Abstract
Introduction
Incidence of massive transfusion after birth was high in the Netherlands between 2004 and 2006 compared with other high‐income countries. This study investigated incidence, causes, management and outcome of women receiving massive transfusion due to postpartum hemorrhage in the Netherlands in more recent years.
Material and methods
Data for all pregnant women who received eight or more units of packed red blood cells from a gestational age of 20 weeks and within the first 24 hours after childbirth, during 2011 and 2012, were obtained from a nationwide retrospective cohort study, including 61 hospitals with a maternity unit in the Netherlands.
Results
Incidence of massive transfusion due to postpartum hemorrhage decreased to 65 per 100 000 births (95% CI 56‐75) between 2011 and 2012, from 91 per 100 000 births (95% CI 81‐101) between 2004 and 2006, while median blood loss increased from 4500 mL (interquartile range 3250‐6000) to 6000 mL (interquartile range 4500‐8000). Uterine atony remained the leading cause of hemorrhage. Thirty percent (53/176) underwent peripartum hysterectomy between 2011 and 2012, compared with 25% (83/327) between 2004 and 2006. Case fatality rate for women who received massive transfusion due to postpartum hemorrhage was 2.3% (4/176) between 2011 and 2012, compared with 0.9% (3/327) between 2004 and 2006.
Conclusions
The incidence of postpartum hemorrhage with massive transfusion decreased in the Netherlands between both time frames, but remained an important cause of maternal mortality and morbidity, including peripartum hysterectomy. National surveillance of maternal morbidity and mortality due to postpartum hemorrhage through an improved and continuous registration with confidential enquiries may lead to the identification of clear improvements of maternal care.
Keywords: blood transfusion, hysterectomy, morbidity, mortality, postpartum hemorrhage
Abbreviations
- BMI
body mass index
- CI
confidence interval
- IQR
interquartile rage
- LEMMoN
Landelijke studie naar Etnische determinanten van Maternale Morbiditeit in Nederland
- MBRRACE‐UK
Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK
- OR
odds ratio
- TeMpOH
transfusion strategies in women during Major Obstetric Hemorrhage
Key message.
Despite a decreasing incidence, postpartum hemorrhage leading to massive transfusion remained an ongoing burden of maternal morbidity and mortality in the Netherlands. Improved registration of major obstetric hemorrhage with confidential enquiries could lead to better maternal care.
1. INTRODUCTION
Postpartum hemorrhage (PPH) is a serious obstetric complication and a major contributor to maternal morbidity and mortality worldwide.1 Its incidence seems to be increasing in high‐income countries accompanied by increasing rates of severe adverse outcomes.2 In obstetrics, massive transfusion (defined as 8 or more units of packed red blood cells transfused) after birth is associated with high rates of morbidity and hysterectomy.3, 4 Incidence of massive transfusion due to PPH was notably high in the Netherlands between 2004 and 2006 (91 per 100 000 births) compared with the United Kingdom between 2012 and 2013 (23 per 100 000 births) and with the state of New York between 1998 and 2007 (60 per 100 000 births).3, 4, 5
A nationwide study based on the national perinatal database in the Netherlands showed an increased incidence of PPH (defined as ≥ 1000 mL blood loss following the first 24 h after birth) between 2004 and 2013 (from 4.1% to 6.4% of women giving birth), but a decreased incidence of any number of obstetric‐related transfusion of packed red blood cells (from 23% to 3.9% of all women with PPH).6 It is unknown whether the number of women receiving massive transfusion due to PPH followed this same decreasing pattern. Assessing such a pattern and discerning possible differences over time in incidence, causes, management and outcome of PPH leading to massive transfusion could help to evaluate maternity care. Moreover, identifying antepartum risk factors may also raise awareness for women at high risk of receiving massive transfusion after birth.
The aim of this study was to describe incidence, causes, management and outcome of women who received massive transfusion due to PPH in the Netherlands between 2011 and 2012 and compare these with the same parameters previously described in the Netherlands between 2004 and 2006 and with the Dutch general pregnant population of 2012.3
2. MATERIAL AND METHODS
2.1. Study design
We performed a secondary analysis of women who received massive transfusion due to PPH as part of the Transfusion strategies in women during Major Obstetric Hemorrhage study (TeMpOH‐1). TeMpOH‐1 is a nationwide retrospective cohort study in 61 hospitals in the Netherlands (71% of all hospitals in the country at the time) that collected data from women of at least 18 years old, who received 4 units of packed red blood cells or any transfusion of fresh frozen plasma and/or platelets in addition to packed red blood cells because of obstetric hemorrhage (≥ 1000 mL blood loss during pregnancy or during the first 24 h following childbirth) between 1 January 2011 and 1 January 2013.
2.2. Population
For the present analysis, women were selected from the TeMpOH‐1 cohort who had experienced PPH and received massive transfusion at a gestational age of at least 20 weeks. Massive transfusion was defined as 8 or more units of packed red blood cells transfused within the first 24 h after childbirth. All results were compared with our previous observations from the LEMMoN cohort (Landelijke studie naar Etnische determinanten van Maternale Morbiditeit in Nederland) between 2004 and 2006; nationwide statistics obtained from the Netherlands Perinatal Registry (PRN, 2012) were used as national reference values.3, 7, 8
2.3. Data collection
The TeMpOH‐1 study identified eligible women by cross‐referencing data from hospitals’ blood transfusion services with local birth registers in participating hospitals. Trained medical students and research nurses obtained available data from maternity units, operating theaters and intensive care units. We recorded the following parameters: maternal age at time of birth, body mass index (BMI) at beginning of pregnancy, parity, ethnicity (Caucasian/non‐Caucasian), obstetric history (previous cesarean section and/or previous PPH), gestational age, mode of birth (vaginal birth, instrumental vaginal birth, elective cesarean section or emergency cesarean section), induction of labor, multiple pregnancy, preeclampsia in current pregnancy, blood loss (measured by weighing gauzes and by use of a suction system in the operating theater), number of packed red blood cells transfused, cause of hemorrhage (uterine atony, uterine rupture, placental pathology [including retained placenta, placental remnants, placenta previa, abnormally invasive placenta and placental abruption], laceration of the birth canal, uterine inversion and clotting disorder with or without amniotic fluid embolism) and management of hemorrhage (uterotonic agents [oxytocin, sulprostone, ergometrine, misoprostol], non‐uterotonic agents [tranexamic acid], intrauterine balloon tamponade, surgical interventions [B‐Lynch sutures, uterine artery ligation, hysterectomy] and uterine artery embolization). Furthermore, as major PPH can be the result of concurrent causes, we re‐examined all cases of massive transfusion due to PPH within the TeMpOH‐1 cohort, and only included multiple causes for an individual woman if those causes contributed significantly to the bleeding, as was previously done in the LEMMoN study.3 Causes of PPH in women who received massive transfusion were further analyzed by mode of birth and the number of packed red blood cells transfused, using the same cut‐off points described by Green et al4 in the UK: “moderate” (8‐12 units of packed red blood cells), “high” (13‐20 units of packed red blood cells) and “immense” (> 20 units of packed red blood cells). Adverse maternal outcome was defined as the need for hysterectomy, admission to an intensive care unit and/or maternal death.
2.4. Statistical analyses
All statistical analyses were performed with IBM SPSS Statistics (version 22.0, IBM Corp, Armonk, NY, USA). Categorical data were presented as frequencies with percentages and continuous data as medians with the 25th and 75th interquartile ranges (IQR). The association between possible risk factors and occurrence of PPH leading to massive transfusion was analyzed by comparing available characteristics from the TeMpOH‐1 cohort with characteristics of the general pregnant population in 2012, as obtained from the PRN database.8 Given that the PRN database only has summary denominator data, odds ratios (OR) were calculated by means of univariate logistic regression models, resulting in crude odds ratios with 95% CI.8 Women with missing values for a specific parameter were excluded from analyses that required that parameter. Furthermore, the number of births of the TeMpOH‐1 cohort comprised women who gave birth under the guidance of obstetricians, but did not include women with low‐risk pregnancies who had given birth under guidance of their midwives or family physicians (primary care), which represented about 29% of all births in the Netherlands between 2011 and 2012.8, 9 To estimate a population‐based incidence of massive transfusion due to PPH, the number of births in the TeMpOH‐1 study was multiplied by 100/71 to represent all births, including those under guidance of primary care.
2.5. Ethical approval
The TeMpOH‐1 study was approved by the ethics committee of the Leiden University Medical Center on 31 January 2013 (P12.273) and by the institutional review board of each participating hospital. The study was registered in the Netherlands Trial Register (NTR4079). Need to obtain informed consent was waived by the ethics committee.
3. RESULTS
The TeMpOH‐1 source population comprised 270 101 births (including births under guidance of primary care) in the Netherlands during the 2‐year inclusion period. A total of 176 women experienced PPH and received transfusion of 8 or more units of packed red blood cells, making the incidence of massive transfusion due to PPH in the Netherlands 65 per 100 000 births (95% CI 56‐75) (Figure 1).3, 4, 5
Figure 1.
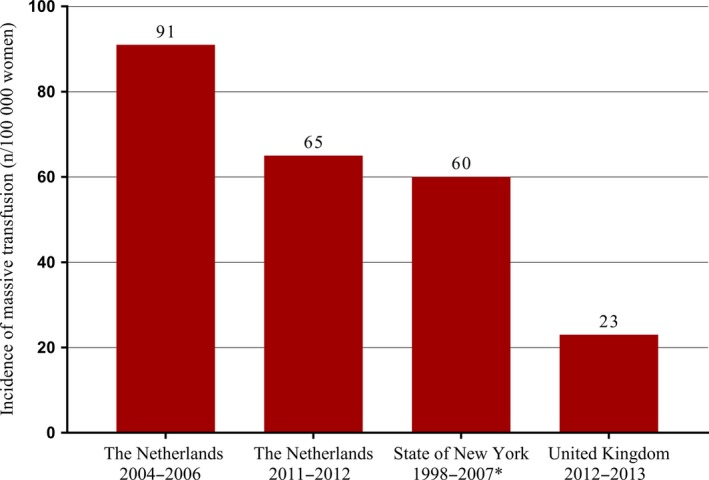
Incidence of women requiring massive transfusion due to postpartum hemorrhage. *Defined massive transfusion as ≥10 packed red cells and included all pregnancy‐realted hemorrhage5 [Color figure can be viewed at wileyonlinelibrary.com]
3.1. Characteristics of women and bleeding
Women who received massive transfusion due to PPH had a median (IQR) age of 32 years (29‐37 years), BMI of 23 kg/m2 (21‐26 kg/m2) and a gestational age of 39 weeks (37‐40 weeks). The characteristics of women, pregnancy and birth are presented in Table 1 and juxtaposed to the characteristics of women who experienced PPH and received massive transfusion between 2004 and 2006 in the Netherlands and to the Dutch general pregnant population in 2012.3, 8, 10, 11 The median (IQR) estimated blood loss was 6000 mL (4500‐8000 mL).
Table 1.
Characteristics of the women, pregnancy and birth
| n (%) | |||
|---|---|---|---|
| 2004‐20063 (n = 327) | 2011‐2012 (n = 176) | General pregnant Dutch population | |
| Age (y) | |||
| < 20 | 0 (0) | 2 (1) | 2257 (1.3)8 |
| 20‐34 | 208 (63) | 114 (65) | 135 406 (78.2)8 |
| 35‐39 | 94 (29) | 44 (25) | 29 562 (17.1)8 |
| ≥ 40 | 25 (8) | 16 (9) | 5860 (3.4)8 |
| BMI (kg/m2) | |||
| < 18.5 | 15 (5) | 6 (3) | N/A (N/A) |
| 18.5‐24.9 | 137 (42) | 84 (48) | N/A (N/A) |
| 25.0‐29.9 | 39 (12) | 30 (17) | N/A (N/A) |
| ≥ 30 | 24 (7) | 10 (6) | N/A (N/A) |
| Missing | 112 (34) | 46 (26) | N/A (N/A) |
| Ethnicity | |||
| Caucasian | N/A (N/A) | 109 (62) | N/A (N/A) |
| Non‐Caucasian | N/A (N/A) | 51 (29) | N/A (N/A) |
| Missing | N/A (N/A) | 16 (9) | N/A (N/A) |
| Parity | |||
| 0 | 158 (48.3) | 82 (47) | 77 647 (44.9)8 |
| 1‐2 | 145 (44.3) | 83 (47) | 93 454 (49.1)8 |
| ≥ 3 | 24 (7.3) | 11 (6) | 1998 (6.0)8 |
| Previous CS | 66 (20) | 45 (26) | 1068 (10.9)10 |
| Previous PPHa | 40 (12) | 15 (9) | N/A (N/A) |
| Gestational age (wk) | |||
| 20‐24 | 6 (2) | 1 (1) | N/A (N/A) |
| 24‐32 | 18 (5) | 10 (6) | N/A (N/A) |
| 32‐37 | 62 (19) | 24 (13) | N/A (N/A) |
| ≥ 37 | 241 (74) | 141 (80) | N/A (N/A) |
| Mode of birth | |||
| Vaginal | 131 (40) | 64 (36) | 131 265 (74.5)8 |
| Instrumental | 43 (13) | 37 (21) | 16 210 (9.2)8 |
| Cesarean section | 151 (46) | 75 (43) | 28 680 (16.3)8 |
| Elective | 46 (14) | 36 (21) | 12 280 (7.1)8 |
| Emergency | 105 (32) | 39 (22) | 16 400 (9.2)8 |
| Induction of labor | 100 (31) | 61 (35) | 37 510 (21.7)8 |
| Multiple pregnancy | 37 (11) | 11 (6) | 2992 (1.7)8 |
| Pre‐eclampsia | 54 (17) | 23 (13) | 31 560 (2.2)11 |
BMI, body mass index; CS, cesarean section; N/A, not available; PPH, postpartum hemorrhage.
Data about previous experienced PPH were missing for 82 women (47%).
3.2. Risk factors and causes of hemorrhage
Women with PPH leading to massive transfusion were more likely to be aged over 35 years (OR 2.01, 95% CI 1.47‐2.74), to have had a previous cesarean section (OR 2.81, 95% CI 2.00‐3.95), to have suffered from preeclampsia (OR 6.71, 95% CI 4.33‐10.41), to have had a multiple pregnancy (OR 3.86, 95% CI 2.10‐7.12), to have had induced labor (OR 1.92, 95% CI 1.41‐2.62), to have had an instrumental vaginal birth (OR 2.63, 95% CI 1.83‐3.78), or had an elective (OR 3.37, 95% CI 2.34‐4.86) or emergency (OR 2.82, 95% CI 1.97‐4.02) cesarean birth. In Figure 2 the proportion of causes of PPH that led to massive transfusion in 2004‐2006 and in 2011‐2012 in the Netherlands are compared.3 The commonest cause of major PPH remained uterine atony, followed by retained placenta and placenta previa. Compared with 2004‐2006, it appears that a larger proportion of women between 2011 and 2012 sustained major PPH due to placenta previa (17% [29/176] vs 11% [37/327]), abnormally invasive placenta (13% [22/176] vs 10% [32/327]) and uterine rupture (11% [19/176] vs 6% [20/327]).3 For 67 women (38%), 2 causes were registered with uterine atony and placental remnants (n = 12) being the commonest combination. The most frequent combination of women with 3 causes (n = 12) was uterine atony with placental remnants and laceration of the birth canal (n = 5). The “other causes” as mentioned in Figure 2 were primary clotting disorder (n = 4), amniotic fluid embolism (n = 1), uterine inversion (n = 1) and liver capsule rupture with uterine atony (n = 1). Categorizing the top three causes of PPH with massive transfusion according to mode of birth showed no noticeable differences over time with placenta previa (22/36; 61%) remaining the commonest cause during elective cesarean section and uterine atony in other modes of birth (see Supplementary material, Table S1). Uterine atony also remained a leading cause when causes were grouped by the total units of packed red blood cells transfused: “moderate” (8‐12 units, n = 113), “high” (13‐20 units, n = 49) and “immense” (≥ 20 units, n = 14).
Figure 2.
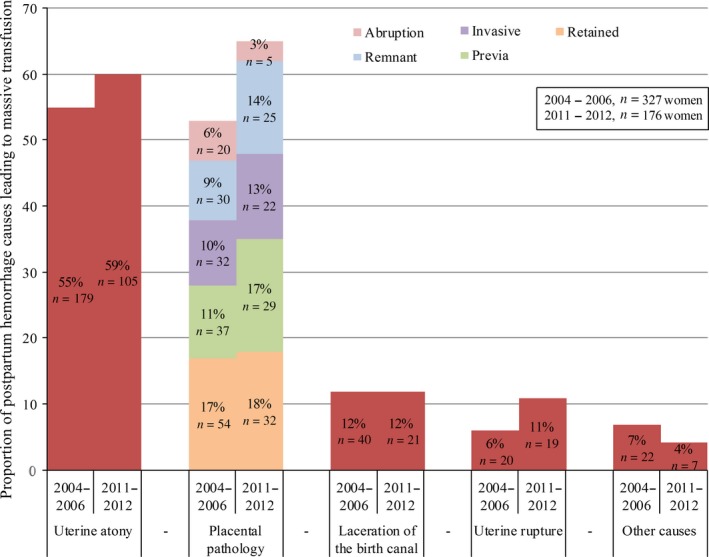
Proportion of causes leading to postpartum hemorrhage with massive transfusion [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Management of PPH
Median (IQR) number of units of packed red blood cells transfused was 11 (9‐16; see Supplementary material, Figure S1). Distribution of obstetric interventions per cause of hemorrhage in 2004‐2006 and in 2011‐2012 are summarized in Table 2.3 Compared with 2004‐2006, it seems that proportionally more women received sulprostone between 2011 and 2012 (82% vs 70%), and sulprostone became the most frequent uterotonic drug administered during PPH leading to massive transfusion.3 Misoprostol (34% vs 11%) and tranexamic acid (74% vs 22%) were seemingly administered more often as well, while it appears that ergometrine (10% vs 18%) and (non‐prophylactic) oxytocin (64% vs 87%) were used less frequently over time.3 Thirty‐six women with uterine atony did not receive postpartum oxytocin infusion and of those who gave birth vaginally (n = 12), all received sulprostone. Of those women without postpartum oxytocin infusion and who gave birth by cesarean section (n = 24), 17 received sulprostone. Among these 24 women, uterine atony co‐occurred frequently with placenta previa (n = 8), uterine rupture (n = 6) and abnormally invasive placenta (n = 4), and the hysterectomy rate was high (10/24; 42%). Furthermore, it appears that proportionally more women between 2011 and 2012 received intrauterine balloon tamponade (56% vs 23% between 2004 and 2006), B‐Lynch suture (14% vs 2% between 2004 and 2006) and embolization of uterine arteries (48% vs 22% between 2004 and 2006).3 Hysterectomy rate among all women receiving massive transfusion due to PPH was allegedly higher in 2011‐2012 compared with 2004‐2006 (30% [53/176] vs 25% [83/327]),3 with highest rates among women who endured bleeding due to abnormally invasive placenta (n = 18/22), placenta previa (n = 20/29) and uterine rupture (n = 12/19).
Table 2.
Distribution of obstetric interventions by cause of postpartum hemorrhage
| Uterine atony | Uterine rupture | Placenta previa | Invasive placenta | Placental abruption | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004‐20063 (n = 179) | 2011‐2012 (n = 105) | 2004‐20063 (n = 20) | 2011‐2012 (n = 19) | 2004‐20063 (N = 37) | 2011‐2012 (n = 29) | 2004‐20063 (N = 32) | 2011‐2012 (n = 22) | 2004‐20063 (N = 20) | 2011‐2012 (n = 5) | 2004‐20063 (n = 327) | 2011‐2012 (n = 176) | |
| Oxytocina | 94% (n = 168) | 66% (n = 69) | 70% (n = 14) | 47% (n = 9) | 87% (n = 32) | 38% (n = 11) | 91% (n = 29) | 55% (n = 12) | 70% (n = 14) | 40% (n = 2) | 84% (n = 275) | 64% (n = 112) |
| Sulprostone | 87% (n = 156) | 88% (n = 92) | 50% (n = 10) | 84% (n = 16) | 54% (n = 20) | 66% (n = 19) | 72% (n = 23) | 73% (n = 16) | 55% (n = 11) | 40% (n = 2) | 70% (n = 228) | 82% (n = 145) |
| Tranexamic acid | 33% (n = 59) | 71% (n = 74) | 5% (n = 1) | 74% (n = 14) | 19% (n = 7) | 62% (n = 18) | 13% (n = 4) | 77% (n = 17) | 5% (n = 1) | 60% (n = 3) | 22% (n = 72) | 74% (n = 130) |
| Ergometrine | 23% (n = 41) | 12% (n = 12) | 15% (n = 3) | 11% (n = 2) | 14% (n = 5) | 7% (n = 2) | 19% (n = 6) | 9% (n = 2) | 20% (n = 4) | 0% (n = 0) | 18% (n = 60) | 10% (n = 18) |
| Misoprostol | 16% (n = 29) | 37% (n = 39) | 5% (n = 1) | 37% (n = 7) | 3% (n = 1) | 21% (n = 6) | 3% (n = 1) | 18% (n = 4) | 20% (n = 4) | 20% (n = 1) | 11% (n = 36) | 34% (n = 60) |
| Intrauterine balloon | 32% (n = 58) | 59% (n = 62) | 10% (n = 2) | 16% (n = 3) | 14% (n = 5) | 45% (n = 13) | 28% (n = 9) | 36% (n = 8) | 5% (n = 1) | 20% (n = 1) | 23% (n = 75) | 56% (n = 99) |
| Uterine artery ligation | 6% (n = 10) | 7% (n = 7) | 5% (n = 1) | 5% (n = 1) | 8% (n = 3) | 3% (n = 1) | 3% (n = 1) | 9% (n = 2) | 5% (n = 1) | 0% (n = 0) | 5% (n = 17) | 5% (n = 9) |
| Uterine artery embolization | 29% (n = 52) | 49% (n = 51) | 10% (n = 2) | 37% (n = 7) | 19% (n = 7) | 35% (n = 10) | 19% (n = 6) | 32% (n = 7) | 5% (n = 1) | 40% (n = 2) | 22% (n = 71) | 48% (n = 84) |
| B‐Lynch suture | 5% (n = 8) | 18% (n = 19) | 0% (n = 0) | 11% (n = 2) | 3% (n = 1) | 21% (n = 6) | 3% (n = 1) | 9% (n = 2) | 10% (n = 2) | 0% (n = 0) | 2% (n = 8) | 14% (n = 25) |
| Hysterectomy | 27% (n = 48) | 24% (n = 25) | 70% (n = 14) | 63% (n = 12) | 38% (n = 14) | 69% (n = 20) | 66% (n = 21) | 82% (n = 18) | 5% (n = 1) | 0% (n = 0) | 25% (n = 83) | 30% (n = 53) |
Prophylactic oxytocin after childbirth excluded.
3.4. Adverse maternal outcome
Of all women, 146 (83%) were admitted to an intensive care unit and 53 (30%) underwent hysterectomy as a last resort to stop bleeding. Four women died (three due to exsanguination caused by uterine atony and one due to liver capsule rupture accompanied by uterine atony), of whom two died after hysterectomy. Case fatality rate of PPH with massive transfusion was one in 44 women (2.27%) and case fatality rate of women who underwent peripartum hysterectomy due to major PPH was two in 53 women (3.77%).
4. DISCUSSION
The incidence of massive transfusion due to PPH decreased in the Netherlands from 91 per 100 000 births (95% CI 81‐101) between 2004 and 2006 to 65 per 100 000 births (95% CI 56‐75) between 2011 and 2012, while median (IQR) blood loss increased from 4500 mL (3250‐6000 mL) to 6000 mL (4500‐8000 mL).3 The leading cause of PPH with massive transfusion remained uterine atony. Sulprostone was the most commonly administered uterotonic agent and almost one‐third (30%) underwent peripartum hysterectomy with the highest rates among women with abnormally invasive placenta, placenta previa and uterine rupture. Case fatality rate was 0.9% (3/327) between 2004 and 2006 vs 2.27% (4/176) between 2011 and 2012.3
Key strengths of this study are that our results are based on a nationwide cohort study (the 71% of the Dutch obstetric units that participated in the TeMpOH‐1 were a representative distribution of all obstetric units in the Netherlands at the time, thereby reflecting the general pregnant population in the Netherlands) and that we included all women with PPH who received massive transfusion by cross‐referencing data from hospitals’ blood transfusion services with birth registers in local participating hospitals. Additionally, using the same inclusion criteria and definition of massive transfusion as our previous cohort and the study conducted in the UK, results are directly comparable.3, 4 However, in applying eight or more units of packed red blood cells as definition of massive transfusion, it must be noted that these numbers can be influenced by clinical decision making. There is still no definite consensus in the literature as to which definition of massive transfusion should be applied.12, 13, 14 Furthermore, number of births used as denominator for calculation of incidence of massive transfusion due to PPH was estimated by multiplying the number of hospital births in the TeMpOH‐1 study by the proportion of births under guidance of primary care. However, since coverage of the PRN registry was about 99% of all births in the Netherlands and all women with hemorrhage receiving massive transfusion will eventually have been referred to a maternity unit, we believe that this estimate of incidence is reliable.8
The decreased incidence of PPH leading to massive transfusion along with an observed increase in median blood loss reflects the more restrictive blood transfusion policy in the Netherlands over time.6 This gradual change followed, among other things, after the introduction of the “4‐5‐6 rule” (depending on the presence of comorbidity, the threshold for transfusion of packed cells varies between 4.0 mmol/L [6.5 g/dL] and 6.0 mmol/L [9.7 g/dL]) in the Dutch national guideline on blood transfusion in 2004, the implementation of which probably took time before the effect on the number of blood transfusions became visible.15 At the same time, the finding that proportionally more women appear to have undergone peripartum hysterectomy and uterine artery embolization in the recent time‐frame could either reflect a larger proportion of high‐risk pregnancies due to more restrictive blood transfusion policy, or more aggressive management of PPH. Nonetheless, the incidence of massive transfusion due to PPH in the Netherlands between 2011 and 2012 became comparable to the state of New York between 1998 and 2007 (notwithstanding the higher threshold of 10 or more units of packed cells applied to define massive transfusion in that setting). The incidence, however, remained considerably higher than in the UK between 2012 and 2013 (23 per 100 000 births, 95% CI 19‐26), even though median blood loss in women receiving massive transfusion due to PPH was similar between the UK and the Netherlands.4, 5
Of the women receiving massive transfusion after birth in the Netherlands between 2011 and 2012, there appears to be an increase in the proportion of women who experienced hemorrhage due to placenta previa, abnormally invasive placenta and uterine rupture, and it seems that proportionally more women underwent hysterectomy to control hemorrhage. Although the cesarean section rate remained relatively stable, around 16%, in the Netherlands,8, 16 these seemingly increased proportions are partly explained by the fact that a larger proportion of women with a prior cesarean birth were present in the TeMpOH‐1 cohort (26% compared with 20% between 2004 and 2006).3 This is known to be associated with increased risks of placenta previa, abnormally invasive placenta and uterine rupture during subsequent pregnancies.17, 18, 19 Furthermore, studies showed that the risk of peripartum hysterectomy increased with the number of previous cesarean births and in the presence of an abnormally invasive placenta.20, 21, 22 Other countries have reported increasing previous cesarean birth rates with an increasing trend in the incidence of placenta previa, abnormally invasive placenta and uterine rupture, which could suggest that more countries experience the high burden of hysterectomy due to PPH with massive transfusion.23, 24, 25
Nevertheless, the hysterectomy rate in the the Netherlands remained substantially lower than in the UK, where the overall rate was 45%.4 This difference could be explained by the lower rates of women with previous cesarean births in the Netherlands (26% vs 40% in the UK) and higher rates of embolization (48% vs 16% in the UK), which may avert hysterectomy in most cases.4 The national guideline from the Netherlands Society of Obstetrics and Gynecology states that in case of ongoing PPH, embolization and/or surgical interventions should not be postponed, while the national guideline in the UK made by Royal College of Obstetricians and Gynaecologists specifically recommends to “resort to hysterectomy sooner rather than later” without explicitly mentioning the option of trying embolization first.26, 27 This difference could lead to a more restrictive policy of peripartum hysterectomy in the Netherlands.
Furthermore, the incidence of PPH also depends on prevalence of risk factors in the population. Our findings confirm previous observations that increased maternal age (≥ 35 years), previous cesarean birth, multiple pregnancy, pre‐eclampsia, labor induction, and instrumental or cesarean birth are associated with major PPH.5, 11, 28, 29 These antepartum risk factors were only present in a certain number of women enduring major PPH. Considering that uterine atony remained the leading cause of PPH that required massive transfusion, demonstrates that all obstetric caregivers should acknowledge the possible severity of uterine atony despite the absence of risk factors. In this respect, the substantial decrease of oxytocin use among women with PPH with massive transfusion due to uterine atony is striking, considering that the Dutch national guideline specifically recommends oxytocin as the uterotonic agent of first choice.26 Although our findings rely on the accuracy of data entered into the TeMpOH‐1 database, such a decrease is worrying and should be reported to all obstetric caregivers by emphasizing the importance of oxytocin in the Dutch national guideline on the management of PPH, which may reduce the need to resort to massive transfusion after childbirth.
Despite all changes over time, the maternal mortality ratio in women with PPH leading to massive transfusion in the Netherlands was 1.48 deaths per 100 000 live births between 2011 and 2012 vs 0.84 deaths per 100 000 live births between 2004 and 2006.3 These results should be viewed with caution, given the substantial uncertainty surrounding these estimates due to the small number of deaths. However, the maternal mortality ratio of PPH requiring massive transfusion was considerably higher than reported in the UK between 2012 and 2013 (0.23 deaths per 100 000 live births),4 and this finding is worrying and merits closer analysis. Our findings are of utmost importance to other high‐income countries, where similar patterns may have occurred. Maternal mortality has become a very rare outcome in these countries, which hampers comparisons over time and between settings. Our findings should encourage researchers in other high‐income settings to critically evaluate clinical management and maternal mortality due to major PPH.
National surveillance of maternal morbidity and mortality due to PPH through improved continuous registration with confidential enquiries and multidisciplinary simulation training of PPH‐related emergencies could improve the quality of maternal care. The MBRRACE‐UK reports (Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK) are likely to have contributed to a steady fall of obstetric hemorrhage‐related deaths in the UK and provide a rational framework for how national surveillance may be applied by other countries.30, 31 Nevertheless, the escalating rates of PPH in other high‐income countries emphasize the importance of nationwide studies into obstetric hemorrhage‐related maternal morbidity and mortality.29, 32, 33 International comparison of data regarding PPH that led to massive transfusion could reveal variations in management and outcome between countries, and consequently lead to improvements in maternal care. Collaboration in networks such as the International Network of Obstetric Survey Systems could help to facilitate population‐based studies.34
5. CONCLUSION
Incidence of PPH leading to massive transfusion decreased in the Netherlands, but continues to be an important cause of maternal morbidity and mortality. Improved continuous registration of major obstetric hemorrhage with confidential enquiries to identify substandard factors could lead towards better maternal care and may prevent morbidity and mortality from major PPH in the Netherlands. International comparison of our findings could provide high‐quality evidence for the best management practices of major PPH.
CONFLICT OF INTERESTS
No competing interests were reported by all authors.
Supporting information
ACKNOWLEDGMENTS
We thank all 61 participating hospitals, the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynecology—Netherlands Society of Obstetrics and Gynecologists Consortium 2.0, medical students R.M. Loeff, R.J. van Goeverden, B. Eijlers, A. Hillebrand, S.E. Spelmin, T.J. Beunder, V. Harskamp, M. Wind, M.D. Koning, R.A. Cramer, A. Veenstra, S.M. Smith and E.E. Ensing; data managers C. Caram‐Deelder, A. Pors, C.J. van Brussel‐de Groot and O. Zouitni; and research nurses C. Kolster‐Bijdevaate, M.S. Bourgonje‐Verhart, C.E. Bleeker Taborh and E. Roos‐van Milligen for their contributions to the TeMpOH‐1 study. Thanks to the TeMpOH‐1 Study Group for its participation in this study; a complete membership list appears in the Supplementary material (Appendix S1).
Ramler PI, van den Akker T, Henriquez DDCA, et al. Women receiving massive transfusion due to postpartum hemorrhage: A comparison over time between two nationwide cohort studies. Acta Obstet Gynecol Scand. 2019;98:795–804. 10.1111/aogs.13542
Contributor Information
Johanna G. van der Bom, Email: j.g.van_der_bom@lumc.nl.
the TeMpOH‐1 study group:
R.M. Loeff, R.J. van Goeverden, B. Eijlers, A. Hillebrand, S.E. Spelmin, T.J. Beunder, V. Harskamp, M. Wind, M.D Koning, R.A. Cramer, A. Veenstra, S.M. Smith, E.E. Ensing, C. Caram‐Deelder, A. Pors, C.J. van Brussel‐de Groot, O. Zouitni, C. Kolster‐Bijdevaate, M.S Bourgonje‐Verhart, C.E. Bleeker Taborh, and E. Roos‐van Milligen
REFERENCES
- 1. Kassebaum NJ, Bertozzi‐Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):980‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramler PI, van den Akker T, Henriquez D, Zwart JJ, van Roosmalen J. Incidence, management and outcome of women requiring massive transfusion after childbirth in the Netherlands: secondary analysis of a nationwide cohort study between 2004 and 2006. BMC Pregnancy Childbirth. 2017;17(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green L, Knight M, Seeney FM, et al. The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: a national cross‐sectional study. BJOG. 2016;123(13):2164‐2170. [DOI] [PubMed] [Google Scholar]
- 5. Mhyre JM, Shilkrut A, Kuklina EV, et al. Massive blood transfusion during hospitalization for delivery in New York State, 1998‐2007. Obstet Gynecol. 2013;122(6):1288‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Stralen G, von Schmidt Auf Altenstadt JF, Bloemenkamp KW, van Roosmalen J, Hukkelhoven CW. Increasing incidence of postpartum hemorrhage: the Dutch piece of the puzzle. Acta Obstet Gynecol Scand. 2016;95(10):1104‐1110. [DOI] [PubMed] [Google Scholar]
- 7. Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population‐based study of 371,000 pregnancies. BJOG. 2008;115(7):842‐850. [DOI] [PubMed] [Google Scholar]
- 8. Stichting Perinatale Registratie Nederland . Perinatale Zorg in Nederland 2012. Utrecht: Stichting Perinatale Registratie Nederland; 2013. https://assets.perined.nl/docs/41346c24-0a52-4a26-9544-7cb3b7d78cc0.PDF. Accessed June 4, 2018. [Google Scholar]
- 9. Stichting Perinatale Registratie Nederland . Perinatale Zorg in Nederland 2011. Utrecht: Stichting Perinatale Registratie Nederland; 2013. https://assets.perined.nl/docs/ec7434e3-c899-4008-8a04-b3068f285b86.PDF. Accessed June 4, 2018. [Google Scholar]
- 10. Schoorel E, Melman S, van Kuijk S, et al.; on behalf of the SIMPLE study project group . 326: Mode of delivery after previous cesarean section in the Netherlands. AJOG. 2012;206(1):S154. [Google Scholar]
- 11. von Schmidt auf Altenstadt JF, Hukkelhoven CW, van Roosmalen J, Bloemenkamp KW. Pre‐eclampsia increases the risk of postpartum haemorrhage: a nationwide cohort study in the Netherlands. PLoS ONE. 2013;8(12):e81959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharpe JP, Weinberg JA, Magnotti LJ, Croce MA, Fabian TC. Toward a better definition of massive transfusion: focus on the interval of hemorrhage control. J Trauma Acute Care Surg. 2012;73(6):1553‐1557. [DOI] [PubMed] [Google Scholar]
- 13. Mitra B, Cameron PA, Gruen RL, Mori A, Fitzgerald M, Street A. The definition of massive transfusion in trauma: a critical variable in examining evidence for resuscitation. Eur J Emerg Med. 2011;18(3):137‐142. [DOI] [PubMed] [Google Scholar]
- 14. Pham HP, Shaz BH. Update on massive transfusion. Br J Anaesth. 2013;111(suppl 1):i71‐i82. [DOI] [PubMed] [Google Scholar]
- 15. The Dutch Institute for Healthcare Improvement (CBO) . Blood Transfusion Guideline (2004). Utrecht, the Netherlands: van Zuiden Communication B.V.; 2004. [Google Scholar]
- 16. Stichting Perinatale Registratie Nederland . Perinatale Zorg in Nederland 2005. Utrecht: Stichting Perinatale Registratie Nederland; 2008. https://assets.perined.nl/docs/2f0a701a-d8f3-4cbd-98a1-2c4e588cf435.pdf. Accessed June 4, 2018. [Google Scholar]
- 17. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226‐1232. [DOI] [PubMed] [Google Scholar]
- 18. Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta‐analysis of observational studies. J Matern Fetal Neonatal Med. 2003;13(3):175‐190. [DOI] [PubMed] [Google Scholar]
- 19. Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am J Obstet Gynecol. 2011;205(3):262.e1‐262.e8. [DOI] [PubMed] [Google Scholar]
- 20. van den Akker T, Brobbel C, Dekkers OM, Bloemenkamp KW. Prevalence, indications, risk indicators, and outcomes of emergency peripartum hysterectomy worldwide: a systematic review and meta‐analysis. Obstet Gynecol. 2016;128(6):1281‐1294. [DOI] [PubMed] [Google Scholar]
- 21. Rossi AC, Lee RH, Chmait RH. Emergency postpartum hysterectomy for uncontrolled postpartum bleeding: a systematic review. Obstet Gynecol. 2010;115(3):637‐644. [DOI] [PubMed] [Google Scholar]
- 22. de la Cruz CZ, Thompson EL, O'Rourke K, Nembhard WN. Cesarean section and the risk of emergency peripartum hysterectomy in high‐income countries: a systematic review. Arch Gynecol Obstet. 2015;292(6):1201‐1215. [DOI] [PubMed] [Google Scholar]
- 23. Al‐Zirqi I, Stray‐Pedersen B, Forsen L, Daltveit AK, Vangen S. Uterine rupture: trends over 40 years. BJOG. 2016;123(5):780‐787. [DOI] [PubMed] [Google Scholar]
- 24. Roberts CL, Algert CS, Warrendorf J, Olive EC, Morris JM, Ford JB. Trends and recurrence of placenta praevia: a population‐based study. Aust N Z J Obstet Gynaecol. 2012;52(5):483‐486. [DOI] [PubMed] [Google Scholar]
- 25. Guleria K, Gupta B, Agarwal S, Suneja A, Vaid N, Jain S. Abnormally invasive placenta: changing trends in diagnosis and management. Acta Obstet Gynecol Scand. 2013;92(4):461‐464. [DOI] [PubMed] [Google Scholar]
- 26. Nederlanse Vereniging voor Obstetrie en Gynaecologie . Haemorrhagia postpartum (HPP). Arnhem: NVOG; 2013. https://www.nvog.nl/wp-content/uploads/2018/02/Hemorrhagia-postpartum-HPP-3.0-14-11-2013.pdf. Accessed May 18, 2018. [Google Scholar]
- 27. Mavrides E, Allard S, Chandraharan E, et al.; on behalf of the Royal College of Obstetricians and Gynaecologists . Prevention and management of postpartum haemorrhage: Green‐Top Guideline No. 52. BJOG. 2017;124(5):e106‐e149. [DOI] [PubMed] [Google Scholar]
- 28. Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449.e1‐449.e7. [DOI] [PubMed] [Google Scholar]
- 29. Marocchini M, Lauferon J, Quantin C, Sagot P. Postpartum hemorrhage with transfusion: trends, near misses, risk factors and management at the scale of a perinatal network. J Gynecol Obstet Hum Reprod. 2017;46(5):455‐460. [DOI] [PubMed] [Google Scholar]
- 30. Knight M, Nair M, Tuffnell D, et al. (Eds.) on behalf of MBBRACE‐UK . Saving Lives, Improving Mothers’ Care – Surveillance of maternal deaths in the UK 2012‐14 and lessons learned to inform maternity care in the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009‐14. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2016. [Google Scholar]
- 31. Kerr RS, Weeks AD. Lessons from 150 years of UK maternal hemorrhage deaths. Acta Obstet Gynecol Scand. 2015;94(6):664‐668. [DOI] [PubMed] [Google Scholar]
- 32. Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994‐2006. Am J Obstet Gynecol. 2010;202(4):353e1‐353e6. [DOI] [PubMed] [Google Scholar]
- 33. Patterson JA, Roberts CL, Bowen JR, et al. Blood transfusion during pregnancy, birth, and the postnatal period. Obstet Gynecol. 2014;123(1):126‐133. [DOI] [PubMed] [Google Scholar]
- 34. Knight M; INOSS . The International Network of Obstetric Survey Systems (INOSS): benefits of multi‐country studies of severe and uncommon maternal morbidities. Acta Obstet Gynecol Scand. 2014;93(2):127‐131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


