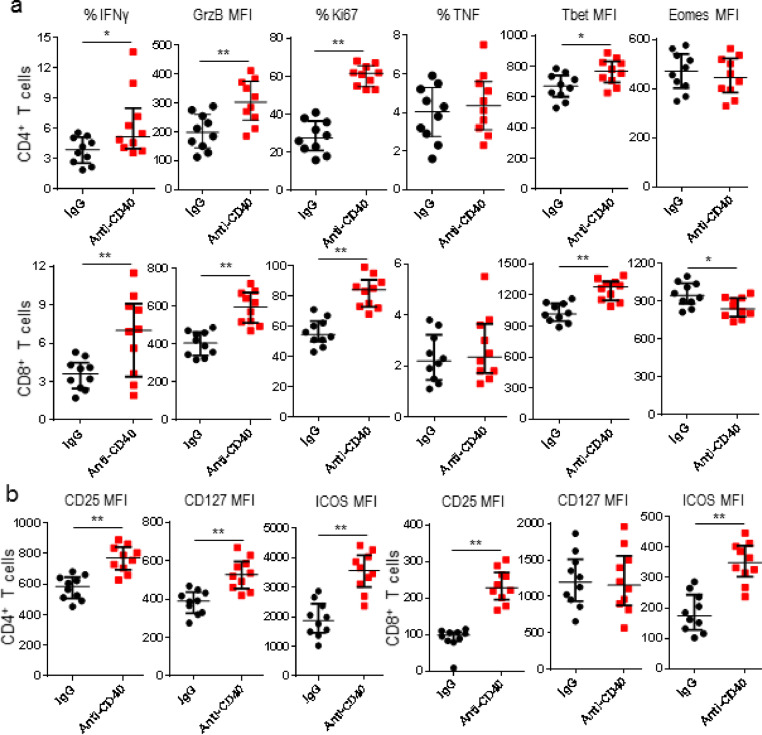
Comparing to effector T cells, exhausted T cells with CD8+PD1hi phenotype are characterized by progressive functional loss of effectors and different transcription factor expression [15]. After anti-CD40 mAb treatment, Ki67-, granzyme B-, and IFNγ-expressing T cells increased, however, frequency of T cells expressing TNF did not change. Consistent with Tbet:Eomes transcriptional profile of PD1hi (TbetloEomeshi) and PD1lo (TbethiEomeslo) CD8+ T cells, Tbet expression increased, but Eomes decreased in intratumor CD8+ T cells isolated from tumor-bearing mice treated with anti-CD40 mAb. Induction of Tbet after anti-CD40 mAb treatment was also observed in intratumor CD4+ T cells, but levels of Eomes did not change. An increase of T cells expressing CD25+ and ICOS was found in tumor tissues after anti-CD40 mAb treatment. Furthermore, an upregulation of CD127 was found in these intratumor CD4+ T cells (Fig. 3). Our results indicated anti-CD40 mAb treatment significantly induced transcriptional changes to T cells in tumor tissues, accompanied by an increase in their capacities of proliferation, cytotoxicity, and cytokine-production.
Fig. 3.
Anti-CD40 mAb reversed exhaustion of intratumor T cells. K7M2 tumor-bearing mice were treated with IgG or anti-CD40 mAb, and tumors were harvested 3 days after treatments for flow cytometric analyses. (a) Frequencies of IFNγ+, TNF+, and Ki67+; and MFIs of granzyme B (GrzB), Tbet, and Eomes in CD4+ and CD8+ TILs between IgG and anti-CD40-treated mice were shown; (b) MFIs of CD25, CD127, and ICOS in CD4+ and CD8+ TILs were shown. Data were expressed as median ± interquartile (n = 10) for scatter plot. *p < 0.05; **p < 0.01.
3.3. Anti-CD40 mAb treatment altered immune checkpoint receptor expression
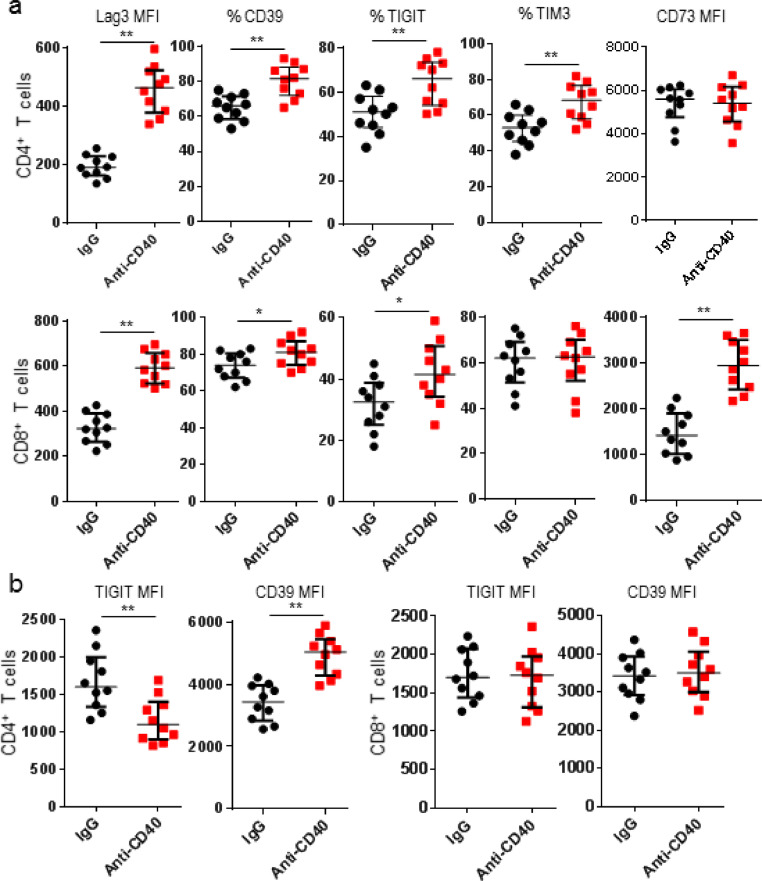
Exhausted T cells are characterized with expression of multiple checkpoint receptors [16]. Therefore expression of other T cell inhibitory receptors and exhaustion-related markers were examined after anti-CD40 mAb treatment. Results showed anti-CD40 mAb treatment changed the expression of TIGIT, Tim3, Lag3 and CD39 on CD4+ T cells, but no significant changes for CD73 expression on CD4+ T cells. Lower TIGIT expression was measured on TIGIT+CD4+ T cells. In contrast, higher CD39 expression was displayed on CD39+CD4+ T cells after anti-CD40 mAb therapy. Just like CD4+ T cells, anti-CD40 mAb treatment also upregulated expression of CD39, Lag3 and CD73 on CD8+ T cells. However, anti-CD40 mAb treatment did not significantly change TIGIT+CD8+ or Tim3+ T cells (Fig. 4). Our data demonstrated anti-CD40 mAb treatment initiated differential regulatory effects for multiple immune receptors on exhausted T cells in tumor tissues.
Fig. 4.
Anti-CD40 mAb regulated multiple intratumor T cell checkpoint expression. K7M2 tumor-bearing mice were treated with IgG or anti-CD40 mAb, and tumors were harvested 3 days after treatments for flow cytometric analyses. (a) MFIs of Lag3 and CD73; and frequencies of Tim3+, TIGIT+, and CD39+ in CD4+ and CD8+ TILs were shown; (b) TIGIT and CD39 MFIs of CD4+ and CD8+ TILs were shown. Data were expressed as median ± interquartile (n = 10) for scatter plot. *p < 0.05; **p < 0.01.
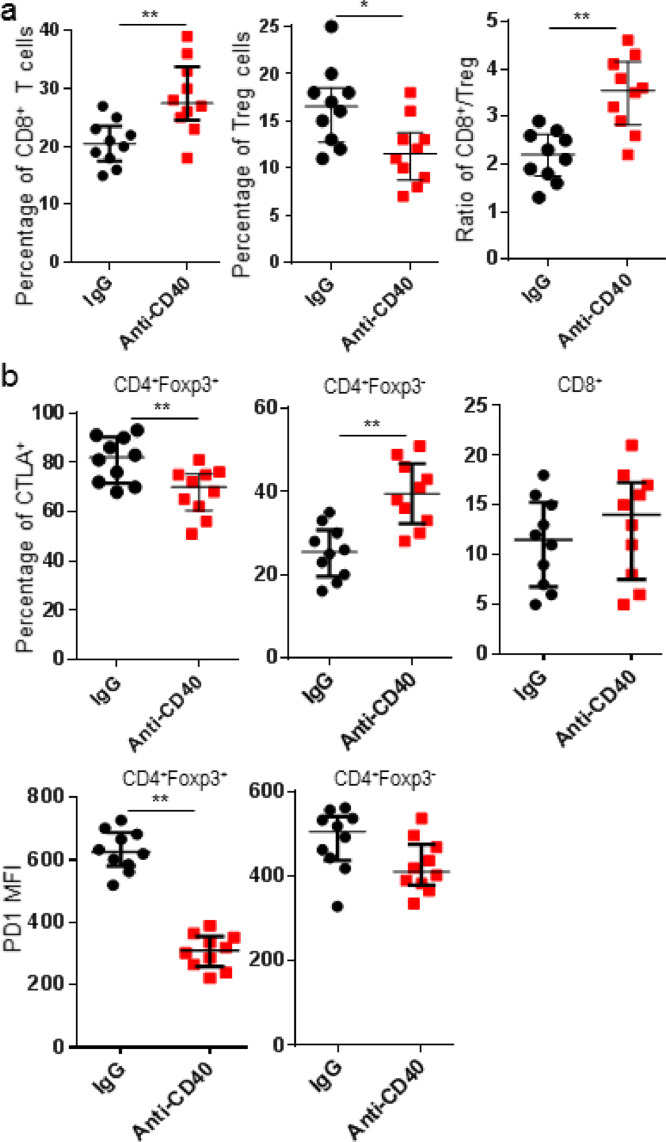
3.4. Anti-CD40 mAb treatment attenuated Treg suppressive phenotype
Treg depletion with anti-CTLA4 mAb could lower the expression of PD1 on CD8+ T cells. The upregulation of CD127, CD25, and ICOS on CD4+ T cells implied the potential modulation of CTLA4 and/or Treg in responding to anti-CD40 mAb treatment [17], [18]. An increase was observed in the proportion of CD8+ T cells and the ratio of CD8/Treg, but decreased Treg (CD4+Foxp3+). We then explored whether intratumor Treg was rendered attenuated suppressor functions after anti-CD40 mAb treatment. Results demonstrated expression levels of PD1 and CTLA4 decreased on intratumor Treg isolated from tumor-bearing mice treated with anti-CD40 mAb, indicating Treg with less suppressive effects. On the other way, the proportion of CD4+Foxp3− T cells expressing CTLA4 significantly increased and no obvious changes for CD8+ T cells expressing CTLA4 after anti-CD40 mAb treatment (Fig. 5). These data indicated anti-CD40 mAb therapy primed tumor microenvironment with less suppressive effects for anti-PD1 treatment.
Fig. 5.
Anti-CD40 mAb induced intratumor Treg with an attenuated suppressive phenotype. K7M2 tumor-bearing mice were treated with IgG or anti-CD40 mAb, and tumors were harvested 3 days after treatments for flow cytometric analyses. (a) Frequencies of CD8+ T cells and Treg (CD4+Foxp3+), and CD8/Treg ratio were shown; (b) Frequencies of CTLA4+ in Treg, CD4+Foxp3− and CD8+ T cells, and MFIs of PD1 in Treg and CD4+Foxp3− were shown. Data were expressed as median ± interquartile (n = 10) for scatter plot. *p < 0.05; **p < 0.01.
4. Discussion
Osteosarcoma is currently treated with combination therapy including surgery, chemotherapy and radiation therapy. However, the efficacy of chemotherapy has reached a plateau with more than 40% of patients ultimately relapsing or developing metastasis [19], [20]. Moreover, few treatment options are available when traditional therapy fails. Therefore, to search for effective and novel therapies is of utmost importance. Immunotherapy has revolutionized the treatment of cancer. Restoring the anti-tumor function of T cells via immune checkpoint blockade has led to a breakthrough in solid tumors [21], [22].
The durable response rate of patients with solid tumors to immune checkpoint blockade is still modest, largely because of tumor-mediated immunosuppression [23]. Comparing to nivolumab alone, combining treatment with nivolumab and ipilimumab was in correlation with a better survival profile in patients with negative PD-L1 staining [24]. However, immunohistochemistry staining of PD1 on T cells in tumor tissues could not distinguish T cells with PD1hi and PD1lo profile, therefore the high positivity of PD-L1 staining might reflect a PD-L1hi and T-cell PD1lo tumor microenvironment. The inverse correlation of T-cell PD1 status to PD-L1 expression (PD1lo and PD-L1hi; PD1hi and PD-L1lo) in the tumor microenvironment was also confirmed [12]. Although this PD1/PD-L1-modulating effects were driven by the Treg-depleting activity of anti-CTLA4 mAb, it has not been proven in the clinic. In our tumor model, a reduction of intratumor Treg was observed, likely mimicking the activity of anti-CTLA4 mAb. Therefore, as a CTLA4-blockade, the immune modulating effects of ipilimumab is likely beneficial in improving the therapeutic efficacy of anti-PD1 mAb treatment [25]. Thereafter, it is important to identify targets lowering PD1 expression on tumor-infiltrating T-cells because it will enable the synergistic effects with PD1 inhibitors, demonstrated by the effects of anti-PD1 and anti-CD40 mAbs in osteosarcoma tumor-bearing mice. Furthermore, results showed a single treatment with anti-CD40 mAb could prime a profound anti-tumor effect. Therefore, it is interesting to identify factors, other than T cell proportion and the PD1 status, which determine synergistic effects of anti-PD1 and anti-CD40 mAbs in controlling tumor growth.
Many inhibitory receptors, including CTLA4 and PD1, express on exhausted CD8+ T cells, which regulate the proliferation, cytotoxicity, and cytokine-production of these T cells to eliminate tumor cells. Although PD1/PD-L1 blockade could partly reinvigorate functions of effector T cell, only CD8+ T cells expressing PD1lo together with a transcriptional profile of Tbethi Eomeslo could respond to this treatment [26]. The transition from PD1hi to PD1lo expression on CD8+ T cells in tumor-bearing mice treated with anti-CD40 mAb, together with changes of function, resulted in a reversion of exhausted T cells and the following anti-tumor activity rendered by anti-PD1 mAb treatment. Anti-CD40 mAb treatment selectively increased expression of CD39, CD73 and Lag3, but not TIGIT or Tim3 expression on CD8+ T cells, together with the increased expression of PD-L1, implying that these expression changes of inhibitory receptors are likely a therapy-induced adaptive resistance [27].
Anti-CD40 treatment induced co-stimulatory ligands on intratumor myeloid cells, however, activated signaling of CD80/CD86/CD28 or CD70/CD27 did not directly down-regulate expression of PD1 on T cells. Although anti-CD27 mAb reduced PD1 expression on intratumor Foxp3+ Treg and CD8+ T cells in melanoma tumors [28], such changes were not observed in our study, likely because of the different analysis time (early response versus late response). Our results did not exclude the potential activity of anti-CD40 mAb in activating tumoricidal macrophages, in driving this PD1 modulatory effects when anti-PD1 mAb is used. Our results also did not exclude the importance of anti-CD40 mAb-driven costimulatory signals to support the expansion of effector T cells [29]. Although anti-CD40 mAb treatment did not significantly delete intratumor Treg, these Treg displayed less suppressive phenotype. These Treg changes could be driven by the induction of Tbet, resulting from the interaction between Treg and antigen presenting cells.
Given the efficacy in patients with melanoma, it is particularly exciting for the development of immune checkpoint inhibitors to treat cancer [30], [31], [32], [33]. However, only a subset of patients exhibit durable responses. Therefore, it is in great need to better understand PD1 biology to prolong its therapeutic efficacy and broaden it to other malignancies [30], [34]. Using tumor harbouring PD1hi T cells as a biomarker of therapeutic resistance to anti-PD1 mAb, multiple immune conditioning agents have been identified to render T cells from insensitive to sensitive upon the anti-PD1 treatment. By assessing the conversion of PD1hi to PD1lo, our work is not limited to immune-based therapeutic agents, but able to be expanded to screen radiotherapy, epigenetic modulators, chemotherapeutic agents and small molecules, which might potentiate the anti-tumor response to anti-PD1 mAb in a previously refractory tumor. Our results provided a scientific rationale to improve the efficacy of immune checkpoint inhibitors in clinic against osteosarcoma.
Conflict of interest statement
The authors declared that there is no conflict of interests in this work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100245.
Appendix. Supplementary materials
References
- 1.Graham-Pole J., Ayass M., Cassano W. Neoadjuvant chemotherapy for patients with osteosarcoma: university of Florida studies. Cancer Treat. Res. 1993;62:339–346. doi: 10.1007/978-1-4615-3518-8_41. [DOI] [PubMed] [Google Scholar]
- 2.Urakawa H., Tsukushi S., Suqiura H. Neoadjuvant and adjuvant chemotherapy with doxorubicin and ifosfamide for bone sarcomas in adult and older patients. Oncol. Lett. 2014;8:2485–2488. doi: 10.3892/ol.2014.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel S.J., Lynch J.W., Jr., Johnson T. Dose-intense ifosfamide/doxorubicin/cisplatin based chemotherapy for osteosarcoma in adults. Am. J. Clin. Oncol. 2002;25:489–495. doi: 10.1097/00000421-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Massari F., Santoni M., di Nunno V. Adjuvant and neoadjuvant approaches for urothelial cancer: updated indications and controversies. Cancer Treat. Rev. 2018;68:80–85. doi: 10.1016/j.ctrv.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Ansell S.M., Lesokhin A.M., Borrello I. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tumeh P.C., Harview C.L., Yearley J.H. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C., Long G.V., Brady B. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 8.Rizvi N.A., Hellmann M.D., Snyder A. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGranahan N., Furness A.J., Rosenthal R. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gubin M.M., Zhang X., Schuster H. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngiow S.F., Young A., Jacquelot N. A threshold level of intratumor CD8+ T-cell PD1 expression dictates therapeutic response to anti-PD1. Cancer Res. 2015;75:3800–3811. doi: 10.1158/0008-5472.CAN-15-1082. [DOI] [PubMed] [Google Scholar]
- 13.Penaloza-MacMaster P., Kamphorst A.O., Wieland A. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakuishi K., Ngiow S.F., Sullivan J.M. TIM3+FOXP3+ regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. OncoImmunology. 2013;2:e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry E.J., Kurachi M M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauken K.E., Wherry E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley J.L., Blair P.J., Musser J.T. ICOS costimulation requires IL-2 and can be prevented by CTLA-4 engagement. J. Immunol. 2001;166:4943–4948. doi: 10.4049/jimmunol.166.8.4943. [DOI] [PubMed] [Google Scholar]
- 18.Rudd C.E., Schneider H. Unifying concepts in CD28, ICOS and CTLA4 coreceptor signaling. Nat. Rev. Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 19.Saraf A.J., Fenger J.M., Roberts R.D. Osteosarcoma: accelerating progress makes for a hopeful future. Front. Oncol. 2018;8:4. doi: 10.3389/fonc.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Judson I., Verweij J., Gelderblom H. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 21.Wolchok J.D., Kluger H., Callahan M.K. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou W., Wolchok J.D., Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkie K.P., Hahnfeldt P. Tumor-immune dynamics regulated in the microenvironment inform the transient nature of immune-induced tumor dormancy. Cancer Res. 2013;73:3534–3544. doi: 10.1158/0008-5472.CAN-12-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin J., Chiarion-Sileni V., Gonzalez R. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romano E., Kusio-Kobialka M., Foukas P.G. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl. Acad. Sci. U.S.A. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackburn S.D., Shin H., Freeman G.J., Wherry E.J. Selective expansion of a subset of exhausted CD8 T cells by PD-L1 blockade. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zippelius A., Schreiner J., Herzig P., Muller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol. Res. 2015;3:236–244. doi: 10.1158/2326-6066.CIR-14-0226. [DOI] [PubMed] [Google Scholar]
- 28.Roberts D.J., Franklin N.A., Kingeter L.M. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8+ T cells. J. Immunother. 2010;33:769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beatty G.L., Chiorean E.G., Fishman M.P. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth M.J., Ngiow S.F., Ribas A., Teng M.W.L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 31.Khalil D.N., Smith E.L., Brentjens R.J., Wolchok J.D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016;13:394. doi: 10.1038/nrclinonc.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamid O., Robert C., Daud A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng MW., Ngiow S.F., Ribas A., Smyth M.J. Classifying cancers based on T cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.