Abstract
Ewing sarcoma (ES) family of tumors includes bone and soft tissue tumors that are often characterized by a specific translocation between chromosome 11 and 22, resulting in the EWS-FLI1 fusion gene. With the advent of multi-modality treatment including cytotoxic chemotherapy, surgery, and radiation therapy, the prognosis for patients with ES has substantially improved. However, a therapeutic plateau is now reached for both localized and metastatic disease over the last two decades. Burdened by the toxicity limits associated with the current frontline systemic therapy, there is an urgent need for novel targeted therapeutic strategies. In this review, we discuss the current treatment paradigm of ES, and explore preclinical evidence and emerging treatments directed at tumor signaling pathways and immune targets.
Keywords: Ewing sarcoma, antibodies, immunotherapy, targeted therapy, pediatric sarcomas
Introduction
Ewing sarcoma (ES) family of tumors is a family of small round blue cell tumors that arise from bone or soft tissue. It represents the second most common malignant bone tumor in children and young adults, with an incidence of more than 200 cases per year in the United States (1). ES is characterized by a specific translocation involving EWS (Ewing sarcoma gene) on chromosome 22 with one of the E26 transformation-specific transcription factory family genes. The EWS-FLI1 (Friend Leukemia Integration 1 transcription factor) fusion gene, t(11;22)(q24;q12) is found in ~85% of ES tumors. The fusion protein plays a key role in the pathogenesis and proliferation of ES (2, 3), with EWS-FLI1 knockdown cells showing decreased proliferation in vitro and tumor regression in vivo (4, 5). Although the fusion protein has multiple functions, one of its primary roles is as a transcription factor, increasing the expression of many downstream targets involved in tumor survival and growth [for example, IGF1 (6), GLI1 (7), Myc (8), ID2 (9)], while decreasing expression of cell cycle regulators and pro-apoptotic genes [for example, TGFB2 (10), p21 (11), IGFBP3 (12)]. In addition, the fusion protein plays an important role in promoting cell differentiation by upregulating such genes as EZH2 (13) and SOX2 (14). Although ES cells were originally thought to arise from primitive neuroectodermal cells, there is now growing evidence (although not conclusive) that ES cells arise instead from mesenchymal stem cells (15, 16), and that the neuroectodermal phenotype of ES is secondary to EWS-FLI1 expression (17).
With the introduction of multi-disciplinary management and specifically cytotoxic chemotherapy, survival for localized ES has improved from <20 to 70–80% by the 1990's. However, over the last two decades, there has been no further advancement in survival, witnessing the limit of further intensification of cytotoxic chemotherapy to cure children and young adults with localized disease. Additionally, the current frontline systemic therapy is aggressive and carries with it significant morbidity. For patients with metastatic disease, prognosis has remained poor, with survival rates of <30% in those with isolated lung metastases and <20% for those with bone and bone marrow involvement (18, 19). Outcomes for patients with relapsed disease is even poorer, with a 5-year survival rate of only 13%. Given these considerations of toxicity and suboptimal survival from metastatic disease, there is an urgent unmet need to develop novel therapies for ES (20).
Molecularly targeted therapy and immunotherapy are promising approaches for attacking these tumors without a significant increase in overlapping toxicity with chemoradiation (21, 22). A good example of the potential for immunotherapy in children is the use of anti-GD2 antibody in metastatic high risk neuroblastoma where cures beyond 10 years are now possible in the majority of patients without appreciable late effects from the anti-GD2 antibody (23, 24). Although the EWS-FLI1 fusion protein is present only in ES tumor cells and not in normal tissue (providing an ideal target for drug development), EWS-FLI1 targeted therapy has so far been unsuccessful in the clinic. In this review, we summarize the current treatment paradigm of ES, and emerging therapies for ES, including molecularly targeted therapy and immunotherapy.
Frontline Therapy
Localized Disease
Although <25% of patients present with gross metastatic disease, ES is considered a systemic disease with subclinical spread (25). In fact, patients with ES who undergo local therapy alone experience relapse rates approaching 90% (26). Thus, the current treatment paradigm for ES consists of multimodality therapy with chemotherapy, surgery, and/or radiation therapy (RT). Chemotherapy is considered the backbone of therapy for ES, and is typically given both neoadjuvantly and adjuvantly. Induction therapy is specifically recommended for ES to address micrometastatic disease as well as to reduce the size of the tumor, potentially allowing for a less extensive or less morbid surgery (and/or smaller radiation volumes).
The first two Intergroup Ewing sarcoma studies (IESS) established the use of vincristine, doxorubicin, cyclophosphamide, and actinomycin A (VDCA) with dose-intensive doxorubicin as the standard of care (27, 28). IESS-III was a phase III randomized clinical trial that showed a relapse-free survival benefit with the addition of ifosfamide and etoposide to VDCA (18). Subsequent trials omitted actinomycin D with no deleterious effect on outcomes. Given these findings, standard chemotherapy for ES now consists of vincristine, doxorubicin, and cyclophosphamide, with the addition of ifosfamide and etoposide (VDC/IE). Although dose intensification of the alkylating agents did not improve outcomes for patients with localized ES in a large Children's Oncology Group (COG) study (29), interval compressed-therapy (cycles given every 14 days rather than every 21 days) did result in improved outcomes (30). As such, interval-compressed chemotherapy with alternating cycles of VDC/IE is recommended as first-line systemic therapy for localized disease.
Surgical resection and/or RT are done for local control of the primary tumor, with considerations given to the location of the tumor, the response to induction therapy, and the degree of morbidity associated with resection vs. RT. Typically, for patients with ES arising from dispensable bones (i.e., fibula, ribs) or from the axial skeleton in which a margin-negative resection can be performed without excessive morbidity, surgical resection is the preferred choice of local control, sparing children the risk of second malignant neoplasms after RT. However, for patients with tumors arising from the pelvis, spine, or other locations in which function-preserving (or limb-sparing), margin-negative surgery cannot be performed, definitive radiation is the preferred modality of treatment. There are no randomized trials directly comparing the efficacy of surgery vs. RT for local control, but some retrospective studies and a systematic review have shown that local control may be superior with surgery (31–33). However, retrospective studies can be flawed because of selection bias, as tumors that are easily surgically resectable are often smaller tumors in more favorable locations.
Metastatic Disease
For patients with metastatic disease, outcomes remain poor and are dependent on the site of metastatic disease as described above, with the worst prognosis for those that spread to bone and bone marrow. Systemic chemotherapy used for patients who present with metastatic disease is similar to that used for patients with localized disease. However, unlike patients with localized disease in which the addition of ifosfamide and etoposide to vincristine, doxorubicin, and cyclophosphamide has been shown to improve survival, the addition of ifosfamide and etoposide has not shown benefit for those with metastatic disease (18, 34). Despite these concerns, front-line treatment for patients with metastatic disease still employs VDC/IE. While dose-intensified VDC/IE with augmented alkylator dose does not seem to improve outcomes for patients with metastatic disease (35), the role of interval-compressed chemotherapy remains under investigation on COG AEWS1221 (NCT02306161). The role of high-dose chemotherapy with hematopoietic cell support for patients with metastatic disease has also been explored. Some studies have shown excellent event-free survival rates in patients who received high-dose chemotherapy followed by autologous transplant (36), while others have shown no benefit (37, 38). The role of autologous stem cell rescue was further explored on the EURO-EWING 99 trial, with interim results showing no improvement in survival with stem cell rescue for patients with metastatic disease to the lung compared to conventional chemotherapy with whole lung irradiation (39). Of note, stem cell rescue on this trial did improve survival for patients with localized disease at high risk of relapse, though (40). Given different inclusion criteria and stratification approaches, it is difficult to compare these results to those from the COG showing a benefit of interval-compressed chemotherapy in the localized setting. However, the outcomes appear similar after either dose-intensification approach (stem-cell transplant vs. intensively timed chemotherapy), and there is no consensus across continents regarding front-line approach.
Treatment of metastatic sites of disease with radiation and sometimes even surgery has been considered as part of consolidation. For example, for patients with pulmonary metastases, non-randomized studies supported the delivery of whole lung irradiation after completion of chemotherapy; (41) and for patients with residual lung metastases after completion of chemotherapy, further surgical resection has been advocated (42). Consideration has also been given to irradiating bone and soft tissue metastases, especially in the setting of oligometastatic disease. On the ongoing COG metastatic ES protocol, AEWS1221, patients can be treated with stereotactic body RT to up to five sites of metastatic disease. Local control of the primary site is also important for patients with metastatic disease, with RT often preferred over surgical resection unless there has been a significant response to chemotherapy.
Relapsed Disease
As is true for patients with metastatic disease, the prognosis of patients with local relapse remains poor. Phase II clinical trials have shown activity of camptothecin-based approaches with either topotecan or irinotecan for recurrent disease (43–45). Other agents utilized in the recurrent setting such as gemcitabine, docetaxel, bortezomib, and ecteinascidin-743 have not improved survival (46–49). Given the dose-limiting toxicities of cytotoxic chemotherapy and the overall poor outcomes, patients with recurrent ES should be considered for clinical trials using novel molecularly targeted therapies or immune-based approaches as discussed in more detail below.
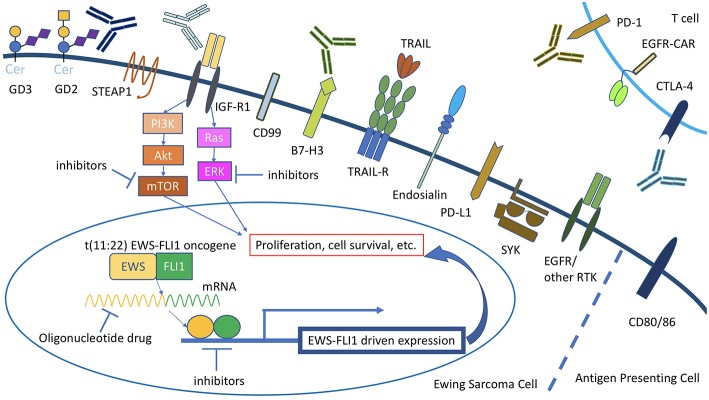
Molecularly Targeted Therapy (Figure 1)
Figure 1.
Overview of current molecularly targeted therapy for Ewing sarcoma. For STEAP1 and CD99, antibodies have been tested in mouse models of Ewing sarcoma but not in the clinical trial setting. Cer, ceramide.
EWS-FLI1 Pathway (Clinical Data)
The EWS-FLI1 fusion protein results in the production of a unique tumor driver only found in tumor cells. Given this specificity, targeted therapy directed at the EWS-FLI1 protein should avoid non-specific toxicities. Additionally, tumorigenesis in ES is dependent on EWS-FLI1 fusion protein expression, with deletion resulting in ES cell death in pre-clinical studies (5, 50, 51). This mechanistic dependency on the fusion protein further supports EWS-FL11 as an obvious therapeutic target. Despite these considerations, there is no available drug that can directly inhibit the fusion protein. Studies in the 1960's and 1970's utilizing various peptides and natural products to target the EWS-FL1I fusion protein showed activity in the preclinical setting (Table 1), although their translation into the clinical setting was limited by toxicity. For example, mithramycin is a natural product known to repress the EWS-FLI1 protein in vitro. A phase I/II study including eight patients with refractory ES treated with mithramycin showed no clinical responses with an inability to safely achieved the desired dose secondary to hepatotoxicity (63).
Table 1.
Preclinical studies targeting the EWS-FLI1 pathway.
| Drug | Drug type | Molecular target | Mechanism | References |
|---|---|---|---|---|
| EC-8042 and EC-8105 | Chemicals (natural product, mithramycin analogs) | EWS-FLI1 protein | Represses EWS-FLI1 activity by decreasing expression of EWS-FLI1 downstream targets | (52) |
| Englerin A | Chemicals (natural product) | EWS-FLI1 protein | Inhibits cellular proliferation through a decrease in EWS-FLI1 phosphorylation and reduction its DNA binding ability | (53) |
| ESAP1 (TMRGKKKRTRAN) | Chemicals (synthetic peptide) | EWS-FLI1 protein | Impairs the transcriptional activity of EWS-FLI1 and blocks cell cycle progression | (54) |
| Romidepsin, Depsipeptide, FK228, entinostat (MS-27-275) | Chemicals (natural product, synthetic peptide) | Histone deacetylase | Reverses EWS-FLI1 mediated histone deacetylation, decreases EWS-FLI1 mRNA and protein levels, inhibits cell proliferation, and induces TRAIL-dependent apoptosis of ES cells | (55–57) |
| LSD1 inhibitor HCI-2509 | Chemicals (benzoic hydrazide) | Lysine specific demethylase 1 (Histone demethylase) | Comprehensively reverses the transcriptional profiles driven by both EWS-FLI and EWS-ERG, and markedly delays tumorigenesis in vivo | (58) |
| JIB-04 | Chemicals (pyridine hydrazone) | Jumonji domain containing histone demethylases | Deregulates oncogenic programs and increases DNA damage, resulting in impaired cell proliferation and survival, and reduced tumor growth | (59) |
| Arsenic trioxide | Chemicals (inorganic arsenic compound) | Glioma-Associated Oncogene Homolog 1 (GLI1) | Inhibits ES tumor growth via the inhibition of GLI1 | (60) |
| Methylseleninic acid (MSA) | Chemicals (organic selenium compounds) | FOXO1, Forkhead box family protein | Increases FOXO1 expression in the presence of EWS-FLI1, induces massive cell death and decreases xenograft tumor growth dependent on FOXO1 | (61, 62) |
Strategies to inactivate or decrease the expression or function of the EWS-FLI1 protein have shown some promise, including inhibitory oligonucleotides and small-molecule inhibitors that are able to disrupt its transcriptional complex. Inhibitory oligonucleotides are short nucleotide sequences that can be designed to hybridize to single-stranded mRNA molecules and subsequently inhibit protein translation (64). Both antisense oligonucleotides and inhibitory RNA can be utilized for this purpose. For ES, inhibitory oligonucleotides have been designed that can bind to selected sequences coding for the EWS-FLI1 fusion protein, consequently decreasing expression of the fusion protein and resulting in decreased tumor growth in preclinical models (65, 66). Although inhibitory oligonucleotides have been successfully used to treat ES in vitro, translation to humans has proven difficult partly because of the inefficiency of drug transport intracellularly for maximal activity (67). Thus, inhibitory oligonucleotides for ES are not currently in the clinic.
The Toretsky lab at Georgetown has pioneered the inhibition of the EWS-FLI1 protein via disruption of protein-protein interactions. Specifically, they have created a peptide that competes with wild type RNA helicase A for a specific binding site on the EWS-FLI1 protein. This interaction between RNA helicase A and EWS-FLI1 is necessary for the function of EWS-FLI1 (68, 69). The small-molecule inhibitor of RNA helicase A, YK-4-279, has shown activity against ES in vitro (70), and an analog of YK-4-279, TK216, is currently being tested in a Phase 1 trial in relapsed or refractory ES (NCT02657005).
Poly (ADP-ribose) polymerase 1 (PARP1) is an enzyme involved in transcriptional regulation and DNA repair. PARP1 interacts with the EWS-FLI1 protein to create a positive feedback loop for transcriptional activation. Given the disruption of this critical interaction with PARP inhibitors, ES is highly responsive to PARP inhibition in preclinical models (71). Other studies have found that the concurrent administration of the PARP inhibitor, olaparib, with radiation results in increased lethal DNA damage and as a result, increased cell death in vitro (72). A phase II study including 12 patients with refractory ES showed that olaparib was well-tolerated, although no significant responses were seen, with patients progressing at a median time of 5.7 weeks from initiation of therapy (73). Given the preclinical efficacy data of combining olaparib with temozolomide (74), there is now an ongoing phase I study testing the safety and efficacy of this combination in patients with ES (NCT01858168).
EWS-FLI1 Pathway (Preclinical Data)
Other approaches involve targeting downstream signaling molecules driven by the EWS-FLI1 fusion protein such as Glioma-Associated Oncogene Homolog 1 (GLI1). GLI1 is a transcription factor that is upregulated by EWS-FLI1 and plays an important role in the Hedgehog pathway (7). In ES specifically, when upregulated by EWS-FLI1, GLI1 plays a major role in maintaining the malignant phenotype and cell growth (75). In mice, antineoplastic arsenic trioxide (ATO) inhibits ES tumor growth via the inhibition of GLI1 (60). Anecdotal reports using a combination of ATO and standard chemotherapy (VP-16 and paclitaxel) have encountered minimal toxicities (76). A second candidate of the EWS-FLI1 pathway is the Forkhead box (FOX) gene family of proteins. The EWS-FLI1 gene regulates, though indirectly, the expression of FOXO1 which controls tumor growth and differentiation (61). In an orthotopic xenograft mouse model, methyl-imino selenium acid (MSA) was found to reactivate endogenous FOXO1, thereby significantly decreasing tumor growth (62). Unlike FOXO1, the FOXM1 protein is upregulated by EWS-FLI1 and serves as an oncogenic mediator that results in tumor proliferation; a reduction of FOXM1 protein results in decreased anchorage independent growth (77). Inhibition of FOXM1 can be achieved via thiostrepton both in vitro and in vivo (78), suggesting that FOXM1 may also serve as a potential therapeutic target of the FOX gene family. Tsafou et al. also performed an integrative drug screening analysis to identify mechanisms and compounds that interfere with the EWS-FL11 pathway and EWS-FL1 cell viability (79). Among the druggable targets identified, the authors found that MCL-1 (a known inhibitor of apoptosis) is directly activated by the fusion protein, suggesting a potential role of BLC-2 family inhibitors in ES.
Insulin-Like Growth Factor (IGF) Pathway
The IGF family of ligands and receptors play key roles in normal human growth and development; not surprisingly, they have been implicated in various types of human cancers. Insulin-like growth factor 1 (IGF-1) is required for the growth of fibroblasts, epithelial cells, bone marrow stem cells, and osteoblasts (80). The binding of IGF-1 to its receptor, IGF-1R, initiates a cascade of events that affect protein turnover, exerting potent mitogenic and differentiating effects on most cell types. In preclinical models of ES, the IGF-1R-mediated signaling pathway is constantly active, suggesting its role in the tumorigenesis of ES (4, 81–85). As such, IGF-R is another attractive target for ES, where its inhibition both in vitro and in vivo have impaired the migratory ability of ES cells thereby slowing tumor growth (83, 86, 87).
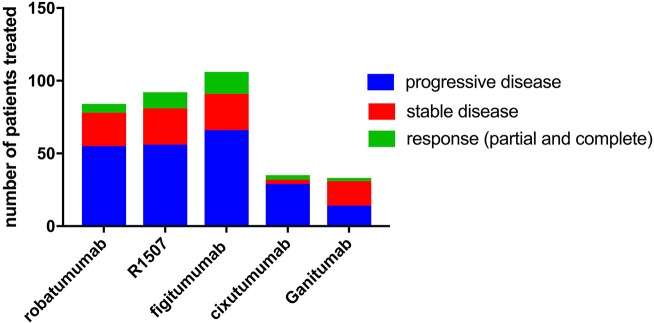
Both small molecule and antibody-mediated approaches to block the IGF pathway have been investigated in early phase clinical trials (Table 2) (89, 97, 108–110). Five human anti-IGF-1R antibodies for ES have been tested in phase II clinical trials: robatumumab (89) (also known as SCH 717454 and MK-7454, Merck and Schering-Plow), R1507 (111) (Roche), ganitumab (98) (NantCell, previously known as AMG 479 by Amgen Inc), cixutumumab (94, 95) (IMC-A12, ImClone systems), and figitumumab (93) (CP-751871, Pfizer). Among them, figitumumab showed the highest objective response rate of 14.2%; R1507 showed a response rate of 10.8%, cixutumumab 8.6%, robatumumab 7.1%, and ganitumab 6.1% (Figure 2). An ongoing phase III trial through the COG is testing the addition of ganitumab to combination chemotherapy in patients with newly diagnosed metastatic ES (NCT02306161). In addition, three other antibodies have been tested in the phase I setting: dalotuzumab (MK-0646) (99), BIIB022 (100), and AVE-1642 (101). In terms of their clinical efficacy, only one of six patients treated with dalotuzumab had a partial response; no patients treated with BIIB022 responded; and three of 40 patients treated with AVE-1642 had a partial response.
Table 2.
Antibody-based approaches, immunotherapy, and small molecule inhibitors tested in clinical trials for Ewing sarcoma.
| Drug | Molecular target | Phase | Clinicaltrial.org identifier | Number of patients | Response rate (RR %) | References |
|---|---|---|---|---|---|---|
| Mithramycin | EWS-FLI1 pathway | 1/2 | NCT01610570 | 8 | 0 | (63) |
| TK216 | EWS-FLI1 pathway | 1 | NCT02657005 | 45 | N/A | Ongoing, recruiting |
| Olaparib | PARP1 | 2 | NCT01583543 | 12 | 0 | (88) |
| Olaparib + temozolomide | PARP1 | 1 | NCT01858168 | 93 | N/A | Ongoing, recruiting |
| Robatumumab | IGF-R1 | 2 | NCT00617890 | 84 | 7.2 | (89) |
| R1507 | IGF-R1 | 1 | NCT00560144 | 9 | 22.2 | (90) |
| R1507 | IGF-R1 | 2 | NCT00642941 | 92 | 10.8 | (91) |
| Figitumumab | IGF-R1 | 1 | NCT00474760 | 16 | 12.5 | (92) |
| Figitumumab | IGF-R1 | 2 | NCT00560235 | 106 | 14.2 | (93) |
| Cixutumumab | IGF-R1 | 1/2 | NCT00668148 | 35 | 8.6 | (94, 95) |
| Cixutumumab + Temsirolimus | IGF-R1 + mTOR | 2 | NCT01614795 | 46 | 0 | (96) |
| Ganitumab | IGF-R1 | 1 | NCT00562380 | 12 | 16.7 | (97) |
| Ganitumab | IGF-R1 | 2 | NCT00563680 | 33 | 6 | (98) |
| Ganitumab + chemotherapy | IGF-R1 | 3 | NCT02306161 | 330 | N/A | Ongoing, recruiting |
| Dalotuzumab (MK-0646) | IGF-R1 | 1 | NCT01431547 | 6 | 16 | (99) |
| BIIB022 | IGF-R1 | 1 | NCT00555724 | 40 | 7.5 | (100) |
| AVE1642 | IGF-R1 | 1 | UK study | 40 | 8 | (101) |
| Ipilimumab | CTLA4 | 1/2 | NCT02304458 | 484 | N/A | Ongoing, recruiting |
| Imatinib | c-KIT + PDGF-R | 2 | NCT00031915 | 185 | 1.6 | (102) |
| Imatinib | c-KIT + PDGF-R | 2 | NCT00062205 | 7 | 14.2 | (103) |
| Imatinib | c-KIT + PDGF-R | 2 | NCT00030667 | 70 | 1.7 | (104) |
| Bevacizumab | VEGF-R | 2 | NCT00516295 | 7 | N/A | Closed |
| Pazopanib | Multi-targeted RTK | 1 | NCT00929903 | 53 | 3.9 | (105) |
| Lexatumumab | TRAIL-R | 1 | NCT00428272 | 24 | 0 | (106) |
| Hu14.18K322A | GD2 | 1 | NCT02159443 | 100 | N/A | Ongoing, recruiting |
| Ontuxizumab | Endosialin | 1 | NCT01748721 | 27 | 0 | (107) |
| Enoblituzumab | B7-H3 | 1 | NCT02982941 | 25 | N/A | Active, not recruiting |
| Nivolumab + ABI-009 | PD1 + mTOR | 1/2 | NCT03190174 | 40 | N/A | Ongoing, recruiting |
| Ipilimumab ± Nivolumab | CTLA4 ± PD1 | 1/2 | NCT02304458 | 484 | N/A | Ongoing, recruiting |
| Ipilimumab + Nivolumab | CTLA4+PD1 | 2 | NCT02982486 | 60 | N/A | Not yet recruiting |
| EGFR806 CAR T Cell | EGFR | 1 | NCT03618381 | 36 | N/A | Ongoing, recruiting |
| Sarcoma-specific CAR-T cells | CD133, GD2, Muc1, CD117 | 1 | NCT03356782 | 20 | N/A | Ongoing, recruiting |
Figure 2.
Clinical trial results of anti-IGF-1R therapy across phase II trials.
These results suggest that anti-IGF-1R antibody treatment may provide therapeutic benefit for a select group of patients, but additional efforts are needed to identify biomarkers that can predict which subset of patients will respond. In the cixutumumab trial, tumor levels of IGF-1, IGF-2, and IGF-1R were evaluated by immunohistochemistry, but there was no correlation between expression of these three proteins and response to cixutumumab treatment (94). On the other hand, in the figitumumab trial, patients with intermediate pretreatment IGF-1 levels had improved survival compared to patients with lower baseline IGF-1 levels (93). Data from the R1507 trial suggest that high baseline IGF-1 levels correlate with improved overall survival but not with response to treatment (91). Taken together, IGF-1 levels may be prognostic but did not show consistent utility in predicting response to anti-IGF-IR therapy (111). In addition, given the relatively low response rates in the anti-IGF-1R clinical trials, it may be necessary to re-examine the mixed results of preclinical and clinical studies as well as the modest biological evidence underlying IGF-1R as a target for ES therapy. Specifically, more rigorous preclinical data may be needed to develop strategies in targeting the IGF family before further development of clinical trials.
Furthermore, mechanism-based molecular approaches utilizing combination strategies may prove more efficacious than IGF-1R antibody monotherapy. For example, the combination of IGF-1R antibodies with mTOR inhibitors has been evaluated in ES, with the rationale that mTOR inhibitors can induce AKT phosphorylation and signaling via an IGF-1R dependent mechanism; (112) given this dependence, it was thought that the combination of an IGF-1R antibody and mTOR inhibitor would have the potential to overcome the resistance seen when either was given as monotherapy. For ES specifically, the combination of ganitumab with rapamycin showed efficacy in preclinical modes (113), and the combination of cixutumumab and temsirolimus in the phase I setting for patients with refractory ES showed durable tumor regression in 29% of patients (114). However, a subsequent phase II study evaluating the efficacy of cixutumumab and temsirolimus among 46 patients with refractory or recurrent pediatric sarcoma (12 of whom had ES) showed no objective responses (96).
Other Tyrosine Kinases
Besides IGF-1R, other receptor tyrosine kinases (RTKs) active in ES have been explored as potential therapeutic targets given the key role of RTKs in tumor growth and survival, and the success of RTK-inhibitors in other cancers. For example, c-KIT and platelet-derived growth factor receptor β are both expressed in ES, and treatment of ES with imatinib (which inhibits phosphorylation of KIT and platelet-derived growth factor receptors) results in decreased proliferation and enhanced antitumor activity of both ES cell lines (115) and xenografts (116). However, three phase II trials treating patients with ES with imatinib showed either no response in all ES patients enrolled (102) or partial response only in one patient (103, 104).
Epithelial growth factor receptor (EGFR) inhibition has also been explored in preclinical models of ES, showing decreased cell growth with high doses of gefintib in vitro (117), but minimal activity in vivo (118). Vascular endothelial growth factor (VEGF) inhibition similarly results in decreased cell growth as well as reduced tumor vessel density in preclinical models (119–121). Bevacizumab (monoclonal antibody targeting the VEGF receptor), has been tested in a phase II study through the COG including seven patients with ES (four of whom completed therapy), with results pending (NCT00516295). Similarly, pazopanib (multi-kinase inhibitor with activity against VEGF) was tested in a phase I study of children with soft tissue sarcoma (including three patients with ES), showing that it was well-tolerated with evidence of anti-angiogenic effects (105). However, as ES is not dependent on the EGFR and VEGF pathways for oncogenesis and proliferation, it is unclear how much patients with refractory disease will ultimately benefit from targeting of these pathways.
Potratz et al. found that nine individual RTKs were more active in ES tumors derived from metastatic disease than localized disease (122). Among these 9 RTKs, the authors further explored the role of ROR1 in ES given its promising results as a therapeutic target in leukemia (123) and metastatic carcinomas in preclinical models (124, 125). The authors showed that silencing of ROR1 resulted in dysfunctional migration of ES cells in vitro, with the conclusion that ROR1 may also be a potential therapeutic target for ES. A second study found that high expression of the RTK, AXL, was a significant predictor of poor survival, and that inhibition of AXL with BGB324 resulted in decreased cell growth, viability, and migratory capabilities of ES cells in vitro (126). The authors concluded that AXL is also a potential target for ES.
Spleen tyrosine kinase (SYK) is a non-receptor tyrosine kinase that may also serve as a targetable oncogene in ES. SYK is known to promote cell survival in a variety of pediatric tumors including leukemia and retinoblastoma (127). Sun et al. recently found that SYK is also highly phosphorylated and active in ES, with inhibition of SYK resulting in decreased cell growth both in vivo and in vitro (128). This study also identified c-MYC as an SYK-promoted gene that in turn could activate transcription of MALAT1, resulting in tumor growth. Given the oncogenicity that results from activation of the SYK/c-MYC/MALAT1 pathway, inhibition of SYK signaling may be a potential treatment strategy for ES, although further preclinical studies testing this hypothesis are needed before translation into the clinical setting.
TRAIL
Tumor necrosis factor-related-apoptosis-inducing ligand (TRAIL) is a member of the tumor necrosis factor (TNF) family that plays a key role in immunosurveillance and apoptosis. The binding of TRAIL to death receptors (TRAIL-R1 and TRAIL-R2) leads to the activation of the extrinsic apoptosis pathway (129). Pediatric soft tissue sarcomas including ES and rhabdomyosarcoma are sensitive to TRAIL-induced apoptosis (130, 131). As TRAIL-R1 and TRAIL-R2 have restricted expression on normal tissue, TRAIL receptors are attractive immune targets. A monoclonal antibody activating TRAIL-R2, lexatumumab, has been tested in the phase I setting for adult solid tumors (132), as well as pediatric solids tumors including four patients with ES, with results showing some anti-tumor activity but no partial or complete responses (106).
Gangliosides
The ganglioside, GD2, is a cell-surface molecule with a highly restricted pattern of expression, found in neuroectoderm-derived tumors and sarcomas, including ES. Although the expression level of GD2 is heterogeneous across different ES cell lines and primary ES cell cultures, GD2 is still a potential cell surface target for treating ES, with expression levels ranging from 40 to 90% from diagnostic biopsy samples (133, 134). In addition, anti-GD2 antibodies have been actively tested in clinical trials for neuroblastoma for over two decades, with proven safety and efficacy (23, 24, 135–137). An ongoing clinical trial study at St. Jude is testing the role of the anti-GD2 antibody, hu14.18K322A, in the treatment of ES (NCT02159443). GD2 remains an attractive target in ES, but more preclinical and clinical data are needed to justify this approach.
GD3 is a ganglioside that is primarily expressed on human melanoma tissues (138), with recent findings of high expression levels on pediatric tumors including osteosarcoma, ES, rhabdomyosarcoma, and desmoplastic small round cell tumor (DSRCT) (134). Although there are no randomized trials testing anti-GD3 antibodies as there are for GD2, the targeting of GD3 has shown activity in phase I trials including patients with melanoma (139, 140). In addition, a phase I trial of a bivalent GD2/GD3 vaccine for neuroblastoma showed encouraging survival benefit (141), with a phase II study ongoing. Like GD2, GD3's expression is largely limited to malignant cells and some activated T cells, making it a potential immune target for ES. N-glycolated GD3 (Neu-Gc-GM3) also has a restricted expression pattern on malignant cells and not normal tissues. A controlled phase II trial in patients with metastasis breast cancer with a Neu-Gc-GM3 based vaccine showed that the vaccine was well-tolerated, immunogenic, and had encouraging efficacy (142). Neu-Gc-GM3 is also expressed on the surface of ES, Wilm's tumor, and neuroblastoma (143), making it another potential target for treatment of ES that could avoid non-specific toxicity.
B7-H3
B7-H3 is a cell surface immunomodulatory glycoprotein that could play a role in tumor progression via the inhibition of T cells and natural killer cells (144). B7-H3 is overexpressed in a variety of adult and pediatric tumors including ES (145), and shown to be a good target for tumor purging before stem cell transplant (146). Radioimmunotherapy directed at B7-H3 using the antibody, 8H9, has been tested in the phase I setting for patients with DSCRT (NCT01099644), neuroblastoma with central nervous involvement (NCT00089245 and NCT03275402), and diffuse intrinsic pontine glioma (NCT01502917). Results have shown minimal toxicity and encouraging efficacy, with the potential to increase survival in patient populations that have very few treatment options (147–149).
Endosialin
Endosialin (also known as tumor endothelial marker-1 or TEM-1) is a cell surface glycoprotein that is found on mural cells, myofibroblasts, as well as a variety of pediatric tumors including ES, rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and neuroblastoma (150–153). Endosialin promotes tumor cell growth and neovascular formation via the platelet-derived growth factor (PDGF) pathway (154). Ontuxizumab is a humanized monoclonal antibody targeting endosialin and has the ability to block PDGF signaling and tumor stroma organization (155). A phase I study of ontuxizumab in relapsed or refractory pediatric solid tumors (including four patients with ES) showed that ontuxizumab was well tolerated, although no objective responses were seen (107).
STEAP1
Another potential therapeutic target for ES includes the six-transmembrane epithelial antigen of the prostate 1 (STEAP 1). STEAP1 is a 339-amino-acid protein named for its six transmembrane spanning regions, and is upregulated in a variety of tumors, including prostate, bladder, ovarian, rhabdomyosarcoma, and ES (156, 157). Grunewald et al. utilized transcriptome and proteome analyses as well as functional studies to show that STEAP1 expression correlates with oxidative stress responses and elevated levels of reactive oxygen species. This in turn regulates redox-sensitive and pro-invasive genes, suggesting that STEAP1 may be associated with an invasive phenotype of ES (158). Grunewald et al. also found that STEAP1 can serve as an immunohistological marker for patients with ES; 71 of 114 (62.3%) ES samples displayed detectable membranous STEAP1 immunoreactivity, making STEAP1 a potential therapeutic target (159). Another genetic profiling study done in ES patients showed that the absence of STEAP1 transcript in the bone marrow was strongly correlated with patient overall survival and survival without new metastases (160). Given the expression of STEAP1 in >60% of ES tumors but with limited expression in normal tissue (secretory tissue of the bladder and prostate) (161, 162), it could be a useful target for antibody-based and T-cell based strategies.
CD99
CD99 antigen, also known as MIC2 or single-chain type-1 glycoprotein, is a heavily O-glycosylated transmembrane protein with a molecular weight of 32 kD. It is expressed on leukocytes and is believed to increase T-cell adhesion and function in apoptosis (88, 163). CD99 is also expressed on the surface of ES cells, making it an attractive tumor target. Reduced CD99 expression leads to neural differentiation of ES cells, suggesting that CD99 may have role in the inhibition of neural differentiation (164). In addition, knockdown of CD99 in ES cell lines results in decreased oncogenic potential, including decreased growth in tissue culture, diminished colony formation in soft agar assays, reduced cell motility, and smaller tumors with less metastasis in xenograft models. Given CD99's involvement in apoptosis, CD99 engagement in ES cell lines have led to caspase-independent cell death (165, 166), making anti-CD99 antibody therapy another attractive therapeutic approach (75). Cu-labeled anti-CD99 antibodies were shown to be superior to FDG-PET in detecting micrometastases in xenograft models (167). A combination of doxorubicin and an anti-CD99 antibody could improve mouse survival (168, 169). Unfortunately, CD99 is not only expressed on ES but also on normal human tissues including the testis, gastric mucosa, prostate, and hematopoietic tissues, with potential of off-tumor, on-target bystander toxicities when used in humans (170).
Methionine Depletion
Cancer cells require methionine for aberrant transmethylation. As a result, cancers develop a dependence on methionine (171), with deprivation of methionine resulting in cell cycle arrest and eventually apoptosis (172). Recombinant methioninase (L-methionine-cleaving enzyme from Pseudomonas putida) acts to deplete methionine and in a variety of tumors including ES, results in arrested cell growth in preclinical models (typically at the S/G2 phase) (173). Although prolonged use of recombinant methioninase is not feasible given the potential liver toxicity, there has been interest in combining recombinant methioninase with standard chemotherapeutic agents, especially those active in S/G2 (171). For example, in preclinical models of neuroblastoma, recombinant methioninase showed synergism with microtubule depolymerization agents (174), and in preclinical models of synovial sarcoma, overcame resistance to doxorubicin monotherapy (175).
Immunotherapy
Immunotherapy is a treatment modality for many human solid tumors. Unfortunately, ES belongs to the majority class called “cold” tumors where little immune and/or inflammatory infiltrates are present, whether as a result of immune privilege, immune escape, or immune inhibition by the tumor microenvironment. Consistent with the absence of tumor infiltrating lymphocytes (TILs), ES tumors typically have low expressions of immune checkpoint molecules including PD-1 and PD-L1 (176). Nevertheless, the observation of an increased number of tumor-infiltrating CD8+ T cells associated with decreased tumor progression of ES might suggest a role for T cell based therapy if only T cells can be educated appropriately (177).
Cell-Based Immunotherapy and Vaccines
Cell-mediated immunotherapy strategies explored in ES include both T cell cancer vaccines and cell-based immunotherapy. Peptides produced from the EWS-FLI1 fusion protein are weakly immunogenic and do not significantly stimulate cytotoxic T-lymphocytes (CTL). Methods to enhance antigen presentation and strengthen the immunogenicity of the EWS-FLI1 peptides have been explored. For example, Evans et al. found that a modified peptide, YLNPSVDSV, induced strong CTL killing in ES cells, and the adoptive transfer of these specific CTLs into mice killed ES xenografts and increased survival (178). A translocation specific peptide vaccine has also been tested a pilot study in humans, although disappointingly with no impact on patient outcomes (179). Membrane-associated phospholipase A1 beta (LIPI) is a cancer/testis antigens (CTA) that is highly tumor specific, making it a potential target for immune-based therapies as well (180). Mahlendorf et al. found that CTLs targeting LIPI-derived peptides, LDYTDAKFV and NLLKHGASL, were able to kill ES cells in vitro (181).
Ghisoli et al. tested the efficacy and safety of a therapeutic vaccine known as FANG immunotherapy, consisting of autologous tumor cells that have been transfected with RNAi bi-shRNA furin and the rhGMCSF transgene. This creates a vaccine that assists in antigen presentation and the recruiting of regional nodal migration of dendritic cells (via GM-CSF), with the possibility of negating the immunosuppressive proteins including TGB1 and TGB2 (via furin). A pilot trial of FANG immunotherapy given to 12 patients with advanced or metastatic ES showed a good safety profile and successful elicitation of a tumor-specific systemic immune response in all patients. Additionally, the 1-year overall survival for this heavily pre-treated cohort was 75% (182). A two-part Phase II study utilizing FANG immunotherapy in patients with refractory ES is now ongoing (NCT02511132). In the first part, patients with refractory ES are randomized to either FANG immunotherapy or chemotherapy with gemcitabine and docetaxel; while in part two, patients with refractory ES receive FANG immunotherapy in combination with irinotecan and temozolomide. Another strategy utilizing dendritic cell vaccination with or without recombinant human IL7 has been tested in patients with metastatic and recurrent ES. The 5-year survival in the intent-to-treat analysis for patients with newly metastatic ES or rhabdomyosarcoma was 77%, with a T cell response to autologous tumor lysate seen in 62% of patients (183).
Natural killer (NK) cell based immunotherapy is another potential treatment strategy for ES. In the absence of tumor specific antibody, NK cells can kill ES tumors. In the presence of specific antibodies, NK cells mediate efficient antibody-dependent cell mediated cytotoxicity (ADCC). Cho et al. tested NK cell cytotoxicity in a variety of pediatric tumors, and found that ES cells are sensitive to the cytotoxicity of expanded, activated NK cells in vitro (184). NK cell killing of ES cells is specifically mediated via NKG2D and DNAM-1 receptor dependent pathways (185), and histone deacetylase inhibitors can enhance expression of NKG2D ligands and increase the sensitivity of ES cell lines to NKG2D-depedent toxicity (186). Additionally, expanded NK cells have shown efficacy in treating immunodeficient mice with ES tumors, resulting in long-lasting disease control (184).
Immune Checkpoint Inhibitors and CAR-T Cells
Immune checkpoint inhibitors have been evaluated in a multitude of clinical trials involving many different cancer types and are currently an area of active investigation in ES (Table 2). Ongoing clinical trials testing checkpoint inhibitors in ES patients include: Ipilimumab (anti-CTLA4, NCT02304458), Nivolumab (Anti-PD1, NCT03190174), Ipilimumab + Nivolumab (NCT02982486), and Enoblituzumab (B7-H3, NCT02982941). Given the paucity of mutations in ES (hence low frequency of neoantigens) accompanied by low expression levels of PD-1 and PD-L1, it remains questionable if patients with ES can derive significant clinical benefit from these agents.
To overcome the low frequency of tumor-specific T cell clones, CAR T cells may provide an alternative option. In one adoptive T cell study, patients are randomized to either EGFR-specific CAR T cells or CAR T cells directed at both EGFR and CD19 (NCT03618381). A second ongoing trial is treating patients with relapsed or metastatic ES with 4th generation CAR T cells (NCT03356782). Another strategy explored in a pilot study of ES patients utilized chondromodulin-I/HLA-A*02:01/antigen-specific allorestricted T cells, with a treatment response seen in one of three patients (187). Given the lack of success of CART across a broad spectrum of human solid tumors, more research is probably needed before the success of CD19 CART could be translated into solid tumor systems. Their clinical application will likely have to wait for more in-depth research.
Future Directions for Combined Modality Treatment
Although the novel therapies discussed above have shown encouraging results in preclinical models of ES, successful integration into the clinical setting remains challenging. Careful consideration must be given to the timing of signaling blockade (with small molecules) and immunotherapy (whether antibody-based or cell-based) in relation to standard chemoradiation in order to maximize clinical benefit. For example, patients with locally advanced non-small cell lung cancer treated on the PACIFIC trial were randomized to receive either placebo or adjuvant durvalumab (PD-L1 inhibitor) after completion of definitive chemoradiation (188). The addition of adjuvant durvalumab significantly improved progression-free survival, with the benefit exclusively seen in patients who received durvalumab within 2 weeks of completion of RT. These findings highlight the importance of the timing of immunotherapy in relation to RT. However, the ideal sequencing of immunotherapy with standard therapy for many adult solid tumors remains unknown, and preclinical data have largely resulted in conflicting data (189). Similarly how the inhibition of signaling pathways could be optimally combined with cytotoxic therapy, either concurrently or sequentially, requires more detailed testing in preclinical models. Perhaps combination therapy targeting multiple signaling pathways to overcome heterogeneity and resistance is one of several principles one can borrow from the past experience in cancer therapeutics.
In ES, where the intensive induction chemotherapy is immunosuppressive, a 6–12 month recovery period is generally needed for any adaptive immunity to be operational (190). As in metastatic neuroblastoma treated with N6/N6-like induction regimens (from which P6 regimen was derived), a viable immunotherapy option immediately post-chemotherapy is the “passive” monoclonal antibody approach (e.g., against GD2). That success in neuroblastoma is predicated on its sensitivity to myeloid-ADCC and cell-mediated cytotoxicity, where the effector cells and proteins (neutrophils and complement) can rapidly recover even after strong chemotherapy, and where myeloid cells can be put into overdrive using growth factors such as GM-CSF. On the other hand, even though CD16(+) NK cells that mediate NK-ADCC recover faster than T and B cells, their numbers are still suboptimal. Furthermore, after many years of testing, IL2 is now proven to have no role in accelerating their recovery or function to impact patient outcome (191). For neuroblastoma, the 6-month immune convalescence has pushed the timing of the GD2/GD3 vaccine to later on during consolidation when patients could make a meaningful anti-ganglioside immune response. It is expected that T cell based therapies using bispecific antibodies (BsAb), T cell vaccines, or checkpoint inhibitors will likely require a similar recovery period. Alternatively, if healthy autologous T cells are cryopreserved before significant chemotherapy damage, or if third party antigen-primed (e.g., EBV) T cells, can be activated ex vivo for arming with BsAb or for viral transduction to make CARTs, they may be ideal for “passive” adoptive cell therapy. KIR-mismatched NK cells may also be used for cell therapy with or without anti-tumor antibodies, although their expansion ex vivo will require cocktails of interleukins (e.g., IL7, IL15, IL21).
For patients with locally advanced ES, administration of “passive” immunotherapy (using tumor-selective antibodies, CART, BsAb, or BsAb armed-T cells), either integrated with or right after the completion of definitive radiation therapy (as done on the PACIFIC trial) may provide the most benefit given the immunomodulatory effects of RT such as increased neoantigen presentation and enhancement of T-cell infiltration (192–195). Again, full immune recovery will not be complete for at least 6–12 months, at which point a vaccine program may be most useful. For patients with metastatic disease, adding immunotherapy to chemotherapies that are not immunosuppressive could be advocated (196–198). Such combination strategies of immunotherapy with RT have been explored in many preclinical and clinical studies of adult solid tumors (195, 198). However, parallel studies in pediatric solid tumors including ES remain largely unexplored. A more in-depth immune profiling and genomic tracing of ES as patients recover from induction chemoradiotherapy, or suffer relapse, is critically important to inform the future design and integration of immunotherapy and small molecules into the standard of care of ES.
Further consideration must also be taken to incorporating ongoing discoveries of the molecularly diverse underpinnings of ES. For many years, the heterogenous clinical presentations and outcomes of ES were at odds with its relative genomic homogeneity, consisting of near-universal EWS-FLI1 fusion alterations and relatively few other recurrent somatic alterations (199). However, recent in-depth epigenomic profiling of ES has uncovered significant inter-individual and intra-tumoral heterogeneity of DNA methylation states, most pronounced in metastatic tumors (200). Additionally, large genomic sequencing efforts in ES have shown that the presence of intra-tumor genetic heterogeneity at diagnosis affects the evolution of recurrent ES tumors, with an ~3-fold increase in number of genetic alterations seen in relapsed samples (199). This genetic and epigenetic intra-tumoral heterogeneity likely plays a substantial role in driving clonal evolution and clinical response to therapy (201). Therefore, incorporating in-depth molecular profiling both at diagnosis and at the time of recurrence may be imperative to the potential design and success of precision-targeted ES therapy.
Conclusion
Proteomics and genomic analysis of normal and tumor tissue have led to the discovery of many genes whose downstream products may provide substrates for targeted therapies. Valuable insights into the role of different targets in ES biology have been gained throughout the past two decades. Antibody-based and cell-based immunotherapy have emerged rapidly as potential modalities for ES (20). Many have shown promising results in preclinical models. The clinical success of GD2-based immunotherapy in neuroblastoma could provide a framework in designing the next generation strategies for metastatic ES, a tumor much less lethal than high risk neuroblastoma historically. The successful integration of biologic targeted therapies and immunotherapy into standard of care will be the future challenge in changing the natural history of a lethal disease.
Author Contributions
DC, T-YL, and N-KC contributed to the conception and design of the review and wrote sections of the manuscript. DC wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest Statement
N-KC reports receiving commercial research grants from Y-mabs Therapeutics and Abpro-Labs Inc.; holding ownership interest/equity/options in Y-Mabs Therapeutics Inc., and in Abpro-Labs, and owning stock options in Eureka Therapeutics. N-KC is the inventor of pending and issued patents filed by MSK, including hu3F8 and 8H9 licensed to Ymabs Therapeutics, beta-glucan to Biotec Pharmacon, and HER2 bispecific antibody to Abpro-labs. N-KC is an advisory board member for Abpro-Labs and Eureka Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. NIH grant P30 CA 008748.
References
- 1.Esiashvili N, Goodman M, Marcus RB. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J Pediatr Hematol Oncol. (2008) 30:425–30. 10.1097/MPH.0b013e31816e22f3 [DOI] [PubMed] [Google Scholar]
- 2.Arvand A, Denny CT. Biology of EWS/ETS fusions in Ewing's family tumors. Oncogene. (2001) 20:5747–54. 10.1038/sj.onc.1204598 [DOI] [PubMed] [Google Scholar]
- 3.May WA, Gishizky ML, Lessnick SL, Lunsford LB, Lewis BC, Delattre O, et al. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci USA. (1993) 90:5752–6. 10.1073/pnas.90.12.5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toretsky JA, Connell Y, Neckers L, Bhat NK. Inhibition of EWS-FLI-1 fusion protein with antisense oligodeoxynucleotides. J Neurooncol. (1997) 31:9–16. 10.1023/A:1005716926800 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K, Iwakuma T, Harimaya K, Sato H, Iwamoto Y. EWS-Fli1 antisense oligodeoxynucleotide inhibits proliferation of human Ewing's sarcoma and primitive neuroectodermal tumor cells. J Clin Invest. (1997) 99:239–47. 10.1172/JCI119152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cironi L, Riggi N, Provero P, Wolf N, Suvà ML, Suvà D, et al. IGF1 is a common target gene of Ewing's sarcoma fusion proteins in mesenchymal progenitor cells. PLoS ONE. (2008) 3:e2634. 10.1371/journal.pone.0002634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zwerner JP, Joo J, Warner KL, Christensen L, Hu-Lieskovan S, Triche TJ, et al. The EWS/FLI1 oncogenic transcription factor deregulates GLI1. Oncogene. (2008) 27:3282–91. 10.1038/sj.onc.1210991 [DOI] [PubMed] [Google Scholar]
- 8.Sollazzo MR, Benassi MS, Magagnoli G, Gamberi G, Molendini L, Ragazzini P, et al. Increased c-myc oncogene expression in Ewing's sarcoma: correlation with Ki67 proliferation index. Tumori. (1999) 85:167–73. 10.1177/030089169908500304 [DOI] [PubMed] [Google Scholar]
- 9.Fukuma M, Okita H, Hata J, Umezawa A. Upregulation of Id2, an oncogenic helix-loop-helix protein, is mediated by the chimeric EWS/ets protein in Ewing sarcoma. Oncogene. (2003) 22:1–9. 10.1038/sj.onc.1206055 [DOI] [PubMed] [Google Scholar]
- 10.Hahm KB, Cho K, Lee C, Im YH, Chang J, Choi SG, et al. Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet. (1999) 23:222–7. 10.1038/13854 [DOI] [PubMed] [Google Scholar]
- 11.Nakatani F, Tanaka K, Sakimura R, Matsumoto Y, Matsunobu T, Li X, et al. Identification of p21WAF1/CIP1 as a direct target of EWS-Fli1 oncogenic fusion protein. J Biol Chem. (2003) 278:15105–15. 10.1074/jbc.M211470200 [DOI] [PubMed] [Google Scholar]
- 12.Prieur A, Tirode F, Cohen P, Delattre O. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. (2004) 24:7275–83. 10.1128/MCB.24.16.7275-7283.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter GH, Plehm S, Fasan A, Rössler S, Unland R, Bennani-Baiti IM, et al. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci USA. (2009) 106:5324–9. 10.1073/pnas.0810759106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riggi N, Suvà ML, De Vito C, Provero P, Stehle JC, Baumer K, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. (2010) 24:916–32. 10.1101/gad.1899710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riggi N, Cironi L, Provero P, Suvà ML, Kaloulis K, Garcia-Echeverria C, et al. Development of Ewing's sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. (2005) 65:11459–68. 10.1158/0008-5472.CAN-05-1696 [DOI] [PubMed] [Google Scholar]
- 16.Riggi N, Suvà ML, Suvà D, Cironi L, Provero P, Tercier S, et al. EWS-FLI-1 expression triggers a Ewing's sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res. (2008) 68:2176–85. 10.1158/0008-5472.CAN-07-1761 [DOI] [PubMed] [Google Scholar]
- 17.Hu-Lieskovan S, Zhang J, Wu L, Shimada H, Schofield DE, Triche TJ. EWS-FLI1 fusion protein up-regulates critical genes in neural crest development and is responsible for the observed phenotype of Ewing's family of tumors. Cancer Res. (2005) 65:4633–44. 10.1158/0008-5472.CAN-04-2857 [DOI] [PubMed] [Google Scholar]
- 18.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. (2003) 348:694–701. 10.1056/NEJMoa020890 [DOI] [PubMed] [Google Scholar]
- 19.Cotterill SJ, Ahrens S, Paulussen M, Jürgens HF, Voûte PA, Gadner H, et al. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the european intergroup cooperative ewing's sarcoma study group. J Clin Oncol. (2000) 18:3108–14. 10.1200/JCO.2000.18.17.3108 [DOI] [PubMed] [Google Scholar]
- 20.Bailey K, Cost C, Davis I, Glade-Bender J, Grohar P, Houghton P, et al. Emerging novel agents for patients with advanced Ewing sarcoma: a report from the Children's Oncology Group (COG) New Agents for Ewing Sarcoma Task Force. F1000Res. (2019) 8:F1000 Faculty Rev-493. 10.12688/f1000research.18139.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp LM, Katsanis E. Targeted immunotherapy for pediatric solid tumors. Oncoimmunology. (2016) 5:e1087637. 10.1080/2162402X.2015.1087637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wedekind MF, Denton NL, Chen CY, Cripe TP. Pediatric cancer immunotherapy: opportunities and challenges. Paediatr Drugs. (2018) 20:395–408. 10.1007/s40272-018-0297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. (2010) 363:1324–34. 10.1056/NEJMoa0911123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. (2012) 30:3264–70. 10.1200/JCO.2011.41.3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am. (2005) 19:501–25. 10.1016/j.hoc.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 26.Jenkin RD. Ewing's sarcoma a study of treatment methods. Clin Radiol. (1966) 17:97–106. 10.1016/S0009-9260(66)80064-8 [DOI] [PubMed] [Google Scholar]
- 27.Burgert EO, Jr, Nesbit ME, Garnsey LA, Gehan EA, Herrmann J, Vietti TJ, et al. Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: intergroup study IESS-II. J Clin Oncol. (1990) 8:1514–24. 10.1200/JCO.1990.8.9.1514 [DOI] [PubMed] [Google Scholar]
- 28.Nesbit ME, Jr, Gehan EA, Burgert EO, Jr, Vietti TJ, Cangir A, Tefft M, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol. (1990) 8:1664–74. 10.1200/JCO.1990.8.10.1664 [DOI] [PubMed] [Google Scholar]
- 29.Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol. (2009) 27:2536–41. 10.1200/JCO.2008.19.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. (2012) 30:4148–54. 10.1200/JCO.2011.41.5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunst J, Sauer R, Burgers JM, Hawliczek R, Kürten R, Winkelmann W, et al. Radiation therapy as local treatment in Ewing's sarcoma. Results of the Cooperative Ewing's Sarcoma Studies CESS 81 and CESS 86. Cancer. (1991) 67:2818–25. [DOI] [PubMed] [Google Scholar]
- 32.DuBois SG, Krailo MD, Gebhardt MC, Donaldson SS, Marcus KJ, Dormans J, et al. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children's Oncology Group. Cancer. (2015) 121:467–75. 10.1002/cncr.29065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werier J, Yao X, Caudrelier JM, Di Primio G, Ghert M, Gupta AA, et al. A systematic review of optimal treatment strategies for localized Ewing's sarcoma of bone after neo-adjuvant chemotherapy. Surg Oncol. (2016) 25:16–23. 10.1016/j.suronc.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 34.Wexler LH, DeLaney TF, Tsokos M, Avila N, Steinberg SM, Weaver-McClure L, et al. Ifosfamide and etoposide plus vincristine, doxorubicin, and cyclophosphamide for newly diagnosed Ewing's sarcoma family of tumors. Cancer. (1996) 78:901–11. [DOI] [PubMed] [Google Scholar]
- 35.Magnan H, Goodbody CM, Riedel E, Pratilas CA, Wexler LH, Chou AJ. Ifosfamide dose-intensification for patients with metastatic Ewing sarcoma. Pediatr Blood Cancer. (2015) 62:594–7. 10.1002/pbc.25373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberlin O, Rey A, Desfachelles AS, Philip T, Plantaz D, Schmitt C, et al. Impact of high-dose busulfan plus melphalan as consolidation in metastatic Ewing tumors: a study by the Societe Francaise des Cancers de l'Enfant. J Clin Oncol. (2006) 24:3997–4002. 10.1200/JCO.2006.05.7059 [DOI] [PubMed] [Google Scholar]
- 37.Meyers PA, Krailo MD, Ladanyi M, Chan KW, Sailer SL, Dickman PS, et al. High-dose melphalan, etoposide, total-body irradiation, and autologous stem-cell reconstitution as consolidation therapy for high-risk Ewing's sarcoma does not improve prognosis. J Clin Oncol. (2001) 19:2812–20. 10.1200/JCO.2001.19.11.2812 [DOI] [PubMed] [Google Scholar]
- 38.Ladenstein R, Lasset C, Pinkerton R, Zucker JM, Peters C, Burdach S, et al. Impact of megatherapy in children with high-risk Ewing's tumours in complete remission: a report from the EBMT Solid Tumour Registry. Bone Marrow Trans. (1995) 15:697–705. [PubMed] [Google Scholar]
- 39.Dirksen U, Deley M-CL, Brennan B, Judson IR, Bernstein ML, Gorlick RG, et al. Efficacy of busulfan-melphalan high dose chemotherapy consolidation (BuMel) compared to conventional chemotherapy combined with lung irradiation in ewing sarcoma (ES) with primary lung metastases: results of EURO-EWING 99-R2pulm randomized trial (EE99R2pul). J Clin Oncol. (2016) 34:11001 10.1200/JCO.2016.34.15_suppl.11001 [DOI] [Google Scholar]
- 40.Whelan J, Le Deley MC, Dirksen U, Le Teuff G, Brennan B, Gaspar N, et al. High-dose chemotherapy and blood autologous stem-cell rescue compared with standard chemotherapy in localized high-risk ewing sarcoma: results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol. (2018) 6:JCO2018782516 10.1200/JCO.2018.78.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunst J, Paulussen M, Jurgens H. Lung irradiation for Ewing's sarcoma with pulmonary metastases at diagnosis: results of the CESS-studies. Strahlenther Onkol. (1993) 169:621–3. [PubMed] [Google Scholar]
- 42.Raciborska A, Bilska K, Rychłowska-Pruszynska M, Duczkowski M, Duczkowska A, Drabko K, et al. Management and follow-up of Ewing sarcoma patients with isolated lung metastases. J Pediatr Surg. (2016) 51:1067–71. 10.1016/j.jpedsurg.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 43.Saylors RL, III, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol. (2001) 19:3463–9. 10.1200/JCO.2001.19.15.3463 [DOI] [PubMed] [Google Scholar]
- 44.Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, Jürgens H. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer. (2006) 47:795–800. 10.1002/pbc.20719 [DOI] [PubMed] [Google Scholar]
- 45.Bisogno G, Riccardi R, Ruggiero A, Arcamone G, Prete A, Surico G, et al. Phase II study of a protracted irinotecan schedule in children with refractory or recurrent soft tissue sarcoma. Cancer. (2006) 106:703–7. 10.1002/cncr.21629 [DOI] [PubMed] [Google Scholar]
- 46.Wagner-Bohn A, Paulussen M, Vieira Pinheiro JP, Gerss J, Stoffregen C, Boos J. Phase II study of gemcitabine in children with solid tumors of mesenchymal and embryonic origin. Anticancer Drugs. (2006) 17:859–64. 10.1097/01.cad.0000217426.82702.74 [DOI] [PubMed] [Google Scholar]
- 47.Zwerdling T, Krailo M, Monteleone P, Byrd R, Sato J, Dunaway R, et al. Phase II investigation of docetaxel in pediatric patients with recurrent solid tumors: a report from the Children's Oncology Group. Cancer. (2006) 106:1821–8. 10.1002/cncr.21779 [DOI] [PubMed] [Google Scholar]
- 48.Maki RG, Kraft AS, Scheu K, Yamada J, Wadler S, Antonescu CR, et al. A multicenter Phase II study of bortezomib in recurrent or metastatic sarcomas. Cancer (2005) 103:1431–8. 10.1002/cncr.20968 [DOI] [PubMed] [Google Scholar]
- 49.Lau L, Supko JG, Blaney S, Hershon L, Seibel N, Krailo M, et al. A phase I and pharmacokinetic study of ecteinascidin-743 (Yondelis) in children with refractory solid tumors. A Children's Oncology Group study. Clin Cancer Res. (2005) 11:672–7. [PubMed] [Google Scholar]
- 50.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Res. (2005) 65:8984–92. 10.1158/0008-5472.CAN-05-0565 [DOI] [PubMed] [Google Scholar]
- 51.Toretsky JA, Kalebic T, Blakesley V, LeRoith D, Helman LJ. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. (1997) 272:30822–7. 10.1074/jbc.272.49.30822 [DOI] [PubMed] [Google Scholar]
- 52.Osgood CL, Maloney N, Kidd CG, Kitchen-Goosen S, Segars L, Gebregiorgis M, et al. Identification of mithramycin analogues with improved targeting of the EWS-FLI1 transcription factor. Clin Cancer Res. (2016) 22:4105–18. 10.1158/1078-0432.CCR-15-2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caropreso V, Darvishi E, Turbyville TJ, Ratnayake R, Grohar PJ, McMahon JB, et al. Englerin a inhibits EWS-FLI1 DNA binding in ewing sarcoma cells. J Biol Chem. (2016) 291:10058–66. 10.1074/jbc.M115.701375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erkizan HV, Scher LJ, Gamble SE, Barber-Rotenberg JS, Sajwan KP, Üren A, et al. Novel peptide binds EWS-FLI1 and reduces the oncogenic potential in Ewing tumors. Cell Cycle. (2011) 10:3397–408. 10.4161/cc.10.19.17734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakimura R, Tanaka K, Nakatani F, Matsunobu T, Li X, Hanada M, et al. Antitumor effects of histone deacetylase inhibitor on Ewing's family tumors. Int J Cancer. (2005) 116:784–92. 10.1002/ijc.21069 [DOI] [PubMed] [Google Scholar]
- 56.Sonnemann J, Dreyer L, Hartwig M, Palani CD, Hong le TT, Klier U, et al. Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing's sarcoma cells. J Cancer Res Clin Oncol. (2007) 133:847–58. 10.1007/s00432-007-0227-8 [DOI] [PubMed] [Google Scholar]
- 57.Jaboin J, Wild J, Hamidi H, Khanna C, Kim CJ, Robey R, et al. MS-27-275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res. (2002) 62:6108–15. [PubMed] [Google Scholar]
- 58.Sankar S, Theisen ER, Bearss J, Mulvihill T, Hoffman LM, Sorna V, et al. Reversible LSD1 inhibition interferes with global EWS/ETS transcriptional activity and impedes Ewing sarcoma tumor growth. Clin Cancer Res. (2014) 20:4584–97. 10.1158/1078-0432.CCR-14-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parrish JK, McCann TS, Sechler M, Sobral LM, Ren W, Jones KL, et al. The Jumonji-domain histone demethylase inhibitor JIB-04 deregulates oncogenic programs and increases DNA damage in Ewing Sarcoma, resulting in impaired cell proliferation and survival, and reduced tumor growth. Oncotarget. (2018) 9:33110–23. 10.18632/oncotarget.26011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beauchamp EM, Ringer L, Bulut G, Sajwan KP, Hall MD, Lee YC, et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. (2011) 121:148–60. 10.1172/JCI42874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L, Hu HM, Zielinska-Kwiatkowska A, Chansky HA. FOXO1 is a direct target of EWS-Fli1 oncogenic fusion protein in Ewing's sarcoma cells. Biochem Biophys Res Commun. (2010) 402:129–34. 10.1016/j.bbrc.2010.09.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niedan S, Kauer M, Aryee DN, Kofler R, Schwentner R, Meier A, et al. Suppression of FOXO1 is responsible for a growth regulatory repressive transcriptional sub-signature of EWS-FLI1 in Ewing sarcoma. Oncogene. (2014) 33:3927–38. 10.1038/onc.2013.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grohar PJ, Glod J, Peer CJ, Sissung TM, Arnaldez FI, Long L, et al. A phase I/II trial and pharmacokinetic study of mithramycin in children and adults with refractory Ewing sarcoma and EWS-FLI1 fusion transcript. Cancer Chemother Pharmacol. (2017) 80:645–652. 10.1007/s00280-017-3382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer. (2005) 5:468–79. 10.1038/nrc1631 [DOI] [PubMed] [Google Scholar]
- 65.Maksimenko A, Malvy C. Oncogene-targeted antisense oligonucleotides for the treatment of Ewing sarcoma. Expert Opin Ther Targets. (2005) 9:825–30. 10.1517/14728222.9.4.825 [DOI] [PubMed] [Google Scholar]
- 66.Mateo-Lozano S, Gokhale PC, Soldatenkov VA, Dritschilo A, Tirado OM, Notario V. Combined transcriptional and translational targeting of EWS/FLI-1 in Ewing's sarcoma. Clin Cancer Res. (2006) 12:6781–90. 10.1158/1078-0432.CCR-06-0609 [DOI] [PubMed] [Google Scholar]
- 67.Kovar H, Ban J, Pospisilova S. Potentials for RNAi in sarcoma research and therapy: Ewing's sarcoma as a model. Semin Cancer Bio.l. (2003) 13:275–81. 10.1016/S1044-579X(03)00041-5 [DOI] [PubMed] [Google Scholar]
- 68.Toretsky JA, Erkizan V, Levenson A, Abaan OD, Parvin JD, Cripe TP, et al. Oncoprotein EWS-FLI1 activity is enhanced by RNA helicase A. Cancer Res. (2006) 66:5574–81. 10.1158/0008-5472.CAN-05-3293 [DOI] [PubMed] [Google Scholar]
- 69.Erkizan HV, Kong Y, Merchant M, Schlottmann S, Barber-Rotenberg JS, Yuan L, et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing's sarcoma. Nat Med. (2009) 15:750–6. 10.1038/nm.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minas TZ, Han J, Javaheri T, Hong SH, Schlederer M, Saygideger-Kont Y, et al. YK-4-279 effectively antagonizes EWS-FLI1 induced leukemia in a transgenic mouse model. Oncotarget. (2015) 6:37678–94. 10.18632/oncotarget.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brenner JC, Feng FY, Han S, Patel S, Goyal SV, Bou-Maroun LM, et al. PARP-1 inhibition as a targeted strategy to treat Ewing's sarcoma. Cancer Res. (2012) 72:1608–13. 10.1158/0008-5472.CAN-11-3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee HJ, Yoon C, Schmidt B, Park DJ, Zhang AY, Erkizan HV, et al. Combining PARP-1 inhibition and radiation in Ewing sarcoma results in lethal DNA damage. Mol Cancer Ther. (2013) 12:2591–600. 10.1158/1535-7163.MCT-13-0338 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Choy E, Butrynski JE, Harmon DC, Morgan JA, George S, Wagner AJ, et al. Phase II study of olaparib in patients with refractory Ewing sarcoma following failure of standard chemotherapy. BMC Cancer. (2014) 14:813. 10.1186/1471-2407-14-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Engert F, Schneider C, Weiβ LM, Probst M, Fulda S. PARP inhibitors sensitize ewing sarcoma cells to temozolomide-induced apoptosis via the mitochondrial pathway. Mol Cancer Ther. (2015) 14:2818–30. 10.1158/1535-7163.MCT-15-0587 [DOI] [PubMed] [Google Scholar]
- 75.Beauchamp E, Bulut G, Abaan O, Chen K, Merchant A, Matsui W, et al. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. (2009) 284:9074–82. 10.1074/jbc.M806233200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo W, Tang XD, Tang S, Yang Y. Preliminary report of combination chemotherapy including Arsenic trioxide for stage III osteosarcoma and Ewing sarcoma. Zhonghua Wai Ke Za Zhi. (2006) 44:805–8. [PubMed] [Google Scholar]
- 77.Christensen L, Joo J, Lee S, Wai D, Triche TJ, May WA. FOXM1 is an oncogenic mediator in Ewing Sarcoma. PLoS ONE. (2013) 8:e54556. 10.1371/journal.pone.0054556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sengupta A, Rahman M, Mateo-Lozano S, Tirado OM, Notario V. The dual inhibitory effect of thiostrepton on FoxM1 and EWS/FLI1 provides a novel therapeutic option for Ewing's sarcoma. Int J Oncol. (2013) 43:803–12. 10.3892/ijo.2013.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsafou K, Katschnig AM, Radic-Sarikas B, Mutz CN, Iljin K, Schwentner R, et al. Identifying the druggable interactome of EWS-FLI1 reveals MCL-1 dependent differential sensitivities of Ewing sarcoma cells to apoptosis inducers. Oncotarget. (2018) 9:31018–31. 10.18632/oncotarget.25760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.LeRoith D, Baserga R, Helman L, Roberts CT. Insulin-like growth factors and cancer. Ann Intern Med. (1995) 122:54–9. 10.7326/0003-4819-122-1-199501010-00009 [DOI] [PubMed] [Google Scholar]
- 81.Yee D, Favoni RE, Lebovic GS, Lombana F, Powell DR, Reynolds CP, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J Clin Invest. (1990) 86:1806–14. 10.1172/JCI114910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Valen F, Winkelmann W, Jurgens H: Type I and type II insulin-like growth factor receptors and their function in human Ewing's sarcoma cells. J Cancer Res Clin Oncol. (1992) 118:269–75. 10.1007/BF01208615 [DOI] [PubMed] [Google Scholar]
- 83.Scotlandi K, Benini S, Sarti M, Serra M, Lollini PL, Maurici D, et al. Insulin-like growth factor I receptor-mediated circuit in Ewing's sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res. (1996) 56:4570–4. [PubMed] [Google Scholar]
- 84.Hofbauer S, Hamilton G, Theyer G, Wollmann K, Gabor F. Insulin-like growth factor-I-dependent growth and in vitro chemosensitivity of Ewing's sarcoma and peripheral primitive neuroectodermal tumour cell lines. Eur J Cancer. (1993) 29A:241–5. 10.1016/0959-8049(93)90183-G [DOI] [PubMed] [Google Scholar]
- 85.Toretsky JA, Thakar M, Eskenazi AE, Frantz CN. Phosphoinositide 3-hydroxide kinase blockade enhances apoptosis in the Ewing's sarcoma family of tumors. Cancer Res. (1999) 59:5745–50. [PubMed] [Google Scholar]
- 86.Hamilton G, Mallinger R, Hofbauer S, Havel M. The monoclonal HBA-71 antibody modulates proliferation of thymocytes and Ewing's sarcoma cells by interfering with the action of insulin-like growth factor I. Thymus. (1991) 18:33–41. [PubMed] [Google Scholar]
- 87.Scotlandi K, Benini S, Nanni P, Lollini PL, Nicoletti G, Landuzzi L, et al. Blockage of insulin-like growth factor-I receptor inhibits the growth of Ewing's sarcoma in athymic mice. Cancer Res. (1998) 58:4127–31. [PubMed] [Google Scholar]
- 88.Choi EY, Park WS, Jung KC, Kim SH, Kim YY, Lee WJ, et al. Engagement of CD99 induces up-regulation of TCR and MHC class I and II molecules on the surface of human thymocytes. J Immunol. (1998) 161:749–54. [PubMed] [Google Scholar]
- 89.Anderson PM, Bielack SS, Gorlick RG, Skubitz K, Daw NC, Herzog CE, et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr Blood Cancer. (2016) 63:1761–70. 10.1002/pbc.26087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. (2010) 16:2458–2465. 10.1158/1078-0432.CCR-09-3220 [DOI] [PubMed] [Google Scholar]
- 91.Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. (2011) 29:4541–7. 10.1200/jco.2010.28.15_suppl.10000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol. (2010) 11:129–35. 10.1016/S1470-2045(09)70354-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Juergens H, Daw NC, Geoerger B, Ferrari S, Villarroel M, Aerts I, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol. (2011) 29:4534–40. 10.1200/JCO.2010.33.0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malempati S, Weigel B, Ingle AM, Ahern CH, Carroll JM, Roberts CT, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. (2012) 30:256–62. 10.1200/JCO.2011.37.4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asmane I, Watkin E, Alberti L, Duc A, Marec-Berard P, Ray-Coquard I, et al. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur J Cancer. (2012) 48:3027–35. 10.1016/j.ejca.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 96.Wagner LM, Fouladi M, Ahmed A, Krailo MD, Weigel B, DuBois SG, et al. Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. (2015) 62:440–4. 10.1002/pbc.25334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. (2009) 27:5800–7. 10.1200/JCO.2009.23.6745 [DOI] [PubMed] [Google Scholar]
- 98.Tap WD, Demetri G, Barnette P, Desai J, Kavan P, Tozer R, et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol. (2012) 30:1849–56. 10.1200/JCO.2011.37.2359 [DOI] [PubMed] [Google Scholar]
- 99.Frappaz D, Federico SM, Pearson AD, Gore L, Macy ME, DuBois SG, et al. Phase 1 study of dalotuzumab monotherapy and ridaforolimus-dalotuzumab combination therapy in paediatric patients with advanced solid tumours. Eur J Cancer. (2016) 62:9–17. 10.1016/j.ejca.2016.03.084 [DOI] [PubMed] [Google Scholar]
- 100.von Mehren M, Britten CD, Pieslor P, Saville W, Vassos A, Harris S, et al. A phase 1, open-label, dose-escalation study of BIIB022 (anti-IGF-1R monoclonal antibody) in subjects with relapsed or refractory solid tumors. Invest N Drugs. (2014) 32:518–25. 10.1007/s10637-014-0064-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Macaulay VM, Middleton MR, Protheroe AS, Tolcher A, Dieras V, Sessa C, et al. Phase I study of humanized monoclonal antibody AVE1642 directed against the type 1 insulin-like growth factor receptor (IGF-1R), administered in combination with anticancer therapies to patients with advanced solid tumors. Ann Oncol. (2013) 24:784–91. 10.1093/annonc/mds511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chugh R, Wathen JK, Maki RG, Benjamin RS, Patel SR, Meyers PA, et al. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol. (2009) 27:3148–53. 10.1200/JCO.2008.20.5054 [DOI] [PubMed] [Google Scholar]
- 103.Chao J, Budd GT, Chu P, Frankel P, Garcia D, Junqueira M, et al. Phase II clinical trial of imatinib mesylate in therapy of KIT and/or PDGFRalpha-expressing Ewing sarcoma family of tumors and desmoplastic small round cell tumors. Anticancer Res. (2010) 30:547–52. [PubMed] [Google Scholar]
- 104.Bond M, Bernstein ML, Pappo A, Schultz KR, Krailo M, Blaney SM, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer. (2008) 50:254–8. 10.1002/pbc.21132 [DOI] [PubMed] [Google Scholar]
- 105.Glade Bender JL, Lee A, Reid JM, Baruchel S, Roberts T, Voss SD, et al. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. J Clin Oncol. (2013) 31:3034–43. 10.1200/JCO.2012.47.0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merchant MS, Geller JI, Baird K, Chou AJ, Galli S, Charles A, et al. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J Clin Oncol. (2012) 30:4141–7. 10.1200/JCO.2012.44.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Norris RE, Fox E, Reid JM, Ralya A, Liu XW, Minard C, et al. Phase 1 trial of ontuxizumab (MORAb-004) in children with relapsed or refractory solid tumors: a report from the Children's Oncology Group Phase 1 Pilot Consortium (ADVL1213). Pediatr Blood Cancer. (2018) 65:e26944. 10.1002/pbc.26944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock R, Carol H, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. (2008) 50:1190–7. 10.1002/pbc.21450 [DOI] [PubMed] [Google Scholar]
- 109.Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. (2009) 69:7662–71. 10.1158/0008-5472.CAN-09-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jully B, Rajkumar T: Potential molecular targets for Ewing's sarcoma therapy. Indian J Med Paediatr Oncol. (2012) 33:195–202. 10.4103/0971-5851.107074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ho AL, Schwartz GK. Targeting of insulin-like growth factor type 1 receptor in Ewing sarcoma: unfulfilled promise or a promising beginning? J Clin Oncol. (2011) 29:4581–3. 10.1200/JCO.2011.38.2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. (2007) 26:1932–40. 10.1038/sj.onc.1209990 [DOI] [PubMed] [Google Scholar]
- 113.Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, et al. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing's and osteogenic sarcoma models. J Pharmacol Exp Ther. (2011) 337:644–54. 10.1124/jpet.110.178400 [DOI] [PubMed] [Google Scholar]
- 114.Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing's sarcoma family tumors. Clin Cancer Res. (2012) 18:2625–31. 10.1158/1078-0432.CCR-12-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.González I, Andreu EJ, Panizo A, Inogés S, Fontalba A, Fernández-Luna JL, et al. Imatinib inhibits proliferation of Ewing tumor cells mediated by the stem cell factor/KIT receptor pathway, and sensitizes cells to vincristine and doxorubicin-induced apoptosis. Clin Cancer Res. (2004) 10:751–61. 10.1158/1078-0432.CCR-0778-03 [DOI] [PubMed] [Google Scholar]
- 116.Merchant MS, Woo CW, Mackall CL, Thiele CJ. Potential use of imatinib in Ewing's Sarcoma: evidence for in vitro and in vivo activity. J Natl Cancer Inst. (2002) 94:1673–9. 10.1093/jnci/94.22.1673 [DOI] [PubMed] [Google Scholar]
- 117.Andersson MK, Aman P. Proliferation of Ewing sarcoma cell lines is suppressed by the receptor tyrosine kinase inhibitors gefitinib and vandetanib. Cancer Cell Int. (2008) 8:1. 10.1186/1475-2867-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chernoguz A, Crawford K, Donovan E, Vandersall A, Berglund C, Cripe TP, et al. EGFR inhibition fails to suppress vascular proliferation and tumor growth in a Ewing's sarcoma model. J Surg Res. (2012) 173:1–9. 10.1016/j.jss.2011.04.041 [DOI] [PubMed] [Google Scholar]
- 119.Guan H, Zhou Z, Wang H, Jia SF, Liu W, Kleinerman ES. A small interfering RNA targeting vascular endothelial growth factor inhibits Ewing's sarcoma growth in a xenograft mouse model. Clin Cancer Res. (2005) 11:2662–9. 10.1158/1078-0432.CCR-04-1206 [DOI] [PubMed] [Google Scholar]
- 120.Zhou Z, Bolontrade MF, Reddy K, Duan X, Guan H, Yu L, et al. Suppression of Ewing's sarcoma tumor growth, tumor vessel formation, and vasculogenesis following anti vascular endothelial growth factor receptor-2 therapy. Clin Cancer Res. (2007) 13:4867–73. 10.1158/1078-0432.CCR-07-0133 [DOI] [PubMed] [Google Scholar]
- 121.Ackermann M, Morse BA, Delventhal V, Carvajal IM, Konerding MA. Anti-VEGFR2 and anti-IGF-1R-Adnectins inhibit Ewing's sarcoma A673-xenograft growth and normalize tumor vascular architecture. Angiogenesis. (2012) 15:685–95. 10.1007/s10456-012-9294-9 [DOI] [PubMed] [Google Scholar]
- 122.Potratz J, Tillmanns A, Berning P, Korsching E, Schaefer C, Lechtape B, et al. Receptor tyrosine kinase gene expression profiles of Ewing sarcomas reveal ROR1 as a potential therapeutic target in metastatic disease. Mol Oncol. (2016) 10:677–92. 10.1016/j.molonc.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bicocca VT, Chang BH, Masouleh BK, Muschen M, Loriaux MM, Druker BJ, et al. Crosstalk between ROR1 and the Pre-B cell receptor promotes survival of t(1;19) acute lymphoblastic leukemia. Cancer Cell. (2012) 22:656–67. 10.1016/j.ccr.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang S, Chen L, Cui B, Chuang HY, Yu J, Wang-Rodriguez J, et al. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS ONE. (2012) 7:e31127. 10.1371/journal.pone.0031127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cui B, Zhang S, Chen L, Yu J, Widhopf GF, Fecteau JF, et al. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. (2013) 73:3649–60. 10.1158/0008-5472.CAN-12-3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fleuren ED, Hillebrandt-Roeffen MH, Flucke UE, Te Loo DM, Boerman OC, van der Graaf WT, et al. The role of AXL and the in vitro activity of the receptor tyrosine kinase inhibitor BGB324 in Ewing sarcoma. Oncotarget. (2014) 5:12753–68. 10.18632/oncotarget.2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Geahlen RL. Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends Pharmacol Sci. (2014) 35:414–22. 10.1016/j.tips.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun H, Lin DC, Cao Q, Pang B, Gae DD, Lee VKM, et al. Identification of a novel SYK/c-MYC/MALAT1 signaling pathway and its potential therapeutic value in Ewing sarcoma. Clin Cancer Res. (2017) 23:4376–87. 10.1158/1078-0432.CCR-16-2185 [DOI] [PubMed] [Google Scholar]
- 129.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. (2002) 168:1356–61. 10.4049/jimmunol.168.3.1356 [DOI] [PubMed] [Google Scholar]
- 130.Kontny HU, Hämmerle K, Klein R, Shayan P, Mackall CL, Niemeyer CM. Sensitivity of Ewing's sarcoma to TRAIL-induced apoptosis. Cell Death Differ. (2001) 8:506–14. 10.1038/sj.cdd.4400836 [DOI] [PubMed] [Google Scholar]
- 131.Petak I, Douglas L, Tillman DM, Vernes R, Houghton JA. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. (2000) 6:4119–27. [PubMed] [Google Scholar]
- 132.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. (2010) 21:376–81. 10.1093/annonc/mdp292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kailayangiri S, Altvater B, Meltzer J, Pscherer S, Luecke A, Dierkes C, et al. The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Cancer. (2012) 106:1123–33. 10.1038/bjc.2012.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dobrenkov K, Ostrovnaya I, Gu J, Cheung IY, Cheung NK. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr Blood Cancer. (2016) 63:1780–5. 10.1002/pbc.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahmed M, Cheung NK. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. (2014) 588:288–97. 10.1016/j.febslet.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 136.Dobrenkov K, Cheung NK: GD2-targeted immunotherapy and radioimmunotherapy. Semin Oncol. (2014) 41:589–612. 10.1053/j.seminoncol.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. (2017) 18:946–957. 10.1016/S1470-2045(17)30355-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hamilton WB, Helling F, Lloyd KO, Livingston PO. Ganglioside expression on human malignant melanoma assessed by quantitative immune thin-layer chromatography. Int J Cancer. (1993) 53:566–73. 10.1002/ijc.2910530407 [DOI] [PubMed] [Google Scholar]
- 139.Minasian LM, Yao TJ, Steffens TA, Scheinberg DA, Williams L, Riedel E, et al. A phase I study of anti-GD3 ganglioside monoclonal antibody R24 and recombinant human macrophage-colony stimulating factor in patients with metastatic melanoma. Cancer. (1995) 75:2251–7. [DOI] [PubMed] [Google Scholar]
- 140.Scott AM, Lee FT, Hopkins W, Cebon JS, Wheatley JM, Liu Z, et al. Specific targeting, biodistribution, and lack of immunogenicity of chimeric anti-GD3 monoclonal antibody KM871 in patients with metastatic melanoma: results of a phase I trial. J Clin Oncol. (2001) 19:3976–87. 10.1200/JCO.2001.19.19.3976 [DOI] [PubMed] [Google Scholar]
- 141.Kushner BH, Cheung IY, Modak S, Kramer K, Ragupathi G, Cheung NK. Phase I trial of a bivalent gangliosides vaccine in combination with beta-glucan for high-risk neuroblastoma in second or later remission. Clin Cancer Res. (2014) 20:1375–82. 10.1158/1078-0432.CCR-13-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mulens V, de la Torre A, Marinello P, Rodríguez R, Cardoso J, Díaz R, et al. Immunogenicity and safety of a NeuGcGM3 based cancer vaccine: Results from a controlled study in metastatic breast cancer patients. Hum Vaccin. (2010) 6:736–44. 10.4161/hv.6.9.12571 [DOI] [PubMed] [Google Scholar]
- 143.Scursoni AM, Galluzzo L, Camarero S, Lopez J, Lubieniecki F, Sampor C, et al. Detection of N-glycolyl GM3 ganglioside in neuroectodermal tumors by immunohistochemistry: an attractive vaccine target for aggressive pediatric cancer. Clin Dev Immunol. (2011) 2011:245181. 10.1155/2011/245181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scursoni AM, Galluzzo L, Camarero S, Lopez J, Lubieniecki F, Sampor C, et al. B7-H3-mediated tumor immunology: friend or foe? Int J Cancer. (2014) 134:2764–71. 10.1002/ijc.28474 [DOI] [PubMed] [Google Scholar]
- 145.Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. (2001) 61:4048–54. [PubMed] [Google Scholar]
- 146.Merino ME, Navid F, Christensen BL, Toretsky JA, Helman LJ, Cheung NK, et al. Immunomagnetic purging of Ewing's sarcoma from blood and bone marrow: quantitation by real-time polymerase chain reaction. J Clin Oncol. (2001) 19:3649–59. 10.1200/JCO.2001.19.16.3649 [DOI] [PubMed] [Google Scholar]
- 147.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. (2010) 97:409–18. 10.1007/s11060-009-0038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kramer K, Smith M, Souweidane MM. Safety profile of long-term intraventricular access devices in pediatric patients receiving radioimmunotherapy for central nervous system malignancies. Pediatr Blood Cancer. (2014) 61:1590–2. 10.1002/pbc.25080 [DOI] [PubMed] [Google Scholar]
- 149.Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. (2018) 19:1040–50. 10.1016/S1470-2045(18)30322-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D, et al. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol. (2008) 172:486–94. 10.2353/ajpath.2008.070623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rouleau C, Curiel M, Weber W, Smale R, Kurtzberg L, Mascarello J, et al. Endosialin protein expression and therapeutic target potential in human solid tumors: sarcoma versus carcinoma. Clin Cancer Res. (2008) 14:7223–36. 10.1158/1078-0432.CCR-08-0499 [DOI] [PubMed] [Google Scholar]
- 152.Rouleau C, Smale R, Fu YS, Hui G, Wang F, Hutto E, et al. Endosialin is expressed in high grade and advanced sarcomas: evidence from clinical specimens and preclinical modeling. Int J Oncol. (2011) 39:73–89. [DOI] [PubMed] [Google Scholar]
- 153.Rouleau C, Smale R, Sancho J, Fu YS, Kurtzberg L, Weber W, et al. Endosialin: a novel malignant cell therapeutic target for neuroblastoma. Int J Oncol. (2011) 39:841–51. [DOI] [PubMed] [Google Scholar]
- 154.Tomkowicz B, Rybinski K, Sebeck D, Sass P, Nicolaides NC, Grasso L, et al. Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol Ther. (2010) 9:908–15. 10.4161/cbt.9.11.11731 [DOI] [PubMed] [Google Scholar]
- 155.Tomkowicz B, Rybinski K, Foley B, Ebel W, Kline B, Routhier E, et al. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc Natl Acad Sci USA. (2007) 104:17965–70. 10.1073/pnas.0705647104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. (1999) 96:14523–8. 10.1073/pnas.96.25.14523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rodeberg DA, Nuss RA, Elsawa SF, Celis E. Recognition of six-transmembrane epithelial antigen of the prostate-expressing tumor cells by peptide antigen-induced cytotoxic T lymphocytes. Clin Cancer Res. (2005) 11:4545–52. 10.1158/1078-0432.CCR-04-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grunewald TG, Diebold I, Esposito I, Plehm S, Hauer K, Thiel U, et al. STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol Cancer Res. (2012) 10:52–65. 10.1158/1541-7786.MCR-11-0524 [DOI] [PubMed] [Google Scholar]
- 159.Grunewald TG, Ranft A, Esposito I, da Silva-Buttkus P, Aichler M, Baumhoer D, et al. High STEAP1 expression is associated with improved outcome of Ewing's sarcoma patients. Ann Oncol. (2012) 23:2185–90. 10.1093/annonc/mdr605 [DOI] [PubMed] [Google Scholar]
- 160.Cheung IY, Feng Y, Danis K, Shukla N, Meyers P, Ladanyi M, et al. Novel markers of subclinical disease for Ewing family tumors from gene expression profiling. Clin Cancer Res. (2007) 13:6978–83. 10.1158/1078-0432.CCR-07-1417 [DOI] [PubMed] [Google Scholar]
- 161.Machlenkin A, Paz A, Bar Haim E, Goldberger O, Finkel E, Tirosh B, et al. Human CTL epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as candidates for prostate cancer immunotherapy. Cancer Res. (2005) 65:6435–42. 10.1158/0008-5472.CAN-05-0133 [DOI] [PubMed] [Google Scholar]
- 162.Alves PM, Faure O, Graff-Dubois S, Cornet S, Bolonakis I, Gross DA, et al. STEAP, a prostate tumor antigen, is a target of human CD8+ T cells. Cancer Immunol Immunother. (2006) 55:1515–23. 10.1007/s00262-006-0165-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dworzak MN, Fritsch G, Buchinger P, Fleischer C, Printz D, Zellner A, et al. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood. (1994) 83:415–25. [PubMed] [Google Scholar]
- 164.Rocchi A, Manara MC, Sciandra M, Zambelli D, Nardi F, Nicoletti G, et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J Clin Invest. (2010) 120:668–80. 10.1172/JCI36667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cerisano V, Aalto Y, Perdichizzi S, Bernard G, Manara MC, Benini S, et al. Molecular mechanisms of CD99-induced caspase-independent cell death and cell-cell adhesion in Ewing's sarcoma cells: actin and zyxin as key intracellular mediators. Oncogene. (2004) 23:5664–74. 10.1038/sj.onc.1207741 [DOI] [PubMed] [Google Scholar]
- 166.Sohn HW, Choi EY, Kim SH, Lee IS, Chung DH, Sung UA, et al. Engagement of CD99 induces apoptosis through a calcineurin-independent pathway in Ewing's sarcoma cells. Am J Pathol. (1998) 153:1937–45. 10.1016/S0002-9440(10)65707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.O'Neill AF, Dearling JL, Wang Y, Tupper T, Sun Y, Aster JC, et al. Targeted imaging of Ewing sarcoma in preclinical models using a 64Cu-labeled anti-CD99 antibody. Clin Cancer Res. (2014) 20:678–87. 10.1158/1078-0432.CCR-13-1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Scotlandi K, Perdichizzi S, Bernard G, Nicoletti G, Nanni P, Lollini PL, et al. Targeting CD99 in association with doxorubicin: an effective combined treatment for Ewing's sarcoma. Eur J Cancer. (2006) 42:91–6. 10.1016/j.ejca.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 169.Guerzoni C, Fiori V, Terracciano M, Manara MC, Moricoli D, Pasello M, et al. CD99 triggering in Ewing sarcoma delivers a lethal signal through p53 pathway reactivation and cooperates with doxorubicin. Clin Cancer Res. (2015) 21:146–56. 10.1158/1078-0432.CCR-14-0492 [DOI] [PubMed] [Google Scholar]
- 170.Pasello M, Manara MC, Scotlandi K. CD99 at the crossroads of physiology and pathology. J Cell Commun Signal. (2018) 12:55–68. 10.1007/s12079-017-0445-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hoffman RM. Development of recombinant methioninase to target the general cancer-specific metabolic defect of methionine dependence: a 40-year odyssey. Expert Opin Biol Ther. (2015) 15:21–31. 10.1517/14712598.2015.963050 [DOI] [PubMed] [Google Scholar]
- 172.Guo H, Lishko VK, Herrera H, Groce A, Kubota T, Hoffman RM. Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res. (1993) 53:5676–9. [PubMed] [Google Scholar]
- 173.Murakami T, Li S, Han Q, Tan Y, Kiyuna T, Igarashi K, et al. Recombinant methioninase effectively targets a Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. (2017) 8:35630–8. 10.18632/oncotarget.15823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hu J, Cheung NK. Methionine depletion with recombinant methioninase: in vitro and in vivo efficacy against neuroblastoma and its synergism with chemotherapeutic drugs. Int J Cancer. (2009) 124:1700–6. 10.1002/ijc.24104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Igarashi K, Kawaguchi K, Li S, Han Q, Tan Y, Gainor E, et al. Recombinant methioninase combined with doxorubicin (DOX) regresses a DOX-resistant synovial sarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. (2018) 9:19263–72. 10.18632/oncotarget.24996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Machado I, López-Guerrero JA, Scotlandi K, Picci P, Llombart-Bosch A. Immunohistochemical analysis and prognostic significance of PD-L1, PD-1, and CD8+ tumor-infiltrating lymphocytes in Ewing's sarcoma family of tumors (ESFT). Virchows Arch. (2018) 472:815–824. 10.1007/s00428-018-2316-2 [DOI] [PubMed] [Google Scholar]
- 177.Berghuis D, Santos SJ, Baelde HJ, Taminiau AH, Egeler RM, Schilham MW, et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8(+) T-lymphocyte infiltration and affect tumour progression. J Pathol. (2011) 223:347–57. 10.1002/path.2819 [DOI] [PubMed] [Google Scholar]
- 178.Evans CH, Liu F, Porter RM, O'Sullivan RP, Merghoub T, Lunsford EP, et al. EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors. Clin Cancer Res. (2012) 18:5341–51. 10.1158/1078-0432.CCR-12-1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dagher R, Long LM, Read EJ, Leitman SF, Carter CS, Tsokos M, et al. Pilot trial of tumor-specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: an inter-institute NIH study. Med Pediatr Oncol. (2002) 38:158–64. 10.1002/mpo.1303 [DOI] [PubMed] [Google Scholar]
- 180.Foell JL, Hesse M, Volkmer I, Schmiedel BJ, Neumann I, Staege MS. Membrane-associated phospholipase A1 beta (LIPI) Is an Ewing tumour-associated cancer/testis antigen. Pediatr Blood Cancer. (2008) 51:228–34. 10.1002/pbc.21602 [DOI] [PubMed] [Google Scholar]
- 181.Mahlendorf DE, Staege MS. Characterization of Ewing sarcoma associated cancer/testis antigens. Cancer Biol Ther. (2013) 14:254–61. 10.4161/cbt.23298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ghisoli M, Barve M, Schneider R, Mennel R, Lenarsky C, Wallraven G, et al. Pilot Trial of FANG Immunotherapy in Ewing's Sarcoma. Mol Ther. (2015) 23:1103–9. 10.1038/mt.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Merchant MS, Bernstein D, Amoako M, Baird K, Fleisher TA, Morre M, et al. Adjuvant Immunotherapy to Improve Outcome in High-Risk Pediatric Sarcomas. Clin Cancer Res. (2016) 22:3182–91. 10.1158/1078-0432.CCR-15-2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. (2010) 16:3901–9. 10.1158/1078-0432.CCR-10-0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Verhoeven DH, de Hooge AS, Mooiman EC, Santos SJ, ten Dam MM, Gelderblom H, et al. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol Immunol. (2008) 45:3917–25. 10.1016/j.molimm.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 186.Berghuis D, Schilham MW, Vos HI, Santos SJ, Kloess S, Buddingh' EP, et al. Histone deacetylase inhibitors enhance expression of NKG2D ligands in Ewing sarcoma and sensitize for natural killer cell-mediated cytolysis. Clin Sarcoma Res. (2012) 2:8. 10.1186/2045-3329-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thiel U, Schober SJ, Einspieler I, Kirschner A, Thiede M, Schirmer D, et al. Ewing sarcoma partial regression without GvHD by chondromodulin-I/HLA-A*02:01-specific allorestricted T cell receptor transgenic T cells. Oncoimmunology. (2017) 6:e1312239. 10.1080/2162402X.2017.1312239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 189.Gunderson AJ, Young KH. Exploring optimal sequencing of radiation and immunotherapy combinations. Adv Radiat Oncol. (2018) 3:494–505. 10.1016/j.adro.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Oncologist. (1999) 4:370–8. [PubMed] [Google Scholar]
- 191.Ladenstein R, Pötschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19:1617–29. 10.1016/S1470-2045(18)30578-3 [DOI] [PubMed] [Google Scholar]
- 192.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. (2015) 1:1325–32. 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- 193.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. (2015) 3:345–55. 10.1158/2326-6066.CIR-14-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jiang W, Chan CK, Weissman IL, Kim BYS, Hahn SM. Immune Priming of the tumor microenvironment by radiation. Trends Cancer. (2016) 2:638–45. 10.1016/j.trecan.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 195.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. (2017) 14:365–79. 10.1038/nrclinonc.2016.211 [DOI] [PubMed] [Google Scholar]
- 196.Lake RA, Robinson BW. Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer. (2005) 5:397–405. 10.1038/nrc1613 [DOI] [PubMed] [Google Scholar]
- 197.Lazzari C, Karachaliou N, Bulotta A, Viganó M, Mirabile A, Brioschi E, et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther Adv Med Oncol. (2018) 10:1758835918762094. 10.1177/1758835918762094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. (2015) 3:436–43. 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Crompton BD, Stewart C, Taylor-Weiner A, Alexe G, Kurek KC, Calicchio ML, et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. (2014) 4:1326–41. 10.1158/1538-7445.AM2014-999 [DOI] [PubMed] [Google Scholar]
- 200.Sheffield NC, Pierron G, Klughammer J, Datlinger P, Schönegger A, Schuster M, et al. DNA methylation heterogeneity defines a disease spectrum in Ewing sarcoma. Nat Med. (2017) 23:386–395. 10.1038/nm.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mazor T, Pankov A, Song JS, Costello JF. Intratumoral heterogeneity of the epigenome. Cancer Cell. (2016) 29:440–451. 10.1016/j.ccell.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]