Version Changes
Revised. Amendments from Version 1
Based upon the comments of reviewers the following changes have been made:
The conclusions which previously read: "…primary follicular occlusion as a pathogenic paradigm and the principal driver of HS is not consistent with the findings of this review…", has been altered to now read: "… primary follicular occlusion as a pathogenic paradigm and the principal driver of HS is unclear based upon the findings of this review…"
Minor points raised including the sentence which read: "Increased K16 expression was increased....", has now been corrected to read: "K16 expression was increased...."
Abstract
Background: Hidradenitis suppurativa (HS) is a chronic inflammatory disease with significant morbidity and impact on quality of life. Our understanding of the pathophysiology is incomplete, impairing efforts to develop novel therapeutic targets. Immunohistochemistry studies have produced conflicting results and no systematic evaluation of study methods and results has been undertaken to date.
Methods: This systematic review aimed to collate and describe all reports of immunohistochemical staining in HS. This systematic review was registered with PROSPERO and conducted in line with the PRISMA reporting guidelines. Potential bias was assessed using the NIH Criteria and antibodies used across various studies were tabulated and compared.
Results: A total of 22 articles were identified describing results from 494 HS patients and 168 controls. 87 unique immunohistochemical targets were identified. The overall quality of studies was sub-optimal with staining intensity confounded by active treatment. Conflicting data was identified and able to be reconciled through critical evaluation of the study methodology.
Conclusions: Keratinocyte hyperplasia with loss of cytokeratin markers co-localizes with inflammation comprising of dendritic Cells, T-lymphocytes and macrophages, which are known to play central roles in inflammation in HS. Primary follicular occlusion as a pathogenic paradigm and the principal driver of HS is unclear based upon the findings of this review. Inflammation as a primary driver of disease with secondary hyperkeratosis and follicular occlusion is more consistent with the current published data.
Keywords: Hidradenitis Suppurativa, Cytokeratin, Immunohistochemistry, Pathogenesis, Inflammation, Follicular Occlusion
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory disease, the exact pathophysiology of which remains incompletely defined 1. Numerous inflammatory mediators including TNF-α 2, IL-17 2, 3, IL-32 4 and IL-36 subtypes 5, 6 have been implicated in the disease. However, there is an incomplete understanding of the source and triggers of these mediators and how they sustain the chronic inflammation that characterizes this disease 1, 2. The pathogenic paradigm of HS has evolved dramatically since the first description by Velpau in 1839 7. First thought of as an apocrinitis of infectious aetiology, it is now considered a disorder of follicular occlusion and more recently an inflammatory disease characterised by a keratinocyte mediated inflammatory response 6. However, the variable response to topical, systemic and biologic therapies in HS 8 indicate our understanding of disease pathophysiology is incomplete when compared to other cutaneous inflammatory diseases such as psoriasis 9 and atopic dermatitis 10. Existing studies examining the histology and immunohistochemical profiling of HS tissues represent conflicting results, for example in the degree of dermal dendritic cell infiltration 11, 12 and the production of TNF-alpha in the follicular unit 13, 14. These results may be influenced by heterogeneous sampling methods, laboratory processing methods and data analysis 15. An additional complicating factor is that clinical comorbidities which are strongly associated with disease activity in HS, such as obesity 16, diabetes 17, inflammatory bowel disease 18, and smoking 19 also impact inflammatory cell activity in the skin 18, 20– 22. Hence it remains unclear whether the presence or absence of these conditions may confound the findings of immunohistochemical studies in HS 15, 23 and whether clinical stratification of patients is required to identify distinct pathogenic pathways, which may be amenable to pharmacological intervention. This variability across studies makes comparing data problematic. To date no systematic analysis of immunohistochemical studies has been undertaken to compare results, methodology and analytical techniques.
Objectives
The objectives of this systematic review are:
-
1)
To collate and describe all published reports of immunohistochemical studies in HS
-
2)
To critically evaluate the sampling, laboratory and analysis techniques used in each study to determine if comparisons can be made across studies.
Methods
This systematic review was registered with PROSPERO 24 (Registration number CRD42018104763) and was conducted in line with the PRISMA 25. The STROBE statement 26 was used to assess the observational studies included in this study.
Data sources
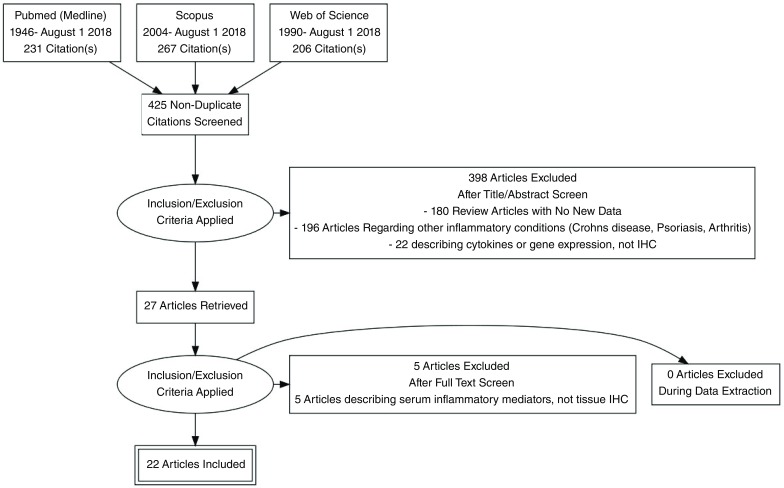
Information Sources for this review encompassed Pubmed (1946-July 1 2018), Scopus (2004- July 1 2018) and Web of Science (1990-July 1 2018) as shown in Figure 1. Search strategy is presented as Table 1.
Figure 1. PRISMA flowchart.
Table 1. Search Strategy for Systematic Review Entitled “A Systematic Review and Critical Evaluation of Immunohistochemisty Studies in Hidradenitis Suppurativa.
| Resources: | |
|---|---|
| 1) Pubmed (1946-July 1 2018),
2) Scopus (2004- July 1 2018) 3) Web of Science (1990-July 1 2018) 4) Published Abstracts 5) Contact with Authors for abstracts without full text for clarification of data and methodology |
|
| Pubmed Search Strategy: | |
| acne inversa OR apocrine acne OR apocrinitis OR Fox-den disease OR hidradenitis axillaris
OR HS OR pyodermia sinifica fistulans OR Velpeau’s disease OR Verneuil’s disease OR Hidradenitidis Suppurative AND IHC OR Immunohistochemistry OR Histology |
|
Study eligibility criteria
Eligibility criteria for this review included cohort studies, case-control studies and other observational studies with no restrictions of patient age, sex, ethnicity or language of publication. Eligible studies included those reporting the results of immunohistochemical findings in HS. Studies deemed not eligible included articles which provided no new data, only a review or summary of previously published data.
Appraisal and synthesis methods
Data collection was performed independently by 2 authors (JWF & JEH), with any disagreements regarding inclusion of citations being referred to a third author (JGK) for mediation. Information was collected using a standardized data collection form (available as Extended data 27) with the principal outcomes of interest being the immunohistochemical stain of interest, the site and rated intensity of staining (as described by authors), and comparison with perilesional/ unaffected/ control tissue. If data from individual patients was not available then the aggregate data was collected.
Potential sources of bias in the identified studies are acknowledged including the small size of patient cohorts, the variability in sampling and laboratory techniques, antibodies published and reactants used. Therefore these variables (where available) were collated to assess the heterogeneity of studies. Bias was also assessed using the NIH quality assessment tool for observational studies 28.
Results
A total of 425 non-duplicated citations were identified in the literature review ( Figure 1). 398 of these articles were removed upon review of titles and abstracts against the pre-defined eligibility criteria. Full text review of the remaining 27 articles excluded 5 articles providing no new data. The remaining 22 studies 4– 6, 11– 14, 29– 43 reporting the results of 494 individual HS patients and 168 control patients were used as the basis of this systematic review.
Descriptive analysis
The demographics of the patients of the included studies are presented in Table 2. Of 494 HS patients, 180 were male (38.3%) and 290 female (61.7%) with 24 cases unreported. Ages ranged from 15–72 years. 47/50 (94%) of reported cases were smokers, 12/30 (40%) had a BMI >30, and there was no information pertaining to diabetes or family history of HS. Of the 200 documented biopsy sites 93 were axillae (46.5%), 69 were inguinal (34.5%), and 38 were genital (19%) ( Table 3). 64 patients had Hurley staging with 7/64 (10.9%) Stage 1, 40/64 (62.5%) Stage 2 and 17/64 (26.6%) Stage 3. No individual Sartorius scores were reported. Where current treatment was reported, 6 patients (4.5%) were on adalimumab, 42 (31.6%) were untreated, 85 patients (63.9%) had treatment withheld prior to biopsy, and 357 cases were unreported. Lesional biopsies were taken from all studies, with 3 individual studies also taking perilesional biopsies 6, 30, 41. Age and Sex matched controls were present in 3 studies 29, 31, 43 and results were stratified in a minority of studies. 2 studies stratified by disease severity 4, 12, 7 studies stratified by lesion site 13, 33– 37, 39, 5 studies stratified by treatment 4, 5, 12, 29, 32, and no studies stratified by comorbidities. Analysis of immunohistochemical staining methodology varied and included quantitative analysis (3 studies) 14, 30, 32, semi-quantitative analysis (14 studies) 4, 5, 11– 13, 29, 34, 37– 43, and the presence or absence of staining (5 studies) 6, 31, 33, 35, 36. A total of 87 distinct immunohistochemical staining targets were identified ( Table 4, Table 5 and Table 6).
Table 2. Demographic data of included studies.
| Number
of HS Patients |
Male | Female | Mean Age
(Years) |
Comorbidities | Biopsy Sites | Hurley Staging | mHSS
Score (Mean) |
Therapy | Study
Reference |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoking | Obesity
(BMI>30) |
Diabetes | Family
History |
Axillae | Groin | Genital | ||||||||
| 18 | 11 | 7 | (Range 19–62) | NR | NR | NR | NR | NR | NR | NR | 14 | |||
| 15 | 6 | 9 | 38.7 | NR | NR | NR | NR | 9 | 4 | 2 | Stage 1=0
Stage 2=10 Stage 3=5 |
NR | Nil | 6 |
| 24 | 8 | 16 | 36.5 (range 21–51) | NR | NR | NR | NR | NR | NR | NR | Mean=2.29
(SD=0.62) |
NR | Untreated | 29 |
| 22 | 10 | 12 | 38.2 (Range 19–60) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 30 |
| 10 | 5 | 5 | 42 (Range 21–49) | NR | NR | NR | NR | Y | Y | N | Stage 2 (100%) | NR | Treatment
Withheld |
32 |
| 20 | 8 | 12 | 37.5 (Range 21–51) | N=18 | N=10 | NR | NR | NR | NR | NR | NR | NR | Treatment
Withheld (8 weeks) |
4 |
| 25 | 9 | 16 | 36 (Range 18–51) | NR | NR | NR | NR | NR | NR | NR | Mean =2.16
(SD=0.55) |
NR | Treatment
Withheld (3 weeks) |
5 |
| 47 | 19 | 28 | 42.3 (Range 22–54) | NR | NR | NR | NR | 48.3
(Range 8–144) |
NR | 31 | ||||
| 11 | 9 | 2 | 39.6 (Range 18–61) | NR | NR | NR | NR | NR | NR | NR | “Mod-Severe
Disease” |
NR | NR | |
| 20 | 6 | 14 | 40 (SD=15) | 19 | 27.6 (4.1) | NR | NR | 7 | 12 | 1 | Stage 1=4
Stage 2=11 Stage 3=5 |
NR | Treatment
withheld 3 weeks prior |
12 |
| 10 | 1 | 9 | 38 (SD=15) | 10 | 28.9 (SD 4.5) | NR | NR | 3 | 7 | 0 | Stage1=2
Stage2=7 Stage3=1 |
NR | Treatment
Withheld 3 weeks prior |
|
| 14 | 1 | 30 | NR | NR | NR | NR | 1 | 0 | NR | NR | NR | 13 | ||
| 1 | 42 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 25 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 22 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 45 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 27 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 38 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 34 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 59 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 41 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 33 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 46 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 49 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 1 | 31 | NR | NR | NR | NR | 1 | NR | NR | NR | |||||
| 60 | 26 | 34 | 37.3 (Range 15–67) | NR | NR | NR | NR | 1 | 6 | 1 | NR | NR | NR | 33 |
| 9 | 1 | 47 | NR | Y | NR | NR | 1 | 3 | NR | adalimumab | 11 | |||
| 1 | 31 | NR | N | NR | NR | 1 | 1 | NR | adalimumab | |||||
| 1 | 24 | NR | N | NR | NR | 1 | 3 | NR | adalimumab | |||||
| 1 | 32 | NR | N | NR | NR | 1 | 3 | NR | adalimumab | |||||
| 1 | 58 | NR | N | NR | NR | 1 | 3 | NR | adalimumab | |||||
| 1 | 58 | NR | N | NR | NR | 1 | 2 | NR | adalimumab | |||||
| 1 | 36 | NR | Y | NR | NR | 1 | 2 | NR | Nil | |||||
| 1 | 39 | NR | N | NR | NR | 1 | 3 | NR | Nil | |||||
| 1 | 67 | NR | N | NR | NR | 1 | 3 | NR | Nil | |||||
| 16 | 1 | 15 | NR | NR | N | NR | NR | 3 | 13 | NR | NR | NR | 34 | |
| 5 | 1 | 4 | 18-36 | NR | NR | NR | NR | 2 | 3 | NR | NR | NR | 35 | |
| 50 | 18 | 32 | 11-70 | NR | NR | NR | NR | 39 | 6 | 5 | NR | NR | NR | 36 |
| 14 | 11 | 3 | 16-72 | NR | NR | NR | NR | 2 | 12 | 0 | NR | NR | NR | 37 |
| 15 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 38 |
| 22 | 6 | 16 | 45.6 (Range 29–69) | NR | NR | NR | NR | 13 | 7 | 2 | NR | NR | NR | 39 |
| 9 | 3 | 6 | 44 (Range 32–70) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 40 |
| 12 | 0 | 12 | 29.4 (Range 19–42) | NR | NR | NR | NR | 3 | 0 | 9 | NR | NR | NR | 41 |
| 36 | 13 | 23 | 25 (Range 20–69) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 42 |
| 10 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 43 |
| 494 | 180 | 290 |
47/50
Reported |
12/30
Reported |
None
Reported |
None
Reported |
93/200 | 69/200 | 38/200 |
Hurley 1= 7
Hurley 2= 40 Hurley 3= 17 Unknown= 430 |
No
Individual Scores Reported |
adalimumab=6,
untreated=42, treatment withheld=- 85, not reported= 357 |
||
BMI= Body Mass Index mHSS= modified Hidradenitis Suppurativa Score (Sartorius Score) NR= Not Reported
Table 3. Critical Evaluation of Methodology of Studies Included in This Review.
| IHC Targets | Number
of HS Patients |
Number
of Controls |
Samples
Analyzed |
Age/Sex
Matched Controls |
Stratified by
severity |
Stratified by
lesion site |
Stratified
by Co- morbidities |
Stratified by
Treatment |
Immunostaining Intensity
Assessment |
Study
Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| α-MSH, LL37, S100A7, MIF, TNF-α, hBD3,
lysozyme |
18 | 12 | L | N | NR | N | N | N | Quantitiative
Immunohistomorphometry (Image J Software) |
14 |
| IL36 | 15 | 15 | L, PL | NR | NR | N | N | N | Present/ Absent | 6 |
| CD3, CD56 LL37 | 24 | 9 | L | Y | NR | NR | N | Y (untreated) | Semiquantitative (0-3) | 29 |
| CD1a, CD4, CD8 CD20, CD56, Factor XIIIa, IL17,
NLRP3, Caspase-1 |
22 | Yes (NR) | L, PL, U, C | NR | N | N | N | N | Cell Counting square grid
x400 magnification |
30 |
| IL-23, IL-12, CD68, CD4 | 10 | 8 | L, C | N | N | N | N | Y (ceased
3/52 prior) |
Positive stained cells per
mm 2 |
32 |
| IL-32 | 20 | 10 | L, C, S | N | Y | N | N | Y (ceased
8/52 prior) |
Semiquantitative (+ to ++++) | 4 |
| IL-36 | 25 | 7 | L, C, S | N | N | N | N | Y (ceased
3/25 prior) |
Semiquantitative (+ to ++++) | 5 |
| LCN2 | 10 | 16 | L | Y | N | N | N | N | Present/ Absent | 31 |
| CD11c | 20 | 6 | L | N | Y | N | N | Y | Semiquantitative (+ to ++++) | 12 |
| MMP2 hBD2 TNF-α | 14 | 2 | L, C | N | N | Y | N | N | Semiquantitative ((+ to
++++) |
13 |
| CD3, CD4, CD8, CD68 CD79 CD56 | 60 | Yes (NR) | L,C | N | N | Y | N | N | Present or Absent | 33 |
| CD3, CD4, CD8, CD20, CD138, CD14, CD68,
CD11c |
9 | Yes (NR) | L,C | N | N | N | N | N | Semiquantitative (+ to ++++) | 11 |
| GCDFP-15, CD15, Lysosyme, S100, Ca19-9,
HMB45 |
13 | 3 | L,C | N | N | Y | N | N | Semiquantitative (+ to ++++) | 34 |
| CD29, CTx-FITC | 5 | 4 | L,C | N | N | Y | N | N | Present or Absent | 35 |
| AE1/AE3/PKC26/ Enhanced Alkaline
Phosphatase |
50 | Y (NR) | L,C | N | N | Y | N | N | Present or Absent | 36 |
| K1, K10, K14, K16,k17, K19, | 14 | 1 | L,C | N | N | Y | N | N | Semiquantitative (+ to ++++) | 37 |
| Desmoplakin 1,2, Plakoglobin, Plakophilin 1,2,
Desmoglein 1,2,3, Desmocollin 1,2,3,K2e, K4, K5, K6, K7, CK8,CK9, CK10, K13, K13/15/16, K14, K17,K19, K20 Ki67 |
15 | Y (NR) | L,C | N | N | N | N | N | Semiquantitative (+ to ++++) | 38 |
| ER, AR | 22 | 10 | L,C | N | N | Y | N | N | Semiquantitative (+ to ++++) | 39 |
| TLR2, CD3, CD19, CD56,CD68, CD11c, CD1a,
CD206, CD207, CD209 |
9 | Y (NR) | L,C | N | N | N | N | N | Semiquantitative (+ to ++++) | 40 |
| TLR 2,3,4,7,9,ICAM-1, TNF-α, IL-6, IL-10, TGF-β,
α-MSH, hBD2, hBD4 IGF-1 |
12 | Y (NR) | L,PL | N | N | N | N | N | Semiquantitative (+ to ++++)
Epidermis Only |
41 |
| hBD3, S100A7, RNase7 | 36 | 57 | L,C | N | N | N | N | N | Semiquantitative (+ to ++++) | 42 |
| MMP8 | 10 | 8 | L,C | Y | N | N | N | N | Semiquantitative (+ to ++++) | 43 |
| 494 | 168 | 3/22 | 2/22 | 7/22 | 0/22 | 5/22 |
Table 3: Critical Evaluation of Methodology of Studies Included in This Review Key:L= Lesional, PL= Perilesional, U= Uninvolved, C= Control S=Serum, Y=Yes, N=No, NR= Not Reported, CTx-FITC =Cholera Toxin
Table 4. Data pertaining to distribution of cells expressing Immunohistochemical markers described in this review.
| Cell Type | Study | Results |
|---|---|---|
| Basal Keratinocytes | 13 | MMP2 Expressed |
| Suprabasal Keratinocytes | 31 | LCN 2 staining of suprabasal keratinocuytes |
| Dermal Fibroblasts | 13 | MMP2 expressed |
| 7 | 33/51 specimens associated with ++ fibrosis | |
| Neutrophils | 13 | MMP2 in keratinocytes, fibroblasts, macrophages and lymphocytes, |
| 30 | Significant increase in number of neutrophils in dermis Dermis> Perifollicular | |
| 31 | LCN2 in neutrophils – epidermis and dermis | |
| 7 | +++ infiltrate in 29/51 specimens | |
| Plasma Cells | 7 | +++ Plasma cell infiltrate in 2/51 specimens |
| Eosinophils | 7 | +++ Eosinophilic infiltrate ++ in 3/51 specimens |
| Histiocytes | 7 | +++ Infiltrate 24/51 speciments |
| Lymphocytes (NOS) | 13 | MMP2 expressed TNF alpha positivity in dermis |
| 44 | Lymphocytes, giant cell and necrosis in established lesions | |
| T Cells | 4 | CD3 + Dermis producing IL32C D 56 + NK T cells producing IL32 |
| 33 | lymphocytic mixed infiltrate perifollicular (with unruptured terminal follicles). This consisted of CD-3 (39%), CD-4 (30%), CD-8 (14%), and positive
cells (CD-4 ⁄ CD-8 ratio: 2.1:1). CD-56 (0.1%) and UCHL-1 (0%) brought no conclusive results. Conspicuous was a CD-8 cell positive folliculotropism in all immuno- histologies (Figure 3). CD-8 positive lymphocytes were loosely distributed not only in the stratum basale but also in the suprabasal epithelial areas. The subepidermal inflammatory infiltrate in the area of interfollicular epidermal hyperplasia showed a comparable cellular composition: CD-3 (38%), CD-4 (26%), CD-8 (19%), CD-56 (0.2%) and UCHL-1 (0%), CD-4 ⁄ CD-8 Ratio: 1.4:1. Here too, a CD-8 positive pronounced epidermotropism was impressive |
|
| 30 | At perifollicular sites, quantitative analysis showed a significant increase in the mean number of CD3+, CD4+ and CD8+ T lymphocytes (CD3+, 34 `
20 per HPF; CD4+, 38 ` 21; CD8+, 12 ` 8) compared with healthy control skin (CD3+, 9`4; CD4+, 2`1; CD8+, 1`1; |
|
| 3 | CD4 T cells producing IL17 in dermis | |
| 32 | CD4 T cells producing IL17 in dermis | |
| B Cells | 11, 12 | Psuedolymphomatous nests (see cytokine studies) |
| 33 | Perifollicular infiltrate with unruptured terminal follicles: CD-79 (35%) Subepidermal interfollicular Infiltrate: CD-79 (33%), | |
| Dendritic Cells | 11 | Successful Adalimumab treatment reduced influx of CD11c+ dendritic cells in lesional skin |
| 12 | Number of dendritic cells stable in skin- mild elevation only | |
| 4 | Dermis producing IL32 | |
| Macrophages | 13 | MMP2 expressed TNF alpha positivity |
| 30 | Significant increase in deep infiltrate | |
| 32 | Increase with co-staining of CD68/CD32 and IL12/ IL23 | |
| 33 | Perifollicular infiltrate with unruptured terminal follicles: CD-68 (12%) Subepidermal interfollicular Infiltrate: CD-68 (19%), | |
| 4 | Dermis producing IL32 | |
| Mast Cells | 30 | Significant increase in deep infiltrate |
| 12 | Significant increase in deep infiltrate |
Table 5. Reported Immunohistochemical Staining Results Identified in this Systematic Review.
|
IHC Staining
Target |
Epidermis | Dermis | Hair Follicles | Sinus Tracts | Subcutis |
Apocrine/
Eccrine Glands |
Study
Reference |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Suprabasal
Staining |
Basal
Staining |
Dermal
Staining |
Infundibular
Staining |
ORS
Staining |
Type 1 | Type 2 | Type 3 | |||||
| Type A | Type B | Type C | ||||||||||
| CD1a | + | 30 | ||||||||||
| ++ | + | 40 | ||||||||||
| +++ | 12 | |||||||||||
| CD3 | ++ | ++ | ++ | 30 | ||||||||
| + | + | + | 40 | |||||||||
| 33 | ||||||||||||
| CD4 | ++ | 12 | ||||||||||
| Interfollicular and perifollicular | 33 | |||||||||||
| + | + | 30 | ||||||||||
| 32 | ||||||||||||
| CD8 | + | 12 | ||||||||||
| Epidermotropism | Interfollicular and perifollicular | 33 | ||||||||||
| + | + | 30 | ||||||||||
| CD11c | +++ | 12 | ||||||||||
| +++ | 40 | |||||||||||
| CD14 | 12 | |||||||||||
| CD15 | + | 34 | ||||||||||
| CD19 | - | + | 40 | |||||||||
| CD20 | +++ | 30 | ||||||||||
| +++ | 12 | |||||||||||
| CD29 | + | + | + | + (NOS) | 35 | |||||||
| CD32 | 32 | |||||||||||
| CD56 | 30 | |||||||||||
| - | + | 40 | ||||||||||
| CD68 | Deep> Perifollicular | ++ | + | 30 | ||||||||
| Interfollicular and perifollicular | 33 | |||||||||||
| + | +++ | 40 | ||||||||||
| 32 | ||||||||||||
| CD79 | Interfollicular and perifollicular | 33 | ||||||||||
| CD138 | Mild infiltrate | 12 | ||||||||||
| CD206 | + | +++ | 40 | |||||||||
| CD207 | +++ * | + | 40 | |||||||||
| CD209 | ++ | ++++ | 40 | |||||||||
| Cytokeratins | ||||||||||||
| AE1 | Single K | 36 | ||||||||||
| AE3 | Single K | 36 | ||||||||||
| PKC26 | Single K | 36 | ||||||||||
| Factor XIIIa | DC + | 12 | ||||||||||
| + | + | 30 | ||||||||||
| K1 | Present in acanthotic epidermis | + | - | + | + | - | - | 37 | ||||
| K2e | ++ | - | - | - | 38 | |||||||
| K4 | - | - | - | - | 38 | |||||||
| K5 | + | + | + | + | 38 | |||||||
| K6 | - | + | + | + | 38 | |||||||
| K5/6 § | ++++ | 30 | ||||||||||
| K7 | - | - | - | + | 7 | |||||||
| K8 | - | - | - | - | 38 | |||||||
| K9 | - | - | - | - | 38 | |||||||
| K10 | - | 36 | ||||||||||
| Present in acanthotic Epidermis | + | - | + | + | - | - | 37 | |||||
| ++ | + | ++ | - | - | 38 | |||||||
| K13 | - | + | + | - | 38 | |||||||
| K13+15+16 § | + | + | + | + | + | 38 | ||||||
| K14 | Highly positive in acanthotic
epidermis |
+ | + | + | + | ++ | Sebaceous
Duct and Gland + |
37 | ||||
| + | + | + | 38 | |||||||||
| K15 | Stained
apocrine glands |
34 | ||||||||||
| K16 | Weakly positive in acanthotic
epidermis |
- | + | - | + | + | - | 37 | ||||
| K17 | Weakly positive in acanthotic
epidermis |
- | + | - | + | + | - | 37 | ||||
| K18 | 38 | |||||||||||
| K19 | + NOS | + | 36 | |||||||||
| Weakly positive in acanthotic
epidermis |
- | + | - | - | - | - | 37 | |||||
| - | - | ++ | 38 | |||||||||
| K20 | - | - | - | 38 | ||||||||
| Ki67 | + | ++ | ++ | ++ | 38 | |||||||
| ER | - | + | 39 | |||||||||
| AR | - | NC | 39 | |||||||||
| GCDFP-15 | Apocrine
glands |
34 | ||||||||||
| S100 | Eccrine
glands |
34 | ||||||||||
| Lysozyme | Vulval
cases only |
34 | ||||||||||
| ↓ in scarred cases | 14 | |||||||||||
| HMB45 | - | Negative
all cases |
34 | |||||||||
| TLR2 | ++ | ++++ | 40 | |||||||||
| ↓ | 41 | |||||||||||
| TLR3 | ↓ | 41 | ||||||||||
| TLR4 | ↓ | 41 | ||||||||||
| TLR7 | ↓ | 41 | ||||||||||
| TLR9 | ↓ | 41 | ||||||||||
| ICAM-1 | ↓ | 41 | ||||||||||
| TGF-β | ↓ | 41 | ||||||||||
| IGF-1 | ↓ | 41 | ||||||||||
| RNase7 | +++ | 42 | ||||||||||
| MMP2 | +++/++++ | +++/++++ | + | +++ | + NOS | 13 | ||||||
| MMP8 | (Neutrophils) | +++ | (Neutrophils) | 43 | ||||||||
| Cholera Toxin | Slopes of papillae suprabasal
epidermis, |
hair follicles | + NOS | 35 | ||||||||
| Desmoplakin
1 |
++ | ++ | ++ | + | 38 | |||||||
| Desmoplakin
2 |
++ | ++ | ++ | + | 38 | |||||||
| Plakoglobin | ++ | ++ | ++ | + | 38 | |||||||
| Plakophilin 1 | ++ | ++ | ++ | + | 38 | |||||||
| Plakophilin 2 | - | - | - | 38 | ||||||||
| Desmoglein 1 | ++ | + | ++ | ++ | - | 38 | ||||||
| Desmoglein 2 | + | + | ++ | ++ | 38 | |||||||
| Desmoglein 3 | ++ | ++ | ++ | + | 38 | |||||||
| Desmocollin 1 | ++ | + | ++ | - | - | 38 | ||||||
| Desmocollin 2 | ++ | + | ++ | ++ | + | 38 | ||||||
| Desmocollin 3 | ++ | ++ | ++ | + | 38 | |||||||
| hBD2 | ↓ | Negative in
12/14 |
1 | |||||||||
| ↓ | 41 | |||||||||||
| hBD3 | ++ (suprabasal) | - | ++ | 14 | ||||||||
| +++ | + | 42 | ||||||||||
| hBD4 | ↓ | 41 | ||||||||||
| TNF- α | ++/+++ (macrophage/
lymphocytes) |
++/+++ | +++ | 13 | ||||||||
| ++ | ++ | + | NC | ↓ | 14 | |||||||
| IL-6 | ↓ | 41 | ||||||||||
| IL-10 | ↓ | 41 | ||||||||||
| IL-12 | ++++ | 32 | ||||||||||
| IL-23 | ++++ | 32 | ||||||||||
| IL-17 | Diffuse | 30 | ||||||||||
| +++ | 32 | |||||||||||
| IL-32 | ++ | +++ | 4 | |||||||||
| IL-36 | + | +++ | 6 | |||||||||
| Suprabasal | +++ | 5 | ||||||||||
| Caspase1 | ++ | 30 | ||||||||||
| NLRP3 | ++ | 30 | ||||||||||
| MIF | ++ | ++ | 14 | |||||||||
| S100A7 | ++ | +++ | ++ | 14 | ||||||||
| ++ | 41 | |||||||||||
| LL-37 | ++ | ++ | NC | 14 | ||||||||
| ++ | +++ | ++ | 29 | |||||||||
| α -MSH | ++ | NC | 14 | |||||||||
Key: + to ++++ = Degree of positive staining, - = reported negative staining, NC= No Change; ↓ Decreased, NOS= Not Otherwise Specified, *= Statistically significant result compared with healthy controls, §=Pan Cytokeratin Stain, DC= Dendritic Cells, Single K= Single keratinocytes,
Table 6. Immunohistochemistry stains/antibodies used in included reviews.
| Target | Details | Study
Reference |
|---|---|---|
| CD1a | CloneO10; Dako Cytomartion | 30 |
| CloneO10; Dako Cytomartion | 40 | |
| O10 1:20 Immmunotech, Prague, Czech Republic | 12 | |
| CD3 | clone F7.2.38; Dako) | 30 |
| Polyclonal rabbit anti-human CD-3 dilution 1:25; Dako Cytomation Denmark A ⁄ S, Glostrup, Denmark), | 33 | |
| Polyclonal 1:150 Dako, Glostrup, Denmark | 12 | |
| Clone PC3/188A; DakoCytomation, Glostrup, Denmark | 40 | |
| CD4 | 4B12 1:160 Monosan Uden The Netherlands | 12 |
| monoclonal mouse anti-human CD-4 dilution 1:10; Vision Biosystems Novocastra, Newcastle, UK | 33 | |
| clone 4B12; Dako | 30 | |
| MT310 Dako | 32 | |
| CD8 | C9/144B 1:100 Dako | 12 |
| monoclonal mouse anti-human CD-8 dilution 1:50; Dako Cytomation Denmark A ⁄ S | 33 | |
| clone C8/144B; Dako | 30 | |
| CD11c | 5D11 1:60 Novocastra Newcastle Upon Tyne, UK | 12 |
| Clone KB90 DakoCytomation | 40 | |
| CD14 | MY4 1:100 Novocastra Newcastle Upon Tyne, UK | 12 |
| CD15 | Not Reported | 34 |
| CD19 | Clone HD37; DakoCytomation | 40 |
| CD20 | clone L26 (1,4); Dako | 30 |
| L 26 1:400 Dako | 12 | |
| CD29 | fluorescein-tagged B-subunit of cholera toxin (CTx-FITC) + CyChrome (Pharmingen BD Biosciences, Franklin Lakes, NJ, USA) | 35 |
| CD32 | KB61 Dako | 32 |
| CD56 | clone 123C3; Dako | 30 |
| Clone MOC-1; DakoCyotmation | 40 | |
| monoclonal mouse anti-human CD-56 1:50; Dako Cytomation Denmark A ⁄ S), | 33 | |
| 123C3.D5 1:25 Thermo Fisher Scientific Altrincham UK | 12 | |
| CD68 | Clone PG- M1; Dako) | 30 |
| monoclonal mouse anti-human CD-68 dilution 1:50; Dako Cytomation Denmark A ⁄ S) | 33 | |
| Clone EBM11; Dako Cytomation | 40 | |
| KP1 1:160 Dako | 12 | |
| EBM11 Dako | 32 | |
| CD79a | monoclonal mouse anti-human CD-79 dilution 1:25; Dako Cytomation Denmark A ⁄ S) | 33 |
| JCB117 1:100 Dako | 12 | |
| CD138 | B-A38 1:25 IQ Products Groningen, The Netherlands | 12 |
| CD206 | Clone 19.2; BD Biosciences Pharmingen | 40 |
| CD207 | Clone DCGM4; Immunotech, Marseilles, France | 40 |
| CD209 | Clone DCN46; BD Biosciences Pharmingen, San Diego Ca USA | 40 |
| Cytokeratins | ||
| Pankeratin | AE1/AE3/PKC26; Ventana Medical Systems SA, Illkirch, Cedex, France | 36 |
| AE1/AE3 1:200 Thermo Fisher Scientific | 12 | |
| Factor XIIIa | AC-1A1 1:200 Thermo Fisher Scientific | 12 |
| clone E980.1; Leica Biosystems Newcastle, Newcastle upon Tyne, U.K.) | 30 | |
| K1 | 34 Beta B4 Novo Castra Laboratories Ltd, Newcastle- upon-Tyne, UK) | 37 |
| K2e | Ks2́ 342́ 7.1 against CK 2e (Dr L.Langbein, Heidelberg, Germany), | 38 |
| K4 | 6B10 against CK 4, | 38 |
| K5 | AE 14 against CK 5, | 38 |
| K6 | Ks6.KA12 against CK 6, | 38 |
| K5/6 | clone M7237; Dako | 30 |
| CK7 | OV-TL 12/30 and Ks7 ´18 against CK 7, | 38 |
| K8 | CAM 5 ´2 against CK 8, | 38 |
| K9 | HK9TY1 (guinea-pig polyclonal) against CK 9 (Dr L.Langbein) | 38 |
| K10 | Not Reported | 36 |
| LHP1 Novo Castra Laboratories Ltd, Newcastle- upon-Tyne, UK) | 37 | |
| MoAbs K8́ 60 and DE-K10 against CK 10, | 38 | |
| K13 | Ks13 ´1 against CK 13, | 38 |
| K13+15+16 | Ks8 ´12 against CK 13 15 16, | 38 |
| K14 | LL001 Novo Castra Laboratories Ltd, Newcastle- upon-Tyne, UK) | 37 |
| LL001 against CK 14, | 38 | |
| K15 | Not Reported | 34 |
| K16 | LL025 Novo Castra Laboratories Ltd, Newcastle- upon-Tyne, UK) | 37 |
| K17 | E3 Novo Castra Laboratories Ltd, Newcastle- upon-Tyne, UK) | 37 |
| Ks17.E3 against CK 17, | 38 | |
| K19 | Not reported | 36 |
| B170 Novo Castra Laboratories Ltd, Newcastle- upon-Tyne, UK) | 37 | |
| Ks19́1 against CK 19, | 38 | |
| K20 | IT-Ks20́10 against CK 20 | 38 |
| Ki67 | MIB 1 against Ki-67 | 38 |
| MIB1 1:100 Dako | 12 | |
| ER | ER (Thermo Scientific; pretreatment EDTA, pH 9.0, dilution 1:80). | 39 |
| AR | AR (Santa Cruz; pretreatment citrate, pH 6.0, dilution 1:100) | 39 |
| GCDFP-15 | Not Reported | 34 |
| S100 | Not Reported | 34 |
| Lysozyme | Not Reported | 34 |
| A0099 pAbG 1:100 rabbit antihuman Dako Corporation | 14 | |
| HMB45 | Not Reported | 34 |
| TLR2 | Clone TL2.3; Alexis Corp. San Diego Ca USA | 40 |
| Santa Cruz Biotechnology, Inc, Santa Cruz, California | 41 | |
| TLR3 | Santa Cruz Biotechnology, Inc, Santa Cruz, California | 41 |
| TLR4 | Santa Cruz Biotechnology, Inc, Santa Cruz, California | 41 |
| TLR7 | Santa Cruz Biotechnology, Inc, Santa Cruz, California | 41 |
| TLR9 | Santa Cruz Biotechnology, Inc, Santa Cruz, California | 41 |
| ICAM-1 | Beckman Coulter, Inc, Brea, California | 41 |
| TGF-β | AbD Serotec | 41 |
| IGF-1 | R&D Systems, Inc, Lille, France | 41 |
| RNase7 | Dako | 42 |
| MMP2 | MMP-2 (cat no. AF902, LOT DUB034081, obtained from goat, 1:100 dilution, R&D Systems | 13 |
| MMP8 | Dako | 43 |
| Cholera Toxin | fluorescein-tagged B-subunit of cholera toxin (CTx-FITC) + CyChrome (Pharmingen BD Biosciences, Franklin Lakes, NJ, USA) | 35 |
| Desmoplakin 1 | DP 1 2±2́15 and DP 1±2́17 against DP I II, | 38 |
| Desmoplakin 2 | DP 1 2±2́15 and DP 1±2́17 against DP I II, | 38 |
| Plakoglobin | PG 5́1 and PG 11E4 (Dr M.J.Wheelock, Toledo, OH, U.S.A.) against PG, | 38 |
| Plakophilin 1 | PP1-9E7 and PP1-5C2 against PP 1, | 38 |
| Plakophilin 2 | PP2- 150 against PP 2, | 38 |
| Desmoglein 1 | Dsg1E-P124 and Dsg1E-P23 against Dsg1, | 38 |
| Desmoglein 2 | Dsg2E-G129 and Dsg2E- G96 against Dsg2, | 38 |
| Desmoglein 3 | Dsg3-G194 and 5G11 against Dsg3, | 38 |
| Desmocollin 1 | Dsc1-U100 against Dsc1, | 38 |
| Desmocollin 2 | DC-Rab 36 (rabbit polyclonal) against Dsc2, | 38 |
| Desmocollin 3 | MoAb Dsc3-U114 against Dsc3, | 38 |
| hBD2 | Human beta-defensin 2 (cat no. AF 2758, LOT VJU015051, obtained from goat, 1:100 dilution, R&D Systems, Germany) | 13 |
| Abcam, San Francisco, California | 41 | |
| hBD3 | 1:400 rabbit antihuman Donated by Prof Schroders Labor Kiel germany | 14 |
| 1:1000 rabbit ani-human PeproTech, Rocky Hill, N J | 42 | |
| hBD4 | Abcam, San Francisco, California | 41 |
| TNF-α | TNF-α (code ab 6671, obtained from rabbit, 1:100 dilution, Abcam, Cambridge, UK | 13 |
| 559071 mAbG 1:10 mouse antihuman R&D Systems | 14 | |
| IL-6 | AbD Serotec, Oxford, England | 41 |
| IL-10 | R&D Systems, Inc, Minneapolis, Minnesota | 41 |
| IL-12 | IL-12p7024945 R&D Systems | 32 |
| IL-23 | IL23p19 HLT2736 Biolegend | 32 |
| IL-17 | clone AF-317-NA; R&D Systems, Wiesbaden Germany | 30 |
| Polyclonal R& D Systems | 32 | |
| IL-32 | NBP-76684, Novus (Littleton, CO, U.S.A.) | 4 |
| IL-36 | rabbit polyclonal anti-IL-36a (C-terminal; ab180909), rabbit polyclonal anti- IL-36b (C-terminal; ab180890) and mouse
monoclonal anti-IL-36c (ab156783; all from Abcam, Cambridge, U.K. |
6 |
| AF 1078,1099,2320,1275 RnD | 5 | |
| Caspase1 | clone 14F468; Imgenex/Novus Biologicals, Littleton, CO, U.S.A.) | 30 |
| NLRP3 | clone Ab17267; Abcam, Cambridge, U.K. | 30 |
| MIF | MAB289 mAbG 1:100 mouse antihuman | 14 |
| S100A7 | HL15-4 mAbG 1: 20,000 mouse antihuman Donated by Prof Schroders Labor Kiel germany | 14 |
| LL37/
Cathelicidin |
Ab64892 pAbG 1:1000 rabbit antihuman Abcam | 14 |
| Rabbit anti-human LL-37 [Abcam, Cambridge, UK | 29 | |
| α-MSH | M0939 1:500 Rabbit Anithuman Sigma | 14 |
| PROGEN Biotechnik GmbH, Heidelberg, Germany | 41 | |
| Tryptase | AA1 1:800 Dako | 12 |
| clone AA1; Dako | 30 |
Immunohistochemistry results
Epidermis. The epidermis of HS lesional tissue expressed the normal array of keratins (K) in the basal (K5, K14) and suprabasal (K1, K2e, K10) layers. K6, K16 and K17 staining were increased compared to healthy controls in the suprabasal epidermis in one study 30, however, K6 and K17 staining was not increased in the epidermis (only in non-keratinized portions of sinus tracts) in a second study 38. Where K6 and K17 were positive in suprabasal epidermis, K17 staining was more pronounced than K6 staining 30. K19 was weakly positive in acanthotic epidermis 37. Ki67 staining was elevated in basal and suprabasal epidermis. Normal staining patterns of desmoplakin, plakophilin and plakoglobin were seen 38. Cells staining positive for CD1a, CD206, CD207 and CD209 were seen throughout the epidermis 40. CD3, CD4, CD8 and to a lesser degree CD68 positive cells demonstrated epidermotropism in sites of epidermal acanthosis 30, 33. CD29 and cholera toxin (double positive) staining cells were seen on the slopes of papillae of the epidermis 35. hBD2 (human beta defensin) staining was decreased throughout the epidermis in two studies 13, 41 whilst hBD3 staining was increased throughout the suprabasal epidermis 14, 42, however only significantly in Hurley Stage 1 and 2 patients (p=0.045) 42. hBD4 was decreased in suprabasal epidermis compared to healthy controls (p=0.001) 41. Contradictory findings were seen in toll like receptor (TLR) 2 staining with an increase in the epidermis co-localizing with dendritic cells and macrophages in one study 40 but suppressed in a second study 41. Levels of TLR3, TLR4, TLR7, TLR9, ICAM-1, TGF-Beta and IGF-1 were only assessed by one study and all were suppressed throughout the epidermis compared with controls 41. RNAase7 was increased in expression compared to healthy controls (p<0.05) 42. MMP2 was positively expressed in keratinocytes throughout the epidermis 13 and MMP8 in neutrophils within the epidermis 43. TNF-α was highly expressed in macrophages and lymphocytes present in the epidermis, particular in the basal layers 13, 14 and NLRP3, MIF, S100A7, LL37/Cathelicidin and α-MSH all positive in suprabasal keratinocytes 30, 41. IL-6 and IL-10 were reported as suppressed compared to healthy control skin 41, however, IL-36 subtypes were highly expressed in epidermal keratinocytes (more suprabasal than basal) 5, 6 with IL-32 also positive in the stratum granulosum 4.
Dermis. CD1a, CD11c, CD206, CD207, CD209 and Factor XIIIa positive cells were identified in the dermis in three separate studies 12, 30, 40, however the degree of infiltration varied. Dermal infiltrates of CD3, CD4, and CD8 positive cells, continuous with the epidermal infiltrates were a consistent feature of lesional HS dermis and were increased over controls 30, 33. The distribution of these cells was most pronounced in the interfollicular dermis (ie. towards the papillary slopes) and perifollicularly (ie. peri-infundibularly) 30, 33. CD56, CD68 and CD138 positive cells were diffusely seen throughout the dermis 40. CD19 and CD20 positive pseudolymphoid follicles have been noted in other studies 30, 40. Single keratinocytes have also been identified in the dermis which stain with pancytokeratin markers (AE1/AE3/PKC26) 36. Inflammatory cells in the dermis co-localized with TNF-α 13, 14, LL-37/cathelicidin 29, IL-12 32, IL-23 32, IL-17 30, 32, IL-32 4, TLR2 40 and MMP8 43. MMP2 co-localized with macrophages and fibroblasts 13. IL-36 was not identified in the dermis 5, 6.
Hair follicle. Cytokeratin staining of the follicular apparatus is consistent with normal K14, K16 and K17 staining. CD29 positive cells were identified in the infundibulum 35. CD3, CD4, CD8, CD68, Factor XIIIa positive cells were seen within the outer root sheath (ORS) contiguous with dense peri-follicular inflammation in the adjacent dermis 30, 33. The presence of inflammatory cells co-localized with MMP2 13, TNF- α 13, 14, and LL37/cathelcidicin 13, 29. hBD3 13, 42 and MIF 13 also stained positive in the ORS. One conflicting study reported no change in TNF-α staining of the follicular unit 13.
Sinus tracts. Staining patterns differed between superficial keratinized sinus tracts and deeper, inflamed non-keratinized sinus tracts. Normal epidermal cytokeratin staining was seen in the keratinized superficial portion of sinus tracts including K1, K10, K14 36– 38. Ki67 was elevated and CD29 positive cells were also identified in sinus tracts 35. Ki67 stained in both keratinized and non-keratinised portions of the sinus tract 38. K19 staining was absent in keratinized portions of sinus tracts 37. In deeper, inflamed, non-keratinized portions of the sinus tracts, K16, K17 and K19 were positive, with loss of K1, K10 and adhesions molecules including DG1 (desmoglein 1)and DSC1 (desmocollin 1) 37, 38. Apocrine gland nuclei stained weakly positive for estrogen receptor 39 and androgen receptor 39, and these results were reported as no different from control specimens 39. Lysozyme staining of apocrine glands was seen in cases of vulval HS only 34.
Immunohistochemistry methods. The list of antibodies used for IHC staining is presented in Table 6. Consistent antibodies were used for CD1a; CD20 and tryptase staining, whilst different antibodies were used for other staining targets. Antibodies used were not described in two studies 34, 36.
Assessment of Bias. The result of bias assessment using NIH criteria is presented in Table 7. All 22 articles clearly stated the research question of interest with well-defined study populations. The application of inclusion and exclusion criteria, or the calculation of sample size, or effect estimates were not described in any study. Exposures (ie. the presence of disease) were established and measured in all studies prior to the outcome measures (IHC staining) being assessed and the disease was established for such a time that a relationship between exposure and outcome would be identified if one existed. Different levels of exposure (severity of disease) was taken into account in only two studies 4, 12 and was consistently measured using Hurley staging across all studies. No articles accounted for all possible confounding variables such as obesity, diabetes, family history or smoking status ( Table 3).
Table 7. NIH Risk of Bias.
| Study
Reference |
1. Was
the research question or objective in this paper clearly stated? |
2. Was
the study population clearly specified and defined? |
3. Was the
participation rate of eligible persons at least 50%? |
4. Were all
the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? |
5. Was a
sample size justification, power description, or variance and effect estimates provided? |
6. For the
analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? |
7. Was the
timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? |
8. For
exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? |
9. Were the
exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? |
10. Was the
exposure(s) assessed more than once over time? |
11. Were the
outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? |
12. Were the
outcome assessors blinded to the exposure status of participants? |
13. Was
loss to follow- up after baseline 20% or less? |
14. Were key
potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emelianov
et al. 14 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Hessam
et al. 6 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Thomi
et al. 29 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Lima et al. 30 | Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Schlapbach
et al. 32 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Thomi
et al. 4 |
Y | Y | N/A | N | N | Y | Y | Y | Y | N | Y | NR | N/A | N |
| Thomi
et al. 5 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Wolk et al. 31 | Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Van der Zee
et al. 12 |
Y | Y | N/A | N | N | Y | Y | Y | Y | Y | Y | NR | N/A | N |
| Mozeika
et al. 13 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Von Laffert
et al. 33 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Van der Zee
et al. 11 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Heller
et al. 34 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Gniadecki
et al. 35 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Fismen
et al. 36 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Kurokawa
et al. 37 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Kurzen
et al. 38 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Buiner
et al. 39 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Hunger
et al. 40 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Derno
et al. 41 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Hofmann
et al. 42 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Tsaousi
et al. 43 |
Y | Y | N/A | N | N | Y | Y | N | Y | N | Y | NR | N/A | N |
| Total | 22/22 | 22/22 | N/A | 0/22 | 0/22 | 22/22 | 22/22 | 2/22 | 22/22 | 1/22 | 22/22 | NR | N/A | 0/22 |
Key: Y = Yes; N= No, NR= Not Reported N/A = Not Applicable
Discussion
Quality of data and risk of bias
The overall quality of data in this systematic review was sub-optimal with poor correction for potential confounding factors with only two of the 22 studies using objective measurement systems for IHC staining intensity 4, 12. The proportion of smokers was elevated (94%) compared to the rates of smoking in the HS population at large (70–89%) 45. A number of studies (17/22) did not stratify results by treatment therefore there is a risk that staining intensity of pro-inflammatory mediators may be reduced due to concomitant treatment at the time of biopsy. The use of de-paraffinized tissue in retrospective studies 30, 33, 34 can lead to false negatives in IHC dependent upon the preparation method of the original sample and the de-paraffinization process 15. Hence there are factors in the population studied in this review which may bring into question the reliability of staining quantification. However, the presence or absence of IHC staining, particularly when confirmed in multiple studies is still considered reliable despite the risks of bias.
Conflicting results
Conflicting results were identified in dermal CD1a staining 12, 30, 40, dermal CK19 staining 36– 38, Epidermal TLR2 staining 40, 41 and TNF alpha staining in the follicular infundibulum 13, 14. Regarding CD1a staining, two of the studies reported only a mild dermal infiltrate of CD1a positive cells 30, 40, with a third study demonstrating a significant infiltration of these cells 12. This third study clearly documented all treatment was withheld 3 weeks prior to the biopsies being taken 12, whereas there is no description in the other two articles regarding the discontinuation or ongoing use of treatments 30, 40. Therefore, with the possibility of partially treated disease, an artificial reduction in the number of dermal dendritic cells is a possibility as treatment for HS (such as adalimumab) has been demonstrated to effectively reduce the infiltration of dendritic cells in vivo 12. Similarly, studies examining TNF-alpha staining also differed in their stratification of patient based upon active treatment 13, 14. Significant reductions in TNF alpha staining were seen in the study with no documentation of treatment cessation 14 when compared to the one study with clear documentation that all patients had treatment ceased prior to biopsy 13. K19 staining was reported negative in all areas of the sinus tracts in one study 37, whereas two additional studies 36, 38 described positive K19 staining in sinus tracts (one study non-specifically 36 and the second in the deep inflamed, non-keratinized epithelium of the tract 38). The difference between these staining patterns may be explained by the presence of inflammation. Kurzen et al. 38 described the presence of K19 staining in non-keratinized epithelium of the deep sinus tracts only when associated with inflammation (Type 3 epithelia), staining was negative when no inflammation was present (Type 2 epithelia) 38. Kurokawa et al. did not differentiate between inflamed and non-inflamed non-keratinized epithelium in their study 37, and noted that the lesser degree of inflammation seen histologically may explain their differing results in comparison to Kurzen’s study 37.
Localization of production of inflammatory mediators
IHC staining, in particular co-staining with cellular markers and cytokines has enabled the localization of inflammatory mediators in order to ascertain the functional aspects of infiltrating inflammatory cells in HS, particularly highlighting the strong T h17 polarity of inflammation in HS 3. A schematic representation of the pathogenesis of HS based upon the findings of this review is presented in Figure 2. This highlights the inter-relationship between inflammation and hyperkeratinization. Localization of TNF-α 13, IL-12 32, IL-23 32 and IL-32 4, TLR2 40, MMP2 13, MMP8 43 and LL-37/cathelicidin 14, 29 production to infiltrating dermal macrophages and lymphocytes as well as localization of IL-36 subtypes 5, LL-37/cathelicidin 14, 29, IL-1β 32 and IL-22 32 to keratinocytes illustrate the feed forward mechanisms similar to those seen in psoriasis 9 and atopic dermatitis 10 which likely contribute to persistent inflammation in HS. Rather than keratinocytes being innocent bystanders, these IHC findings demonstrate the central role keratinocytes play as producers of key inflammatory mediators as well as mediators of products (such as TGF-β and ICAM) 41 that may contribute to fibroblast dysregulation and hypertrophic scarring 46. A remaining unanswered question includes the temporal relationship between keratinocyte hyperproliferation and the activation of inflammatory cells infiltrating the dermis and epidermis in HS.
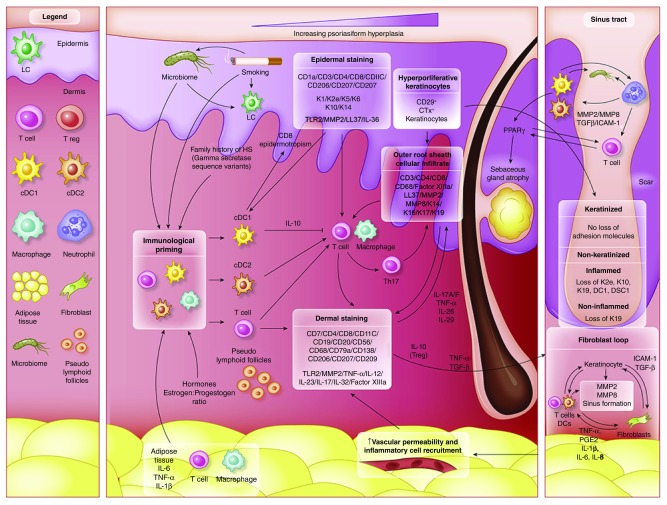
Figure 2. Schematic Representation of Immunohistochemical findings in hidradenitis suppurativa.
Immunological ‘priming’ occurs due to the contribution of adipose tissue, genetic susceptibility, smoking-related inflammatory mediators and obesity related pro-inflammatory signals and the composition of the microbiome. Increased activity of cDC1, cDC2 and T cells lead to both keratinocyte hyperplasia via the actions of IL-12 and IL-23, as well as a T h17 predominant immune response. Alterations of antimicrobial peptides (AMP’s) also occur throughout the epidermis. IHC staining localize Langerhan cells and activated dendritic cells to the epidermis and the dermo-epidermal junction. A population of epidermotropic CD8 T cells are also present. IHC staining indicates a mixed inflammatory infiltrate in the dermis, with contributions from Dendritic cells, B cells, T cells and plasma cells. Within sinus tracts, adhesion molecules are preserved, but inflammation in associated with non-keratinised sinus tracts leads to a loss of K19. The development of scarring and sinus tracts is associated with MMP2, ICAM-1 and TGF-Beta, with possible augmentation of ICAM-1 and TGF-B signaling via specific components of the microbiome. TNF-a, PGE2 and CXCL2 then lead to additional feed forward mechanisms perpetuating the inflammatory cycle.
Insights into pathophysiology of HS
The current pathophysiological paradigm of HS is one of follicular infundibular occlusion leading to follicle rupture and a resultant inflammatory cascade 1. This paradigm was based on the pivotal work of Shelley and Cahn in 1955 47, whom demonstrated the induction of HS after application of belladonna impregnated tape to manually epilated axillae of 12 men. Only 3 of the 12 men developed the lesions described, and infection from the manual epilation procedure could not be excluded as a cause of the lesions, but this study enabled the paradigm to slowly shift away from one of apocrinitis, which had been in place since the original descriptions of the disease 7. Detailed descriptions of infundibular hyperkeratosis (also termed poral occlusion) were made by Jemec et al. 7 and demonstrated the secondary involvement of apocrinitis in HS lesions. Jemec noted that poral occlusion was seen to occur alongside inflammation, but there was no suggestion of causation in one direction or another 7.
Although individual cases of epidermal hyperkeratosis in the absence of inflammation are noted 7, 33, these cases are established or chronic lesions associated with significant fibrosis which is documented to be associated with reduce inflammatory infiltrate 7, 33. A consistent finding in all studies of this review is the co-localization of infundibular ORS keratinocyte hyperplasia with CD3, CD4, CD8 and CD68 positive inflammatory cells expressing TNF-α, IL-12, IL-23 and IL-32 4, 13, 32, 40, 43. K19 is also documented as positive in the infundibulum suggesting keratinocyte hyperplasia 36– 38. However, it remains unclear whether keratinocyte hyperplasia induces the inflammatory cascade or if the inflammatory cascade induces the keratinocyte hyperplasia. The presence of inflammation in clinically normal, peri-lesional HS skin is well documented 4, 30, 33 implying the existence of a pre-clinical inflammation preceding symptoms of follicular occlusion. This is consistent with recent findings in acne pathogenesis that suggest that inflammation precede follicular hyperkeratosis and development of microcomedones 48 and is also pivotal in the ongoing development of nodulocystic acne and acne scars 49. This pre-clinical inflammation is also consistent with the pathogenic paradigm in psoriasis and atopic dermatitis 9, 10 with inflammation driving epidermal hyperkeratosis and alterations in keratinocyte maturation, consistent with the spongiform infundibulfolliculitis seen in established lesions of HS 50. Our disparate findings in K19 staining in deep non-keratinized sinus tract epithelia with and without inflammation 37, 38 also fit with this paradigm. In contrast, findings which would hold consistency with the current follicular occlusion paradigm would include infundibular occlusion preceding the development of inflammation, as well as alterations to desmosomal and hemidesmosomal proteins which would allow for rupture of the occluded follicles in order to drive the development of dermal inflammation and sinus tract formation. Although Danby et al. 51 reports reduced PAS positivity in the basement membrane zone at the sebo-follicular junction associated with inflammation in HS, it is likely that the reduced basement membrane integrity is secondary to inflammation and release of TGF-β and MMP2 52 (cytokines known to be altered in HS lesional skin and consistent with an abnormal wound healing response) rather than the follicular rupture being the primary driver of inflammation.
A more consistent hypothesis which accounts for the observed results of this review would be that of subclinical inflammation (due to a variety of triggers and immunological primers as illustrated in Figure 2) driving keratinocyte proliferation in the interfollicular epidermis and the follicular ORS, with follicular occlusion being a secondary phenomenon (mediated by TLR2 and IL-1α as documented in the development of comedones) 53. The development of sinus tracts and hypertrophic scarring may also be mediated by the keratinocyte inflammatory response given the alterations in important wound healing mediators including TGF-β, ICAM-1 and comparisons by other authors of an altered wound healing response 8 in HS. This comparison would be appropriate given the high levels of dermal MMP2 13 and MMP8 43; the loss of keratinocyte maturation markers (K2e, K10, K19) 36– 38 adhesion molecules (DG1 and DCN2) 38 in the non keratinized inflamed epithelium of the deep dermis; suppressed levels of ICAM-1 41 (seen impaired wound healing 54) and TGF-β 41 which leads to the dysregulation of TGF- β receptor ratio on fibroblasts which is linked with the development of hypertrophic scarring 46, 54 seen in HS. These alterations to keratinocyte maturation are reminiscent of epithelial mesenchymal transition (EMT) 52 which may also explain the presence of free keratinocytes in the dermis in established lesions of HS 7, 36. Indeed, as ICAM-1 is up-regulated by pro-inflammatory mediators 54, the low level of ICAM-1 noted appears paradoxical, however specific bacteria (including Porphyromonas species) which have been associated with HS 44, 55 can suppress ICAM-1 production as an immune evasion strategy 56. This implies that exogenous triggers (possibly including bacterial stimuli) can be a common cause for the initial inflammatory cascade as well as the development of tunneling and hypertrophic scarring in HS.
Conclusions
This systematic review of immunohistochemical staining of lesions in HS has highlighted the heterogeneity of studies and the methodological issues, which bring into question some of the results of IHC staining in HS lesions. The design of studies and variable reporting of potential confounding factors (such as ongoing or previous treatments) makes it impossible to compare staining intensity across studies. The results of existing studies suggest a florid inflammatory reaction comprising of T-lymphocytes, macrophages and dendritic cells with a strong Th-17 signature along with a keratinocyte mediated IL-36 inflammatory loop associated with keratinocyte hyperproliferation. The follicular occlusion paradigm as a primary driver of HS is unclear given the findings of this review and other histological and cytokine studies and inflammation as a primary driver of disease with secondary hyperkeratosis and occlusion is a plausible hypothesis.
Data availability
All data underlying the results are available as part of the article and no additional source data are required
Extended data
OSF: Extended data. Data collection sheet. https://doi.org/10.17605/OSF.IO/2JKPW 27
Licence: CC0 1.0 Universal
Reporting guidelines
OSF: PRISMA Checklist for ‘A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa’. https://doi.org/10.17605/OSF.IO/2JKPW 27
Licence: CC0 1.0 Universal
Funding Statement
Supported in part by a grant from the National Center for Advancing Translational Sciences (NCATS) [UL1 TR001866], National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Hoffman LK, Ghias MH, Lowes MA: Pathophysiology of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36(2):47–54. 10.12788/j.sder.2017.017 [DOI] [PubMed] [Google Scholar]
- 2. Melnik BC, John SM, Chen W, et al. : T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa/acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. Br J Dermatol. 2018;179(2):260–272. 10.1111/bjd.16561 [DOI] [PubMed] [Google Scholar]
- 3. Moran B, Sweeney CM, Hughes R, et al. : Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which is Corrected by Anti-TNF Therapy. J Invest Dermatol. 2017;137(11):2389–2395. 10.1016/j.jid.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 4. Thomi R, Yerly D, Yawalkar N, et al. : Interleukin-32 is highly expressed in lesions of hidradenitis suppurativa. Br J Dermatol. 2017;177(5):1358–1366. 10.1111/bjd.15458 [DOI] [PubMed] [Google Scholar]
- 5. Thomi R, Kakeda M, Yawalkar N, et al. : Increased expression of the interleukin-36 cytokines in lesions of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2017;31(12):2091–2096. 10.1111/jdv.14389 [DOI] [PubMed] [Google Scholar]
- 6. Hessam S, Sand M, Gambichler T, et al. : Interleukin-36 in hidradenitis suppurativa: evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br J Dermatol. 2018;178(3):761–767. 10.1111/bjd.16019 [DOI] [PubMed] [Google Scholar]
- 7. Jemec GB, Hansen U: Histology of hidradenitis suppurativa. J AM Acad Dermatol. 1996;34(6):994–999. 10.1016/S0190-9622(96)90277-7 [DOI] [PubMed] [Google Scholar]
- 8. Negus D, Ahn C, Huang W: An update on the pathogenesis of hidradenitis suppurativa: implications for therapy. Expert Rev Clin Immunol. 2018;14(4):275–283. 10.1080/1744666X.2018.1449647 [DOI] [PubMed] [Google Scholar]
- 9. Hawkes JE, Chan TC, Krueger JG: Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653. 10.1016/j.jaci.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brunner PM, Guttman-Yassky E, Leung DY: The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4S):S65–S76. 10.1016/j.jaci.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Zee HH, de Ruiter L, van den Broecke DG, et al. : Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164(6):1292–1298. 10.1111/j.1365-2133.2011.10254.x [DOI] [PubMed] [Google Scholar]
- 12. van der Zee HH, Laman JD, de Ruiter L, et al. : Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br J Dermatol. 2012;166(2):298–305. 10.1111/j.1365-2133.2011.10698.x [DOI] [PubMed] [Google Scholar]
- 13. Mozeika E, Pilmane M, Nürnberg BM, et al. : Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm Venereol. 2013;93(3):301–304. 10.2340/00015555-1492 [DOI] [PubMed] [Google Scholar]
- 14. Emelianov VU, Bechara FG, Glasera R, et al. : Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. Br J Dermatol. 2012;166(5):1023–1034. 10.1111/j.1365-2133.2011.10765.x [DOI] [PubMed] [Google Scholar]
- 15. Wester K, Wahlund E, Sundström C, et al. : Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000;8(1):61–70. [PubMed] [Google Scholar]
- 16. Ergun T: Hidradenitis suppurativa and the metabolic syndrome. Clin Dermatol. 2018;36(1):41–47. 10.1016/j.clindermatol.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 17. Garg A, Birabaharan M, Strunk A: Prevalence of type 2 diabetes mellitus among patients with hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2018;79(1):71–76. 10.1016/j.jaad.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 18. Lukach AJ, Saul MI, Ferris LK, et al. : Risk Factors for Hidradenitis Suppurativa in Patients with Inflammatory Bowel Disease. Dig Dis Sci. 2018;63(3):755–760. 10.1007/s10620-018-4919-5 [DOI] [PubMed] [Google Scholar]
- 19. Garg A, Papagermanos V, MIdura M, et al. : Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178(3):709–714. 10.1111/bjd.15939 [DOI] [PubMed] [Google Scholar]
- 20. Sgambato JA, Jones BA, Caraway JW, et al. : Inflammatory profile analysis reveals differences in cytokine expression between smokers, moist snuff users, and dual users compared to non-tobacco consumers. Cytoine. 2018;107:43–51. 10.1016/j.cyto.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 21. Spoto B, Di Betta E, Mattace-Raso F, et al. : Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr Metab Cardiovasc Dis. 2014;24(10):1137–43. 10.1016/j.numecd.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 22. Rehman K, Akash MS: Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23(1):87. 10.1186/s12929-016-0303-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou X, Fragala MS, McElhaney JE, et al. : Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care. 2010;13(5):541–547. 10.1097/MCO.0b013e32833cf3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frew J, Hawkes J, Krueger J: A Systematic Review of Immunohistochemical studies in Hidradenitis Suppurativa. PROSPERO. 2018; CRD42018104763. Accessed 27 August 2018. Reference Source [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Elm E, Altman DG, Egger M, et al. : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 27. Frew J: A Systematic Review and Critical Evaluation of Immunohistochemical Associations in Hidradenitis Suppurativa.2018. 10.17605/OSF.IO/2JKPW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. NIH NIoH: Quality Assessment Tool for Observational Cohort and Cross Sectional Studies.2018. Accessed 30 July 2018. Reference Source [Google Scholar]
- 29. Thomi R, Schlapbach C, Yawalkar N, et al. : Elevated levels of the antimicrobial peptide LL-37 in hidradenitis suppurativa are associated with a Th1/Th17 immune response. Exp Dermatol. 2018;27(2):172–177. 10.1111/exd.13482 [DOI] [PubMed] [Google Scholar]
- 30. Lima AL, Karl I, Giner T, et al. : Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174(3):514–521. 10.1111/bjd.14214 [DOI] [PubMed] [Google Scholar]
- 31. Wolk K, Wenzel J, Tsaousi A, et al. : Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. British J Dermatol. 2017;177(5):1385–1393. 10.1111/bjd.15424 [DOI] [PubMed] [Google Scholar]
- 32. Schlapbach C, Hanni T, Yawalkar N, et al. : Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790–8. 10.1016/j.jaad.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 33. von Laffert M, Stadie V, Wohlrab J, et al. : Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164(2):367–371. 10.1111/j.1365-2133.2010.10034.x [DOI] [PubMed] [Google Scholar]
- 34. Heller DS, Haefner HK, Hameed M, et al. : Vulvar hidradenitis suppurativa. Immunohistochemical evaluation of apocrine and eccrine involvement. J Reprod Med. 2002;47(9):695–700. [PubMed] [Google Scholar]
- 35. Gniadecki R, Jemec GB: Lipid raft-enriched stem cell-like keratinocytes in the epidermis, hair follicles and sinus tracts in hidradenitis suppurativa. Exp Dermatol. 2004;13(6):361–363. 10.1111/j.0906-6705.2004.00166.x [DOI] [PubMed] [Google Scholar]
- 36. Fismen S, Ingvarsson G, Moseng D, et al. : A clinical-pathological review of hidradenitis suppurativa: using immunohistochemistry one disease becomes two. APMIS. 2012;120(6):433–440. 10.1111/j.1600-0463.2011.02771.x [DOI] [PubMed] [Google Scholar]
- 37. Kurokawa I, Nishijima S, Kusomoto K, et al. : Immunohistochemical study of cytokeratins in hidradenitis suppurativa (acne inversa). J Int Med Res. 2002;30(2):131–136. 10.1177/147323000203000205 [DOI] [PubMed] [Google Scholar]
- 38. Kurzen H, Jung EG, Hartschuh W, et al. : Forms of epithelial differentiation of draining sinus in acne inversa (hidradenitis suppurativa). Br J Dermatol. 1999;141(2):231–239. 10.1046/j.1365-2133.1999.02970.x [DOI] [PubMed] [Google Scholar]
- 39. Buiner MG, Wobbes T, Klinkenbilj JH, et al. : Immunohistochemical analysis of steroid hormone receptors in hidradenitis suppurativa. Am J Dermatopathol. 2015;37(2):129–132. 10.1097/DAD.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 40. Hunger RE, Surovy AM, Hassan AS, et al. : Toll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptor. Br J Dermatol. 2008;158(4):691–697. 10.1111/j.1365-2133.2007.08425.x [DOI] [PubMed] [Google Scholar]
- 41. Dreno B, Khammari A, Brocard A, et al. : Hidradenitis suppurativa: the role of deficient cutaneous innate immunity. Arch Dermatol. 2012;148(2):182–186. 10.1001/archdermatol.2011.315 [DOI] [PubMed] [Google Scholar]
- 42. Hofmann SC, Saborowski V, Lange S, et al. : Expression of innate defense antimicrobial peptides in hidradenitis suppurativa. J Am Acad Dermatol. 2012;66(6):966–974. 10.1016/j.jaad.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 43. Tsaousi A, Witte E, Witte K, et al. : MMP8 Is Increased in Lesions and Blood of Acne Inversa Patients: A Potential Link to Skin Destruction and Metabolic Alterations. Mediators Inflamm. 2016;2016:4097574. 10.1155/2016/4097574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ring HC, Thorsen J, SAunte DM, et al. : The Follicular Skin Microbiome in Patients With Hidradenitis Suppurativa and Healthy Controls. JAMA Dermatol. 2017;153(9):897–905. 10.1001/jamadermatol.2017.0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Micheletti RG: Hidradenitis suppurativa: current views on epidemiology, pathogenesis, and pathophysiology. Semin Cutan Med Surg. 2014;33(3 Suppl):S48–S50. 10.12788/j.sder.0091 [DOI] [PubMed] [Google Scholar]
- 46. Zhu Z, Ding J, Tredget EE: The molecular basis of hypertrophic scars. Burns Trauma. 2016;4:2. 10.1186/s41038-015-0026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shelley WB, Cahn MM: The pathogenesis of hidradenitis suppurativa in man; experimental and histologic observations. AMA Arch Derm. 1955;72(6):562–565. 10.1001/archderm.1955.03730360068008 [DOI] [PubMed] [Google Scholar]
- 48. Del Rosso JQ, Kircik LH: The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 Suppl):s109–115. [PubMed] [Google Scholar]
- 49. Holland DB, Jeremy AH: The role of inflammation in the pathogenesis of acne and acne scarring. Semin Cutan Med Surg. 2005;24(2):79–83. 10.1016/j.sder.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 50. Boer J, Weltvreden EF: Hidradenitis suppurativa or acne inversa. A clinicopathological study of early lesions. Br J Dermatol. 1996;135(5):721–5. 10.1046/j.1365-2133.1996.d01-1069.x [DOI] [PubMed] [Google Scholar]
- 51. Danby FW, Jemec GB, Marsch WCh, et al. : Preliminary findings suggest hidradenitis suppurativa may be due to defective follicular support. Br J Dermatol. 2013;168(5):1034–1039. 10.1111/bjd.12233 [DOI] [PubMed] [Google Scholar]
- 52. Kalluri R, Weinberg RA: The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Selway JL, Kurczab T, Kealey T, et al. : Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10. 10.1186/1471-5945-13-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koivisto L, Heino J, Hakkinne L, et al. : Integrins in Wound Healing. Adv Wounds Care (New Rochelle). 2014;3(12):762–783. 10.1089/wound.2013.0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guen-Revillet H, Jais JP, Ungeheuer MN, et al. : The Microbiological Landscape of Anaerobic Infections in Hidradenitis Suppurativa: A Prospective Metagenomic Study. Clin Infec Dis. 2017;65(2):282–291. 10.1093/cid/cix285 [DOI] [PubMed] [Google Scholar]
- 56. Huang GT, Kim D, Lee JK, et al. : Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect Immun. 2001;69(3):1364–1372. 10.1128/IAI.69.3.1364-1372.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]