Abstract
Background
The increasing interest in replacing petroleum-based products by more sustainable materials in the packaging sector gives relevance to cellulose as a biodegradable natural resource. Moreover, its properties can be modified physically, chemically or biotechnologically in order to obtain new bioproducts. Refined cotton linters with high cellulose content were treated with hydrolytic (cellulases) and oxidative (LPMO and Laccase_Tempo) enzymes to evaluate their effect on fibre properties and in improving mechanical fibrillation.
Results
Cellulases released cellooligosaccharides, reducing fibre length and partially degrading cellulose. They also improved mechanical fibrillation yielding up to 18% of nanofibrillated cellulose (NFC). LPMO introduced a slight amount of COOH groups in cellulose fibres, releasing cellobionic acid to the effluents. The action of cellulases was improved after LPMO treatment; however, the COOH groups created disappeared from fibres. After mechanical fibrillation of LPMO–cellulase-treated cotton linters a 23% yield of NFC was obtained. Laccase_Tempo treatment also introduced COOH groups in cellulose fibres from cotton, yielding 10% of NFC. Degree of polymerization was reduced by Laccase_Tempo, while LPMO treatment did not significantly affect it but produced a higher reduction in fibre length. The combined treatment with LPMO and cellulase provided films with higher transparency (86%), crystallinity (92%), smoothness and improved barrier properties to air and water than films casted from non-treated linters and from commercial NFC.
Conclusions
The combined enzymatic treatment with LPMO and cellulases boosted mechanical fibrillation of cotton linters, improving the NFC production and providing bioproducts with high transparency and high barrier properties.
Electronic supplementary material
The online version of this article (10.1186/s13068-019-1502-z) contains supplementary material, which is available to authorized users.
Keywords: Cellulose, Cotton linters, LPMO, Laccase_Tempo, Cellulases, NFC
Background
Cotton linters are an important by-product of the textile industry, being the short fibre fraction that cannot be used in the textile process [1]. They are obtained from cotton plant (Gossypium sp.), an annual shrub harvested for their high industrial interest. Cotton linters consist of high-quality cellulose fibres presenting very high cellulose content (98%) [2]. They are typically used in special applications such as the production of cellulose derivatives, regenerated cellulose, or the manufacture of high added-value papers [3].
In order to construct new materials and products based on renewable resources, the interest in functionalizing cellulose has gained importance in the last years. In fact, there is an increasing interest in replacing synthetic polymers by more sustainable materials to replace petroleum-based products in the packaging sector [4]. Modification of cellulose by chemical or biotechnological means has been reported [5]. Hydrolytic enzymes like cellulases can successfully modify cellulose, improving its reactivity and also altering fibre morphology [6]. On the other hand, the oxidative enzymatic system Laccase_Tempo (2,2,6,6-tetramethyl-1-piperidinyloxy) can create new functional groups to cellulose converting primary hydroxyl groups to aldehyde or carboxyl forms [7, 8]. In this system, laccase, having a redox potential in the range of 0.7–0.9 V, can easily oxidize the stable oxyl-radical form of Tempo to oxoammonium ion (E° 0.2 V). This ion is the actual oxidant of cellulose, which can be regenerated by laccase oxidation or by acid-induced disproportionation.
A new generation of enzymes that also create functional groups in cellulose and other crystalline polysaccharides such as chitin, lytic polysaccharide monooxygenases (LPMO), have been discovered [9, 10]. They oxidatively cleave glycosidic linkages, leading to the formation of oxidized glucose units at different positions, resulting in the formation of aldonic acids at the C1 position and/or 4-ketoaldoses (gemdiols) at the C4 position [11]. This oxidation renders the substrate more susceptible to be hydrolysed by conventional cellulases and is considered as a breakthrough in the enzymatic degradation of cellulose [12]. The enzymatic effects that LPMO produce in cellulose have been mainly evaluated through their increase in cellulose degradation [13, 14]. However, the effect that LPMO produces on pulp fibres has been poorly investigated [15–17]. These authors demonstrated that LPMO weakens fibres cohesion, promoting their disruption during mechanical fibrillation.
The production of nanocrystalline cellulose (NCC) from cotton linters has been reported, and also the ability of cellulases to improve its yield [2, 18]. However, little knowledge exists about the production of nanofibrillated cellulose (NFC) from these fibres [19–21]. Interestingly, cotton provides fibres with promising interest in nanocellulose production due to its high purity and highly crystalline cellulose [2]. NFC is usually produced by high-pressure homogenization, being major impediments for its commercial success the very high energy consumption of the production process and the clogging of homogenizers. Therefore, some pretreatments are needed in order to facilitate this process [22]. The ability of cellulases to improve this process has been demonstrated [23–26]. Also, the improvement of mechanical fibrillation produced with Tempo–NaBr–NaClO system is well known [20]. In order to replace the halide-based co-oxidizer system, laccase can be used to oxidize Tempo.
In this work, several enzymes were applied on cotton linters in order to analyse their effects on sugar release and on cellulose and fibre modifications. Four hydrolytic enzymes (cellulases) and also two oxidative enzymatic systems (a new bacterial LPMO and the Laccase_Tempo system) were used for this purpose. The effects that enzymes produced during mechanical fibrillation were also analysed, together with the optical, physical and barrier properties of the films casted from these treated linters.
Materials and methods
Raw material
Cotton linters obtained from the second cut were supplied by CELESA (Celulosa de Levante S.A.), Tortosa, Spain. The initial fibres had an average of 0.47 mm of length, 19.67 μm of width and 38.87% fine content. Their drainability, measured as ºSR, was 12. Prior to the enzymatic pretreatments, cotton linters were refined in a valley mill for 24 h in order to reduce their average length. Refined linters, named as “R”, had an average fibre length of 0.25 mm, fibre with 25.5 μm and 52.58% of fines; their drainability was increased to 77ºSR.
A commercial NFC (Com) supplied by the University of Maine, with 90% of fines, was used for comparison.
Enzymes
Four hydrolytic enzymes (cellulases) and two oxidative enzymes (LPMO and Laccase_Tempo) were used as pretreatments in cotton linters. Cel9B from Paenibacillus barcinonensis BP-23 [27] was a monocomponent processive endoglucanase named as “C9”. A commercial cellulase from Sertec20 was named as “C50”, whereas two commercial cellulases supplied by Novozymes® (Fibercare and Celluclast) were named as “CF” and “Cll”, respectively. Their initial enzymatic activities were 5.5, 383, 99 and 536 U mL−1 for C9, C50, CF and Cll, respectively. The commercial cellulase preparations used were not monocomponent and contained mixtures of several enzymes. Enzymatic activity was assayed by measuring the amount of reducing sugars released from carboxymethylcellulose (CMC) by the dinitrosalicylic (DNS) reagent method [28]. The standard assay (100 µL reaction volume) was performed at 50 °C in 50 mM potassium acetate buffer at pH 5 for 15 min. One unit of enzymatic activity (U) was defined as the amount of enzyme that releases 1 µmol of reducing sugar equivalent per min under the assay conditions described. A standard curve of glucose was used to calculate activity units. All determinations of enzyme activity were made in triplicate.
For the oxidative treatments, an LPMO from Streptomyces ambofaciens (SamLPMO10C) [29] and a laccase from Trametes villosa in combination with Tempo (2,2,6,6-tetramethyl-1-piperidinyloxy) were used. They were named as “S” and “L_Tempo”, respectively. Laccase was supplied by Novozymes® (Denmark) and had an activity of 746 U mL−1. Tempo was purchased from Sigma-Aldrich. The laccase activity was measured as the extent of oxidation of 5 mM 2,20-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) to its cation radical (ε436 = 29,300 M−1 cm−1) in 0.1 M sodium acetate buffer (pH 5) at 24 °C. One activity unit (U) was defined as the amount of enzyme converting 1 µmol of ABTS per min.
Enzymatic pretreatments on cotton linters
Pretreatments with cellulases were performed with 5 g odp (oven-dried pulp) at 10% consistency, with 10 U g−1 odp of enzyme in 50 mM potassium acetate buffer, pH 5, at 50 °C for 18 h. A combined treatment with CF and Cll, named as “Cmix”, was also performed. This pretreatment was performed as described above but with 10 U g−1 odp of CF and 10 U g−1 odp of Cll. Treatment with LPMO (S treatment) was performed with 5 g odp and 4 mg of enzyme g−1 odp at 5% consistency, for 72 h at 50 °C in 10 mM of ammonium acetate buffer at pH 6, with 2 mM ascorbic acid and 20 μM hydrogen peroxide. L_Tempo oxidation treatments were performed at room temperature, at 5% consistency, using 50 mM potassium acetate buffer at pH 5, 60 U g−1 odp laccase and 8% odp of Tempo for 18 h, according to previous works [7, 8].
All the enzymatic treatments were conducted in polyethylene bags that were placed in a laboratory water bath. After treatment, liquors were recovered and the resulting pulp was extensively washed as reported elsewhere for eucalyptus pulp [30] in order to remove the enzymes and their degradation products. In the case of L_Tempo treatments, pulp was also washed with ethanol. Control treatments with potassium acetate buffer and ammonium acetate buffer were also performed at the same application conditions but without the addition of enzymes. They were named “CK” and “SK”.
Effects on effluent properties
Released cellooligosaccharides were quantified by the dinitrosalicylic (DNS) reagent method and analysed by thin-layer chromatography (TLC) and HPAEC-PAD (high-performance anion-exchange chromatography with pulsed amperometric detection). For reducing sugar quantification, 100 μL of DNS was added to 100 μL samples and mixtures were incubated at 100 °C for 5 min. Then, 40 μL of reaction mixtures was placed in ELISA plates, 260 μL of distilled water was added, and the absorbance at 540 nm was measured. Samples were analysed in triplicate. A standard curve of glucose was used to calculate the glucose reducing sugar equivalent of the different samples [31].
For TLC analysis 10–15 μL of samples was applied on a silica gel plate (Merck, Germany) constituting the solid phase. 10 μL of an oligomer standard mixture containing cellooligosaccharides at a concentration of 20 mg mL−1 was applied as migration standards. The mobile phase was a mixture of chloroform, acetic acid and H2O in 6:7:1 ratio, respectively. The migration was repeated twice, and the silica gel plate was then sprayed (Fungilab S.A., Spain) with a developing solution, consisting of 5% H2SO4 in ethanol. Finally, the plate was heated in the oven at 100 °C for 5 min, where the spots corresponding to different cellooligosaccharides were visualized [31]. For HPAEC-PAD sample preparation, after removing insoluble substrates by centrifugation, supernatants were centrifuged and diluted in water 1/20 and analysed by HPAEC-PAD using Dionex GS50, gradient pump, Dionex AS50 Autosample and electrochemical detector Waters 2465. In brief, 40-μL samples were injected on a CarboPac PA1 2 × 250 mm analytical column (Dionex). Cellooligosaccharides were eluted at 0.25 mL min−1 using a stepwise linear gradient from 100% eluent A (0.1 M NaOH) towards 10% eluent B (0.6 M NaOAc in 0.1 M NaOH) 10 min after injection and to 40% eluent B 15 min after injection, followed by a 5-min exponential gradient to 100% B. The column was reconditioned between each run by running initial conditions for 10 min. Standards were generated using 1, 2, 4 and 8 μg mL−1 cellobiose and cellobionic acid [17].
Pulp characterization
The morphological properties of the fibres (viz. length and width) and the content in fines of the pulp samples were determined in accordance with TAPPI T 271 on a Metso kajaani FS300 fibre analyser. All samples were analysed in duplicate. Viscosity was determined according to ISO 5351:2010. The degree of polymerization (DP) was calculated from the intrinsic viscosity (Ƞ), using the equation of (SCAN-CM15:88): DP0.085 = 1.1 × [Ƞ]. Carboxyl groups were determined by measuring methylene blue adsorption onto cellulose fibres according to Davidson [32]. To measure aldehyde groups samples were further oxidized with NaClO2 for selective conversion of aldehyde groups into carboxyl groups at room temperature for 48 h. The carboxyl content was determined with the above-described method. The carboxyl groups formed by effect of NaClO2 oxidation were assumed to derive from aldehyde groups originally present in the pulp. Three measures per sample were performed, and the 95% confidence interval was calculated.
High-pressure homogenization
Prior to fibrillation, 2 g of oven-dried pulp (odp) at 1% consistency was disintegrated for 1 min at 11,200 rpm with a homogenizer (Homogenizing System UNIDRIVE X1000). Then, samples were diluted until 0.5% consistency and homogenized through the PANDA GEA 2000 homogenizer by 5 passes at 300 bar and 10 passes at 900 bar.
The yield of fibrillation (Eq. 1) was calculated after centrifuging 10 mL of a sample at 0.1% consistency at 2200×g for 20 min, removing the supernatant (containing the nanofibrillated fraction) and drying pellet (C) at 85 °C until constant weight.
| 1 |
Transmittance measurements were taken on samples with 0.1% of solid content. The sample was introduced in quartz cuvettes, and the transmittance was obtained with a T92 + UV spectrophotometer (PG instruments) set in the range between 400 and 800 nm. Milli-Q water was used as blank.
Fibre morphology and DP were measured as previously described in pulp samples. Electrophoretic mobility of aqueous suspensions (zeta potential) was determined using a Zetamaster model ZEM (Malvern Instruments, UK). Data were averaged over 10 measurements. All samples were analysed at room temperature.
Film characterization
After fibrillation, films with a grammage around 45–50 g m−2 were obtained by the film casting technique [33]. Their optical and physical–mechanical properties were determined in accordance with the standards in brackets as follows: transparency (22891:2013), apparent density (ISO 534:2005), Bekk smoothness (5627:1995), and dry and wet zero-span index (ISO 15361:2000). Fibres zero-span tensile index was determined in a Zero-span 1000 Pulmac tester. For analysis of wet zero-span index, films were previously soaked in distilled water for 120 s.
Barrier properties to air and water were also analysed. Air permeance was measured with Bekk equipment. Water impermeability was measured by the water drop test (WDT) according to TAPPI standard T835 om-08. The WDT involved placing a drop of deionized water on the surface of paper and recording the time needed for complete absorption, which was signalled by vanishing of the drop specular gloss. Ten measurements per treated film sample were made and averaged. Six measures per sample were performed, and the 95% confidence interval was calculated.
The crystallinity index (CrI) of different cellulosic substrates was measured by XRD (X-ray powder diffraction). Samples were dried directly on an aluminium plate of 32 mm of diameter and 3.0 mm of thickness, that were mounted in standard sample holders for bulk samples of thickness ≤ 7 mm (PW1812/00) by means of plasticine. A PANalytical X’Pert PRO MPD Alpha1 powder diffractometer in Bragg–Brentano θ/2θ geometry of 240 mm of radius with Cu Kα1 radiation (λ = 1.5406 Å) at 45 kV and 40 mA, focalizing Ge (111) primary monochromator, with sample spinning at 2 revolutions per s, fixed divergence slit of 0.25º, was used. The measurement range (2θ) was from 2º to 50º with a step size of 0.033º and measuring time of 100 s per step. To calculate the CrI of cellulose from the XRD spectra, the peak height method used elsewhere was applied [34].
Total crystallinity index (TCI) was measured using Fourier transform infrared (FTIR) spectra as previously described [35].
Morphological characterization of film surface was performed by field emission scanning electron microscopy (FESEM) (JSM 7100 F) using a LED filter and a backscattered electron detector (BED).
Results and discussion
The initial cotton linters were long fibres, with an average length of 0.47 mm, which had been hornified (stiffened) during the drying inherent to their production. These traits made them difficult to process because they usually clog in the high-pressure homogenization apparatus. For this reason, they were mechanically refined by beating in a valley mill, which reduced fibre length to 0.25 mm and facilitated their homogenization.
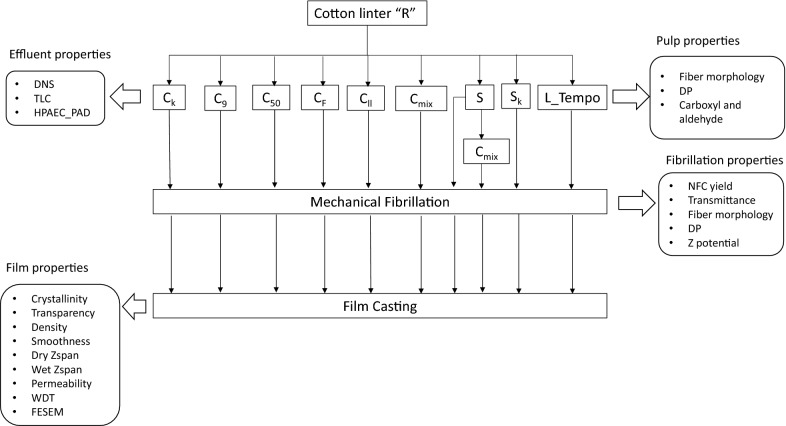
The effect of enzymes on the refined cotton linters was firstly assessed on the properties of effluents released. Then, modifications produced by enzymes in fibre morphology and cellulose were analysed, together with their effect on the fibrillation improvement. Finally, the optical, physical and barrier properties of the films casted from the treated fibres were evaluated (Fig. 1) and compared with films obtained from commercial NFCs.
Fig. 1.
General schema of the experimental work performed
Effect of enzymes on sugar release
Cotton linters were treated with different cellulases: endoglucanase Cel9B (C9) and commercial cellulases C50, CF or Cll, and the amount of neutral sugars released was analysed by DNS (Table 1). C9 and CF produced a similar sugar release, much lower than that released by C50 and Cll. When CF and Cll were applied in the same treatment (Cmix) the sugar release was the same as that with Cll alone.
Table 1.
Neutral sugar and cellobionic acid release produced by the enzymatic pretreatments
| Sugar release (mg g−1 pulp) | Cellobionic acid release (mg g−1 pulp) | |
|---|---|---|
| Ck | 0 | 0 |
| C9 | 22 ± 4 | 0 |
| C50 | 90 ± 10 | 0 |
| CF | 34 ± 5 | 0 |
| Cll | 141 ± 11 | 0 |
| Cmix | 126 ± 13 | 0 |
| S | – | 0.8 ± 0.2 |
| SCmix | 242 ± 2 | 6.1 ± 1.1 |
Ck (cellulase control treatment), C9 (Cel9B), C50 (Sertec20 cellulase), CF (Fibercare cellulase), Cll (Celluclast cellulase), Cmix (cellulase mixture consisting in Fibercare and Celluclast), S (LPMO), SCmix (LPMO and Cmix)
TLC analysis showed that C9 released mainly glucose and cellobiose (Additional file 1), being cellobiose the most abundant cellooligosaccharide released in accordance with its processive endoglucanase activity [27]. Similar product pattern was reported by Garcia-Ubasart et al. [36] when treating flax pulp with this enzyme. Commercial cellulases released a wider pattern of products from cotton linters, neutral sugars from glucose to cellotetraose, without noticeable differences among the enzymes (Additional file 1).
The action of LPMO, SamLPMO10C (S), was analysed determining the production of oxidized sugars in the effluents by HPAEC-PAD. S treatment released cellobionic acid and other aldonic acid oligosaccharides of higher molecular weight, together with a small fraction of neutral sugars (Fig. 2). Although the amount of cellobionic acid released to the effluents was low (Table 1), the ability of SamLPMO10C to oxidize cotton linters was demonstrated. Our results are in accordance with the production of C1-oxidized oligosaccharides from phosphoric acid-swollen cellulose (PASC) by SamLPMO10C, which was also able to release aldonic acids from flax fibres [17, 29]. On the contrary, in other reported works, the production of aldonic acids when an LPMO belonging to the AA9 family was applied to softwood kraft pulp was not observed [15].
Fig. 2.
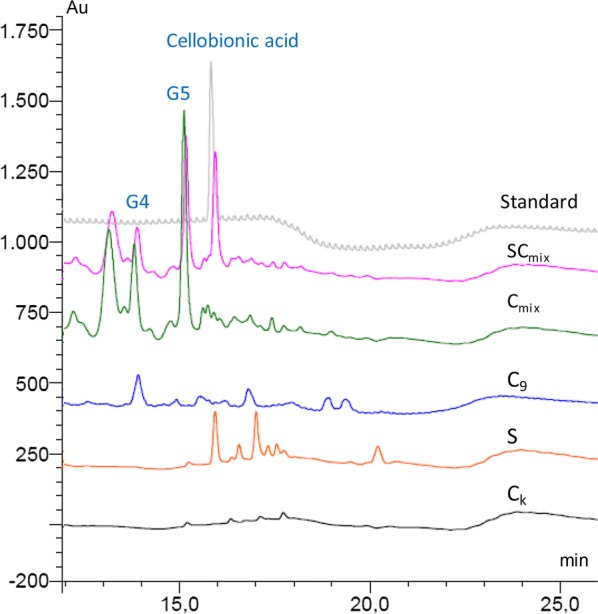
Sugar release produced by Ck (control treatment), C9 (Cel9B), Cmix (cellulase mixture consisting in Fibercare and Celluclast), S (LPMO) and SCmix (LPMO and Cmix) analysed by HPAEC-PAD. Enzymatic treatments with cellulases were performed at 50 °C, pH 5, for 18 h with 10 U g−1 odp of enzyme. (In the case of Cmix the enzymatic dose was 20 U g−1 odp.) Enzymatic treatment with LPMO (S) was performed at 50 °C, pH 6, for 72 h with 4 mg of enzyme g−1 odp in the presence of ascorbic acid and hydrogen peroxide
LPMO have extensively been reported to promote the efficiency of cellulases [12], and in our hands, an increase in sugar release by the combined SCmix treatment, that was twice more than in the single treatment with cellulases, Cmix, confirmed this statement. Furthermore, the cellobionic acid release in the SCmix treatment was eightfold higher than with S, suggesting that the oxidized fractions of cellulose created during S were cleaved and released to the effluent during the Cmix treatment (Table 1). Cmix treatment was not applied after L_Tempo treatment since some authors state that cellulose oxidation produced by L_Tempo impairs the action of these enzymes [37].
Effect of enzymes on fibre morphology and cellulose modification
The refined cotton linter fibres used were short (0.25 mm average length) and had a large amount (more than 50%) of fibres lower than 0.2 mm (fines) (Additional file 2a). Enzyme treatment changed the morphology and size distribution of fibres. Cellulases acted on the longer fibres (around 0.2–7.6 mm) creating high amounts of fines, which showed the highest increase in their shortest fraction, fibres lower than 0.1 mm. Among the cellulases, C9 produced the smaller morphology change. It slightly reduced fibre length, with only 3% increase in the fine content, and it did not produce a significant effect on fibre width (Table 2). Fibre degradation by Cll was higher (16% fines increase), in accordance with its higher sugar release. Although C50 released more quantity of sugars than CF, they produced a similar fibre degradation (fines increased in 10%). Combined cellulase treatment (Cmix) produced the highest increase in fines content (31%), although sugar release was not increased in the combined treatment. Fibre width was slightly reduced by Cll and Cmix probably because of the degradation of the surface fibrillation of fibres.
Table 2.
Effects of enzymatic pretreatments on fibre morphology and on mechanical fibrillation
| Pulp | After mechanical fibrillation | ||||
|---|---|---|---|---|---|
| Fibre width (μm) | Fibre length (mm) | Fines (%) | Yield (%) | Transmittance at 700 nm (%) | |
| R | 25.5 | 0.25 | 52.58 | 0 | 2.4 ± 0.5 |
| Ck | 24.9 | 0.25 | 53.7 | 0 | 3.2 ± 0.7 |
| C9 | 24.7 | 0.24 | 55.43 | 0 | 7.8 ± 0.3 |
| C50 | 24.2 | 0.22 | 59.25 | 0 | 45.6 ± 1.2 |
| CF | 24.8 | 0.22 | 59.01 | 0 | 32.9 ± 0.9 |
| Cll | 23.2 | 0.21 | 62.18 | 0 | 27.9 ± 0.8 |
| Cmix | 21.2 | 0.18 | 70.44 | 18 ± 1 | 52.7 ± 0.9 |
| Sk | 25.1 | 0.25 | 52.8 | 0 | 3.8 ± 0.5 |
| S | 23.3 | 0.21 | 61.58 | 2.5 ± 2 | 4.3 ± 0.4 |
| SCmix | 23.2 | 0.16 | 73.5 | 23 ± 1 | 51.8 ± 0.5 |
| L_Tempo | 23.0 | 0.23 | 57.13 | 10 ± 2 | 4.5 ± 0.6 |
| Com | – | – | – | 11 ± 2 | 5.7 ± 0.7 |
The confidence interval of the fibre width, fibre length and fines was less than 2% in all cases
R (initial refined pulp), Ck (cellulase control treatment), C50 (Sertec20 cellulase), CF (Fibercare cellulase), Cll (Celluclast cellulase) C9 (Cel9B), Cmix (cellulase mixture consisting in Fibercare and Celluclast), Sk (LPMO control treatment), S (LPMO), SCmix (LPMO and Cmix), L_Tempo (Laccase_Tempo treatment) and Com (commercial NFC)
Whereas hydrolytic treatments with cellulases are well known to act on fibre morphology [38], little knowledge exists about the fibre modification produced by oxidative treatments, particularly with LPMO enzymes. Interestingly, the two oxidative treatments performed affected fibre morphology, reducing its fibre length and width and consequently increasing fines content (Table 2). The increase in fines content was more pronounced with S (16%) than with L_Tempo (6%). These results contrast with those reported by Aracri et al. [8] reporting that no effect on fines content was produced by L_Tempo treatment of sisal pulps. Finally, SCmix treatment produced the highest increase in fines (37%) and a large amount of fines lower than 0.1 mm (42%) (Additional file 2b), in agreement with the highest sugar release of SCmix treatment, confirming that fibre degradation by cellulases was boosted by LPMO action. These results are in accordance with the proposed mechanism of LPMO that create nicking points where the cohesion of the fibres was decreased, improving the attack of cellulases [15].
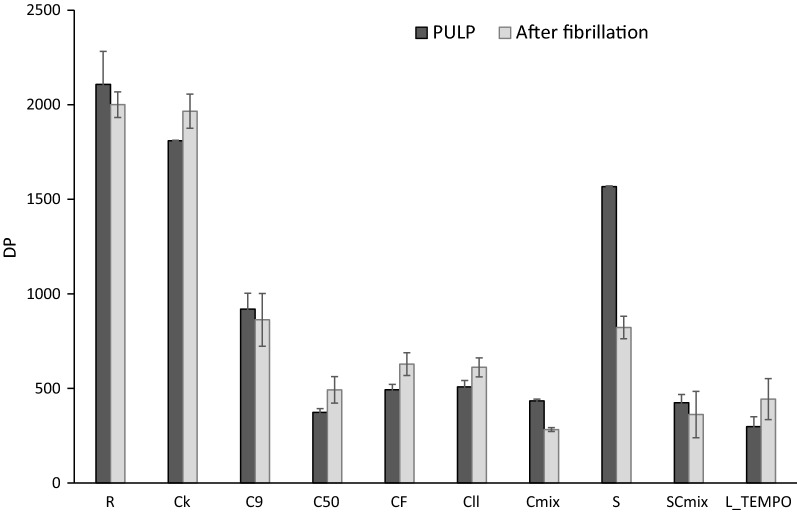
Changes in cellulose polymerization were assessed via intrinsic viscosity measurements (Fig. 3). Similarly to what has been reported [26, 39] all the tested cellulases decreased DP. In correlation with the effects of the cellulases on fibre morphology and sugar release described above, C9 produced lower cellulose degradation (52% decrease in DP) than the commercial cellulases applied (around 73–79%). A similar cellulose depolymerization was observed by Qing et al. in 2013 [24] when CF and Cll were applied to a bleached eucalyptus kraft pulp at lower enzymatic doses. Contrary to our results, previous authors reported that DP of softwood and flax pulps was not significantly affected by endoglucanase C9 [36, 38], but in our case, the higher cellulose degradation produced by C9 in cotton linters can be due to the longer treatment applied (18 h vs. 1–2 h in previous works).
Fig. 3.
Effect of enzymatic treatments on cellulose degree of polymerization. R (initial refined pulp), Ck (control treatment), C9 (Cel9B), C50 (Sertec20 cellulase), CF (Fibercare cellulase), Cll (Celluclast cellulase), Cmix (cellulase mixture consisting in Fibercare and Celluclast), S (LPMO), SCmix (LPMO and Cmix) and L_Tempo (Laccase_Tempo treatment). Enzymatic treatments with cellulases were performed at 50 °C, pH 5, for 18 h with 10 U g−1 odp of enzyme. (In the case of Cmix the enzymatic dose was 20 U g−1.) Enzymatic treatment with LPMO (S) was performed at 50 °C, pH 6, for 72 h with 4 mg of enzyme g−1 odp in the presence of ascorbic acid and hydrogen peroxide. Enzymatic treatment with Laccase_Tempo was performed at room temperature, pH 5, for 18 h at 60 U g−1 odp of laccase and 8% odp of Tempo
Concerning the oxidative treatments, cellulose DP was highly affected by L_Tempo, while S treatment produced a small decrease (Fig. 3). Depolymerization of cellulose by L_Tempo has been described to be produced by active species such as hydroxyl radicals formed in situ by side reactions of the hydroxylamine structure with oxygen during the oxidative treatment [40]. Moreover, the presence of aldehyde groups produced by L_Tempo treatment can provide underestimation of viscosity values. These aldehyde groups can give depolymerization reactions through β-elimination during the viscosity determination method, with cupriethylenediamine at alkaline conditions. To avoid this problem, viscosity was also measured after treating the pulp samples with sodium borohydride (borohydride viscosity) in order to inactivate carbonyl groups by reduction to hydroxyl groups [8]. Borohydride viscosity was measured in all the samples (data not shown) obtaining DP values similar to those shown in Fig. 3, with the exception of L_Tempo sample that showed an increased DP, indicating that aldehyde groups were formed in this treatment. However, even after the reductive treatment, DP of L_Tempo sample was low (572), indicating degradation of cotton linters cellulose by L_Tempo, similarly to previous results described for sisal pulps, although with less intense degradation [8]. On the contrary, the low depolymerization produced by LPMO suggested that this enzyme affects fibre morphology without significantly degrading cellulose. Villares et al. also reported a slight decrease in DP by LPMO although fibre morphology was not affected [15]. Interestingly, despite the high fibre modification and cellobionic acid release by the S treatment, cellulose was not significantly degraded. Subsequent treatment with the cellulase mixture, SCmix, did not increase cellulose depolymerization by cellulases.
Finally, the creation of functional groups on cellulose was evaluated by measuring the carboxyl and aldehyde content of fibres. Results showed a significant increase of these groups only with the oxidative treatments, where the L_Tempo treated pulps exhibited the highest content (Table 3), as also appreciated by FTIR spectra (Additional file 3). A different mechanism of creating COOH groups was produced among the oxidative treatments: whereas L_Tempo oxidized cellulose as a result of the conversion of C6 primary hydroxyl groups in cellulose via an aldehyde group [41], S created COOH through oxidation of alcohol in C1 position [29]. A small fraction of aldehydes were also produced during L_Tempo in accordance with the previous results on borohydride viscosity. The modest increase in carboxyl group content provided by the L_Tempo system in comparison with other works under the conditions used is probably due to the absence of added oxygen during the treatment [7].
Table 3.
Effects of enzymatic pretreatments in the creation of COOH and CHO groups in cellulose fibres
| COOH (µmol g−1 odp) | CHO (µmol g−1 odp) | |
|---|---|---|
| R | 18 ± 4 | 0 |
| Ck | 13 ± 1 | 0 |
| C9 | 20 ± 1 | 1 ± 1 |
| Cmix | 17 ± 3 | 1 ± 1 |
| S | 29 ± 4 | 0 |
| SCmix | 20 ± 1 | 0 |
| L_Tempo | 85 ± 9 | 4 ± 1 |
R (initial refined pulp), Ck (cellulase control treatment), C9 (Cel9B), Cmix (cellulase mixture consisting in Fibercare and Celluclast), S (LPMO), SCmix (LPMO and Cmix) and L_Tempo (Laccase_Tempo treatment)
The oxidative cleavage of cellulose by S treatment leads to the formation of a small quantity of aldonic acids (COOH groups) at the C1 position. However, a fraction of these COOH groups disappeared when Cmix was applied after S, suggesting they were removed. This result is in accordance with the higher cellobionic acid release in the SCmix treatment previously shown, corroborating that the oxidized fractions of cellulose created by S activity were cleaved by the Cmix treatment.
Effect of enzymes on fibrillation improvement
Enzyme-treated samples were homogenized at high pressure, and their properties were analysed. NFC was only obtained in the hydrolytic treatment with the cellulase mixture Cmix (simultaneous application of CF and Cll), while none of the other cellulase treatments gave significant amount of NFC (Table 2). Nanofibrillation of this sample may have been promoted by its low fibre length (70% of fines) or by the decrease of hornification produced by these cellulases [19, 42]. A lower yield of NFC was obtained with oxidative S and L_Tempo treatments. In these treatments, nanofibrillation was probably stimulated by the presence of COOH groups, as it has been reported [43, 44]. The highest yield of NFC (23%) was produced with the SCmix pretreatment, in concordance with the best performance observed in the other parameters evaluated, where a higher effect of cellulases after an LPMO treatment was achieved. This yield increase produced by LPMO can be related to the introduction of nicks in the most crystalline regions of cellulose molecules (as suggested by Villares et al. and Valenzuela et al. [15, 17]), rather than to the small increase of COOH that are left on fibres after cellulase treatment. Recently, it has been reported the nanofibrillation of flax pulp after a sequential pretreatment of SamLPMO10C and C9, obtaining a similar yield of 24% [17]. Remarkably, the NFC yield obtained with Cmix and SCmix was higher than the NFC content of a commercial nanocellulose (Table 2).
Despite the fact that in some samples no NFC was obtained, other parameters were measured in order to analyse the fibrillation improvements produced by the enzymes. For example, transmittance is a simple mean to get an approximate idea about the width of the ensuing fibrils. In fact, when light passes through a medium containing randomly dispersed particles, it is scattered by the particles causing a reduction in the transparency degree, as previously reported [24]. Transmittance at 700 nm was strongly improved by hydrolytic treatments in all the samples (Table 2), suggesting a decrease in the amount of non-fibrillated and partially fibrillated fractions responsible for light scattering phenomenon. In accordance with the NFC yield results, the highest improvement in transmittance was produced with Cmix and SCmix samples. Although fibres of lower length were created with Cll treatment, a higher transmittance value was obtained with C50, followed by CF, Cll and C9. Concerning the oxidative treatments, they only improved transmittance to less than 5%. The higher carboxyl content of L_Tempo sample did not produce a significant increase in transmittance, in accordance with the observations of Besbes et al., 2011, who reported that COOH content has to be higher than 300 μmol g−1 odp to produce a significant increase in transmittance [44].
Zeta potential is a measure of the magnitude of the electrostatic or charge repulsion/attraction between particles and is one of the fundamental parameters known to affect stability. All the samples obtained after mechanical fibrillation had a Z potential around − 30 mV, that indicates that there is no agglomeration, which means sufficient mutual repulsion resulting in colloidal stability. This value was slightly increased with the oxidative treatments to − 40 mV probably due to the COOH groups (Additional file 4). However, it was reduced in the SCmix treatments, correlating again the removal of the LPMO-produced COOH groups by the cellulase treatment. A similar result has been reported in NFC from flax and bleached kraft pulp [16, 17].
Although it has been reported that DP can be reduced during fibrillation [24, 25, 45], in our results DP was not affected after the pass through the high-pressure homogenizer (Fig. 3). In fact, only in the S sample the DP decreased. Maybe the oxidation of glycosidic linkages during the treatment with LPMO rendered cellulose more susceptible to be degraded during fibrillation. Finally, it has to be pointed out the low DP of Cmix and SCmix samples, indicating that cellulose chains were only formed by ≈ 300 glucose units. This value was only slightly higher than in cellulose nanocrystals (≈ 200 glucose units) obtained from cotton linters [35].
Effect of enzymes on film properties
Films of ~ 45 μm thickness were prepared, and their optical, physico-mechanical and barrier properties were measured (Table 4). Crystallinity of films was determined by XRD. It was high in all the samples (around 90%), as expected for cotton linters, although they had suffered multiple passes through the homogenizer, a process that has been reported to reduce crystallinity [46]. Values obtained are similar to that reported by Hideno et al. in 2016 and higher to that obtained by Saito et al. in 2006 [19, 47]. Cellulases treatment slightly increased crystallinity of films, probably due to their action on the amorphous zones of cellulose more susceptible to be attacked by these enzymes [48], a phenomenon observed also when commercial cellulases were applied to bleached wood pulps [24, 26]. Crystallinity is also an important parameter that affects the action of LPMO enzymes, where, on the contrary, higher crystalline cellulose seems to be a better substrate to be oxidized [17, 49]. Interestingly, in our experiments, this property was not negatively affected by the S treatment, similarly to what has been reported for NFC from flax pulps [17]. The other oxidative treatment, L_Tempo, did not affect to this property neither, as previously reported [47]. The lower DP produced with the enzymatic treatments did not affect cellulose crystallinity. This property was also measured from the FTIR spectra obtaining the total crystallinity index (TCI) (Additional file 3). It had a value around 1.2, without significant differences between samples, in accordance with values obtained by XRD.
Table 4.
Effects of enzymatic pretreatments in crystallinity and physical properties of the films obtained after mechanical fibrillation
| Crystallinity (%) | Transparency (%) | Density (g cm−3) | Bekk smoothness (s) | ||
|---|---|---|---|---|---|
| Upper face | Lower face | Upper face | |||
| R | 89.1 ± 0.1 | 27.8 ± 0.9 | 28.0 ± 0.1 | 0.8 ± 0.1 | 15 ± 4 |
| Ck | 88.5 ± 0.5 | 35.9 ± 3.7 | 36.0 ± 3.7 | 0.8 ± 0.1 | 10 ± 1 |
| C9 | 90.3 ± 0.2 | 54.3 ± 0.4 | 52.9 ± 0.2 | 1.0 ± 0.1 | 29 ± 5 |
| C50 | 91.4 ± 0.1 | 83.4 ± 0.5 | 83.2 ± 1.1 | 1.1 ± 0.0 | 46 ± 6 |
| CF | 91.0 ± 0.3 | 74.9 ± 0.7 | 73.8 ± 0.5 | 1.1 ± 0.1 | 53 ± 2 |
| Cll | 91.4 ± 0.2 | 75.7 ± 0.9 | 74.7 ± 0.8 | 1.1 ± 0.0 | 34 ± 2 |
| Cmix | 92.4 ± 0.2 | 86.1 ± 0.5 | 85.9 ± 1.5 | 0.9 ± 0.0 | 50 ± 6 |
| Sk | 89.0 ± 0.2 | 52.9 ± 1.8 | 51.5 ± 2.3 | 0.8 ± 0.1 | 11 ± 2 |
| S | 90.5 ± 0.5 | 60.0 ± 0.4 | 60.9 ± 0.3 | 0.9 ± 0.0 | 10 ± 2 |
| SCmix | 92.5 ± 0.1 | 85.6 ± 0.5 | 85.3 ± 0.5 | 1.1 ± 0.1 | 53 ± 1 |
| L_Tempo | 89.8 ± 0.1 | 38.9 ± 3.2 | 37.8 ± 1.8 | 0.9 ± 0.1 | 14 ± 3 |
| Com | 81.1 ± 0.2 | 66.2 ± 0.7 | 66.7 ± 0.4 | 1.0 ± 0.0 | 19 ± 3 |
R (initial refined pulp), Ck (cellulase control treatment), C9 (Cel9B), C50 (Sertec20 cellulase), CF (Fibercare cellulase), Cll (Celluclast cellulase), Cmix (cellulase mixture consisting in Fibercare and Celluclast), Sk (LPMO control treatment), S (LPMO), SCmix (LPMO and Cmix), L_Tempo (Laccase_Tempo treatment) and Com (commercial NFC)
Transparency of films was determined, showing agreement with transmittance of homogenized suspensions previously shown, and no significant differences were found between upper and lower faces of films (Table 4). The highest transparency was achieved with Cmix and SCmix, where the increase in transparency was around 50 points (see Additional file 5). C50, CF, Cll and C9 increased this property in 47, 39, 39 and 18 points, respectively. Transparency obtained with Cmix treatments was similar to that reported by Hideno et al. in 2016 with cotton linters and cellulase, and also to Chen et al. in 2014 in a NFC/acrylic resin composite sheet [19, 21]. S showed a lower increase in transparency of 7 points, being these films of higher transparency than L_Tempo films. Interestingly, crystallinity and transparency of films from cellulase treatments were higher than those of the films made from commercial NFC.
The density of the films obtained (Table 4) was comparable to that of films obtained from bacterial cellulose and considerably higher than papers from wood fibres [50]. Enzymatic treatment with cellulases produced the films of higher density probably due to the lower fibre size. Smoothness of non-treated films (R) was similar to films obtained from commercial NFC (Table 4). Whereas smoothness was not affected by the oxidative treatments, this property was significantly increased with all the cellulases applied, particularly with C50, CF and Cmix treatments. No differences were appreciated if Cmix was applied after S. Increased values of smoothness were obtained in the lower face of films (data not shown). The high values of smoothness and transparency of the obtained films give them the potential to be applied for printed electronics [51].
Mechanical resistance of films was determined (Fig. 4). Non-treated films (R) showed dry zero-span index of 153 Nm g−1, similar to that of commercial NFC films (185 Nm g−1) and higher than that of the paper from unbleached kraft pulp reinforced with cotton linters NFC [52] (8 Nm g−1). As previously said, DP was affected by the action of enzymes, and consequently, this could affect the physical properties of the resulting films. Interestingly, although cellulose was partially degraded with all the treatments, dry zero-span index of C9, S and L_Tempo samples was not significantly affected, while a significant reduction in this property was produced with all the commercial cellulases used (Fig. 4). In order to evaluate the resistance offered by a single fibre, wet zero-span index was also measured. The resistance in all the samples was reduced around 55–84%, including the one from commercial NFC. Whereas non-treated films had values around 70 Nm g−1, this value was reduced to 25 Nm g−1 in all the enzymatic treated samples, without significant differences between them. Films from commercial NFC had a slightly higher wet zero-span index (42 Nm g−1). These wet zero-span values obtained were significantly lower than those reported for bacterial cellulose films (around 100 Nm g−1) [50], probably as a result of the higher crystallinity of bacterial nanocellulose.
Fig. 4.
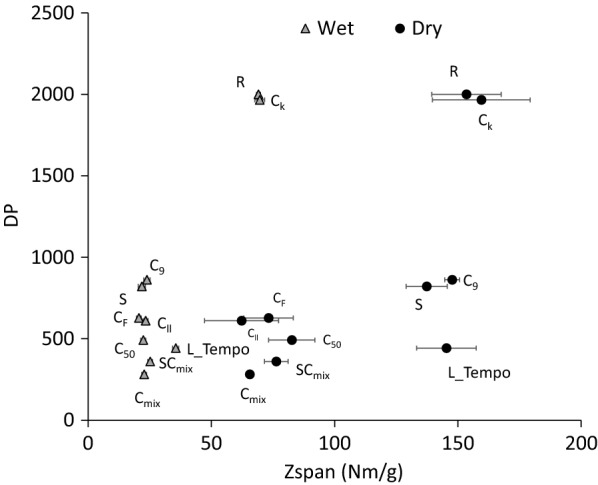
DP of cellulose vs. mechanical resistance of NFC films. R (initial refined pulp), Ck (control treatment), C9 (Cel9B), C50 (Sertec20 cellulase), CF (Fibercare cellulase), Cll (Celluclast cellulase), Cmix (cellulase mixture consisting in Fibercare and Celluclast), S (LPMO), SCmix (LPMO and Cmix) and L_Tempo (Laccase_Tempo treatment). Enzymatic treatments with cellulases were performed at 50 °C, pH 5, for 18 h with 10 U g−1 odp of enzyme. (In the case of Cmix the enzymatic dose was 20 U g−1.) Enzymatic treatment with LPMO (S) was performed at 50 °C, pH 6, for 72 h with 4 mg of enzyme g−1 odp in the presence of ascorbic acid and hydrogen peroxide. Enzymatic treatment with Laccase_Tempo was performed at room temperature, pH 5, for 18 h at 60 U g−1 odp of laccase and 8% odp of Tempo
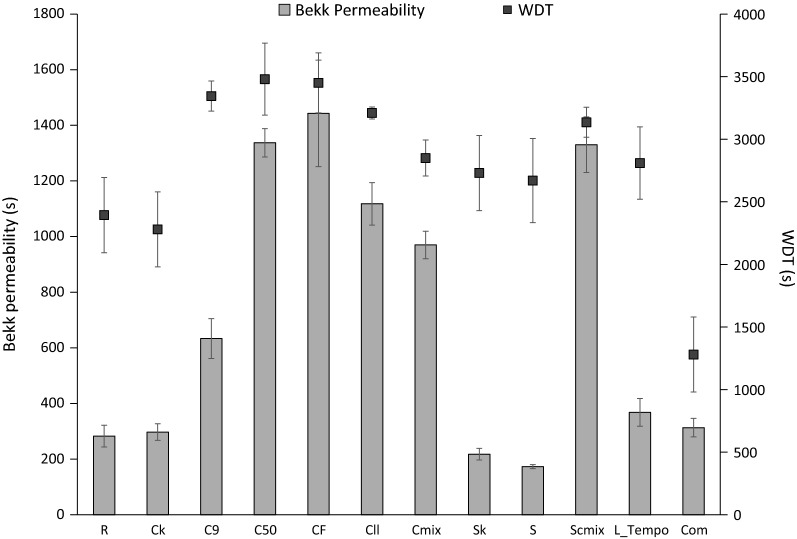
Barrier properties to air and water of the obtained films were also measured. Air permeability was measured by the Bekk method (Fig. 5). The non-treated sample (R) had a similar value than films from commercial NFC. Interestingly, cellulases strongly increased the seconds that the air needed to pass through the films, i.e. decreased permeability. The most notable effect was produced with CF, C50 and SCmix followed by Cll, Cmix and C9. On the other hand, oxidative treatments did not produce significant effects. Permeability of films from cellulase-treated samples was threefold higher than that of commercial NFC films, indicating that a strongly closed structure was formed after the enzymatic treatments. The increased fine content and fibrillation obtained with the cellulase treatments are consistent with an increased cohesion between fibre surfaces and responsible for the decreased paper permeability. These results are consistent with those of Cadena et al. who found cellulase treatments to reduce the air permeance of paper [53]. Similar to smoothness, permeability was strongly decreased in the lower face of the film (data not shown).
Fig. 5.
Barrier properties to air (Bekk permeability) and water (water drop test) of NFC films. R (initial refined pulp), Ck (cellulase control treatment), C9 (Cel9B), C50 (Sertec20 cellulase), CF (Fibercare cellulase), Cll (Celluclast cellulase), Cmix (cellulase mixture consisting in Fibercare and Celluclast), Sk (LPMO control treatment), S (LPMO), SCmix (LPMO and Cmix), L_Tempo (Laccase_Tempo treatment) and Com (commercial NFC). Enzymatic treatments with cellulases were performed at 50 °C, pH 5, for 18 h with 10 U g−1 odp of enzyme. (In the case of Cmix the enzymatic dose was 20 U g−1.) Enzymatic treatment with LPMO (S) was performed at 50 °C, pH 6, for 72 h with 4 mg of enzyme g−1 odp in the presence of ascorbic acid and hydrogen peroxide. Enzymatic treatment with Laccase_Tempo was performed at room temperature, pH 5, for 18 h at 60 U g−1 odp of laccase and 8% odp of Tempo
Film permeability was intensely related to the barrier property to water, measured by the WDT (Fig. 5). All the films from enzyme-treated samples showed an increased water impermeability although the effect was more noticeable with cellulase treatments, which showed a maximum value of 3150 s in CF sample. In spite of the high impermeability compared to current cellulosic papers, it was lower than that provided by bacterial cellulose films (4000 s) [50]. Interestingly, non-treated films had lower permeability to water than commercial NFC films, maybe because of the higher crystallinity of the cotton linters used.
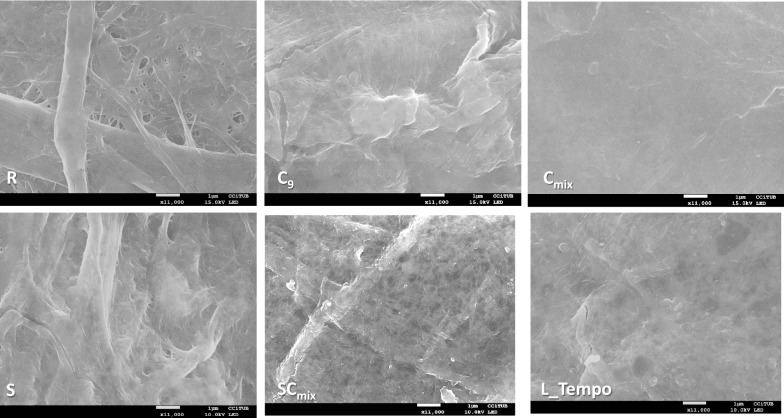
Finally, film surface morphology was analysed by FESEM (Fig. 6). Non-treated films (R) showed fibres of different lengths and fibre widths with fibrillation. A highly entangled nano- and/or micro-fibre network was observed in enzyme-treated samples, similarly to that reported by Hu et al. in 2018 and Tarrés et al. in 2017 [16, 39]. Surface morphology of films demonstrates that the enzymatic treatments performed boosted mechanical delamination, since those films showed a compact structure and their structure was difficult to be visualized. Moreover, in SCmix films a thin layer of nanofibres surrounding bigger fibres was appreciated.
Fig. 6.
FESEM images of NFC films from non-treated and enzymatically treated samples. R (initial refined pulp), C9 (Cel9B), Cmix (cellulase mixture consisting in Fibercare and Celluclast), S (LPMO), SCmix (LPMO and Cmix) and L_Tempo (Laccase_Tempo treatment). Enzymatic treatments with cellulases were performed at 50 °C, pH 5, for 18 h with 10 U g−1 odp of enzyme. (In the case of Cmix the enzymatic dose was 20 U g−1.) Enzymatic treatment with LPMO (S) was performed at 50 °C, pH 6, for 72 h with 4 mg of enzyme g−1 odp in the presence of ascorbic acid and hydrogen peroxide. Enzymatic treatment with Laccase_Tempo was performed at room temperature, pH 5, for 18 h at 60 U g−1 odp of laccase and 8% odp of Tempo
Although the presence of NFC material was not detected in films from individual cellulases and oxidative enzymes, the film properties obtained clearly show that these treatments improved fibrillation. Moreover, according to the optical, physical and barrier properties obtained, the films from enzymatically treated cotton linters seem very promising to obtain biomaterials that could replace petrol-based products.
Conclusions
Four hydrolytic enzymes (cellulases) were applied on cotton linters, affecting fibre morphology and degrading cellulose differently. Improved mechanical fibrillation and 18% NFC yield were obtained with a cellulase mixture (Cmix). Application of oxidative enzymes (LPMO and L_Tempo) introduced COOH groups into cellulose. The amount of COOH groups created with L_Tempo allowed the production of NFC during mechanical fibrillation (10%). However, the smaller quantity of these groups introduced by LPMO was not enough to produce NFC. The main difference between the two oxidative treatments was that L_Tempo degraded cellulose, whereas LPMO had more effect on fibre degradation. LPMO (S) boosted the action of cellulases although the COOH groups created were released to effluents after the hydrolytic treatment. Films with high crystallinity (92%) and transparency (86%), increased smoothness, and high air and water barrier properties were obtained after cellulase treatment and mechanical fibrillation on cotton linters. The introduction of an LPMO treatment before cellulase mixture (SCmix treatment) produced higher NFC yield (23%) without a further improvement in film properties.
Additional files
Additional file 1. TLC analysis of sugars released during enzymatic treatments performed at 50 °C, pH 5, during 18 h with 10 U g−1 odp of enzyme (in the case of Cmix the enzymatic dose was 20 U g−1 odp). C9 (Cel9B), C50 (Sertec20 cellulase), CF (Fibercare cellulase), Cll (Celluclast cellulase), Cmix (cellulase mixture consisting in Fibercare and Celluclast). M) size markers of glucose (G), cellobiose (G2), cellotriose (G3), cellotetraose (G4) and cellopentaose (G5).
Additional file 2. Effect of the hydrolytic (a) and oxidative and Cmix (b) enzymatic pretreatments on fibre length distribution. The confidence interval of the length fibre distribution was less than 2% in all cases.
Additional file 3. FTIR spectra of obtained films from control treatment (Ck) and Laccase_Tempo treatment (L_Tempo). TCI was calculated from the ratio of the absorptions at 1372 and 2900 cm−1. The peak detected at 1750 cm−1 with L_Tempo correspond to the COOH groups.
Additional file 4. Z potential values of the samples obtained after mechanical fibrillation. R (initial refined pulp), Ck (control treatment), C9 (Cel9B), Cmix (cellulase mixture consisting in Fibercare and Celluclast), S (LPMO), SCmix (LPMO and Cmix) and L_Tempo (Laccase_Tempo treatment).
Additional file 5. Obtained films from cellulase control Ck (a) and Cmix (b) pretreatments after mechanical fibrillation.
Acknowledgements
We thank Serveis Científics Tècnics of University of Barcelona for HPAEC, XRD and FESEM analysis, and Novozymes® and Sertec20 for supplying cellulases. Cotton linters were kindly supplied by CELESA.
Abbreviations
- NFC
nanofibrillated cellulose
- R
refined cotton linter
- Ck
cellulase control treatment
- LPMO
lytic polysaccharide monooxygenases
- S
treatment with LPMO enzyme
- Sk
LPMO control treatment
- C9
treatment with endoglucanase Cel9B
- Cll
treatment with commercial cellulase Celluclast
- CF
treatment with commercial cellulase Fibercare
- C50
treatment with commercial cellulase from Sertec20
- Cmix
combined treatment with Celluclast and Fibercare
- SCmix
treatment with LPMO enzyme followed by Cmix
- L_Tempo
treatment with laccase and Tempo
- Com
commercial NFC
- DNS
dinitrosalicylic
- TLC
thin-layer chromatography
- HPAEC-PAD
high-performance anion-exchange chromatography with pulsed amperometric detection
- DP
degree of polymerization
- WDT
water drop test
- FESEM
field emission scanning electron microscopy
- Odp
oven-dried pulp
- XRD
X-ray powder diffraction
Authors’ contributions
CV conducted most of the experiments and wrote the manuscript; JP supervised the experiments and revised the manuscript; BR refined the cotton linters used and performed the analysis of fibre morphology; TV and PD took part in planning the study, checking the results and revising the work; JM performed FESEM experiments; SV obtained the LPMO and Cel9B enzymes, performed HPAEC and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the Spanish MINECO FILMBIOCEL CTQ2016-77936-R (funding also from the “Fondo Europeo de Desarrollo Regional FEDER”), MICROBIOCEL CTQ2017-84966-C2-1-R and BIOFABCEL CTQ2017-84966-C2-2-R projects. Special thanks are due to the consolidated research group AGAUR 2017 SGR 30 and to the Serra Húnter Fellow to Cristina Valls.
Availability of data and materials
The data sets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cristina Valls, Phone: +34 937398176, Email: cristina.valls@upc.edu.
F. I. Javier Pastor, Email: fpastor@ub.edu.
M. Blanca Roncero, Email: blanca.roncero@upc.edu.
Teresa Vidal, Email: teresa.vidal@upc.edu.
Pilar Diaz, Email: pdiaz@ub.edu.
Josefina Martínez, Email: jmartinez@ub.edu.
Susana V. Valenzuela, Phone: +34 937398176, Email: susanavalenzuela@ub.edu
References
- 1.Morais JPS, Rosa MDF, de Souza Filho MDSM, Nascimento LD, do Nascimento DM, Cassales AR. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr Polym. 2013;91:229–235. doi: 10.1016/j.carbpol.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Beltramino F, Roncero MB, Vidal T, Valls C. Facilitating the selection of raw materials: evaluation of the effects of TCF and ECF bleaching sequences on different wood and non-wood pulps. Afinidad. 2018;75:91–96. [Google Scholar]
- 3.Sczostak A. Cotton linters: an alternative cellulosic raw material. Macromol Symp. 2009;280:45–53. doi: 10.1002/masy.200950606. [DOI] [Google Scholar]
- 4.Tarrés Q, Oliver-Ortega H, Ferreira PJ, Àngels Pèlach M, Mutjé P, Delgado-Aguilar M. Towards a new generation of functional fiber-based packaging: cellulose nanofibers for improved barrier, mechanical and surface properties. Cellulose. 2018;25:683–695. doi: 10.1007/s10570-017-1572-7. [DOI] [Google Scholar]
- 5.Quintana E, Ago M, Valls C, Roncero MB, Rojas OJ. Alternative chemo-enzymatic treatment for homogeneous and heterogeneous acetylation of wood fibers. Cellulose. 2018;25:5323–5336. doi: 10.1007/s10570-018-1947-4. [DOI] [Google Scholar]
- 6.Quintana E, Valls C, Vidal T, Roncero MB. Comparative evaluation of the action of two different endoglucanases. Part I: on a fully bleached, commercial acid sulfite dissolving pulp. Cellulose. 2015;22:2067–2079. doi: 10.1007/s10570-015-0623-1. [DOI] [Google Scholar]
- 7.Quintana E, Roncero MB, Vidal T, Valls C. Cellulose oxidation by Laccase_TEMPO treatments. Carbohydr Polym. 2017;157:1488–1495. doi: 10.1016/j.carbpol.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Aracri E, Valls C, Vidal T. Paper strength improvement by oxidative modification of sisal cellulose fibers with laccase_TEMPO system: influence of the process variables. Carbohydr Polym. 2012;88:830–837. doi: 10.1016/j.carbpol.2012.01.011. [DOI] [Google Scholar]
- 9.Quinlan RJ, Sweeney MD, Lo Leggio L, Otten H, Poulsen J-CN, Johansen KS, et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc Natl Acad Sci. 2011;108:15079–15084. doi: 10.1073/pnas.1105776108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaaje-Kolstad G, Westereng B, Horn SJ, Liu Z, Zhai H, Sørlie M, Eijsink VGH. An oxidative enzyme boosting the the enzymatic conversion of recalcitrant polysaccharides. Science. 2010;330:219–222. doi: 10.1126/science.1192231. [DOI] [PubMed] [Google Scholar]
- 11.Vaaje-Kolstad G, Forsberg Z, Loose JS, Bissaro B, Eijsink VGH. Structural diversity of lytic polysaccharide monooxygenases. Curr Opin Struct Biol. 2017;44:67–76. doi: 10.1016/j.sbi.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Horn SJ, Vaaje-kolstad G, Westereng B, Eijsink VGH. Novel enzymes for the degradation of cellulose novel enzymes for the degradation of cellulose. Biotechnol Biofuels. 2012;5:45. doi: 10.1186/1754-6834-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eibinger M, Ganner T, Bubner P, Rošker S, Kracher D, Haltrich D, et al. Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency. J Biol Chem. 2014;289:35929–35938. doi: 10.1074/jbc.M114.602227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, Pribowo A, Saddler JN. Oxidative cleavage of some cellulosic substrates by auxiliary activity (AA) family 9 enzymes influences the adsorption/desorption of hydrolytic cellulase enzymes. Green Chem. 2016;18:6329–6336. doi: 10.1039/C6GC02288J. [DOI] [Google Scholar]
- 15.Villares A, Moreau C, Bennati-Granier C, Garajova S, Foucat L, Falourd X, et al. Lytic polysaccharide monooxygenases disrupt the cellulose fibers structure. Sci Rep. 2017;7:1–9. doi: 10.1038/srep40262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Tian D, Renneckar S, Saddler JN. Enzyme mediated nanofibrillation of cellulose by the synergistic actions of an endoglucanase, lytic polysaccharide monooxygenase (LPMO) and xylanase. Sci Rep. 2018;8:4–11. doi: 10.1038/s41598-018-21016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valenzuela SV, Valls C, Schink V, Sánchez D, Roncero MB, Diaz P, et al. Differential activity of lytic polysaccharide monooxygenases on celluloses of different crystallinity. Effectiveness in the sustainable production of cellulose nanofibrils. Carbohydr Polym. 2019;207:59–67. doi: 10.1016/j.carbpol.2018.11.076. [DOI] [PubMed] [Google Scholar]
- 18.Beltramino F, Roncero MB, Torres AL, Vidal T, Valls C. Optimization of sulfuric acid hydrolysis conditions for preparation of nanocrystalline cellulose from enzymatically pretreated fibers. Cellulose. 2016;23:1777–1789. doi: 10.1007/s10570-016-0897-y. [DOI] [Google Scholar]
- 19.Hideno A, Abe K, Uchimura H, Yano H. Preparation by combined enzymatic and mechanical treatment and characterization of nanofibrillated cotton fibers. Cellulose. 2016;23:3639–3651. doi: 10.1007/s10570-016-1075-y. [DOI] [Google Scholar]
- 20.Saito T, Nishiyama Y, Putaux JL, Vignon M, Isogai A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules. 2006;7:3–7. doi: 10.1021/bm060154s. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Abe K, Uetani K, Yu H, Liu Y, Yano H. Individual cotton cellulose nanofibers: pretreatment and fibrillation technique. Cellulose. 2014;21:1517–1528. doi: 10.1007/s10570-014-0172-z. [DOI] [Google Scholar]
- 22.Lavoine N, Desloges I, Dufresne A, Bras J. Microfibrillated cellulose—its barrier properties and applications in cellulosic materials: a review. Carbohydr Polym. 2012;90:735–764. doi: 10.1016/j.carbpol.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Pääkkö M, Ankerfors M, Kosonen H, Nykänen A, Ahola S, Österberg M, et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules. 2007;8:1934–1941. doi: 10.1021/bm061215p. [DOI] [PubMed] [Google Scholar]
- 24.Qing Y, Sabo R, Zhu JY, Agarwal U, Cai Z, Wu Y. A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr Polym. 2013;97:226–234. doi: 10.1016/j.carbpol.2013.04.086. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Mozuch MD, Sabo RC, Kersten P, Zhu JY, Jin Y. Production of cellulose nanofibrils from bleached eucalyptus fibers by hyperthermostable endoglucanase treatment and subsequent microfluidization. Cellulose. 2015;22:351–361. doi: 10.1007/s10570-014-0465-2. [DOI] [Google Scholar]
- 26.Long L, Tian D, Hu J, Wang F, Saddler J. A xylanase-aided enzymatic pretreatment facilitates cellulose nanofibrillation. Bioresour Technol. 2017;243:898–904. doi: 10.1016/j.biortech.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 27.Chiriac AI, Cadena EM, Vidal T, Torres AL, Díaz P, Pastor FIJ. Engineering a family 9 processive endoglucanase from Paenibacillus barcinonensis displaying a novel architecture. Appl Microbiol Biotechnol. 2010;86:1125–1134. doi: 10.1007/s00253-009-2350-8. [DOI] [PubMed] [Google Scholar]
- 28.Miller GL. Use of dinitrosalicyclic reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 29.Valenzuela SV, Ferreres G, Margalef G, Pastor FIJ. Fast purification method of functional LPMOs from Streptomyces ambofaciens by affinity adsorption. Carbohydr Res. 2017;448:205–211. doi: 10.1016/j.carres.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Valls C, Vidal T, Roncero MB. Enzymatic strategies to improve removal of hexenuronic acids and lignin from cellulosic fibers. Holzforschung. 2014;68:229–237. doi: 10.1515/hf-2012-0114. [DOI] [Google Scholar]
- 31.Valls C, Pastor FIJ, Vidal T, Roncero MB, Díaz P, Martínez J, et al. Antioxidant activity of xylooligosaccharides produced from glucuronoxylan by Xyn10A and Xyn30D xylanases and eucalyptus autohydrolysates. Carbohydr Polym. 2018;194:43–50. doi: 10.1016/j.carbpol.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Davidson GF. 6—the acidic properties of cotton cellulose and derived oxycelluloses. Part II. The absorption of methylene blue. J Text Inst Trans. 1948;39:T65–T86. doi: 10.1080/19447024808659403. [DOI] [Google Scholar]
- 33.Nanocellulose DA. A new ageless bionanomaterial. Mater Today. 2013;16:220–227. doi: 10.1016/j.mattod.2013.06.004. [DOI] [Google Scholar]
- 34.Segal L, Creely JJ, Martin AE, Conrad CM. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J. 1959;29:786–794. doi: 10.1177/004051755902901003. [DOI] [Google Scholar]
- 35.Beltramino F, Roncero MB, Vidal T, Valls C. A novel enzymatic approach to nanocrystalline cellulose preparation. Carbohydr Polym. 2018;189:39–47. doi: 10.1016/j.carbpol.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Ubasart J, Torres AL, Vila C, Pastor FIJ, Vidal T. Biomodification of cellulose flax fibers by a new cellulase. Ind Crops Prod. 2013;44:71–76. doi: 10.1016/j.indcrop.2012.10.019. [DOI] [Google Scholar]
- 37.Moilanen U, Kellock M, Várnai A, Andberg M, Viikari L. Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol Biofuels. 2014;7:1–13. doi: 10.1186/s13068-014-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintana E, Valls C, Vidal T, Roncero MB. Comparative evaluation of the action of two different endoglucanases. Part II: on a biobleached acid sulphite pulp. Cellulose. 2015;22:2081–2093. doi: 10.1007/s10570-015-0631-1. [DOI] [Google Scholar]
- 39.Tarrés Q, Boufi S, Mutjé P, Delgado-Aguilar M. Enzymatically hydrolyzed and TEMPO-oxidized cellulose nanofibers for the production of nanopapers: morphological, optical, thermal and mechanical properties. Cellulose. 2017;24:3943–3954. doi: 10.1007/s10570-017-1394-7. [DOI] [Google Scholar]
- 40.Shibata I, Isogai A. Depolymerization of cellouronic acid during TEMPO-mediated oxidation. Cellulose. 2003;10:151–158. doi: 10.1023/A:1024051514026. [DOI] [Google Scholar]
- 41.Aracri E, Vidal T, Ragauskas AJ. Wet strength development in sisal cellulose fibers by effect of a laccase_TEMPO treatment. Carbohydr Polym. 2011;84:1384–1390. doi: 10.1016/j.carbpol.2011.01.046. [DOI] [Google Scholar]
- 42.García O, Torres AL, Colom JF, Pastor FIJ, Díaz P, Vidal T. Effect of cellulase-assisted refining on the properties of dried and never-dried eucalyptus pulp. Cellulose. 2002;9:115–125. doi: 10.1023/A:1020191622764. [DOI] [Google Scholar]
- 43.Fillat Ú, Wicklein B, Martín-Sampedro R, Ibarra D, Ruiz-Hitzky E, Valencia C, et al. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr Polym. 2018;179:252–261. doi: 10.1016/j.carbpol.2017.09.072. [DOI] [PubMed] [Google Scholar]
- 44.Besbes I, Alila S, Boufi S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: effect of the carboxyl content. Carbohydr Polym. 2011;84:975–983. doi: 10.1016/j.carbpol.2010.12.052. [DOI] [Google Scholar]
- 45.Zimmermann T, Bordeanu N, Strub E. Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr Polym. 2010;79:1086–1093. doi: 10.1016/j.carbpol.2009.10.045. [DOI] [Google Scholar]
- 46.Besbes I, Vilar MR, Boufi S. Nanofibrillated cellulose from Alfa, Eucalyptus and Pine fibres: preparation, characteristics and reinforcing potential. Carbohydr Polym. 2011;86:1198–1206. doi: 10.1016/j.carbpol.2011.06.015. [DOI] [Google Scholar]
- 47.Saito T, Okita Y, Nge TT, Sugiyama J, Isogai A. TEMPO-mediated oxidation of native cellulose: microscopic analysis of fibrous fractions in the oxidized products. Carbohydr Polym. 2006;65:435–440. doi: 10.1016/j.carbpol.2006.01.034. [DOI] [Google Scholar]
- 48.Igarashi K, Uchihashi T, Koivula A, Wada M, Kimura S, Okamoto T, et al. Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Science. 2011;333:1279–1282. doi: 10.1126/science.1208386. [DOI] [PubMed] [Google Scholar]
- 49.Johansen KS. Lytic polysaccharide monooxygenases: the microbial power tool for lignocellulose degradation. Trends Plant Sci. 2016;21:926–936. doi: 10.1016/j.tplants.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Fillat A, Martínez J, Valls C, Cusola O. Bacterial cellulose for increasing barrier properties of paper products. Cellulose. 2018;25:6093–6105. doi: 10.1007/s10570-018-1967-0. [DOI] [Google Scholar]
- 51.Fang Z, Zhu H, Preston C, Han X, Li Y, Lee S, et al. Highly transparent and writable wood all-cellulose hybrid nanostructured paper. J Mater Chem C. 2013;1:6191–6197. doi: 10.1039/c3tc31331j. [DOI] [Google Scholar]
- 52.Bharimalla AK, Patil PG, Deshmukh SP, Vigneshwaran N. Energy efficient production of nano-fibrillated cellulose (NFC) From cotton linters by tri-disc refining and its characterization. Cellul Chem Technol. 2017;51:5–6. [Google Scholar]
- 53.Cadena EM, Chiriac AI, Pastor FIJ, Díaz P, Vidal T, Torres AL. Use of cellulases and recombinant cellulose binding domains for refining TCF kraft pulp. Biotechnol Prog. 2010;26:960–967. doi: 10.1002/btpr.411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. TLC analysis of sugars released during enzymatic treatments performed at 50 °C, pH 5, during 18 h with 10 U g−1 odp of enzyme (in the case of Cmix the enzymatic dose was 20 U g−1 odp). C9 (Cel9B), C50 (Sertec20 cellulase), CF (Fibercare cellulase), Cll (Celluclast cellulase), Cmix (cellulase mixture consisting in Fibercare and Celluclast). M) size markers of glucose (G), cellobiose (G2), cellotriose (G3), cellotetraose (G4) and cellopentaose (G5).
Additional file 2. Effect of the hydrolytic (a) and oxidative and Cmix (b) enzymatic pretreatments on fibre length distribution. The confidence interval of the length fibre distribution was less than 2% in all cases.
Additional file 3. FTIR spectra of obtained films from control treatment (Ck) and Laccase_Tempo treatment (L_Tempo). TCI was calculated from the ratio of the absorptions at 1372 and 2900 cm−1. The peak detected at 1750 cm−1 with L_Tempo correspond to the COOH groups.
Additional file 4. Z potential values of the samples obtained after mechanical fibrillation. R (initial refined pulp), Ck (control treatment), C9 (Cel9B), Cmix (cellulase mixture consisting in Fibercare and Celluclast), S (LPMO), SCmix (LPMO and Cmix) and L_Tempo (Laccase_Tempo treatment).
Additional file 5. Obtained films from cellulase control Ck (a) and Cmix (b) pretreatments after mechanical fibrillation.
Data Availability Statement
The data sets used and analysed during the current study are available from the corresponding author on reasonable request.