Abstract
Objectives
This study was conducted to establish a method for early, quick and cheap screening of iron excess tolerance in rice (Oryza sativa L.) cultivars.
Results
Based on the experiments, iron excess leads to reduction in shoot length (SL) and this can be a useful characteristic for adequate screening of tolerant genotypes. The sensitive genotypes Nipponbare and BR-IRGA 409 indicated higher accumulation of iron in their tissues while BRS-Agrisul and Epagri 108 also accumulated iron, but at lower concentrations. BR-IRGA 410 displayed an intermediate phenotype regarding iron accumulation. No changes in shoot Cu content can be observed when comparing treatments. On the other hand, an increase was seen for Zn and Mn when shoots are subjected to Fe2+ excess. Fe stress at a lower concentration than 7 mM increased Zn but decreased Mn contents in shoots of BR-IRGA 409. Strong positive correlations were found here for Fe × Zn (0.93); Fe × Mn (0.97) and Zn × Mn (0.92), probably due to the Fe-induced activation of bivalent cation transporters. Results show that genotypes scored as sensitive present higher concentration of Fe in shoots and this is an efficient method to characterize rice cultivars regarding iron response.
Electronic supplementary material
The online version of this article (10.1186/s13104-019-4362-5) contains supplementary material, which is available to authorized users.
Keywords: Lowland rice, Iron toxicity, Hydroponic culture, Efficient method
Introduction
Rice (Oryza sativa L.) is an important cereal used to feed more than two-thirds of the worlds population, being the source of more than 20% of the calories consumed by humankind [1]. In this scenario, Brazil, where rice cultivation represents an important economic activity, is the largest rice producer in the Western hemisphere [2].
One of the major abiotic stresses that affect irrigated rice production and expansion is iron toxicity. Iron (Fe) is an essential nutrient for plant metabolic processes such as respiration and photosynthesis. However, when in excess, it becomes a highly toxic element [3–5]. Even though most world’s rice production comes from flood-irrigated farms, flooded soils constitute a hypoxic condition which favors the reduction of iron, increasing the concentration of Fe2+ in solution [5, 6]. Iron excess can cause rusty leaf spots, stained leaf edges, reduction of plant growth, tillering and spikelet fertility. Also, reductions in root system development are observed, which can present dark brown color and stunted growth, with few thick roots. In severe cases, these symptoms associate with yield losses up to 100% [7, 8].
Rice genotypes greatly vary in their response to iron toxicity and the use of tolerant cultivars is one of the effective strategies for preventing yield loss, especially for farmers with low income [8, 9].
Considering such background, in this report we aim to evaluate the efficiency of an early, quick and easy method for detection of iron excess tolerance using different rice cultivars.
Main text
Four Brazilian lowland rice (Oryza sativa L.) genotypes were used in this study. These varieties are recommended by the Southern Brazilian Society of Irrigated Rice and are known to be tolerant to iron toxicity by field experiment results [10]: BRS-Agrisul (tolerant), Epagri 108 (tolerant), BR-IRGA 410 (sensitive) and BR-IRGA 409 (sensitive). Nipponbare, the Japanese variety used for the first rice genome sequencing project, is reported as sensitive to iron toxicity. Here Nipponbare was used due to its available molecular data and as a reference for comparisons between different studies [11, 12].
Seeds were disinfected with 20% sodium hypochlorite for 10 min, rinsed for three times in ultrapure water and placed in germination paper for 72 h (25 °C; 16 h of photoperiod; relative humidity of 100%).
Iron stress was performed through the modification of early reports [6]. Seedlings presenting uniform root length were placed in nylon nets fixed on top of plastic pots (2 L), containing modified nutrient [13]: 40 mg L−1 of (NH4)2.SO4; 10 mg L−1 of KH2PO4; 40 mg L−1 of KNO3; 40 mg L−1 CaNO3; 40 mg L−1 of MgSO4·7H2O; 0.5 mg L−1 of MnSO4·H2O; 0.05 mg L−1 of Na2MoO4·2H2O; 0.58 mg L−1 of NaCl; 0.2 mg L−1 of H3BO3; 0.01 mg L−1 of ZnSO4·7H2O, 0.01 mg L−1 of CuSO4·5H2O and 2 mg L−1 of FeSO4·7H2O. Seedlings were kept at 25 °C, 16 h of photoperiod for 28 days, with changing the solution every 7 days.
After this period, the seedlings were subjected to different treatments: Control (T1) with standard nutrient solution (2 mg L−1 of FeSO4·7H2O with pH 4.0 ± 0.1); iron excess (T2) with modified nutrient solution (2000 mg L−1 of FeSO4.7H2O with pH 4.0 ± 0.1). Seedlings were kept under these conditions for 3 days. The visual evaluations were performed following the standard evaluation system for rice.
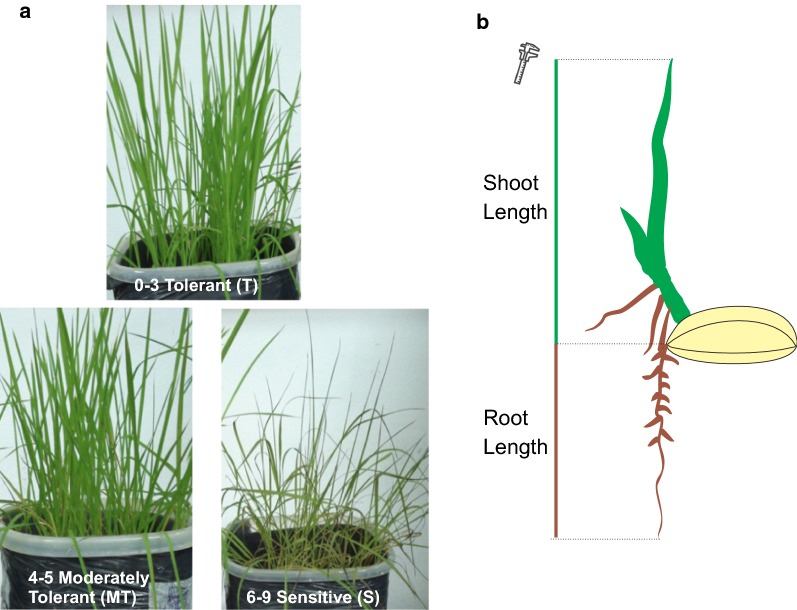
The visual symptoms were based in leaf death and symptom intensity, compared to control (Fig. 1a). The grades ranged from 0 to 9. Tolerant (T) plants received grades 0–3, moderately tolerant (MT) 4–5 and the sensitive (S) 6–9 [14]. After the treatment, root (RL) and shoot (SL) lengths were measured (Fig. 1b). Copper (Cu), zinc (Zn), manganese (Mn) and iron (Fe) contents accumulated in shoots were also evaluated [15].
Fig. 1.
Information about the evaluation procedures on a visual symptoms for scoring and b shoot and root length measurements
A completely randomized design in a double factorial 2 × 5 (treatment × genotype) scheme with three replications, where the observation unit consisted on 20 plants per genotype. The data was subjected to analysis of variance (ANOVA) and then to Tukey HSD test and a Pearson’s correlation, both with p ≤ 0.05. Path analysis was performed as described [16, 17]. Data from path analysis are not completely shown here, but most important results are described.
Visual symptoms observed on plants subjected to iron excess toxicity were yellowing, brown spots along the leaves and leaf tip necrosis. The scores based on genotype performance under excess iron are shown on Table 1.
Table 1.
Leaf bronzing score (LBS) of five genotypes/varieties at 3 days in hydroponic culture under iron toxicity condition
| Genotype | LBS1 | LBS2 | LBS3 | Classification |
|---|---|---|---|---|
| Epagri 108 | 2 | 1 | 2 | T |
| BRS-Agrisul | 2 | 4 | 3 | T |
| BR-IRGA 410 | 5 | 4 | 6 | MT |
| Nipponbare | 7 | 8 | 8 | S |
| BR-IRGA 409 | 6 | 7 | 7 | S |
The LBS numbers follow a scale from 0 to 9, adapted from standard evaluation system for Rice used by IRRI
Classification regarding iron tolerance levels, T (tolerant 0–3), MT (moderately tolerant 4–5) and S (sensitive 6–9)
Epagri 108 (tolerant in field conditions) barely presented Fe2+ toxicity symptoms while the genotype BR-IRGA 409 (sensitive in field conditions) presented easily identifiable Fe2+ toxicity symptoms under iron excess conditions (T2). These results agree with field assays [10]. Nipponbare presented higher scores, being ranked as sensitive to iron stress, classification in agreement with previous reports obtained in hydroponic systems [12]. The use of bronzing scores, measured in the field or in hydroponic systems, has shown to be efficient on the discrimination of tolerant genotypes, being associated to grain yield [8, 9, 18]. However, since in early developmental stages changes in SL, RL and nutrient accumulation in tissues have been reported to constitute an objective form of evaluation that can be used in conjunction with bronzing scores, these were also evaluated during this study [19–21].
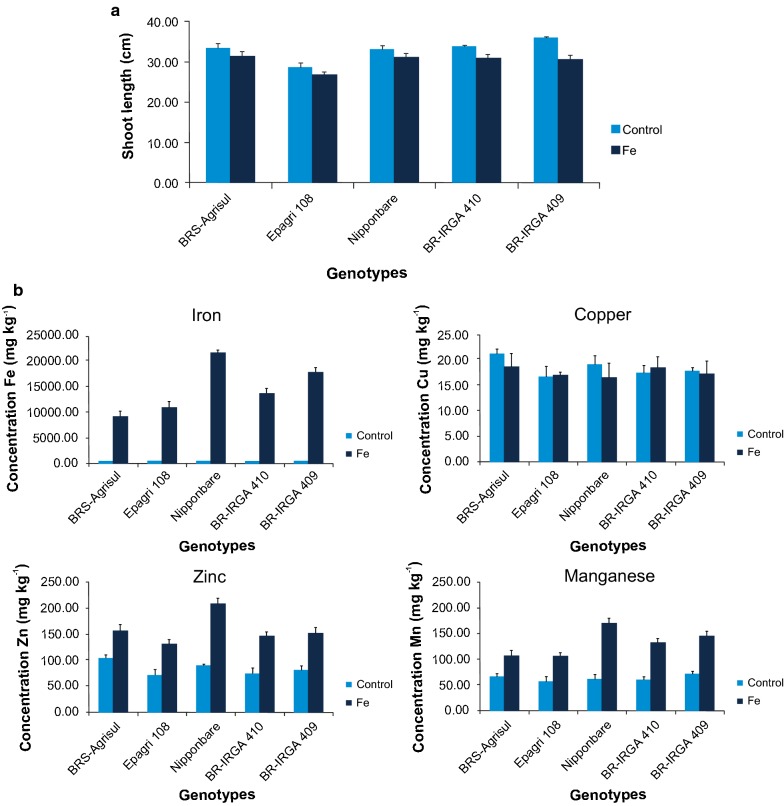
It is shown here that iron excess can lead to reduction in SL (Fig. 2a) and, as other previous studies suggest, this can be a useful characteristic for helping the screening of tolerant genotypes [19, 21].
Fig. 2.
a Shoot length (SL) of each genotype, of the five lowland rice genotypes subjected to standard conditions (control) and to iron excess; b micronutrient content in shoots of five lowland rice genotypes subjected to standard conditions (control) and to iron excess. A graph showing the relative performance of these cultivars and Tukey’s pairwise comparisons is available in the Additional file 1
The sensitive genotypes Nipponbare and BR-IRGA 409 indicated higher accumulation of iron in their tissues (Fig. 2b), while BRS-Agrisul and Epagri 108 (both previously characterized as tolerant) also accumulated iron, but at lower concentrations (i.e., ca. 50% less). It is shown that BR-IRGA 410 display an intermediate phenotype regarding iron accumulation.
It is also shown that BRS-Agrisul (a medium cycle genotype; 121–130 days) accumulated lower amounts of iron than other medium cycle genotypes such as BR-IRGA 410 and BR-IRGA 409, showing that the time from germination to grain production is not the cause of differences in the amount of iron accumulated in tissues.
No changes in shoot Cu content can be observed when comparing treatments (Fig. 2b). On the other hand, an increase was seen for Zn and Mn (Fig. 2b) when shoots are subjected to Fe2+ excess (T2). Fe stress at a lower concentration than 7 mM increased Zn but decreased Mn contents in shoots of BR-IRGA 409 [22].
Strong positive correlations were found here for Fe × Zn (0.93); Fe × Mn (0.97) and Zn × Mn (0.92) (Table 2), probably due to the Fe-induced activation of bivalent cation transporters [23].
Table 2.
Pearson’s correlation coefficient within and between traits (SL and RL) and micronutrients (iron, copper, zinc and manganese) for five genotypes/varieties under control and iron toxicity conditions in hydroponic culture
| Variables | Fe | Cu | Zn | Mn | SL | RL |
|---|---|---|---|---|---|---|
| Fe | 1 | − 0.19 | 0.93* | 0.97* | − 0.37* | − 0.42* |
| Cu | 1 | − 0.02 | − 0.19 | 0.24 | 0.26 | |
| Zn | 1 | 0.92* | − 0.27 | − 0.38* | ||
| Mn | 1 | − 0.30 | − 0.49* | |||
| SL | 1 | 0.08 | ||||
| RL | 1 |
* Significant at p ≤ 0.05
Iron content is negatively correlated with SL (− 0.37) and RL (− 0.42), highlighting the impact that excessive accumulation of this metal has on rice growth and development (Table 2). Although similar results are observed for correlations between Mn and Zn with SL and RL, these are only significant for RL (Table 2) and, according to the path analysis (data not shown), it seems to be an indirect effect of iron accumulation.
This quick and easy modification of the protocol described by [6] proved to be an efficient method to select tolerant Brazilian lowland rice genotypes for iron excess tolerance. Plants showing higher Fe2+ accumulation in shoots (BR-IRGA 409 and Nipponbare) were the same identified as sensitive to Fe2+ by the bronzing score. Besides, the genotype BR-IRGA 409, characterized as sensitive, is the one showing the highest reduction of SL due to iron toxicity. BR-IRGA 410, an intermediate phenotype for shoot Fe2+ accumulation, is characterized as moderately tolerant in symptom score evaluation. BRS-Agrisul and Epagri 108 which are the genotypes displaying the lowest Fe2+ accumulation levels in shoots, are characterized as tolerant in visual symptom evaluation.
In Brazil the search for iron tolerant genotypes has been performed for many years. The methods used involve field tests during different years/growing seasons [24]. Even today, this is the most acceptable method, being necessary to have credibility when registering a cultivar. However, for a quick and inexpensive initial selection in breeding programs, efficient protocols to predict genotype performance have not yet been achieved [25]. Different methods have been tested, these include pot- or tank-based screening procedures that have been presented over time until very recently [26–28].
It is common for the methods tested to find an adequate correlation with the field experiments using soils of the site of interest [29]. The method presented here is useful for an initial selection of genotypes, without considering the soil, since the removal of this from its place of origin does not guarantee perfect reproducibility of the results, either by the modification of the structure or by the lack of local climatic elements. Thus, a prior soil-independent evaluation can be useful to reduce the number of genotypes to be tested and the cost, taking only the most promising ones to field.
Limitations
The limitations of this work are the use of just five genotypes. Although they are contrasting for iron response and should be sufficient to explain the responses, it could be considered a limitation.
Additional files
Additional file 1: Figure S1. Relative performance obtained by the division of the values of plants under stress by the values of the control treatment. Columns followed by the same letters do not differ significantly (Tukey’s pairwise comparisons, p < 0.05).
Acknowledgements
Not applicable.
Abbreviations
- ANOVA
analysis of variance
- MT
moderately tolerant
- RL
root length
- S
sensitive
- SL
shoot length
- T
tolerant
- Tukey HSD
Tukey honest significant difference
Authors’ contributions
APSB: performed the experiments and wrote the MS; RSS: helped with analyses and to write the MS; RCDW: helped with nutrient analyses; ROS: suggested modifications in the analysis protocols and corrected manuscript; LCM: sought information on field experiments and corrected manuscript; ACO: conceived the idea and corrected the manuscript. All authors read and approved the final manuscript.
Funding
The financial support was provided by Coordination for the Improvement of Higher Education Personnel (CAPES) (fellowships to graduate students), Research Support Foundation of Rio Grande do Sul (FAPERGS) (fellowships to undergraduates) and The Brazilian National Council for Scientific and Technological Development (CNPq) (grants and fellowships).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adriana Pires Soares Bresolin, Email: adrianabresolin@unipampa.edu.br.
Railson Schreinert dos Santos, Email: rschsan@hotmail.com.
Roberto Carlos Doring Wolter, Email: robertowolter@gmail.com.
Rogério Oliveira de Sousa, Email: rosousa@ufpel.edu.br.
Luciano Carlos da Maia, Email: lucianoc.maia@gmail.com.
Antonio Costa de Oliveira, Email: acostol@terra.com.br.
References
- 1.Wogu MD, Omoruyi MI, Odeh HO, Guobadia JN. Microbial load in ready-to-eat rice sold in Benin City. J Microbiol Antimicrob. 2011;3:29–33. [Google Scholar]
- 2.Santos CE. Brazilian rice yearbook. Santa Cruz do Sul: Editora Gazeta Santa Cruz; 2015. [Google Scholar]
- 3.Santos RS, Krüger MM, Pegoraro C, Madabula FP, et al. Transcriptional regulation of seven ERFs in rice under oxygen depletion and iron overload stress. Trop Plant Biol. 2013;6:16–25. doi: 10.1007/s12042-013-9117-1. [DOI] [Google Scholar]
- 4.Finatto T, de Oliveira AC, Chaparro C, da Maia LC, et al. Abiotic stress and genome dynamics: specific genes and transposable elements response to iron excess in rice. Rice. 2015;8:1–13. doi: 10.1186/s12284-015-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos RS, Araujo Júnior AT, Pegoraro C, Oliveira AC. Dealing with iron metabolism in rice: from breeding for stress tolerance to biofortification. Genet Mol Biol. 2017;40:312–325. doi: 10.1590/1678-4685-gmb-2016-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asch F, Becker M, Kpongor DS. A quick and efficient screen for resistance to iron toxicity in lowland rice. J Plant Nutr Soil Sci. 2005;168:764–773. doi: 10.1002/jpln.200520540. [DOI] [Google Scholar]
- 7.Sahrawat KL. Iron toxicity in wetland rice and the role of other nutrients. J Plant Nutr. 2004;27:1471–1504. doi: 10.1081/PLN-200025869. [DOI] [Google Scholar]
- 8.Elec V, Quimio CA, Mendoza R, Sajise AGC, et al. Maintaining elevated Fe2+ concentration in solution culture for the development of a rapid and repeatable screening technique for iron toxicity tolerance in rice (Oryza sativa L.) Plant Soil. 2013;372:253–264. doi: 10.1007/s11104-013-1739-4. [DOI] [Google Scholar]
- 9.Audebert A, Fofana M. Rice yield gap due to iron toxicity in West Africa. J Agron Crop Sci. 2009;195:66–76. doi: 10.1111/j.1439-037X.2008.00339.x. [DOI] [Google Scholar]
- 10.SOSBAI . Sociedade Sul-Brasileira de Arroz Irrigado. Arroz Irrigado: Recomendações técnicas da pesquisa para o sul do Brasil. Pelotas: SOSBAI; 2016. p. 197. [Google Scholar]
- 11.International Rice Genome Sequencing Project The map-based sequence of the rice genome. Nature. 2005;436:793–800. doi: 10.1038/nature03895. [DOI] [PubMed] [Google Scholar]
- 12.Engel K, Asch F, Becker M. Classification of rice genotypes based on their mechanisms of adaptation to iron toxicity. J Plant Nutr Soil Sci. 2012;175:871–881. doi: 10.1002/jpln.201100421. [DOI] [Google Scholar]
- 13.Yoshida S. Laboratory manual for physiological studies of rice. 3. Manila: IRRI; 1976. p. 83. [Google Scholar]
- 14.IRRI, International Rice Research Institute . Standard evaluation system for rice. Los Banõs: IRRI; 1975. [Google Scholar]
- 15.Tedesco MJ. Análises de Solo, Planta e Outros Materiais. 2. Porto Alegre: Departamento de Solos, Faculdade de Agronomia; UFRGS; 1995. [Google Scholar]
- 16.Wright S. Correlation and causation. J Agric Res. 1921;20:557–585. [Google Scholar]
- 17.Li CC. Path analysis: a primer. Pacific Grove: Boxwood Press; 1975. p. 346. [Google Scholar]
- 18.Chérif M, Audebert A, Fofana M, Zouzou M. Evaluation of iron toxicity on lowland irrigated rice in West Africa. Tropicultura. 2009;27:88–92. [Google Scholar]
- 19.Crestani M, da Silva JAG, Souza VQ, Hartwig I, et al. Irrigated rice genotype performance under excess iron stress in hydroponic culture. Crop Breed Appl Biotechnol. 2009;9:85–93. doi: 10.12702/1984-7033.v09n01a12. [DOI] [Google Scholar]
- 20.Quinet M, Vromman D, Clippe A, Bertin P, et al. Combined transcriptomic and physiological approaches reveal strong differences between short- and long-term response of rice (Oryza sativa) to iron toxicity. Plant Cell Environ. 2012;35:1837–1859. doi: 10.1111/j.1365-3040.2012.02521.x. [DOI] [PubMed] [Google Scholar]
- 21.Onaga G, Egdane J, Edema R, Abdelbagi I. Morphological and genetic diversity analysis of rice accessions (Oryza sativa L) differing in iron toxicity tolerance. J Crop Sci Biotechnol. 2013;16:53–62. doi: 10.1007/s12892-012-0104-0. [DOI] [Google Scholar]
- 22.Müller C, Kuki KN, Pinheiro DT, de Souza LR, et al. Differential physiological responses in rice upon exposure to excess distinct iron forms. Plant Soil. 2015;391:123–138. doi: 10.1007/s11104-015-2405-9. [DOI] [Google Scholar]
- 23.Palmer C, Guerinot ML. A question of balance: facing the challenges of Cu, Fe and Zn homeostasis. Nat Chem Biol. 2011;5:333–340. doi: 10.1038/nchembio.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EMBRAPA. Anais da I Reunião sobre ferros em solos inundados; 1988.
- 25.Sikirou M, Saito K, Achigan-Dako EG, Drame KN, Ahanchédé A, Venuprasad R. Genetic improvement of iron toxicity tolerance in rice-progress, challenges and prospects in West Africa. Plant Prod Sci. 2015;18:423–434. doi: 10.1626/pps.18.423. [DOI] [Google Scholar]
- 26.Abifarin AO. Progress in breeding rice for tolerance to iron toxicity. In: WARDA, editor. WARDA Annual report for 1990. Bouaké: West Africa Rice Development Association; 1989.
- 27.Onaga G, Edema R, Asea G. Tolerance of rice germplasm to iron toxicity stress and the relationship between tolerance, Fe2+, P and K content in the leaves and roots. Arch Agron Soil Sci. 2012;59:213–229. doi: 10.1080/03650340.2011.622751. [DOI] [Google Scholar]
- 28.Sikirou M. Agro-morphological characterization of lowland rice collection for tolerance to iron toxicity. Abomey-Calavi: Faculty of Agricultural Sciences (FSA); 2009. [Google Scholar]
- 29.Sikirou M, Saito K, Dramé KN, Saidou A, Dieng I, Ahanchédé A, Venuprasad R. Soil-based screening for iron toxicity tolerance in rice using pots. Plant Prod Sci. 2016;19(4):489–496. doi: 10.1080/1343943X.2016.1186496. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Relative performance obtained by the division of the values of plants under stress by the values of the control treatment. Columns followed by the same letters do not differ significantly (Tukey’s pairwise comparisons, p < 0.05).
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.