Abstract
Aims
The present study aimed at investigating the association between left ventricular (LV) mechanical dispersion measured with speckle tracking echocardiography and severity of aortic stenosis (AS) and its impact on prognosis.
Methods and results
This retrospective study included 630 patients [age 72 (62–78) years, 61.4% men] with various grades of AS (mild AS, 19.8%; moderate AS, 37.0%; severe AS, 43.2%). LV mechanical dispersion (defined as standard deviation of time from Q/R on electrocardiogram to peak longitudinal strain in 17 LV segments) was assessed by speckle tracking echocardiography. Clinical, electrocardiographic, and echocardiographic determinants of increased LV mechanical dispersion were evaluated. During a follow-up of 107 (43–133) months, the independent association between LV mechanical dispersion and all-cause mortality (n = 302, 48%) was evaluated including aortic valve replacement as time-dependent co-variate. LV mechanical dispersion increased significantly with increasing severity of AS (mild AS, 54.5 ± 17.2 ms; moderate AS, 56.7 ± 19.3 ms; severe AS, 70.9 ± 24.3 ms; P < 0.001). Independent determinants of increased mechanical dispersion included older age (β = 0.28; P = 0.003), lower LV ejection fraction (β = −0.24; P = 0.020), smaller aortic valve area (β = −8.55; P = 0.001), larger LV mass index (β = 0.20; P < 0.001), and longer QRS duration (β = 1.12 per each 10 ms increase; P = 0.012). LV mechanical dispersion showed incremental prognostic value for all-cause mortality (hazard ratio 1.10 per each 10 ms increase, 95% confidence interval 1.04–1.15; P < 0.001).
Conclusion
LV mechanical dispersion assessed by speckle tracking echocardiography increases significantly with severity of AS and is significantly associated with all-cause mortality.
Keywords: left ventricular, mechanical dispersion, aortic stenosis, prognosis
Introduction
Calcific aortic stenosis (AS) is one of the most prevalent valvular heart disease in the Western world.1,2 The increased afterload imposed by the narrow aortic valve area on the left ventricle induces a remodelling process, which is initially characterized by adaptive left ventricular (LV) hypertrophy. Long-standing AS further induces myocardial fibrosis, heart failure, and poor outcome.3,4 Therefore, alongside presence of symptoms and severity of AS, timely assessment of LV systolic dysfunction plays a pivotal role in the decision for referral to aortic valve replacement (AVR).5,6 Left ventricular ejection fraction (LVEF) is the benchmark parameter to define LV systolic function and is included in current guideline recommendations.5,6 However, LVEF may not recover after AVR and patients may remain symptomatic. Speckle tracking echocardiographic parameters of LV shortening and mechanical dispersion have been proposed to detect LV systolic dysfunction at an earlier stage than LVEF7 and are related to the presence of myocardial fibrosis on cardiac magnetic resonance.8 The increase in myocardial fibrosis may lead to slow conduction and heterogeneous activation of the left ventricle which can be quantified by measuring the LV mechanical dispersion on speckle tracking echocardiography. Prolonged LV mechanical dispersion has been associated with worse outcome in various cardiomyopathies,9,10 after myocardial infarction,11,12 and (as shown more recently) in patients with severe AS.13 However, the underlying mechanisms and relationship between LV mechanical dispersion, LV hypertrophy and QRS duration in various grades of AS are currently lacking. We hypothesize that LV mechanical dispersion increases with the severity of AS and may impact outcome.
Therefore, we aimed at evaluating the correlates and prognostic value of LV mechanical dispersion across various grades of AS.
Methods
Patients
Patients with any grade (mild, moderate, and severe) of native AS identified from the departmental echocardiographic database of the Leiden University Medical Center (Leiden, The Netherlands) were included consecutively. Patients with prosthetic aortic valves, subvalvular or supravalvular AS, dynamic subaortic obstruction, more than moderate aortic or mitral regurgitation and any grade of mitral stenosis, ventricular pacing, and active endocarditis were excluded.
Clinical parameters including medical history, cardiovascular risk factors, cardiac symptoms, and physical examination were collected from patients’ medical records. The first two-dimensional (2D) transthoracic echocardiography showing diagnosis of AS was considered the baseline reference and the resting 12-lead electrocardiogram (ECG) obtained at the moment of the baseline echocardiography was evaluated. Patients were followed for the occurrence of AVR and all-cause mortality.
Data entered in the departmental Cardiology Information System (EPD-Vision®; Leiden University Medical Centre, Leiden, the Netherlands) was retrospectively collected and analysed. The institutional ethical committee approved this retrospective analysis of clinically acquired data and waived the need for patient written informed consent.
Echocardiography
Transthoracic echocardiography was performed at rest in all patients using commercially available ultrasound systems (Vivid 7 and E9 systems; GE-Vingmed, Horten, Norway). Data were stored digitally and analysed offline (EchoPAC version 113.0.3; GE-Vingmed, Horten, Norway). From parasternal, apical, subcostal and suprasternal views, 2D, colour, pulsed, and continuous-wave Doppler data were recorded according to current recommendations.6 Using the Simpson’s biplane method of discs, LVEF was calculated from the LV end-diastolic and end-systolic volumes and expressed as a percentage.14 LV mass was calculated from the 2D LV linear measurements obtained on the parasternal LV long-axis view using the formula as recommended.14 LV end-diastolic and end-systolic volumes and LV mass were indexed to body surface area. On a zoomed parasternal long-axis view, the LV outflow tract diameter was measured and the cross-sectional area was derived. From the apical LV long-axis or five-chamber views, continuous wave and pulsed wave Doppler spectral recordings were obtained through the aortic valve and at the LV outflow tract, respectively. The peak and mean aortic pressure gradients were estimated with the modified Bernoulli equation. The continuity equation was used to calculate the aortic valve area. Severity of AS was categorized based on current recommendations.5,6 Severe AS was defined as peak velocity >4.0 m/s, mean gradient >40 mmHg, or aortic valve area <1.0 cm2; moderate AS was defined as peak velocity 3.0–4.0 m/s, mean gradient 30–40 mmHg, or aortic valve area 1.0–1.5 cm2; and mild AS was defined as peak velocity 2.6–2.9 m/s, mean gradient <20 mmHg, or aortic valve area >1.5 cm2. Diastolic function was assessed according to recent guidelines.15 LV mechanical dispersion according to speckle tracking echocardiography was calculated as the standard deviation of time from Q/R on the ECG, to peak longitudinal strain in 17 LV segments and expressed in milliseconds (ms).16
Electrocardiography
Standard resting 12-lead ECGs performed within a time window of 12 months prior to or after the date of the baseline echocardiogram were included in the analysis and retrospectively assessed. Calibration of the ECG was set at 0.1 mV/mm and the paper speed was 25 mm/s. Sinus rhythm and atrial fibrillation were defined as recommended by current guidelines.17 QRS duration was measured in ms in the ECG lead with the greatest QRS width and the QRS morphology (left bundle branch block, right bundle branch block, and intraventricular conduction disorder) was defined according to current recommendations.18
Follow-up
Patients were followed-up for the occurrence of all-cause mortality. Survival data were complete for all subjects and collected from the departmental cardiology information system, which is linked to the governmental death registry database. In addition, the occurrence and timing of AVR during follow-up was noted.
Statistical analysis
Continuous variables were presented as mean ± standard deviation if the distribution was Gaussian, whereas non-Gaussian distributed continuous variables were presented as median and interquartile range (IQR). Comparisons of continuous variables across patient groups were performed using the one-way ANOVA-test, with post hoc Bonferroni analysis when appropriate. All categorical variables were presented as percentages and compared with the χ2 test. Univariable and multivariable linear regression analyses were performed to identify clinical, electrocardiographic, and echocardiographic correlates of LV mechanical dispersion. The level of significance for univariable analysis was set at P < 0.10.
The cumulative event rates for the clinical endpoint of all-cause mortality were estimated with the Kaplan–Meier curves and the log-rank test was used to compare two groups dichotomized according to the mean value of LV mechanical dispersion in the entire population. To investigate the independent associates of all-cause mortality, a multivariable Cox proportional hazards regression analysis was performed. Clinical and echocardiographic parameters known to influence mortality in patients with AS were included in the univariable analysis on a priori manner based on previous studies.19–21 The level of significance for univariable analysis was set at P < 0.10. Subsequent AVR was included in the model as a time-dependent co-variate. Additionally, the potential incremental value of LV mechanical dispersion for the association with all-cause mortality was evaluated by the change in χ2 and the likelihood ratio. Furthermore a IDI (integrated discrimination improvement) and NRI (net reclassification improvement) was performed. A tolerance level of >0.5 was set to avoid multicollinearity between the univariable determinants. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. A two-tailed P-value of <0.05 was considered significant. Inter-observer and intra-observer variability of mechanical dispersion was tested by reanalysing 45 random studies (15 cases of mild, moderate, and severe AS) by two cardiologists (E.A.P. and E.M.V.) who were blinded for the results of the first analysis. All statistical analyses were performed using SPSS for Windows version 23 (SPSS Inc.; Armonk, NY, USA: IBM Corp).
Results
Baseline characteristics
A total of 630 patients (61.4% men) with a median age of 72 (IQR 62–78) years were included in this study. Table 1 summarizes the clinical characteristics of the study population. Mild, moderate, and severe AS was noted in 125 (19.8%), 233 (37.0%), and 272 (43.2%) patients, respectively. Patients with severe AS were more likely to be older (P < 0.001) and present with worse New York Heart Association functional class (P < 0.001), compared with their counterparts. No significant differences in the usage of cardiovascular medications between the three groups were found. On the ECG, the QRS duration increased (from 99 ms in mild AS to 107 ms in severe AS, P < 0.001) and QRS conduction disturbances became more prevalent with increasing AS severity. Furthermore, atrial fibrillation was more frequently observed among patients with severe AS. Table 2 shows the echocardiographic characteristics according to the different AS groups. Patients with severe AS had significantly larger LV volumes, higher LV mass index, and higher percentage of reduced LVEF.
Table 1.
Clinical and electrocardiographic characteristics according to severity of aortic stenosis
| Variables | Mild AS | Moderate AS | Severe AS | P-value |
|---|---|---|---|---|
| (n = 125) | (n = 233) | (n = 272) | ||
| Clinical | ||||
| Age (years) | 69 (61–77)‡ | 71 (60–77)‡ | 74 (65–80)*,† | <0.001 |
| Male sex (%) | 59.8 | 65.8 | 58.8 | 0.222 |
| Body mass index (kg/m2) | 25.9 ± 4.8† | 26.5 ± 4.2*,‡ | 25.5 ± 3.7† | 0.014 |
| Systolic blood pressure (mmHg) | 148 ± 30 | 145 ± 24 | 144 ± 25 | 0.327 |
| Diastolic blood pressure (mmHg) | 81 ± 14 | 81 ± 12 | 79 ± 13 | 0.237 |
| Hypertension (%) | 53.7 | 49.8 | 52.3 | 0.761 |
| Diabetes mellitus (%) | 17.4 | 15.7 | 20.7 | 0.344 |
| Previous myocardial infarction (%) | 13.6 | 10.0 | 16.3 | 0.114 |
| Dyslipidaemia (%) | 25.7 | 27.5 | 32.7 | 0.305 |
| Familial history (%) | 18.9 | 24.1 | 19.8 | 0.487 |
| NYHA function class (%) | ||||
| III/IV | 9.6 | 16.4 | 31.8 | <0.001 |
| Medication | ||||
| Beta-blockers (%) | 34.6 | 37.0 | 41.1 | 0.438 |
| Calcium channel blockers (%) | 22.0 | 25.9 | 18.6 | 0.155 |
| Renin–angiotensin–aldosterone inhibitor (%) | 37.6 | 42.5 | 40.3 | 0.681 |
| Statins (%) | 30.8 | 40.4 | 44.2 | 0.061 |
| Diuretics (%) | 24.3 | 30.1 | 32.6 | 0.294 |
| Electrocardiography | ||||
| QRS duration (ms) | 99 ± 18†,‡ | 100 ± 17*,‡ | 107 ± 23*,† | <0.001 |
| QRS morphology | <0.001 | |||
| Narrow QRS (%) | 68.8 | 60.8 | 50.7 | |
| Right bundle branch block (%) | 7.3 | 6.2 | 6.3 | |
| Left bundle branch block (%) | 1.0 | 1.9 | 10.8 | |
| Intraventricular conduction delay (%) | 22.9 | 31.1 | 32.2 | |
| Atrial fibrillation (%) | 2.1 | 1.4 | 5.5 | 0.005 |
Values are presented as mean ± standard deviation or median (95% confidence interval).
P-value by ANOVA with Bonferroni-correction (*P < 0.05 vs. mild AS, †P < 0.05 vs. moderate AS, ‡P < 0.05 vs. severe AS).
AS, aortic stenosis; NYHA, New York Heart Association.
Table 2.
Echocardiographic characteristics according to the severity of aortic stenosis
| Variables | Mild AS | Moderate AS | Severe AS | P-value |
|---|---|---|---|---|
| (n = 125) | (n = 233) | (n = 272) | ||
| Echocardiography | ||||
| Heart rate (bpm) | 72 ± 15 | 73 ± 13 | 74 ± 14 | 0.231 |
| LV mass index (g/m2) | 113.1 ± 25.5 | 114.0 ± 30.0 | 137.5 ± 39.8*,† | <0.001 |
| LV EDVI (mL/m2) | 50.8 ± 14.9 | 51.6 ± 14.8 | 60.0 ± 25.3*,† | <0.001 |
| LV ESVI (mL/m2) | 20.6 ± 8.0 | 21.3 ± 8.9 | 29.6 ± 21.6*,† | <0.001 |
| LVEF (%) | 60.0 ± 6.0 | 59.4 ± 7.8 | 54.5 ± 12.7*,† | <0.001 |
| LVEF <55% (%) | 22.8 | 26.8 | 38.6 | <0.001 |
| Stroke volume index (mL/m2) | 47.0 ± 13.0‡ | 46.0 ± 11.0‡ | 38.7 ± 10.2*,† | <0.001 |
| Transmitral E/A ratio | 0.90 ± 0.43 | 0.89 ± 0.32 | 1.04 ± 0.74 | 0.007 |
| Pulmonary S/D ratio | 1.45 ± 0.42 | 1.46 ± 0.42 | 1.36 ± 0.48 | 0.038 |
| E/E′ | 12.0 (9.7–16.6) | 13.0 (10.4–17.1) | 17.4 (12.4–24.7) | <0.001 |
| Peak aortic valve jet velocity (m/s) | 2.3 ± 0.4†,‡ | 3.1 ± 0.7*,‡ | 4.0 ± 0.8*,† | <0.001 |
| Mean aortic valve gradient (mmHg) | 12.8 ± 5.3†,‡ | 24.4 ± 11.6*,‡ | 42.0 ± 17.2*,† | <0.001 |
| Aortic valve area (cm2) | 1.69 ± 0.23†,‡ | 1.18 ± 0.16*,‡ | 0.72 ± 0.16*,† | <0.001 |
| Mechanical dispersion (ms) | 54.5 ± 17.2†,‡ | 56.7 ± 19.3*,‡ | 70.9 ± 24.3*,† | <0.001 |
Values are presented as mean ± standard deviation, median (interquartile range) or percentages.
P-value by ANOVA with Bonferroni-correction (*P < 0.05 vs. mild AS, †P < 0.05 vs. moderate AS, ‡P < 0.05 vs. severe AS).
AS, aortic stenosis; EDVI, end-diastolic volume index; EF, ejection fraction; ESVI, end-systolic volume index; LV, left ventricular.
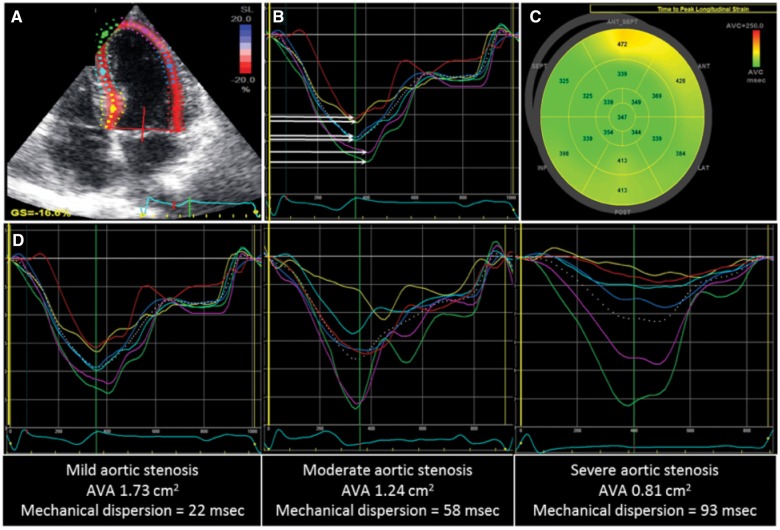
Speckle tracking echocardiographic analysis showed a mean LV mechanical dispersion of 62.4 ± 22.5 ms in the total population. LV mechanical dispersion increased along worsening severity in AS (54.5 ± 17.2 ms in mild AS, 56.7 ± 19.3 ms in moderate AS, and 70.9 ± 24.3 ms in severe AS, P < 0.001) (Figure 1). Table 3 shows the characteristics of the population dichotomized according to the mean value of LV mechanical dispersion [<62 ms: indicating less mechanical dispersion (homogeneous LV contraction), ≥62 ms: indicating more pronounced mechanical dispersion (heterogeneous LV contraction)]. Compared with patients with LV mechanical dispersion <62 ms (n = 352), patients with LV mechanical dispersion ≥62 ms (n = 278) had more frequently a history of myocardial infarction, were older and had more prolonged QRS duration on the ECG. Furthermore, patients with LV mechanical dispersion ≥62 ms showed larger LV volumes and mass index, worse LVEF and more severe AS on echocardiography.
Figure 1.
Evaluation of mechanical dispersion in patients with various degrees of aortic stenosis. Speckle tracking echocardiography of the left ventricle from the apical four-chamber view (A), long-axis view and two-chamber view is performed. The time durations (in ms) from Q/R on the ECG (B, yellow line) to peak longitudinal strain (B, white arrows) in 17 LV segments are automatically generated by the software (C) and LV mechanical dispersion is defined as the standard deviation of these 17 time durations. (D) Different examples of increasing AS severity where mechanical dispersion becomes more increased is shown. AVA, aortic valve area; ECG, electrocardiogram; LV, left ventricle.
Table 3.
Population characteristics according to LV mechanical dispersion
| Variables | LV mechanical dispersion <62 ms | LV mechanical dispersion ≥62 ms | P-value |
|---|---|---|---|
| (n = 352) | (n = 278) | ||
| Clinical | |||
| Age (years) | 66.6 ± 13.4 | 73.4 ± 10.6 | <0.001 |
| Male sex (%) | 60.8 | 62.2 | 0.742 |
| Body mass index (kg/m2) | 26.2 ± 4.1 | 25.6 ± 4.1 | 0.048 |
| Hypertension (%) | 48.9 | 54.8 | 0.163 |
| Diabetes mellitus (%) | 15.5 | 21.4 | 0.071 |
| Previous myocardial infarction (%) | 9.1 | 18.7 | 0.001 |
| Dyslipidaemia (%) | 28.9 | 30.0 | 0.784 |
| NYHA functional class (%) | |||
| III/IV | 19.0 | 25.6 | 0.076 |
| Electrocardiography | |||
| QRS duration (ms) | 99 ± 17 | 107 ± 23 | <0.001 |
| Atrial fibrillation (%) | 2.0 | 5.0 | 0.030 |
| Echocardiography | |||
| Heart rate (bpm) | 74 ± 14 | 72 ± 13 | 0.177 |
| LV mass index (g/m2) | 115.4 ± 31.4 | 134.9 ± 38.1 | <0.001 |
| LV EDVI (mL/m2) | 52.8 ± 16.3 | 57.9 ± 24.5 | 0.002 |
| LV ESVI (mL/m2) | 21.8 ± 10.3 | 28.5 ± 20.8 | <0.001 |
| LVEF (%) | 59.7 ± 7.9 | 54.1 ± 12.0 | <0.001 |
| Stroke volume index (mL/m2) | 44.8 ± 11.3 | 40.8 ± 11.9 | <0.001 |
| Peak aortic valve jet velocity (m/s) | 3.3 ± 0.9 | 3.4 ± 1.0 | 0.020 |
| Mean aortic valve gradient (mmHg) | 28.1 ± 17.1 | 31.7 ± 18.4 | 0.013 |
| Aortic valve area (cm2) | 1.17 ± 0.38 | 0.96 ± 0.41 | <0.001 |
| Mechanical dispersion (ms) | 47.0 ± 9.5 | 81.9 ± 18.7 | <0.001 |
Values are presented as mean ± standard deviation or percentages.
P-value by independent sample t-test or χ2.
EDVI, end-diastolic volume index; EF, ejection fraction; ESVI, end-systolic volume index; LV, left ventricular; NYHA, New York Heart Association.
Analysis of LV mechanical dispersion showed a low inter-observer variability (intraclass correlation coefficient = 0.776) and intra-observer variability (intraclass correlation coefficient = 0.847).
Independent correlates of mechanical dispersion
To investigate the independent factors associated with an increase in LV mechanical dispersion, a multivariable linear regression analysis model was constructed including clinical, electrocardiographic, and echocardiographic variables (Table 4). Univariable clinical associates of LV mechanical dispersion included older age, previous myocardial infarction, and the use of diuretics and statins. Furthermore, lower LVEF, higher LV mass index, lower aortic valve area and lower stroke volume index, and QRS prolongation were significant echocardiographic and electrocardiographic associates of LV mechanical dispersion. On multivariable analysis, older age [β = 0.28; (95% CI 0.15–0.42), P = 0.003], lower LVEF [β = −0.24; (95% CI −0.44 to −0.04), P = 0.020], smaller aortic valve area [β = −8.55; (95% CI −13.39 to −3.72), P = 0.001], larger LV mass index [β = 0.20; (95% CI 0.15–0.26), P < 0.001], and longer QRS duration [β = 1.12 per each 10 ms increase; (95% CI 0.25–1.99), P = 0.012] were independently associated with an increase in LV mechanical dispersion (Table 4).
Table 4.
Univariable and multivariable correlates of mechanical dispersion in aortic stenosis
| Variables | Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | |
| Age (years) | 0.47 | 0.34 to 0.61 | <0.001 | 0.28 | 0.15 to 0.42 | 0.003 |
| Sex (female) | −0.70 | −4.32 to 2.92 | 0.704 | |||
| Diabetes mellitus | 3.16 | −1.58 to 7.90 | 0.191 | |||
| Arterial hypertension | 2.50 | −1.15 to 6.15 | 0.179 | |||
| Previous myocardial infarction | 9.87 | 4.59 to 15.15 | <0.001 | 4.36 | −0.63 to 9.34 | 0.087 |
| Dyslipidaemia | −0.36 | −4.46 to 3.75 | 0.865 | |||
| History of atrial fibrillation | 2.05 | −3.78 to 7.88 | 0.490 | |||
| Beta-blockers | 2.15 | −1.63 to 5.93 | 0.265 | |||
| RAAS-inhibitors | 2.08 | −1.66 to 5.82 | 0.275 | |||
| Calcium blockers | 2.93 | −1.50 to 7.36 | 0.195 | |||
| Diuretics | 6.26 | 2.27 to 10.24 | 0.002 | 3.65 | −0.03 to 7.262 | 0.058 |
| Statins | 4.12 | 0.39 to 7.85 | 0.031 | 2.42 | −0.96 to 5.80 | 0.160 |
| LVEF (%) | −0.77 | −0.93 to −0.61 | <0.001 | −0.24 | −0.44 to −0.04 | 0.020 |
| LV mass index (g/m2) | 0.25 | 0.20 to 0.29 | <0.001 | 0.20 | 0.15 to 0.26 | <0.001 |
| Aortic valve area (cm2) | −19.12 | −23.20 to −15.04 | <0.001 | −8.55 | −13.39 to −3.72 | 0.001 |
| Stroke volume index (mL/m2) | −0.35 | −0.49 to −0.19 | <0.001 | −0.03 | −0.19 to 0.13 | 0.734 |
| QRS-duration (per 10 ms) | 3.24 | 2.35 to 4.14 | <0.001 | 1.12 | 0.25 to 1.99 | 0.012 |
Bold are those with a P<0.05 (significant).
CI, confidence interval; EF, ejection fraction; LV, left ventricular; RAAS, renin–angiotensin–aldosterone system.
Long-term follow-up
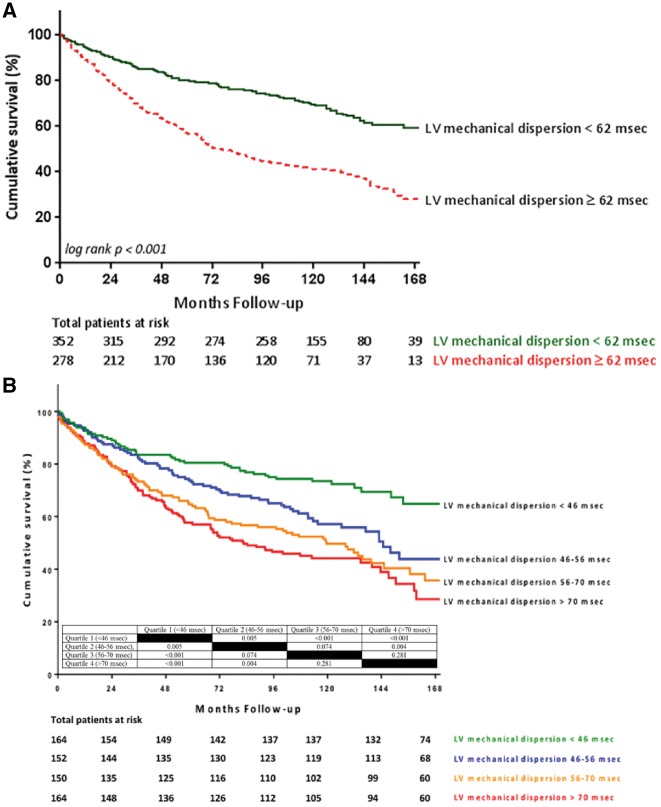
During a median follow-up of 107 (IQR 43–133) months, 246 patients received AVR and 302 died. Of those who died, 48.7% had severe AS, 33.1% had moderate AS, and 18.2% had mild AS at baseline. The cumulative event-free survival was significantly worse among patients with LV mechanical dispersion ≥62 ms, compared with patients with LV mechanical dispersion <62 ms (log rank P < 0.001) (Figure 2A). Similar results were observed when dividing the population into quartiles of LV mechanical dispersion (<46, 46–56, 56–70, and >70 ms) (Figure 2B). On univariable analysis, older age, previous myocardial infarction, diabetes mellitus, and QRS duration were associated with worse outcome, whereas AVR (as a time-dependent covariate) was related with improved outcome (Table 6). Furthermore, smaller aortic valve area, lower LVEF and stroke volume index, and larger LV mass index and LV mechanical dispersion were the echocardiographic parameters associated with worse outcome on univariable analysis. On multivariable analysis, older age (HR 1.05, 95% CI 1.04–1.06), smaller aortic valve area (HR 0.37, 95% CI 0.26–0.53), and lower LVEF (HR 0.98, 95% CI 0.97–0.99) were independently associated with worse outcome, whereas AVR as a time-dependent co-variate was associated with better survival (HR 0.42, 95% CI 0.32–0.56) (Table 5). The addition of LV mechanical dispersion to the baseline clinical model increased by 10% the risk of all-cause mortality (HR 1.10 per each 10 ms increase, 95% CI 1.04–1.15; P < 0.001) and resulted in a significant increment of the discrimination power of the model (difference in χ2 = +20.5, difference in log likelihood ratio = −12; P < 0.001 for both). Similar results for the multivariable analysis were found after excluding patients with prior myocardial infarction (n = 107) from the study population (Table 6). When performing IDI and NRI analyses, the addition of LV mechanical dispersion to a model containing age, LVEF, aortic valve area, and AVR as time dependent covariate did not result in significant change.
Figure 2.
Kaplan–Meier survival curve for the population dichotomized according to mechanical dispersion (A) and quartiles of mechanical dispersion (B). Table in B summarizes the inter-group P-value for survival rates between the different quartiles of mechanical dispersion.
Table 6.
Univariable and multivariable Cox proportional hazard models for the study population (n = 523), after exclusion of patients with prior myocardial infarction (n = 107)
| Variables | Baseline Model |
Baseline model + mechanical dispersion |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (years) | 1.08 | 1.03–1.09 | <0.001 | 1.07 | 1.04–1.08 | 0.012 |
| AVRa | 0.37 | 0.32–0.58 | <0.001 | 0.39 | 0.34–0.59 | <0.001 |
| Aortic valve area (cm2) | 0.42 | 0.25–0.54 | <0.001 | 0.44 | 0.32–0.78 | <0.001 |
| LVEF (%) | 0.96 | 0.93–0.98 | <0.001 | 0.95 | 0.93–0.98 | 0.038 |
| LV mechanical dispersion (per 10 ms) | 1.08 | 1.04–1.12 | <0.001 | |||
AVR, aortic valve replacement; EF, ejection fraction; LV, left ventricular.
Time dependent covariate.
Table 5.
Univariable and multivariable Cox proportional hazard models for the total population
| Variables | Univariables |
Baseline model |
Baseline model + mechanical dispersion |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (years) | 1.06 | 1.05–1.07 | <0.001 | 1.05 | 1.04–1.06 | <0.001 | 1.05 | 1.03–1.06 | <0.001 |
| Male sex | 0.94 | 0.75–1.19 | 0.612 | ||||||
| Previous myocardial infarction | 2.03 | 1.52–2.72 | <0.001 | ||||||
| Diabetes mellitus | 1.48 | 1.12–1.96 | 0.005 | ||||||
| Hypertension | 1.19 | 0.95–1.50 | 0.131 | ||||||
| QRS duration per 10 ms | 1.01 | 1.00–1.02 | 0.001 | ||||||
| AVRa | 0.40 | 0.28–0.56 | <0.001 | 0.42 | 0.32–0.56 | <0.001 | 0.43 | 0.32–0.57 | <0.001 |
| Aortic valve area (cm2) | 0.39 | 0.29–0.53 | <0.001 | 0.37 | 0.26–0.53 | <0.001 | 0.42 | 0.29–0.60 | <0.001 |
| LVEF (%) | 0.98 | 0.97–0.99 | <0.001 | 0.98 | 0.97–0.99 | <0.001 | 0.99 | 0.98–0.99 | 0.041 |
| LV mass index (per g/m2) | 1.01 | 1.00–1.01 | <0.001 | ||||||
| LV stroke volume index (mL/m2) | 0.98 | 0.97–0.99 | 0.001 | ||||||
| LV mechanical dispersion (per 10 ms) | 1.20 | 1.15–1.26 | <0.001 | 1.10 | 1.04–1.15 | <0.001 | |||
| Model discrimination Statistics | χ2 | 160.9 | 181.4b | ||||||
| −2 Log likelihood | 3478 | 3466b | |||||||
AVR, aortic valve replacement; EF, ejection fraction; LV, left ventricular.
Time-dependent co-variate.
Difference with baseline model P < 0.001.
Discussion
In a large unselected group of patients with various grades of AS, LV mechanical dispersion by speckle tracking echocardiography increased significantly with the severity of AS. Older age, lower LVEF, larger LV mass index, smaller aortic valve area, and more prolonged QRS duration were independently associated with increasing LV mechanical dispersion. Furthermore, LV mechanical dispersion was independently associated with increased all-cause mortality.
Determinants of LV mechanical dispersion in AS
LV mechanical dispersion reflects regional heterogeneity in myocardial contraction throughout the cardiac cycle. Among several factors, one of the underlying substrates of increased LV mechanical dispersion is the abnormally increased amount of myocardial fibrosis. After myocardial infarction, the amount of dispersion in myocardial contraction22 and ventricular dyssynchrony23,24 is related to the presence and size of myocardial scar. In patients with hypertrophic cardiomyopathy, an increase in mechanical dispersion has been correlated with the presence of fibrosis on cardiac magnetic resonance.25 In AS, there is progressive LV hypertrophy to reduce the wall stress and maintain the LV systolic function in response to the increased pressure afterload. If left untreated, severe AS is characterized by myocyte apoptosis and myocardial fibrosis.26 The increased fibrosis leads to slow conduction and heterogeneous myocardial activation which may be detected by speckle tracking echocardiography. An earlier study by Klaeboe et al.13 on the use of speckle tracking echocardiography in AS patients, was not powered enough to identify the independent correlates of increased LV mechanical dispersion. The current study, which includes a larger population with various grades of AS allowed us to investigate the independent determinants of increased LV mechanical dispersion. Non-modifiable factors associated with myocardial fibrosis, such as older age,27 parameters reflecting increased myocardial fibrosis such as low LVEF, and prolonged QRS duration,28,29 or associated with increased myocardial fibrosis such as severe AS and increased LV mass index30 were independent correlates of prolonged LV mechanical dispersion. These factors have also been associated with increased myocardial fibrosis assessed on histology or with late gadolinium contrast enhanced cardiac magnetic resonance.28–30 Accordingly, LV mechanical dispersion could potentially be used as a surrogate of myocardial fibrosis in patients with AS, however, this needs further prospective validation with cardiac magnetic resonance-derived fibrosis data.
Prognostic relevance of increased LV mechanical dispersion in AS
Current guidelines still advocate the use of LVEF as the main LV functional parameter to decide on AVR in severe AS.5 However, accumulating evidence has shown that other indirect markers (such as LV global longitudinal strain) or direct markers (late gadolinium enhancement on cardiac magnetic resonance) of myocardial fibrosis provide incremental prognostic value over LVEF.7,31–35 Those studies suggest that timely detection of myocardial fibrosis rather than deterioration of LVEF may be preferred to determine the optimal timing of AVR. The prognostic implications of LV mechanical dispersion in patients with AS have not been evaluated extensively.
In 162 patients with severe AS, Klaeboe et al.13 identified increased LV mechanical dispersion as an independent predictor of worse prognosis, which provided incremental prognostic value over LVEF. Our study provides more evidence in a much larger population with various grades of AS. LV mechanical dispersion had incremental prognostic value over LVEF, when corrected for age and AS severity at baseline and timing and occurrence of AVR during follow-up. The inclusion of patients with less than severe AS is clinically relevant, as concomitant LV systolic dysfunction in moderate AS is not infrequent and is associated with worse prognosis.36 It has been suggested that patients with moderate AS and reduced LVEF may also benefit from AVR.36,37 Whether prolonged LV mechanical dispersion can be used to justify AVR in this subset of patients, or, perhaps, as an indication for cardiac resynchronization therapy to try to correct it,38 needs to be investigated in randomized prospective studies. Furthermore, in a watchful waiting strategy in patients with significant AS, progressive increase of mechanical dispersion over time may be a more sensitive parameter for referral to AVR than decline in LVEF.
Limitations
The present retrospective evaluation has limitations inherent to its observational design. A potential selection bias cannot be excluded as these patients were referred to a tertiary referral centre. Further, this study included unselected patients with AS presenting with any grade of LV dysfunction at baseline. Although LVEF <55% was more prevalent with increasing severity of AS, this could also be attributed to a more frequent history of myocardial infarction in patients with more severe AS. However, on multivariable analysis, prior myocardial infarction was not significantly associated with an increase in LV mechanical dispersion. Also, after exclusion of these patients with prior myocardial infarction, the results of the multivariable analysis remained unchanged. Furthermore, all-cause mortality was chosen as the primary endpoint as these data were readily available. More specific causes of death, such as ventricular arrhythmias or sudden cardiac death, were not available for all patients and could have strengthened the results of the current study.
Conclusions
LV mechanical dispersion by speckle tracking echocardiography increases significantly along with the severity of AS and is associated with well-known reflectors of increased myocardial fibrosis. Furthermore, LV mechanical dispersion was independently associated with increased all-cause mortality.
Conflict of interest: The Department of Cardiology of the Leiden University Medical Center received research grants from Medtronic, Biotronik, Boston Scientific, GE Healthcare and Edwards Lifesciences. J.B. and V.D. received speaking fees from Abbott Vascular. All other authors declared no conflict of interest.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M.. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. [DOI] [PubMed] [Google Scholar]
- 2. d'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson-Stuttard J, Birks J. et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J 2016;37:3515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dweck MR, Boon NA, Newby DE.. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol 2012;60:1854–63. [DOI] [PubMed] [Google Scholar]
- 4. Chin CW, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G. et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017;10:1320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA. et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159–95. [DOI] [PubMed] [Google Scholar]
- 6. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ. et al. ; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 7. Ng AC, Delgado V, Bertini M, Antoni ML, van Bommel RJ, van Rijnsoever EP. et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011;32:1542–50. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann R, Altiok E, Friedman Z, Becker M, Frick M.. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography in comparison to late gadolinium enhancement cardiac magnetic resonance for analysis of myocardial fibrosis in severe aortic stenosis. Am J Cardiol 2014;114:1083–8. [DOI] [PubMed] [Google Scholar]
- 9. Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A. et al. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr 2012;25:667–73. [DOI] [PubMed] [Google Scholar]
- 10. Sarvari SI, Haugaa KH, Anfinsen OG, Leren TP, Smiseth OA, Kongsgaard E. et al. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J 2011;32:1089–96. [DOI] [PubMed] [Google Scholar]
- 11. Haugaa KH, Grenne BL, Eek CH, Ersbøll M, Valeur N, Svendsen JH. et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 2013;6:841–50. [DOI] [PubMed] [Google Scholar]
- 12. Ersbøll M, Valeur N, Andersen MJ, Mogensen UM, Vinther M, Svendsen JH. et al. Early echocardiographic deformation analysis for the prediction of sudden cardiac death and life-threatening arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 2013;6:851–60. [DOI] [PubMed] [Google Scholar]
- 13. Klaeboe LG, Haland TF, Leren IS, Rma TB, Brekke PH, Rosjo H. et al. Prognostic value of left ventricular deformation parameters in patients with severe aortic stenosis: a pilot study of the usefulness of strain echocardiography. J Am Soc Echocardiogr 2017;30:727–35.e1. [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 16. Haugaa KH, Smedsrud MK, Steen T, Kongsgaard E, Loennechen JP, Skjaerpe T. et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc Imaging 2010;3:247–56. [DOI] [PubMed] [Google Scholar]
- 17. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA. et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 18. Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A. et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:976–81. [DOI] [PubMed] [Google Scholar]
- 19. Sebag FA, Lellouche N, Chaachoui N, Dubois-Rande JL, Gueret P, Monin JL.. Prevalence and clinical impact of QRS duration in patients with low-flow/low-gradient aortic stenosis due to left ventricular systolic dysfunction. Eur J Heart Fail 2014;16:639–47. [DOI] [PubMed] [Google Scholar]
- 20. Minamino-Muta E, Kato T, Morimoto T, Taniguchi T, Inoko M, Haruna T. et al. Impact of the left ventricular mass index on the outcomes of severe aortic stenosis. Heart 2017;103:1992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lonnebakken MT, De Simone G, Saeed S, Boman K, Rossebo AB, Bahlmann E. et al. Impact of stroke volume on cardiovascular risk during progression of aortic valve stenosis. Heart 2017;103:1443–8. [DOI] [PubMed] [Google Scholar]
- 22. Muser D, Tioni C, Shah R, Selvanayagam JB, Nucifora G.. Prevalence, correlates, and prognostic relevance of myocardial mechanical dispersion as assessed by feature-tracking cardiac magnetic resonance after a first ST-segment elevation myocardial infarction. Am J Cardiol 2017;120:527–33. [DOI] [PubMed] [Google Scholar]
- 23. Nucifora G, Bertini M, Marsan NA, Delgado V, Scholte AJ, Ng AC. et al. Impact of left ventricular dyssynchrony early on left ventricular function after first acute myocardial infarction. Am J Cardiol 2010;105:306–11. [DOI] [PubMed] [Google Scholar]
- 24. Chang SA, Chang HJ, Choi SI, Chun EJ, Yoon YE, Kim HK. et al. Usefulness of left ventricular dyssynchrony after acute myocardial infarction, assessed by a tagging magnetic resonance image derived metric, as a determinant of ventricular remodeling. Am J Cardiol 2009;104:19–23. [DOI] [PubMed] [Google Scholar]
- 25. Haland TF, Almaas VM, Hasselberg NE, Saberniak J, Leren IS, Hopp E. et al. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 2016;17:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee SP, Lee W, Lee JM, Park EA, Kim HK, Kim YJ. et al. Assessment of diffuse myocardial fibrosis by using MR imaging in asymptomatic patients with aortic stenosis. Radiology 2015;274:359–69. [DOI] [PubMed] [Google Scholar]
- 27. Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ. et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;62:1280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazzoleni A, Curtin ME, Wolff R, Reiner L, Somes G.. On the relationship between heart weights, fibrosis, and QRS duration. J Electrocardiol 1975;8:233–6. [DOI] [PubMed] [Google Scholar]
- 29. Yamada T, Fukunami M, Ohmori M, Iwakura K, Kumagai K, Kondoh N. et al. New approach to the estimation of the extent of myocardial fibrosis in patients with dilated cardiomyopathy: use of signal-averaged electrocardiography. Am Heart J 1993;126:626–31. [DOI] [PubMed] [Google Scholar]
- 30. Lee SP, Park SJ, Kim YJ, Chang SA, Park EA, Kim HK. et al. Early detection of subclinical ventricular deterioration in aortic stenosis with cardiovascular magnetic resonance and echocardiography. J Cardiovasc Magn Reson 2013;15:72.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO. et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010;56:278–87. [DOI] [PubMed] [Google Scholar]
- 32. Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G. et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging 2012;13:827–33. [DOI] [PubMed] [Google Scholar]
- 33. Kamperidis V, van Rosendael PJ, Ng AC, Katsanos S, van der Kley F, Debonnaire P. et al. Impact of flow and left ventricular strain on outcome of patients with preserved left ventricular ejection fraction and low gradient severe aortic stenosis undergoing aortic valve replacement. Am J Cardiol 2014;114:1875–81. [DOI] [PubMed] [Google Scholar]
- 34. Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G. et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017;10:1320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vassiliou VS, Perperoglou A, Raphael CE, Joshi S, Malley T, Everett R. et al. Midwall fibrosis and 5-year outcome in moderate and severe aortic stenosis. J Am Coll Cardiol 2017;69:1755–6. [DOI] [PubMed] [Google Scholar]
- 36. van Gils L, Clavel MA, Vollema EM, Hahn RT, Spitzer E, Delgado V. et al. Prognostic implications of moderate aortic stenosis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2017;69:2383–92. [DOI] [PubMed] [Google Scholar]
- 37. Stewart WJ. Aortic stenosis is still very tricky, especially when it is moderate. J Am Coll Cardiol 2017;69:2393–6. [DOI] [PubMed] [Google Scholar]
- 38. Senechal M. What is the best therapeutic strategy in patients with low flow, low-gradient aortic stenosis, and wide QRS? Eur J Heart Fail 2014;16:598–600. [DOI] [PubMed] [Google Scholar]