ABSTRACT
Objectives
Congenital heart disease (CHD) has been associated with reduced fetal head circumference (HC), although the underlying pathophysiology remains undetermined. We aimed to define trends in fetal growth and cerebroplacental Doppler flow, and to investigate their relationship, in fetuses with CHD.
Methods
This was a retrospective study in two fetal medicine units in The Netherlands. We included all fetuses with CHD in whom Doppler flow patterns (middle cerebral artery (MCA) pulsatility index (PI), umbilical artery (UA) PI and cerebroplacental ratio (CPR)) and biometry (HC and abdominal circumference (AC)) had been measured serially after 19 weeks' gestation between January 2010 and November 2016. Fetuses were categorized into three groups based on the expected cerebral arterial oxygen saturation of their particular type of CHD: normal; mild to moderately reduced; severely reduced. Trends over time in Z‐scores were analyzed using a linear mixed‐effects model.
Results
A total of 181 fetuses fulfilled the inclusion criteria. Expected cerebral arterial oxygen saturation in CHD was classified as normal in 44 cases, mild to moderately reduced in 84 and severely reduced in 53. In the cohort overall, average trends over time were significant for both HC and AC Z‐scores. HC Z‐scores showed a tendency to decrease until 23 weeks, then to increase until 33 weeks, followed by another decrease in the late third trimester. AC Z‐scores increased progressively with advancing gestation. MCA‐PI and UA‐PI Z‐scores showed significant trends throughout pregnancy, but CPR Z‐scores did not. There were no associations between expected cerebral arterial oxygen saturation and fetal growth. Average trends in MCA‐PI Z‐scores were significantly different between the three subgroups, whereas those in UA‐PI Z‐scores and in CPR Z‐scores were similar between the subgroups. There was no significant association between MCA‐PI and HC Z‐scores.
Conclusions
Fetal biometry and Doppler flow patterns are within normal range in fetuses with CHD, but show trends over time. Head growth in fetuses with CHD is not associated with cerebral blood flow pattern or placental function and HC is not influenced by the cerebral arterial oxygen saturation. © 2018 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of the International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: congenital heart disease, Doppler flow patterns, growth, head circumference, oxygenation
INTRODUCTION
A number of abnormal extracardiac findings have been described in fetuses with congenital heart disease (CHD). They are often small‐for‐gestational age, they have smaller head circumference (HC) and they show signs of abnormal magnetic resonance imaging (MRI), such as smaller brain volume, delayed development and white‐matter injuries, increasing the risk of neurodevelopmental deficiencies1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12. Regarding their growth impairment, Rosenthal12 and Rudolph13, 14 proposed two underlying pathophysiological mechanisms: either fetuses with intrinsic growth disturbances are more at risk for developmental errors during cardiogenesis, or CHD might lead to circulatory alterations (such as retrograde flow in the aortic arch), low cerebral blood flow and reduced cerebral oxygen or energy substrate delivery, which are incompatible with optimal growth.
Doppler flow patterns reflecting circulatory alterations in fetuses with CHD have been observed: a lower middle cerebral artery (MCA) pulsatility index (PI), a higher umbilical artery (UA) PI and a lower cerebroplacental ratio (CPR) have been reported in these fetuses compared with healthy ones1 , 2 , 4, 5, 6, 7 , 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25. However, the precise relationship between circulatory alterations and fetal growth, particularly of HC and, by implication, brain development, remains unclear. Only a few studies have assessed the relationship between prenatal Doppler flow patterns and fetal growth in CHD fetuses and they were unable to demonstrate a clear association. However, these studies used a single Doppler measurement in a heterogeneous cohort of CHD fetuses2, 5, 7.
The association between fetal growth and Doppler flow patterns may be influenced by gestational age1, 2 and by the nature of the cardiac defect1, 5, 6, 16, 19, 21. Our aim, therefore, was to define trends in fetal growth and cerebroplacental Doppler flow and to investigate the relationship between fetal head growth and cerebroplacental flow in fetuses with CHD classified according to the expected cerebral arterial oxygen saturation.
METHODS
Study population
This was a retrospective collaborative study in two fetal medicine units in The Netherlands (University Medical Center Groningen and Academic Medical Center Amsterdam). All fetuses with CHD in whom Doppler flow patterns and biometry had been measured serially after 19 weeks' gestation between January 2010 and November 2016 were included. Fetuses with chromosomal and genetic abnormalities or extracardiac malformations were excluded.
Study design
As part of routine clinical care, a fetal medicine expert performed fetal biometry and assessed Doppler flow, including HC, abdominal circumference (AC), MCA‐PI and UA‐PI. CPR was calculated as MCA‐PI divided by UA‐PI. All available measurements of HC, AC, MCA‐PI and UA‐PI were retrieved starting from 19 weeks' gestation, which is the usual referral time after the routine 19–21‐week scan. All fetal measurements were converted into Z‐scores to adjust for differences in gestational age based on previously published normative data26, 27, 28, 29. In addition, at the first examination following referral, a complete fetal echocardiogram, as well as measurement of the PI of the uterine arteries (UtA‐PI), was performed by a fetal medicine expert and/or an experienced pediatric cardiologist using a standardized protocol to assess fetal cardiac anatomy and function. All cardiac diagnoses were reviewed by a single pediatric cardiologist (S.C.). Postnatal echocardiographic examinations and surgical reports were reviewed for those fetuses which were liveborn and postmortem reports were reviewed in cases of termination of pregnancy or intrauterine fetal demise. Cases with unknown pregnancy outcome were included only if fetal echocardiographic examination was sufficient to confirm the CHD diagnosis. Additional information collected from the maternal and neonatal medical files included gestational age at birth, HC at birth, Apgar score at 5 min and outcome (liveborn, intrauterine fetal demise, termination of pregnancy or neonatal/infant death). We also collected information on maternal complications (e.g. CHD, diabetes, hypertensive disorder, hypothyroidism) and maternal body mass index.
Classification of congenital heart disease
Each fetus was categorized by a pediatric cardiologist into one of three groups according to the expected cerebral arterial oxygen saturation for the particular type of CHD. Much of our knowledge of the fetal circulation is derived from studies performed in fetal lambs30. Subsequent human fetal ultrasound studies have shown that blood flow patterns are similar in human and lamb fetuses13. Human fetal MRI studies are currently expanding our knowledge of oxygen delivery to the brain and oxygen consumption in normal hearts and in CHD, but precise knowledge of cerebral arterial oxygen saturation in fetal CHD is lacking11, 31. CHD may cause abnormal blood volume and flow patterns, altered chamber and great‐vessel sizes and positions, altered oxygen delivery to organs and increased venous pressure. Blood flow in the ductus venosus and arterial duct is also important. All of these factors were taken into consideration when defining three CHD categories based on the expected cerebral arterial oxygen saturation: expected normal cerebral arterial oxygen saturation, expected mild to moderately reduced cerebral arterial oxygen saturation and expected severely reduced cerebral arterial oxygen saturation. Figure 1 shows the hemodynamics and saturation levels, as reported by Rudolph13, 14, 30, of three examples of CHD allocated to each of these three groups. Fetuses with different degrees of left ventricular outflow tract obstruction sometimes fell into different categories depending on the intracardiac anatomy and degree of obstruction. In mild aortic stenosis, a normal level of cerebral arterial oxygen saturation is expected. In critical aortic stenosis, retrograde flow over the aortic arch occurs, reducing the cerebral arterial oxygen saturation, hence its allocation to the group with expected mild to moderately reduced cerebral arterial oxygen saturation. Hypoplastic left heart syndrome (HLHS), in which the entire cerebral blood flow may be dependent on retrograde flow in the aortic arch, was allocated to the group with expected severely reduced cerebral arterial oxygen saturation.
Figure 1.
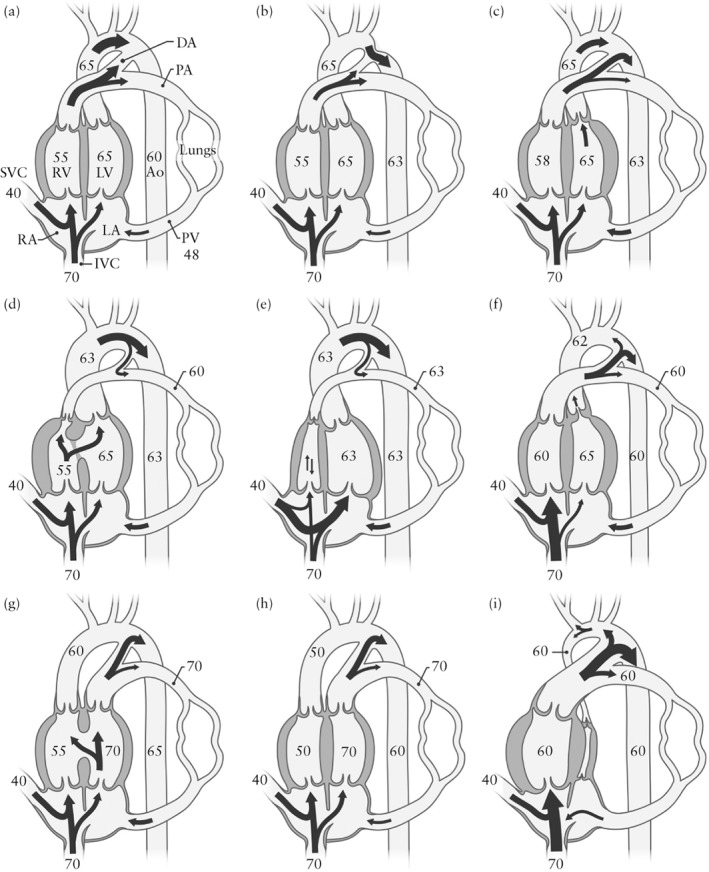
Classification of fetal congenital heart defects according to expected cerebral arterial oxygen saturation, with three examples from each category (adapted from Rudolph)13, 14, 30. Numbers are saturations (in percent) based on lamb experiments; values may be lower in human fetuses. Arrows indicate flow hemodynamics. In (a–c) are examples of those classified as having expected normal cerebral arterial oxygen saturation: (a) normal heart; (b) coarctation of the aorta; (c) mild to moderate aortic stenosis. In (d–f) are those classified as having expected mild to moderately reduced cerebral arterial oxygen saturation: (d) tetralogy of Fallot; (e) pulmonary atresia with intact septum; (f) critical aortic stenosis. In (g–i) are those classified as having expected severely reduced cerebral arterial oxygen saturation: (g) transposition of the great arteries (TGA) with ventricular septal defect; (h) TGA with intact interventricular septum; (i) hypoplastic left heart syndrome (aortic and mitral atresia). Ao, descending aorta; DA, arterial duct; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PA, pulmonary artery; PV, pulmonary veins; RA, right atrium; RV, right ventricle; SVC, superior vena cava.
Sensitivity analyses
In the MRI study of Sun et al.11, the mean aortic oxygen saturation in human fetuses with CHD was lower than that in normal hearts. Furthermore, there were differences in aortic saturation between different types of CHD. Fetuses with tetralogy of Fallot (ToF) had the highest ascending aortic saturation levels of the cyanotic CHDs, followed by transposition of the great arteries (TGA) (including both TGA with intact ventricular septum (IVS) and ventricular septal defect) and single‐ventricle lesions (predominantly HLHS and tricuspid atresia). With this in mind, we repeated the analysis, further subdividing the intermediate (mild to moderately reduced) group into two subgroups, with HLHS reallocated from the severely reduced to the moderately reduced group and only cases of TGA with IVS allocated to the severely reduced group (Table S1).
As Jansen et al.32 reported that fetuses with ToF had a smaller HC compared with reference values, starting from 20 weeks' gestation onwards, we also repeated the analyses separately for fetuses with ToF. We performed two different analyses, one with three subgroups (normal, mild to moderately reduced, severely reduced) plus ToF and one with four subgroups (normal, mildly reduced, moderately reduced, severely reduced) plus ToF.
Statistical analysis
All data were entered into an IBM SPSS statistics database (version 23.0, IBM Corp., Armonk, NY, USA). Study population characteristics were described as frequencies (percentage) for categorical variables, mean (± SD) for continuous parameters with an approximately symmetric distribution and median (interquartile range) for continuous data with a skewed distribution. Average trends over time for fetal growth (HC and AC Z‐scores) and Doppler flow (MCA‐PI, UA‐PI and CPR Z‐scores) were estimated using a linear mixed‐effects model and analyzed with statistical software R (version 3.4.0, Foundation for Statistical Computing, Vienna, Austria). A linear mixed‐effects model takes into account repeat measurements over time and allows the number and timing of the measurements to vary per fetus. To avoid selection bias, all fetuses with at least one measurement were included in the analyses33. Average age trends (‘fixed effects’) were allowed to differ by CHD classification and were modeled by restricted cubic splines. The restricted cubic spline function allowed us to explore the effect of age without making restrictive assumptions about the shape of the time trends. Knots were placed at five fixed quintiles of the predictor's distribution, as suggested by Stone and Koo34. Fetuses in the study population were considered to be a random sample of the total fetal CHD population. Therefore, we allowed the intercept (i.e. value at birth) and slope to differ per fetus, and assumed these parameters to follow a multivariate normal distribution (‘random effects’). In addition, specific time intervals were tested based on the averaged trend. This enabled analysis of the difference in MCA‐PI Z‐score trends over time between fetuses with a normal HC and those with an abnormal HC (i.e. HC < –2.0 SD or > 2.0 SD) at the last measurement before birth in the total cohort and in the three different CHD categories. It also enabled the analysis of the difference in MCA‐PI trends over time between fetuses with a normal HC/AC ratio and those with an abnormal HC/AC ratio (abnormal HC/AC ratio: < 5th percentile or > 95th percentile). Sampling uncertainty was quantified via 95% CI and P‐values. P < 0.05 was considered to be statistically significant.
RESULTS
Patient characteristics
Included in the study were 181 fetuses with CHD, of which 164 had known outcome. Of these, 144 were liveborn and 122 survived the first 3 months. Maternal characteristics and pregnancy outcome are presented in Table 1. Six (3%) fetuses suffered an intrauterine death, 13 (7%) pregnancies were terminated and 22 (12%) infants died during the neonatal period or within the first 3 months after birth. Cardiac diagnosis was confirmed by postnatal echocardiographic examination or surgical reports in 156 (86%) cases, by postmortem reports in seven (4%) cases and by fetal echocardiographic examination in 18 (10%) cases. Forty‐four fetuses were allocated to the group with expected normal cerebral arterial oxygen saturation, 84 to the group with expected mild to moderately reduced cerebral oxygen saturation and 53 to the group with expected severely reduced cerebral oxygen saturation (Table 2). In total, 745 ultrasound examinations were performed in the 181 CHD fetuses. The median number of ultrasound observations per fetus was three, with an interquartile range of two–four and a maximum of 16 observations. Mean (± SD) UtA‐PI Z‐scores were –0.44 (± 1.87) for the left and –0.75 (± 2.14) for the right UtA. Fifteen percent of the fetuses had an abnormal UtA‐PI Z‐score (> 2.0).
Table 1.
Maternal characteristics and pregnancy outcome for study population of 181 cases of fetal congenital heart disease (CHD)
| Characteristic | Value |
|---|---|
| Maternal | |
| Body mass index at first examination (kg/m2) | 24.8 ± 4.6 |
| Smoker | 17 (9) |
| Comorbidity | |
| None | 151 (83) |
| CHD | 2 (1) |
| Diabetes | 6 (3) |
| Hypothyroidism | 1 (1) |
| Other* | 9 (5) |
| Unknown | 12 (7) |
| Nulliparous | 61 (34) |
| Fetal | |
| Outcome | |
| Survived† | 122 (68) |
| IUFD | 6 (3) |
| TOP | 13 (7) |
| NND/ID | 22 (12) |
| Unknown | 18 (10) |
| Gestational age at birth (weeks)‡ | 37.5 ± 4.32 |
| Birth weight (g)§ | 3055 ± 802 |
| Head circumference at birth (cm)¶ | 33.4 ± 3.16 |
| 5‐min Apgar score** | 9 ± 1.27 |
Data are presented as mean ± SD or n (%).
Hyperthyroidism, pituitary gland pathology, congenital hepatic fibrosis, migraine.
Beyond 3 months of age.
n = 148.
n = 142.
n = 93.
n = 126.
IUFD, intrauterine fetal demise; NND/ID, neonatal/infant death; TOP, termination of pregnancy.
Table 2.
Classification of congenital heart disease (CHD) according to expected cerebral arterial oxygen saturation in study population of 181 fetuses
| Type of CHD | n |
|---|---|
| Expected normal cerebral arterial oxygen saturation | 44 |
| AVSD (1 with left isomerism, 1 with ASD) | 10 |
| Isolated VSD/VSDs | 13 |
| VSD + ASD (1 with absent ductus venosus) | 2 |
| Moderate AS (1 with PS) | 2 |
| CoA | 6 |
| PS and dysplastic pulmonary valve | 5 |
| Tricuspid valve abnormalities (antegrade flow over aortic arch) | 2 |
| Double discordance with Ebstein's anomaly (antegrade flow over aortic arch) | 1 |
| Mitral stenosis including small LV (antegrade flow over aortic arch) | 3 |
| Expected mild to moderately reduced cerebral arterial oxygen saturation | 84 |
| AS (critical, retrograde flow over aortic arch) | 1 |
| CoA with VSD (with or without AS or hypoplastic aortic arch) | 10 |
| Interrupted aortic arch with VSD | 2 |
| Ebstein's anomaly (retrograde flow in arterial duct (with or without PS/PA)) (1 with small VSD) | 5 |
| PS with VSD (n = 1)/tetralogy of Fallot (n = 14)/DORV with PS (n = 4) | 19 |
| PA with VSD | 4 |
| Atrioventricular discordance with DORV and CoA | 1 |
| Atrioventricular concordance with mal/transposed great arteries and PS | 4 |
| Atrioventricular concordance with mal/transposed great arteries and/or mitral hypoplasia and/or CoA, no PS or Taussig–Bing | 4 |
| Transposition of the great arteries with PS and mitral stenosis | 1 |
| Common arterial trunk (1 with AVSD) | 7 |
| PA with IVS | 5 |
| TA (with or without mal/transposition of the great arteries, hypoplastic aortic arch, PS or DORV) (1 with infracardiac TAPVD) | 7 |
| Hypoplastic right heart (TA or tricuspid stenosis with PA or critical PS with IVS) | 6 |
| Double inlet left ventricle (with or without mal/transposition of the great arteries with hypoplastic aortic arch or PA) (1 with AVSD and right isomerism) | 7 |
| Complex: right atrial isomerism with unbalanced AVSD, criss‐cross atrioventricular junction, PA with solitary outlet (Ao) from RV and PAPVC | 1 |
| Expected severely reduced cerebral arterial oxygen saturation | 53 |
| HLHS (aortic atresia with or without mitral atresia/stenosis) | 16 |
| Transposition of the great arteries with VSD | 10 |
| DORV with transposition of the great arteries (Taussig–Bing) with or without hypoplastic aortic arch | 3 |
| Transposition of the great arteries with IVS | 24 |
AS, aortic stenosis; ASD, atrial septal defect; AVSD, atrioventricular septal defect; CoA, coarctation of the aorta; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; IVS, intact ventricular septum; LV, left ventricle; PA, pulmonary atresia; PAPVC, partial anomalous pulmonary venous connection; PS, pulmonary stenosis; RV, right ventricle; TA, tricuspid atresia; TAPVD, total anomalous pulmonary venous drainage; VSD, ventricular septal defect.
Trends in fetal biometry and Doppler flow
Entire cohort
The study included 611 HC, 632 AC, 396 MCA‐PI, 465 UA‐PI and 362 CPR measurements. UtA‐PI was assessed in 86 (48%) cases. All fetuses had at least two HC measurements and 60% had at least two MCA‐PI measurements. There were no differences in baseline characteristics between fetuses with one and fetuses with more than one MCA‐PI measurement. The average trends in Z‐scores of fetal biometry (HC and AC) and Doppler flow (MCA‐PI, UA‐PI and CPR) for the entire cohort are shown in Figure 2. Average trends over time were significant for both HC (P < 0.0001) and AC (P < 0.0001) Z‐scores. The HC Z‐scores decreased from 20 weeks until 23 weeks (P = 0.005), then increased thereafter until 33 weeks (P < 0.0001). After 33 weeks, HC Z‐scores decreased again. The AC Z‐scores increased progressively during gestation. Doppler flow patterns showed a statistically significant average trend for MCA‐PI (P = 0.010) and UA‐PI (P < 0.0001) Z‐scores, but not for CPR (P = 0.161) Z‐scores. The Z‐scores of MCA‐PI showed a slight increase between 25 and 30 weeks of gestation, although this trend was not statistically significant (P = 0.102), and a decrease after 30 weeks until approximately 35 weeks (P = 0.001). Irrespective of the observed trends, the averaged trend lines of all parameters fell within normal ranges (Z‐scores > –2.00 and < 2.00).
Figure 2.
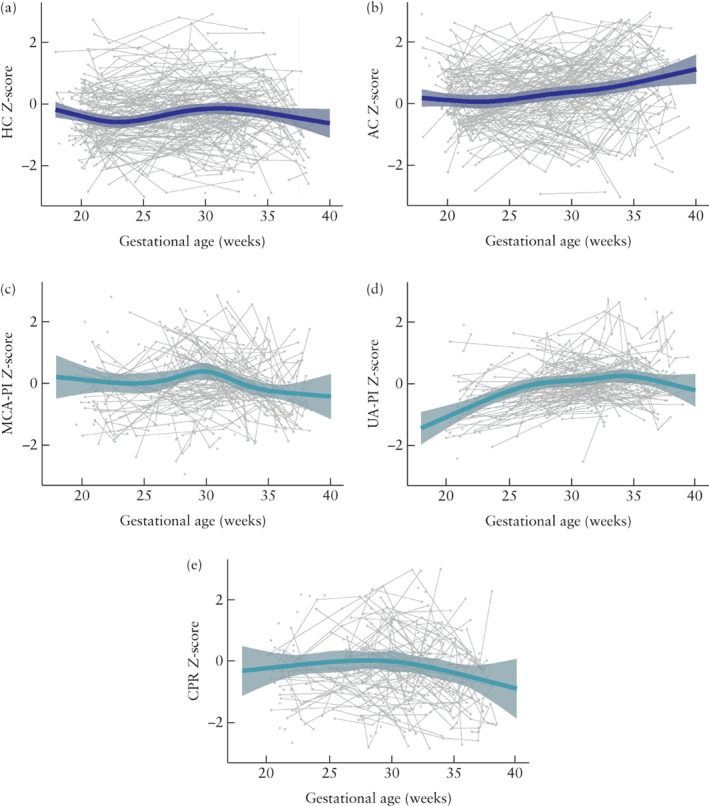
Averaged trends in Z‐scores of fetal growth and Doppler flow patterns in fetuses with congenital heart disease. (a) Head circumference (HC); (b) abdominal circumference (AC); (c) middle cerebral artery pulsatility index (MCA‐PI); (d) umbilical artery pulsatility index (UA‐PI); (e) cerebroplacental ratio (CPR). Gray dots are individual measurements, gray lines are individual trends and dark gray shading shows 95% CIs for fitted model.
Subgroups
In Figure 3, the average trends in Z‐scores are shown for the three CHD groups categorized according to the expected cerebral arterial oxygen saturation. There were no statistically significant differences in average trends among the CHD groups for HC (P = 0.884) or for AC (P = 0.879) Z‐scores. There were also no differences in the average trends among the groups for UA‐PI (P = 0.530) or for CPR (P = 0.285) Z‐scores. The average trends in MCA‐PI Z‐scores were significantly different between the three subgroups (P = 0.010): fetuses with an expected mild to moderately reduced or severely reduced cerebral arterial oxygen saturation level showed relatively more fluctuations in MCA‐PI Z‐scores (P = 0.019 and P < 0.0001, respectively) throughout pregnancy, while fetuses with an expected normal cerebral arterial oxygen saturation level showed no trend in MCA‐PI Z‐scores (P = 0.831).
Figure 3.
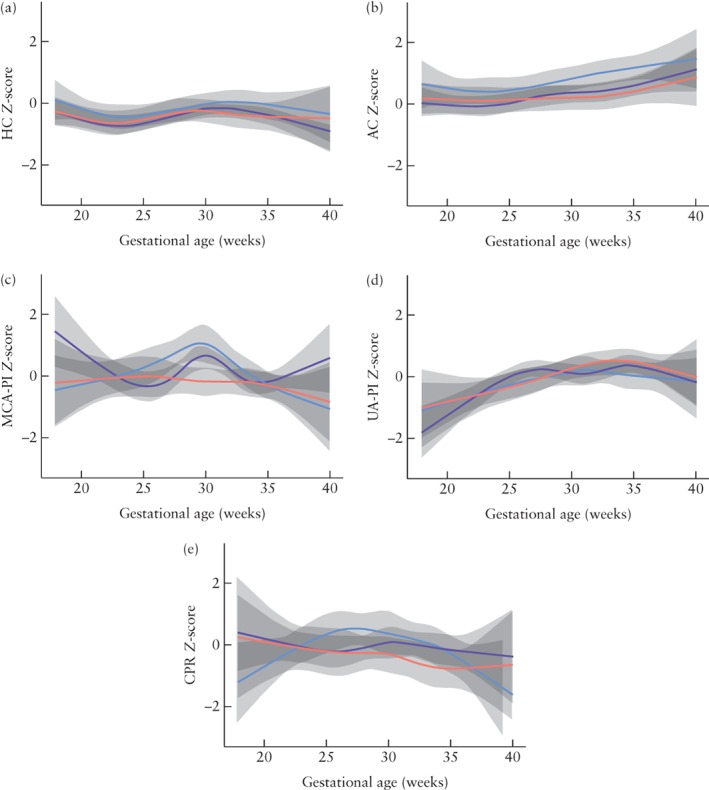
Averaged trends in Z‐scores of fetal growth and Doppler flow patterns in three subgroups of fetuses with congenital heart disease, classified according to whether cerebral arterial oxygen saturation would be expected to be normal ( ), mild to moderately reduced (
), mild to moderately reduced ( ) or severely reduced (
) or severely reduced ( ). 95% CIs of fitted model are shaded gray. (a) Head circumference (HC); (b) abdominal circumference (AC); (c) middle cerebral artery pulsatility index (MCA‐PI); (d) umbilical artery pulsatility index (UA‐PI); (e) cerebroplacental ratio (CPR).
). 95% CIs of fitted model are shaded gray. (a) Head circumference (HC); (b) abdominal circumference (AC); (c) middle cerebral artery pulsatility index (MCA‐PI); (d) umbilical artery pulsatility index (UA‐PI); (e) cerebroplacental ratio (CPR).
Trends in MCA‐PI according to HC before birth
Figure 4 shows the average trend in MCA‐PI Z‐scores in fetuses with normal and those with abnormal HC Z‐scores at the last measurement, both for the total cohort and for the three CHD subgroups. Nineteen (10%) fetuses with various types of CHD had an abnormal HC Z‐score at the last measurement before birth. There was no statistically significant difference in the average trend of MCA‐PI Z‐scores between fetuses with normal HC and those with abnormal HC for the total cohort (P = 0.284) or for the CHD subgroups (P = 0.363). There was also no difference in the average trend of MCA‐PI Z‐scores between fetuses with normal and those with abnormal HC/AC ratio (P = 0.505).
Figure 4.
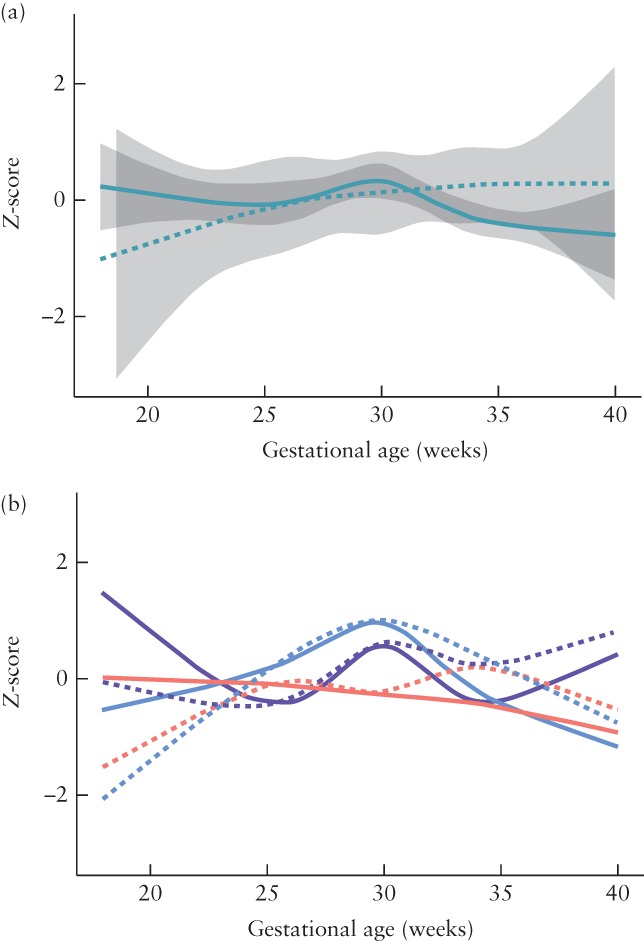
Averaged trends in Z‐score of middle cerebral artery pulsatility index (MCA‐PI) for normal head circumference (HC) (solid lines) and for abnormal HC (dashed lines) at last measurement before birth in fetuses with congenital heart disease (CHD): (a) entire cohort; (b) three subgroups according to whether CHD type would be expected to have normal ( ), mild to moderately reduced (
), mild to moderately reduced ( ) or severely reduced (
) or severely reduced ( ) cerebral arterial oxygen saturation. 95% CIs of fitted model are shaded gray for total cohort only; those for CHD subgoups were broad and overlapping.
) cerebral arterial oxygen saturation. 95% CIs of fitted model are shaded gray for total cohort only; those for CHD subgoups were broad and overlapping.
Sensitivity analyses
The average trends in Z‐scores of fetal biometry and Doppler flow patterns from the repeated analysis using four different subgroups (normal, mildly reduced, moderately reduced and severely reduced cerebral arterial oxygen saturation) are shown in Figure S1. The average trends for HC (P = 0.656), AC (P = 0.707) and CPR (P = 0.513) Z‐scores did not differ between the four subgroups, whereas trends in MCA‐PI and UA‐PI Z‐scores were significantly different between the four subgroups (P = 0.028 and P = 0.020, respectively). When ToF was analyzed separately from the three subgroups (normal, mild to moderately reduced and severely reduced cerebral arterial oxygen saturation), similar trends in Z‐scores of fetal biometry and Doppler flow patterns were observed (Figure S2). The average trends for HC (P = 0.950), AC (P = 0.701) and CPR (P = 0.152) Z‐scores did not differ between the subgroups, while the average trends for MCA‐PI (P = 0.024) and UA‐PI (P < 0.0001) Z‐scores were significantly different between the subgroups. When ToF cases were analyzed separately from the four subgroups (normal, mildly reduced, moderately reduced and severely reduced cerebral arterial oxygen saturation), the results were also similar, with the exception that the MCA‐PI Z‐score trend (P = 0.073) was no longer significant (Figure S3).
DISCUSSION
We found that, although HC, AC, MCA‐PI, UA‐PI and CPR remained within normal range in fetuses with CHD, there were significant trends over time in these variables, with the exception of CPR. Trends in MCA‐PI also differed between fetuses grouped according to their expected cerebral arterial oxygen saturation (normal, mild to moderately reduced or severely reduced), while trends in fetal growth did not differ between these subgroups. Fetuses with CHD with expected mild to moderately reduced or severely reduced cerebral arterial oxygen saturation showed more fluctuations in MCA‐PI Z‐scores throughout pregnancy than did those with expected normal oxygen saturation levels. Furthermore, we found no significant associations between MCA‐PI and HC Z‐scores in fetuses with various types of CHD, subdivided according to the expected cerebral arterial oxygen saturation.
It has been suggested that, in fetuses with CHD, there is preferential blood redistribution to the brain, in order to guarantee an optimal cerebral oxygen supply, as happens in cases of fetal hypoxemia due to placental insufficiency1,2,4–7,15–25. Most of these studies used a single Doppler measurement, made during the second or third trimester4–7,15–25. We were unable to confirm circulatory redistribution in favor of the brain; in our cohort, Doppler parameters remained within normal ranges. However, in contrast to the situation in chronic hypoxemia, HC Z‐scores showed a tendency to decrease at the end of the third trimester, while AC Z‐scores continued to increase with advancing gestation. This late head growth impairment suggests that fetuses with CHD may be unable to meet the increased metabolic demands of the developing brain at the end of pregnancy35. This hypothesis is further supported by the lack of circulatory compensation, inferred by the constant MCA‐PI Z‐scores, despite the lower HC Z‐scores.
Previous studies on serial fetal biometry and Doppler measurements in fetuses with CHD show striking discrepancies. While the majority of studies report significant trends over time in both fetal biometry and cerebroplacental Doppler flow, the directions of trends differ significantly between studies1, 2, 32. Such discrepancies might be caused by differences in study methodology. For instance, while we used the same statistical approach as that of Ruiz et al.1, they assumed that the effect of gestational age followed a quadratic time trend, which may have produced the tendency for fetal parameters to increase during pregnancy. We made no assumptions regarding the shape of the time trend as we cannot be certain that use of a quadratic trend is justified. Furthermore, discrepancies might be caused by differences in study populations, although the populations are fairly representative of CHD found most commonly during fetal life1, 2, 32.
Since fetal brain development is dependent on adequate oxygen and nutrient supply, it has been hypothesized that fetuses with CHD in which umbilical venous blood, rich in nutrients and oxygen, is partly shunted away from the brain, have impaired cerebral development1, 5, 6, 19, 22. Several studies have reported lower MCA‐PI and CPR and higher UA‐PI in CHD cases with low expected cerebral oxygen supply1, 5, 6, 19, 22. We were unable to confirm this circulatory redistribution, but found more fluctuations in MCA‐PI Z‐scores throughout pregnancy in CHD fetuses with mild to moderately reduced or low expected cerebral oxygen supply compared with fetuses with CHD with normal expected oxygen supply, a potentially important observation. Although we cannot exclude the possibility that this was due to physiological variation, as longitudinal studies in healthy fetuses are lacking, we speculate that, if a manifestation of abnormality, these fluctuations might affect adversely development of the brain. They might suggest impaired hemodynamic autoregulation, which, similar to the situation in preterm infants36, 37, may have a harmful effect on the vulnerable developing brain.
There were no associations between MCA‐PI and HC Z‐scores, neither in the entire cohort nor in any of the three CHD subgroups. Previous studies were also unable to demonstrate a clear association between MCA‐PI and HC. Furthermore, Jansen et al.32 found no association between the flow in the ascending aorta and HC. These studies, however, either were based on univariate statistical analyses or used only theoretical hemodynamics. We used multivariate statistical analyses and measured the MCA‐PI. Therefore, we believe that our study is more robust, and we speculate that head growth might be more dependent on other factors, such as maternal or (epi)genetic factors, or nutrients (glucose)31, than on blood flow to the brain.
This study has several strengths and limitations. It is the first study to assess the association between trends in Doppler flow and fetal growth in a relatively large cohort of consecutive fetuses with CHD. Furthermore, we included an unselected population (the assessment of fetal growth and cerebroplacental Doppler flow was part of routine clinical care in both institutions) and we excluded all cases with chromosomal abnormalities, including microdeletions. However, this was a retrospective study, operators were not blinded to the presence of CHD, which might have affected Doppler and biometry measurements, and the assessment of Doppler flow was not standardized, resulting in various numbers of measurements across the range of gestational ages. Nonetheless, there were no indications that missing data were not at random. Therefore, our linear mixed‐effects model provides an unbiased estimate of the age trends. In addition, we were unable to confirm cardiac diagnosis using postnatal echocardiograms, surgery reports or postmortem reports in 10% of the cases. Also, comparison with other studies is limited by differences in study populations, reference charts used and study design. We believe that our classification of fetuses into normal, mild to moderately reduced and severely reduced expected cerebral arterial oxygen saturation is based on the best data available11, 13, 14, 30, 31; however, this remains speculative, as it is impossible to measure directly oxygen levels prenatally. We thus also performed a sensitivity analysis with slightly different classification of CHD, but the results remained unchanged. Finally, we were unable to analyze most types of CHD separately due to the relatively small numbers; the exceptions were ToF, previously reported to be associated with smaller HC32, and TGA + IVS, for which the lowest level of cerebral arterial oxygen saturation is expected. Multicenter studies providing larger numbers with the same type of CHD are necessary to provide insights into possible mechanisms responsible for suboptimal intrauterine cerebral development in a specific heart lesion.
In conclusion, this study demonstrates that, while there are significant trends in biometry and Doppler flow throughout pregnancy in fetuses with CHD, these measurements are within normal range. Fetal head growth is not associated with the expected cerebral arterial oxygen saturation or fetal Doppler flow, confirming that mechanisms other than circulatory modifications may influence the cerebral development in fetuses with CHD.
Supporting information
Table S1 Alternative classification of congenital heart disease according to expected cerebral arterial oxygen saturation, including four subgroups
Figures S1–S3 Averaged trends in Z‐scores of fetal growth and Doppler flow patterns in different subgroups of fetuses with congenital heart disease, classified according to expected level of cerebral arterial oxygen saturation: normal, mildly reduced, moderately reduced or severely reduced (Figure S1); normal, mildly to moderately reduced, severely reduced or tetralogy of Fallot (Figure S2); and normal, mildly reduced, moderately reduced, severely reduced or tetralogy of Fallot (Figure S3).
ACKNOWLEDGMENTS
We thank Prof. A. M. Rudolph for his inspiring discussions that helped in finalizing the CHD classification. This study was part of the research program of the Graduate School of Medical Sciences, Research Institutes BCN‐BRAIN and GUIDE. M.J.M. and W.S.K. were supported financially by a University of Groningen Junior Scientific Masterclass grant.
REFERENCES
- 1. Ruiz A, Cruz‐Lemini M, Masoller N, Sanz‐Cortes M, Ferrer Q, Ribera I, Martínez JM, Crispi F, Arévalo S, Gómez O, Pérez‐Hoyos S, Carreras E, Gratacós E, Llurba E. Longitudinal changes in fetal biometry and cerebroplacental hemodynamics in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2017; 49: 379–386. [DOI] [PubMed] [Google Scholar]
- 2. Hahn E, Szwast A, Cnota J, Levine JC, Fifer CG, Jaeggi E, Andrews H, Williams IA. Association between fetal growth, cerebral blood flow and neurodevelopmental outcome in univentricular fetuses. Ultrasound Obstet Gynecol 2016; 47: 460–465. [DOI] [PubMed] [Google Scholar]
- 3. Zeng S, Zhou QC, Zhou JW, Li M, Long C, Peng QH. Volume of intracranial structures on three‐dimensional ultrasound in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2015; 46: 174–181. [DOI] [PubMed] [Google Scholar]
- 4. Masoller N, Martinez JM, Gomez O, Bennasar M, Crispi F, Sanz‐Cortés M, Egaña‐Ugrinovic G, Bartrons J, Puerto B, Gratacós E. Evidence of second‐trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2014; 44: 182–187. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto Y, Khoo NS, Brooks PA, Savard W, Hirose A, Hornberger LK. Severe left heart obstruction with retrograde arch flow influences fetal cerebral and placental blood flow. Ultrasound Obstet Gynecol 2013; 42: 294–299. [DOI] [PubMed] [Google Scholar]
- 6. Arduini M, Rosati P, Caforio L, Guariglia L, Clerici G, Di Renzo GC, Scambia G. Cerebral blood flow autoregulation and congenital heart disease: possible causes of abnormal prenatal neurologic development. J Matern Fetal Neonatal Med 2011; 24: 1208–1211. [DOI] [PubMed] [Google Scholar]
- 7. Itsukaichi M, Kikuchi A, Yoshihara K, Serikawa T, Takakuwa K, Tanaka K. Changes in fetal circulation associated with congenital heart disease and their effects on fetal growth. Fetal Diagn Ther 2011; 30: 219–224. [DOI] [PubMed] [Google Scholar]
- 8. Wallenstein MB, Harper LM, Odibo AO, Roehl KA, Longman RE, Macones GA, Cahill AG. Fetal congenital heart disease and intrauterine growth restriction: a retrospective cohort study. J Matern Fetal Neonatal Med 2012; 25: 662–665. [DOI] [PubMed] [Google Scholar]
- 9. Paladini D, Alfirevic Z, Carvalho JS, Khalil A, Malinger G, Martinez JM, Rychik J, Ville Y, Gardiner H, ISUOG Clinical Standards Committee . ISUOG consensus statement on current understanding of the association of neurodevelopmental delay and congenital heart disease: impact on prenatal counseling. Ultrasound Obstet Gynecol 2017; 49: 287–288. [DOI] [PubMed] [Google Scholar]
- 10. Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH Jr, Li J, Smith SE, Bellinger DC, Mahle WT; American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council . Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 2012; 126: 1143–1172. [DOI] [PubMed] [Google Scholar]
- 11. Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, Grosse‐Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, Hickey E, Miller S, Seed M. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015; 131: 1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol 1996; 143: 505–513. [DOI] [PubMed] [Google Scholar]
- 13. Rudolph AM. Congenital cardiovascular malformations and the fetal circulation. Arch Dis Child Fetal Neonatal Ed 2010; 95: F132–136. [DOI] [PubMed] [Google Scholar]
- 14. Rudolph AM. Impaired cerebral development in fetuses with congenital cardiovascular malformations; Is it the result of inadequate glucose supply? Pediatr Res 2016; 80: 172–177. [DOI] [PubMed] [Google Scholar]
- 15. Chen Y, Lv G, Li B, Wang Z. Cerebral vascular resistance and left ventricular myocardial performance in fetuses with Ebstein's anomaly. Am J Perinatol 2009; 26: 253–258. [DOI] [PubMed] [Google Scholar]
- 16. Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol 2005; 25: 32–36. [DOI] [PubMed] [Google Scholar]
- 17. Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, Cetta F, Falkensammer CB, Huhta JC, Kleinman CS. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol 2003; 24: 436–443. [DOI] [PubMed] [Google Scholar]
- 18. Jouannic JM, Benachi A, Bonnet D, Fermont L, Le Bidois J, Dumez Y, Dommergues M. Middle cerebral artery Doppler in fetuses with transposition of the great arteries. Ultrasound Obstet Gynecol 2002; 20: 122–124. [DOI] [PubMed] [Google Scholar]
- 19. Masoller N, Sanz‐Cortes M, Crispi F, Gómez O, Bennasar M, Egaña‐Ugrinovic G, Bargalló N, Martínez JM, Gratacós E. Severity of fetal brain abnormalities in congenital heart disease in relation to the main expected pattern of in utero brain blood supply. Fetal Diagn Ther 2016; 39: 269–278. [DOI] [PubMed] [Google Scholar]
- 20. Masoller N, Sanz‐CorteS M, Crispi F, Gómez O, Bennasar M, Egaña‐Ugrinovic G, Bargalló N, Martínez JM, Gratacós E. Mid‐gestation brain Doppler and head biometry in fetuses with congenital heart disease predict abnormal brain development at birth. Ultrasound Obstet Gynecol 2016; 47: 65–73. [DOI] [PubMed] [Google Scholar]
- 21. Szwast A, Tian Z, McCann M, Soffer D, Rychik J. Comparative analysis of cerebrovascular resistance in fetuses with single‐ventricle congenital heart disease. Ultrasound Obstet Gynecol 2012; 40: 62–67. [DOI] [PubMed] [Google Scholar]
- 22. Berg C, Gembruch O, Gembruch U, Geipel A. Doppler indices of the middle cerebral artery in fetuses with cardiac defects theoretically associated with impaired cerebral oxygen delivery in utero: is there a brain‐sparing effect? Ultrasound Obstet Gynecol 2009; 34: 666–672. [DOI] [PubMed] [Google Scholar]
- 23. Guorong L, Shaohui L, Peng J, Huitong L, Boyi L, Wanhong X, Liya L. Cerebrovascular blood flow dynamic changes in fetuses with congenital heart disease. Fetal Diagn Ther 2009; 25: 167–172. [DOI] [PubMed] [Google Scholar]
- 24. Modena A, Horan C, Visintine J, Chanthasenanont A, Wood D, Weiner S. Fetuses with congenital heart disease demonstrate signs of decreased cerebral impedance. Am J Obstet Gynecol 2006; 195: 706–710. [DOI] [PubMed] [Google Scholar]
- 25. Meise C, Germer U, Gembruch U. Arterial Doppler ultrasound in 115 second‐ and third‐trimester fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2001; 17: 398–402. [DOI] [PubMed] [Google Scholar]
- 26. Arduini D, Rizzo G. Normal values of pulsatility index from fetal vessels: a cross‐sectional study on 1556 healthy fetuses. J Perinat Med 1990; 18: 165–172. [DOI] [PubMed] [Google Scholar]
- 27. Baschat AA, Gembruch U. The cerebroplacental Doppler ratio revisited. Ultrasound Obstet Gynecol 2003; 21: 124–127. [DOI] [PubMed] [Google Scholar]
- 28. Gomez O, Figueras F, Fernandez S, Bennasar M, Martinez JM, Puerto B, Gratacos E. Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet Gynecol 2008; 32: 128–132. [DOI] [PubMed] [Google Scholar]
- 29. Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW, Witteman JC. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population‐based cohort study. Ultrasound Obstet Gynecol 2008; 31: 388–396. [DOI] [PubMed] [Google Scholar]
- 30. Rudolph AM. Congenital Diseases of the Heart: Clinical‐Physiological Considerations. Wiley Blackwell: New York, 2009. [Google Scholar]
- 31. Prsa M, Sun L, van Amerom J, Yoo SJ, Grosse‐Wortmann L, Jaeggi E, Macgowan C, Seed M. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase‐contrast magnetic resonance imaging. Circ Cardiovasc Imaging 2014; 7: 663–670. [DOI] [PubMed] [Google Scholar]
- 32. Jansen FA, van Zwet EW, Rijlaarsdam ME, Pajkrt E, van Velzen CL, Zuurveen HR, A1 Kragt, Bax CL, Clur SA, van Lith JM, Blom NA, Haak MC. Head growth in fetuses with isolated congenital heart defects: lack of influence of aortic arch flow and ascending aorta oxygen saturation. Ultrasound Obstet Gynecol 2016; 48: 357–364. [DOI] [PubMed] [Google Scholar]
- 33. Thiebaut R, Walker S. When it is better to estimate a slope with only one point. QJM 2008; 101: 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stone CJ, Koo CY. Additive splines in statistics In Proceedings of the Statistical Computing Section ASA. American Statistical Association: Washington, DC, 1985. [Google Scholar]
- 35. Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr, Guizard N, McGrath E, Geva J, Annese D, Dunbar‐Masterson C, Trainor B, Laussen PC, du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010; 121: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Volpe JJ. Neurobiology of periventricular leukomalacia in the pre‐mature infant. Pediatr Res 2001; 5: 553–562. [DOI] [PubMed] [Google Scholar]
- 37. Volpe JJ. Neurology of the Newborn. Chapter 11. Saunders: Philadelphia, PA, 2008; 524. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Alternative classification of congenital heart disease according to expected cerebral arterial oxygen saturation, including four subgroups
Figures S1–S3 Averaged trends in Z‐scores of fetal growth and Doppler flow patterns in different subgroups of fetuses with congenital heart disease, classified according to expected level of cerebral arterial oxygen saturation: normal, mildly reduced, moderately reduced or severely reduced (Figure S1); normal, mildly to moderately reduced, severely reduced or tetralogy of Fallot (Figure S2); and normal, mildly reduced, moderately reduced, severely reduced or tetralogy of Fallot (Figure S3).


