Abstract
Physical activity has many health benefits for individuals with and without history of brain injury. Here, we evaluated in a large cohort study the impact of physical activity on global and cognitive health as measured by the PROMIS global health and NeuroQoL cognitive function questionnaires. A nested case control study assessed the influence of a history of traumatic brain injury (TBI) on the effects of physical activity since underlying pathophysiology and barriers to physical activity in individuals with TBI may mean the effects of physical activity on perceived health outcomes differ compared to the general population. Those with a history of TBI (n = 81) had significantly lower Global health (β = −1.66, p = 0.010) and NeuroQoL cognitive function (β = −2.65, p = 0.006) compared to healthy adults (n = 405). A similar proportion of individuals in both groups reported being active compared to being insufficiently active ( = 0.519 p = 0.471). Furthermore, the effect of physical activity on global health (β = 0.061, p = 0.076) and particularly for NeuroQoL (β = 0.159, p = 0.002) was greater in those with a history of TBI. Individuals with a history of TBI can adhere to a physically active lifestyle, and if so, that is associated with higher global and cognitive health perceptions. Adhering to a physically active lifestyle is non-trivial, particularly for individuals with TBI, and therefore adapted strategies to increase participation in physical activity is critical for the promotion of public health.
Keywords: physical activity, cognition, global health, traumatic brain injury, health-related quality of life
Introduction
Physical activity is associated with a 20%–30% lower risk in all-cause mortality and incidence of multiple chronic conditions (James et al., 2016). Numerous governing bodies including the World Health Organization, American Heart Association and the American College of Sports Medicine have focused much attention on the beneficial effects of a physically active lifestyle (Warburton et al., 2006; Haskell et al., 2007; WHO, 2019a). The effects span multiple bodily systems from cardiovascular benefits (James et al., 2016) to mental health (Blumenthal et al., 1999; Babyak et al., 2000) and cognitive function (Colcombe and Kramer, 2003; Gomes-Osman et al., 2018; Northey et al., 2018). The effects of physical activity on cognitive function has received particular attention in recent decades across distinct age groups, including adolescents (Donnelly et al., 2016), older adults (Colcombe and Kramer, 2003) and patients with dementia (Gomes-Osman et al., 2018). Physical activity represents a modifiable lifestyle factor capable of improving global and cognitive health across the lifespan. However, engaging in a physically active lifestyle is non-trivial. Globally, one in four adults are classified as insufficiently active (WHO, 2019b), which is estimated to contribute to 9% of all premature deaths worldwide (or 5.3 million; Lee et al., 2012). Physical inactivity is exacerbated in those with a disability, with almost half of US adults with a disability being physically inactive and having a greater likelihood of having a chronic disease (Carroll et al., 2014).
Individuals who have acquired brain injuries can become prone to a sedentary lifestyle which places them at risk of secondary health complications (Shavelle et al., 2001). A number of studies have reported that individuals with a history of traumatic brain injury (TBI) are insufficiently active enough to receive a health benefit (Reavenall and Blake, 2010; Hamilton et al., 2015), according to current guidelines (Larry Durstine et al., 2012). The long-term health consequences of TBI include cognitive, sensorimotor, behavioral and social problems that can negatively affect quality of life (Stocchetti and Zanier, 2016), and in the US alone, an estimated 3.2 million individuals live with residual effects of TBI (Benedictus et al., 2010). Cognitive dysfunction following TBI can last for decades post-injury (Draper and Ponsford, 2008) and physical exercise is a potential therapeutic intervention for those living with residual effects of an injury (Hoffman et al., 2010; Wise et al., 2012; Chin et al., 2015). Whilst the feasibility of dedicated and professional-led, short-to-mid-term (3 months) aerobic exercise programs in community-dwelling individuals with moderate-to-severe TBI has been demonstrated (Devine et al., 2016), the association between a physically active lifestyle and global and cognitive brain health in this population has not been assessed. Distinct barriers to performing physical activity (Rimmer et al., 2004) and underlying pathophysiology (Werner and Engelhard, 2007) may mean that this relationship differs in individuals with a history of TBI.
We performed a nested case-control study to assess the impact of a history of TBI on the associations between physical activity and perceived global and cognitive health. We hypothesized that physical activity would be predictive of higher global and cognitive health in both those with and without a history of TBI.
Materials and Methods
Study Design
A cohort of community-dwelling adults, mainly in the Catalonia region of Spain, was established as part of the Barcelona Brain Health Initiative1, starting in 2017. Adults aged 40–65 were invited to participate in an online questionnaire-based survey via television and local advertisements. The study protocol is described in Cattaneo et al. (2018). All methods described were approved by the education and ethics committee of Institut Guttmann. This manuscript was prepared under the strengthening the reporting of observational studies in epidemiology (STROBE) guidelines.
At the time of analysis (June 2018), a total of 4,624 individuals had enrolled. Of those, 81 individuals (1.8%) answered positively to the question: Have you ever had a TBI with loss of consciousness? A control group (N = 405, 49% female) was randomly selected from the total cohort using Microsoft Excel’s “rand” function. The total cohort was organized by this random number sequence and then filtered per the age and gender criteria. Whereby five adults free from any neurological or psychological disorders were selected for every participant with a history of TBI at random in blocks of 5 years with an even male to female ratio.
Outcomes and Covariates
Our main outcome variables were perceived global health and perceived cognitive function. To measure these constructs, the PROMIS global health questionnaire (Cella et al., 2010) and the NeuroQoL cognitive function questionnaire (Gershon et al., 2012) were used. PROMIS global health is a 10-item 5-point Likert scale (1-poor, 5-excellent) that probes respondents physical, mental and social health. Higher scores mean more of the construct is being measured (i.e., higher global health). NeuroQoL cognitive function is 5-point Likert scale (1-very often, 5-never) with 12 items that probes respondents thinking, attention, planning, new task learning and comprehension. Higher scores mean more of the construct is being measured (i.e., better perceived cognitive function).
Our main predictor variable was physical activity levels. We chose to implement the Godin-Shepard leisure time physical activity questionnaire (Godin and Shephard, 1985) to measure this. The GSLTPAQ probes the number of times spent performing moderate (not exhausting) or strenuous (heart beats rapidly) physical activity of at least 15-min during a typical 7-day period (Godin, 2011). The frequency score is multiplied by a corresponding metabolic equivalent for task (MET) value (moderate = *5; strenuous = *9) and summed to obtain an arbitrary leisure score index (LSI). Cut-off points can be created using the LSI, the rationale for which originate from the World Organization (WHO, 2018) and American College of Sports Medicine (Ferguson, 2014) guidelines for weekly physical activity associated with significant health benefits (combination of moderate and strenuous activity 3–5 times per week). An LSI of ≥24 is active where as those ≤23 are insufficiently active. Consequently, those culminating in a score of ≥24 using questions that pertain to moderate and strenuous physical activity and LSI calculations based on both frequency and energy expenditure will likely meet the physical activity guidelines. The utility and accuracy of these cut-off scores have been validated in healthy adults (Amireault and Godin, 2015). Raw scores above 7 for each question were excluded (N = 13) as they were considered to be derived through misinterpretation of the question. Those without responses were set to missing (N = 12).
Co-variates included gender and age, which was asked in years and three categories were created; 40–49, 50–59 and 60 and above. BMI was calculated as body weight (in kilograms) divided by height (in meters squared). Self-perceived negative affect in depression, anxiety and stress was also assessed using the 21-item sub-scale version of the Depression Anxiety Stress Scale (Brown et al., 1997). This is a 4-point Likert scale (1-never, 4-always) where higher scores represent higher negative affective state. This was chosen as a potential confounder as perceived negative effect can affect how one reports cognitive and global health (Bartrés-Faz et al., 2018).
Statistical Analysis
We tested age, gender and negative affective status as potential confounding variables in our analysis. Variables which predicted the outcome measure with a p ≤ 0.05 were defined as confounders and added to the final model. The proportion of individuals who reported being insufficiently active compared to active was tested using Pearson’s chi squared test of proportions, as was gender. A Kruskal-Wallis test was used to assess differences in BMI between groups. Generalized linear models with a Gaussian family function and identity link function were used to assess the independent associations between physical activity levels and diagnosis history (history of TBI or not) on perceived cognitive function and perceived global health, controlling for all other significant variables in the model. To assess whether the associations between physical activity and perceived cognitive and global health differed between those with and without a history of TBI, an interaction term between physical activity and diagnosis history was added to the model. Post estimation tests were performed using marginal effects and the estimated slopes were plotted. All statistical analyses were performed in Stata version 15 (StataCorp LLC, College Station, TX, USA).
Results
Table 1 describes all participant characteristics and questionnaire scores for each group. There was no significant difference between the age of the participants in each group (t(116) = −0.3, p ≥ 0.740) and the proportion of male and female participants was similar across groups [49% female ( = 0.004 p = 0.951)]. BMI was not significantly different between each group ( = 0.042 p = 0.838).
Table 1.
Participant characteristics and questionnaire scores between groups.
| Healthy adults | History of TBI | |
|---|---|---|
| Age (years) | 51.8 (7.2) | 51.7 (7.1) |
| BMI (kg/m2) | 24.2 (3.5) | 24.4 (4.0) |
| PROMIS Global Health | 34.9 (4.6) | 32.2 (6.2)* |
| PROMIS NeuroQoL | 52.3 (6.5) | 47.5 (11.0)* |
| DASS21 | 13.4 (11.3) | 20.4 (17.5)* |
| GSLTPAQ | 19.5 (19.2) | 18.8 (18.3) |
*Significant at p < 0.05. GSLTPAQ, Godin-Shepard leisure time physical activity questionnaire.
Physical Activity Level
Using the LSI cut-off scores to classify participants into active and insufficiently active, a similar proportion of those with and without and history of TBI were classified as active ( = 0.519 p = 0.471), compared to insufficiently active (Figure 1).
Figure 1.
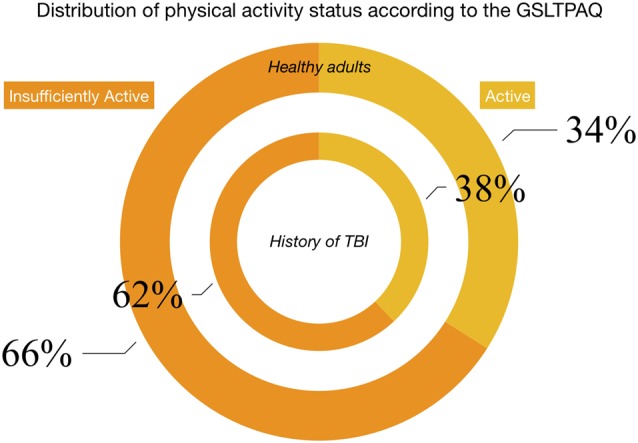
Hollow pie charts show the proportion of individuals who report being active (yellow bars) compared to insufficiently active (orange bars) based on the Godin-Shepard leisure time physical activity questionnaire (GSLTPAQ). The Godin questionnaire classifies people as active or insufficiently active based on the number of times per week spent performing either moderate or strenuous physical activity of at least 15 min. The inner bars represent the group with a history of traumatic brain injury (TBI) and the outer bars represent those free from any neurological or neuropsychiatric disease. A numerically higher proportion of individuals in both groups report being insufficiently active compared to those reporting being active.
PROMIS Global Health
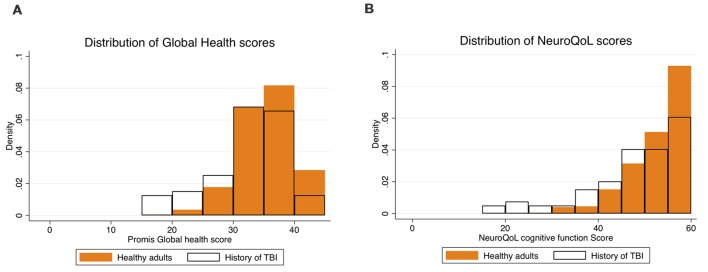
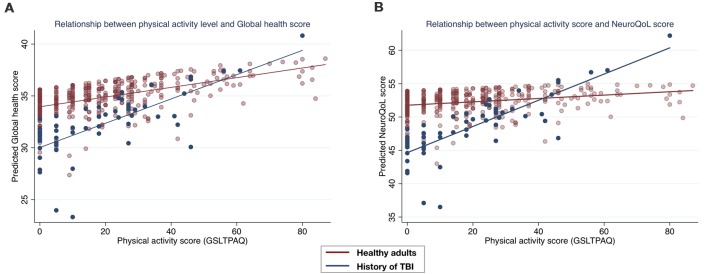
The distribution of Promis global health scores in both groups is shown in Figure 2A. Diagnosis was a significant predictor of global health (β = −1.66, SE = 0.644, p = 0.010, 95% CI’s = −2.927, −0.403) with those with a history of TBI reporting lower perceived global health (32.2 ± 0.70) than those without (34.9 ± 0.22). Physical activity level significantly predicted Promis global health (β = 0.049, SE = 0.011, p ≤ 0.001, 95% CI’s = 0.027, 0.071). A non-significant interaction between diagnosis and physical activity level was shown (β = 0.061, SE = 0.034, p = 0.076, 95% CI’s = −0.006, 0.128). Post estimation tests showed that the relationship between physical activity and global health was marginally greater in the group with a history of TBI (β = 0.103, SE = 0.032, p = 0.001, 95% CI’s = 0.040, 0.166), than in the healthy adults (β = 0.042, SE = 0.011, p ≤ 0.001, 95% CI’s = 0.019, 0.065; Figure 3A).
Figure 2.
Histogram plots show the distribution of scores on the global health scale (A) and the NeuroQoL cognitive function scale (B) for both participants in the healthy group (orange filled bars) and the group with a history of TBI (black outlined bars).
Figure 3.
Scatter plots of the relationship between physical activity levels and the predicted Global health and NeuroQoL cognitive function scores as a result of the generalized linear models, by group. (A) A trending interaction (p = 0.076) was observed between groups whereby the group with a history of TBI had a greater effect of physical activity level on global health (navy line). In (B) a significant interaction between groups is observed (p = 0.002) whereby the group with a history of TBI shows a significant effect of physical activity on NeuroQoL cognitive function, which is not significant for the group without a history of TBI (red line).
NeuroQoL Cognitive Function
The distribution of NeuroQoL cognitive function scores in both groups is shown in Figure 2B. Diagnosis was a significant predictor of NeuroQoL cognitive function (β = −2.65, SE = 0.974, p = 0.006, 95% CI’s = −4.561, −0.743), with those with a history of TBI reporting lower perceived cognitive function health (47.45 ± 1.20) than those without (52.26 ± 3.29). Physical activity level was predictive of NeuroQoL cognitive function (β = 0.037, SE = 0.016, p ≤ 0.023, 95% CI’s = 0.005, 0.070). A significant interaction between diagnosis and physical activity level was shown (β = 0.159, SE = 0.051, p = 0.002, 95% CI’s = 0.058, 0.260) whereby post estimation tests showed that the relationship between physical activity and NeuroQoL cognitive function was significant in the group with a history of TBI (β = 0.179, SE = 0.049, p ≤ 0.001, 95% CI’s = 0.083, 0.274), but not in the healthy adults (β = 0.019, SE = 0.017, p = 0.260, 95% CI’s = −0.014, 0.054; Figure 3B).
Discussion
In this study, we aimed to assess the impact of a history of TBI on the relationship between physical activity levels and global and cognitive brain health. Not surprisingly, we found that healthy adults reported higher global and cognitive brain health compared to those with a history of TBI. However, we also found that individuals with a history of TBI were as likely to be physically active as those without a history of TBI and physical activity levels were even stronger predictors of both global and cognitive function health perceptions than in individuals without a history of TBI.
Self-reported levels of physical activity have been associated with numerous protective health benefits such as reduced risk of cognitive impairment (Laurin et al., 2001) and cognitive decline (Sofi et al., 2011), mortality due to cardiovascular disease (Nocon et al., 2008) and reduced incident rates of dementia (Larson et al., 2006). Self-report physical activity has also been associated with better health-related quality of life (HRQOL; Brown et al., 2003). These findings from the 2001 behavioral risk factor surveillance system survey found that adhering to recommended levels of physical activity was significantly associated with less days of poor perceived mental and physical health. Our results corroborate these findings in so much as higher physical activity levels are related to higher perceptions of global (mental, physical, social and pain) health. Thereupon, physical activity appears not only associated with improved health outcomes but with higher perceptions of health also.
Numerous randomized-control trials have shown positive associations between physical activity and physical exercise and cognitive function (Colcombe and Kramer, 2003; Gomes-Osman et al., 2018). Additionally, a number of these studies have also shown improvements in the structure and function of cortical networks associated with cognitive functioning (Erickson et al., 2011; Voss et al., 2013; Weng et al., 2017). Whilst dose-response studies are limited (Vidoni et al., 2015; Chen et al., 2018), a linear relationship between physical activity and health status has been reported, such that increases in physical activity leads to greater health benefits, especially in previously sedentary individuals (Warburton et al., 2006). Our results, however, failed to show any association between physical activity levels and perceptions of cognitive functioning in middle to older aged adults free from any neurological or neuropsychiatric disease. This may be explained by the difference in the characteristics of the included participants in our analysis whereby those with a history of TBI had significantly lower NeuroQoL scores compared to those without a history of TBI. This could have been further exacerbated by a ceiling effect in the NeuroQoL scale for the healthy adult population. This scale was developed to assess cognitive complaints in those with neurological afflictions and so may fail to capture a large enough variance in cognitive health perceptions amongst healthy adults (Gershon et al., 2012). Additionally, perceptions of cognitive function may not correlate well with objective measures of cognitive functioning, which is the case in a number of clinical populations (Schiehser et al., 2011; Hutchinson et al., 2012). Consequently, whilst it may be the case that those with lower perceptions of cognitive health receive a greater benefit of adhering to a physically active lifestyle, studies with objective measures of cognitive functioning may further help delineate this relationship.
Some concepts of HRQOL in TBI overlap with those of the general population yet research suggests that HRQOL following TBI may be more complex (Carlozzi et al., 2011). We saw that individuals with a history of TBI had significantly lower self-reported global and cognitive brain health compared to neurologically healthy adults. Whilst we cannot be certain that this lower perception of global and cognitive brain health is derived from the injury, previous reports have shown many individuals with a history of TBI live with residual negative effects of the injury (Benedictus et al., 2010). Cognitive dysfunction is prevalent post-injury and deficits can be seen at 6 months (Dikmen et al., 2009) and for as long as 10 years after injury (Draper and Ponsford, 2008). Long-term lifestyle interventions aimed at reducing these deficits are therefore of great importance to those living with residual effects of TBI.
We found that a similar proportion of those adults with a history of TBI reported being active as those without a history of TBI. However, a numerical majority of participants (both in the history of TBI and healthy group) were classified as insufficiently active. Previous research has suggested that those with a disability or a TBI have distinct barriers to engaging in physical activity (Rimmer et al., 2004; Reavenall and Blake, 2010; Pinto et al., 2018), including but not limited to having health concerns and lack of counselling by a physician (Pinto et al., 2018). Therefore, strategies to increase adherence to a physically active lifestyle, such as the WHO’s Global Strategy on Diet and Physical Activity (Bauman and Craig, 2005), are not only critical for both the general population and community-dwelling adults with a history of TBI but may need to be adapted to those living with a history of TBI. Promising results from a feasibility study of aerobic exercise programs in community-dwelling individuals with a history of moderate-to-severe TBI showed that greater adherence to exercise was achieved when free access to local gymnasiums was provided (Devine et al., 2016). Our results suggest that strategies like these that will increase adherence to a physically active lifestyle, will likely lead to improved perceptions of global and cognitive health.
Our study has certain limitations that may limit the interpretation of the results. Self-reported health outcomes, specifically TBI can be problematic and whilst sports concussion research has improved the self-reporting of concussion through better descriptions of concussion definitions (Robbins et al., 2014), this type of description is less well defined for the general public. Though the accuracy of self-reported measures of certain health outcomes (including stroke) have been documented (Okura et al., 2004), self-reported measures are often the best tool available, especially for large cohort studies. Given this constraint, we did not assess the severity of an individual’s TBI nor the time since injury in our cohort of TBI. Whilst this should not affect the exposure/outcome relationship, it means that we cannot be certain whether different injury severities are more or less associated with the results found. This might be of interest to future studies. Additionally, if co-morbid medical conditions were reported in the history of TBI group, they were not excluded from the analysis. Nevertheless, the number of participants in this group who had multiple conditions was small (headache (Lee et al., 2012), chronic pain (Babyak et al., 2000), anxiety (Gomes-Osman et al., 2018), depression (Blumenthal et al., 1999), memory loss (James et al., 2016), heart attack (Warburton et al., 2006), sleep apnea (Haskell et al., 2007), arthritis (James et al., 2016) and so are unlikely to have significantly affected the results.
Conclusion
Adhering to a physically active lifestyle is associated with higher global and cognitive health perceptions, especially in individuals with a history of TBI. Notwithstanding, a majority of individuals are insufficiently active and therefore it is critical to develop strategies to increase adherence to and participation in a physically active lifestyle in both those with and without a history of TBI.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher. The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was carried out in accordance with the recommendations of the ethics and education committee of Institut Guttmann with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the “Institut Guttmann.”
Author Contributions
AP-L, DB-F and J-MTM performed the initial conception of the project. GC and JS-S collected the data. TM analyzed the data. TM drafted the manuscript and all authors critically revised it for intellectual content.
Conflict of Interest Statement
AP-L serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Constant Therapy, Cognito, and Neosync, and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
A special thanks is extended to all study participants and other partners of the BBHI (Ad-Salutem Institute, Sodexo, I.C.A. Informática y Comunicaciones Avanzadas, Neuroelectrics, Corporació Catalana de Mitjans Audiovisuals, Club Metropolitan, Casa Ametller, and Agéncia de Qualitat i Avaluació Sanitáries de Catalunya-AQuAS) for their invaluable collaboration.
Funding. AP-L was partly supported by the Sidney R. Baer Jr. Foundation, the National Institutes of Health (NIH; R01MH100186, R01HD069776, R01NS073601, R21 NS082870, R21 MH099196, R21 NS085491, R21 HD07616), the Football Players Health Study at Harvard University, and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Institutes of Health, the Sidney R. Baer Jr. Foundation. The BBHI receives funding from “Obra Social La Caixa” (grant agreement n° LCF/PR/PR16/11110004) and the Abertis foundation, as well as internal funding from the Institut Guttmann (Institut Guttmann and Fundació Abertis).
References
- Amireault S., Godin G. (2015). The Godin-Shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Skills 120, 604–622. 10.2466/03.27.pms.120v19x7 [DOI] [PubMed] [Google Scholar]
- Babyak M., Blumenthal J. A., Herman S., Khatri P., Doraiswamy M., Moore K., et al. (2000). Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom. Med. 62, 633–638. 10.1097/00006842-200009000-00006 [DOI] [PubMed] [Google Scholar]
- Bartrés-Faz D., Cattaneo G., Solana J., Tormos J. M., Pascual-Leone A. (2018). Meaning in life: resilience beyond reserve. Alzheimers Res. Ther. 10:47. 10.1186/s13195-018-0381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman A., Craig C. L. (2005). The place of physical activity in the WHO global strategy on diet and physical activity. Int. J. Behav. Nutr. Phys. Act. 2:10. 10.1186/1479-5868-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedictus M. R., Spikman J. M., van der Naalt J. (2010). Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch. Phys. Med. Rehabil. 91, 1436–1441. 10.1016/j.apmr.2010.06.019 [DOI] [PubMed] [Google Scholar]
- Blumenthal J. A., Babyak M. A., Moore K. A., Craighead W. E., Herman S., Khatri P., et al. (1999). Effects of exercise training on older patients with major depression. Arch. Intern. Med. 159, 2349–2356. 10.1001/archinte.159.19.2349 [DOI] [PubMed] [Google Scholar]
- Brown D. W., Balluz L. S., Heath G. W., Moriarty D. G., Ford E. S., Giles W. H., et al. (2003). Associations between recommended levels of physical activity and health-related quality of life Findings from the 2001 Behavioral Risk Factor Surveillance System (BRFSS) survey. Prev. Med. 37, 520–528. 10.1016/s0091-7435(03)00179-8 [DOI] [PubMed] [Google Scholar]
- Brown T. A., Chorpita B. F., Korotitsch W., Barlow D. H. (1997). Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav. Res. Ther. 35, 79–89. 10.1016/s0005-7967(96)00068-x [DOI] [PubMed] [Google Scholar]
- Carlozzi N. E., Tulsky D. S., Kisala P. A. (2011). Traumatic brain injury patient-reported outcome measure: identification of health-related quality-of-life issues relevant to individuals with traumatic brain injury. Arch. Phys. Med. Rehabil. 92, S52–S60. 10.1016/j.apmr.2010.12.046 [DOI] [PubMed] [Google Scholar]
- Carroll D. D., Courtney-Long E. A., Stevens A. C., Sloan M. L., Lullo C., Visser S. N., et al. (2014). Vital signs: disability and physical activity—United States, 2009–2012. Morb. Mortal. Wkly. Rep. 63, 407–413. [PMC free article] [PubMed] [Google Scholar]
- Cattaneo G., Bartrés-Faz D., Morris T. P., Sánchez J. S., Macià D., Tarrero C., et al. (2018). The barcelona brain health initiative: a cohort study to define and promote determinants of brain health. Front. Aging Neurosci. 10:321. 10.3389/fnagi.2018.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., et al. (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 63, 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.-T., Etnier J. L., Wu C.-H., Cho Y.-M., Hung T.-M., Chang Y.-K. (2018). Dose-response relationship between exercise duration and executive function in older adults. J. Clin. Med. 7:E279. 10.3390/jcm7090279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L. M., Keyser R. E., Dsurney J., Chan L. (2015). Improved cognitive performance following aerobic exercise training in people with traumatic brain injury. Arch. Phys. Med. Rehabil. 96, 754–759. 10.1016/j.apmr.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S., Kramer A. F. (2003). Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 14, 125–130. 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- Devine J. M., Wong B., Gervino E., Pascual-Leone A., Alexander M. P. (2016). Independent, community-based aerobic exercise training for people with moderate-to-severe traumatic brain injury. Arch. Phys. Med. Rehabil. 97, 1392–1397. 10.1016/j.apmr.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Dikmen S. S., Corrigan J. D., Levin H. S., Machamer J., Stiers W., Weisskopf M. G. (2009). Cognitive outcome following traumatic brain injury. J. Head Trauma Rehabil. 24, 430–438. 10.1097/HTR.0b013e3181c133e9 [DOI] [PubMed] [Google Scholar]
- Donnelly J. E., Hillman C. H., Castelli D., Etnier J. L., Lee S., Tomporowski P., et al. (2016). Physical activity, fitness, cognitive function and academic achievement in children: a systematic review. Med. Sci. Sports Exerc. 48, 1197–1222. 10.1249/MSS.0000000000000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper K., Ponsford J. (2008). Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology 22, 618–625. 10.1037/0894-4105.22.5.618 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U S A 108, 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B. (2014). ACSM’s guidelines for exercise testing and prescription 9th Ed. 2014. J. Can. Chiropr. Assoc. 58:328. [Google Scholar]
- Gershon R. C., Lai J. S., Bode R., Choi S., Moy C., Bleck T., et al. (2012). Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qual. Life Res. 21, 475–486. 10.1007/s11136-011-9958-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G. (2011). The Godin-Shephard leisure-time physical activity Questionnaire. Health Fit. J. Can. 4, 18–22. 10.14288/hfjc.v4i1.82 [DOI] [Google Scholar]
- Godin G., Shephard R. J. (1985). A simple method to assess exercise behavior in the community. Can. J. Appl. Sport Sci. 10, 141–146. [PubMed] [Google Scholar]
- Gomes-Osman J., Cabral D. F., Morris T. P., McInerney K., Cahalin L. P., Rundek T., et al. (2018). Exercise for cognitive brain health in aging: a systematic review for an evaluation of dose. Neurol. Clin. Pract. 8, 257–265. 10.1212/CPJ.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M., Williams G., Bryant A., Clark R., Spelman T. (2015). Which factors influence the activity levels of individuals with traumatic brain injury when they are first discharged home from hospital? Brain Inj. 29, 1572–1580. 10.3109/02699052.2015.1075145 [DOI] [PubMed] [Google Scholar]
- Haskell W., Lee I.-M., Pate R., Powell K., Blair S., Franklin B., et al. (2007). Physical activity and public health: updated recommendation for adults from the American college of sports medicine and the American heart association. Circulation 116, 1081–1093. 10.1161/circulationaha.107.185649 [DOI] [PubMed] [Google Scholar]
- Hoffman J. M., Bell K. R., Powell J. M., Behr J., Dunn E. C., Dikmen S., et al. (2010). A randomized controlled trial of exercise to improve mood after traumatic brain injury. PM R 2, 911–919. 10.1016/j.pmrj.2010.06.008 [DOI] [PubMed] [Google Scholar]
- Hutchinson A. D., Hosking J. R., Kichenadasse G., Mattiske J. K., Wilson C. (2012). Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat. Rev. 38, 926–934. 10.1016/j.ctrv.2012.05.002 [DOI] [PubMed] [Google Scholar]
- James M., Lithwick D. J., Morrison B. N., Nazzari H., Isserow S., Heilbron B., et al. (2016). The health benefits of physical activity and cardiorespiratory fitness. BCMJ 58, 131–137. [Google Scholar]
- Larry Durstine J., Moore G. E., Painter P. L., Roberts S. O. (2012). ACSM’s exercise management for persons with chronic diseases and disabilities. Act. Adapt. Aging 36, 182–183. 10.1080/01924788.2012.677794 [DOI] [Google Scholar]
- Larson E. B., Wang L., Bowen J. D., McCormick W. C., Teri L., Crane P., et al. (2006). Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 144, 73–81. 10.7326/0003-4819-144-2-200601170-00004 [DOI] [PubMed] [Google Scholar]
- Laurin D., Verreault R., Lindsay J., MacPherson K., Rockwood K. (2001). Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 58, 498–504. 10.1001/archneur.58.3.498 [DOI] [PubMed] [Google Scholar]
- Lee I.-M., Shiroma E. J., Lobelo F., Puska P., Blair S. N., Katzmarzyk P. T. (2012). Impact of physical inactivity on the world’s major non-communicable diseases. Lancet 380, 219–229. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocon M., Hiemann T., Müller-Riemenschneider F., Thalau F., Roll S., Willich S. N. (2008). Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur. J. Cardiovasc. Prev. Rehabil. 15, 239–246. 10.1097/hjr.0b013e3282f55e09 [DOI] [PubMed] [Google Scholar]
- Northey J. M., Cherbuin N., Pumpa K. L., Smee D. J., Rattray B. (2018). Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br. J. Sports Med. 52, 154–160. 10.1136/bjsports-2016-096587 [DOI] [PubMed] [Google Scholar]
- Okura Y., Urban L. H., Mahoney D. W., Jacobsen S. J., Rodeheffer R. J. (2004). Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J. Clin. Epidemiol. 57, 1096–1103. 10.1016/j.jclinepi.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Pinto S. M., Newman M. A., Hirsch M. A. (2018). Perceived barriers to exercise in adults with traumatic brain injury vary by age. J. Funct. Morphol. Kinesiol. 3:47 10.3390/jfmk3030047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavenall S., Blake H. (2010). Determinants of physical activity participation following traumatic brain injury. Int. J. Ther. Rehabil. 17, 360–369. 10.12968/ijtr.2010.17.7.48893 [DOI] [Google Scholar]
- Rimmer J. H., Riley B., Wang E., Rauworth A., Jurkowski J. (2004). Physical activity participation among persons with disabilities: barriers and facilitators. Am. J. Prev. Med. 26, 419–425. 10.1016/j.amepre.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Robbins C. A., Daneshvar D. H., Picano J. D., Gavett B. E., Baugh C. M., Riley D. O., et al. (2014). Self-reported concussion history: impact of providing a definition of concussion. Open Access. J. Sports Med. 5, 99–103. 10.2147/oajsm.s58005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiehser D. M., Delis D. C., Filoteo J. V., Delano-Wood L., Han S. D., Jak A. J., et al. (2011). Are self-reported symptoms of executive dysfunction associated with objective executive function performance following mild to moderate traumatic brain injury? J. Clin. Exp. Neuropsychol. 33, 704–714. 10.1080/13803395.2011.553587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavelle R. M., Strauss D., Whyte J., Day S. M., Yu Y. L. (2001). Long-term causes of death after traumatic brain injury. Am. J. Phys. Med. Rehabil. 80, 510–516; quiz 517–519. 10.1097/00002060-200107000-00009 [DOI] [PubMed] [Google Scholar]
- Sofi F., Valecchi D., Bacci D., Abbate R., Gensini G. F., Casini A., et al. (2011). Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J. Intern. Med. 269, 107–117. 10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Stocchetti N., Zanier E. R. (2016). Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit. Care 20:148. 10.1186/s13054-016-1318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni E. D., Johnson D. K., Morris J. K., van Sciver A., Greer C. S., Billinger S. A., et al. (2015). Dose-Response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial. PLoS One 10:e0131647. 10.1371/journal.pone.0131647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M., Heo S., Prakash R. S., Erickson K. I., Alves H., Chaddock L., et al. (2013). The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervetion. Hum. Brain Mapp. 34, 2972–2985. 10.1002/hbm.22119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D. E. R., Nicol C. W., Bredin S. S. D. (2006). Health benefits of physical activity: the evidence. CMAJ 174, 801–809. 10.1503/cmaj.051351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T. B., Pierce G. L., Darling W. G., Falk D., Magnotta V. A., Voss M. W. (2017). The acute effects of aerobic exercise on the functional connectivity of human brain networks. Brain Plast. 2, 171–190. 10.3233/bpl-160039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C., Engelhard K. (2007). Pathophysiology of traumatic brain injury. Br. J. Anaesth. 99, 4–9. 10.1093/bja/aem131 [DOI] [PubMed] [Google Scholar]
- WHO (2018). WHO | Physical Activity and Adults. World Health Organization; Available online at: http://www.who.int/dietphysicalactivity/factsheet_adults/en/. Accessed April 15, 2018. [Google Scholar]
- WHO (2019a). Global Recommendations on Physical Activity for Health. World Health Organization; Available online at: https://www.who.int/dietphysicalactivity/publications/9789241599979/en/. Accessed May 30, 2019. [Google Scholar]
- WHO (2019b). Physical activity. Available online at: https://www.who.int/news-room/fact-sheets/detail/physical-activity. Accessed January 18, 2019.
- Wise E. K., Hoffman J. M., Powell J. M., Bombardier C. H., Bell K. R. (2012). Benefits of exercise maintenance after traumatic brain injury. Arch. Phys. Med. Rehabil. 93, 1319–1323. 10.1016/j.apmr.2012.05.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher. The datasets generated for this study are available on request to the corresponding author.