Abstract
A wide variety of the roots and tubers plays a major role in human diet, animal feed, and industrial raw materials. Sweet potatoes (SPs) play an immense role in human diet and considered as second staple food in developed and underdeveloped countries. Moreover, SP production and management need low inputs compared to the other staple crops. The color of SP flesh varied from white, yellow, purple, and orange. Scientific studies reported the diversity in SP flesh color and connection with nutritional and sensory acceptability. Among all, orange‐fleshed sweet potato (OFSP) has been attracting food technologists and nutritionists due to its high content of carotenoids and pleasant sensory characteristics with color. Researchers reported the encouraging health effects of OFSP intervention into the staple food currently practicing in countries such as Uganda, Mozambique, Kenya, and Nigeria. Scientific reviews on the OFSP nutritional composition and role in vitamin A management (VAM) are hardly available in the published literature. So, this review is conducted to address the detailed nutritional composition (proximate, mineral, carotenoids, vitamins, phenolic acids, and antioxidant properties), role in vitamin A deficiency (VAD) management, and different food products that can be made from OFSP.
Keywords: antioxidants, orange‐fleshed sweet potato, polyphenols, sweet potato, β‐carotene
1. INTRODUCTION
Root and tuber crops play a significant role in agriculture and facilitate food security in many developing countries. In the year 2017 worldwide, 494.6 million tons of roots and tubers (including potato) are produced (FAOSTAT, 2019). Roots and tubers are part of diet for majority of the global population, with world average per capita consumption of 19.4 kg/year (2013–2015) and projecting to achieve 21.0 kg/year by 2025 (OCED‐FAO, 2016) and also contributing to animal feeds and industrial needs (starch source) (Scott, Rosegrant, & Ringler, 2000). Among the roots and tubers, SP (Ipomoea batatas) is very important after potato on the basis of production and consumption. SP is a dicotyledon associated with Convolvulaceae family and ranks worlds' seventh most important food crop (Ahn et al., 2010); it is a potential energy contributor and considered as fifth essential crop (fresh weight basis) after rice, wheat, maize, and sorghum (Ndolo, Nungo, Kapinga, & Agili, 2007). Currently, SP cultivation was reported in more than 115 nations (FAOSTAT, 2019), and SP was recognized as the secondary staple food and possess significant role in diet of humans in many underdeveloped countries (Van Jaarsveld et al., 2005). In contrast to the other staple food crops, Trancoso‐Reyes et al. (2016) defined that SP possess special attributes such as adoptability in wider topography, ability to grow in subsidiary circumstance, good productivity in short durations, and balanced nutritional composition. Sweet potato was reported to have good sensory acceptability due to the eye‐pleasing colors and sweet taste. This high sensory acceptability of some SP varieties was suitable in malnutrition management and facilitating food security in underdeveloped nations (Julianti, Rusmarilin, Ridwansyah, & Yusraini, 2017).
Central American countries are considered as the center of SP origin, but recent times they are extensively cultivated in the tropical, in the subtropical zone, and in few temperate locations with divergent agroclimatic conditions (Chandrasekara & Josheph Kumar, 2016). The roots of SP plants are primary storage organs and act as sinks to photosynthetic products, and it resulted in the deposition of different micro (phytochemicals)‐ and macro (carbohydrates)nutrients (Lemoine et al., 2013). SP was reported to have highest dry matter content due to its sink strength (Rukundo, Shimelis, Laing, & Gahakwa, 2013), and this crop was highly appreciated for the positive agronomic and nutritive characteristics (Abidin, Carey, Mallubhotla, & Sones, 2017).
Sweet potato production was reported to be 112.8 million tons (in 115 countries) in 2017, and China is the leading producer, followed by Nigeria and Tanzania, Indonesia, and Uganda (FAOSTAT, 2019). SP production and consumption in Africa, Asia, South American continents, and Caribbean islands are increased tremendously in recent times (Figures 1 and 2). SP is the most abundantly grown root crops in Africa. International Potato Center (2017) reported that sweet potato is 3rd vital food crop in seven central and eastern African countries, 4th priority crop in six South African nations, and 8th in four West African countries. SP is a key conventional crop, growing traditionally in limited area for domestic consumption purpose. SP is praised as a “poor man's” crop as it characteristically grown and consumed by meager communities especially by women‐headed families (Githunguri & Migwa, 2004; Ndolo et al., 2001). SP considered as the food security crop due to its low agriculture input requirements and high yields in wider climatic conditions (Ziska et al., 2009). SP crop is recently changing from a sustainable low‐input, low‐output crop to a significant cash crop. As a food security crop, it can be harvested at the point of demand as gradually (Tairo et al., 2005), also contributing to a reliable source of food and revenue to pastoral farmers who are frequently susceptible to regular crop damages.
Figure 1.
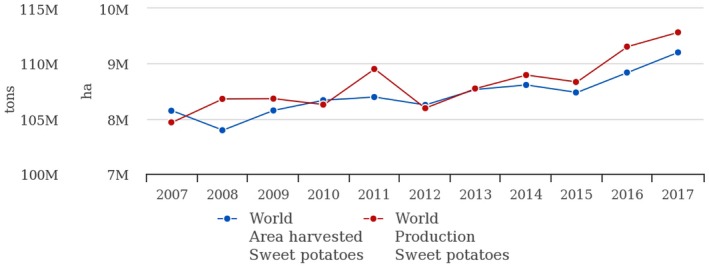
World area of harvested and production of sweet potato from 2017 to 2017 (source: FAOSTAT, 2019)
Figure 2.
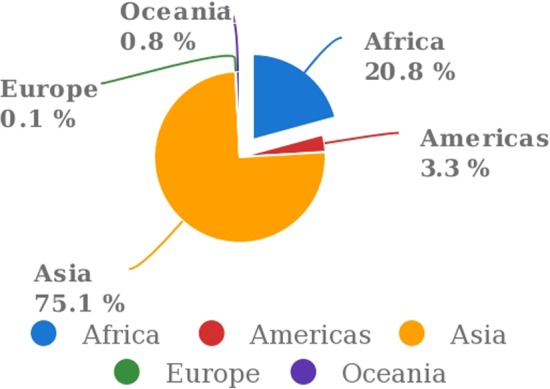
Production share of sweet potatoes by region from 2007 to 2017 (FAOSTAT, 2019)
Even though SP is a good source of carbohydrates (20%), the World Health Food Organization (WHFO) has acknowledged as the root crop with “antidiabetic” activity (Anbuselvi, Kumar, Selvakumar, Rao, & Anshumita, 2012). In vivo studies concluded that carbohydrate from SP stabilizes the sugar levels in blood and decreases the resistance to insulin (Mohanraj & Sivasankar, 2014). Hernández Suárez et al. (2016) reported that SP also provides the substantial quantities of selected vitamins (Vit C and PVA), specific minerals (potassium, magnesium, and calcium), and various bioactive compounds (phenolic acids and anthocyanins [ACN]) for consumers.
Researchers reported the clear role of variety difference in physical properties and chemical compositions of SP; for instance, van Jaarsveld, Marais, Harmse, Nestel, and Rodriguez‐Amaya (2006) reported that purple‐ and orange‐fleshed cultivars possess higher quantities of ACN and carotenes in comparison with white‐fleshed cultivars. In recent time, demand and attention on orange‐fleshed sweet potato (OFSP) are raised due to the high concentrations of β‐carotene (BC) and non‐pro‐vitamin A carotenoids (NPVAC). Kurabachew (2015) reported that OFSP has high potential to address VAD by food‐based intervention programs in targeted nations (Kurabachew, 2015). OFSP has given credit in recent scientific reports for its role in bakery, snack, and confectionery foods (Chen, Schols, & Voragen, 2003).
There are many scientific studies reported on the nutritional properties of the OFSP in different countries and varieties. Differences in nutritional contents are noticed among the varieties, and this created ambiguity in researchers. The review on the nutritional and bioactive composition of the OFSP is hardly available. So, this article is initiated to review the nutritional composition, bioactive components of OFSP, and role in VAD handling.
2. OFSP
From a dietary point of view and nutritional perspective, OFSP ranked as number 1 among all vegetables. OFSP tubers are considered as an significant dietary resource of VAC and NPVAC (Mohammad, Ziaul, & Sheikh, 2016). OFSP is appreciated due to the VA contribution and role in VAD eradication in developing countries (Girard et al., 2017; Kurabachew, 2015; Van Jaarsveld et al., 2005). Due to the many positive aspects related to agriculture, nutritional security and food security are resulted in intensified research on OFSP in present decade to augment its production and consumption in different countries. The OFSP possesses the characteristic of attractive sweet taste and eye‐pleasing yellow to orange color to children in comparison with white‐fleshed sweet potato (WFSP; Kaguongo, 2012); hence, OFSP has reported potential role to tackle calorific and VAD malnutrition problems of children in targeted communities.
Orange‐fleshed sweet potato is a good source of nondigestible dietary fiber, specific minerals, different vitamins, and antioxidants (Endrias, Negussie, & Gulelat, 2016; Rodrigues, Barbosa, & Barbosa, 2016). Phenolic compounds and carotenoids are responsible for distinguishing flesh and skin colors (deep yellow, red to orange, purple, and pale) of SP along with antioxidant properties (Steed & Truong, 2008). Scientists established the role of OFSP in health, and this accredited to its rich nutritional components with anticarcinogenic and cardiovascular disease (CVD)‐preventing attributes (Chandrasekara & Josheph Kumar, 2016; Jung, Lee, Kozukue, Levin, & Friedman, 2011). Recent scientific reports concluded the antioxidative and free radical scavenging activity of phenolic acid components in OFSP with beneficial health‐promoting activities (Bovell Benjamin, 2007; Rumbaoa, Cornago, & Geronimo, 2009; Teow et al., 2007). However, OFSP varieties with identical flesh color reported variations in phenolic content, individual phenolic acid profile, and antioxidant activity (AA) agents' concentrations. Reports on the OFSP incorporation in staple foods and its role in national food security and well‐being are readily available (Donado‐Pestana, Salgado, de Oliveira Rios, dos Santos, & Jablonski, 2012; Oki, Nagai, Yoshinaga, Nishiba, & Suda, 2006; Rumbaoa et al., 2009; Tang, Cai, & Xu, 2015; Teow et al., 2007).
3. NUTRITIONAL COMPOSITION OF OFSP
The OFSP is a good source of the basic nutrients and different vitamins, minerals, polyphenols, antioxidants, and ACN.
3.1. Proximate composition
Orange‐fleshed sweet potato is a second staple food crop in many countries of African and Asian continents for most of the population, so the proximate composition is very important to understand the role of this tuber crop in basic nutrition. In this section summarized is the proximate composition of the OFSP reported by different researchers. The proximate analyses of the OFSP from different countries and varieties are depicted in Table 1.
Table 1.
Proximate composition of orange‐fleshed sweet potato reported by different authors
| S. no. | Component | Rodrigues et al. (2016)a | $Huang et al. (1999)b | Endrias et al. (2016)c | Lyimo et al. (2010)d | Nascimento et al. (2015)e | Nicanuru et al. (2015)f | Mohammad et al. (2016)g | ||
|---|---|---|---|---|---|---|---|---|---|---|
| $Fresh | Flour driedh | Fresh | Un pealed | Pealed | Dry weight | Dry weight | Wet base | Wet base | ||
| 1 | Moisture | 69.42 | 10.97 | 62.2–78.2 | 76.97 | 74.84 | NR | NR | 64.5–70.4 | 70.97–72.96 |
| 2 | Ash | 2.04 | 2.11 | NR | 4.94 | 4.33 | 0.87–0.98 | 0.85 | NR | 1.17–1.31 |
| 3 | Protein | 3.69 | 4.80 | NR | 2.84 | 2.48 | 1.44–2.50 | 0.58 | 1.9–2.7 | 1.91–5.83 |
| 4 | Fats | 0.42 | 0.39 | NR | 1.00 | 1.12 | 0.03–0.95 | 0.19 | 1.1–1.7 | 0.17–0.63 |
| 5 | Starch | 65.41 | 33.66 | NR | NR | NR | NR | 26.34 | NR | NR |
| 6 | Crude fiber (total) | 3.68 | 2.57 | 2.01–3.23 | 4.52 | 3.83 | NR | NR | 3.0–3.6 | 0.35–0.54 |
| Soluble | NR | NR | 0.15–1.00 | NR | NR | NR | NR | NR | NR | |
| insoluble | NR | NR | 1.35–2.64 | NR | NR | NR | NR | NR | NR | |
| 7 | Total carbohydrate | 90.17 | 90.13 | NR | 86.72 | 88.01 | 23.91–33.45 | NR | 18.3–26.1 | 21.10–24.50 |
| 9 | pH | 6.55 | 6.52 | NR | NR | NR | NR | NR | NR | |
| 10 | Acidity | 1.08 | 0.91 | NR | NR | NR | NR | NR | NR | |
| 11 | Energy (Kcal/100g.) | NR | NR | NR | 373.97 | 373.05 | NR | 344.52 | NR | NR |
Abbreviation: NR, not reported.
Samples from Rio de Janeiro, Brazil.
Sample from Hawaii, range taken from seven varieties (Excel, Jewel, Kona, Makua yam, Regal, South Delite, and UH 71‐5).
Samples from Ethiopia.
Sample from three districts (Meatu, Sengerema, and Misungwi) of Lake Zone in Tanzania. (The varieties are Carrot Dar, Japon, Zapallo, and Mafuta.)
Samples from Brazil.
Samples from Tanzania (range of Jewel, Karoti Dar, Kabode, Ejumula varieties).
Range of different varieties (Tripti, Kamala Sundari, Daulatpuri, Misti Alu, Lalkothi, and Kalmegh) from Bangladesh.
Product from blanched at 100°C for 20 min and hot air oven dried at 65°C for 24 hr.
Moisture is the major component in OFSP, which accounts for >62% and <75% (Endrias et al., 2016; Lyimo, Gimbi, & Kihinga, 2010; Nicanuru, Laswai, & Sila, 2015). Other root and tubers also reported similar moisture contents such as potato (79%), cassava (60%), WFSP (77%), and yam (70%) (USDA, 2018). Usually, roots and tubers are consumed in fresh after boiling or minimal processing with traditional practices. Different researchers reported the various drying techniques for OFSP and converted into flour to the moisture content of <11% (Rodrigues et al.., 2016). The low moisture content is very important for OFSP flour to maintain long shelf life. These similar trends were reported in case of staple grains also, and usually at the time of harvesting, cereals possess moisture contents >22% to 25% and dried to 9%–13% for long‐term storage (Liu, Chen, Wu, & Peng, 2007). The moisture content of OFSP reported variation in different studies; this may be related to the diversity in variety, agroclimatic conditions, agriculture practices employed, etc.
Ash is an inorganic residue in any food substance, which directly denotes the mineral content. The ash values were reported from the range of 1.17%–4.33% (Mohammad et al., 2016), this broad range is obvious, and it happened due to the varietal and agrogeological differences. Also, processes such as peeling and blanching were followed by drying reported on the ash contents (Endrias et al., 2016). The ash contents of OFSP are comparable with other roots and tubers.
Proteins are very important nutrients for structural, functional performers of different biomolecules in human body, and they provide the essential amino acids required for metabolism. The protein in the range of 1.91%–5.83% was reported in OFSP (Mohammad et al., 2016). Usually, roots and tuber are the poor source for protein, which is similar in case of the OFSP. The protein contents of the OFSP are in similar to those of potato (2%), cassava (1.4%), WFSP (1.6%), and yam (1.5). In contrast, the protein compositions of OFSP are very low as compared to the staple food grains, such as maize (9.4%), rice (7.1%), wheat (12.6%), and sorghum (11.6%) (USDA, 2018). So, to combat the Protein Energy Malnutrition (PEM), it is very important to consume the protein‐rich pulses and animal foods among communities where OFSP is considered as second staple (Neumann, Harris, & Rogers, 2002).
Very low concentration (<1%) of the fat was reported in OFSP; usually, this trend is the property of roots and tubers (Lyimo et al., 2010; Mohammad et al., 2016; Rodrigues et al., 2016). The fat concentration of the OFSP is even little better than other roots and tubers such as potato (0.09%), cassava (0.28%), WFSP (0.05%), and yam (0.17%) (USDA, 2018). Usually, staple crops contain less concentration of the oils and fats; this is true in case of rice (0.66%) and wheat (1.54%). The fat concentrations directly influence the energy density of the food, but people with limited energy to avoid certain disease can consume OFSP (Hu, van Dam, & Liu, 2001). However, people in underdeveloped countries in Africa are consuming OFSP more so, and supplementation of the oilseeds and high‐energy foods is highly recommended to certain vulnerable groups (need high energy) (Butte, 2000).
The high starch (65.41%) concentration was reported in OFSP on fresh weight basis (Rodrigues et al., 2016). This is similar to other staple cereal, roots, and tubers. Starch is one of the very important energy sources for the consumers. So, OFSP can consume as the staple crop because of high concentration of carbohydrates (Jobling, 2004).
Crude fiber is one of the nondigestible carbohydrates, which provides the fecal bulkiness, less intestinal transit, role in cholesterol level reduction, and trapping dangerous substance like cancer‐causing agents, and also encourages the growth of natural microbial flora in gut (Dhingra, Michael, Rajput, & Patil, 2012; Sánchez‐Zapata, Viuda‐Martos, Fernández‐LÓpez, & Pérez‐Alvarez, 2015; Satija & Hu, 2012; Slavin, 2013). The total dietary fiber of 3.6% (maximum) was reported in OFSP, but the lower concentrations like 0.35% were reported in different varieties of OFSP (Endrias et al., 2016). These variations may be related to the varietal and agroclimatic differences of the crop. The crude fiber of OFSP is higher than potatoes (2.2%) and cassava (1.8%); however, the comparable concentrations of yam were reported in OFSP. In case of the staple cereals such as maize (7.3%) and wheat (12.2%), they reported high concentration of the fiber than OFSP (USDA, 2018). Usually, cereals contain outer bran layers, which are high source of the crude fibers. The good concentration of crude fiber is an advantage for OFSP consumers. Very limited studies are reported on soluble and insoluble fibers of OFSP, Huang, Tanudjaja, and Lum (1999) reported high concentration of the insoluble dietary fiber (1.35%–2.64%) compared to soluble (0.15%–1.00%), and this is a positive nutritional aspect of the OFSP.
Energy density is a very important property of the staple crops, for example, 344.52–375.05 kcal/100 g in OFSP (Endrias et al., 2016; Nascimento, Dhiego, Cristina, José, & Maria, 2015). The energy of OFSP is comparable with the cereals such as maize (365.2 kcal/100 g) and rice (365 kcal/100 g); in comparison with the other roots and tubers, the energy composition of the OFSP was reported better. Due to this property, the OFSP is one of the choices for staple foods (Prentice & Jebb, 2003).
pH and TSS are very important parameters that influence the taste and overall sensory acceptability of foods. The almost neutral pH (6.52) and TSS (acidity) of 1% (Rodrigues et al., 2016) were reported in the OFSP. It denotes the plain flavor of the OFSP, which is suitable for all age groups as a staple food.
4. MINERAL COMPOSITION OF THE OFSP
Minerals are the inorganic components, having very specific and important role in metabolism (Soetan, Olaiya, & Oyewole, 2010). Consumption of optimum concentration of minerals is recommended (Soetan et al., 2010). The mineral compositions of the OFSP from different researchers are tabulated in Table 2.
Table 2.
Minerals (mg/100 g) in orange‐fleshed Sweet potato reported by different authors from varieties around the world
| S. no. | Mineral | RDA mg | Laurie et al. (2012)a | Endrias et al. (2016)b | Sanoussi et al. (2016)c | Ukom et al. (2009)d | |
|---|---|---|---|---|---|---|---|
| Un pealed | Pealed | ||||||
| 1 | Calcium | 1,000 | 34–39 | 47.00 | 45.54 | 24.40–29.97 | 50.20–90.40 |
| 2 | Magnesium | 130 | 15–37 | 3.00 | UD | 23.50–25.40 | 12.20–30.40 |
| 3 | Phosphorous | 500 | 28–51 | 22.11 | 20.67 | 42.00–43.67 | 18.80–27.50 |
| 4 | Potassium | 3,000 | 191–334 | NR | NR | 310.67–315.33 | 138.00–260.00 |
| 5 | Iron | 10 | 0.73–1.26 | 15.26 | 11.45 | 0.63–0.73 | NR |
| 6 | Zinc | 5 | 0.51–0.69 | 1.30 | 0.93 | 0.24–0.73 | NR |
| 7 | Sodium | 1,500 | NR | NR | NR | 30.33–33.10 | 23.00–59.00 |
Abbreviation: NR, not reported.
Range mean was taken from study done by nine OFSP varieties 3 (Khano, Serolane, and Impilo), from South Africa.
Sample from Ethiopia, UD, undetected, NR, not reported.
Samples from Benin, range of 4 orange‐fleshed varieties (Carrotti, Loki kpikpa, Dokouin C, and Mansawin).
Two varieties (CIP‐Tanzania and Ex‐lgbarium) with different conc. of fertilizer.
Calcium plays a major role in muscle function, formation and strengthening of bones, teeth, conducting nerve impulses, blood clotting, and maintaining a normal heartbeat (Zemel, 2009). Humans in the ages of 18–50 require 1,000 mg of calcium per day as recommended daily allowance (RDA). Individuals younger than 18 years need superior concentration (1,300 mg) of Ca for developing bones and teeth (Wosje & Specker, 2000). The calcium content of 24.40–45.54 mg/100 g was reported in OFSP, and this variation in the Ca concentration attributed to varietal and agrogeological conditions (Endrias et al., 2016; Laurie, Jaarsveld, Faber, Philpott, & Labuschagne, 2012; Sanoussi et al., 2016; Ukom, Ojimelukwe, & Okpara, 2009). The Ca content in OFSP was reported as superior to common staple cereals, roots, and tubers. Sorghum (28 mg/100 g), maize (7 mg/100 g), rice (28 mg/100 g), wheat (29 mg/100 g), potato (12 mg/100 g), cassava (6 mg/100 g), WFSP (30 mg/100 g), and yam (17 mg/100 g) are reported to have superior concentration of Ca than common staples (USDA, 2018). In all the common roots and tubers, high concentration of the Ca, high amount of OFSP has to consume, or the food rich in Ca (animal‐based food) has to incorporate into the staple diets to achieve RDA of Ca was reported in OFSP.
Magnesium is one of the six important key macrominerals and essential mineral in >300 metabolic functions and possesses role in strong bones, appropriate muscle tasks, optimal blood pressure, and appropriate cardiac tempo (Saris, Mervaala, Karppanen, Khawaja, & Lewenstam, 2000). Sales and Pedrosa (2006) reported the DNA synthesis and stability depend on magnesium. The RDA of Mg to men and women is 420 and 320 mg, respectively. The Mg concentration of 3–37 mg/100 g was reported in OFSP, and this variation was attributed to the varietal and agroclimatic conditions (Endrias et al., 2016; Laurie et al., 2012; Sanoussi et al., 2016; Ukom et al., 2009). Staple cereals are good source of the Mg than OFSP and other roots and tubers. Maize and wheat contain high concentration of the Mg (125 mg/100 g), but rice (25 mg/100 g) and other tuber crops such as potato (23 mg/100 g), cassava (21 mg/100 g), WFSP (31 mg/100 g), and yam (21 mg/100 g) are similar to OFSP (USDA, 2018). The Mg‐rich food supplementation is highly recommended in the consumers of OFSP to meet RDA of magnesium.
Phosphorus is a necessary mineral in human body after calcium and possesses a pivotal role in abundant metabolic process, including energy metabolism and bone mineralization, and DNA and RNA framework (Karp, Vaihia, Kärkkäinen, Niemistö, & Lamberg‐Allardt, 2007). The RDA of 700 mg phosphorus is for healthy adults, and phosphorous of 15–51 mg/100 g was reported in OFSP, which is almost equal to the other roots and tubers such as potato (57 mg/100 g), cassava (27 mg/100 g), WFSP (47 mg/100 g), and yam (55 mg/100 g) (Endrias et al., 2016; Lyimo et al., 2010; Nicanuru et al., 2015). In contrast, staple grains contain high concentration of the phosphorous, maize, rice, and wheat are reported to have 210 mg/100 g, 115 mg/100 g, and 288 mg/100 g of phosphorous, respectively (USDA, 2018). The consumption of whole grains is recommended achieving RDA of phosphorous along with the OFSP.
Potassium along with Na and Ca regulates the fluid balance, maintains normal heart rhythm (Khaw & Barrett‐Connor, 1987), and is accountable for nerve signals and muscle functions. Potassium in the range of 138–334 mg/100 g was reported in OFSP (Endrias et al., 2016; Lyimo et al., 2010; Nicanuru et al., 2015), and these amounts are better than rice (115 mg/100 g) and maize (287 mg/100 g), but wheat (363 mg/100 g) contains similar amount of K with OFSP (USDA, 2018). In case of roots and tubers, cassava (271 mg/100 g) contains lesser concentration of potassium than OFSP, but potato (421 mg/100 g) and yam (816 mg/100 g) contain higher amount of K, and among all, yam is rich source of potassium. Children older than 13 years and adults RDA of K are 4,700 mg, but lactating women require 5,100 mg. The roots and tubers are good source of K, and OFSP is moderately providing the RDA of potassium for consumers (Constán‐Aguilar et al., 2014).
About 6% of iron in humans are present in certain proteins, which are crucial for respiration and energy metabolism process and implicated in the amalgamation of collagen and certain neurotransmitters (Bashiri, Burstein, Sheiner, & Mazor, 2003). About quarter of the Fe in humans is deposited as ferritin, located in cells, and circulated in the blood circulation system. Iron deficiency is known as erythropoiesis, and worst Fe deficiency leads to anemia (Abbaspour, Hurrell, & Kelishadi, 2014). The RDA of iron is 1.8 mg in adults, and merely 10%–30% of the Fe in diet is bioavailable (Endrias et al., 2016; Lyimo et al., 2010; Nicanuru et al., 2015). OFSP was reported to be 0.63–15.26 mg/100 g of iron, whereas maize (2.71 mg/100 g), rice (0.8 mg/100 g), wheat (3.19 mg/100 g), potato (0.78 mg/100 g), cassava (0.27 mg/100 g), WFSP (0.61 mg/100 g), and yam (0.54 mg/100 g) reported to be lesser than the OFSP (USDA, 2018). So, OFSP is a good source for providing the RDA of iron.
Zn plays an important role in body where deficiency symptoms are shown in many ways (Powell, 2000). Antinutritional factors are prime known inhibitor of zinc, which abundantly present in cereals and grains (Janet, 2003). Zinc is required for good immune system function, cell growth, wound healing, and insulin function (Chausmer, 1998). OFSP was reported to be 0.24–0.93 mg/100 g of zinc, but staple food grains such as maize (2.21 mg/100 g), rice (1.09 mg/100 g), and wheat (2.65 mg/100 g) have high concentration of the Zn, whereas potatoes (0.29 mg/100 g), cassava (0.34 mg/100 g), WFSP (0.3 mg/100 g), and yam (0.24 mg/100 g) contain the zinc in the range of OFSP (Endrias et al., 2016; Lyimo et al., 2010; Nicanuru et al., 2015). RDA of Zn varied depends on the age, 14 years old and above need 11 mg/day, and women in age 19 and above need 8 mg/day (Lutter & Rivera, 2003), whereas pregnant and lactating women need 13 and 14 mg/day, respectively. OFSP provides very small quantities of the Zn, but the bioavailability is more comparative to the cereals and grains because of no or less antinutritional factors (phytate) (Wanasundera & Ravindran, 1994).
Human body acquires sodium from food and drink, and losses through sweat and urine; kidneys play a crucial role in sodium‐level adjustments (Reynolds, Padfield, & Seckl, 2006). OFSP was reported to be 23–59 mg/100 mg, staples foods such as maize (35 mg/100 mg) contain the Na within the range of OFSP; however, rice (5 mg/100 mg), wheat (2 mg/100 mg), potato (6 mg/100 mg), cassava (14 mg/100 mg), and yam (9 mg/100 mg) contain less concentrations (Endrias et al., 2016; Lyimo et al., 2010; Nicanuru et al., 2015). RDA of sodium is 1,500 mg; less concentrations of the sodium in food source may not have any health problem, because the addition of sodium in form of table salt is a common practice in human food preparation for sake of taste.
5. CAROTENES IN OFSP
Carotenoids are natural pigments; more than 750 types are present in divergent algae and photosynthetic bacteria. These carotenoids are accountable for yellow, orange, and red colors in different plant products (fruits, vegetables, and flowers), distinct aromatic scents, and flavors (Namitha & Negi, 2010).
In plants, carotenoids are located in chromoplasts and chloroplasts, and possess pivotal roles in photosynthetic guard from photo‐oxidative injuries (Carol & Kuntz, 2001). Carotenoids are metabolized in the plastid by methylerythritol‐4‐phosphate (MEP) pathway (Phillips, León, Boronat, & Rodríguez‐Concepción, 2008). This biopathway is composed of two branches behind lycopene to produce AC and BC by lycopene ε‐cyclase (LCYE) and lycopene β‐cyclase (LCYB) (Fraser & Bramley, 2004).
5.1. β‐Cryptoxanthin
β‐Cryptoxanthin (BCX) is the most common dietary carotenoids found in the fruits and vegetables, and it is a PVAC along with AC and BC (Berni, Chitchumroonchokchai, Canniatti‐Brazaca, De Moura, & Failla, 2015; Rao & Rao, 2007). 24 µg of BCX in food will metabolize to 1 μg of retinol in human body (Weber & Grune, 2012). Medical studies reported the risk of pulmonary cancer was estimated to reduce 20% with consumption of BCX (Lian, Hu, Russell, & Wang, 2006; Yuan, Ross, Chu, Gao, & Yu, 2001). Research reports also concluded that superior consumption of carotenoids (BCX and lycopene) possesses optimistic connection with a reduced mouth cancer risk (Terry, Lagergren, Hansen, Wolk, & Nyrén, 2001). Researchers proved that high concentration of NPVAC (lutein, zeaxanthin and lycopene) to VAC (AC, BC, BCX) in plasma has positive role in reducing diabetic retinopathy in type 2 diabetes mellitus patients (Brazionis, Rowley, Itsiopoulos, & O'dea, 2009). Lower concentrations of high‐density lipid (HDL) concentration in body was found with BCX intake by food, and also recent experimental study reported the BCX positive correlation with osteoporosis prevention (Gammone, Riccioni, & D'Orazio, 2015).
Limited studies were reported on concentration of BCX in OFSP as 21.2 (µg/g db) (Kim et al., 2015). So, consumption of OFSP with BCX concentration will contribute to the 0.5 µg of VA by 1 g consumption. So, 100 g of OFSP will provide 50 µg of VA, and it contributes to the VA RDA requirements with BCX, AC, and BC. Some foods such as pumpkin, papaya, oranges, carrots, and yellow corn are rich sources of BCX. The comparable BCX concentrations in agreement with other foods is reported in OFSP (Breithaupt & Bamedi, 2001). The composition of different carotenoids and their concentrations in OFSP are reported in Table 3.
Table 3.
Composition of different carotenoids and vitamins in orange‐fleshed sweet potato (OFSP) reported by different authors
| Reference | α‐Carotene | β‐Carotene | Total carotene | Anthocyanin (mg/g fw) | Ascorbic acid | β‐Cryptoxanthin | α‐Tocopherol | Lutein | Zeaxanthin |
|---|---|---|---|---|---|---|---|---|---|
| Stinco et al. (2014)a | 13.11 (µg/g db) | 48.66 (µg/g db) | 61.77 (µg/g db) | NR | NR | NR | NR | NR | NR |
| Teow et al. (2007)b | NR | 44.9–226 (µg/g fw) | NR | 17–38 (µg/g fw) | NR | NR | NR | NR | NR |
| Shih et al. (2009)c | NR | 34.6–83.3 (µg/g db) | NR | 4.0–8.5 (µg/g db) | NR | NR | NR | NR | NR |
| Tomlins et al. (2012)d | NR | 20–364 (µg/g db) | 41.7–251 (µg/g db) | NR | NR | NR | NR | NR | NR |
| Grace et al. (2014)e | NR | NR | NR | ND | 870 (µg/g db) | NR | NR | NR | NR |
| Islam et al. (2016)f | NR | NR | 19.31–61.94 (µg/g fw) | NR | NR | NR | NR | NR | NR |
| van Jaarsveld et al. (2006)g | NR | 132–194 (µg/gfw) | NR | NR | NR | NR | NR | NR | NR |
| Kim et al. (2015)h | NR | NR | 570 (µg/g db) | NR | NR | 21.2 (µg/g db) | NR | NR | NR |
| Lebot et al. (2016)i | NR | NR | NR | 0–1558 (mean 243 [AU, area units]) | NR | NR | NR | NR | NR |
| Wu et al. (2008)j | NR | 6.2–231 (µg/gfw) | NR | NR | NR | NR | NR | NR | NR |
| Tang et al. (2015)k | NR | NR | NR | 2.9 (µ/g) | NR | NR | NR | NR | NR |
| Brown et al. (1993)l | NR | NR | NR | NR | NR | NR | NR | 120–148 (µg/g fw) | 242–2,055 (µg/g fw) |
| Donado‐Pestana et al. (2012)m | NR | NR | NR | NR | NR | NR | NR | 1–4 (µg/g db) | 1–2 (µg/g db) |
| (Mohammad et al. (2016)n | NR | NR | 5.5–72.4 (µg/g fw) | NR | NR | NR | NR | NR | NR |
| Huang et al. (1999)o | 1–15 (µg/g fw) | 67–131 (µg/g fw) | NR | NR | NR | NR | NR | NR | NR |
| Oki et al. (2006)p | NR | 85.36–177.16 (µg/g fw) | NR | NR | NR | NR | 2.61–8.48 (µg/g fw) | NR | NR |
| Tumuhimbise et al. (2009)q | NR | 67.33–315.71 (µg/g db) | NR | NR | NR | NR | NR | NR | |
| Vimala, Nambisan, and Hariprakash (2011)r | NR | 0.96–13.6 (µg/g fw) | 15–155 (µg/g fw) | NR | NR | NR | NR | NR |
Abbreviation: NR, not reported.
Values from (Rubina, Diane, California Organic, Evangeline, Darby, Beauregard varieties) from the United States and Israel.
Ranges from Beauregard (Beau), Hernandez (Hern), Covington (Covin), 11–5, 11–20,13–14,13–15 from the United States.
Tainong 66, from Taiwan.
Range from different varieties (Ejumula, Kakamega, SPK004/1, SPK004/6/6, and SPK004/ 1/1) from Uganda.
Covington variety fresh sample from the United States.
Ranges from Kamalasundari, BARI SP 4, BARI SP 5, BARI SP 6.
Raw Resisto, from the same harvest batch.
Sinhwangmi, from Korea.
OFSP from France.
Samples procured from Shandong, Sichuan, Jiangsu, Fujian, Sichuan places, and range was taken from the varieties of Ji03314, Ji03468, S01009, S01056, Xushu 22‐5, xushu, Yanshu, 200730 from China.
Guineng 05‐6 (orange), cyanidin‐3‐glucoside (cyE).
Ranges taken from D6.11, 90B10.2, 90B 10.20, 90B 10.35 varieties.
The values that are taken range from varieties of CNPH1007, CNPH1194, CNPH1202, CNPH 1205 from Brazil.
BARI SP1 to 9 varieties from Bangladesh.
Range taken from varieties Excel, Jewel, Kona B, Makua yam, Regal, South Delite, UH 71‐5 from Hawaii.
Range taken from Kyushu, 144, 1243, 241; sunnyred, Hamakomachi, J‐Red, Ayakomachi from Japan.
Ejumula, SPK004/6/6, SPK004/6, SPK004, SPK0041 from Uganda.
Range taken from the varieties KS‐7, ST‐14‐1, ST‐14‐16, ST‐14‐34, ST‐14‐49, ST‐14‐53, ST‐14‐6, ST14‐9, SV‐3‐17, SV‐3‐22, from India.
5.2. Lutein and zeaxanthin
Lutein (β,ε‐carotene‐3′,‐diol) is naturally present with zeaxanthin (β, β‐carotene‐3,3′‐diol) as stereoisomer and available in large quantities in leafy vegetables (Perry, Rasmussen, & Johnson, 2009). Lutein and zeaxanthin are structured with 40 carbon atoms and named as tetraterpenoids, and they are synthesized only in plant kingdom (Shegokar & Mitri, 2012). Lutein and zeaxanthin belong to xanthophyll family and do not possess any VA activity (Abdel‐Aal, Akhtar, Zaheer, & Ali, 2013). The yellow colors of these two xanthophylls are connected to their structural properties and responsible for orange to yellow color of different vegetables and animal products (egg yolk, animal fat). The animal retina is exceptional and contains a yellow colored region positioned in its ocular center known as “macula lutea” (Kalariya, Ramana, & vanKuijk, 2012). This yellow color attributed to the accumulation of lutein, zeaxanthin, and meso‐zeaxanthin, and they are very important in eye well‐being (Kijlstra, Tian, Kelly, & Berendschot, 2012; Nwachukwu, Udenigwe, & Aluko, 2016). Xanthophylls play a critical role in the defense of skin against sunlight (Stahl & Sies, 2007). Lutein and zeaxanthin protect eye from high‐energy blue light, and inhibit and repair the photoinduced oxidative damage (Bian et al., 2012).
Limited reports were available on the concentration of the lutein and zeaxanthin in OFSP. Brown, Edwards, Yang, and Dean (1993) reported the 120–148 (µg/g fw) and 242–2,055 (µg/g fw) of lutein and zeaxanthin respectively, whereas Donado‐Pestana et al. (2012) reported 1–4 (µg/g db) and 1–2 (µg/g db) of lutein and zeaxanthin in OFSP, respectively. The orange color of the OFSP is devoted to the presence of this lutein and zeaxanthins. The differences in the reported concentrations of the two reports may be attributed to the variety‐related issues. Researchers reported the intensity variations of orange color among the cultivars in different countries, and this was directly linked to the concentration of lutein + zeaxanthin. However, the lutein + zeaxanthin by Brown et al. (1993) reported high concentration in OFSP, which is comparable with the other leafy vegetables. Vegetables such as baby carrots, peaches, corn, papaya, and oranges possess <1 mg of lutein + zeaxanthin in 100 g of fresh weight (Holden et al., 1999). Ziegler et al. (2015) reported the limited quantity of lutein esters in cereals; in contrast, xanthophylls are rich in fruits and vegetables such as collards, capsicum, yellow corn, and spinach (Sajilata, Singhal, & Kamat, 2008). Consumption of OFSP also provides the required xanthophylls to the consumers. Recent studies reported that these two xanthophylls possess very important role in different disease management such as skin damages, reduce the chance of pancreatic cancer, reducing the CHD, and increase in overall antioxidant activity (Gordon, 1996; Mares‐Perlman, Millen, Ficek, & Hankinson, 2002).
5.3. α, β‐Carotenes (AC and BC)
AC and BC are prime bases for VA; they metabolize to retinol in humans. Fruits and vegetables with orange, yellow, and green color are rich source of these two carotenoids (Saini, Nile, & Park, 2015). The VA conversion efficacy of BC is double in comparison with AC, one portion of BC yields two portions of retinol, but AC yields only half portion (Thurnham, 2007). Due to the large concentrations of the BC shares with AC in plant sources, its medicinal values are not well established (Murakoshi et al., 1992).
Variations in polar groups determine the metabolic roles of carotenoids (Britton, 2008). Carotenoids are very significant for AA, intercellular signal transmission and body defense mechanisms (Chew & Park, 2004; Edge, Mc Garvey, & Truscott, 1997; Kumar, Singh, & Ekavali, 2015), lung cancer (Ziegler, 1989), and coronary health and vision problems (Meyers et al., 2013; Sharoni et al., 2012). Shortage of carotenoid consequences with broader medical symptoms related to the eye and vision (Sommer, 2008) weaken innate, adaptive immunity (Stephensen, 2001). Levy et al. (1995) reported that BC is very effective in the suppression of malignant tumor cells.
The OFSP is considered as the good source of the carotenoids, and many researches are recommended to use the OFSP to combat the problems of VAD in developing countries. Stinco, Benítez‐González, Hernanz, Vicario, and Meléndez‐Martínez (2014) reported 13.11 (µg/g db), and Huang et al. (1999) reported 1–15 (µg/g fw) of AC in OFSP. Many researchers reported the BC concentrations are higher than AC in fresh and dry basis. Among the all, Tomlins, Owori, Bechoff, Menya, and Westby (2012) reported the highest range (20–364 (µg/g db)) of the BC in OFSP from different varieties grown in Uganda, whereas Teow et al. (2007) reported the 44.9–226 (µg/g fw) of the BC in fresh base from US varieties of OFSP. Overall, the presence of high concentration of BC in the OFSP is a good sign as previously discussed as BC yields high amount of the PVA. Both the carotenes are very high in OFSP compared to the common consuming yellow to orange vegetables and fruits. As reported by the Gul et al. (2015) the carotenoid concentration in different foods, such as carrot (43.5–88.4 µg/g), mango (10.9–12.1 µg/g), and tomato (2.17–2.83 µg/g), contain the lower concentration of the BC than the OFSP. In case of the total carotenoids, 570 µg/g (db) of the carotenoids are reported by Kim et al. (2015) in OFSP from Korea. These concentrations are much higher than any other fruits and vegetables.
5.4. Carotenoid isomers in OFSP
Carotenoids naturally exist in different isomers; obviously, the majority of the carotenoids happen in trans‐isomers in vegetative source. Nevertheless, rise in cis‐isomers attributed to food processing conditions (Schieber & Carle, 2005). Many authors, Mertz, Brat, Caris‐Veyrat, and Gunata (2010), Shi et al. (2010), and Zepka and Mercadante (2009) reported the different isomers in the dietary carotenoids, whereas some researchers are focused on geometric isomerization of carotenoids (Niedzwiedzki, Enriquez, Lafountain, & Frank, 2010; Qiu, Chen, & Li, 2009).
Food processing conditions such as application of heat, exposure of radiation, and innate structural variations are responsible for variation in carotenoid isomer formations (Chwartz, 2002; De Rigal, Gauillard, & Richard‐Forget, 2000; Kumar et al., 2015; Parker, 1996).
Carotenoids with seven and more double bonds (conjugated) are identified for good AA (Borsarelli & Mercadante, 2009). Trans‐BC is highly sensitive to heat and light and isomerizes to cis‐isomers when exposed; for instance, Vásquez‐Caicedo, Schilling, Carle, and Neidhart (2007) reported the processing of fruits leads to cis‐trans isomer formation and this processes is very particular in case of 13‐cis‐BC formations (Lozano‐Alejo, Carrillo, Pixley, & Palacios‐Rojas, 2007). 9‐cis‐BC in various foods was reported when exposed to light (Lozano‐Alejo et al., 2007; Schieber & Carle, 2005), while 13‐cis‐AC and BC isomers are identified in extended storage durations (Tang & Chen, 2000).
Chen and Huang (1998) reported that the reflux heating converts BC to all‐trans‐BC and 13‐cis‐BC. Pasteurized and sterilized carrot juice reported the presence of high concentration of 13‐cis‐BC, while 9‐cis‐BC was reported in blanched conditions. Moreover, 9‐cis‐ and 13‐cis‐BC were reported to produce independently from cis bases due to the nonenzymatic actions of all‐trans forms (Marx, Stuparic, Schieber, & Carle, 2003). In contrast, heating and exposure to air of cis‐BC found no difference in all‐trans‐isomer (Qiu et al., 2009); however, studies conclude that BC undergoes to the isomerization rather degradation when exposed to adverse condition. Recent studies reported that all‐trans forms contain higher biological availability than cis‐counterpart, while BC and β‐apo‐12′‐carotenal have the highest bioconversion rate (Castenmiller & West, 1998).
In case of OFSP, Islam, Nusrat, Begum, and Ahsan (2016) reported cis‐BC of 3.5–23.44 µg/g (fw), Liu, Lin, and Yang (2009) reported di‐cis‐BC 1.5–5.7 µg/g (fw), whereas Donado‐Pestana et al. (2012) reported 70–113 µg/g (db) of 5,6 epoxy‐BC. Berni et al. (2015) reported 13‐ or 13′‐cis‐AC of 3.8–9.0 µg/g (fw), 15‐ or 15′‐cis‐BC 1.9–3.5 µg/g (fw), but 0.004–0.39 µg/g (fw). Comparatively other isomers of carotenoids, 13‐ or 13′‐cis‐BC concentrations in OFSP, were reported to be 4.7–94 µg/g (db) from the studies reported by Bengtsson, Namutebi, Alminger, and Svanberg (2008), Donado‐Pestana et al. (2012), Kim et al. (2015), and Tumuhimbise, Namutebi, and Muyonga (2009). 1.14–4.7 µg/g (fw) in range of the 13‐ or 13′‐cis‐BC in OFSP was reported by Berni et al. (2015) and Liu et al. (2009). The concentration of 9‐ or 9′‐cis‐BC was identified in the range of 0.006–9.8 µg/g (fw) in the studies of Berni et al. (2015) and Liu et al. (2009). In case of dry OFSP, samples were reported to be 7.4–618 (µg/g) range of 9‐ or 9′‐cis‐ BC by Donado‐Pestana et al. (2012) and Kim et al. (2015). All‐trans‐AC was reported to be 1.2–2.7 µg/g (fw) by only Liu et al. (2009), whereas All‐trans‐BC concentration was reported to be in the range of 0.01–1285 µg/g (fw) by studies of Berni et al. (2015), Donado‐Pestana et al. (2012), Kim et al. (2015), Liu et al. (2009), and Tumuhimbise et al. (2009), whereas in dry base, it was shown that all‐trans‐BC was reported to be 0.01–114.4 µg/g by, Berni et al. (2015), Islam et al. (2016), and Liu et al. (2009). Liu et al. (2009) reported total cis‐isomers of AC and total cis‐isomers of BC of 3.8–0.9.0 and 14.4–33.1 µg/g (fw), respectively. The concentrations of carotenoid isomers in OFSP from different locations, and authors are presented in Table 4.
Table 4.
Concentrations of carotenoid isomers in orange‐fleshed sweet potatoes reported by different authors
| Isomers of carotene | Liu et al. (2009)a (µg/g fw) | Islam et al. (2016)b (µg/g fw) | Bengtsson et al. (2008)c (µg/g db) | Kim et al. (2015)d (µg/g db) | Berni et al. (2015)e (µg/g fw) | Donado‐Pestana et al. (2012)f (µg/g db) | Tumuhimbise et al. (2009)g (µg/g db) |
|---|---|---|---|---|---|---|---|
| cis‐β‐Carotene | 4.3–9.3 | 3.5–23.44 | NR | NR | NR | NR | NR |
| di‐cis‐β‐Carotene | 1.5–5.7 | NR | NR | NR | NR | NR | NR |
| 5,6 epoxy‐β‐ carotene | NR | NR | NR | NR | NR | 70–113 | NR |
| 13‐ or 13′‐cis‐α‐Carotene | 3.8–9.0 | NR | NR | NR | NR | NR | NR |
| 15‐ or 15′‐cis‐ β‐Carotene | 1.9–3.5 | NR | NR | NR | 0.004–0.39 | NR | NR |
| 13‐ or 13′‐cis‐ β‐Carotene | 3.0–4.7 | NR | 4.7–15.9 | 11.6 | 1.14–2.30 | 88–94 | 0.75–1.22 |
| 9‐ or 9′‐cis‐β‐Carotene | 7–9.8 | NR | NR | 7.4 | 0.006–2.82 | 55–61 | NR |
| All‐trans‐α‐Carotene | 1.2–2.7 | NR | NR | NR | NR | NR | |
| All‐trans‐β‐Carotene | 2.7–37.6 | 76.59–96.49 | 108.1–314.5 | 530 | 0.01–114.4 | 791–1285 | 66.79–314.48 |
| Total cis‐isomers of α‐carotene | 3.8–0.9.0 | NR | NR | NR | 0.11–0.27 | NR | NR |
| Total cis‐isomers of β‐carotene | 14.4–33.1 | NR | NR | NR | NR | NR | NR |
Tainung 66 (with orange flesh) range from harvested from January to October, from Taiwan.
Ranges from Kamalasundari, BARI SP 4, BARI SP 5, BARI SP 6.
SPK004/1, SPK004/6, SPK004, Sowola 6/94/9, SPK 004/1, SPK 004/6/6, Ejumula.
Sinhwangmi, from Korea.
Brazlandia, Amelia, Beauregard.
The values that are taken range from varieties of CNPH1007, CNPH1194, CNPH1202, CNPH1205 from Brazil.
Ejumula, SPK004/6/6, SPK004/6, SPK004, SPK0041 from Uganda.
6. α‐TOCOPHEROL
Vitamin E represents eight isomers of fat‐soluble compounds (α‐, β‐, γ‐,δ‐tocopherol and tocotrienol) metabolized in plants (Bjorneboe, Bjorneboe, & Drevon, 1990; Helmut, Wilhelem, & Alfred, 1992; Netscher, 2007), available in various concentrations in fat‐rich foods (edible oils and seeds) (Reboul et al., 2006; Yanishlieva & Marinova, 2001). α‐Tocopherol (Pryor, 2000) is appreciated for cytoprotective nature, anti‐inflammatory, and liver protection (Galli et al., 2017), and also considered as potential AA and immune system booster, to fight against viruses and pathogenic bacteria (Traber & Atkinson, 2007). Tocopherol also helps to synthesize new red blood cells and widens blood vessels (Evelyne et al., 1994), potentially lowering risk of developing blood clots (Krem & Cera, 2002). Vit. E RDA is between 3,000 and 15,000 µg in different countries, and the variation reported depends on the age (Jiang, Christen, Mark, & Bruce, 2001). Limited studies were reported on the concentration of the α‐tocopherol (Table 3) in OFSP, and the range of 2.61–8.48 (µg/g fw) was reported by Oki et al. (2006). Consumption of 100–200 gm of OFSP requires achieving around 20%–25% RDA of Vit. E. However, the OFSP is not considered as the best source of the α‐tocopherol because less concentration of fats was reported in OFSP. So, supplementation of Vit. E‐rich foods such as oilseeds is very important for the consumers of OFSP as second staple.
7. ASCORBIC ACID
Vitamin C naturally presents in two isomers as ascorbic and dehydroascorbic acid, and they are metabolites in various plants, animals, and fungi (Drouin, Godin, & Page, 2011). The biological activity of ascorbic acid depends on redox state, acting as cofactor for eight human enzymes and different antioxidants (Padayatty & Levine, 2016). Vit. C is known as one of the safest and most effective nutrients, and involves in immune system functions (Padayatty et al., 2003), cardiovascular disease (Simon, 1992), prenatal health problems, eye disease, and skin wrinkling (Vitamin C & Eye Health, 2007).
Vitamin C is extremely essential in synthesis of collagen (Varani et al., 2000), carnitine (Johnston, Solomon, & Corte, 1996), and neurotransmitters (Lee, Lee, Jung, & Lee, 2001), and in the formation and maintenance of bone material (Wang, Villa, Marcus, & Kelsey, 1997), to fight against the oxidative stress (Bendich & Langseth, 1995). Adult men and women require 90 and 75 mg of the Vit. C as the RDA, respectively (Naidu, 2003). Grace et al. (2014) reported (Table 3) Vit. C concentration in OFSP as 870 µg/g (db) of ascorbic acid, which is very less than the different fruits and vegetables.
8. ANTHOCYANINS
Anthocyanins are very essential pigments in vascular plants, responsible for glittery orange, pink, red, violet, and blue colors in plants (Glover & Cathie, 2012); they are safe and effortless to apply in aqueous media as natural water‐soluble dyes (Wrolstad, 2000). They naturally occur as glycosides, and anthocyanidins are bound with different sugar groups (Kong, Chia, Goh, Chia, & Brouillard, 2003). Anthocyanidins are highly appreciated due to the antioxidant, anti‐inflammatory, anticarcinogenic activities, protection against heart disease (Bowen‐Forbes, Zhang, & Nair, 2010; Miguel, 2011), and diabetes reduction (Sancho & Pastore, 2012).
Anthocyanin consumption in worldwide reported in high variation depends on the diet composition and eating patterns of the individuals. Studies identified the deviations in the anthocyanin intake in different countries such as the United States (12.5 mg/day) (Wu et al., 2006), the Netherlands (19.8 mg/day), Italy (64.9 mg/day), and Spain (18.4 mg/day) (Zamora‐Ros et al., 2013). In case of OFSP, different authors reported the concentration of ACN. Teow et al. (2007) reported 17–38 (µg/g fw), Shih, Kuo, and Chiang (2009) reported 4.0–8.5 (µg/g db), and Tang et al. (2015) reported 2.9 (µCyE/g) anthocyanins in OFSP (Table 3). The variation in the concentrations reported in OFSP by different researchers may be due to the agrogeological conditions. However, the OFSP is not the good source of the anthocyanin compared to the other fruits and vegetables.
9. PHENOLIC ACIDS
Phenolic acids, especially caffeic (CA), ferulic (FA), sinapic (SA), and p‐coumaric acids (PCAs) are present in good quantities in variety of fruits and vegetables (Naczk & Shahidi, 2006). Chlorogenic acid (CGA) is an important hydroxycinnamic acid in food with high AA (Acosta‐Estrada, Gutiérrez‐Uribe, & Serna‐Saldívar, 2014), and its bioavailability depends on absorption and metabolism in human alimentary tract. Gonthier, Verny, Besson, Rémésy, and Scalbert (2003) identified the resultant aromatic products (m‐coumaric acid and derivatives of phenylpropionic and benzoic acids) by gut microbial action on CGA. These resultant metabolites are very important to activity of polyphenols poorly absorbed in the alimentary tract.
CGA and CA are reported for good AA, inhibition of N‐nitroso compounds (Kono, Shibata, Kodama, & Sawa, 1995), protection of DNA from damage (Cinkilic et al., 2013), cardiovascular disease (Mirella et al.., 1995), antimutagenic properties (Yamada & Tomita, 1996), scavenge of reactive oxygen species (Sato et al., 2011), and inhibition of 8‐dehydroxy‐deoxyguanosine (involved in DNA breakage) (Kasai, Fukada, Yamaizumi, Sugie, & Mori, 2000). CGA reported as novel insulin sensitizer limits the blood glucose concentrations by inhibiting G‐6‐Pase enzyme action (Meng, Cao, Feng, Peng, & Hu, 2013).
The most common form of CGA is 5‐caffeoylquinic acid (5‐CQA). Three major subclasses of CGAs are caffeoylquinic (CQA), feruloylquinic (FQA), and dicaffeoylquinic (diCQA) acids. The major CGA isomers are 3‐, 4‐, and 5‐CQA; 3‐,4‐; 3, 5‐, and 4,5‐CQA; 3,4‐; 3,5‐ and 4,5‐diCQA; 3‐,4‐, and 5‐FQA; 3‐,4‐, and 5‐p‐coumaroylquinic acids (Catherine, Nicholas, & George, 2014; Clifford, 2000).
Highest concentration of the CA was reported to be 113.4–490 (µg/g db) by Grace et al. (2014) and Padda and Picha (2008) in OFSP. Lebot, Michalet, and Legendre (2016) reported CA concentration of 0–16,000 µg/g (fw) with mean of 610 (µg/g fw). Other authors reported CA concentration of 4.7–50 (µg/g db) (Grace et al., 2014; Padda & Picha, 2008), but they were not reported FA. In case of the total diCQA, researchers reported 900 (µg/g db) (Grace et al. (2014)). In case of fresh weight base, researchers reported 0–27,791 (AU) of total diCQA with mean of 11,020 (AU). The isomers of diCQA, 4,5‐diCQA were reported to be 9.2–32.4 (µg/g db) (Padda & Picha, 2008), but Lebot et al. (2016) reported (0–1030) mean of 310 (µg/g fw) (e.g., 3,4 diCQA). Other isomer 3,5‐diCQA is reported in dry weight base to be from 56.3 to 300 (µg/g db) as reported by Grace et al. (2014) and Padda and Picha (2008), whereas in wet base weight (0–430) with mean of 170 (µg/g fw), it was reported by Lebot et al. (2016). 3,4‐diCQA was reported to be in the range of 1.9–20 (µg/g db) by Grace et al. (2014) and Padda and Picha (2008). Comparative to the other fruits and vegetables, the low concentration of the phenolic acids was reported in OFSP. Researchers reported wide variation in the total phenolic acid concentration in OFSP in different varieties and grown in wider geographical locations. The concentration of individual phenolic acids reported by the different phenolic acids is tabulated in Table 5.
Table 5.
Individual phenolic acid contents in orange‐fleshed sweet potato of different varieties
| Author | Chlorogenic acid | Caffeic acid | 4,5‐Dicaffeoylquinic acid | 3,5‐Dicaffeoylquinic acid | 3,4‐Dicaffeoylquinic acid | Total dicaffeoylquinic acid |
|---|---|---|---|---|---|---|
| Padda and Picha (2008)a (µg/g db) | 113.4–357.8 | 4.7–13.4 | 9.2–32.4 | 56.3–268.4 | 1.9–12.3 | NR |
| Grace et al. (2014)b (µg/g db) | 490 | 50 | 20 | 300 | 20 | 900 |
| Lebot et al. (2016)c (µg/g fw) |
(0–1,600) Mean 610 |
NR |
(0–1030) Mean 310 (include 3,4 diCQA) |
(0–430) Mean 170 |
NR |
(0−27,791 AU) Mean 11,020 (AU) |
Abbreviations: CafA, caffeic acid; ChlA, chlorogenic acid; diCQA, dicaffeoylquinic acid.
OFSP values (Rubina, Diane, California Organic, Evangeline, Darby, Beauregard varieties) from the United States and Israel.
Covington variety fresh samples from the United States.
OFSP from France (n = 64).
The total phenolic acid concentration of 1.8–310.7 mg/100 g was reported by Donado‐Pestana et al. (2012), Grace et al. (2014), Padda and Picha (2008), and Rumbaoa et al. (2009). Different scientists reported the 0.130–136.05 mg in 100 g of the phenolic acids (Mohammad et al., 2016; Rumbaoa et al., 2009; Teow et al., 2007). CGA accumulation identified high levels in potatoes (2004), and Andre et al. (2007) and Brown (2005) reported the agriculture trails to boost up the phenolic acids and/or carotenoids. Eichhorn and Winterhalter (2005) reported that potato tubers are rich source of flavonoids and CAN available in the form of acylated glycosides located in vacuoles (Kosieradzka, Borucki, Matysiak‐Kata, Szopa, & Sawosz, 2004). High positive correlations were found between total phenolic acids, and anthocyanin presence with antioxidant capacity was reported by many studies (Moyer, Hummer, Finn, Frei, Ronald & Wrolstad, 2002; Pantelidis, Vasilakakis, Manganaris, & Diamantidis, 2007; Prior et al., 1998; Tsai, McIntosh, Pearce, Camden, & Jordan, 2002; Velioglu, Mazza, Gao, & Oomah, 1998).
10. ANTIOXIDANT ACTIVITY OF OFSP
Oxidation response leads to the many problems in humans, and they are connected with pathophysiology problems of many diseases (Blokhina, Virolainen, & Fagerstedt, 2003; Lipinski, 2001), which includes inflammation (Halliwell, 2006), cancer (Reuter, Gupta, Chaturvedi, & Aggarwal, 2010), atherosclerosis, and aging (Finkel & Holbrook, 2000). Antioxidants occur naturally or chemically synthesizing, and foods from the higher plants are rich source of natural antioxidants (tocopherols and polyphenols) (Imark, Kneubühl, & Bodmer, 2000). Naturally occurring antioxidant isolation and application in food and pharmaceutical industries are already existed (Suhaj, 2006).
In case of OFSP, free radical scavenging activity (µmol TE/kg fw) was reported to be 493–969 by Oki et al. (2006) samples from Japan, and in case of iron‐chelating ability, Rumbaoa et al. (2009) reported 310.7 (mg/mg, fw). In case of DPPH scavenging capacity, Shih et al. (2009) reported 889–1,372 (µg/ml) from OFSP in the Taiwan. Inhibition at 50 mg/ml of 92.0 was reported by Rumbaoa et al. (2009). Potato tubers with increased levels of flavonoids and high antioxidant capacities were developed by Lukaszewicz et al. (2004). According to Lachman, Hamouz, Orsák, Pivec, and Dvořák (2008) total polyphenolic acid and ACN content of different colored SP depend on the environmental factors and application of the fertilizer in agriculture practices.
11. ROLE OF OFSP IN HANDLING VITAMIN A MALNUTRITION
Black, Morris, and Bryce (2003) reported the role of retinol deficiency in night blindness, susceptibility to infections, cognitive development problems and immune system function, susceptibility to respiratory infection, diarrhea, measles, and malaria (Thurnham, McCabe, Northrop‐Clewes, & Nestel, 2003). UNICEF (2018) identified the retinol deficiency in 140 million children illness and death. WHO reported VDA is a critical community problem, and it influenced the health of one third of children aged from 6 to 59 months in 2013; children in sub‐Saharan Africa (SSA) (48%) and South Asia (44%) are the major affected. It also reported that 250,000–500,000 VAD children are in fate of blind in each year due to the vitamin A deficiencies, and 50% of them died within 12 months of vision loss (WHO, 2018).
One of the easiest ways to introduce more VA into the diet is by consuming the carotene‐rich plant‐based foods like OFSP. This is a good source of carotenoids, and they readily convert into retinol in human body. International Potato Center (2017) reported that supplementation of 100–150 g of the OFSP in human diet can prevent VAD in younger's and also radically diminish maternal mortality. Low et al. (2007) reported that in Mozambique 50 million children <6 year got benefited by consumption of WFSP along with OFSP. OFSP is popularizing to combat VAD in several SSA countries (Kapinga, Byaruhanga, Zschocke, & Tumwegamire, 2009), including South Africa (Faber, Venter, & Benadé, 2002; Laurie & Faber, 2008) through community‐dependent food intervention programs. In different strategies, incorporation of OFSP resulted in VA increase in different targeted groups (Haskell et al., 2004; Low et al., 2007).
Hortz et al. (2012) reported in their studies that introduction of OFSP in SSA countries such as Uganda and Mozambique is raised in VA intakes among children and women. Jones and de Brauw (2015) reported that biofortified OFSP in Mozambique facilitated the improved child health status and also reported the decrease in incidence and extent of diarrhea in children <5 years. Low et al. (2007) concluded that amplified production and utilization of the OFSP improved the nutritional status of the consumers in African countries, especially in Nigeria (Fetuga et al., 2013). With recent introduction of OFSP in East African countries such as Nigeria, peoples and government and nongovernment agencies concern on growth and utilization (Hagenimana & Low, 2000).
Still more efforts are required from the agriculture and health extension officers to encourage production and utilization of OFSP connecting households with young children in pastoral areas of VAD populations (Low et al., 2007). Prolonged storage of OFSP is usually achieved by drying (sun drying), and during the traditional drying (sun drying) techniques, microbial contamination was reported as the major problem due to the high moisture content of the fresh OFSP. Amajor et al. (2014) reported that OFSP flours can produce by sun drying and milling of the fresh OFSP roots. OFSP flour prepared by milling of dried slices can also utilize as entire flour or as a combined with others. Some of the reported studies on animal or human studies related to the role of OFSP in VAD management are summarized in Table 6.
Table 6.
Some human and animal studied on the orange‐fleshed sweet potato (OFSP) in vitamin A‐related disease management
| S. no. | Country/target group | Study | Results | Reference |
|---|---|---|---|---|
| 1 | Mozambique/Children | Longitudinal study reported from three deliberately selected districts, two are selected for intervention and one as control. Information was collected on demographic, agricultural, and anthropological issues. Blood samples were collected from all respondents for biochemical analysis | Intervention children reported higher intake of OFSP, reported high VA. Mean serum retinol level increased by 0.100 mM in intervention children, which are not increased in control subjects | Low et al. (2007) |
| 2 | Uganda/Children and women | Study was conducted to know impact of the intensive (IP) and reduced practices (RP) with a control on OFSP and VA intakes among children aged 6−35 months and 3–5 years and women, and IP compared with control on VA status of 3‐ to 5‐year‐old children and women with serum retinol <1.05 mM at baseline | 9.5% point reduction in prevalence of serum retinol <1.05 mM identified. At follow‐up, VA intake from OFSP was correlated with VA status. Use of OFSP increased VA intakes of children and women | Hotz, Loechl, Lubowa, et al. (2012) |
| 3 | Bangladesh/women |
Daily consumption of OFSP with or without added fat, on the VA status of Bangladeshi women with low initial VA status was done. Women received one of the following for 6 days/week over 10 weeks. 1. 10 mg RAE/day (WFSP and a corn oil) 2. 600 mg RAE/day (OFSP and a corn oil) 3. Fried OFSP and a corn oil capsule 4. Boiled WFSP and a retinyl palmitate capsule in addition to their home diets. Retinol and BC and VA were assessed before and after the 60 days |
BC concentrations in plasma found in this study were high in groups consumed OFSP and plasma BC was higher in the consumed fried OFSP compared with boiled OFSP. Initial and final total body VA pool sizes were 0.060–0.047 mmol and 0.091–0.070 mmol. Concluded that, the impact of OFSP on VA status in Bangladeshi women was marginal | Jamil et al. (2012) |
| 4 | Mozambique/general consumers |
Reported study on OFSP consumption. The two intervention models were compared: 1. Low intensity (1 year) 2. High‐intensity (nearly 3 years) training model |
OFSP consumption raised VA among consumers. OFSP accounted for 47%–60% of all SP consummation provided 80% of total VA | Hotz, Loechl, de Brauw, et al. (2012) |
| 5 | South Africa/school children |
Effect of boiled and mashed OFSP in improving VA levels in school children studied. Dewarmed 5–10 years kids were randomly assigned to following two sections for 53 days. 1. Treatment group consumed 125 g boiled and mashed OFSP 2. Control group given same portion of WFSP |
High amount of VA reported in OFSP consumed group than control. The proportions of children with normal VA status in the treatment group increased and did not change in the control group | Van Jaarsveld et al. (2005) |
| 6 | Mozambique/children | Health benefits of biofortification in reducing VAD reported in rural area of north Mozambique | Children <5 years, biofortification reduced diarrhea prevalence by 11.4% and by 18.9% in children <3 years | Jones and de Brauw (2015) |
| 7 | Kenya/women | Role of OFSP nutrition and health‐promoting activity reported. VA intakes were assessed with multipass 24‐hr recalls in a subsample of 206 mothers at 8–10 months postpartum | 22.9% of women had VA <1.17 mM. By 9 months of postpartum, intervention women had significantly higher intakes of VA‐rich OFSP in the previous 7 days | Girard et al. (2017) |
12. PRODUCTS FORM OFSP
Orange‐fleshed sweet potato consumed frequently in family meal of different SSA countries by boiling, steaming, roasting, and drying (Low et al., 2007). Traditional sweet potato products are having significant role in income generation in small‐scale businesses and entrepreneurs run by women. In developing nations such as Uganda and other SSA countries, dehydrated and minimally processed foodstuffs from OFSP have recognized as the significant for domestic utilization and for small‐scale commerce in domestic markets (Sweet Potato Knowledge Portal, 2018). OFSP and their products are highly promoting in the different African countries such as Kenya, Uganda, Ethiopia, Mali by local governments and NGOs with the help of international research organizations (Abidin et al., 2015). Semiprocessed products from OFSP have been extensively studied in some SSA countries such as Kenya were reported. Complementary food in form of porridge by OFSP flour was highly accepted by the assessors (Stathers, Bechoff, Sindi, Low, & Ndyetabula, 2013). Researchers are concentrated on the methods to develop retention of the carotenes by the processing and are trying to develop the traditional foods by incorporating OFSP such as bread (Nzamwita, Duodu, & Minnaar, 2017), cookies (Kolawole, Akinwande, & Ade‐Omowaye, 2018), juices (Muhammad, Aminah, & Abbas, 2012), and porridge (Pillay, Khanyile, & Siwela, 2018) Table 7 shows the detailed explanation of different foods prepared by OFSP.
Table 7.
Different food products from orange‐fleshed sweet potato (OFSP)
| S. no. | Product group | Product | Description | Findings | Reference |
|---|---|---|---|---|---|
| 1. | Baked products | Cookies | Cookies were produced from OFSP, mushroom powder with different blending ratios; only wheat flour was used as control | Cookies are identified with high protein, ash, crude fiber, and mineral as compared to control. Concluded that nutrient dense cookies with best sensory attributes can produce with blend OFSP and mushroom | Kolawole et al. (2018) |
| Swahili Buns (Mandazi) | Developed and determine quality parameters in OFSP–wheat composite buns (Mandazi) at different levels of wheat flour substitutions with OFSP | Specific volume of the buns decreased significantly with increasing OFSP levels. Proximate composition, sensory acceptability, and VA content of product significantly increase by OFSP powder | Mongi, Simbano, Ruhembe, and Majaliwa (2015) | ||
| Composite bread | Stability of BC during baking of OFSP–wheat breads | Baking causes the degradation of all‐trans‐BC, and breads containing 20% and 30% of OFSP flours can potentially be used for reducing the VAD in children | Nzamwita et al. (2017) | ||
| Cakes | Evaluated the acceptance and preference of cakes prepared with OFSP flour | The cakes prepared with 40% OFSP flour had high acceptability among school students. Cake containing 40% OFSP can reach up to 22% of the RDA of VA to children between 10 and 13 years old | Rangel et al. (2011) | ||
| Bread | OFSP–wheat flour enriched bread prepared and analyzed for different nutritional properties and BC | 30% OFSP flour in bread can contribute 83.3 and 74.2% of VA to 3‐ to 6‐year‐old children's RDA | Kidane, Abegaz, Mulugeta, and Singh (2013) | ||
| Sourdough panettones | OFSP flour on sourdough and prepared panettones by fermentation was reported. Also evaluated OFSP flour on the technological properties and volatile compounds of the final products | OFSP flour suitable for panettones with good moisture and strong yellow color with new volatile compounds such as 2‐octenal‐2‐butyl, dimethyl‐decane, and 2‐chlorooctane | Paula et al. (2018) | ||
| 2. | Extruded products | Noodles | Noodles prepared up to 40% OFSP paste blending with domestic wheat flours and physical, chemical, and sensory properties were assessed | Noodles with OFSP showed quality of moisture and protein and concluded as OFSP is a promising for noodle ingredient | Ginting and Yulifianti (2015) |
| Flours (extruded and nonextruded) | OFSP flour produced by extruded and nonextrusion. Effect of process on carotenoid contents of raw flours was determined | Extrusion reported stabilization of OFSP flours but reported in carotenoids losses due to moisture and screw speed with fixed screw configuration, barrel temperature, and formulation | Waramboi, Gidley, and Sopade (2013) | ||
| Pasta | OFSP processed into flour using different processing methods with pretreatment (blanching, steaming, grilling) and produced pasta by extrusion | Pretreatments and processing methods had a significant effect on functional properties and the chemical properties of the OFSP. Concluded that OFSP could be used for pasta production with rich BC | Olubunmi, Abraham, Mojirade, Afolake, and Kehinde (2017) | ||
| The extruded product with rice and OFSP flours | Evaluated and compared the total carotenoid content of two cultivars and the losses on the dehydrated extruded OFSP flour with different concentrations of rice flour | Losses of total carotenoids higher in the extruded products. The values of VA are very high, indicating that this product is a very good source of PVA. Total carotenoids content of 50% OFSP mixing with 50% of rice flour were reported very good source of PVA | Fonseca, Soares, Freire Junior, Almeida, and Ascheri (2008) | ||
| Instant noodle | Evaluated the noodles prepared using wheat–OFSP–African yam bean flour | OFSP and African yam bean flour up to 20% and 30% resulted in a nutritious instant noodle | Effiong, Maduka, and Essien (2018) | ||
| 3. | Dried products and flours | Powder (spray‐dried) | Determined effect of amylase and maltodextrin on OFSP pure drying. The nutrient composition and rheological properties of rehydrated powder determined | BC and vitamin C reduction reported. All‐trans form of BC was changed to cis. OFSP powders act like pregelatinized starch. Also concluded this product can use as thickener | Grabowski, Truong, and Daubert (2008) |
| Flour (sun‐dried) | Microbiological, chemical, functional, and sensory properties of the fermented, sun‐dried OFSP flour determined | OFSP flours are microbiologically safe, and BC was reduced. Recommended a good processing method to retain PVA | Amajor et al. (2014) | ||
| Dried chips (low temp.) | Determined the nature of PVA losses during drying at low temperature | 16% and 34% reduction in trans‐BC. Both drying method and shape have effect on the BC. Sun drying slightly destroying PVA content compared to solar and hot air drying | Bechoff et al. (2009) | ||
| Dried and stored OFSP | Preservation of carotenoids in OFSP chips determined. Impact of pretreatments to retain carotenoids after drying and storage for 6 months at room temperature was verified | Pretreated and soaked samples had higher content of carotenoid than the control. Also, researchers concluded that applying chemical pretreatment is effective | Bechoff, Westby, Menya, and Tomlins (2011) | ||
| 4 | Complementary and other foods | Complementary food | Assessed the acceptance of complementary foods made from OFSP | OFSP complementary food was well accepted in its color and soft texture, concluded that OFSP has the potential to use in complementary feeding to improve VA status | Pillay et al. (2018) |
| Complementary food | Nutrient composition of OFSP complementary with maize–soybean–groundnut was done | OFSP complementary food is a good source of BC and VA status of infants. OFSP complementary food meets all the energy and macronutrient densities in the Codex | Amagloh and Coad (2014) | ||
| Weaning foods | Effect of OFSP–cereal–legume blend of maize fortified with soybeans on weaning food determined | 25% replacement levels with maize and OFSP are highly acceptable in nutritional | Bonsi, Plahar, and Zabawa (2014) | ||
| Porridge (OFSP‐mangoes) | OFSP boiled and mixed with mangoes puree. Samples pasteurized (80°C for 5 min), packaged, hot filled, and cooled | BC and vitamin C loss identified after pasteurization. Sensory changes were reported after 6 months of the storage at room temperature | Muchoki and Imungi (2017) | ||
| Blended foods | This study determined the BC degradation in the ready‐to‐eat OFSP‐derived products made under local processing conditions. The preparation (i.e., drying) and cooking process (either by boiling or frying) were conducted under noncontrolled conditions in Uganda | All‐trans‐BC contents were varied in porridges and chapati depends on processors. The all‐trans‐BC was greater in products cooked with oil, as compared to the boiled ones | Bechoff, Poulaert, et al. (2011) | ||
| Complimentary food | OFSP‐based infant food developed in SSA with soybean, soybean oil, and fishmeal was processed as complementary food by oven toasting | The OFSP formulation meets energy, protein, fructose, and fat specifications but very less in amino acid compositions | Amagloh et al. (2012) | ||
| OFSP and haricot bean food | Formulated foods from OFSP and haricot bean in different proportions and analyzed nutritional composition | Proximate composition, VA, and minerals are providing RDA for children. The food formulated from 70% OFSP and 30% haricot bean provides the highest protein, fat and fiber, energy, and minerals | Haile and Getahun (2018) | ||
| Bambara groundnut–OFSP snacks | Ready‐to‐eat extruded snack prepared using a combination of OFSP and bambara groundnut | Snacks showed good source of nutrients for RDA for school children. Snack food is a good source of PVA with good sensory properties | Buzo, Mongi, and Mukisa (2016) | ||
| Sweet potato amala | A traditional processing method of sweet potato flour for amala (a stiff paste meal) in Southwest Nigeria was done | Different methods resulted in sensory variations of amala | Fetuga et al. (2013) | ||
| 5 | Drinks | Radish and OFSP juice | Stability of color and pigment obtained from red radish and OFSP performed in a juice model system during 65 weeks | At room temperature, high stability was obtained in juices colored with C‐18 purified radish anthocyanins and lowest with OFSP. Refrigerated temperatures increased the half‐life of the pigment to more than one year | Rodríguez‐Saona, Glusti, and Wrolstad (1999) |
| OFSP‐based juice drink | OFSP‐pineapple drink prepared from 50% to 10%, and different physico‐chemical and sensory properties were studies | OFSP‐based juice was prepared successfully with high overall acceptability. The drink also had substantial quantities of VA | Muhammad et al. (2012) | ||
| 6 | Other products | Natural colorants | OFSP anthocyanin‐based dye compared to synthetic red 40 and red 3 colorants as well as purple carrot and red grape commercial colorants | OFSP‐based dye has high‐to‐moderate resistance for pH, temp., and light. OFSP colors showed red‐violet hue for extended periods of time in comparison with synthetics | Cevallos‐Casals and Cisneros‐Zevallos (2004) |
| Starch | The functional and structural properties of starches from six OFSP varieties were studied | Tainan 18 variety is good source of starch with high‐amylose with properties of high setback and breakdown viscosities, high water solubility at 85°C but low swelling volume at 65°C, and high hardness and adhesiveness | Lai et al. (2016) | ||
| Bioethanol (raw materials) | Suitability to produce bioethanol of 50 varieties of the OFSP was determined | Reported that selected OFSP varieties are with good yields ranged between 23.6 and 49.0 t/ha and capable to produce ethanol between 3,320.1 and 5,364.5 L/ha | Waluyo, Roosda, Istifadah, Ruswandi, and Karuniawan (2015) |
13. CONCLUSION
An eye pleasant color and good flavor make OFSP possesses acceptable by different age groups in many processed forms. The OFSP contained high moisture and starch contents; in contrast, protein and fats are present in very less concentrations. However, the ash and fiber contents are present in moderate concentrations. The minerals are moderately present in OFSP, calcium, magnesium, zinc, and sodium are reported in very less concentrations, but phosphorus and potassium are reported in moderate concentrations, and iron is reported in good concentrations. PVA and NPVA carotenoids are reported in the OFSP; in case of BCX, lutein, zeaxanthin, and AC and BC are in good concentrations. Among the all, very high concentrations of the BC are reported, which is a good source for the VA. Very low concentration of the Vit. E and Vit. C is reported in OFSP. In case of phenolic acids, such as CGA, CA, 4, 5‐; 3, 5‐; 3, 4‐diCQA isomers are reported in good concentrations. The presence of different carotenoids and phenolic acids with good AA is reported by different scientists. The role of OFSP is successfully reported in the VAM in developing countries. Scientists successfully prepared the different food products both in liquid and in solid forms by OFSP base. Finally, it is concluded that OFSP is a good secondary staple food for the developed countries.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STATEMENT
This study does not involve any neither human nor animal testing.
ACKNOWLEDGMENTS
Authors are thankful to the Dean, Faculty of Chemical and Food Engineering, Scientific Director, Bahir Dar Institute of Technology, Bahir Dar University, Ethiopia, for their considerable help to complete this review successfully.
Satheesh N, Workneh S. Review on nutritional composition of orange‐fleshed sweet potato and its role in management of vitamin A deficiency. Food Sci Nutr. 2019;7:1920–1945. 10.1002/fsn3.1063
REFERENCES
- Abbaspour, N. , Hurrell, R. , & Kelishadi, R. (2014). Review on iron and its importance for human health. Journal of Research in Medical Sciences, 19(2), 164–174. [PMC free article] [PubMed] [Google Scholar]
- Abdel‐Aal, E. S. M. , Akhtar, H. , Zaheer, K. , & Ali, R. (2013). Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients, 5(4), 1169–1185. 10.3390/nu5041169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidin, P. E. , Carey, E. , Mallubhotla, S. , & Sones, K. (2017). Sweetpotato cropping guide, Africa soil health consortium. Nairobi, Kenya: CAB International; 10.1063/1.1689294 [DOI] [Google Scholar]
- Abidin, P. E. , Dery, E. , Amagloh, F. K. , Asare, K. , Amoaful, E. F. , & Carey, E. E. (2015). Training of trainers' module for orange‐fleshed sweetpotato (OFSP). Sub-Saharan Africa: Nutrition Department of the Ghana Health Service; https://cgspace.cgiar.org/handle/10568/64872 [Google Scholar]
- Acosta‐Estrada, B. A. , Gutiérrez‐Uribe, J. A. , & Serna‐Saldívar, S. O. (2014). Bound phenolics in foods, a review. Food Chemistry, 152, 46–55. 10.1016/j.foodchem.2013.11.093 [DOI] [PubMed] [Google Scholar]
- Ahn, Y. O. , Kim, S. H. , Kim, C. Y. , Lee, J. S. , Kwak, S. S. , & Lee, H. S. (2010). Exogenous sucrose utilization and starch biosynthesis among sweetpotato cultivars. Carbohydrate Research, 345(1), 55–60. 10.1016/j.carres.2009.08.025 [DOI] [PubMed] [Google Scholar]
- Amagloh, F. K. , & Coad, J. (2014). Orange‐fleshed sweet potato‐based infant food is a better source of dietary vitamin A than a maize‐legume blend as complementary food. Food and Nutrition Bulletin, 35(1), 51–59. 10.1177/156482651403500107 [DOI] [PubMed] [Google Scholar]
- Amagloh, F. K. , Hardacre, A. , Mutukumira, A. N. , Weber, J. L. , Brough, L. , & Coad, J. (2012). A household‐level sweet potato‐based infant food to complement vitamin A supplementation initiatives. Maternal and Child Nutrition, 8(4), 512–521. 10.1111/j.1740-8709.2011.00343.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amajor, J. U. , Oti, E. , Ekeledo, N. , Omodamiro, R. , Amajor, E. E. , & Aniedu, C. (2014). Studies on the characteristic properties of fermented, sun‐dried orange‐fleshed sweet potato flour. Nigerian Food Journal, 32(1), 45–53. 10.1016/S0189-7241(15)30095-3 [DOI] [Google Scholar]
- Anbuselvi, S. , Kumar, M. S. , Selvakumar, S. , Rao, M. R. K. , & Anshumita, D. (2012). A comparative study on biochemical constituents of sweet potatoes from Orissa and Tamilnadu and its curd formation. Journal of Chemical and Pharmaceutical Research, 4(11), 4879–4882. [Google Scholar]
- Andre, C. M. , Ghislain, M. , Bertin, P. , Oufir, M. , Herrera, M. D. R. , Hoffmann, L. , & Evers, D. (2007). Andean potato cultivars (Solarium tuberosum L.) as a source of antioxidant and mineral micronutrients. Journal of Agricultural and Food Chemistry, 55(2), 366–378. 10.1021/jf062740i [DOI] [PubMed] [Google Scholar]
- Bashiri, A. , Burstein, E. , Sheiner, E. , & Mazor, M. (2003). Anemia during pregnancy and treatment with intravenous iron: Review of the literature. European Journal of Obstetrics Gynecology and Reproductive Biology, 110(1), 2–7. 10.1016/S0301-2115(03)00113-1 [DOI] [PubMed] [Google Scholar]
- Bechoff, A. , Dufour, D. , Dhuique‐Mayer, C. , Marouzé, C. , Reynes, M. , & Westby, A. (2009). Effect of hot air, solar and sun drying treatments on provitamin A retention in orange‐fleshed sweetpotato. Journal of Food Engineering, 92(2), 164–171. 10.1016/j.jfoodeng.2008.10.034 [DOI] [Google Scholar]
- Bechoff, A. , Poulaert, M. , Tomlins, K. I. , Westby, A. , Menya, G. , Young, S. , & Dhuique‐Mayer, C. (2011). Retention and bioaccessibility of β‐carotene in blended foods containing orange‐fleshed sweet potato flour. Journal of Agricultural and Food Chemistry, 59(18), 10373–10380. 10.1021/jf201205y [DOI] [PubMed] [Google Scholar]
- Bechoff, A. , Westby, A. , Menya, G. , & Tomlins, K. I. (2011). Effect of pretreatments for retaining total carotenoids in dried and stored orange‐fleshed‐sweet potato chips. Journal of Food Quality, 34(4), 259–267. 10.1111/j.1745-4557.2011.00391.x [DOI] [Google Scholar]
- Bendich, A. , & Langseth, L. (1995). The health effects of vitamin C supplementation: a review. Journal of the American College of Nutrition, 14(2), 124–136. 10.1080/07315724.1995.10718484 [DOI] [PubMed] [Google Scholar]
- Bengtsson, A. , Namutebi, A. , Alminger, M. L. , & Svanberg, U. (2008). Effects of various traditional processing methods on the all‐trans‐β‐carotene content of orange‐fleshed sweet potato. Journal of Food Composition and Analysis, 21(2), 134–143. 10.1016/j.jfca.2007.09.006 [DOI] [Google Scholar]
- Berni, P. , Chitchumroonchokchai, C. , Canniatti‐Brazaca, S. G. , De Moura, F. F. , & Failla, M. L. (2015). Comparison of content and in vitro bioaccessibility of provitamin A carotenoids in home cooked and commercially processed orange fleshed sweet potato (Ipomea batatas Lam). Plant Foods for Human Nutrition, 70(1), 1920–8. 10.1007/s11130-014-0458-1 [DOI] [PubMed] [Google Scholar]
- Bian, Q. , Gao, S. , Zhou, J. , Qin, J. , Taylor, A. , Johnson, E. J. , … Shang, F. U. (2012). Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation‐related genes in retinal pigment epithelial cells. Free Radical Biology and Medicine, 53(6), 1298–1307. 10.1016/j.freeradbiomed.2012.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorneboe, A. , Bjorneboe, G.‐E. , & Drevon, C. A. (1990). Absorption, transport and distribution of vitamin E. Journal of Nutrition, 120, 233–242. 10.1002/9780470720622.ch3 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Morris, S. S. , & Bryce, J. (2003). Child survival I where and why are 10 million children dying every year? The Lancet, 361, 2226–2234. 10.1016/S0140-6736(03)13779-8 [DOI] [PubMed] [Google Scholar]
- Blokhina, O. , Virolainen, E. , & Fagerstedt, K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: A review. Annals of Botany, 91, 179–194. 10.1093/aob/mcf118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm, V. , Puspitasari‐Nienaber, N. L. , Ferruzzi, M. G. , & Schwartz, S. J. (2002). Trolox equivalent antioxidant capacity of different geometrical isomers of α‐carotene, β‐carotene, lycopene, and zeaxanthin. Journal of Agricultural and Food Chemistry, 50, 221–226. 10.1021/jf010888q [DOI] [PubMed] [Google Scholar]
- Bonsi, E. A. , Plahar, W. A. , & Zabawa, R. (2014). Nutritional enhancement of Ghanaian weaning foods using the orange flesh sweetpotato (Ipomea batatas). African Journal of Food, Agriculture, Nutrition and Development, 14(5), 2036–2056. [Google Scholar]
- Borsarelli, C. D. , & Mercadante, A. Z. (2009). Thermal and photochemical degradation of carotenoids In Landrum J. T. (Ed.). Carotenoids: Physical, chemical, and biological functions and properties (1st edn). (p. 229). Boca Raton, FL: CRC Press. [Google Scholar]
- Bovell Benjamin, A. C. (2007). Sweet potato: A review of its past, present, and future role in human nutrition. Advances in Food and Nutrition Research, 52, 1920–59. [DOI] [PubMed] [Google Scholar]
- Bowen‐Forbes, C. S. , Zhang, Y. , & Nair, M. G. (2010). Anthocyanin content, antioxidant, anti‐inflammatory and anticancer properties of blackberry and raspberry fruits. Journal of Food Composition and Analysis, 23(6), 554–560. 10.1016/j.jfca.2009.08.012 [DOI] [Google Scholar]
- Brazionis, L. , Rowley, K. , Itsiopoulos, C. , & O'dea, K. (2009). Plasma carotenoids and diabetic retinopathy. British Journal of Nutrition, 101(2), 270–277. 10.1017/S0007114508006545 [DOI] [PubMed] [Google Scholar]
- Breithaupt, D. E. , & Bamedi, A. (2001). Carotenoid esters in vegetables and fruits: A screening with emphasis on β‐cryptoxanthin esters. Journal of Agricultural and Food Chemistry, 49(4), 2064–2070. [DOI] [PubMed] [Google Scholar]
- Britton, G. (2008). Functions of intact carotenoids In Britton G., Jensen S. L. & Pfander H. (Eds.). Carotenoids (pp. 189–212). Berlin, Germany: Springer; https://link.springer.com/book/10.1007/978-3-7643-7499-0 [Google Scholar]
- Brown, C. R. (2005). Antioxidants in potato. American Journal of Potato Research, 82(2), 163–172. 10.1007/BF02853654 [DOI] [Google Scholar]
- Brown, C. R. , Culley, D. , Yang, C.‐P. , Durst, R. , & Wrolstad, R. (2005). Variation of anthocyanin and carotenoid contents and associated antioxidant values in potato breeding lines. Journal of the American Society for Horticultural Science, 130(2), 174–180. 10.1016/S0306-2619(03)00106-5 [DOI] [Google Scholar]
- Brown, C. R. , Edwards, C. G. , Yang, C.‐P. , & Dean, B. B. (1993). Orange flesh trait in potato: Inheritance and carotenoid content. Journal of the American Society for Horticultural Science, 118(1), 145–150. 10.21273/JASHS.118.1.145 [DOI] [Google Scholar]
- Butte, N. F. (2000). Fat intake of children in relation to energy requirements. American Journal of Clinical Nutrition, 72(Suppl. 5), 1246–1252. 10.1207/s15374424jccp2604 [DOI] [PubMed] [Google Scholar]
- Buzo, H. , Mongi, R. , & Mukisa, I. (2016). Development and assessment of nutritional quality and sensory properties of orange‐fleshed sweetpotato and bambara groundnut‐based snacks for school children. Fifth RUFORUM Biennial Regional Conference, 14(2), 1067–1073. [Google Scholar]
- Carol, P. , & Kuntz, M. (2001). A plastid terminal oxidase comes to light: Implications for carotenoid biosynthesis and chlororespiration. Trends in Plant Science, 6(1), 31–36. 10.1016/S1360-1385(00)01811-2 [DOI] [PubMed] [Google Scholar]
- Castenmiller, J. J. M. , & West, C. E. (1998). Bioavailability and bioconversion of carotenoids. Annual Review of Nutrition, 18(1), 19–38. 10.1146/annurev.nutr.18.1.19 [DOI] [PubMed] [Google Scholar]
- Catherine, A. E. , Nicholas, J. M. , & George, P. (2014). Structure‐antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology & Medicine, 31(03), 525–556. 10.1017/S0952675714000244 [DOI] [PubMed] [Google Scholar]
- Cevallos‐Casals, B. A. , & Cisneros‐Zevallos, L. (2004). Stability of anthocyanin‐based aqueous extracts of Andean purple corn and red‐fleshed sweet potato compared to synthetic and natural colorants. Food Chemistry, 86(1), 69–77. 10.1016/j.foodchem.2003.08.011 [DOI] [Google Scholar]
- Chandrasekara, A. , & Josheph Kumar, T. (2016). Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. International Journal of Food Science, 2016, 1920–15. 10.1155/2016/3631647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausmer, A. B. (1998). Zinc, insulin and diabetes. Journal of the American College of Nutrition, 17(2), 109–115. 10.1080/07315724.1998.10718735 [DOI] [PubMed] [Google Scholar]
- Chen, B. H. , & Huang, J. H. (1998). Degradation and isomerization of chlorophyll a and β‐carotene as affected by various heating and illumination treatments. Food Chemistry, 62(3), 299–307. [Google Scholar]
- Chen, B. H. , & Tang, Y. C. (1998). Processing and stability of carotenoid powder from carrot pulp waste. Journal of Agricultural and Food Chemistry, 46(6), 2312–2318. 10.1021/jf9800817 [DOI] [Google Scholar]
- Chen, Z. , Schols, H. A. , & Voragen, A. G. J. (2003). Starch granule size strongly determines starch noodle processing and noodle quality. Food Chemistry and Toxicology Starch, 68(5), 1584–1589. 10.1111/j.1365-2621.2003.tb12295.x [DOI] [Google Scholar]
- Chew, B. P. , & Park, J. S. (2004). Carotenoid action on the immune response. Journal of Nutrition, 135(1), 257S–261S. 10.1093/jn/134.1.257S [DOI] [PubMed] [Google Scholar]
- Cinkilic, N. , Cetintas, S. K. , Zorlu, T. , Vatan, O. , Yilmaz, D. , Cavas, T. , … Bilaloglu, R. (2013). Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X‐ray induced DNA damage in human blood lymphocytes in vitro. Food and Chemical Toxicology, 53, 359–363. 10.1016/j.fct.2012.12.008 [DOI] [PubMed] [Google Scholar]
- Clifford, M. N. (2000). Chlorogenic acids and other cinnamates—nature, occurence, dietary burden, absorption and metabolism. Journal of the Science of Food and Agriculture, 80, 1033–1043. [Google Scholar]
- Constán‐Aguilar, C. , Leyva, R. , Blasco, B. , Sánchez‐Rodríguez, E. , Soriano, T. , & Ruiz, J. M. (2014). Biofortification with potassium: Antioxidant responses during postharvest of cherry tomato fruits in cold storage. Acta Physiologiae Plantarum, 36(2), 283–293. 10.1007/s11738-013-1409-4 [DOI] [Google Scholar]
- De Rigal, D. , Gauillard, F. , & Richard‐Forget, F. (2000). Changes in the carotenoid content of apricot (Prunus armeniaca, var Bergeron) during enzymatic browning: β‐carotene inhibition of chlorogenic acid degradation. Journal of the Science of Food and Agriculture, 80(6), 763–768. [DOI] [PubMed] [Google Scholar]
- Dhingra, D. , Michael, M. , Rajput, H. , & Patil, R. T. (2012). Dietary fibre in foods: A review. Journal of Food Science and Technology, 49(3), 255–266. 10.1007/s13197-011-0365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donado‐Pestana, C. M. , Salgado, J. M. , de Oliveira Rios, A. , dos Santos, P. R. , & Jablonski, A. (2012). Stability of carotenoids, total phenolics and in vitro antioxidant capacity in the thermal processing of orange‐fleshed sweet potato (Ipomoea batatas Lam.) cultivars grown in Brazil. Plant Food for Human Nutrition, 67(1), 262–270. 10.1007/s11130-012-0298-9 [DOI] [PubMed] [Google Scholar]
- Drouin, G. , Godin, J.‐R. , & Page, B. (2011). The genetics of vitamin C loss in vertebrates. Current Genomics, 12(5), 371–378. 10.2174/138920211796429736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge, R. , Mc Garvey, D. J. , & Truscott, T. G. (1997). The carotenoids as anti‐oxidants – A review. Journal of Photochemistry and Photobiology B: Biology, 41(3), 189–200. 10.1016/S1011-1344(97)00092-4 [DOI] [PubMed] [Google Scholar]
- Effiong, B. , Maduka, N. , & Essien, A. (2018). Evaluation of wheat and orange‐fleshed sweet potato composite flour fortified with African yam bean flour for instant noodle production. Archives of Current Research International, 13(4), 1920–15. 10.9734/ACRI/2018/41174 [DOI] [Google Scholar]
- Eichhorn, S. , & Winterhalter, P. (2005). Anthocyanins from pigmented potato (Solanum tuberosum L.) varieties. Food Research International, 38(8–9), 943–948. 10.1016/j.foodres.2005.03.011 [DOI] [Google Scholar]
- Endrias, D. , Negussie, R. , & Gulelat, D. (2016). Comparison of three sweet potato (Ipomoea Batatas (L.) Lam) varieties on nutritional and anti‐nutritional factors. Global Journal of Science Frontier Research: D Agriculture and Veterinary, 16(4), 1920–11 10.1016/j.trstmh.2006.05.010 [DOI] [Google Scholar]
- Evelyne, P. , Marie, A. C. , Liliane, D. , Marie, J. T. , Annie, P. , Catherine, V. , & Michel, C. (1994). Lipoperoxidation in plasma and red blood cells of patients undergoing haemodialysis: Vitamins A, E and iron status. Pergamon, Free Radical Biology and Medicine, 16(3), 339–346. 10.1016/0891-5849(94)90035-3 [DOI] [PubMed] [Google Scholar]
- Faber, M. , Venter, S. L. , & Benadé, A. S. (2002). Increased vitamin A intake in children aged 2–5 years through targeted home‐gardens in a rural South African community. Public Health Nutrition, 5(01), 11–16. 10.1079/PHN2001239 [DOI] [PubMed] [Google Scholar]
- FAOSTAT (2019). Food Agriculture and Organization (FAOSTAT). Retrieved from http://www.fao.org/faostat/en/#data/QC [Google Scholar]
- Fetuga, G. O. , Tomlins, K. , Bechoff, A. , Henshaw, F. O. , Idowu, M. A. , & Westby, A. (2013). A survey of traditional processing of sweet potato flour for amala, consumption pattern of sweet potato amala and awareness of orange‐fleshed sweet potato (OFSP) in South West Nigeria. Journal of Food, Agriculture and Environment, 11(3–4), 67–71. [Google Scholar]
- Finkel, T. , & Holbrook, N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature, 408, 239 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Fonseca, M. J. , de Soares, A. G. , Freire Junior, M. , Almeida, D. L. , & de Ascheri, J. L. R. (2008). Effect of extrusion‐cooking in total carotenoids content in cream and orange flesh sweet potato cultivars. Horticultura Brasileira, 26(1), 112–115. 10.1590/S0102-05362008000100022 [DOI] [Google Scholar]
- Fraser, P. D. , & Bramley, P. M. (2004). The biosynthesis and nutritional uses of carotenoids. Progress in Lipid Research, 43(3), 228–265. 10.1016/j.plipres.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Galli, F. , Azzi, A. , Birringer, M. , Cook‐Mills, J. M. , Eggersdorfer, M. , Frank, J. , … Özer, N. K. (2017). Vitamin E: Emerging aspects and new directions. Free Radical Biology and Medicine, 102, 16–36. 10.1016/j.freeradbiomed.2016.09.017 [DOI] [PubMed] [Google Scholar]
- Gammone, M. A. , Riccioni, G. , & D'Orazio, N. (2015). Carotenoids: Potential allies of cardiovascular health? Food and Nutrition Research, 59, 26762 10.3402/fnr.v59.26762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginting, E. , & Yulifianti, R. (2015). Characteristics of noodle prepared from orange‐fleshed sweet potato, and domestic wheat flour. Procedia Food Science, 3, 289–302. 10.1016/j.profoo.2015.01.032 [DOI] [Google Scholar]
- Girard, A. W. , Grant, F. , Watkinson, M. , Okuku, H. S. , Wanjala, R. , Cole, D. , … Low, J. (2017). Promotion of orange‐fleshed sweet potato increased vitamin A intakes and reduced the odds of low retinol‐binding protein among postpartum Kenyan women. Journal of Nutrition, 147(5), 955–963. 10.3945/jn.116.236406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githunguri, C. M. , & Migwa, Y. N. (2004). Performance, foliage and root yield of sweet potato clones from a preliminary yield trial at kiboko in semiarid eastern Kenya. NHFRC‐Katumani: Kenya Agricultural Research Institute. [Google Scholar]
- Glover, B. J. , & Cathie, M. (2012). Anthocyanins. Current Biology, 22(5), R147–R150. 10.1016/j.cub.2012.01.021 [DOI] [PubMed] [Google Scholar]
- Gonthier, M.‐P. , Verny, M.‐A. , Besson, C. , Rémésy, C. , & Scalbert, A. (2003). Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. Journal of Nutrition, 133(6), 1853–1859. 10.1093/jn/133.6.1853 [DOI] [PubMed] [Google Scholar]
- Gordon, M. H. (1996). Dietary antioxidants in disease prevention. Natural Product Reports, 13(4), 265 10.1039/np9961300265 [DOI] [PubMed] [Google Scholar]
- Grabowski, J. A. , Truong, V. D. , & Daubert, C. R. (2008). Nutritional and rheological characterization of spray dried sweetpotato powder. LWT – Food Science and Technology, 41(2), 206–216. 10.1016/j.lwt.2007.02.019 [DOI] [Google Scholar]
- Grace, M. H. , Yousef, G. G. , Gustafson, S. J. , Truong, V. D. , Yencho, G. C. , & Lila, M. A. (2014). Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweetpotato storage and impacts on bioactive properties. Food Chemistry, 145, 717–724. 10.1016/j.foodchem.2013.08.107 [DOI] [PubMed] [Google Scholar]
- Gul, K. , Tak, A. , Singh, A. K. , Singh, P. , Yousuf, B. , & Wani, A. A. (2015). Chemistry, encapsulation, and health benefits of beta‐carotene – A review. Journal Cogent Food and Agriculture, 1(1), 1920–12. 10.1080/23311932.2015.1018696 [DOI] [Google Scholar]
- Hagenimana, V. , & Low, J. (2000). Potential of orange‐fleshed sweet potatoes for raising vitamin A intake in Africa. Food and Nutrition Bulletin, 21(4), 414–418. 10.1177/156482650002100414 [DOI] [Google Scholar]
- Haile, A. , & Getahun, D. (2018). Evaluation of nutritional and anti nutrition factors of orange‐fleshed sweet potato and haricot bean blended mashed food for pre‐school children : The case of Dale. Food Science and Technology, 6(1), 10–19. 10.13189/fst.2018.060102 [DOI] [Google Scholar]
- Halliwell, B. (2006). Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology, 141, 312–322. 10.1104/pp.106.077073.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell, M. J. , Jamil, K. M. , Hassan, F. , Peerson, J. M. , Hossain, M. I. , Fuchs, G. J. , & Brown, K. H. (2004). Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total‐body vitamin A stores in Bangladeshi men. American Journal of Clinical Nutrition, 80(3), 705–714. 10.1093/ajcn/80.3.705 [DOI] [PubMed] [Google Scholar]
- Helmut, S. , Wilhelem, S. , & Alfred, R. S. (1992). Antioxidant functions of vitamins. Annals of the New York Academy of Sciences, 669(1), 7–20. 10.1111/j.1749-6632.1992.tb17085.x [DOI] [PubMed] [Google Scholar]
- Hernández Suárez, M. , Montes Hernández, A. I. , Rodríguez Galdón, B. , Hernández Rodríguez, L. , Medina Cabrera, C. E. , Ríos Mesa, D. , … Díaz Romero, C. (2016). Application of multidimensional scaling technique to differentiate sweet potato (Ipomoea batatas (L.) Lam) cultivars according to their chemical composition. Journal of Food Composition and Analysis, 46, 43–49. 10.1016/j.jfca.2015.10.008 [DOI] [Google Scholar]
- Holden, J. M. , Eldridge, A. L. , Beecher, G. R. , Marilyn Buzzard, I. , Bhagwat, S. , Davis, C. S. , … Schakel, S. (1999). Carotenoid content of U.S. foods: An update of the database. Journal of Food Composition and Analysis, 12(3), 169–196. 10.1006/jfca.1999.0827 [DOI] [Google Scholar]
- Hortz, C. , Loechl, C. , Lubowa, A. , Tumwine, J. K. , Ndeezi, G. , Masawi, A. N. , & Meenakshi, V. J. (2012). Introduction of beta carotene‐rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. Journal of Nutrition, 142, 1871–1880. [DOI] [PubMed] [Google Scholar]
- Hotz, C. , Loechl, C. , de Brauw, A. , Eozenou, P. , Gilligan, D. , Moursi, M. , … Meenakshi, J. V. (2012). A large‐scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. British Journal of Nutrition, 108(1), 163–176. 10.1017/S0007114511005174 [DOI] [PubMed] [Google Scholar]
- Hotz, C. , Loechl, C. , Lubowa, A. , Tumwine, J. K. , Ndeezi, G. , Nandutu Masawi, A. , & Gilligan, D. O. (2012). Introduction of ‐carotene‐rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. Journal of Nutrition, 142(10), 1871–1880. 10.3945/jn.111.151829 [DOI] [PubMed] [Google Scholar]
- Hu, F. B. , van Dam, R. M. , & Liu, S. (2001). Diet and risk of type II diabetes: The role of types of fat and carbohydrate. Diabetologia, 44(7), 805–817. 10.1007/s001250100547 [DOI] [PubMed] [Google Scholar]
- Huang, A. S. , Tanudjaja, L. , & Lum, D. (1999). Content of alpha‐, beta‐carotene, and dietary fiber in 18 sweetpotato varieties grown in Hawaii. Journal of Food Composition and Analysis, 12(2), 147–151. 10.1006/jfca.1999.0819 [DOI] [Google Scholar]
- Imark, C. , Kneubühl, M. , & Bodmer, S. (2000). Occurrence and activity of natural antioxidants in herbal spirits. Innovative Food Science and Emerging Technologies, 1(4), 239–243. 10.1016/S1466-8564(00)00018-7 [DOI] [Google Scholar]
- International Potato Center (2017). Sweet potato in Africa. Retrieved from https://cipotato.org/research/sweetpotato-in-africa/ [Google Scholar]
- Islam, S. N. , Nusrat, T. , Begum, P. , & Ahsan, M. (2016). Carotenoids and β‐carotene in orange fleshed sweet potato: A possible solution to vitamin A deficiency. Food Chemistry, 199, 628–631. 10.1016/j.foodchem.2015.12.057 [DOI] [PubMed] [Google Scholar]
- Jamil, K. M. , Brown, K. H. , Jamil, M. , Peerson, J. M. , Keenan, A. H. , Newman, J. W. , & Haskell, M. J. (2012). Daily consumption of orange‐fleshed sweet potato for 60 days increased plasma β‐carotene concentration but did not increase total body vitamin a pool size in Bangladeshi women. Journal of Nutrition, 142(10), 1896–1902. 10.3945/jn.112.164830 [DOI] [PubMed] [Google Scholar]
- Janet, H. (2003). Bioavailability of iron, zink, and other trace minerals from vegetarian diets. American Journal of Clinical Nutrition, 78(Suppl), 633S–S639. [DOI] [PubMed] [Google Scholar]
- Jiang, Q. , Christen, S. , Mark, K. , & Bruce, N. A. (2001). α‐Tocopherol, the major form of vitamin E in the US diet, deserves. American Journal of Clinical Nutrition, 74, 714–722. [DOI] [PubMed] [Google Scholar]
- Jobling, S. (2004). Improving starch for food and industrial applications. Current Opinion in Plant Biology, 7(2), 210–218. 10.1016/j.pbi.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Johnston, C. S. , Solomon, R. E. , & Corte, C. (1996). Vitamin C depletion is associated with alterations in blood histamine and plasma free carnitine in adults. Journal of the American College of Nutrition, 15(6), 586–591. 10.1080/07315724.1996.10718634 [DOI] [PubMed] [Google Scholar]
- Jones, K. M. , & de Brauw, A. (2015). Using agriculture to improve child health: Promoting orange sweet potatoes reduces diarrhea. World Development, 74, 15–24. 10.1016/j.worlddev.2015.04.007 [DOI] [Google Scholar]
- Julianti, E. , Rusmarilin, H. , Ridwansyah, , & Yusraini, E. (2017). Functional and rheological properties of composite flour from sweet potato, maize, soybean and xanthan gum. Journal of the Saudi Society of Agricultural Sciences, 16(2), 171–177. 10.1016/j.jssas.2015.05.005 [DOI] [Google Scholar]
- Jung, J. K. , Lee, S. U. , Kozukue, N. , Levin, C. E. , & Friedman, M. (2011). Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. Journal of Food Composition and Analysis, 24(1), 29–37. 10.1016/j.jfca.2010.03.025 [DOI] [Google Scholar]
- Kaguongo, W. (2012). Factors influencing adoption and intensity of adoption of orange flesh sweet potato varieties: Evidence from an extension intervention in Nyanza and Western provinces, Kenya. African Journal of Agricultural Research, 7(3), 493–503. 10.5897/AJAR11.062 [DOI] [Google Scholar]
- Kalariya, N. M. , Ramana, K. V. , & vanKuijk, F. J. G. M. (2012). Focus on molecules: Lutein. Experimental Eye Research, 102, 107–108. 10.1016/j.exer.2011.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinga, R. , Byaruhanga, P. , Zschocke, T. , & Tumwegamire, S. (2009). Growing orange‐fleshed sweetpotato for a healthy diet: A supplementary learners' resource book for upper primary schools. Apartado, Lima: International Potato Center; https://pdfs.semanticscholar.org/f736/03003f8214a9b46fdd3924ff5a5257eba8c6.pdf [Google Scholar]
- Karp, H. J. , Vaihia, K. P. , Kärkkäinen, M. U. M. , Niemistö, M. J. , & Lamberg‐Allardt, C. J. E. (2007). Acute effects of different phosphorus sources on calcium and bone metabolism in young women: A whole‐foods approach. Calcified Tissue International, 80(4), 251–258. 10.1007/s00223-007-9011-7 [DOI] [PubMed] [Google Scholar]
- Kasai, H. , Fukada, S. , Yamaizumi, Z. , Sugie, S. , & Mori, H. (2000). Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8‐hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food and Chemical Toxicology, 38(5), 467–471. 10.1016/S0278-6915(00)00014-4 [DOI] [PubMed] [Google Scholar]
- Khaw, K.‐T. , & Barrett‐Connor, E. (1987). Dietary potassium and stroke‐associated mortality. New England Journal of Medicine, 316(5), 235–240. 10.1056/NEJM198701293160502 [DOI] [PubMed] [Google Scholar]
- Kidane, G. , Abegaz, K. , Mulugeta, A. , & Singh, P. (2013). Nutritional analysis of vitamin A enriched bread from orange flesh sweet potato and locally available wheat flours at Samre Woreda, Northern Ethiopia. Current Research in Nutrition and Food Science Journal, 1(1), 49–57. 10.12944/CRNFSJ.1.1.05 [DOI] [Google Scholar]
- Kijlstra, A. , Tian, Y. , Kelly, E. R. , & Berendschot, T. T. J. M. (2012). Lutein: More than just a filter for blue light. Progress in Retinal and Eye Research, 31(4), 303–315. 10.1016/j.preteyeres.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Kim, H. J. , Park, W. S. , Bae, J.‐Y. , Kang, S. Y. , Yang, M. H. , Lee, S. , … Ahn, M.‐J. (2015). Variations in the carotenoid and anthocyanin contents of Korean cultural varieties and home‐processed sweet potatoes. Journal of Food Composition and Analysis, 41, 188–193. 10.1016/j.jfca.2015.01.012 [DOI] [Google Scholar]
- Kolawole, F. L. , Akinwande, B. A. , & Ade‐Omowaye, B. I. O. (2018). Physicochemical properties of novel cookies produced from orange‐fleshed sweet potato cookies enriched with sclerotium of edible mushroom (Pleurotus tuberregium). Journal of the Saudi Society of Agricultural Sciences, 1920–5. 10.1016/j.jssas.2018.09.001 [DOI] [Google Scholar]
- Kong, J. M. , Chia, L. S. , Goh, N. K. , Chia, T. F. , & Brouillard, R. (2003). Analysis and biological activities of anthocyanins. Phytochemistry, 64(5), 923–933. 10.1016/S0031-9422(03)00438-2 [DOI] [PubMed] [Google Scholar]
- Kono, Y. , Shibata, H. , Kodama, Y. , & Sawa, Y. (1995). The suppression of the N‐nitrosating reaction by chlorogenic acid. Biochemical Journal, 312, 947–953. 10.1042/bj3120947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosieradzka, I. , Borucki, W. , Matysiak‐Kata, I. , Szopa, J. , & Sawosz, E. (2004). Influence of dietary fatty acids and fat level on morphological changes in the liver of nase (Chondrostoma nasus L.) Transgenic potato tubers as a source of phenolic compounds. Journal of Animal and Feed Sciences, 13(2), 87–92. 10.22358/jafs/70299/2004 [DOI] [Google Scholar]
- Krem, M. M. , & Cera, E. D. (2002). Evolution of enzyme cascades from embryonic development to blood coagulation. Trends in Biochemical Sciences, 27(2), 67–74. 10.1016/S0968-0004(01)02007-2 [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Singh, A. , & Ekavali (2015). A review on Alzheimer's disease pathophysiology and its management: An update. Pharmacological Reports, 67(2), 195–203. 10.1016/j.pharep.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Kurabachew, H. (2015). The role of orange fleshed sweet potato (Ipomea batatas) for combating vitamin A deficiency in Ethiopia: A review. International Journal of Food Science and Nutrition Engineering, 5(3), 141–146. [Google Scholar]
- Lachman, J. , Hamouz, K. , Orsák, M. , Pivec, V. , & Dvořák, P. (2008). The influence of flesh colour and growing locality on polyphenolic content and antioxidant activity in potatoes. Scientia Horticulturae, 117(2), 109–114. 10.1016/j.scienta.2008.03.030 [DOI] [Google Scholar]
- Lai, Y. C. , Wang, S. Y. , Gao, H. Y. , Nguyen, K. M. , Nguyen, C. H. , Shih, M. C. , & Lin, K. H. (2016). Physicochemical properties of starches and expression and activity of starch biosynthesis‐related genes in sweet potatoes. Food Chemistry, 199, 556–564. 10.1016/j.foodchem.2015.12.053 [DOI] [PubMed] [Google Scholar]
- Laurie, S. M. , & Faber, M. (2008). Integrated community‐based growth monitoring and vegetable gardens focusing on crops rich in β‐carotene: Project evaluation in a rural community in the Eastern Cape, South Africa. Journal of the Science of Food and Agriculture, 88(12), 2093–2101. 10.1002/jsfa.3319 [DOI] [Google Scholar]
- Laurie, S. M. , Van Jaarsveld, P. J. , Faber, M. , Philpott, M. F. , & Labuschagne, M. T. (2012). Trans‐β‐carotene, selected mineral content and potential nutritional contribution of 12 sweetpotato varieties. Journal of Food Composition and Analysis, 27, 151–159. 10.1016/j.jfca.2012.05.005 [DOI] [Google Scholar]
- Lebot, V. , Michalet, S. , & Legendre, L. (2016). Identification and quantification of phenolic compounds responsible for the antioxidant activity of sweet potatoes with different flesh colours using high performance thin layer chromatography (HPTLC). Journal of Food Composition and Analysis, 49, 94–101. 10.1016/j.jfca.2016.04.009 [DOI] [Google Scholar]
- Lee, H. O. , Lee, B. , Jung, I. K. , & Lee, L. (2001). Effect of supplementation of vitamin E and vitamin C on brain acetylcholinesterase activity and neurotransmitter levels in rats treated with scopolamine, an inducer of dementia. Journal of Nutritional Science and Vitaminology, 47, 323–328. [DOI] [PubMed] [Google Scholar]
- Lemoine, R. , Camera, S. L. , Atanassova, R. , Dédaldéchamp, F. , Allario, T. , Pourtau, N. , … Durand, M. (2013). Source‐to‐sink transport of sugar and regulation by environmental factors. Frontiers in Plant Science, 4, 1920–21. 10.3389/fpls.2013.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, J. , Bosin, E. , Feldman, B. , Giat, Y. , Miinster, A. , Danilenko, M. , & Sharoni, Y. (1995). Lycopene is a more potent inhibitor of human cancer cell proliferation than either α‐carotene or β‐carotene. Nutrition and Cancer, 24(3), 257–266. 10.1080/01635589509514415 [DOI] [PubMed] [Google Scholar]
- Lian, F. , Hu, K. Q. , Russell, R. M. , & Wang, X. D. (2006). β‐Cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non‐small‐cell lung cancer cells and up‐regulates retinoic acid receptor β expression. International Journal of Cancer, 119(9), 2084–2089. 10.1002/ijc.22111 [DOI] [PubMed] [Google Scholar]
- Lipinski, B. (2001). Pathophysiology of oxidative stress in diabetes mellitus. Journal of Diabetes and Its Complications, 15(4), 203–210. 10.1016/S1056-8727(01)00143-X [DOI] [PubMed] [Google Scholar]
- Liu, S. C. , Lin, J. T. , & Yang, D. J. (2009). Determination of cis‐ and trans‐ α‐ and β‐carotenoids in Taiwanese sweet potatoes (Ipomoea batatas (L.) Lam.) harvested at various times. Food Chemistry, 116(3), 605–610. 10.1016/j.foodchem.2008.09.037 [DOI] [Google Scholar]
- Liu, X. , Chen, X. , Wu, W. , & Peng, G. (2007). A neural network for predicting moisture content of grain drying process using genetic algorithm. Food Control, 18(8), 928–933. 10.1016/j.foodcont.2006.05.010 [DOI] [Google Scholar]
- Low, J. W. , Arimond, M. , Osman, N. , Cunguara, B. , Zano, F. , & Tschirley, D. (2007). A food‐based approach introducing orange‐fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. Journal of Nutrition, 137(5), 1320–1327. 10.4081/pb.2010.e10 [DOI] [PubMed] [Google Scholar]
- Lozano‐Alejo, N. , Carrillo, G. V. , Pixley, K. , & Palacios‐Rojas, N. (2007). Physical properties and carotenoid content of maize kernels and its nixtamalized snacks. Innovative Food Science and Emerging Technologies, 8(3), 385–389. 10.1016/j.ifset.2007.03.015 [DOI] [Google Scholar]
- Lukaszewicz, M. , Matysiak‐Kata, I. , Skala, J. , Fecka, I. , Cisowski, W. , & Szopa, J. (2004). Antioxidant capacity manipulation in transgenic potato tuber by changes in phenolic compounds content. Journal of Agricultural and Food Chemistry, 52(6), 1526–1533. 10.1021/jf034482k [DOI] [PubMed] [Google Scholar]
- Lutter, C. K. , & Rivera, J. A. (2003). Nutritional status of infants and young children and characteristics of their diets. Journal of Nutrition, 133(9), 2941S–2949S. 10.1093/jn/133.9.2941S [DOI] [PubMed] [Google Scholar]
- Lyimo, M. , Gimbi, D. , & Kihinga, T. (2010). Effect of processing methods on nutrient contents of six sweet potato varieties grown in lake zone of Tanzania. Tanzania Journal of Agricultural Sciences, 10(1), 55–61. [Google Scholar]
- Mares‐Perlman, J. A. , Millen, A. E. , Ficek, T. L. , & Hankinson, S. E. (2002). The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. Journal of Nutrition, 132(3), 518S–524S. 10.1093/jn/132.3.518S [DOI] [PubMed] [Google Scholar]
- Marx, M. , Stuparic, M. , Schieber, A. , & Carle, R. (2003). Effects of thermal processing on trans‐cis‐isomerization of β‐carotene in carrot juices and carotene‐containing preparations. Food Chemistry, 83(4), 609–617. 10.1016/S0308-8146(03)00255-3 [DOI] [Google Scholar]
- Meng, S. , Cao, J. , Feng, Q. , Peng, J. , & Hu, Y. (2013). Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evidence‐Based Complementary and Alternative Medicine, 2013, 1920–11. 10.1155/2013/801457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz, C. , Brat, P. , Caris‐Veyrat, C. , & Gunata, Z. (2010). Characterization and thermal lability of carotenoids and vitamin C of tamarillo fruit (Solanum betaceum Cav.). Food Chemistry, 119(2), 653–659. 10.1016/j.foodchem.2009.07.009 [DOI] [Google Scholar]
- Meyers, K. J. , Mares, J. A. , Igo, R. P. , Truitt, B. , Liu, Z. , Millen, A. E. , … Iyengar, S. K. (2013). Genetic evidence for role of carotenoids in age‐related macular degeneration in the carotenoids in age‐related eye disease study (CAREDS). Investigative Ophthalmology and Visual Science, 55(1), 587–599. 10.1167/iovs.13-13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel, M. G. (2011). Anthocyanins: Antioxidant and/or anti‐inflammatory activities. Journal of Applied Pharmaceutical Science, 01(06), 7–15. [Google Scholar]
- Mirella, N. , Massimo, D. A. , Gianni, T. , Vincenzo, G. , Maurizio, D. F. , & Cristina, S. (1995). Inhibition of human low‐density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radical Biology & Medicine, 13(8), 1133–1136. 10.1016/j.freeradbiomed.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Mohammad, K. A. , Ziaul, H. R. , & Sheikh, N. I. (2016). Comparison of the proximate composition, total carotenoids and total polyphenol content of nine orange‐fleshed sweet potato varieties grown in Bangladesh. Foods, 5, 2–10. 10.3390/foods5030064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanraj, R. , & Sivasankar, S. (2014). Sweet potato (Ipomoea batatas [L.]) – A valuable medicinal food: A review. Journal of Medicinal Food, 17(7), 733–741. 10.1089/jmf.2013.2818 [DOI] [PubMed] [Google Scholar]
- Mongi, R. J. , Simbano, M. , Ruhembe, C. , & Majaliwa, N. (2015). Development and assessment of frying characteristics, chemical composition, descriptive sensory properties and preference mapping of wheat‐orange fleshed sweet potato composite Swahili buns. An International Journal of Basic and Applied Research, 14(2), 129–142. [Google Scholar]
- Moyer, R. A. , Hummer, K. E. , Finn, C. E. , Frei, B. , Ronald, E. , & Wrolstad, R. E. (2002). Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. Journal of Agriculture and Food Chemistry, 50(3), 519–525. 10.1021/jf011062r [DOI] [PubMed] [Google Scholar]
- Muchoki, C. N. , & Imungi, J. K. (2017). Properties of baby food developed from orange‐fleshed sweetpotato and mangoes. Food and Nutrition Sciences, 08(11), 979–988. 10.4236/fns.2017.811070 [DOI] [Google Scholar]
- Muhammad, L. , Aminah, Y. , & Abbas, K. (2012). Development of orange fleshed sweet potato (OFSP) based juice drink to help reduce on vitamin A deficiency. International Journal of Science and Research, 3(7), 283–289. [Google Scholar]
- Murakoshi, M. , Iwasaki, R. , Satomi, Y. , Takayasu, J. , Hasegawa, T. , Tokuda, H. , & Iwashima, A. (1992). Potent preventive action of α‐carotene against carcinogenesis: Spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by α‐carotene than by β‐carotene. Cancer Research, 52(23), 6583–6587. [PubMed] [Google Scholar]
- Naczk, M. , & Shahidi, F. (2006). Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. Journal of Pharmaceutical and Biomedical Analysis, 41(5), 1523–1542. 10.1016/j.jpba.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Naidu, K. A. (2003). Vitamin C in human health and disease is still a mystery? An overview. BMC Nutrition, 1, 1920–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namitha, K. K. , & Negi, P. S. (2010). Chemistry and biotechnology of carotenoids. Critical Reviews in Food Science and Nutrition, 50(8), 1040–8398. 10.1080/10408398.2010.499811 [DOI] [PubMed] [Google Scholar]
- Nascimento, K. D. O. D. , Dhiego, S. L. , Cristina, Y. T. , José, L. B. J. , & Maria, I. M. J. B. (2015). Physicochemical characteristics of tubers from organic sweet potato roots. Universidade Federal Rural do Semi‐Árido, 28(2), 225–234. [Google Scholar]
- Ndolo, P. J. , Mcharo, T. , Carey, E. E. , Gichuki, S. T. , Ndinya, C. , & Maling'a, J. (2001). Participatory on‐farm selection of sweetpotato varieties in western Kenya. African Crop Science Journal, 9(1), 41–48. 10.4314/acsj.v9i1.27623 [DOI] [Google Scholar]
- Ndolo, P. J. , Nungo, R. A. , Kapinga, R. E. , & Agili, S. (2007). Development and promotion of orange‐fleshed sweetpotato varieties in Western KenyaIn Proceedings of the 13th ISTRC Symposium (pp. 689–695). [Google Scholar]
- Netscher, T. (2007). Synthesis of vitamin E. Vitamins and Hormones, 76(07), 155–202. 10.1016/S0083-6729(07)76007-7 [DOI] [PubMed] [Google Scholar]
- Neumann, C. , Harris, D. M. , & Rogers, L. M. (2002). Contribution of animal source foods in improving diet quality and function in children in the developing world. Nutrition Research, 22(1–2), 193–220. 10.1016/S0271-5317(01)00374-8 [DOI] [Google Scholar]
- Nicanuru, C. , Laswai, H. S. , & Sila, D. N. (2015). Effect of sun‐ drying on nutrient content of orange fleshed sweet potato tubers in Tanzania. Sky Journal of Food Science, 4(7), 91–101. [Google Scholar]
- Niedzwiedzki, D. M. , Enriquez, M. M. , Lafountain, A. M. , & Frank, H. A. (2010). Ultrafast time‐resolved absorption spectroscopy of geometric isomers of xanthophylls. Chemical Physics, 373(1–2), 80–89. 10.1016/j.chemphys.2010.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggeweg, R. , Michael, A. J. , & Martin, C. (2004). Engineering plants with increased levels of the antioxidant chlorogenic acid. Nature Biotechnology, 22, 746 10.1038/nbt966 [DOI] [PubMed] [Google Scholar]
- Nwachukwu, I. D. , Udenigwe, C. C. , & Aluko, R. E. (2016). Lutein and zeaxanthin: Production technology, bioavailability, mechanisms of action, visual function, and health claim status. Trends in Food Science and Technology, 49, 74–84. 10.1016/j.tifs.2015.12.005 [DOI] [Google Scholar]
- Nzamwita, M. , Duodu, K. G. , & Minnaar, A. (2017). Stability of β‐carotene during baking of orange‐fleshed sweet potato‐wheat composite bread and estimated contribution to vitamin A requirements. Food Chemistry, 228, 85–90. 10.1016/j.foodchem.2017.01.133 [DOI] [PubMed] [Google Scholar]
- OCED‐FAO (2016). OECD‐FAO agricultural outlook 2016–2025. https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2016-2025/roots-and-tubers-projections-production-and-food-consumption_agr_outlook-2016-table166-en [Google Scholar]
- Oki, T. , Nagai, S. , Yoshinaga, M. , Nishiba, Y. , & Suda, I. (2006). Contribution of β‐carotene to radical scavenging capacity varies among orange‐fleshed sweet potato cultivars. Food Science and Technology Research, 12(2), 156–160. 10.3136/fstr.12.156 [DOI] [Google Scholar]
- Olubunmi, A. A. , Abraham, I. O. , Mojirade, L. A. , Afolake, B. , & Kehinde, O. E. (2017). Development, evaluation and sensory quality of orange fleshed sweet potato (Ipomoea batatas Lam) extruded pasta products. Croatian Journal of Food Technology, Biotechnology and Nutrition, 12(1–2), 83–89. [Google Scholar]
- Padayatty, S. J. , Katz, A. , Wang, Y. , Eck, P. , Kwon, O. , Lee, J. -H. , Chen, S. … Corpe, C. ( 2003). Vitamin C as an antioxidant: Evaluation of its role in disease prevention. Journal of the American College of Nutrition, 22( 1), 18 – 35. 10.1080/07315724.2003.10719272 [DOI] [PubMed] [Google Scholar]
- Padayatty, S. J. , & Levine, M. (2016). Vitamin C: The known and the unknown and Goldilocks. Oral Diseases, 22(6), 463–493. 10.1111/odi.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padda, M. S. , & Picha, D. H. (2008). Quantification of phenolic acids and antioxidant activity in sweetpotato genotypes. Scientia Horticulturae, 119(1), 17–20. 10.1016/j.scienta.2008.07.008 [DOI] [Google Scholar]
- Pantelidis, G. E. , Vasilakakis, M. , Manganaris, G. A. , & Diamantidis, G. (2007). Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chemistry, 102(3), 777–783. 10.1016/j.foodchem.2006.06.021 [DOI] [Google Scholar]
- Parker, R. S. (1996). Absorption, metabolism, and transport of carotenoids. FASEB Journal, 10(5), 542–551. 10.1096/fasebj.10.5.8621054 [DOI] [PubMed] [Google Scholar]
- Paula, A. , Pereira, A. , Teresa, M. , Silva, P. , Schmiele, M. , Carlos, L. , & Nabeshima, E. H. (2018). Orange‐fleshed sweet potato flour as a precursor of aroma and color of sourdough panettones. LWT‐Food Science and Technology, 101, 145–151. [Google Scholar]
- Perry, A. , Rasmussen, H. , & Johnson, E. J. (2009). Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. Journal of Food Composition and Analysis, 22(1), 9–15. 10.1016/j.jfca.2008.07.006 [DOI] [Google Scholar]
- Phillips, M. A. , León, P. , Boronat, A. , & Rodríguez‐Concepción, M. (2008). The plastidial MEP pathway: Unified nomenclature and resources. Trends in Plant Science, 13(12), 619–623. 10.1016/j.tplants.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Pillay, K. , Khanyile, N. , & Siwela, M. (2018). Acceptance of an orange‐fleshed sweet potato complementary food by infant caregivers in KwaZulu‐Natal Province – A preliminary study. The south African journal of child health, 12(3), 100–104. 10.7196/SAJCH.2018.v12i3.1469 [DOI] [Google Scholar]
- Powell, S. R. (2000). Zinc and health: Current status and future directions. Journal of Nutrition, 130, 1447–1454. 10.1093/jn/130.5.1437S [DOI] [Google Scholar]
- Prentice, A. M. , & Jebb, S. A. (2003). Fast foods, energy density and obesity: A possible mechanistic link. Obesity Reviews, 4(4), 187–194. 10.1046/j.1467-789X.2003.00117.x [DOI] [PubMed] [Google Scholar]
- Prior, R. L. , Cao, G. , Martin, A. , Sofic, E. , McEwen, J. , O'Brien, C. , & Mainland, C. M. (1998). Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. Journal of Agricultural and Food Chemistry, 46(7), 2686–2693. 10.1021/jf980145d [DOI] [Google Scholar]
- Pryor, W. A. (2000). Vitamin E and coronary heart disease: Basic science to clinical intervention trials. Free Radical Biology and Medicine, 28(1), 141–164. [DOI] [PubMed] [Google Scholar]
- Qiu, D. , Chen, Z. R. , & Li, H. R. (2009). Effect of heating on solid β‐carotene. Food Chemistry, 112(2), 344–349. 10.1016/j.foodchem.2008.05.071 [DOI] [Google Scholar]
- Rangel, C. , Moreira da Silva, E. , Salvador, L. , Figueredo, R. , Watanabe, R. , Carvalho da Silva, J. , & Nutti, M. (2011). Sensory evaluation of cakes prepared with orange‐fleshed sweet potato flour (Ipomoea batatas L.). Perspectivas en Nutrición Humana, 13(2), 203–211. [Google Scholar]
- Rao, A. V. , & Rao, L. G. (2007). Carotenoids and human health. Pharmacological Research, 55, 207–216. 10.1007/978-1-62703-203-2 [DOI] [PubMed] [Google Scholar]
- Reboul, E. , Richelle, M. , Perrot, E. , Desmoulins‐Malezet, C. , Pirisi, V. , & Borel, P. (2006). Bioaccessibility of carotenoids and vitamin E from their main dietary sources. Journal of Agricultural and Food Chemistry, 54(23), 8749–8755. 10.1021/jf061818s [DOI] [PubMed] [Google Scholar]
- Reuter, S. , Gupta, S. C. , Chaturvedi, M. M. , & Aggarwal, B. B. (2010). Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biology and Medicine, 49(11), 1603–1616. 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, R. M. , Padfield, P. L. , & Seckl, J. R. (2006). Disorders of sodium balance. British Medical Journal, 332(7543), 702–705. 10.1136/bmj.332.7543.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, N. R. , Barbosa, J. L. , & Barbosa, M. I. M. J. (2016). Determination of physico‐chemical composition, nutritional facts and technological quality of organic orange and purple‐fleshed sweet potatoes and its flours. International Food Research Journal, 23(5), 1920–11. [Google Scholar]
- Rodríguez‐Saona, L. E. , Glusti, M. M. , & Wrolstad, R. E. (1999). Color and pigment stability of red radish and red‐fleshed potato anthocyanins in juice model systems. Journal of Food Science, 64(3), 451–456. 10.1111/j.1365-2621.1999.tb15061.x [DOI] [Google Scholar]
- Rukundo, P. , Shimelis, H. , Laing, M. , & Gahakwa, D. (2013). Storage root formation, dry matter synthesis, accumulation and genetics in sweetpotato. Australian Journal of Crop Science, 7(13), 2054. [Google Scholar]
- Rumbaoa, R. G. O. , Cornago, D. F. , & Geronimo, I. M. (2009). Phenolic content and antioxidant capacity of Philippine potato (Solanum tuberosum) tubers. Journal of Food Composition and Analysis, 22(6), 546–550. 10.1016/j.jfca.2008.11.004 [DOI] [Google Scholar]
- Saini, R. K. , Nile, S. H. , & Park, S. W. (2015). Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Research International, 76, 735–750. 10.1016/j.foodres.2015.07.047 [DOI] [PubMed] [Google Scholar]
- Sajilata, M. G. , Singhal, R. S. , & Kamat, M. Y. (2008). The carotenoid pigment zeaxanthin – A review. Comprehensive Reviews in Food Science and Food Safety, 7(1), 29–49. 10.1111/j.1541-4337.2007.00028.x [DOI] [Google Scholar]
- Sales, C. H. , & Pedrosa, L. F. C. (2006). Magnesium and diabetes mellitus: Their relation. Clinical Nutrition, 25(4), 554–562. 10.1016/j.clnu.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Sánchez‐Zapata, E. , Viuda‐Martos, M. , Fernández‐LÓpez, J. , & Pérez‐Alvarez, J. A. (2015). Resistant starch as functional ingredient. Polysaccharides: Bioactivity and Biotechnology, 43, 1911–1931. 10.1007/978-3-319-16298-0_34 [DOI] [Google Scholar]
- Sancho, R. A. S. , & Pastore, G. M. (2012). Evaluation of the effects of anthocyanins in type 2 diabetes. Food Research International, 46(1), 378–386. 10.1016/j.foodres.2011.11.021 [DOI] [Google Scholar]
- Sanoussi, A. F. , Adjatin, A. , Dansi, A. , Adebowale, A. , Sanni, L. O. , & Sanni, A. (2016). Mineral composition of ten elites sweet potato (Ipomoea batatas [L.] Lam.) Landraces of Benin. International Journal of Current Microbiology and Applied Sciences, 5(1), 103–115. [Google Scholar]
- Saris, N. E. L. , Mervaala, E. , Karppanen, H. , Khawaja, J. A. , & Lewenstam, A. (2000). Magnesium: An update on physiological, clinical and analytical aspects. Clinica Chimica Acta, 294(1–2), 1920–1945. 10.1016/S0009-8981(99)00258-2 [DOI] [PubMed] [Google Scholar]
- Satija, A. , & Hu, F. B. (2012). Cardiovascular benefits of dietary fiber. Current Atherosclerosis Reports, 14(6), 505–514. 10.1007/s11883-012-0275-7 [DOI] [PubMed] [Google Scholar]
- Sato, Y. , Itagaki, S. , Kurokawa, T. , Ogura, J. , Kobayashi, M. , Hirano, T. , … Iseki, K. (2011). In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. International Journal of Pharmaceutics, 403(1–2), 136–138. 10.1016/j.ijpharm.2010.09.035 [DOI] [PubMed] [Google Scholar]
- Schieber, A. , & Carle, R. (2005). Occurrence of carotenoid cis‐isomers in food: Technological, analytical, and nutritional implications. Trends in Food Science and Technology, 16(9), 416–422. 10.1016/j.tifs.2005.03.018 [DOI] [Google Scholar]
- Scott, G. J. , Rosegrant, M. W. , & Ringler, C. (2000). Global projections for root and tuber crops to the year 2020. Food Policy, 25(5), 561–597. 10.1016/S0306-9192(99)00087-1 [DOI] [Google Scholar]
- Sharoni, Y. , Linnewiel‐Hermoni, K. , Khanin, M. , Salman, H. , Veprik, A. , Danilenko, M. , & Levy, J. (2012). Carotenoids and apocarotenoids in cellular signaling related to cancer: A review. Molecular Nutrition and Food Research, 56(2), 259–269. 10.1002/mnfr.201100311 [DOI] [PubMed] [Google Scholar]
- Shegokar, R. , & Mitri, K. (2012). Carotenoid lutein: A promising candidate for pharmaceutical and nutraceutical applications. Journal of Dietary Supplements, 9(3), 183–210. 10.3109/19390211.2012.708716 [DOI] [PubMed] [Google Scholar]
- Shi, J. , Yi, C. , Ye, X. , Xue, S. , Jiang, Y. , Ma, Y. , & Liu, D. (2010). Effects of supercritical CO2 fluid parameters on chemical composition and yield of carotenoids extracted from pumpkin. LWT – Food Science and Technology, 43(1), 39–44. 10.1016/j.lwt.2009.07.003 [DOI] [Google Scholar]
- Shih, M. C. , Kuo, C. C. , & Chiang, W. (2009). Effects of drying and extrusion on colour, chemical composition, antioxidant activities and mitogenic response of spleen lymphocytes of sweet potatoes. Food Chemistry, 117(1), 114–121. 10.1016/j.foodchem.2009.03.084 [DOI] [Google Scholar]
- Simon, J. A. (1992). Vitamin C and cardiovascular disease: A review. Journal of the American College of Nutrition, 11(2), 107–125. [PubMed] [Google Scholar]
- Slavin, J. (2013). Fiber and prebiotics: Mechanisms and health benefits. Nutrients, 5(4), 1417–1435. 10.3390/nu5041417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetan, K. O. , Olaiya, C. O. , & Oyewole, O. E. (2010). The importance of mineral elements for humans, domestic animals and plants. African Journal of Food Science, 4(5), 200–222. 10.1186/s12302-017-0116-y [DOI] [Google Scholar]
- Sommer, A. (2008). Vitamin A deficiency and clinical disease: An historical overview. Journal of Nutrition, 138(10), 1835–1839. 10.1093/jn/138.10.1835 [DOI] [PubMed] [Google Scholar]
- Stahl, W. , & Sies, H. (2007). Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Molecular Biotechnology, 37(1), 26–30. 10.1007/s12033-007-0051-z [DOI] [PubMed] [Google Scholar]
- Stathers, T. , Bechoff, A. , Sindi, K. , Low, J. , & Ndyetabula, D. (2013). Everything you ever wanted to know about sweetpotato: Reaching agents of change ToT manual. 5: Harvesting and postharvest management, processing and utilisation, marketing and entrepreneurship (volume 5). Nairobi, Kenya: International Potato Center. [Google Scholar]
- Steed, L. E. , & Truong, V. D. (2008). Anthocyanin content, antioxidant activity, and selected physical properties of flowable purple‐fleshed sweetpotato purees. Journal of Food Science, 73(5), 215–221. 10.1111/j.1750-3841.2008.00774.x [DOI] [PubMed] [Google Scholar]
- Stephensen, C. B. (2001). Vitamin A, infection, and immune function. Group, 1–7. https://academic.oup.com/jn/article/138/10/1835/4669996 [DOI] [PubMed] [Google Scholar]
- Stinco, C. M. , Benítez‐González, A. M. , Hernanz, D. , Vicario, I. M. , & Meléndez‐Martínez, A. J. (2014). Development and validation of a rapid resolution liquid chromatography method for the screening of dietary plant isoprenoids: Carotenoids, tocopherols and chlorophylls. Journal of Chromatography A, 1370, 162–170. 10.1016/j.chroma.2014.10.044 [DOI] [PubMed] [Google Scholar]
- Suhaj, M. (2006). Spice antioxidants isolation and their antiradical activity: A review. Journal of Food Composition and Analysis, 19(6–7), 531–537. 10.1016/j.jfca.2004.11.005 [DOI] [Google Scholar]
- Sweet Potato Knowledge Portal (2018). Sweet potato processing and marketing. https://www.sweetpotatoknowledge.org/topics/processing-and-marketing/ [Google Scholar]
- Tairo, F. , Mukasa, S. B. , Jones, R. A. C. , Kullaya, A. , Rubaihayo, P. R. , & Valkonen, J. P. T. (2005). Unravelling the genetic diversity of the three main viruses involved in sweet potato virus disease (SPVD), and its practical implications. Molecular Plant Pathology, 6(2), 199–211. 10.1111/J.1364-3703.2005.00267.X [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Cai, W. , & Xu, B. (2015). Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin, China. Food Science and Human Wellness, 4(3), 123–132. 10.1016/j.fshw.2015.07.003 [DOI] [Google Scholar]
- Tang, Y. C. , & Chen, B. H. (2000). Pigment change of freeze‐dried carotenoid powder during storage. Food Chemistry, 69(1), 11–17. 10.1016/S0308-8146(99)00216-2 [DOI] [Google Scholar]
- Teow, C. C. , Truong, V. D. , McFeeters, R. F. , Thompson, R. L. , Pecota, K. V. , & Yencho, G. C. (2007). Antioxidant activities, phenolic and β‐carotene contents of sweet potato genotypes with varying flesh colours. Food Chemistry, 103(3), 829–838. 10.1016/j.foodchem.2006.09.033 [DOI] [Google Scholar]
- Terry, P. , Lagergren, J. , Hansen, H. , Wolk, A. , & Nyrén, O. (2001). Fruit and vegetable consumption in the prevention of oesophageal and cardia cancers. European Journal of Cancer Prevention, 10(4), 365–369. 10.1097/00008469-200108000-00010 [DOI] [PubMed] [Google Scholar]
- Thurnham, D. I. (2007). Bioequivalence of β‐carotene and retinol. Journal of the Science of Food and Agriculture, 87(2), 13–39. [Google Scholar]
- Thurnham, D. I. , McCabe, G. P. , Northrop‐Clewes, C. A. , & Nestel, P. (2003). Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin a deficiency: Meta‐analysis. Lancet, 362(9401), 2052–2058. 10.1016/S0140-6736(03)15099-4 [DOI] [PubMed] [Google Scholar]
- Tomlins, K. , Owori, C. , Bechoff, A. , Menya, G. , & Westby, A. (2012). Relationship among the carotenoid content, dry matter content and sensory attributes of sweet potato. Food Chemistry, 131(1), 14–21. 10.1016/j.foodchem.2011.07.072 [DOI] [Google Scholar]
- Traber, M. G. , & Atkinson, J. (2007). Vitamin E, antioxidant and nothing more. Free Radical Biology and Medicine, 43(1), 4–15. 10.1016/j.freeradbiomed.2007.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trancoso‐Reyes, N. , Ochoa‐Martínez, L. A. , Bello‐Pérez, L. A. , Morales‐Castro, J. , Estévez‐Santiago, R. , & Olmedilla‐Alonso, B. (2016). Effect of pre‐treatment on physicochemical and structural properties, and the bioaccessibility of β‐carotene in sweet potato flour. Food Chemistry, 200, 199–205. 10.1016/j.foodchem.2016.01.047 [DOI] [PubMed] [Google Scholar]
- Tsai, P. J. , McIntosh, J. , Pearce, P. , Camden, B. , & Jordan, B. R. (2002). Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Research International, 35(4), 351–356. 10.1016/S0963-9969(01)00129-6 [DOI] [Google Scholar]
- Tumuhimbise, G. A. , Namutebi, A. , & Muyonga, J. H. (2009). Microstructure and in vitro beta carotene bioaccessibility of heat processed orange fleshed sweet potato. Plant Foods for Human Nutrition, 64(4), 312–318. 10.1007/s11130-009-0142-z [DOI] [PubMed] [Google Scholar]
- Ukom, A. , Ojimelukwe, P. C. , & Okpara, D. (2009). Nutritional composition of selected sweet potato. Pakistan Journal of Nutrition, 8(11), 1791–1795. [Google Scholar]
- UNICEF (2018). Vitamin A deficiency in children – UNICEF data. Retrieved from https://data.unicef.org/topic/nutrition/vitamin-a-deficiency/ [Google Scholar]
- USDA (2018). USDA food compositional database. Retrieved from https://ndb.nal.usda.gov/ndb/foods/show/11601?fgcd=&manu=&format=&count=&max=25&offset=&sort=default&order=asc&qlookup=yam&ds=&qt=&qp=&qa=&qn=&q=&ing= [Google Scholar]
- Van Jaarsveld, P. J. , Faber, M. , Tanumihardjo, S. A. , Nestel, P. , Lombard, C. J. , & Benadé, A. J. S. (2005). Beta‐carotene‐rich orange‐fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified‐relative‐dose‐response test. American Journal of Clinical Nutrition, 81(5), 1080–1087. 10.1109/DSN.2004.1311885 [DOI] [PubMed] [Google Scholar]
- van Jaarsveld, P. J. , Marais, D. W. , Harmse, E. , Nestel, P. , & Rodriguez‐Amaya, D. B. (2006). Retention of β‐carotene in boiled, mashed orange‐fleshed sweet potato. Journal of Food Composition and Analysis, 19(4), 321–329. 10.1016/j.jfca.2004.10.007 [DOI] [Google Scholar]
- Varani, J. , Warner, R. L. , Gharaee‐Kermani, M. , Phan, S. H. , Kang, S. , Chung, J. H. , & Voorhees, J. J. (2000). Vitamin A antagonizes decreased cell growth and elevated collagen‐ degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. Journal of Investigative Dermatology, 114(3), 480–486. 10.1046/j.1523-1747.2000.00902.x [DOI] [PubMed] [Google Scholar]
- Vásquez‐Caicedo, A. L. , Schilling, S. , Carle, R. , & Neidhart, S. (2007). Effects of thermal processing and fruit matrix on β‐carotene stability and enzyme inactivation during transformation of mangoes into purée and nectar. Food Chemistry, 102(4), 1172–1186. 10.1016/j.foodchem.2006.07.005 [DOI] [Google Scholar]
- Velioglu, Y. S. , Mazza, G. , Gao, L. , & Oomah, B. D. (1998). Antioxidant activity and total phenolics in selected fruits, and cereals. Journal of Agriculture and Food Chemistry, 46(10) 4113–4117. https://pubs.acs.org/doi/10.1021/jf9801973 [Google Scholar]
- Vimala, B. , Nambisan, B. , & Hariprakash, B. (2011). Retention of carotenoids in orange‐fleshed sweet potato during processing. Journal of Food Science and Technology, 48(4), 520–524. 10.1007/s13197-011-0323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitamin C and Eye Health (2007). Handbook of nutrition and ophthalmology (pp. 371–390). Totowa, NJ: Humana Press; 10.1007/978-1-59259-979-0_9 [DOI] [Google Scholar]
- Waluyo, B. , Roosda, A. A. , Istifadah, N. , Ruswandi, D. , & Karuniawan, A. (2015). Identification of fifty sweetpotato (Ipomoea batatas (L.) Lam.) promising clones for bioethanol raw materials. Energy Procedia, 65, 22–28. 10.1016/j.egypro.2015.01.024 [DOI] [Google Scholar]
- Wanasundera, J. P. D. , & Ravindran, G. (1994). Nutritional assessment of yam (Dioscorea alata) tubers. Plant Foods for Human Nutrition, 46(1), 33–39. 10.1007/BF01088459 [DOI] [PubMed] [Google Scholar]
- Wang, M. C. , Villa, M. L. , Marcus, R. , & Kelsey, J. L. (1997). Associations of vitamin C, calcium and protein with bone mass in postmenopausal Mexican American women. Osteoporosis International, 7(6), 533–538. 10.1007/BF02652558 [DOI] [PubMed] [Google Scholar]
- Waramboi, J. G. , Gidley, M. J. , & Sopade, P. A. (2013). Carotenoid contents of extruded and non‐extruded sweetpotato flours from Papua New Guinea and Australia. Food Chemistry, 141(3), 1740–1746. 10.1016/j.foodchem.2013.04.070 [DOI] [PubMed] [Google Scholar]
- Weber, D. , & Grune, T. (2012). The contribution of β‐carotene to vitamin A supply of humans. Molecular Nutrition and Food Research, 56(2), 251–258. 10.1002/mnfr.201100230 [DOI] [PubMed] [Google Scholar]
- WHO (2018). Micronutrient deficiencies. Retrieved from https://www.who.int/nutrition/topics/vad/en/ [Google Scholar]
- Wosje, K. S. , & Specker, B. L. (2000). Role of calcium in bone health during childhood. Nutrition Reviews, 58(9), 253–268. 10.1111/j.1753-4887.2000.tb01879.x [DOI] [PubMed] [Google Scholar]
- Wrolstad, R. E. (2000). Anthocyanin pigments – Bioactivity and coloring properties. Journal of Food Science, 14(5), C419–C425. 10.1111/j.1365-2621.2004.tb10709.x [DOI] [Google Scholar]
- Wu, X. , Beecher, G. R. , Holden, J. M. , Haytowitz, D. B. , Gebhardt, S. E. , & Prior, R. L. (2006). Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. Journal of Agricultural and Food Chemistry, 54(11), 4069–4075. 10.1021/jf060300l [DOI] [PubMed] [Google Scholar]
- Wu, X. , Sun, C. , Yang, L. , Zeng, G. , Liu, Z. , & Li, Y. (2008). β‐carotene content in sweet potato varieties from China and the effect of preparation on β‐carotene retention in the Yanshu No. 5. Innovative Food Science and Emerging Technologies, 9, 581–586. 10.1016/j.ifset.2008.06.002 [DOI] [Google Scholar]
- Yamada, J. , & Tomita, Y. (1996). Antimutagenic activity of caffeic acid and related compounds. Bioscience, Biotechnology, and Biochemistry, 60(2), 328–329. 10.1271/bbb.60.328 [DOI] [PubMed] [Google Scholar]
- Yanishlieva, N. V. , & Marinova, E. M. (2001). Stabilisation of edible oils with natural antioxidants. European Journal of Lipid Science and Technology, 103(11), 752–767. [DOI] [Google Scholar]
- Yuan, J. M. , Ross, R. K. , Chu, X. D. , Gao, Y. T. , & Yu, M. C. (2001). Prediagnostic levels of serum β‐cryptoxanthin and retinol predict smoking‐related lung cancer risk in Shanghai, China. Cancer Epidemiology Biomarkers and Prevention, 10(7), 767–773. [PubMed] [Google Scholar]
- Zamora‐Ros, R. , Rothwell, J. A. , Scalbert, A. , Knaze, V. , Romieu, I. , Slimani, N. , … González, C. A. (2013). Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. British Journal of Nutrition, 110(8), 1500–1511. 10.1017/S0007114513000688 [DOI] [PubMed] [Google Scholar]
- Zemel, M. B. (2009). Proposed role of calcium and dairy food components in weight management and metabolic health. Physician and Sports Medicine, 37(2), 29–39. 10.3810/psm.2009.06.1707 [DOI] [PubMed] [Google Scholar]
- Zepka, L. Q. , & Mercadante, A. Z. (2009). Degradation compounds of carotenoids formed during heating of a simulated cashew apple juice. Food Chemistry, 117(1), 28–34. 10.1016/j.foodchem.2009.03.071 [DOI] [Google Scholar]
- Ziegler, J. U. , Wahl, S. , Würschum, T. , Longin, C. F. H. , Carle, R. , & Schweiggert, R. M. (2015). Lutein and lutein esters in whole grain flours made from 75 genotypes of 5 Triticum species grown at multiple sites. Journal of Agricultural and Food Chemistry, 63(20), 5061–5071. 10.1021/acs.jafc.5b01477 [DOI] [PubMed] [Google Scholar]
- Ziegler, R. G. (1989). A review of epidemiologic evidence that carotenoids reduce the risk of cancer. Journal of Nutrition, 119(1), 116–122. 10.1093/jn/119.1.116 [DOI] [PubMed] [Google Scholar]
- Ziska, L. H. , Runion, G. B. , Tomecek, M. , Prior, S. A. , Torbet, H. A. , & Sicher, R. (2009). An evaluation of cassava, sweet potato and field corn as potential carbohydrate sources for bioethanol production in Alabama and Maryland. Biomass and Bioenergy, 33(11), 1503–1508. 10.1016/j.biombioe.2009.07.014 [DOI] [Google Scholar]


