Abstract
There is a need to develop more animal species for assessing toxicity in marine environments. Cyst‐based toxicity tests using invertebrates are especially fast, technically simple, cost‐effective, and sensitive to a variety of toxicants. Over the past 30 years, a variety of toxicity endpoints have been measured using the marine rotifer Brachionus plicatilis hatched from cysts, including mortality, reproduction, ingestion, swimming, enzyme activity, and gene expression. A consensus has developed that the most ecologically relevant toxicity measurements should be made using more than one species. Furthermore, it has been noted that the rotifer species toxicant sensitivity distribution is much broader than which endpoint is measured. This implies that toxicity should be measured with the simplest, fastest, least expensive test available on as many species as feasible. If a battery of test species is to be used to estimate toxicity, diapause egg‐based toxicity tests that do not require culturing of test animals will be key. In this paper, we describe how diapause eggs of a new marine rotifer, Proales similis, can be produced, stored and hatched under controlled conditions to produce animals for toxicity tests. Methods are described for quantifying the toxicity of copper, mercury and cadmium based on mortality, ingestion, reproduction, and diapause egg hatching endpoints. We found that reproduction and ingestion endpoints were generally more sensitive to the metals than mortality or diapause egg hatching. When the copper sensitivity of P. similis was compared to Brachionus manjavacas and B. plicatilis using an ingestion test, similar EC50s were observed. In contrast, the B. rotundiformis ingestion EC50 for copper was about 4× more sensitive. Although diapause egg hatching was not the most sensitive endpoint, it is the most ecologically relevant for assessing sediment toxicity. Our discovery of diapausing eggs in the P. similis life cycle has created a conundrum. We have not observed males or sex in P. similis populations, which is a direct contradiction to the orthodox view of the monogonont rotifer life cycle. Work is needed to clarify how diapause egg production is accomplished by P. similis and whether sexual reproduction is involved.
Keywords: diapause egg hatching, ingestion, marine, metals, mortality, reproduction, rotifer, toxicity testing
1. INTRODUCTION
Rotifers are popular model animals for toxicity assessment as evidenced by recent reviews.1, 2, 3 Assessing toxicity in marine environments has fewer options than freshwater, so a need exists to expand marine ecotoxicology animal models.4, 5, 6 Rapid, cost‐effective, and convenient toxicity assessment based on diapausing eggs(DEs) (cysts) of aquatic invertebrates like rotifers and the brine shrimp Artemia has become widespread for a variety of reasons.7, 8 These include their technical simplicity and the elimination of the need to master culture techniques to produce test animals and their food. Standard protocols have been validated and published for a rotifer acute9 and a reproductive toxicity test.10 These are based on the rotifer Brachionus calyciflorus in freshwater and Brachionus plicatilis in seawater. However, it is well appreciated that different rotifer species have different sensitivities to toxicants,11 so the employing more rotifer biodiversity in toxicity assessment could be advantageous.
In this paper, we describe the production and hatching of DEs by the monogonont rotifer Proales similis De Beauchamp 1907, which inhabits estuaries and coastal marine environments. The biology of this rotifer is being investigated because of its usefulness in aquaculture.12, 13, 14 As a marine rotifer, Proales also has value in ecotoxicology for toxicity assessment.15 In recognition of this, we have developed rapid toxicity tests based on hatching of DE to provide animals to initiate tests. We describe methods for a 3‐h test based on particle ingestion, a 6 h mortality test, a 72 h reproductive test, and a 24 h DE hatching inhibition test. We used the metals copper, mercury and cadmium as example toxicants and present dose‐response data with EC50s and LC50s estimated from linear regression. The data show that P. similis has great utility for marine toxicity assessment and is a valuable addition to the marine ecotoxicology toolkit.
2. METHODS
2.1. Rotifer and algae cultures
Proales similis was described from the Urias estuary in Mazatlan, Mexico by Reyes et al.16 Our culture was a gift from Dr. S. Nandini, National Autonomous University of Mexico, Campus Iztacala. We verified the taxonomy of this species by collecting genomic DNA and sequencing the cytochrome oxidase I gene using universal invertebrate primers.17 Proales was maintained in serial dilution culture in 250 mL flasks under continuous fluorescent illumination at 25°C. Flasks contained 200 mL 15 ppt artificial seawater (ASW) and about 10% Tetraselmis suecica algae in modified F medium.18 All T. suecica was centrifuged to remove the algae growth medium before using in any rotifer experiments as F media inhibited P. similis reproduction. Additional T. suecica was added as needed to stock cultures to maintain a density of approximately 2 x 105 cells/mL.
Proales similis DEs are morphologically indistinguishable from asexual eggs, so eggs were preserved by allowing rotifers to naturally deposit them in microcentrifuge tubes. A dense population of P. similis (>100 rotifers per mL) was fed concentrated T. suecica, and then transferred to 0.6 mL microcentrifuge tubes in 0.5 mL aliquots. Tubes were left open at 22°C and allowed to desiccate via evaporation for approximately 1 week. When approximately 50 μL of liquid was left, tubes were capped and stored in the dark at 4°C. The salinity of the remaining medium with the DEs was approximately 100 ppt and contained no active rotifers. DEs were hatched by adding 500 μL of 15 ppt ASW to the tube and incubating at 22°C in constant fluorescent light overnight. After about 18–21 h, neonates hatched and were ready to use to initiate experiments or inoculate new cultures.
We also discovered that the green alga T. suecica could be preserved desiccated in microcentrifuge tubes. T. suecica was grown in modified F media at 25°C for 1–2 weeks. Cells then were concentrated 2–3x by centrifuging at 3 000 rpm for 2 min and removing the excess growth medium. The concentrated algae then was transferred to 1.5 mL microcentrifuge tubes in 1 mL aliquots. Tubes were left open at 22°C and allowed to desiccate completely via evaporation. Tubes were capped and stored at 4°C. To reactivate algae cells, 1 mL F media was added to a tube, and the tube was incubated at 22°C in constant light overnight. Motile cells were visible within 24 h. After 48 h, the full volume of the centrifuge tube was transferred to a 250 mL flask with 200 mL F media. The flask was incubated at 25°C with aeration and constant fluorescent illumination. T. suecica in the flask grew dense enough to use for feeding P. similis cultures within 1 week.
2.2. Proales similis size growth curve
To determine the growth rate of P. similis females, a tube of DEs was hydrated and incubated for 24 h as described above. Neonates were collected and transferred to a six‐well plate with 5 mL ASW and T. suecica was fed ad libidum. Each day, images of 10 rotifers were taken at 45× using a PixeLink camera attached to a stereomicroscope. Length and width of each animal was measured using ImageJ.
2.3. Proales similis population growth curve
To determine the population growth rate of P. similis, a tube of DEs was hydrated and incubated for 24 h as described above. After 24 h, all hatched neonates (several hundred) were collected and transferred to a 250 mL flask containing 200 mL 15 ppt ASW with approximately 2 × 105 T. suecica cells per mL. The flask was incubated at 25°C under constant fluorescent illumination. Each day, five 20–1 000 μL aliquots were taken from the flask and counted to calculate density of rotifers per mL. Additional T. suecica was added to the flask as needed.
Age‐specific reproduction of P. similis females was measured on isolated animals in 0.5 mL in a well of a 48‐well plate. Neonates were hatched from DEs and transferred to experimental wells within 4 h of their birth. Experimental conditions were 25°C, 15 ppt ASW and a diet of 6 × 105 T. suecica cells per mL. Wells were checked daily and offspring counted and removed, leaving only the maternal female in each well. Females were followed until their deaths and population statistics calculated.
2.4. Preparation of metal stocks
Toxicity was characterized for three metals: copper (cupric sulfate), cadmium (cadmium chloride), and mercury (mercury [II] chloride). All stocks were prepared at 1 mg/mL metal in 100 mL of research grade deionized water. Inductively coupled plasma mass spectrometry (ICP‐MS, Agilent 7500a series) was used to verify the concentration of each stock, with each stock measured in triplicate. Cu, Cd, and Hg stocks were found to be within 39.7%, 18.3%, and 7.6% of their nominal values, respectively. The metal concentrations reported are adjusted to their actual measured concentrations.
2.5. Toxicity tests
Toxicity tests were performed at a range of concentrations to measure the effects of the metals on four different P. similis endpoints: survival, population growth rate, ingestion, and DE hatching. Range‐finding tests were first conducted to determine the concentration range over which a linear dose response was observed. Toxicant test ranges were 0.09–9.00 mg/L for copper, 0.08–225 mg/L for cadmium, and 0.005–0.350 mg/L for mercury. Microsoft EXCEL was used to calculate linear regressions which were used to determine LC50s (acute tests) or EC50s (reproductive, ingestion and DE hatching tests). Each test was repeated in triplicate for each toxicant to calculate average LC50 or EC50, coefficients of variation, and 95% confidence limits. Acute and ingestion tests were conducted using mixed age rotifers filtered from an established culture using 25 μm mesh, and reproductive assays used neonates hatched from DEs.
2.6. Acute toxicity assay
To measure the effects of the toxicants on survival, 6‐h acute assays were performed. Approximately 10 adult P. similis filtered from established population were placed into each well of a 24‐well plate containing 1 mL 15 ppt ASW and the appropriate metal concentration. Each experiment consisted of a control with no metal and five increasing metal concentrations, each with four replicate wells per treatment. Plates were incubated at 25°C in the dark for 6 h. After the incubation, the number of live and dead rotifers was counted in each well and used to calculate the percent survival for each concentration and an LC50 from a linear regression of the dose–response data was estimated.
2.7. Reproductive assay
To measure the effects of the metals on population growth rate, 3‐day reproductive assays were performed. Two P. similis neonates hydrated from tubes were placed into each well of a 48‐well plate containing 500 μL of 15 ppt ASW, 1 x 105 cells/mL T. suecica, and the appropriate metal concentration. Each plate consisted of a control with no metal and five increasing metal concentrations with eight replicate wells for each treatment. Plates were incubated at 25°C in the dark. After 72 h, the total number of animals in each well was counted and used to calculate the average population growth rate (r, offspring per rotifer per day) for each treatment. Population growth rate was calculated using the formula r = (ln(N t) − ln(N 0))/T, where ln(N t) is the natural log of the total number of P. similis in a well after 3 days, ln(N 0) is the natural log of the initial number of P. similis (two), and T is the time (3 days).
2.8. Ingestion assay
To measure the effects of the toxicants on rotifer ingestion rates, 3‐h ingestion assays were performed. Approximately 10 adult P. similis filtered from an established population were placed into each well of a 24‐well plate containing 500 μL of 15 ppt ASW and the appropriate concentration of metal. Each plate consisted of a control containing no metal and five different concentrations of metal, each with four replicate wells per treatment. Plates were incubated at 25°C in the dark for 1 h. After 1 h, 30 μL of a suspension of carmine particles (1 mg/mL in 15 ppt ASW) was added to each well and mixed with gentle shaking. Plates were then incubated at 25°C in the dark for an additional 2 h. Then the number of clear animals and the number of animals with red carmine in their guts was counted to calculate the percentage of rotifers actively ingesting in each treatment.
2.9. Hatching inhibition assay
To examine the effects of copper, cadmium, and mercury on P. similis DE hatching, we employed a 24‐h hatching inhibition assay. 500 μL aliquots of a dense P. similis culture in 15 ppt ASW and T. suecica were condensed in 0.6 mL microcentrifuge tubes by evaporation to approximately 50 μL of high salinity seawater (~100 ppt). At this salinity no active animals were observed, but eggs deposited on the bottom could be hatched after several months stored at 4°C. For each metal, 24 P. similis tubes were hydrated with 500 mL 15 ppt ASW containing the proper metal concentrations. Each assay contained a control and five metal concentrations, with four replicates each. Tubes were incubated at 25°C for 24 h under fluorescent light. After 24 h, the liquid in each tube was transferred into a well in a 24‐well plate. The contents of each tube were washed with an additional 250 μL of 15 ppt ASW, and the wash was added to the well. Based on an initial examination of the density of hatched animals in each well, the number of animals was either counted directly or estimated from subsamples from the wells. Density estimates were based on four subsamples ranging in volume from 10 to 30 μL.
2.10. Comparative rotifer sensitivity to copper—ingestion assays
To determine the relative metal sensitivity of P. similis compared to other rotifer species, ingestion assays were performed using several brachionid species. These ingestion assays were performed following the protocol described by Snell.19 Eight Brachionus geographical isolates were hatched from DEs and tested: two B. manjavacas (Bm Russian and Bm Gaynor Pond), two B. plicatilis (Bp China and Bp Salebrejo), two B. rotundiformis (Br Italy and Br Hawaii), and two unnamed Brachionus species (B sp. Nevada 2 and B sp. Lost Lake 1). All DE hatched for these assays were part of a DE collection produced for another project.17
Approximately 15 Brachionus neonates were placed into each well of a 24‐well plate, containing 750 μL 15 ppt ASW and the appropriate concentration of copper. Each plate consisted of a control containing no copper and five increasing concentrations of copper, each with four replicate wells. Plates were incubated at 25°C in the dark for 45 min. Then 10 μL of a carmine suspension (1 mg/2 mL in 15 ppt ASW) was added to each well and mixed by gentle shaking. Plates were then incubated at 25°C in the dark for 15 min. After 15 min in carmine, 5 μL of 20% formalin solution was added to each well to fix the rotifers. The number of clear rotifers and rotifers with red carmine in their guts was scored to calculate percent rotifers ingesting for each treatment. Each assay was repeated in triplicate to determine the average EC50 for each Brachionus population.
2.11. Comparative rotifer sensitivity to copper—DE hatching assays
Hatching of Proales DE in the presence of copper was estimated as described above. For Brachionus, about 50 DE of six geographical isolates (two B. manjavacas [Bm Russian and Bm Gaynor Pond], two B. plicatilis [Bp China and Bp Salebrejo], and two B. rotundiformis [Br Italy and Br Hawaii]) were dried onto the bottom of each well of a 48‐well plate. Experiments were initiated by hydrating the wells with ASW (control) or ASW with a series of five increasing copper concentrations. There were eight replicates for each of the six treatments from which mean number of rotifers hatching per well was recorded. A dose–response curve was calculated using a linear regression and the EC50, the concentration with 50% less hatching than control, was determined.
2.12. Statistical analysis
The non‐linear model for the Proales population growth curve was fit and dose–response linear regressions used to estimate LC50s and EC50s. A one‐way anova was calculated to compare Proales and Brachionus ingestion and DE hatching EC50s, followed by a Tukey–Kramer HSD test to compare means.
3. RESULTS
Populations of P. similis consist of females averaging 88.3 μM long and 47.5 μM wide as adults (Figure 1). Males have not been observed in our laboratory cultures. Eggs are produced parthenogenetically, are carried internally by females, and average 50.8 μM long and 36.3 μM wide (Figure 2). DEs cannot be discriminated from directly developing eggs. The eggs pictured in Figure 2C survived complete desiccation, yet they are morphologically identical to all other P. similis eggs we have observed.
Figure 1.
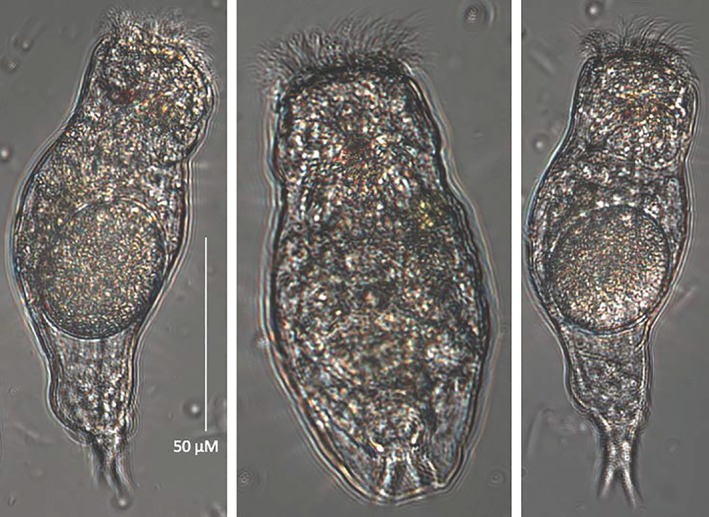
DIC photomicrograph of Proales females with eggs, 400× [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
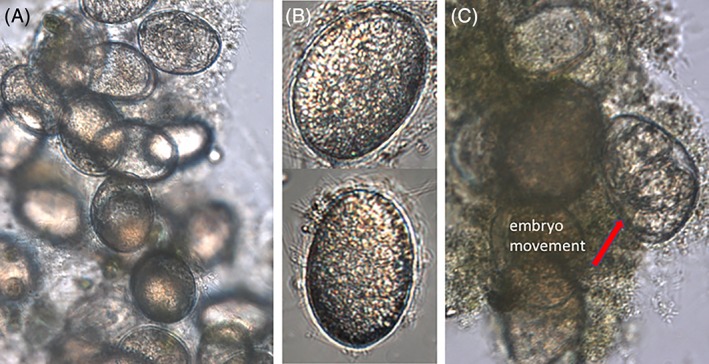
(A) Proales simils aggregation of eggs (200×); (B) Individual P. similis eggs (400×); (C) P. similis eggs that were desiccated, stored in a refrigerator for several days, and rehydrated. The embryo in the egg at the red arrow had extensive movement. This demonstrates that this egg was able to withstand desiccation and remain viable. It is morphologically indistinguishable from other eggs that develop without diapause [Color figure can be viewed at wileyonlinelibrary.com]
Newborn females average 63.3 μM long and 34.9 μM wide (Figure 3). Over the next 3 days at 25°C they grow to adult size and begin producing eggs parthenogenetically. By age 5 days, growth ceases and they maintain this size for the remainder of their lifespan (8 days).
Figure 3.
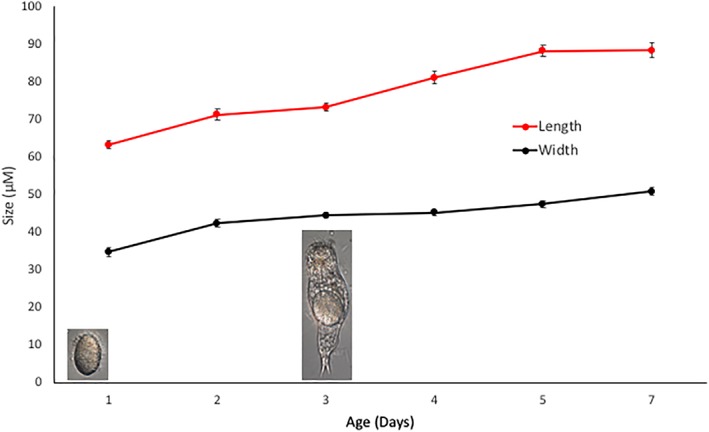
Growth in the size of Proales females from egg hatching to adulthood at 25°C. Vertical lines at each data point represent standard errors [Color figure can be viewed at wileyonlinelibrary.com]
Females produce their first offspring parthenogenetically when they are 3 days old (Figure 4). Their reproduction peaks at four offspring per day at age 5 days and then declines with age for the remainder of their lifespan. Population growth in abundant food follows an exponential function (Y = 0.802X 2.972), growing from a density of about 1 mL−1 on day 1 to 188 mL−1 on day 6 to 947 mL−1 on day 11.
Figure 4.
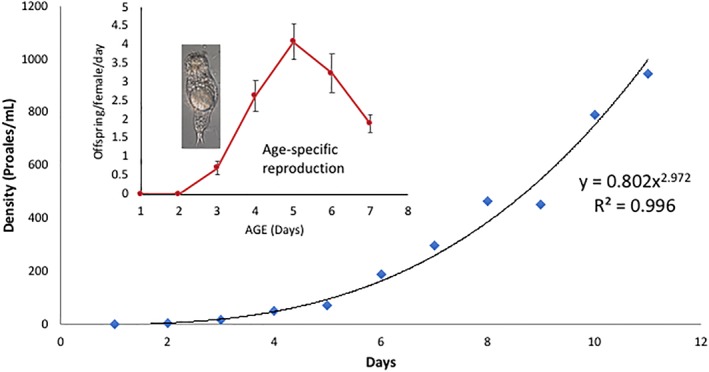
Age‐specific reproduction of Proales females (inset) at 25°C. Population growth curve for a P. similis population in a 250 mL flask inoculated at one female ml−1 [Color figure can be viewed at wileyonlinelibrary.com]
We have summarized the characteristics of Proales simils in Table 1 because they are quite different from those of brachionids, the primary ecotoxicological model for monogonont rotifers. P. similis shares with the B. plicatilis species complex an estuarine and coastal marine habitat, as well as broad temperature and salinity tolerances. Likewise, their diets of bacteria and microalgae are similar. What distinguishes P. similis is the fact that males have yet to be observed and DEs are not morphologically distinguishable from eggs with direct development. P. similis DEs can tolerate desiccation and high salinity and remain viable for months at 4°C. When placed in light at 25°C, DE hatching was initiated after 18 h and proceeded rapidly to an asymptotic value by 21 h. Additional hatching was not observed through 24 h. However, much work needs to be done to clarify the role of sexual reproduction in this monogonont rotifer life cycle.
Table 1.
The ecological characteristics of Proales similis
| Category | Traits |
|---|---|
| Classification | Rotifera, Monogononta, Ploima, Proalidae, Proales similis |
| Habitat | Estuaries and coastal marine |
| Size | Neonates: 63 μM long by 35 wide; adults: 88 μM by 48 |
| Diet | Bacteria and microalgae (Nanochloropsis, Chlorella, Tetraselmis), S. parkle |
| Temperature range | 20–35°C |
| Salinity range | 2–35 ppt |
| Males | Never observed, populations all females |
| Copulation | Never observed |
| Diapausing eggs | Produced, but not distinguishable from non‐diapausing eggs, survive desiccation, remain viable after months of storage at 4°C, hatch in 18–21 h into asexual females |
Copper is toxic to P. similis in the low ppm range, depending on the endpoint used to characterize the dose–response relationship (Figure 5). A 72 h reproductive test produced the lowest EC50 of 0.15 mg Cu L−1. This was followed by a 3 h ingestion test (EC50 = 0.26) and a 6 h mortality test (EC50 = 1.06). The least sensitive endpoint was a 24 h diapause egg hatching test which yielded an EC50 of 3.40 mg Cu L−1. Dose–response relationships for all four endpoints were linear over the test ranges, with R 2s ranging from 0.93 to 0.98.
Figure 5.
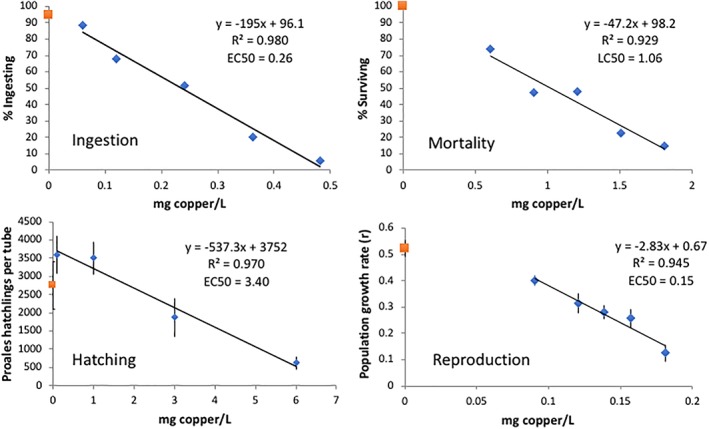
Characterization of P. similis copper toxicity using various endpoints (mg/L). Each data point in the hatching test is the mean of four replicates and eight replicates in the reproductive test. Data points in the ingestion and mortality tests represent one replicate. The EC50s are the mean of three replicate experiments. Data shown is from only one of the three plots. The red square data point on the y‐axis is the control and the blue diamond data points are from the copper treatments. Vertical lines indicate standard errors [Color figure can be viewed at wileyonlinelibrary.com]
We used an ingestion test to compare P. similis' sensitivity to copper to several Brachionus species (Figure 6). The copper EC50 of P. similis of 0.26 mg Cu L−1 was significantly lower than Bm RUS (P = 0.008), but similar to those of Bp China, and Bp SAL. Bm GP copper EC50 was only about 1/5 of P. similis (P = 0.0003), and similar to that of Br HAW, Br Italy, B sp. Nev2, and B sp. LL1. It is interesting to note that there is more variation in copper toxicity in the genus Brachionus than between Brachionus and P. similis, which are in different rotifer families.
Figure 6.
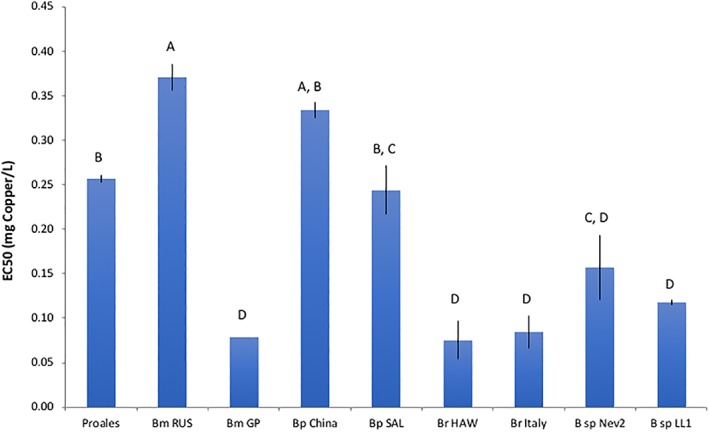
Comparison of P. similis copper toxicity in an ingestion test to several Brachionus species. Bm—Brachionus manjavacas (RUS—Russia, GP—Gaynor Pond, CO), Bp—B. plicatilis (SAL—Salebrejo, Spain), Br—B. rotundiformis (HAW—Hawaii), B. sp.—unnamed species, Nev2—from Nevada, LL1—from Lost Lake, Connecticut. Vertical lines indicate standard errors. The EC50 values of rotifer species sharing the same letter are not significantly different by the Tukey–Kramer HSD test [Color figure can be viewed at wileyonlinelibrary.com]
Similarly, we compared toxicant sensitivity of the hatching of Proales DEs to those of several Brachionus species (Figure 7). The Proales copper EC50 for DE hatching of 3.4 mg Cu L−1 was not significantly different from the EC50s of Bm RUS, Bm GP, Bp SAL, and Bp TOKYO. Only the EC50s of Br HAW (0.91 mg Cu L−1, P = 0.009) and Br ITALY (0.88, P = 0.008) were significantly lower.
Figure 7.
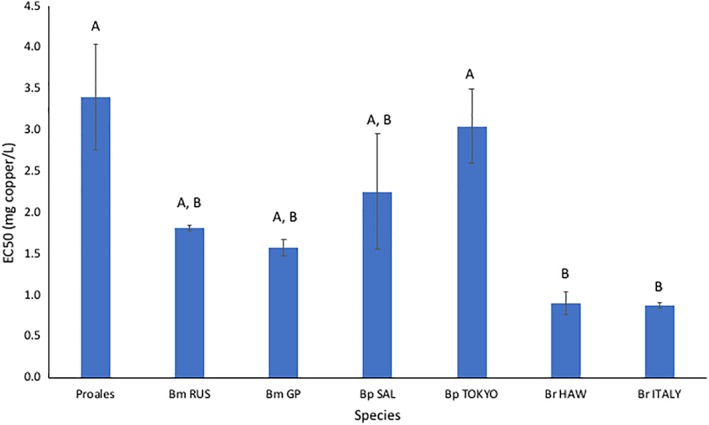
Comparison of P. similis copper toxicity in a diapausing egg hatching inhibition test to several Brachionus species. Bm—Brachionus manjavacas (RUS—Russia, GP—Gaynor Pond, Colorado), Bp—B. plicatilis (SAL—Salebrejo, Spain, TOKYO—Tokyo, Japan), Br—B. rotundiformis (HAW—Hawaii, ITALY—Italy). Vertical lines indicate standard errors. The EC50 values of rotifer species sharing the same letter are not significantly different by the Tukey–Kramer HSD test [Color figure can be viewed at wileyonlinelibrary.com]
Mercury is toxic to P. similis in the low ppb range, depending on the endpoint used to characterize the dose‐response relationship (Figure 8). A 3 h ingestion test yielded the lowest average EC50 (11 μg Hg L−1), followed by a 6 h mortality test (EC50 = 26). A 72 h reproductive test produced an EC50 of 52 μg Hg L−1. The least sensitive endpoint was a 24 h diapause egg hatching test which yielded an EC50 of 101 μg Hg L−1. Dose‐response relationships for all four endpoints were linear over the test ranges, with R 2s ranging from 0.82 to 0.99.
Figure 8.
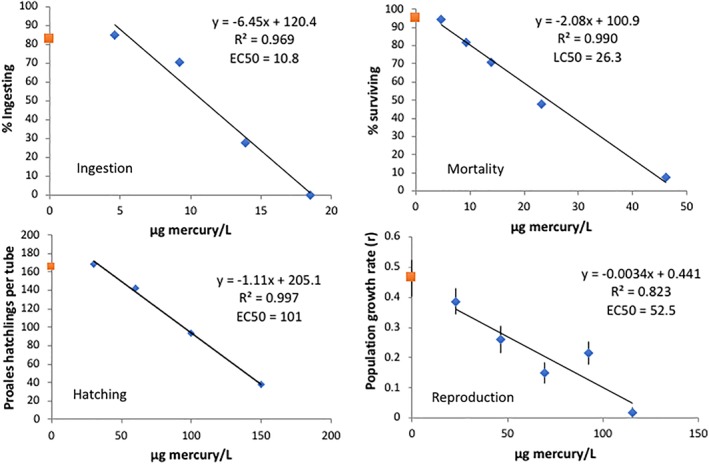
Characterization of P. similis mercury toxicity using various endpoints (μg L−1). Each data point in the hatching test is the mean of four replicates and eight replicates in the reproductive test. Data points in the ingestion and mortality tests represent one replicate. The EC50s are the mean of three replicate experiments. Data shown is from only one of the three plots. The red square data point on the y‐axis is the control and the blue diamond data points are from the mercury treatments. Vertical lines indicate standard errors [Color figure can be viewed at wileyonlinelibrary.com]
Cadmium is toxic to P. similis in the low ppm range, depending on the endpoint used to characterize the dose–response relationship (Figure 9). A 72 h reproductive test produced the lowest EC50 of 1.01 mg Cd L−1, followed by a 3 h ingestion test which yielded an EC50 of 2.2 mg Cd L−1. A 24 h diapause egg hatching test produced an EC50 of 31.3 mg Cd L−1. The least sensitive endpoint was a 6 h mortality test with an EC50 of 50.1 mg Cd L−1. Dose–response relationships for all four endpoints were linear over the test ranges, with R2s ranging from 0.89 to 0.99.
Figure 9.
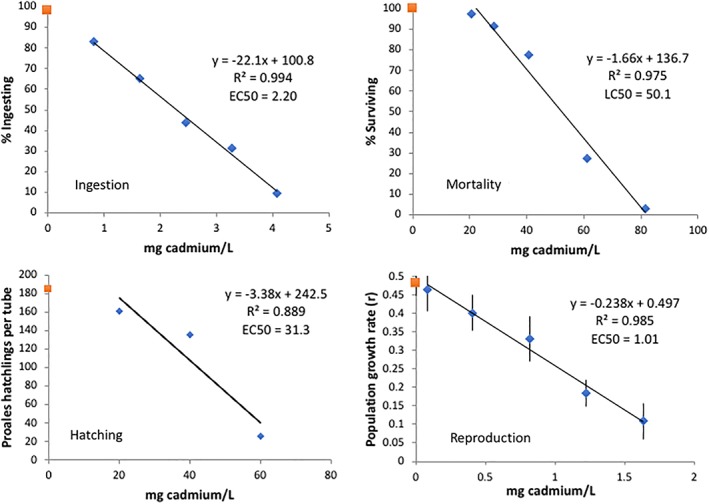
Characterization of P. similis cadmium toxicity using various endpoints. Each data point in the hatching test is the mean of four replicates and eight replicates in the reproductive test. Data points in the ingestion and mortality tests represent one replicate. The EC50s are the mean of three replicate experiments. Data shown is from only one of the three plots. The red square data point on the y‐axis is the control and the blue diamond data points are from the cadmium treatments. Vertical lines indicate standard errors [Color figure can be viewed at wileyonlinelibrary.com]
The means, standard deviations, and 95% confidence limits for all four endpoints (mortality, reproduction, ingestion and hatching) and all three metals (copper, mercury, and cadmium) are summarized in Table 2. The endpoint with the highest variation among replicates was hatching and the lowest was mortality. Coefficients of variation ranged from 2.74% for copper ingestion to 39.1% for mercury hatching.
Table 2.
Summary of toxicity endpoints (LC50s and EC50s) for copper, mercury, and cadmium and their 95% confidence limits
| Metal | Endpoint | N | Mean (mg/L) | Std. deviation | Coef. Var. | Upper 95% CL | Lower 95% CL |
|---|---|---|---|---|---|---|---|
| Copper | Mortality (LC50) | 3 | 1.06 | 0.04 | 3.69 | 1.11 | 1.02 |
| Reproduction (EC50) | 3 | 0.15 | 0.01 | 4.68 | 0.16 | 0.14 | |
| Ingestion (EC50) | 3 | 0.26 | 0.01 | 2.74 | 0.28 | 0.24 | |
| Hatching (EC50) | 3 | 3.40 | 0.66 | 19.5 | 4.64 | 2.15 | |
| Mercury | Mortality (LC50) | 3 | 0.026 | 0.001 | 2.85 | 0.027 | 0.025 |
| Reproduction (EC50) | 3 | 0.052 | 0.010 | 18.62 | 0.064 | 0.041 | |
| Ingestion (EC50) | 3 | 0.011 | 0.001 | 11.56 | 0.012 | 0.009 | |
| Hatching (EC50) | 4 | 0.101 | 0.043 | 39.1 | 0.149 | 0.052 | |
| Cadmium | Mortality (LC50) | 3 | 50.09 | 1.84 | 3.67 | 52.17 | 48.01 |
| Reproduction (EC50) | 3 | 1.01 | 0.07 | 6.49 | 1.08 | 0.94 | |
| Ingestion (EC50) | 3 | 2.20 | 0.29 | 13.23 | 2.53 | 1.87 | |
| Hatching (EC50) | 7 | 31.29 | 13.07 | 35.97 | 46.90 | 15.71 |
4. DISCUSSION
Our principal finding is that the marine rotifer P. similis can be a valuable tool for marine toxicity assessment. We have developed P. similis as another diapause egg‐based, no culture toxicity test that is convenient, fast and sensitive to a variety of toxicants. Ecologically relevant toxicity assessment requires multiple species with different sensitivities, so having an array of species that can be hatched from diapause eggs for rapid, simple toxicity measurements is especially important. Snell20 argued that the rotifer species toxicant sensitivity distribution is much broader than which endpoint is measured. He suggested that toxicity should be measured with the simplest, fastest, least expensive test available on as many species as feasible. This conclusion makes diapause egg‐based toxicity tests that do not require culturing of test animals key for toxicity assessment. Development of such tests based on hatching of diapause eggs of P. similis is an important advance since it expands the species of rotifers that can be easily deployed in marine toxicity tests beyond the traditional B. plicatilis.
We described production of P. similis DEs in our lab, but this has introduced another puzzle. We have observed no evidence of males or sex in P. similis, which is a direct contradiction to the orthodox view of the monogonont rotifer life cycle. Work is needed to clarify how DE production is accomplished by P. similis and whether sexual reproduction is involved. We also showed that reproduction and ingestion endpoints are generally more sensitive to the metals copper, cadmium and mercury than mortality or DE hatching. P. similis has similar copper sensitivity to Brachionus manjavacas and B. plicatilis, but its ingestion EC50 for copper is about 4× higher than that of B. rotundiformis. DE hatching is an ecologically relevant endpoint for assessing sediment toxicity.
Copper toxicity using mortality and DE hatching endpoints has been characterized in B. plicatilis by other authors. Aránguiz‐Acuña and Pérez‐Portilla21 reported that a 48 h LC50 for copper in a Chilean strain of B. plicatilis was about 1 mg/L. Reduced diapause egg hatching also was observed when exposed to 0.2 mg copper L−1. In contrast to their findings, we found P. similis mortality (LC50 = 1.1 mg copper L−1) was a more sensitive endpoint than diapause egg hatching (EC50 = 3.4 mg copper L−1).
Rebolledo et al.15 explored mercury toxicity to B. plicatilis and P. similis. They reported significant reproductive effects at 2 μg mercury L−1 in P. similis using life table analysis. This value is more closely related to a no observed effect concentration (NOEC) than the EC50 that we measured. In comparison, we found a mercury EC50 for P. similis population growth rate of 53 μg L−1.
The basic biology of P. similis has been explored because it is important in larviculture of marine fish like grouper12, 13, 22 rusty angelfish, humphead wrasse and Japanese eels.14 Variables like optimal temperature, salinity and diet for mass culture has been reported by these authors. No mention is made of diapause egg production, storage or hatching in these studies. We have confirmed that the rotifer that we are culturing is indeed P. similis species by DNA sequence analysis of the COI mitochondrial gene.17
There is substantial evidence that P. similis produces DEs that are capable of withstanding desiccation and able to hatch after several months of dormancy. In our cultures we have observed that adult P. similis do not survive desiccation or high salinity brines. In contrast, at least some eggs laid and aggregated on the bottom of culture tubes are capable of surviving these conditions. Moreover, many of these eggs remain viable after months of storage at 4°C and hatch in 18–21 h after transfer to 25°C and light. Unlike other monogononts, we have not been able to discriminate any morphological differences between DE and eggs developing directly without diapause. Nor have we observed any males or copulation in any of our P. similis populations. A survey of 12 monogonont species found males and described copulation in all taxa, providing the first documentation of sexual reproduction in the families Collothecidae, Trochosphaeridae, and Notommatidae.23 Unfortunately no members of the Proalidae were investigated, so we have no comparisons for P. similis within this family.
These observations suggest that P. similis is producing its DEs asexually. There is precedent for asexual DE production in Synchaeta pectinata, 24, 25 but we do not know how widespread it is among monogonont species. The role of sex in DE production of P. similis is important since DE production is used as evidence of sexual reproduction.26 If DE could be produced asexually by some monogonont species, we would have to re‐think our interpretation of this life cycle.
The DEs of Proales have been observed hatching from natural sediments at several sites. Moscatello and Belmonte27 described a hypersaline temporary pond (42.5–264 g L−1) in southeast Italy where they collected sediment cores. They found that resting stages of several zooplankton species hatched from these cores, including P. similis which was one of most abundant zooplankters at certain times of year. Moreno et al.28 reported that Proales sp. hatched from DEs collected from sediments of Tinaja Lake located in Ruidera Natural Park in Central Spain. A Mexican strain of P. similis native to northwestern Mexico was described by Reyes et al.16 This strain hatched from diapause eggs deposited in shrimp pond sediments from a farm south of Mazatlan, Sinaloa, Mexico. They reported rapid population growth of this strain, reaching maximum densities of 2 560/mL in 8 days at 25°C from an inoculation of 50/mL, for a population growth rate of 0.5/day. Rebolledo et al.29 reported DEs of Brachionus ibericus and P. similis hatched from sediment samples from a shrimp farm in the same area of Mexico. Desiccated propagules of P. similis were rehydrated in dustfall experiments by Rivas et al.30 They showed that desiccated DE of several micrometazoan species are dispersed widely by winds throughout the Chihuahuan desert. Dispersal of hundreds of kilometers by wind is apparently a common feature of inhabitants of ephemeral aquatic ecosystems that produce propagules tolerant of desiccation.
DE banks in sediments of aquatic habitats are critical to rotifer population adaptation, persistence, and genetic diversity.31 Moreover, sediments are often where the highest toxicant concentrations are found.3 Development of non‐brachionid rotifers for toxicity testing has been motivated by the desire to have test animals that are ecologically relevant for sediment toxicity assessment.1 Rotifer species that spend most of their life cycle in the benthos and have been used to assess sediment toxicity include Euchlanis dilatata, 32 Lecane quadridentata, 33 Lecane inermis, 34 and Philodina rapida.35 All of these efforts have exposed active adult rotifers to waterborne toxicants and measured endpoints like mortality, swimming, enzyme activity, and reproduction. Natural deposition of rotifer DE into aquatic sediments as part of their life cycle produces prolonged exposure to sediment toxicants. Consequently, hatching of DE is perhaps the most ecologically relevant endpoint for assessing sediment toxicity. In this paper we have described methods for performing such tests with P. similis DE. It should be noted, however, that similar tests could likewise be performed with brachiond DE.
In conclusion, P. similis is a useful animal for marine toxicity testing because of its small size, rapid reproduction, toxicant sensitivity, and production of diapause eggs. Proales DE enable the execution of toxicity tests without having to master techniques for culturing test animals. Furthermore, Proales can become part of a battery of cyst‐based marine toxicity tests available for assessing toxicity that previously has been based solely on the rotifer B. plicatilis. Because they naturally reside in sediments, rotifer DE are especially ecologically relevant for sediment toxicity testing. A mystery remains about the role of sex in DE production by P. similis and will require further research.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert assistance of Dr. David Mark Welch in analyzing the Proales COI gene DNA sequences. Dr. Martial Taillefert performed the inductively coupled plasma mass spectrometry to quantify the concentrations of the metal stock solutions. This work was supported in part by the Georgia Tech School of Biological Sciences and the Elizabeth Smithgall Watts endowment.
Snell TW, Johnston RK, Matthews AB, Park N, Berry S, Brashear J. Using Proales similis (Rotifera) for toxicity assessment in marine waters. Environmental Toxicology. 2019;34:634–644. 10.1002/tox.22729
REFERENCES
- 1. Snell TW, Marcial HS. Using rotifers to diagnose the ecological impacts of toxicants In: Hagiwara A, Yoshinaga T, eds. Rotifers, Fisheries Science Series. Singapore: Springer Nature Singapore Pte Ltd.; 2017:129‐147. [Google Scholar]
- 2. Won EJ, Han J, Kim DH, Dahms HU, Lee JS. Rotifers in ecotoxicology In: Hagiwara A, Yoshinaga T, eds. Rotifers, Fisheries Science Series. Singapore: Springer Nature Singapore Pte Ltd.; 2017:149‐176. [Google Scholar]
- 3. Rico‐Martinez R, Arzate‐Cardenas MA, Alvarado‐Flores J, Perez‐Legaspi IA, Santos‐Medrano GE. Rotifers as models for ecotoxicology and genotoxicology In: Larramendy ML, ed. Issues in Toxicology No. 33, Ecotoxicology and Genotoxicology: Non‐traditional Aquatic Models. London: Royal Society of Chemistry; 2017:48‐69. [Google Scholar]
- 4. Persoone G, Jaspers E, Claus C. Ecotoxicological Testing for the Marine Environment. State University of Ghent and Institute for Marine Scientific Research; 1984; vol I, 722 p, and vol II, 580 p. [Google Scholar]
- 5. Snell TW, Persoone G. Acute toxicity bioassays using rotifers. I. A test for brackish and marine environments with Brachionus plicatilis . Aquat Toxicol. 1989;14:65‐80. [Google Scholar]
- 6. Burgess RM. Characterizing and identifying toxicants in marine waters. A review of marine toxicity identification evaluations. Int J Environ Pollut. 2000;13:13‐26. [Google Scholar]
- 7. Wells PG, Lee K, Blaise C. Microscale Testing in Aquatic Toxicology. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 8. Snell TW, Johnston RK, Matthews AB. Freshwater toxicity testing using rehydrated Philodina sp. (Rotifera) as test animals. Environ Toxicol. 2017;32:2267‐2276. [DOI] [PubMed] [Google Scholar]
- 9. American Society of Testing and Materials (ASTM) . A standard practice for performing acute toxicity tests using rotifers in the genus Brachionus . Am Soc Testing Mater. 1991;11.04:1210‐1216. [Google Scholar]
- 10.Standard Methods for the Examination of Water and Wastewater. Estimating Chronic Toxicity Using Rotifers. Standard Methods for the Examination of Water and Wastewater. Vol 20, 8420; 1998; 8‐62–8‐65.
- 11. McDaniel M, Snell TW. Probability distributions of toxicant sensitivity for freshwater rotifer species. Environ Toxicol. 1999;14:361‐366. [Google Scholar]
- 12. Wullur S, Sakakura Y, Hagiwara A. The minute monogonont rotifer Proales similis de Beauchamp: Culture and feeding to small mouth marine fish larvae. Aquaculture. 2009;293:62‐67. [Google Scholar]
- 13. Wullur S, Sakakura Y, Hagiwara A. Application of the minute monogonont rotifer Proales similis de Beauchamp in larval rearing of seven‐band grouper Epinephelus septemfasciatus . Aquaculture. 2011;315:355‐360. [Google Scholar]
- 14. Le DVB, Nguyen PN, Dierckens K, et al. Growth performance of the very small rotifer Proales similis is more dependent on proliferating bacterial community than the bigger rotifer Brachionus rotundiformis . Aquaculture. 2017;476:185‐193. [Google Scholar]
- 15. Rebolledo UA, Nandini S, Sánchez OE, Sarma SSS. Combined effects of temperature and salinity on the demographic response of Proales similis (Beauchamp, 1907) and Brachionus plicatilis (Müller, 1786) (Rotifera) to mercury. Chemosphere. 2018;202:312‐321. [DOI] [PubMed] [Google Scholar]
- 16. Reyes JCR, Monteón CJL, Urreta HC, Dosta MCM, Montes de Oca GAR. Population growth and protein and energy content of Proales similis (Rotifera: Monogononta) reared at different salinities. Turk J Fisher Aquat Sci. 2017;17:767‐775. [Google Scholar]
- 17. Snell TW, Johnston RK, Matthews AB. Utilizing Brachionus biodiversity in marine finfish larviculture. Hydrobiologia. 2018. 10.1007/s10750-018-3776-8 [DOI] [Google Scholar]
- 18. Guillard RRL. Culture of phytoplankton for feeding marine invertebrates In: Berg CJ, Jr, ed. Culture of Marine Invertebrates. Stroudsburg, PA: Hutchinson Ross; 1983. [Google Scholar]
- 19. Snell TW. Rotifer ingestion test for rapid assessment of toxicity In: Blaise C, Férard JF, eds. Small‐Scale Freshwater Toxicity Investigations. Dordrecht: Springer; 2005. [Google Scholar]
- 20. Snell TW. The distribution of endpoint chronic values for freshwater rotifers In: Persoone G, Janssen CR, de Coen W, eds. New Microbiotests for Routine Toxicity Screening and Biomonitoring. New York: Kluwer Academic/Plenum Publishers; 2000:185‐190. [Google Scholar]
- 21. Aránguiz‐Acuña A, Pérez‐Portilla P. Metal stress in zooplankton diapause production: post‐hatching response. Ecotoxicology. 2017;26:329‐339. [DOI] [PubMed] [Google Scholar]
- 22. Hagiwara A, Wullur S, Marcial HS, Hirai N, Sakakura Y. Euryhaline rotifer Proales similis as initial live food for rearing fish with small mouth. Aquaculture. 2014;432:470‐474. [PubMed] [Google Scholar]
- 23. Alvarado‐Flores J, Guerrero‐Jiménez G, Silva‐Briano M, et al. Sexual reproductive biology of twelve species of rotifers in the genera: Brachionus, Cephalodella, Collotheca, Epiphanes, Filinia, Lecane, and Trichocerca . Marine Freshwater Behav Physiol. 2017;50(2):141‐163. [Google Scholar]
- 24. Gilbert JJ. Structure, development and induction of a new diapause stage in rotifers. Fresh Biol. 1995;34:263‐270. [Google Scholar]
- 25. Gilbert JJ, Schreiber DK. Asexual diapause induced by food limitation in the rotifer Synchaeta pectinata . Ecology. 1998;79:1371‐1381. [Google Scholar]
- 26. Walsh EJ, May L, Wallace RL. A metadata approach to documenting sex in phylum Rotifera: diapausing embryos, males, and hatchlings from sediments. Hydrobiologia. 2017;796:265‐276. [Google Scholar]
- 27. Moscatello S, Belmonte G. Active resting stages of zooplankton and its seasonal evolution in a hypersaline temporary pond of the Mediterranean coast (the “Vecchia Salina”, SE Italy). Scientia Marina. 2004;68:491‐500. [Google Scholar]
- 28. Moreno E, Conde‐Porcuna JM, Gómez A. Barcoding rotifer biodiversity in Mediterranean ponds using diapausing egg banks. Ecol Evol. 2017;7:4855‐4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rebolledo UA Nandini S, Sarma SSS, Reyes JCR, Montes de Oca GAR. Demographic and competition studies on Brachionus ibericus and Proales similis in relation to salinity and algal (Nannochloropsis oculata) density. Aquacult Int. 2018b;26:629‐644. [Google Scholar]
- 30. Rivas JAJ, Mohl JE, Van Pelt RS, et al. Evidence for regional aeolian transport of freshwater micrometazoans in arid regions. Limnol Oceanogr Lett. 2018;3:320‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montero‐Pau J, Serra M, Gómez A. Diapausing egg banks, lake size, and genetic diversity in the rotifer Brachionus plicatilis Muller (Rotifera, Monogononta). Hydrobiologia. 2017;796:77‐91. [Google Scholar]
- 32. Arias‐Almeida JC, Rico‐Martínez R. Toxicity of cadmium, lead, mercury and methyl parathion on Euchlanis dilatata Ehrenberg 1832 (Rotifera: Monogononta). Bull Environ Contam Toxicol. 2011;87:138‐142. [DOI] [PubMed] [Google Scholar]
- 33. Pérez‐Legaspi IA, Rico‐Martínez R. Acute toxicity tests on three species of the genus Lecane (Rotifera: Monogononta). Hydrobiologia. 2001;446(447):375‐381. [Google Scholar]
- 34. Klimek B, Fialkowska E, Kocerba‐Soroka W, Fyda J, Sobczyk M, Pajdak‐Stos A. The toxicity of selected trace metals to Lecane inermis rotifers isolated from activated sludge. Bull Environ Contam Toxicol. 2013;91:330‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Esbaugh AJ, Brix KV, Mager EM, De Schamphelaere K, Grosell M. Multi‐linear regression analysis, preliminary biotic ligand modeling, and cross species comparison of the effects of water chemistry on chronic lead toxicity in invertebrates. Comp Biochem Physiol C. 2012;155:423‐431. [DOI] [PubMed] [Google Scholar]


