Abstract
Many Lactobacillus plantarum strains can secrete some antimicrobial substances and be added to food as antimicrobial agents and preservatives. In this study, three L. plantarum strains (P1, S11, and M7) with strong antimicrobial activity against three pathogenic bacteria were isolated from Xinjiang traditional dairy products. Five common organic acids produced by fermentation of strains play a key role in inhibiting three pathogenic bacteria. At the same pH, the antimicrobial activity of the fermentation broth against Escherichia coli and Salmonella is stronger than that of the organic acid alone. Thus, three kinds of antimicrobial agents (P1‐1, M7‐1, and S11‐1) mixed with five common organic acids were produced. Moreover, the antimicrobial activity against Salmonella ASI.1174 of the antimicrobial agents was about 30% higher than that of the fermentation broth. In addition, organic acid antimicrobial agents combined in different proportions can inhibit different pathogenic bacteria. According to this result, it is a potential approach to develop novel antimicrobial agents used in food preservation by mixing different organic acids.
Keywords: food additive, food preservation, Lactobacillus plantarum, organic acid, probiotic
1. INTRODUCTION
Lactobacilli are widespread microorganisms which have numerous applications in both industry and human health, including food preservation and probiotics (Altay, Karbancıoglu‐Güler, Daskaya‐Dikmen, & Heperkan, 2013; Cebeci & Gürakan, 2003). Among many Lactobacillus strains, Lactobacillus plantarum is a functional and important probiotic which can be found in many fermented foods, probiotic foods, and natural foods (Guidone et al., 2014). In recent years, some L. plantarum strains with unique functions such as L. plantarum CCFM8610, L. plantarum C88, L. plantarum strain 21B were discovered (Huang et al., 2017; Lavermicocca et al., 2000; Zhai et al., 2014). In addition, many Lactobacillus strains with strong antimicrobial activity had also been screened. L. plantarum D1 and L. plantarum D2 show significant inhibitory activity against Salmonella (Teneva, Denkova, Goranov, Denkova, & Kostov, 2017). Lactobacillus acidophilus A2, L. acidophilus Ac, etc., can inhibit Candida albicans NBIMCC 74 by cocultivation (Denkova, Yanakieva, Denkova, Nikolova, & Radeva, 2013). Many traditional fermented foods are a rich bacterial library for screening L. plantarum with antimicrobial activities. Ethnic minorities such as the Kazak and Mongolian people in the Xinjiang region of China have kept the habit of producing and eating fermented dairy products since ancient time. After thousands of years of domestication, these traditional dairy products retain many of the lactic acid bacteria that assist in the unique flavor of dairy products.
The conversion of sugar to lactic acid is still the main function of L. plantarum. In addition, production of exopolysaccharides, antimicrobial peptide, and specific beneficial compounds that are beneficial to the human body such as vitamins are other important properties (de Vries, Vaughan, Kleerebezem, & de Vos, 2006; Li, Gu, Yang, Yu, & Wang, 2017). Furthermore, L. plantarum strains can produce sundry antimicrobial compounds such as organic acids (primarily lactic and acetic acid), hydrogen peroxide, and antimicrobial peptides (Denkova, 2017; Lavermicocca et al., 2000; Nealmckinney et al., 2012). What's more, some studies have found that lactic acid bacteria biofilms show the capability to influence the survival and the multiplication of the pathogen (Guerrieri et al., 2009). Concurrently, the increasing attention of consumers for natural food urged scientific research to investigate the application of natural compounds in food to replace synthetic chemicals and additives as preservatives (Castro, Palavecino, Herman, Garro, & Campos, 2011). According to production of antimicrobial substances and harmless characteristics, L. plantarum could be the suitable candidates for natural antimicrobial agent (da Silva Sabo, Vitolo, González, & Oliveira, 2014). In recent years, many L. plantarum strains with the ability to inhibit pathogenic bacteria have been discovered and used for food preservation (Cortés‐Zavaleta, López‐Malo, Hernández‐Mendoza, & García, 2014; Kecerová, Pristas, & Javorský, 2004). A novel antimicrobial against Bacillus spp. produced by L. plantarum JLA‑9 was segregated and studied in its application fields (Zhao et al., 2016). Phenyllactic acid produced by lactic acid bacteria is a potential natural food preserver and preservative (Valerio, Lavermicocca, Pascale, & Visconti, 2004). In fact, antimicrobial activity of L. plantarum is mainly associated with the organic acids production and lowering pH of environment as indicated by references (Ołdak, Zielińska, Rzepkowska, & Kołożyn‐Krajewska, 2017). Some L. plantarum strains producing large amounts of organic acid were added to many fermented foods as a preservative (Li et al., 2016). This preservation method is very suitable for acid‐proof fermented food. Organic acids and their salts are considered weak acids, meaning they do not entirely dissociate in water but do so in a pH‐dependent manner. Organic acids are deemed to affect microbial activity by two primary mechanisms: cytoplasmic acidification with subsequent uncoupling of energy production and accumulation of the dissociated acid anion to toxic levels (Taylor et al., 2012). The antimicrobial mechanism of different organic acids is equally inconsistent. Therefore, different organic acids have different antimicrobial activities. The study showed that different kinds of lactic acid bacteria produce different types of organic acids, and even some of them produced more acetic acid than lactic acid (Rowland et al., 2010; Tejerosariñena, Barlow, Costabile, Gibson, & Rowland, 2012). In addition, Lactobacillus strains usually produce more than one organic acid, and the difference in the proportion of organic acids may be the reason why lactic acid bacteria's antimicrobial activities were inconsistent (Thu, Foo, Loh, & Bejo, 2013). However, the researches on the cooperation of different organic acids with a certain proportion have not been carried out deeply before.
In this study, the first aim was to screen L. plantarum strains with strong antimicrobial activity from traditional dairy products in Xinjiang. Then, the content of organic acids in the fermentation broths of L. plantarum strains was analyzed to study the relationship between organic acid content and its antimicrobial ability. Therefore, the second purpose was to find the suitable organic acid mixture with strong antimicrobial activity.
2. MATERIALS AND METHODS
2.1. Isolation and identification of lactic acid bacteria
Five samples of traditional dairy products, handmade by herdsmen in Xinjiang were collected. Each time 1 g of dairy products was transferred aseptically into 10 ml physiological saline and homogenized thoroughly. Samples were serially diluted in physiological saline. 0.1 ml samples of dilutions ranging between 10−3and 10−7were plated in duplicate on the surface of Ma–Rogosa–Sharpe (MRS Solarbio) agar with supplemented with 0.0025% of bromocresol green (Macklin) (Fhoula et al., 2013). The plates were incubated at 30°C for 48 hr. The different colonies of acid‐producing bacteria were picked on MRS agar by a yellow zone in the media around each colony.
These colonies were initially subjected to Gram stain assay. Gram‐positive strains were transferred to genetic identification using PCR method and 16S rDNA sequencing. The genomic DNA of the LAB strains was extracted using DNA Extraction Kit (TransGen Biotech) following the manufacture's protocol. 16S rDNA gene‐specific fragment primers for identification of strains were prepared in Sangon Biotech. The primers couple was 27F/1492R (5′‐AGA GTT TGA TCC TGG CTC AG‐3′/5′‐GGT TAC CTT GTT ACG ACT T‐3′). PCR conditions consisted of 30 cycles (1 min at 94°C, 45 s at 54°C, 2 min at 72°C) plus one additional cycle at 72°C for 7 min as a final chain elongation (Pennacchia et al., 2004). PCR products were separated from agarose gel (1.5% w/v), and the amplified fragments were visualized by staining with ethidium bromide under UV light. Their 16S rRNA gene sequences were executed with the Majorbio technique, and the BLAST program was used for sequence comparison (Kullen, Sanozkydawes, Crowell, & Klaenhammer, 2010).
2.2. Bacterial strains and growth conditions
Bacterial cultures were stored at −80°C with 20% glycerol (w/v). All L. plantarum strains were growth in MRS broth at 30°C under anaerobic conditions.
Indicator strains used for assessment of antimicrobial activity were S. aureus ATCC12600 (S. aureus), E. coli ATCC35128 (E. coli), and Salmonella ASI.1174 (Salmonella). All pathogens were grown in Luria–Bertani broth (LB, Oxoid) at 37°C, and solid medium was prepared by adding 1.5% agar to the broth media.
2.3. Antimicrobial properties of L. plantarum strains
The antimicrobial activity was evaluated by well diffusion method (Bonev, Hooper, & Parisot, 2008; Yang et al., 2017). The cultures of L. plantarum strains were grown in MRS broth (pH 6.5) for 24 hr to measure the antimicrobial properties of extracellular metabolites of L. plantarum strains. So the cultures were centrifuged (8,000 g for 10 min, 4°C) and filter sterilized through 0.22‐µm hydrophilic Durapore PVDF membrane (Nylon; RephiLe Bioscience). The cell‐free supernatant was recovered and tested for antimicrobial properties. To investigate the chemical nature of the potentially inhibitory substances secreted by each L. plantarum strain, showing the antagonistic effects, the supernatants were submitted to different treatments according to Herreros et al. (2005). First of all, the supernatants were heated at 100°C for 5 min and neutralized with 1 M NaOH to pH 6.5, in order to judge the antibacterial contribution of organic acids. Then, the neutralized supernatants were treated with catalase (1 mg/ml; Sigma‐Aldrich Corporation) at 37°C for 1 hr, and then pH of the supernatants were adjusted back to the original state, in order to rule out inhibiting effects due to hydrogen peroxide. At last, the neutralized supernatants were digested at 37°C for 2 hr with different proteases, that is, proteinase K (1 mg/ml), trypsin (1 mg/ml), and pepsin (1 mg/ml), in order to determine whether strains can produce antimicrobial peptides.
Pathogenic bacteria were grown overnight and diluted into physiological saline. Sterile Petri dishes were poured with LB agar and inoculated with 500 µl cultures of each indicator strain severally (concentration 7 log CFU/ml). After that Oxford plates were lightly placed on the surface of the LB agar plates and all treated supernatants were collected and 200 µl of each was used to fill Oxford plates previously on LB agar plates. The plates were incubated for 2 hr at 4°C in order to permit supernatants diffusion onto LB agar. All plates were incubated at 37°C for 24 hr, and then, the diameters of inhibition zones around the Oxford plates were measured. Antimicrobial activity (x) was calculated as follows: x = D−d, where D is the inhibition zone diameter and d is the Oxford plate diameter. The value of x represents the antimicrobial activity.
2.4. HPLC analysis of organic acids in cell‐free supernatants
Comparing the antimicrobial activities of various L. plantarum strains, three L. plantarum strains were selected to determine the organic acid content of their fermentation broths. According to the pH curves of L. plantarum strains, it can be found that and the pH of L. plantarum strains reaches 3.80 ± 0.05 and tends to stabilize at 24 hr. From this, we can speculate that L. plantarum strains reach the end of the log‐phase and the number of newly formed cells is equal to the number of dying cells after 24 hr. Therefore, the fermentation broths fermented for 24 hr were used as the sample to be tested. Ten milliliter of the fermentation broths obtained was centrifuged (8,000 g for 10 min, 4°C) to obtain cell‐free supernatants. Then, cell‐free supernatants were added to 1 ml of ammonium dihydrogen phosphate buffer with 3% methanol and were homogenized and centrifuged (14,000 g for 15 min, 4°C) to fully precipitate protein. Supernatants were 0.22‐μm‐filtered (Nylon; RephiLe Bioscience) into HPLC amber vials. For controls, unfermented MRS broth was treated under the same conditions.
Seven common organic acids were selected for the determination: oxalic acid, tartaric acid, malic acid, lactic acid, citric acid, acetic acid, and succinic acid. The reagents used were analytically pure, and tartaric acid was L (+), and malic acid was L (−). First, the standard curves of seven organic acids were identified separately. At the same time, the retention time of eight organic acids was determined.
A Shimadzu Nexera LC system with a photodiode array detector (SPD‐M20A) was utilized to detect and quantify the organic acids. The chromatographic separation was performed on a C18 column (250 × 4.6 mm I.D., 5 μm; Teknokroma). The organic acids were eluted using H2O with 11.5% ammonium dihydrogen phosphate (solvent A) and methanol (solvent B). Both solvents were 0.22‐μm‐filtered and degassed before use. Isocratic elution: 97% solvent A and 3% solvent B. The flow rate was set to 0.7 ml/min, the temperature was set to 25°C, and a volume of 10 μl was injected. Organic acids were detected at a UV wavelength of 210 nm.
2.5. Antimicrobial properties of organic acids produced by L. plantarum strains
There were five kinds of organic acids detected in the fermentation broths of three L. plantarum strains, which are lactic acid, acetic acid, tartaric acid, malic acid, and citric acid by HPLC analysis. Using five organic acids, respectively, the pH of MRS broth was adjusted to 3.80 ± 0.05. Then, the pH‐adjusted MRS broths were used to measure the antimicrobial activity using well diffusion method. MRS broths pH adjustment using HCl as control was treated under the same conditions.
According to the content of different organic acids in the fermentation broths, utilizing exogenous organic acids, antimicrobial agents containing organic acids were configured. The antimicrobial agents corresponding to fermentation broths of L. plantarum P1, L. plantarum S11, and L. plantarum M7 were P1‐1, S11‐1, and M7‐1. Then, these antimicrobial agents were centrifuged (8,000 g for 10 min, 4°C) and were 0.22‐μm‐filtered (Nylon; RephiLe Bioscience) to remove bacteria. Then, these antimicrobial agents were used to measure the antimicrobial activity using well diffusion method.
2.6. Statistics analysis
All experiments were performed three times. The results were subjected to Student's t test for the significant difference (p < 0.05) by GraphPad Prism (version 7.01).
3. RESULTS AND DISCUSSION
3.1. Isolation and identification of lactic acid bacteria
The acid‐producing indicator plate was used to screen single colonies with strong acid production ability. Ninety‐six strains were isolated on MRS agar from five kinds of traditional dairy products, depending on the size of the yellow area around a single colony on the MRS plate. Twenty‐six isolates (Table 1) were identified as rod‐shaped Gram‐positive and catalase‐negative bacteria, and all strains selected for the study demonstrated 98%–100% similarity to L. plantarum for 16S rDNA sequence.
Table 1.
Results of strains screening and identification
| Source | Strain | Acid production capacity (size of the yellow area)a | Gram (+/−) | Species |
|---|---|---|---|---|
| Kumis | K1 | + | + | Enterococcus faecium |
| Milk thistle | G4 | + | + | Lactobacillus plantarum |
| Yogurt | S1 | ++ | + | L. plantarum |
| S2 | ++ | + | L. plantarum | |
| S4 | + | + | L. plantarum | |
| S5 | + | + | L. plantarum | |
| S6 | ++ | + | L. plantarum | |
| S7 | + | + | L. plantarum | |
| S8 | ++ | + | L. plantarum | |
| S10 | ++ | + | L. plantarum | |
| S11 | +++ | + | L. plantarum | |
| S12 | + | + | L. plantarum | |
| S13 | ++ | + | L. plantarum | |
| S21 | + | + | L. plantarum | |
| S24 | + | + | L. plantarum | |
| Fermentation of millet | M7 | ++ | + | L. plantarum |
| M13 | + | + | L. plantarum | |
| M14 | + | + | L. plantarum | |
| M15 | ++ | + | L. plantarum | |
| M17 | ++ | + | L. plantarum | |
| M22 | + | + | L. plantarum | |
| Urum | P1 | +++ | + | L. plantarum |
| P2 | + | + | L. plantarum | |
| P5 | + | + | L. plantarum | |
| P8 | ++ | + | L. plantarum | |
| P16 | + | + | L. plantarum | |
| P21 | ++ | + | L. plantarum |
Acid production capacity: + weak ++ medium +++ strong.
3.2. Screening of strains with strong antimicrobial activity
The antimicrobial activity of nine strains (Table 2) was evaluated by well diffusion method. Obvious inhibition zone on the plate was observed, and the inhibition zone diameter ranged from 10 to 20 mm, which represents the strength of antimicrobial activity (Figure S1). Three common pathogens (Table 3) were used to assess the antimicrobial potential (Figure 1). Fermentation broths of different strains had different antimicrobial activities. All tested strains have antimicrobial activity against S. aureus, and antimicrobial activity (x) of L. plantarum P1 was the highest (11.7 mm). On the other hand, fermentation broths of L. plantarum M7 and L. plantarum S11 display high level of antimicrobial activity against E. coli, and x reached 11.4 and 11.2 mm separately. However, all the strains did not show the excellent ability to inhibit the proliferation of Salmonella, and L. plantarum S11 with the highest antimicrobial activity only reached 7.5 mm. After preliminary screening, L. plantarum P1, L. plantarum S11 and L. plantarum M7 were selected for the further analysis.
Table 2.
Lactobacillus used in this work
| Strain | Original source | Identification by 16S rRNA |
|---|---|---|
| P1 | Urum | Lactobacillus plantarum |
| P5 | Urum | L. plantarum |
| P16 | Urum | L. plantarum |
| S1 | Yogurt | L. plantarum |
| S11 | Yogurt | L. plantarum |
| S24 | Yogurt | L. plantarum |
| M7 | Fermentation of millet | L. plantarum |
| M11 | Fermentation of millet | L. plantarum |
| NCFM | DuPont | Lactobacillus acidophilus |
Table 3.
Pathogenic bacteria used in this work
| Strain | Original source | Identification by 16S rRNA |
|---|---|---|
| ATCC12600 | Saved in our lab | Staphylococcus aureus |
| ATCC35128 | Saved in our lab | Escherichia coli |
| ASI.1174 | Saved in our lab | Salmonella typhimurium |
Figure 1.
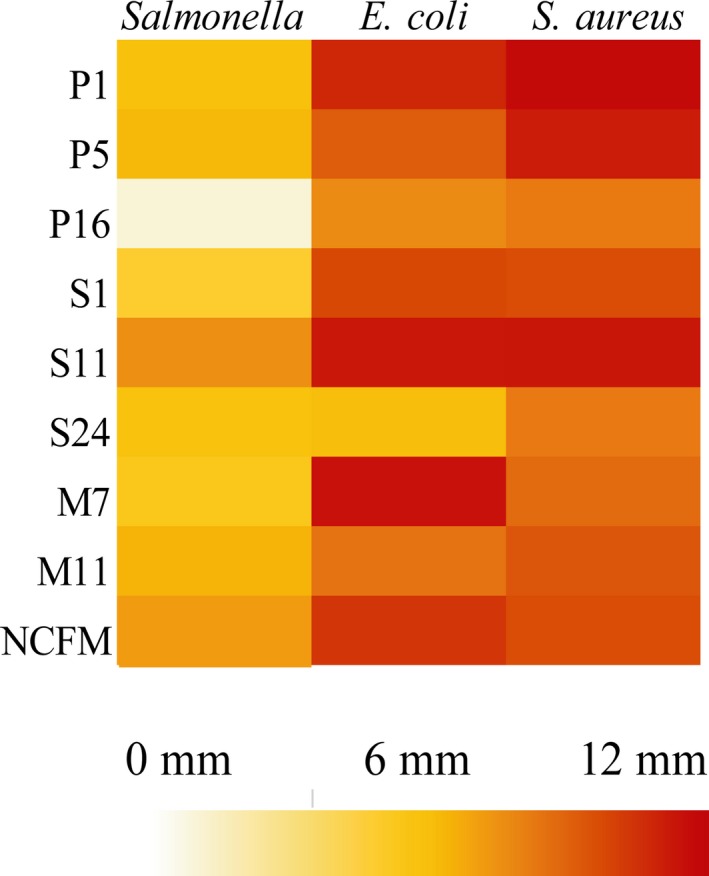
Inhibition zones map. Eight Lactobacillus plantarum strains and Lactobacillus acidophilus NCFM against three common pathogens. S. aureus: S. aureus ATCC12600 as indicator; E. coli: E. coli ATCC35128 as indicator; Salmonella: Salmonella ASI.1174 as indicator. Data are the mean ± SD of at least three independent experiments
3.3. Analysis of antimicrobial substrate in fermentation broths of L. plantarum strains
Lactic acid bacteria are a class of nonspore, Gram‐positive bacteria, whose principal common characteristic is fermenting sugars into organic acids. The decrease in pH can greatly inhibit the growth of other bacteria. Moreover, some other studies have also found that H2O2 produced during the metabolic process can inhibit bacteria (Charlier, Cretenet, Even, Loir, & Loir, 2009). On the other hand, some lactic acid bacteria can produce bacteriocins and bacteriocin‐like compounds to inhibit pathogens (Zhao et al., 2016). In this study, fermentation broths of L. plantarum strains (P1, S11, and M7) were carried out with five treatments to identify the major antimicrobial substrate. As shown in Figure 2, the antimicrobial activities against three pathogenic bacteria were not changed after heating and catalase treatment. Antimicrobial activities (x) of L. plantarum S11 and L. plantarum P1 slightly decreased after treated with proteinase K, pepsin, and trypsin, respectively. The proteinase K treatment reduced the antimicrobial activity of L. plantarum S11 against S. aureus from 10.2 to 5.6 mm (Figure 2a). On the contrary, pepsin and trypsin had no effect on the antimicrobial activity of fermentation broths. Most important of all, the inhibition zone diameter of the three fermentation broths all dropped below 1 mm after adjusting the fermentation broth pH from 3.80 to 6.50.
Figure 2.
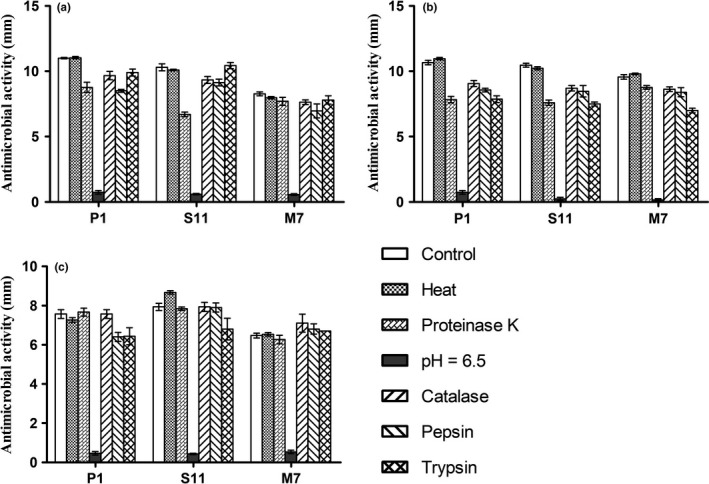
Changes of antimicrobial activity of fermentation broths of three Lactobacillus plantarum strains after various treatments. Control: Untreated fermentation broth; heat: 100°C heat treatment; proteinase K: the fermentation broth treated by proteinase K; pH = 6.5: the fermentation broth with pH adjusted to 6.5; catalase: the fermentation broth treated by catalase; pepsin: the fermentation broth treated by pepsin; trypsin: the fermentation broth treated by trypsin. (a) S. aureus ATCC12600 as indicator; (b) E. coli ATCC35128 as indicator; (c) Salmonella ASI.1174 as indicator. Data are the mean ± SD of at least three independent experiments
This phenomenon indicated that the antimicrobial activity of these three strains is due primarily to organic acids. Other than that, there was no heat‐sensitive antimicrobial substance in fermentation broths, such as macromolecule protein. Three kinds of L. plantarum strains (P1, S11, and M7) did not produce hydrogen peroxide with antimicrobial activity during fermentation. According to the effect of protease on the antimicrobial activity of fermentation broth, there may be polypeptide antimicrobial substances in fermentation broths of L. plantarum P1 and L. plantarum S11.
3.4. Common organic acids in fermentation broths of three L. plantarum strains
Lactobacillus plantarum strains can produce a variety of organic acids, mainly lactic acid. In point of metabolite products of L. plantarum, the main organic acids which own antimicrobial behavior are the acetic acids and lactic (Zalán, Hudáček, Štětina, Chumchalová, & Halász, 2010). In addition, other common organic acids such as tartaric acid and citric acid may also own antimicrobial activity. In our study, tartaric acid, malic acid, lactic acid, citric acid, and acetic acid were selected for the determination. According to pH curves of three L. plantarum strains in MRS, the pH of fermentation broths stabilizes at 3.80 ± 0.05 after 24 hr. Five kinds of organic acids were detected in the fermentation broths of these three strains by HPLC analysis (Figure S2). As shown in Figure 3, the organic acid produced by L. plantarum strains (P1, S11, and M7) was mainly lactic acid which was the highest in L. plantarum S11 (26.4 g/L). Compared to L. plantarum S11 and L. plantarum M7, L. plantarum P1 was detected with the highest level of acetic (3.3 g/L) and lactic acid (2.6 g/L), respectively. Beyond that, a small amount of tartaric acid and malic acid were also detected in all fermentation broths.
Figure 3.
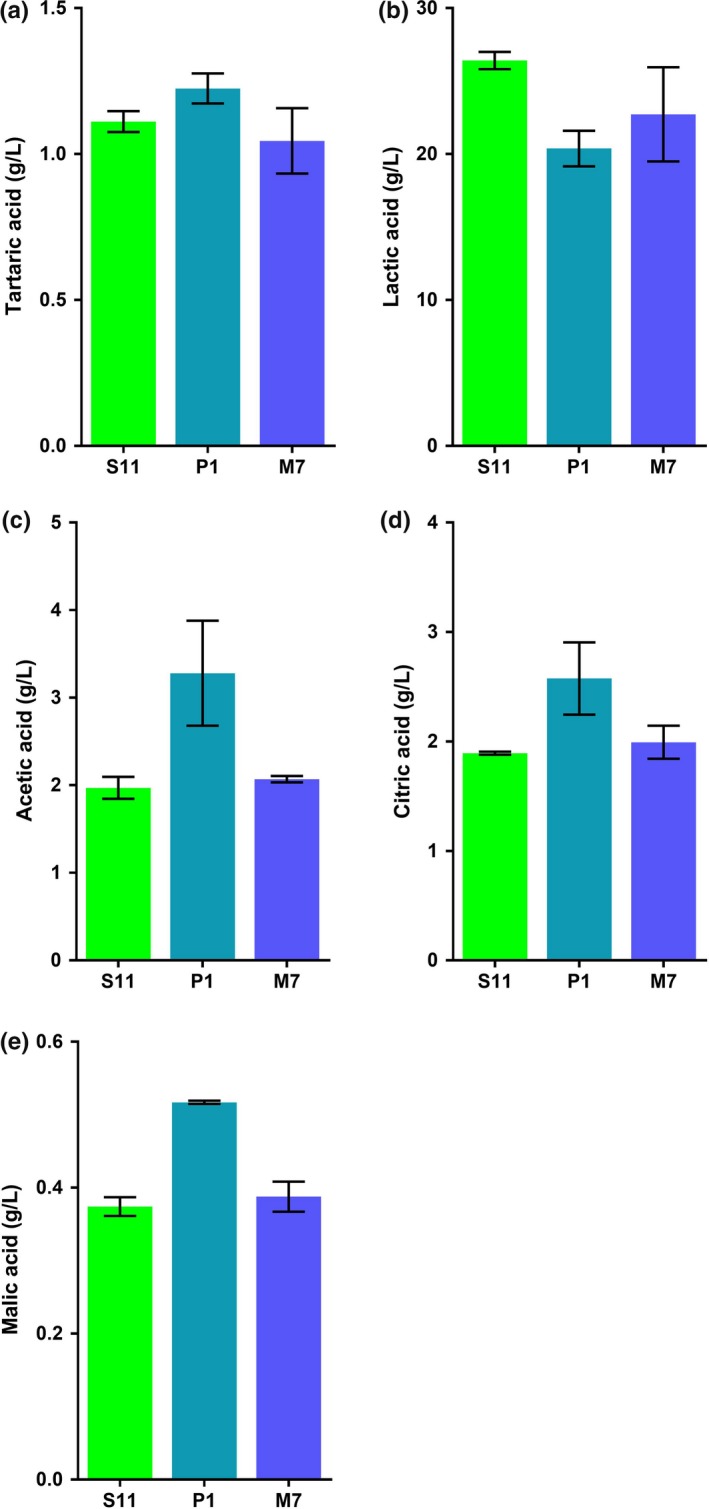
Analysis of organic acids in fermentation broths of three Lactobacillus plantarum strains by HPLC. (a) Analysis of tartaric acid in three fermentation broths; (b) analysis of lactic acid in three fermentation broths; (c) analysis of acetic acid in three fermentation broths; (d) analysis of citric acid in three fermentation broths; (e) analysis of malic acid in three fermentation broths. Data are the mean ± SD of at least three independent experiments
Compared with other organic acids, the content of lactic acid is the highest and it is the main substance that decreases the pH of the broth. The decrease in pH plays a certain inhibitory effect on the pathogenic bacteria growth. However, other organic acids also contribute to the antimicrobial activity. Therefore, the antimicrobial activity of these five organic acids was mainly studied in this study.
3.5. Configuration of antimicrobial agents and determination of their antimicrobial activities
The antimicrobial activities of these five organic acids are presented in Figure 4. Since the pH of three L. plantarum strains stabilized at 3.8 ± 0.05 after 24 hr, pH of MRS mediums was adjusted to 3.80 ± 0.05 using exogenous five organic acids respectively. Then, the antimicrobial activities of these mediums were measured by well diffusion method. When Gram‐positive S. aureus was the indicator, the antimicrobial activity of acetic acid was far higher than that of L. plantarum strains (P1, S11 and M7), reaching 26.2 mm (Figure 4a). However, the antimicrobial activity of the other four organic acids is lower than that of the fermentation broths. When using E. coli and Salmonella as indicator bacteria, the antimicrobial activities of three L. plantarum strains (P1, S11, and M7) were higher than those of five organic acids (Figure 4b,c).
Figure 4.
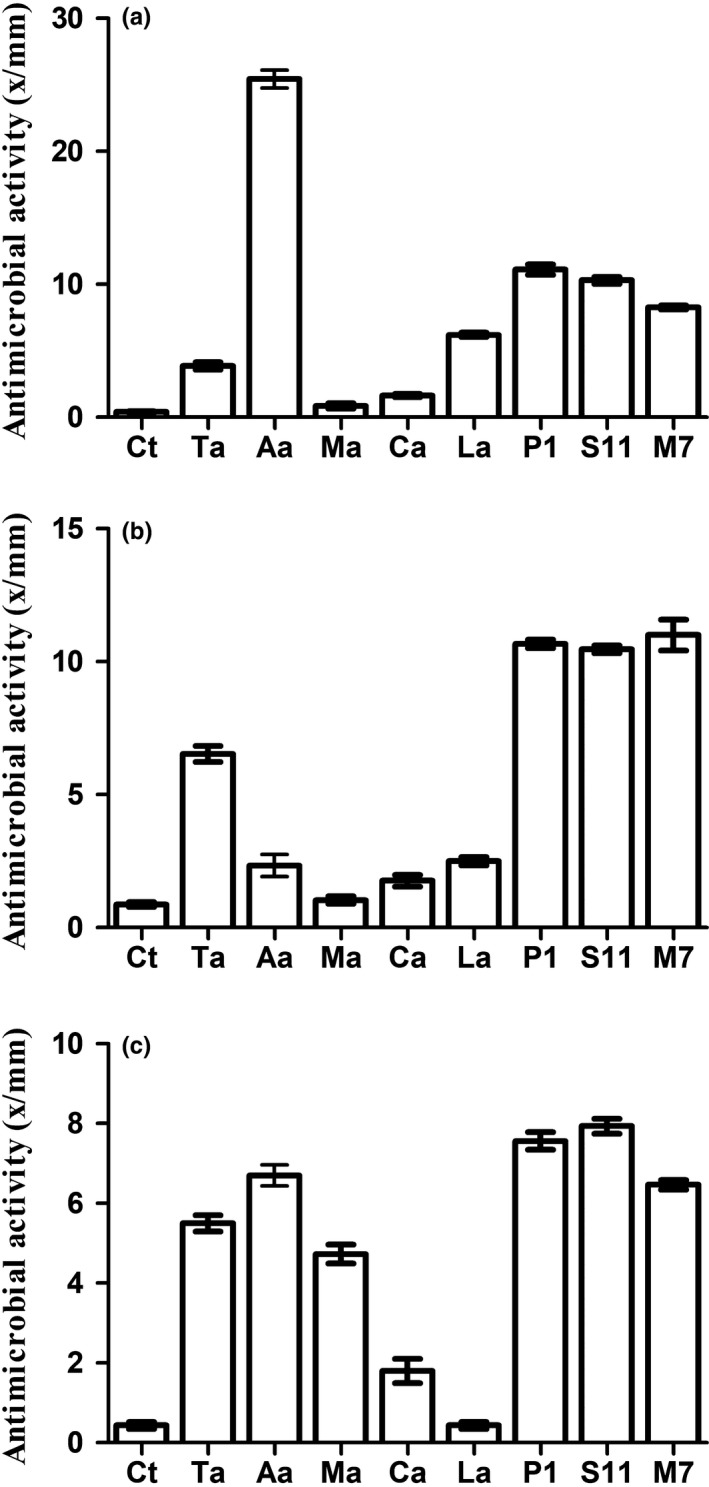
Comparison of the antimicrobial activity of five organic acids and fermentation broths at the same pH value. Ct: MRS (pH 6.2). (a) S. aureus ATCC12600 as indicator; (b) E. coli ATCC35128 as indicator; (c) Salmonella ASI.1174 as indicator. Data are the mean ± SD of at least three independent experiments
Food preservation with organic acids is a very effective method. Fermentation broths of L. plantarum strains (P1, S11, and M7) contained a variety of organic acids, and their antimicrobial activities against E. coli and Salmonella were stronger than any single organic acid at the same pH value. Therefore, we could speculate that the synergistic inhibition of different organic acids may be stronger than that of a single organic acid. Inspired by this, a new type of antimicrobial agent can be configured by mixing common organic acids in different proportions according to the broths.
Depending on the proportion of organic acids shown in Figure 3, antimicrobial agents P1‐1, S11‐1, and M7‐1 were configured by five organic acids. The antimicrobial effect was tested, and the results are shown in Figure 5. The pH of the antimicrobial agents formulated with the exogenous organic acid was kept at 3.80, which was consistent with the fermentation broth. Using S. aureus as an indicator, the antimicrobial activity of P1‐1 is 4 mm which was smaller than that of L. plantarum P1 fermentation broth (Figure 5a). The antimicrobial activity of S11‐1 and M7‐1 is consistent with the corresponding fermentation broths, and among them, S11‐1 had the highest antimicrobial activity, reaching 9.2 mm. Using E. coli as an indicator, fermentation broth of L. plantarum P1 and P1‐1 had the same antimicrobial activity, reaching 12.2 mm, and the antimicrobial activities of S11‐1 and M7‐1 are about 2 mm which were smaller than the fermentation ones (Figure 5b). Using Salmonella as an indicator, the antimicrobial activities of three antimicrobial agents (P1‐1, S11‐1, and M7‐1) are far higher than those of fermentation broths (Figure 5c).
Figure 5.
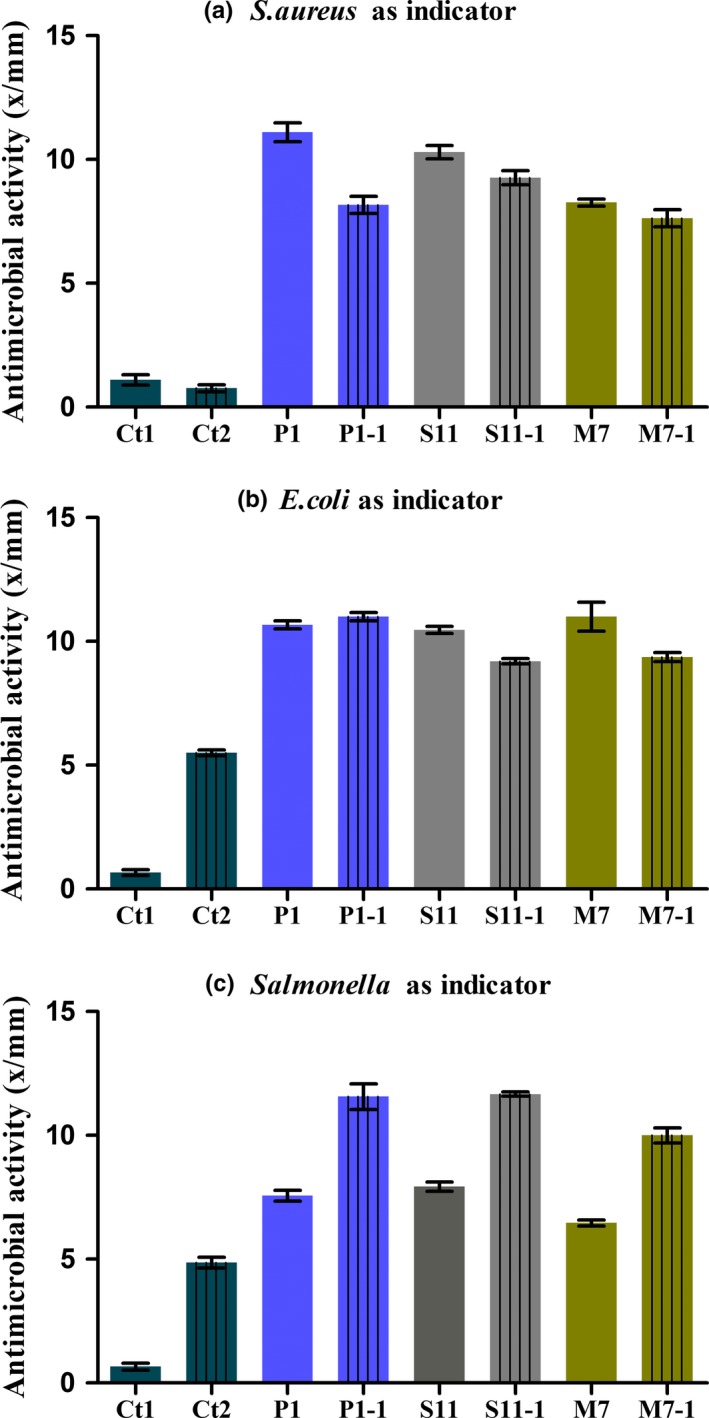
Comparison of antimicrobial activity of the fermentation liquid and exogenous organic acid mixed solution of three Lactobacillus plantarum strains. Ct1: Untreated MRS; Ct2: MRS with pH adjusted with hydrochloric acid. Data are the mean ± SD of at least three independent experiments
Compared with L. plantarum strains (P1, S11, and M7), the antimicrobial activities of antimicrobial agents (P1‐1, S11‐1, and M7‐1) are relatively higher. Making a new antimicrobial agent by mixing organic acids is a potential method for preserving foods effectively. In addition, for different pathogenic bacteria, it can be targeted by adjusting the proportion of organic acids. In conclusion, antimicrobial agents with outstanding antimicrobial effects can be configured by mixing a certain proportion of common organic acids. The antimicrobial activity could be increasing by adjusting the organic acids proportions for the different pathogenic bacteria.
4. CONCLUSIONS
The results of this study showed that three L. plantarum strains (P1, S11, and M7) isolated from Xinjiang traditional dairy products showed strong antimicrobial activities against indicator strains. Moreover, organic acids played a key role in antimicrobial substances in fermentation broths. Five common organic acids were found in the fermentation broth, and the proportion of organic acids in the L. plantarum strains (P1, S11, and M7) fermentation broths was different, matching their different antimicrobial effects. Thus, depending on the proportion of organic acids in the three fermentation broths, five organic acids were mixed to make three artificial antimicrobial agents (P1‐1, S11‐1, and M7‐1). We found that the antimicrobial activity of the three antimicrobial agents against pathogenic bacteria was strong. At the same pH value, the antimicrobial activity of mixed organic acids is sometimes stronger than that of single organic acids. In addition, organic acid antimicrobial agents combined in different proportions can inhibit different pathogenic bacteria. Therefore, it is a simple and effective way to develop new antimicrobial agents through the cooperation of different organic acids.
CONFLICT OF INTEREST
No conflict of interest declared.
ETHICAL STATEMENT
This study does not involve any human or animal testing.
INFORMED CONSENT
Written informed consent was obtained from all study participants.
Supporting information
ACKNOWLEDGMENTS
This research was supported by the Shanghai Municipal Science and Technology Commission Project (18JC1410802) and Fundamental Research Funds for the Central Universities (222201714051).
Hu C‐H, Ren L‐Q, Zhou Y, Ye B‐C. Characterization of antimicrobial activity of three Lactobacillus plantarum strains isolated from Chinese traditional dairy food. Food Sci Nutr. 2019;7:1997–2005. 10.1002/fsn3.1025
REFERENCES
- Altay, F. , Karbancıoglu‐Güler, F. , Daskaya‐Dikmen, C. , & Heperkan, D. (2013). A review on traditional Turkish fermented non‐alcoholic beverages: Microbiota, fermentation process and quality characteristics. International Journal of Food Microbiology, 167(1), 44–56. 10.1016/j.ijfoodmicro.2013.06.016 [DOI] [PubMed] [Google Scholar]
- Bonev, B. , Hooper, J. , & Parisot, J. (2008). Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. Journal of Antimicrobial Chemotherapy, 61(6), 1295–1301. 10.1093/jac/dkn090 [DOI] [PubMed] [Google Scholar]
- Castro, M. P. , Palavecino, N. Z. , Herman, C. , Garro, O. A. , & Campos, C. A. (2011). Lactic acid bacteria isolated from artisanal dry sausages: Characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat Science, 87(4), 321–329. 10.1016/j.meatsci.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Cebeci, A. , & Gürakan, C. (2003). Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiology, 20(5), 511–518. 10.1016/S0740-0020(02)00174-0 [DOI] [Google Scholar]
- Charlier, C. , Cretenet, M. , Even, S. , Loir, Y. L. , & Loir, Y. L. (2009). Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. International Journal of Food Microbiology, 131(1), 30–39. 10.1016/j.ijfoodmicro.2008.06.032 [DOI] [PubMed] [Google Scholar]
- Cortés‐Zavaleta, O. , López‐Malo, A. , Hernández‐Mendoza, A. , & García, H. S. (2014). Antifungal activity of lactobacilli and its relationship with 3‐phenyllactic acid production. International Journal of Food Microbiology, 173(3), 30–35. 10.1016/j.ijfoodmicro.2013.12.016 [DOI] [PubMed] [Google Scholar]
- da Silva Sabo, S. , Vitolo, M. , González, J. M. D. , & Oliveira, R. P. S. (2014). Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Research International, 64, 527–536. 10.1016/j.foodres.2014.07.041 [DOI] [PubMed] [Google Scholar]
- de Vries, M. C. , Vaughan, E. E. , Kleerebezem, M. , & de Vos, W. M. (2006). Lactobacillus plantarum— Survival, functional and potential probiotic properties in the human intestinal tract. International Dairy Journal, 16(9), 1018–1028. 10.1016/j.idairyj.2005.09.003 [DOI] [Google Scholar]
- Denkova, R. , Goranov, B. , Teneva, D. , Denkova, Z. , & Kostov, G. (2017). Antimicrobial activity of probiotic microorganisms: Mechanisms of interaction and methods of examination. Antimicrobial research: Novel bioknowledge and educational programs, 201–212.
- Denkova, R. , Yanakieva, V. , Denkova, Z. , Nikolova, V. , & Radeva, V. (2013). In vitro inhibitory activity of bifidobacterium and Lactobacillus strains against candida albicans . Bulgarian Journal of Veterinary Medicine, 16(3), 186–197. [Google Scholar]
- Fhoula, I. , Najjari, A. , Turki, Y. , Jaballah, S. , Boudabous, A. , & Ouzari, H. (2013). Diversity and antimicrobial properties of lactic acid bacteria isolated from rhizosphere of olive trees and desert truffles of Tunisia. Biomed Research International, 2013(4), 405708 10.1155/2013/405708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri, E. , Niederhäusern, S. D. , Messi, P. , Sabia, C. , Iseppi, R. , Anacarso, I. , & Bondi, M. (2009). Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small‐scale model. Food Control, 20(9), 861–865. 10.1016/j.foodcont.2008.11.001 [DOI] [Google Scholar]
- Guidone, A. , Zotta, T. , Ross, R. P. , Stanton, C. , Rea, M. C. , Parente, E. , & Ricciardi, A. (2014). Functional properties of Lactobacillus plantarum strains: A multivariate screening study. LWT ‐ Food Science and Technology, 56(1), 69–76. 10.1016/j.lwt.2013.10.036 [DOI] [Google Scholar]
- Herreros, M. A. , Sandoval, H. , Gonzalez, L. , Castro, J. M. , Fresno, J. M. , & Tornadijo, M. E. (2005). Antimicrobial activity and antibiotic resistance of lactic acid bacteria isolated from Armada cheese (a Spanish goats' milk cheese). Food Microbiology, 22(5), 455–459. 10.1016/j.fm.2004.11.007 [DOI] [Google Scholar]
- Huang, L. , Duan, C. , Zhao, Y. , Gao, L. , Niu, C. , Xu, J. , & Li, S. (2017). Reduction of aflatoxin B1 toxicity by Lactobacillus plantarum C88: A potential probiotic strain isolated from Chinese traditional fermented food “Tofu”. PLoS ONE, 12(1), e0170109 10.1371/journal.pone.0170109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kecerová, K. , Pristas, P. , & Javorský, P. (2004). Bacteriocin production and sensitivity. Folia Microbiologica, 49(2), 172–174. 10.1007/bf02931395 [DOI] [PubMed] [Google Scholar]
- Kullen, M. J. , Sanozkydawes, R. B. , Crowell, D. C. , & Klaenhammer, T. R. (2010). Use of the DNA sequence of variable regions of the 16S rRNA gene for rapid and accurate identification of bacteria in the Lactobacillus acidophilus complex. Journal of Applied Microbiology, 89(3), 511–516. 10.1046/j.1365-2672.2000.01146.x [DOI] [PubMed] [Google Scholar]
- Lavermicocca, P. , Valerio, F. , Evidente, A. , Lazzaroni, S. , Corsetti, A. , & Gobbetti, M. (2000). Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Applied & Environmental Microbiology, 66(9), 4084–4090. 10.1016/S0038-0717(97)00088-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. , Gu, Q. , Yang, L. , Yu, Y. , & Wang, Y. (2017). Characterization of extracellular vitamin B12 producing Lactobacillus plantarum strains and assessment of the probiotic potentials. Food Chemistry, 234, 494 10.1016/j.foodchem.2017.05.037 [DOI] [PubMed] [Google Scholar]
- Li, X. , Xu, W. , Yang, J. , Zhao, H. , Pan, C. , Ding, X. , & Zhang, Y. (2016). Effects of applying lactic acid bacteria to the fermentation on a mixture of corn steep liquor and air‐dried rice straw. Animal Nutrition, 2(3), 229–233. 10.1016/j.aninu.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealmckinney, J. M. , Lu, X. , Duong, T. , Larson, C. L. , Call, D. R. , Shah, D. H. , & Konkel, M. E. (2012). Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE, 7(9), e43928 10.1371/journal.pone.0043928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ołdak, A. , Zielińska, D. , Rzepkowska, A. , & Kołożyn‐Krajewska, D. (2017). Comparison of antibacterial activity of Lactobacillus plantarum strains isolated from two different kinds of regional cheeses from Poland: Oscypek and Korycinski cheese. Biomed Research International, 2017(2), 6820369 10.1155/2017/6820369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchia, C. , Ercolini, D. , Blaiotta, G. , Pepe, O. , Mauriello, G. , & Villani, F. (2004). Selection of Lactobacillus strains from fermented sausages for their potential use as probiotics. Meat Science, 67(2), 309–317. 10.1016/j.meatsci.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Rowland, I. R. , Capurso, L. , Collins, K. , Cummings, J. , Delzenne, N. , Goulet, O. , … Meier, R. (2010). Current level of consensus on probiotic science–report of an expert meeting–London, 23 November 2009. Gut Microbes, 1(6), 436 10.4161/gmic.1.6.13610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, M. , Joerger, R. , Palou, E. , López‐Malo, A. , Avila‐Sosa, R. , & Calix‐Lara, T. (2012). Alternatives to traditional antimicrobials for organically processed meat and poultry. Hoboken, NJ: Wiley‐Blackwell; 10.1002/9781118229088.ch13 [DOI] [Google Scholar]
- Tejerosariñena, S. , Barlow, J. , Costabile, A. , Gibson, G. R. , & Rowland, I. (2012). In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: Evidence for the effects of organic acids. Anaerobe, 18(5), 530–538. 10.1016/j.anaerobe.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Teneva, D. , Denkova, R. , Goranov, B. , Denkova, Z. , & Kostov, G. (2017). Antimicrobial activity of Lactobacillus plantarum strains against Salmonella pathogens. Ukrainian Food Journal, 6(1), 125–133. 10.24263/2304-974X-2017-6-1-14 [DOI] [Google Scholar]
- Thu, T. V. , Foo, H. L. , Loh, T. C. , & Bejo, M. H. (2013). Inhibitory activity and organic acid concentrations of metabolite combinations produced by various strains of Lactobacillus plantarum . African Journal of Biotechnology, 10(8), 1359–1363. [Google Scholar]
- Valerio, F. , Lavermicocca, P. , Pascale, M. , & Visconti, A. (2004). Production of phenyllactic acid by lactic acid bacteria: An approach to the selection of strains contributing to food quality and preservation. Fems Microbiology Letters, 233(2), 289–295. 10.1111/j.1574-6968.2004.tb09494.x [DOI] [PubMed] [Google Scholar]
- Yang, J. , Wang, J. , Yang, K. , Liu, M. , Qi, Y. , Zhang, T. , … Wei, X. (2017). Antibacterial activity of selenium‐enriched lactic acid bacteria against common food‐borne pathogens in vitro. Journal of Dairy Science, 101, 1930–1942. 10.3168/jds.2017-13430 [DOI] [PubMed] [Google Scholar]
- Zalán, Z. , Hudáček, J. , Štětina, J. , Chumchalová, J. , & Halász, A. (2010). Production of organic acids by Lactobacillus strains in three different media. European Food Research & Technology, 230(3), 395–404. 10.1007/s00217-009-1179-9 [DOI] [Google Scholar]
- Zhai, Q. , Wang, G. , Zhao, J. , Liu, X. , Narbad, A. , Chen, Y. Q. , … Chen, W. (2014). Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Applied & Environmental Microbiology, 80(13), 4063 10.1128/AEM.00762-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Han, J. , Bie, X. , Lu, Z. , Zhang, C. , & Lv, F. (2016). Purification and characterization of plantaricin JLA‐ 9: A novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA‐9 from Suan‐Tsai, a traditional Chinese fermented cabbage. Journal of Agriculture and Food Chemistry, 64(13), 2754–2764. 10.1021/acs.jafc.5b05717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


