Abstract
The efficacy of ibrutinib has been demonstrated in patients with chronic lymphocytic leukemia (CLL), including as first‐line therapy. However, outcomes after ibrutinib discontinuation have previously been limited to higher‐risk populations with relapsed/refractory (R/R) disease. The objective of this study was to evaluate outcomes of ibrutinib‐treated patients based on prior lines of therapy, including after ibrutinib discontinuation. Data were analyzed from two multicenter phase 3 studies of single‐agent ibrutinib: RESONATE (PCYC‐1112) in patients with R/R CLL and RESONATE‐2 (PCYC‐1115) in patients with treatment‐naive (TN) CLL without del(17p). This integrated analysis included 271 ibrutinib‐treated non‐del(17p) patients with CLL (136 TN and 135 R/R). Median progression‐free survival (PFS) was not reached for subgroups with 0 and 1/2 prior therapies but was 40.6 months for patients with ≥3 therapies (median follow‐up: TN, 36 months; R/R, 44 months). Median overall survival (OS) was not reached in any subgroup. Overall response rate (ORR) was 92% in TN and 92% in R/R, with depth of response increasing over time. Adverse events (AEs) and ibrutinib discontinuation due to AEs were similar between patient groups. Most patients (64%) remain on treatment. OS following discontinuation was 9.3 months in R/R patients (median follow‐up 18 months, n = 51) and was not reached in TN patients (median follow‐up 10 months, n = 30). In this integrated analysis, ibrutinib was associated with favorable PFS and OS, and high ORR regardless of prior therapies in patients with CLL. The best outcomes following ibrutinib discontinuation were for patients receiving ibrutinib in earlier lines of therapy.
1. INTRODUCTION
The B‐cell receptor (BCR) signaling pathway has emerged as an important therapeutic target for B‐cell malignancies, including chronic lymphocytic leukemia (CLL).1 Bruton's tyrosine kinase (BTK), a component of signaling via the BCR, plays a role in the survival, proliferation, tissue adhesion, and migration of CLL cells.1, 2, 3, 4, 5 Ibrutinib, a first‐in‐class, once‐daily oral BTK inhibitor, is indicated by the United States Food and Drug Administration and the European Medicines Agency for treating patients with CLL, including del(17p) CLL, and allows for treatment without chemotherapy. Results from the phase 3 RESONATE‐2 study (PCYC‐1115) of ibrutinib versus chlorambucil in treatment‐naive (TN) patients with CLL showed significantly prolonged progression‐free survival (PFS) and overall survival (OS) with ibrutinib.6 In patients with relapsed/refractory (R/R) CLL, the phase 3 RESONATE study (PCYC‐1112) of ibrutinib versus ofatumumab showed superior PFS and OS with ibrutinib.7 Data from RESONATE suggest that outcomes with ibrutinib in the relapsed setting vary by extent of prior therapy; patients treated with ibrutinib after one prior regimen experience significantly longer PFS than patients treated in later lines.8 As BCR signaling inhibitors are only recently available for CLL, and patients discontinue infrequently, few studies have evaluated patient outcomes following cessation of ibrutinib. Recent institutional analyses that included high‐risk, multiply relapsed patients reported poor survival in those who discontinued ibrutinib.9, 10 We conducted an integrated analysis of two phase 3 studies to evaluate outcomes with ibrutinib in CLL based on the number of prior lines of therapy, including after ibrutinib discontinuation.
2. PATIENTS AND METHODS
2.1. Patients, treatment regimen, and clinical end points
Data were analyzed from patients from two multicenter, randomized phase 3 studies of single‐agent ibrutinib: RESONATE‐2 (NCT01722487) in TN patients ≥65 years of age6 and RESONATE (NCT01578707) in patients with CLL treated with ≥1 prior therapy, as previously described.6, 7 RESONATE‐2 excluded patients with del(17p); therefore, this subgroup was also excluded from RESONATE for this analysis to ensure a homogeneous dataset. Patients treated with 1 to 2 prior lines of therapy were combined because the number of patients treated with one prior therapy was small (n = 27).
In both studies, patients on the ibrutinib arm received ibrutinib 420 mg once daily continuously. Patients on the comparator arm received up to 12 cycles of chlorambucil (RESONATE‐2) or 24 weeks of intravenous ofatumumab (RESONATE), and those patients with progression confirmed by an Independent Review Committee were allowed to cross over to ibrutinib.6, 7 Details regarding drug administration have been previously published.6, 7
Clinical end points included PFS, OS, overall response rate (ORR), and safety (grading of severity of adverse events [AEs] based on CTCAE 4.0). In addition, OS post‐discontinuation of ibrutinib and comparator treatments were assessed. PFS and ORR were based on investigator assessment.
Studies were approved by the institutional review boards at each participating institution and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. RESONATE and RESONATE‐2 were registered at www.clinicaltrials.gov as NCT01578707 and NCT01722487.
2.2. Statistical considerations
Patient subgroups were defined according to the number of lines of therapy received before ibrutinib (0, 1‐2, ≥3 prior lines). Descriptive analysis was used to summarize demographics, baseline characteristics, and safety. OS post‐discontinuation was measured from the date of ibrutinib discontinuation to the date of last follow‐up or death. Time‐to‐event end points were estimated using the Kaplan‐Meier method.
3. RESULTS
3.1. Patients
This integrated analysis included 271 ibrutinib‐treated patients: 136 from RESONATE‐2 and 135 from RESONATE (1‐2 prior, 68; ≥3 prior, 67). TN patients were older and had a lower frequency of high‐risk genomic abnormalities, such as del(11q) and complex karyotype relative to the R/R group (Supporting Information Table 1).
3.2. Efficacy
Median follow‐up was 36 months for TN patients and 44 months for R/R patients (44 mo with 1‐2 prior; 44 mo with ≥3 prior). Median PFS was not reached for patients with 0 and 1‐2 prior therapies, and was 40.6 months in patients with ≥3 prior therapies (Figure 1). The differences in PFS between 0 versus ≥3 lines of therapy and 1‐2 versus ≥3 lines of therapy were significant (P < 0.0001 and P = 0.0109, respectively); the difference in PFS between 0 versus 1‐2 lines of therapy was not significant (P = 0.2387). The 36‐month PFS rate for each group was 81%, 74%, and 54%, respectively. In patients with del(11q), median PFS was 38.5 months for patients with ≥3 prior therapies, and was not reached for patients with 0 to 2 prior therapies (data not shown). Median OS was not reached in any subgroup. The difference in OS between 0 versus ≥3 lines of therapy was significant (P = 0.0091); differences in OS between 0 versus 1‐2 lines of therapy and 1‐2 versus ≥3 lines of therapy were not significant (P = 0.2349 and P = 0.2375, respectively). The 36‐month OS rate was 88%, 83%, and 75% for patients with 0, 1‐2, or ≥3 prior therapies, respectively.
Figure 1.
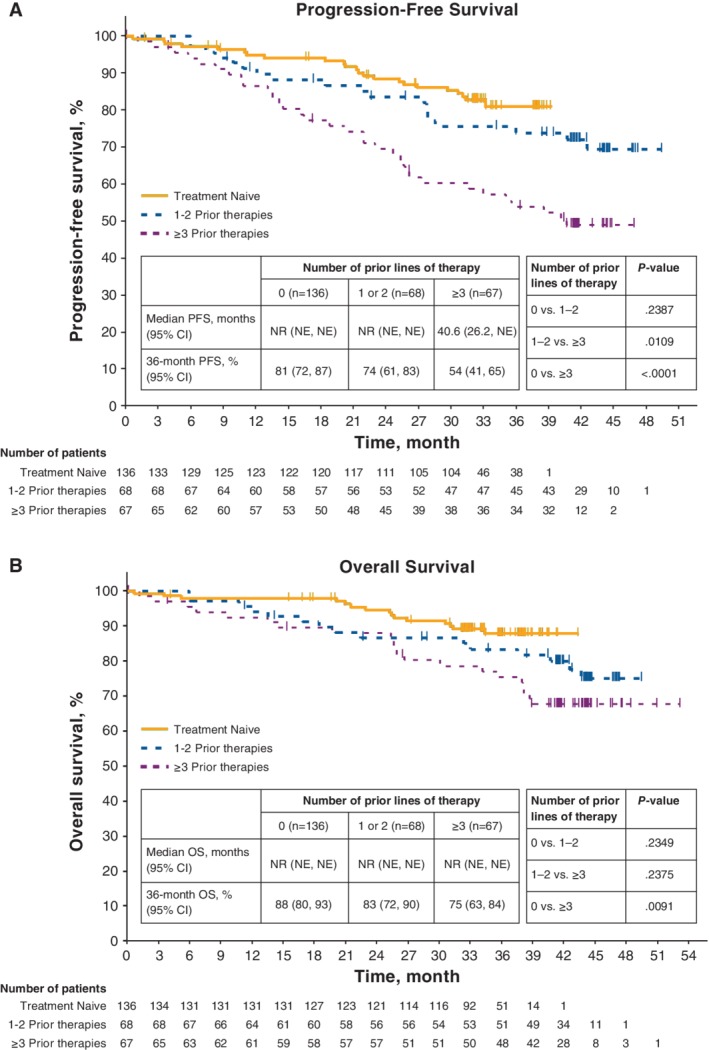
Progression‐free survival and overall survival with ibrutinib by prior lines of therapy. A, Progression‐free survival by investigator assessment. B, Overall survival. Median follow‐up times by subgroups were 36 months for 0 prior and 44 months for 1/2 prior and ≥3 prior. The tick marks indicate patients with censored data. NE, not estimable; NR, not reached; OS, overall survival; PFS, progression‐free survival
The ORR was 92% (complete response [CR], 22%) in TN patients and 92% (CR, 7%) across previously treated subgroups (96% [CR, 6%] for 1‐2 prior treatments and 88% [CR, 7%] for ≥3 prior therapies). Analysis of cumulative response over time showed that response rates (including CRs) increased regardless of lines of therapy (Figure 2), particularly in those who received ibrutinib as their initial treatment. The median time to CR was 20 months (range, 7‐38) for TN patients, 10 months (range, 6‐14) for patients with 1‐2 prior lines, and 17.5 months (range, 5‐36) for patients with ≥3 prior lines of therapy.
Figure 2.
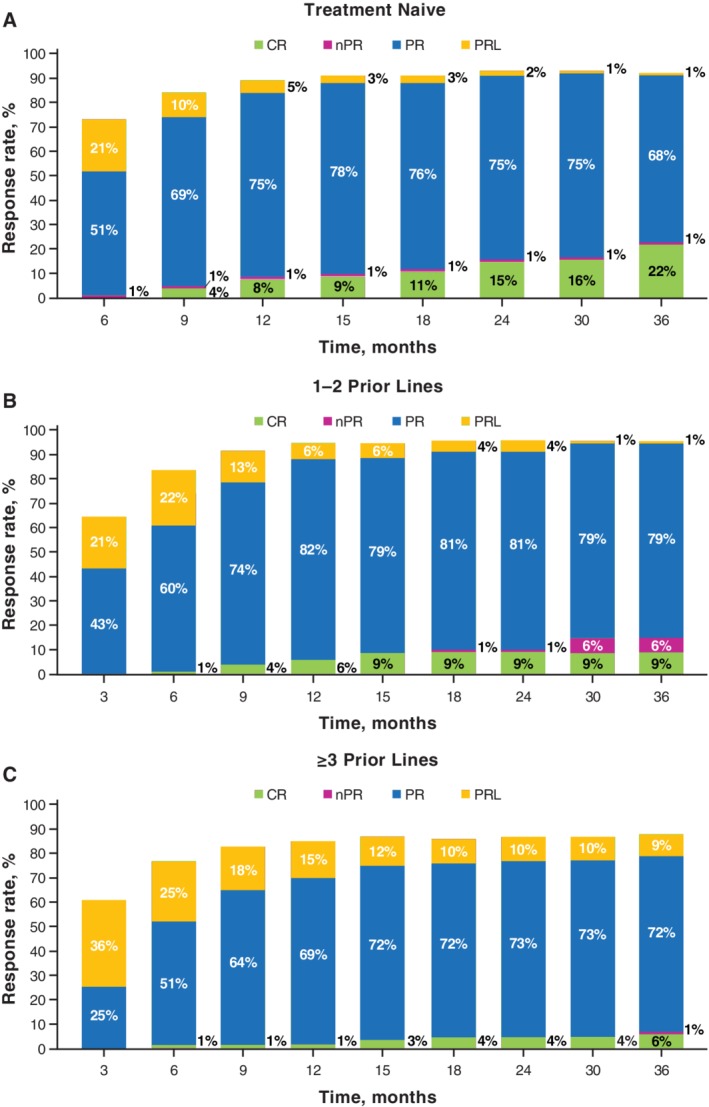
Cumulative best response over time with ibrutinib by prior lines of therapy. Response assessed by investigators by study time points. CR, complete response; PR, partial response; PRL, PR with lymphocytosis; nPR, nodular PR
Most patients with baseline anemia (hemoglobin ≤11 g/dL) or baseline thrombocytopenia (platelet count ≤100 × 109/L) experienced sustained improvement, regardless of previous lines of therapy (Supporting Information Table 2).
3.3. Concomitant hematologic support measures
Patients received hematologic support, including growth factors, transfusions, and intravenous immunoglobulin (IVIG) (Supporting Information Table 3). Rates of neutrophil growth factor use were 10% in the TN subgroup, 28% in patients with 1‐2 prior therapies, and 24% in the ≥3 prior therapies subgroup. Rates of IVIG use were 5% in patients with 0 prior therapies, 24% in the 1‐2 prior therapies subgroup, and 51% in the ≥3 prior therapies subgroup. Red blood cell and platelet transfusions were also more frequent among patients treated with ibrutinib in later lines (Supporting Information Table 3).
3.4. Safety
The rate of common AEs by number of prior lines of therapy is shown in the Supplement for all grade AEs and for ≥3 AEs (Supporting Information Tables 4 and 5). The frequency of AEs generally decreased with time, with AEs in the second and subsequent years of ibrutinib therapy occurring less frequently relative to the first year (Figure 3). Hypertension of any grade occurred more frequently after the first year (8% during first year, 15% in year 2, 20% in year 3, and 19% in years >3) in previously treated patients; more frequent grade ≥3 hypertension events also occurred after the first year (Figure 3B). Fatal AEs occurred in 11 (8%) TN patients and 11 (8%) R/R patients over the 37 months of follow‐up. Fatal AEs included subdural hematoma (n = 1), infections or infectious complications (n = 7), other neoplasms including Richter's transformation (n = 4), myocardial infarction (n = 2), small intestinal obstruction (n = 1), pulmonary fibrosis (n = 1), and other/not specified (n = 6).
Figure 3.
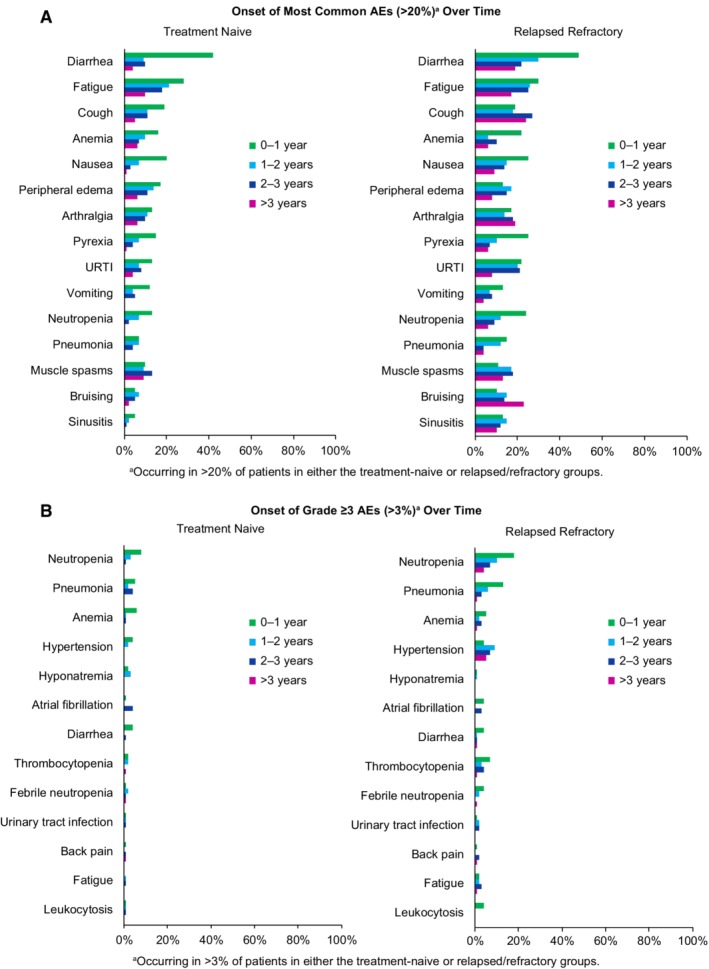
Time‐based analysis of adverse events with ibrutinib treatment. A, Onset of most common AEs (of any grade occurring in ≥20% of patients) by treatment year. B, Onset of most common grade ≥3 AEs (occurring in ≥3% of patients) by treatment year. AEs, adverse events; URTI, upper respiratory tract infection
3.5. Treatment exposure and discontinuation
Median duration of ibrutinib treatment was 36 months (range, 1‐43), 43 months (range, 1‐50), and 39 months (range, 1‐50) in patients with 0, 1‐2, and ≥3 prior treatments, respectively, and the majority of patients continued on ibrutinib (Supporting Information Table 6).
The overall rate of ibrutinib discontinuation was 36%. The main reason for discontinuation was progressive disease (PD) for R/R patients, and was AEs for TN patients (Supporting Information Figure 1). Discontinuation due to Richter's transformation was higher in R/R compared to TN patients. Discontinuation due to AEs was similar for TN and R/R patients and decreased for all patients as treatment duration increased (Supporting Information Table 6). The most common AEs leading to discontinuation (listed as TN or R/R) were infections (4%/5%); malignant, benign, or unspecified neoplasms (2%/5%); nervous system disorders (3%/1%); cardiac disorders (3%/2%); and blood and lymphatic system disorders (1%/2%). The most common AEs leading to dose reductions (in >2 patients) included neutropenia (n = 5; 2%), atrial fibrillation (n = 4; 1%), and anemia, thrombocytopenia, diarrhea, and arthralgia (n = 3; 1% each). Overall, discontinuation due to PD occurred in 32 of 271 patients (12%), including five TN patients (4%; one patient had Richter's transformation) and 27 R/R patients (20%; six patients had Richter's transformation), and occurred less frequently in patients treated with ibrutinib in earlier lines of therapy (Supporting Information Table 6).
3.6. Outcomes following ibrutinib discontinuation
Data from 30 TN and 52 R/R patients were evaluated for outcomes following ibrutinib discontinuation (Table 1). The median OS following discontinuation was not reached for TN patients (n = 30), 9.3 months for patients with 1‐2 prior therapies (n = 22), and 8.9 months for patients with ≥3 prior therapies (n = 30) at median follow‐ups of 10, 15, and 18 months, respectively.
Table 1.
Summary of outcomes following ibrutinib discontinuation
| Subgroups by prior lines of therapy | |||
|---|---|---|---|
| 0 (n = 30) | 1/2 (n = 22) | ≥3 (n = 30) | |
| Median follow‐up, months | 10 | 15 | 18 |
| Median OS,a months (95% CI) | NR (5.9‐NE) | 9.3 (7.8‐22) | 8.9 (4.3‐NE) |
| Median OS post‐DC due to PD, months | 22.7 (0.6‐22.7) [n = 5] | 8.7 (0.5‐16) [n = 8] | 8.9 (2.8‐NE) [n = 19] |
| Median OS post‐DC due to AE, months | NR (5.9‐NE) [n = 22] | 15.5 (6.9‐22) [n = 9] | NR (2.6‐NE) [n = 7] |
Abbreviations: AE, adverse event; DC, discontinuation; NE, not estimable; NR, not reached; OS, overall survival; PD, progressive disease.
Six patients who discontinued study ibrutinib to receive commercial ibrutinib are not included.
For comparison, 117 patients on the comparator arms were evaluated for outcomes following discontinuation of chlorambucil for TN patients and ofatumumab for the R/R subgroup. Median OS following discontinuation was 30.7 months for TN patients, 8 months for patients treated with ≥3 prior therapies, and was not reached in patients with 1‐2 prior therapies (Supporting Information Table 7). When 52 patients who received ibrutinib after crossover were excluded, median OS was 29.4 months for TN patients, versus 2.5 to 2.7 months for patients with ≥1 prior therapy (Supporting Information Table 7).
Data on subsequent therapy following ibrutinib discontinuation were available for nine TN patients and 31 R/R patients (Supporting Information Table 8), with several of these patients receiving multiple subsequent therapies. Data on best responses to the first subsequent therapy after ibrutinib were available for seven TN patients (six PRs following chemotherapy, or chemoimmunotherapy and one SD following radiation therapy).
Patients given ibrutinib as second‐line or third‐line therapy, subsequently received chemoimmunotherapy regimens including rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP), modified R‐CHOP, and rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R‐EPOCH), as well as a range of newer therapies, including idelalisib (with or without rituximab) and venetoclax. Patients given ibrutinib in the fourth line or beyond were subsequently treated with cyclophosphamide‐prednisone chemotherapy; novel therapies, such as BTK inhibitors; or investigational agents under clinical development. For R/R patients, data on best responses to the first subsequent therapy after ibrutinib were available for 26 patients: three CRs, three PRs, and eight SDs were observed, with the other 12 patients having PD. The patients achieving CR received an investigational agent, EPOCH‐R, or doxorubicin, vinblastine, dacarbazine ± bleomycin (AVD ± B); patients with a PR received ofatumumab, cyclophosphamide, doxorubicin, and vincristine (R‐CHO), or venetoclax. The eight patients who had SD received either an anti‐CD20 monoclonal antibody‐based therapy (n = 4), EPOCH (n = 1), or an anti‐CD52 monoclonal antibody‐based regimen (n = 3). The 12 patients who progressed had subsequent therapies that included anti‐CD20 monoclonal antibodies, anti‐CD52 monoclonal antibodies, PI3K inhibitors, or chemotherapy‐based regimens.
4. DISCUSSION
This integrated analysis of two phase 3 studies supports the utilization of ibrutinib as the choice for first‐line therapy and also demonstrates that treatment with ibrutinib does not impair the ability to utilize other active therapies including chemoimmunotherapy or venetoclax, as subsequent lines of therapy. With a median follow‐up of 36 and 44 months for TN and R/R subgroups, respectively, median PFS was not reached for patients with 0 and 1‐2 prior lines of therapy and was 41 months for those with ≥3 prior lines. Median OS was not reached regardless of number of prior lines of therapy. The ORR was high (88%‐96%) for all subgroups with highest CR rates for TN patients, and the proportion of CRs increasing over time. Independent of the number of prior treatments, ibrutinib resulted in sustained improvements in hematologic parameters in most patients with baseline anemia or thrombocytopenia.
AEs with ibrutinib were largely grade 1 to 2, and the safety profile was similar for the TN and R/R subgroups; however, the frequency of AEs differed. For example, grade ≥3 infections and cytopenias occurred less frequently among TN patients compared with R/R patients. These results are consistent with long‐term follow‐up analyses of phase 2 studies of ibrutinib in CLL,11, 12 and are further supported by the infrequent use of growth factors, IVIG, and transfusions among TN patients in this analysis. We also observed that the frequency of most AEs decreased over time. Grade ≥ 3 hypertension occurred more frequently after the first year of ibrutinib among previously treated patients. Although an increase in the prevalence of hypertension would be expected over time in any aging population, the frequency of hypertension was found to be higher with ibrutinib than in the comparator arm in RESONATE‐2.6 Patients in this analysis received ibrutinib for longer durations on average than patients receiving comparator drugs; this may partially explain the higher prevalence of hypertension in the ibrutinib arm.
Discontinuation of ibrutinib due to AEs occurred at a similar rate in the TN patients and R/R subgroups without a specific AE markedly predominant; however, the rate of discontinuations due to AEs among real‐world patients in a retrospective analysis was higher.13 Discontinuation due to PD occurred in 12% of patients, similar to the rate of progression‐related ibrutinib discontinuation previously reported (10%‐12%).9, 10 Patients treated with ibrutinib in earlier lines also tended to have lower rates of progression and experienced better PFS and OS outcomes relative to more heavily treated patients. The risk of progression on ibrutinib has previously been reported to increase with high‐risk factors like del(17p), unmutated IGHV, complex karyotype, or BCL6 abnormalities.9, 10 In our analysis, TN patients tended to have a lower frequency of unmutated IGHV and complex karyotype than patients with R/R disease; data from patients with del(17p) were excluded from our study to more accurately compare the study population in the RESONATE‐2 study. Thus, this analysis cannot evaluate the impact of these higher risk markers.
Among previously treated patients, those receiving ibrutinib in earlier (1‐2 prior) lines of therapy experienced better survival outcomes compared with those receiving ibrutinib in later (≥3 prior) lines of therapy. There was a significant difference in PFS between patients who received 0 versus ≥3 lines of therapy and 1‐2 versus ≥3 lines of therapy; the difference in PFS between patients receiving 0 versus 1‐2 lines of therapy was not significant. These findings are consistent with the results of an integrated analysis of three randomized studies, which included 620 ibrutinib‐treated patients of whom 136 were TN and 66 had received one prior therapy.14 Multivariate analyses showed that patients with one prior therapy had better PFS than patients treated with ibrutinib in later lines. Patients with 0 prior therapies demonstrated longer survival compared with patients with ≥3 prior lines of therapy. This observation is true of most therapies for CLL, and the effect of more prior therapies may be less with ibrutinib than with other therapies. There was no significant difference in OS between patients who received 0 versus 1‐2 or 1‐2 versus 3 lines of therapy. This suggests that patients who have PD on first‐line therapy may be able to receive ibrutinib therapy as second‐line therapy without any adverse consequences.
Moreover, survival following ibrutinib discontinuation was comparable with survival following discontinuation of other single‐agent therapies, as shown in our analysis of OS outcomes in the comparator arms. Although patient numbers are small, those treated after ≥3 prior lines of therapy who discontinued ofatumumab and did not cross over to ibrutinib appeared to have the poorest outcomes with median OS of <3 months.
Analyses of ibrutinib‐treated CLL patients reported poor survival following discontinuation of ibrutinib.9, 10 One single‐institution study reported poor salvageability of 33 patients treated with regimens including chemoimmunotherapy or ofatumumab following ibrutinib discontinuation.9 Another study of pooled data from 308 patients treated with ibrutinib in four clinical studies confirmed the poor prognosis of patients after discontinuation of ibrutinib.10 These analyses also revealed that patients who discontinued because of PD with transformation (Richter's or other) had dismal outcomes with median OS of 2.6 to 3.5 months post‐ibrutinib discontinuation.9, 10 In contrast, median OS ranged from 17.6 months to not reached among patients who discontinued because of PD without transformation.10 Initial reports from these studies indicated that patients who discontinued because of nonrelapse reasons (including AEs) also had a poor prognosis following ibrutinib discontinuation.9, 10 However, these reports primarily included very high‐risk patients with combinations of del(17p), unmutated IGHV, or complex karyotype, who were extensively pretreated, had relapsed early, and/or had exhausted available treatments, including anti‐CD20 therapies, alkylating agents, and purine analogs.9, 10 In addition, few TN patients were included, and follow‐up durations were shorter than in the current study. Subsequently, longer follow‐up of one study indicated that better survival outcomes were observed in patients who discontinued because of treatment‐related toxicity compared with progressive CLL.15 Consistent with these updated findings, in the present analysis, patients who discontinued because of AEs had better survival outcomes than those who discontinued because of PD, particularly in later lines of therapy.
The studies described above were conducted before the availability of venetoclax or idelalisib; recent studies have shown more favorable outcomes when these novel agents were used following discontinuation of ibrutinib.16, 17, 18, 19 A multicenter, retrospective study that evaluated 178 patients following discontinuation of ibrutinib or idelalisib (N = 178) reported an ORR of 50% with an alternative kinase inhibitor, 25% with chemoimmunotherapy, 36% with anti‐CD20 monoclonal antibody, and 76% with BCL‐2 inhibitor therapy.17 Of the 16 patients who received idelalisib, the ORR was 28%.17 Results from a phase 2 study of venetoclax showed an ORR of 70% in a subgroup of 43 patients previously treated with ibrutinib.19 An analysis of patients receiving frontline ibrutinib showed promising results wherein patients responded to chemoimmunotherapy regimens and chlorambucil after discontinuation of ibrutinib.20 In the present analysis, many patients who received therapies following ibrutinib discontinuation remained alive, albeit with short follow‐up. Factors such as number of prior therapies, presence or absence of del(17p), and availability of subsequent therapies, may contribute to the observed differences in post‐ibrutinib salvageability across these various studies. Currently, few patients have progressed on treatment with single‐agent ibrutinib. Evolving data from the RESONATE‐2 study suggest responses with chemoimmunotherapy after treatment with ibrutinib in the first‐line setting is achievable.20
This integrated, retrospective analysis of the randomized RESONATE and RESONATE‐2 studies supports once‐daily treatment with single‐agent ibrutinib leading to high response rates and favorable survival in patients with CLL/SLL, with the best outcomes experienced in those who receive ibrutinib in earlier lines of therapy. Further, the analysis suggests that the best outcomes following discontinuation of ibrutinib were observed in patients who received ibrutinib in the first‐line or second‐/third‐line settings. As follow‐up matures, results of outcomes with subsequent anticancer regimens will allow for a better understanding of salvageability following ibrutinib discontinuation, particularly for those who have not received standard chemoimmunotherapy.
AUTHOR CONTRIBUTIONS
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: S.M. O'Brien, D.F. James, J. Ninomoto.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: S.M. O'Brien, J. Ninomoto, D.F. James.
Critical revision of the manuscript for important intellectual content: J.C. Byrd, P. Hillmen, S. Coutre, J.R. Brown, P.M. Barr, J.C. Barrientos, S. Devereux, T. Robak, N.M. Reddy, T.J. Kipps, A. Tedeschi, F. Cymbalista, P. Ghia, J.A. Burger.
Statistical analysis: S. Chang.
CONFLICT OF INTERESTS
Dr. O'Brien has consulted for and received honoraria from AbbVie, Janssen, and Pharmacyclics LLC, an AbbVie Company; she has also received research funding from Pharmacyclics LLC, an AbbVie company; Dr. Byrd has received research funding from Genentech, Acerta, and Pharmacyclics LLC, an AbbVie Company; Dr. Hillmen has consulted for, received honoraria, and served on the speakers' bureau for AbbVie, Janssen, Gilead, Acerta, and has received research funding from Pharmacyclics LLC, an AbbVie Company, AbbVie, Janssen, Roche, GlaxoSmithKline, and Gilead; Dr. Coutre has consulted for AbbVie, Gilead, Novartis, Celgene, Janssen, and Pharmacyclics LLC, an AbbVie Company, and has received research funding from AbbVie and Pharmacyclics LLC, an AbbVie Company, Gilead, Celgene, and Novartis; Dr. Brown has consulted for Janssen, Gilead, Sun Biopharma, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company, and has received research funding from Gilead and Sun Biopharma; Dr. Barr has consulted for Pharmacyclics LLC, an AbbVie Company, AbbVie, Celgene, Novartis, Seattle Genetics, and Infinity Pharmaceuticals, and has received research funding from Pharmacyclics LLC, an AbbVie Company; Dr. Barrientos has consulted for and received research funding from AbbVie, Gilead, and Pharmacyclics LLC, an AbbVie Company, and has consulted for Janssen; Dr. Devereux has consulted for and received honoraria from Janssen, AbbVie, MSD, and Gilead, and served on the speakers' bureau and received travel accommodations from Janssen and Gilead; Dr. Robak has received research funding from Pharmacyclics LLC, an AbbVie Company; Dr. Reddy has consulted for Celgene, Gilead, AbbVie, and Bristol‐Myers Squibb, and has received research funding from Bristol‐Myers Squibb; Dr. Kipps has consulted for AbbVie, Genentech, and Gilead, and Pharmacyclics LLC, an AbbVie Company, and has received research funding from AbbVie, Genentech, Pharmacyclics LLC, an AbbVie Company, and Oncternal; Dr. Tedeschi has consulted for and received honoraria from Janssen and AbbVie, and has also received honoraria from Gilead and Roche; Dr. Cymbalista has received honoraria from Janssen, Gilead, Abbvie, Sunesis, and Roche, has consulted for AbbVie, has received research funding from Sunesis, and has also received travel accommodations from Roche, Gilead, and Abbvie; Dr. Ghia has consulted for AbbVie, Adaptive, Acerta/AstraZeneca, Gilead, Janssen, and Pharmacyclics LLC, an AbbVie Company, and has received research funding from AbbVie, Gilead, Janssen, and Novartis; he has also served on the speakers' bureau for Gilead; Dr. Chang is employed by Pharmacyclics LLC, an AbbVie Company, and holds stock ownership with AbbVie, Johnson & Johnson, Portola, Abbott, and Ipsen; Dr. Ninomoto is employed with Pharmacyclics LLC, an AbbVie Company, and holds stock ownership with Amgen, Celgene, and AbbVie; Dr. James is employed with Pharmacyclics LLC, an AbbVie Company and her husband is employed with AbbVie; both have equity ownership; she also owns patents/royalties/other intellectual property with AbbVie; Dr. Burger has consulted for and received honoraria and travel accommodations from Gilead, TG Therapeutics, Pharmacyclics LLC, an AbbVie Company, Novartis, and Janssen, and has received research funding from Pharmacyclics LLC, an AbbVie Company.
Supporting information
Supplemental Table 1 Baseline characteristics
Supplemental Table 2. Sustained hematologic improvement in patients with cytopenia
Supplemental Table 3. Summary of concomitant medications of clinical interest
Supplemental Table 4. Most common AEs (≥20% of all patients) by lines of therapy
Supplemental Table 5. Most common grade ≥ 3 AEs (≥3% of all patients) by lines of therapy
Supplemental Table 6. Summary of treatment exposure and discontinuation
Supplemental Table 7. Summary of outcomes following discontinuation in comparator arms
Supplemental Table 8. Summary of patients who received subsequent therapy following ibrutinib discontinuation
Supplemental Figure 1. Reasons for discontinuation of ibrutinib by prior therapy and treatment duration. Six patients who discontinued study ibrutinib to receive commercial ibrutinib are not included. TN, treatment naive; R/R, relapsed refractory.
ACKNOWLEDGMENTS
We thank the patients participating in this study, and their supportive families. Supriya Srinivasan, PhD, funded by Pharmacyclics LLC, an AbbVie Company, wrote the first draft and provided medical writing support in the preparation of this manuscript. PCYC‐1112 was sponsored by Pharmacyclics LLC, an AbbVie Company, and Janssen Pharmaceuticals. PCYC‐1115 was sponsored by Pharmacyclics LLC, an AbbVie Company. Pharmacyclics LLC, an AbbVie Company, was responsible for the study design, data analysis, compilation of data summaries, and confirmation of accuracy of data, and contributed to the data interpretation and writing of the report.
O'Brien SM, Byrd JC, Hillmen P, et al. Outcomes with ibrutinib by line of therapy and post‐ibrutinib discontinuation in patients with chronic lymphocytic leukemia: Phase 3 analysis. Am J Hematol. 2019;94:554–562. 10.1002/ajh.25436
Funding information Pharmacyclics LLC, an AbbVie Company; Janssen Pharmaceuticals
REFERENCES
- 1. de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI‐32765 targets B‐cell receptor‐ and chemokine‐controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590‐2594. [DOI] [PubMed] [Google Scholar]
- 2. Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI‐32765. Blood. 2011;117(23):6287‐6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF‐kappaB signaling and reduces tumor proliferation in tissue‐resident cells of patients with CLL. Blood. 2014;123(21):3286‐3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI‐32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woyach JA, Bojnik E, Ruppert AS, et al. Bruton's tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123(8):1207‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425‐2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown JR, Hillmen P, O'Brien S, et al. Updated efficacy including genetic and clinical subgroup analysis and overall safety in the phase 3 RESONATE trial of ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2014;124(21). Abstract 3331. [Google Scholar]
- 9. Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062‐2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrd JC, Furman RR, Coutre SE, et al. Three‐year follow‐up of treatment‐naive and previously treated patients with CLL and SLL receiving single‐agent ibrutinib. Blood. 2015;125(16):2497‐2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Brien SM, Furman RR, Coutre SE, et al. Five‐year experience with single‐agent ibrutinib in patients with previously untreated and relapsed/refractory chronic lymphocytic leukemia/small lymphocytic leukemia. Blood. 2016;128(22). Abstract 233. [Google Scholar]
- 13. Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 621 ibrutinib‐treated chronic lymphocytic leukemia patients in the United States: a real‐world analysis. Haematologica. 2018;103:874‐879. 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kipps TJ, Fraser C, Coutre SE, et al. Outcomes of ibrutinib‐treated patients with chronic lymphocytic leukemia/small lymphocytic leukemia with high‐risk prognostic factors in an integrated analysis of 3 randomized phase 3 studies. In: XVII International Workshop on Chronic Lymphocytic Leukemia; 2017; New York, New York.
- 15. Jain P, Thompson PA, Keating M, et al. Causes of discontinuation and long‐term outcomes of patients with CLL after discontinuing ibrutinib. Blood. 2016;128(22). Abstract 4390. [Google Scholar]
- 16. Mato A, Nabhan C, Barr PM, et al. Favorable outcomes in CLL pts with alternate kinase inhibitors following ibrutinib or idelalisib discontinuation: results from a large multi‐center study. Blood. 2015;126(23). Abstract 719. [Google Scholar]
- 17. Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18):2199‐2205. [DOI] [PubMed] [Google Scholar]
- 18. Jones J, Mato AR, Coutre S, et al. Preliminary results of a phase 2, open‐label study of venetoclax (ABT‐199/GDC‐0199) monotherapy in patients with chronic lymphocytic leukemia relapsed after or refractory to ibrutinib or idelalisib therapy. Blood. 2015;126(23). Abstract 715. [Google Scholar]
- 19. Jones J, Choi MY, Mato AR, et al. Venetoclax (VEN) monotherapy for patients with chronic lymphocytic leukemia (CLL) who relapsed after or were refractory to ibrutinib or idelalisib. Blood. 2016;128(23). Abstract 637. [Google Scholar]
- 20. Barr PM, Robak T, Owen CJ, et al. Updated efficacy and safety from the phase 3 Resonate‐2 study: ibrutinib as first‐line treatment option in patients 65 years and older with chronic lymphocytic leukemia/small lymphocytic leukemia. Blood. 2016;128(22). Abstract 234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Baseline characteristics
Supplemental Table 2. Sustained hematologic improvement in patients with cytopenia
Supplemental Table 3. Summary of concomitant medications of clinical interest
Supplemental Table 4. Most common AEs (≥20% of all patients) by lines of therapy
Supplemental Table 5. Most common grade ≥ 3 AEs (≥3% of all patients) by lines of therapy
Supplemental Table 6. Summary of treatment exposure and discontinuation
Supplemental Table 7. Summary of outcomes following discontinuation in comparator arms
Supplemental Table 8. Summary of patients who received subsequent therapy following ibrutinib discontinuation
Supplemental Figure 1. Reasons for discontinuation of ibrutinib by prior therapy and treatment duration. Six patients who discontinued study ibrutinib to receive commercial ibrutinib are not included. TN, treatment naive; R/R, relapsed refractory.


