Abstract
Background
Hidradenitis suppurativa (HS) is a chronic, relapsing, inflammatory skin disease characterized by painful inflamed nodules, recurrent abscesses and fistulas located in apocrine gland–bearing body sites. The negative impact of HS on patient's quality of life (QoL) has been reported to be greater than other dermatologic conditions as psoriasis and atopic eczema, and its improvement is an important goal in disease management. Nowadays, there are no specific validated QoL instruments available for HS and generic dermatologic questionnaires are used.
Objective
The objective of this study was to demonstrate the validity, reliability and responsiveness of HIDRAdisk, a new innovative tool designed for rapid assessment of HS burden and, at the same time, an intuitive graphic visualization of the measurement outcome.
Methods
A multicentre, longitudinal, observational study was conducted to validate the HIDRAdisk compared with other validated questionnaires [Skindex‐16, Dermatology Life Quality Index (DLQI), Work Productivity and Activity Impairment–General Health (WPAI:GH)] and to evaluate its correlation with disease severity in Italian patients with any degree of HS severity, as measured by Hurley stage and HS Physician Global Assessment (HS‐PGA).
Results
A total of 140 patients (59% women; mean age 34.9 ± 11.0 years) were enrolled in 27 dermatologic centres. HIDRAdisk showed a strong correlation with Skindex‐16 and DLQI, and a good one with WPAI:GH (correlation coefficient: 0.7568, 0.6651 and 0.5947, respectively) and a statistically significant correlation with both Hurley stage and HS‐PGA. Very good internal consistency (Cronbach coefficient >0.80; intraclass correlation coefficient >0.6), with correlation between the 10 items, good test–retest reliability (Spearman correlation coefficient, 0.8331; P < 0.0001) and responsiveness to changes were demonstrated.
Conclusion
Our study shows that HIDRAdisk, a short and innovative visual HS QoL instrument, has been psychometrically validated in Italian language and it may help improve the management of HS once implemented in routine clinical practice.
Introduction
Hidradenitis suppurativa (HS) is a chronic, relapsing, inflammatory skin disease clinically characterized by painful inflamed nodules, recurrent abscesses and fistulas located in apocrine gland–bearing body sites.1 The negative impact of HS on patients’ quality of life (QoL) has been reported to be greater than other dermatologic conditions such as psoriasis, atopic eczema and chronic urticaria.2 Indeed, involvement of an extensive surface area may interfere with daily activities, such as walking or hugging, due to pain in the groin and/or axillary area, and may cause social embarrassment due to purulent discharge odour.3, 4 HS is associated with high psychiatric morbidity and social impairment,5 in particular with anxiety, depression, social withdrawal and feelings of stigmatization. The effects of chronic skin diseases on the psychosocial, occupational and interpersonal aspects of a patient's QoL are often underestimated by physicians and not taken into consideration by the healthcare system.6, 7 The improvement of QoL in HS patients is an important goal in disease management.
Nowadays, there are no specific and validated QoL instruments available for HS and generic dermatologic questionnaires are used. In this setting, the HIDRAdisk is the first Italian tool designed to assess HS burden.8 Developed by a group of patients and dermatologists using the Delphi method, the questionnaire explores the impact of HS on 10 domains, such as the general state of health, pain, odour, symptom control, skin involvement, personality, social life, sexual life, work and daily activities. The answers are graphically represented on a disc as a polygon, providing an immediate and intuitive representation of the impact of HS on patient's QoL at baseline and the achieved clinical benefit at following visits, since the area of the polygon shrinks when the burden of disease decreases. The HIDRAdisk is designed to be completed by the patient but coloured disc results are discussed with the dermatologist, fostering better patient–physician communication, which may positively influence treatment adherence.7 Furthermore, the HIDRAdisk is administered on electronic devices, offering dermatologists a fast and easy way to measure response in routine clinical practice or in clinical studies.9 The aim of this study was to demonstrate the validity, reliability and responsiveness of the HIDRAdisk as a specific QoL instrument in patients with HS.
Materials and methods
Study
A multicentre, longitudinal, observational study was conducted on Italian HS patients enrolled between July and November 2016. The primary objective was to validate the HIDRAdisk compared with other validated questionnaires [Skindex‐16 and Dermatology Life Quality Index (DLQI)] in patients with HS and to evaluate its correlation with disease severity. Secondary objectives were to assess the influence of the new tool on the patient–physician relationship and to assess patient perception/satisfaction of the new instrument. Clinical HS severity was scored using Hurley stage10 and HS Physician Global Assessment (HS‐PGA).11 Clinical improvement was assessed using the HS Clinical Response12 (HiSCR) and was defined as a reduction of ≥50% in total abscesses (A) and inflammatory nodules (N) count with no increase in abscesses and draining fistulas count compared to basal visit. For study purposes, we also assessed a partial HiSCR defined as a reduction of at least 25% of AN. According to the protocol, an interim analysis for the questionnaire validation was performed when the first 140 enrolled subjects completed the first 3 months of the study or prematurely discontinued from the study.
Centres and subjects
The study was conducted in 27 Italian dermatologic centres selected on the basis of visiting ≥1 subject per month with moderate‐to‐severe HS (according to Hurley or HS‐PGA staging) and availability of internet connection. The participation in the study was proposed consecutively to patients who had an outpatient visit during the study enrolment period. Inclusion criteria included male and female patients aged ≥18 years, affected by HS of any grade/severity, diagnosed ≥6 months before by a dermatology centre and able to understand and complete study‐related questionnaires. Patients with current malignancies or any other important disease (at the physician's discretion) that could impact significantly QoL, those with relevant psychiatric comorbidities, and those in a HS clinical trial were excluded from the study. Patients could be on any therapy for HS. The study protocol was approved by each local ethics committee. All patients provided informed consent for the use of their personal data. Each subject had three visits: visit 2 was performed between 2 and 7 days after visit 1, and visit 3 occurred 3 months after visit 1.
Questionnaires
The HIDRAdisk, Skindex‐16, DLQI and Work Productivity and Activity Impairment–General Health (WPAI‐GH) questionnaires were provided in an electronic format on a tablet device; the HIDRAdisk App used in the study was developed in accordance to the Food and Drug Administration PRO Guidance for Industry.13 The device did not permit unanswered questions. All questionnaires were administered at visit 1 and 3; the HIDRAdisk was also administered at visit 2.
The HIDRAdisk questionnaire is composed of 10 questions (refer to Table S1). Patients answer each question by indicating their perception of HS burden on a visual analogue scale (VAS) ranging from 0 (absolutely not) to 10 (definitely yes). The total score is the sum of the single VAS (0 = no impact of the disease on QoL; 100 = maximum impact of disease on QoL; Fig. 1).8
Figure 1.
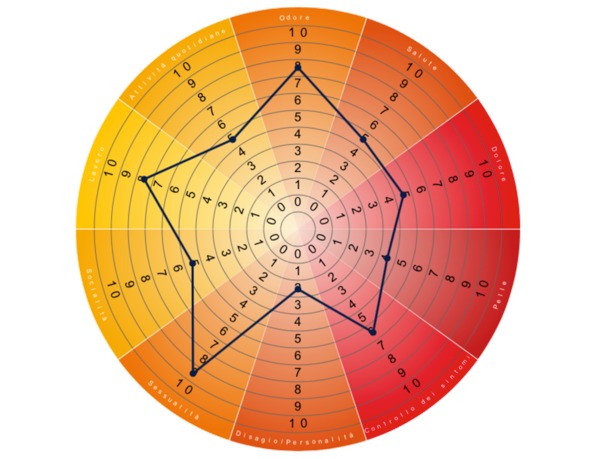
Example of a theoric HIDRAdisk score. Single scores are summed to give the total score and visually linked together to draw a polygon reflecting the extension of the burden of the disease. In this example, the final score is 57.
The Skindex‐16 is a QoL measure for patients with skin diseases. This 16‐item questionnaire is subdivided into three domains: symptoms, emotions and functioning. All scores vary from 0 (no effect) to 100 (effect experienced all the time).14, 15
The DLQI is a 10‐item questionnaire that measures how much the skin disease affected QoL over the past week. The score has a maximum of 30 and a minimum of 0 (the higher the score, the greater the QoL impairment).16
The WPAI‐GH is a 6‐item questionnaire on general health status and health problems’ related fatigue on working activities.17 WPAI outcomes are expressed as impairment percentages due to health, with higher numbers indicating greater impairment and less productivity, as follows: missed work‐time, impairment while working, overall work impairment and activity impairment. The Italian translation was used in this study (Italian for Italy, WPAI:GH V2.3, 3/FEB/2015).18
The Subject Satisfaction Questionnaire was created by the study team in order to evaluate patient perception of the disease and satisfaction with the HIDRAdisk instrument. The first question queries the severity of disease (choices: ‘very mild’, ‘mild’, ‘fair’, ‘high’, ‘very high’); the second question explores whether the HIDRAdisk questionnaire helped the physician understand how each patient experiences the disease; and the third question examines how much – from patient's perception – the HIDRAdisk is useful in providing the physician with a satisfactory overall understanding of their problems due to the disease (both with 5 responses ranging from ‘not at all’ to ‘very much so’).
Sample size
The sample size was determined according to validation criteria19: a total of 10 subjects per each item of the questionnaire were needed. The HIDRAdisk questionnaire consists of 10 items; hence, the study would require a minimum sample size of 100 subjects. Due to the expected rate (20%) of patients lost to follow‐up (based on previous experience from PSOdisk questionnaire validation20), the sample size was increased to 140 subjects. The evaluable population for the validation study was defined as the first 140 patients diagnosed with HS who met the inclusion criteria and performed the first visit.
Psychometric evaluation
The evaluation was performed using the data collected at three different time periods (visits 1, 2 and 3). The face and content validity have already been assessed through focus groups using the Delphi method involving 10 Italian HS treating physicians and nine patients.8
The construct validity was evaluated clinically and psychometrically at visit 1. First, differences in HIDRAdisk total scores were analysed using correlations between HIDRAdisk total score and QoL scores at visit 1 in terms of Spearman's rank correlation, as data were not normally distributed. Correlations between HIDRAdisk total score and severity of HS (Hurley stage and HS‐PGA) at visit 1 were provided using analysis of variance models on ranks, because of non‐normality of residuals. Second, an exploratory factor analysis was performed to identify the factor structure underlying the HIDRAdisk items. Under the hypothesis that the underlying factors are correlated (mainly in a clinical, psychological and a social subdomain), principal axes factor analysis, followed by an oblique rotation, was applied. The number of factors was determined by retaining only those factors with an eigenvalue >1 after factor rotation.
The internal consistency reliability was evaluated at visit 1 using Cronbach's alpha coefficient, a parameter calculated from the pairwise correlations between the 10 items of the HIDRAdisk questionnaire. Internal consistency ranges between 0 and 1: 0.6–0.7 indicates acceptable reliability and ≥0.8 good reliability. Both raw and standardized Cronbach's alpha coefficients were calculated.
The test–retest reliability was determined by administering the same questionnaire to the same respondents in a short term interval (between visit 1 and visit 2). The correlation between the two sets of responses was provided in terms of Spearman's rank correlation and Intraclass Correlation Coefficient (ICC). The ICC was calculated for each item as the ratio between variability due to differences in the visits (intercept covariance parameter estimates) and total variability (intercept covariance parameter estimates + residual covariance parameter estimates). The less scattered the results, the greater the test–retest reliability of the study instrument.
The responsiveness to change was tested using a Wilcoxon signed rank test to compare the change in scores from visit 1 to visit 3 in two groups: patients who did and did not achieve partial HiSCR.
Results
One hundred forty patients (59% women; mean age 34.9 ± 11.0 years) were enrolled in 27 dermatologic centres. Baseline demographics of the study population are described in Table 1. One hundred and thirty‐six patients attended visit 2 (97.1%), and 134 attended visit 3 (95.7%). Five patients were lost to follow‐up and one withdrew consent.
Table 1.
Patient demographics at baseline
| n | % | ||
|---|---|---|---|
| Sex | Male | 57 | 40.7 |
| Female | 83 | 59.3 | |
| Age (years) | Mean ± SD | 34.9 ± 11.0 | |
| Race | White | 138 | 98.6 |
| Asian | 1 | 0.7 | |
| Other | 1 | 0.7 | |
| Civil status | Single | 86 | 61.4 |
| Married | 48 | 34.3 | |
| Divorced | 5 | 3.6 | |
| Widower | 1 | 0.7 | |
| Education level | Primary school | 3 | 2.1 |
| Secondary school | 42 | 30.0 | |
| High school | 78 | 55.7 | |
| University | 17 | 12.1 | |
| Smoking habits | Smoker | 96 | 68.6 |
| Never smoked | 33 | 23.6 | |
| Ex‐smoker (>6 months) | 11 | 7.9 | |
| Alcohol consumption | Yes | 28 | 20.0 |
| No | 108 | 77.1 | |
| Ex‐drinker (>1 month) | 4 | 2.9 | |
| BMI, kg/m2 | Mean ± SD | 28.0 ± 5.8 | |
| BMI <23 | — | 19.3 | |
| 23 ≤ BMI < 25 | — | 17.9 | |
| 25 ≤ BMI < 30 | — | 30.7 | |
| BMI≥ 30 | — | 32.1 | |
| Time from HS diagnosis to visit 1 (years)† | Mean ± SD | 4.2 ± 5.0 | — |
| Duration of illness‡ | <5 years | 101 | 72.1 |
| 5–14 years | 28 | 20.0 | |
| ≥14 years | 11 | 7.9 | |
| Patients with ≥1 major concomitant pathology | Yes§ | 39 | 28.3 |
| Missing | 2 | – | |
| Major concomitant pathology | Obesity | 17 | 12.1 |
| Hypertension | 7 | .0 | |
| Dyslipidaemia | 5 | 3.6 | |
| Diabetes | 5 | 3.6 | |
| Crohn disease | 2 | 1.4 | |
| Spondyloarthropathy | 2 | 1.4 | |
| Metabolic syndrome | 2 | 1.4 | |
| Depression | 2 | 1.4 | |
| Anxiety | 2 | 1.4 | |
| Arthritis | 1 | 0.7 | |
| Other | 10 | 7.1 | |
†Time from HS initial diagnosis to IC signature was calculated in years as the difference between date of IC signature and the date of HS diagnosis + 1 day. ‡Duration of illness was calculated, in years, as the difference between date of IC signature and the date of HS diagnosis + 1 day. §Patients could report >1 major other pathology.
BMI, body mass index; HS, hidradenitis suppurativa; IC, informed consent; SD, standard deviation.
Patients had varying HS severity at baseline (Table 2): Hurley stage 3, 24.6%; Hurley stage 2, 43.5%; Hurley stage 1, 31.9%; HS‐PGA scores were very severe, 9.3%; severe, 11.4%; moderate, 47.1%; mild, 23.6%; minimal, 5.7%; clear, 2.9%. Patients’ perception of HS severity, measured using the Subject Satisfaction Questionnaire, was very high (15.7%), high (41.4%), fair (30.7%), mild (7.1%) and very mild (5%).
Table 2.
HS characteristics and management at visits 1 and 3
| Visit 1 n = 140 | Visit 3 n = 134 | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Hurley stage | 1 | 44 | 31.9 | 54 | 41.5 |
| 2 | 60 | 43.5 | 49 | 37.7 | |
| 3 | 34 | 24.6 | 27 | 20.8 | |
| Missing | 2 | – | 4 | – | |
| Number of inflammatory nodules | Mean ± SD | 5.2 ± 6.6 | — | 3.7 ± 4.7 | — |
| Number of abscesses | Mean ± SD | 2.0 ± 3.4 | — | 1.7 ± 3.1 | — |
| Number of fistulas | Mean ± SD | 2.0 ± 4.6 | — | 1.5 ± 3.8 | — |
| Hidradenitis Suppurativa Physician Global Assessment | Clear | 4 | 2.9 | 4 | 3.0 |
| Minimal | 8 | 5.7 | 19 | 14.2 | |
| Mild | 33 | 23.6 | 40 | 29.9 | |
| Moderate | 66 | 47.1 | 46 | 34.3 | |
| Severe | 16 | 11.4 | 11 | 8.2 | |
| Very severe | 13 | 9.3 | 14 | 10.5 | |
| Patients with ≥1 current pharmacologic treatment for HS at enrolment | — | 86† | 62.3 | — | — |
| Surgical treatment for HS | — | 73‡ | 52.1 | 7§ | 5.2 |
| Number of flares¶ | Mean ± SD | 8.8 ± 8.9†† | — | 3.4 ± 7.6‡‡ | — |
| Need of a professional caregiver (nurse) | — | 22 | 15.7 | 26 | 18.7 |
| Number of medications | Mean ± SD | 38.0 ± 76.5§§ | — | 21.4 ± 47.0¶¶ | — |
| Number of general practitioners + other specialists visits | Mean ± SD | 3. 6 ± 4.0§§ | — | 1.5 ± 2.1¶¶ | — |
†Patients could report >1 treatment: 22.9% reported antiacne preparations, 20.7% reported antibacterials for systemic use, 16.4% reported immunosuppressants, and 12.9% reported antimycobacterials. ‡Patients with ≥1 surgical treatment since HS onset. §Patients with ≥1 surgical treatment for HS in the past 3 months. ¶According to physicians’ opinion. ††Flares occurred in the past 12 months. ‡‡Flares occurred in the past 3 months. §§Summarized considering the past 6 months before the study start. ¶¶Summarized considering the past 3 months.
HS, hidradenitis suppurativa; SD, standard deviation.
At visit 3, 42/134 patients (31.3%) achieved HiSCR and 61/134 (45.5%) patients achieved a partial HiSCR. Patients’ (n = 132) perception of HS severity was very high (13.1%), high (27.7%), fair (36.2%), mild (17.7%) and very mild (5.4%). The most frequent locations at visit 3 were the same reported at visit 1: right and left axilla (reported by 48.5% and 47.0% of patients, respectively), groins (reported by 46.3% of patients for both), genital (31.3%) and perineal (21.6%) area. A reduction in the number of lesions (‘improved’) was observed in 38.8% of patients, while 34.3% had the same number of lesions at both visits (‘stable’), and 26.9% had an increase in the number of lesions at both visits (‘worsened’). The other HS characteristics at visit 3 are described in Table 2. All questionnaires total scores decreased from visit 1 to visit 3 (Table 3) and varied in accordance with disease severity (Fig. 2a according to Hurley and Fig. 2b according to HS‐PGA).
Table 3.
Questionnaire total scores
| Questionnaire total score Mean ± SD (range) | Visit 1 n = 140 | Visit 2 n = 136 | Visit 3 n = 134 | |
|---|---|---|---|---|
| HIDRAdisk† | 68.2 ± 22.6 (1–100) | 65.0 ± 24.0 (1–100) | 62.2 ± 25.3 (0–100) | |
| DLQI‡ | 11.3 ± 8.5 (0–30) | / | 9.6 ± 7.8 (0–30) | |
| Skindex‐16§ | 62.5 ± 25.6 (0–100) | / | 53.4 ± 29.7 (0–100) | |
| WPAI‐GH¶ | Overall work impairment | 21.4 ± 32.3% (0%–100%) | / | 16.6 ± 28.1% (0%–100%) |
| Total activity impairment | 44.0 ± 32.8% (0%–100%) | / | 39.5 ± 32.7% (0%–100%) | |
†Completed by 140 patients at visit 1, 136 patients at visit 2, and by 132/134 at visit 3. ‡Completed by 140 patients at visit 1 and 132 patients at visit 3. §Completed by 140 patients at visit 1 and 128 patients at visit 3. ¶Completed by 140 patients at visit 1 and 132 patients at visit 3.
DLQI, Dermatology Life Quality Index; SD, standard deviation; WPAI‐GH, Work Productivity and Activity Impairment–General Impairment.
Figure 2.
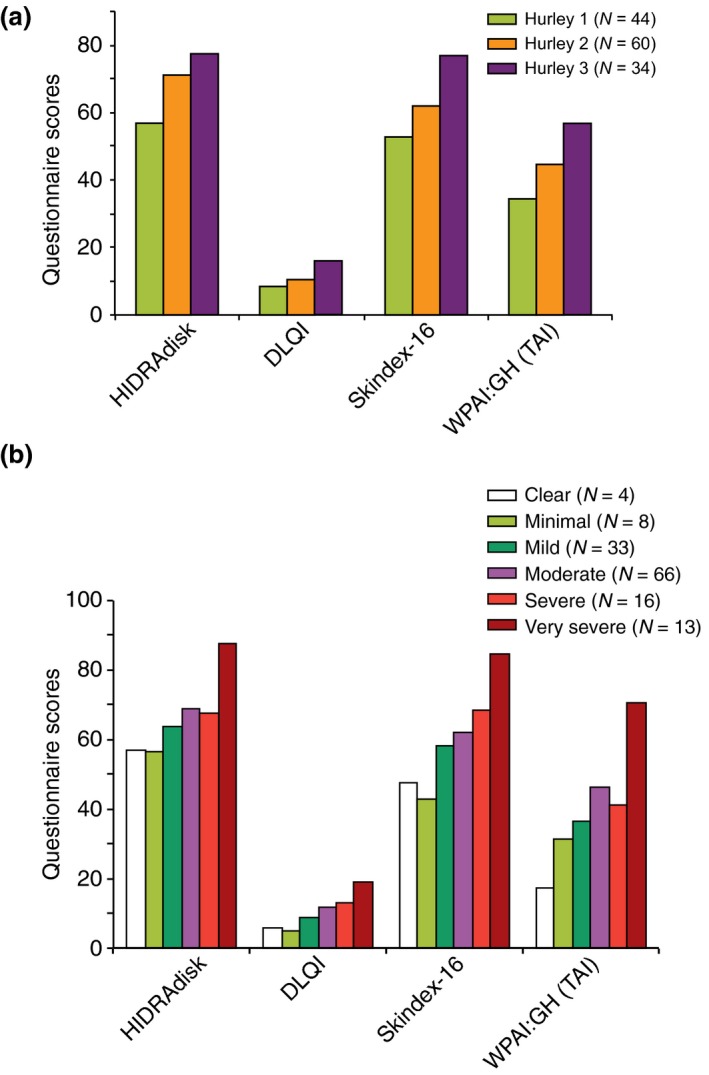
Questionnaires scores stratified by Hurley (a) and Hidradenitis Suppurativa Physician Global Assessment (b).
Psychometric evaluation
Construct validity
All the correlations between HIDRAdisk total score and QoL scores at visit 1 (DLQI total score, Skindex‐16 total score and WPAI:GH total activity impairment) were statistically significant (P < 0.0001) and correlations coefficients were good (0.6651, 0.7568 and 0.5947, respectively). Correlations between HIDRAdisk total score and the severity of HS (Hurley stage and HS‐PGA) at visit 1 were both statistically significant (P = 0.0002 and P = 0.0041, respectively). The factor analysis showed only 1 factor with an eigenvalue >1, therefore, not allowing the grouping of items into domains and a factor rotation.
Internal consistency reliability
Both raw and standardized Cronbach coefficient values were >0.80 (0.894 and 0.898, respectively). Correlation between the 10 items ranged between 0.308 and 0.673.
Test–retest reliability
The correlation between visit 1 and 2 was statistically significant (P < 0.0001) and correlations coefficients were good (>0.70 for 6 of 10 items, Table S2). The Spearman correlation coefficient for the total HIDRAdisk score was 0.8331 (P < 0.0001). All ICC results were >0.6, with the ‘sexuality’ score >0.80.
Responsiveness to change
Among patients who achieved at least the partial HiSCR at visit 3 (45.5%), improvements from visit 1 to visit 3 were statistically significant for most parameters: total HIDRAdisk score (P < 0.0001), odour (P < 0.0001), pain (P < 0.0001), daily activities (P = 0.0020), general health (P = 0.0018), skin (P = 0.0012), work (P = 0.0049) and uneasiness/personality (P = 0.0039). In patients who did not achieve partial HiSCR, change from baseline was statistically significant only for the following parameters: pain (P = 0.0011), uneasiness/personality (P = 0.0194) and daily activities (P = 0.0308).
Secondary analysis
The Subject Satisfaction Questionnaire was completed by 140 patients at visit 1 and 132 out of 134 at visit 3. More than 80% of patients chose the two highest scores at both visits for question 2 and 3 (Fig. 3a,b).
Figure 3.
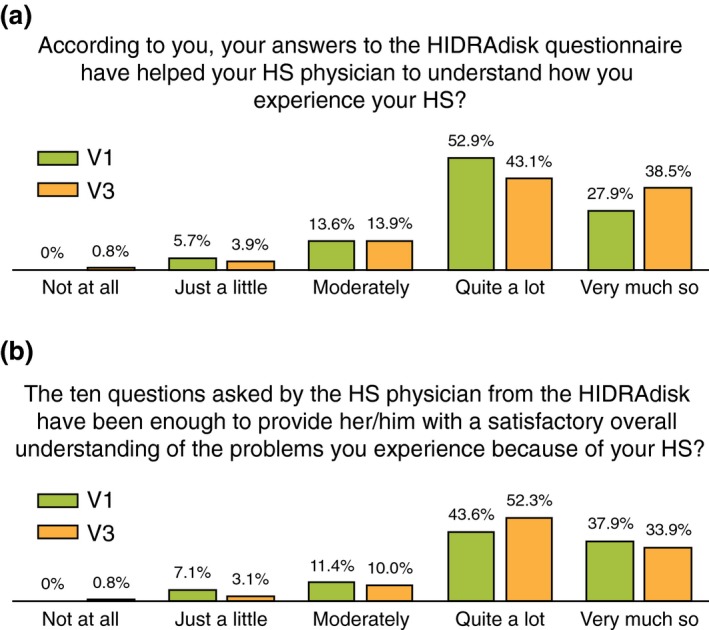
Subject's Satisfaction questionnaire responses. HS, hidradenitis suppurativa.
Discussion
In this multicentric study, the HIDRAdisk was validated psychometrically in a large sample of Italian patients with HS, with varying degrees of disease severity and independently from the medical treatment used.
This new questionnaire showed strong correlation with the dermatologic questionnaires, DLQI and Skindex‐16, and a good one with WPAI:GH (total activity impairment). HIDRAdisk correlation with the severity of HS was statistically significant for both Hurley stage and HS‐PGA, confirming that higher scores on the HIDRAdisk correspond to more severe disease. This evidence and the strong appreciation of HIDRAdisk, as shown by patients through the satisfaction questionnaire, support the use of this HS‐specific tool in clinical practice, together with any current validated severity index, to provide a ‘360‐degree’ assessment of patients burden of disease. The factor analysis showed that HIDRAdisk is composed of a single dimension, as opposed to our hypothesis of three domains (clinical, psychological and social). Its unidimensionality allows the calculation of the total score as the sum of the scores of each question. The HIDRAdisk also demonstrated very good internal consistency reliability and good correlation between the 10 items, showing a high consistency of the results delivered as a unique score and strengthening the unidimensionality of the questionnaire, where all questions address the same underlying construct. The test–retest reliability results highlighted the good HIDRAdisk reproducibility in a short time interval, when the clinical conditions are assumed to be stable and therefore the QoL is expected to be almost the same. Furthermore, HIDRAdisk proved to be responsive to disease changes as assessed over 3 months: the total score changed significantly among patients achieving at least a partial HiSCR. Despite being the most used instrument for HS treatment outcome, the HiSCR may not be able to detect HS severity changes in normal clinical practice; it is plausible that HiSCR is not sensitive for revealing a minimal improvement in HS severity, despite QoL changes from baseline. For this reason, we addressed a medical need for a comprehensive QoL PRO specific for HS.
To date, several QoL questionnaires are in development to study the impact of HS burden on QoL; however, thus far, no patient‐reported outcome measures have demonstrated to be easy to use, quick to score and supporting dermatologists in the management of HS patients. Recently, two QoL HS‐specific tools have been developed: the composite HS Impact Assessment and HS Symptom Assessment tool (HSIA&HSSA)21 (tested in 40 patients) and HS Burden of Disease22 (tested in 29 patients). HSIA&HSSA are currently under validation in a population of 150 subjects. Furthermore, PRO measures are under investigation to be integrated in patients’ clinical evaluation in order to provide a better description of HS burden, as in the recently developed SAHS score.23
From our perspective, a limitation of this validation model could be the use of comparison questionnaires there were not disease‐specific; further analysis comparing HIDRAdisk scores vs. other HS‐specific QoL tools might better support its usefulness. In addition, the effect of the various pharmacologic treatments and surgeries on QoL, as measured by HIDRAdisk, still remains to be evaluated. Data from additional 160 patients with a follow‐up of 9 months will be available shortly, as well as an evaluation of possible cut‐off for patients classification based on disease impact on patient QoL. We also acknowledge a possible selection bias at enrolment due to the fact that routinely HS patients do not come back for a second visit in 1 week interval and therefore some patients might not have accepted to participate in the study. Furthermore, a limitation of this validation study could be its implementation only on the Italian population. For international use, the questionnaire must be translated and its psychometric properties need to be tested in different populations.
In conclusion, the HIDRAdisk is a validated visual instrument administered on electronic devices, completed by patient and dermatologist together, making it an easy‐to‐use QoL tool that, in our wishes, should be implemented soon in routine clinical practice to improve the management of HS, as well as strengthen the patient–physician relationship.
Supporting information
Table S1. HIDRAdisk questionnaire in Italian and in English.
Table S2. HIDRAdisk items test‐retest reliability.
Acknowledgements
We wish to thank Daria Nucciarelli of AbbVie for medical writing and critical review of the manuscript.
Conflicts of interest
K. Peris has served as advisory board member or speaker for AbbVie, Lilly, Novartis and Roche and received research grants from AbbVie and Almirall. G. Fabbrocini, G. Pistone, E. Guanziroli, A. Bertoldi, F. Cusano, A. Casari, M. Fusano, C. Potenza, L.L. Mancini, T. Deboli, F. Rongioletti, M. Ardigò, A. Parodi, V. Dini and M.L. Musumeci declared no conflicts of interest. C. Guarneri has received honoraria from lectures and/or board membership from AbbVie, Pfizer, Janssen, Lilly, and Novartis. A. Offidani has served as a speaker, consultant, and advisory board member for AbbVie, Lilly, Novartis, Pfizer, Sanofi‐Genzyme, UCB, Celgene, and Galderma. L. Stingeni has acted as speaker and consultant for AbbVie, Lilly, Novartis, and Admiral. G. Malara acted as speaker and advisory board member for Janssen, Novartis, Lilly, and Celgene. G. Gualberti and V. Saragaglia are AbbVie employees and may own stocks/options. L. Bianchi acted as speaker or consultant for AbbVie, UCB, Pfizer, Janssen, Celgene. V. Bettoli acted as speaker, consultant and advisory board member for AbbVie, Pierre‐Fabre, Galderma, Mylan, Difa‐Cooper, L'Oreal. C. Franchi acted as speaker for Eli Lilly and AbbVie. F. Prignano acted as speaker for Eli‐Lilly, AbbVie, Novartis, Abiogen Pharma, Leopharma, Novartis. A. Patrizi has acted as speaker or consultant or advisory board member for Abbvie, Lilly, Novartis, Almirall, Celgene, Sanofi‐Genzyme, Leo Pharma, Janssen. A. Lo Schiavo declared conflict of interest with Janssen, Novartis, Abbvie, Lilly. P. Amerio declared speaker and consultant fees from Abbvie, Jannsen, Pfizer, Celgene.
Funding
AbbVie funded the study and participated in the design and study conduct, as well as in interpretation of the data, review and approval of the manuscript.
References
- 1. Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012; 12: 158–164. [DOI] [PubMed] [Google Scholar]
- 2. Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010; 90: 264–268. [DOI] [PubMed] [Google Scholar]
- 3. Onderdijk AJ, van der Zee HH, Esmann S et al Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2013; 27: 473–478. [DOI] [PubMed] [Google Scholar]
- 4. Kurek A, Peters EM, Chanwangpong A, Sabat R, Sterry W, Schneider‐Burrus S. Profound disturbances of sexual health in patients with acne inversa. J Am Acad Dermatol 2012; 67: 422–428, 428.e1. [DOI] [PubMed] [Google Scholar]
- 5. Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol 2011; 91: 328–332. [DOI] [PubMed] [Google Scholar]
- 6. Hong J, Koo B, Koo J. The psychosocial and occupational impact of chronic skin disease. Dermatol Ther 2008; 21: 54–59. [DOI] [PubMed] [Google Scholar]
- 7. Alavi A, Anooshirvani N, Kim WB, Coutts P, Sibbald RG. Quality‐of‐life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol 2015; 16: 61–65. [DOI] [PubMed] [Google Scholar]
- 8. Chiricozzi A, Bettoli V, De Pità O et al HIDRAdisk: an innovative visual tool to assess the burden of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2019; 33: e24–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White A, Danis M. Enhancing patient‐centered communication and collaboration by using the electronic health record in the examination room. JAMA 2013; 309: 2327–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach In Roenigk RK, Roenigk HH, eds. Dermatologic Surgery. Marcel Dekker, New York, NY, 1996: 623–645. [Google Scholar]
- 11. Kimball AB, Kerdel F, Adams D et al Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012; 157: 846–855. [DOI] [PubMed] [Google Scholar]
- 12. Kimball AB, Jemec GB, Yang M et al Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol 2014; 171: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 13. FDA Guidance for Industry . Patient‐Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. U.S. Department of Health and Human Services, Silver Spring, MD, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement of properties of Skindex‐16, a brief quality‐of‐life measure for patients with skin diseases. J Cutan Med Surg 2001; 5: 105–110. [DOI] [PubMed] [Google Scholar]
- 15. Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin 2012; 30: 231–236, xiii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 17. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. [DOI] [PubMed] [Google Scholar]
- 18. Reilly Associates. URL http://www.reillyassociates.net/WPAI_Translations.html (last accessed: 28 June 2018).
- 19. Streiner DL, Norman GR. Validity In Streiner DL, ed. Health Measurement Scales. A Practical Guide to Their Development and Use. Oxford University Press, New York, NY, 1995: 104–162. [Google Scholar]
- 20. Sampogna F, Linder D, Romano GV, Gualberti G, Merolla R, di Luzio PU. Results of the validation study of the Psodisk instrument, and determination of the cut‐off scores for varying degrees of impairment. J Eur Acad Dermatol Venereol 2014; 29: 725–731. [DOI] [PubMed] [Google Scholar]
- 21. Kimball AB, Sundaram M, Banderas B, Foley C, Shields AL. Development and initial psychometric evaluation of patient‐reported outcome questionnaires to evaluate the symptoms and impact of hidradenitis suppurativa. J Dermatolog Treat 2018; 29: 152–164. [DOI] [PubMed] [Google Scholar]
- 22. Pinard J, Vleugels RA, Joyce C, Merola JF, Patel M. Hidradenitis suppurativa burden of disease tool: pilot testing of a disease‐specific quality of life questionnaire. J Am Acad Dermatol 2018; 78: 215–217.e2. . [DOI] [PubMed] [Google Scholar]
- 23. Hessam S, Scholl L, Sand M, Schmitz L, Reitenbach S, Bechara FG. A novel severity assessment scoring system for hidradenitis suppurativa. JAMA Dermatol 2018; 154: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. HIDRAdisk questionnaire in Italian and in English.
Table S2. HIDRAdisk items test‐retest reliability.


