Abstract
At the turn of the century, the pharmaceutical industry began a transition toward a focus on oncology, rare diseases, and other areas of high unmet need that required a new, more complex approach to drug development. For many of these disease states and novel approaches to therapy, traditional approaches to clinical trial design fall short, and a number of innovative trial designs have emerged. In light of these changes, regulators across the globe are implementing new programs to provide regular development program support, facilitate accelerated access, use real‐world data, and use digital tools to improve patients’ lives. Emerging market regulators are also focusing on simplifying their regulatory pathways via regional harmonization schemes with varying levels of ambition. These changes in the external environment imply that biopharma regulatory teams need to adapt and evolve, leveraging digital tools, data, and analytics, and positioning themselves as strategic advisors during development.
Significant changes in the ecosystem for prescription drugs are forcing pharmaceutical companies to reevaluate their approach to drug development. Patients, providers, payers, and regulators are becoming increasingly aligned in seeking products that clearly address unmet need and demand evidence to better allow them to evaluate risks and benefits in the clinical setting.1 Advanced digital technologies offer significant opportunities to capture patient‐reported outcomes (PROs), analyze real‐world data, and to use analytics solutions to improve research and development (R&D) and regulatory operations, but pharma has yet to exploit those opportunities at scale.2, 3
This is, therefore, an opportune moment for pharmaceutical companies to embrace these new areas in order to continue to innovate and refocus their efforts on delivering value. Here, we examine the changing focus of R&D within the industry, how companies are reshaping clinical trial design to bring novel products to market in areas of high unmet need as quickly as possible, and how regulators are responding to the greater complexity of products in the pipeline. We have also charted the extent of digitization in clinical development, how regulators are responding to digitization, and the extent of digital enablement within regulatory teams. We present opportunities for pharma to leverage regulatory flexibilities and digitization to accelerate development timelines and reduce R&D costs.
The last decades saw a major shift of therapeutic area focus
The period since the turn of the century was an era of substantial change for the pharmaceutical industry. After decades of focusing on highly prevalent diseases, such as cardiovascular diseases and psychiatric conditions, the industry shifted its focus increasingly to oncology, immunological conditions, and rare diseases.3, 4 This shift is illustrated in Figure 1 a 5, 6 with the share of revenue for the pharmaceutical industry by therapeutic area for the period from 1996−2016 and Figure 1 b with the number of ongoing commercial clinical trials by therapeutic area in 2017. In many of these therapeutic areas, traditional approaches to clinical trial design and regulatory approval pathways are challenging. This is because many of these disease states are found in small patient populations or involve focusing on specific subpopulations using specific biomarkers. Cancer, with > 5,000 active clinical trials alone (Figure 1 b), is the prime example. This shift has resulted in a far greater share of pharmaceutical sales arising from drugs that treat smaller patient populations (Figure 2),5 fueling further the need to run clinical trials differently to support regulatory approvals.
Figure 1.
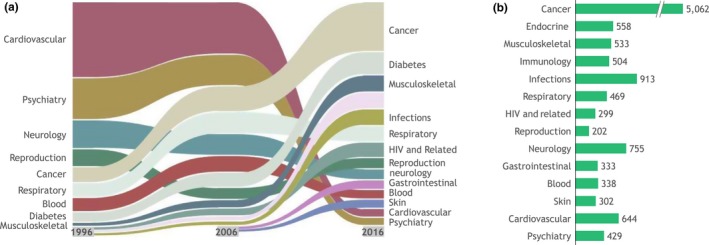
Therapeutic area trends. (a) United States share of revenue by therapeutic area, 1996–2016. (b) Number of ongoing commercial clinical trials by therapeutic area, 2017. Endocrine includes diabetes, growth hormones, and osteoporosis. Source data: EvaluatePharma,5 ClinicalTrials.gov.6
Figure 2.
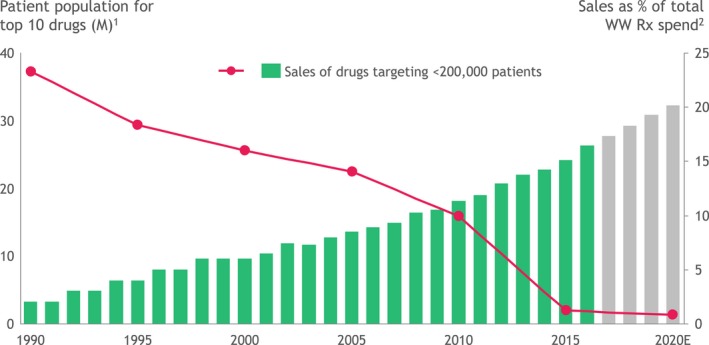
Shift in patient population for top‐selling prescription drugs. 1For top 10 drugs by sales, addressable population calculated based on prevalence of first/major indication marketed. Where prevalence data not available, incidence rates were used instead. 2Percent sales of orphan drugs before 2000 was extrapolated from trend. Source data: EvaluatePharma.5M, millions; WW, worldwide.
More complex modality mix in drug development
Over the period from 2010−2017, the pharmaceutical industry's focus also expanded from small molecule drugs to biologics and to more complex modalities. The proportion of monoclonal antibodies, bioengineered vaccines, and recombination products among new drug approvals doubled over this period from 8 in 2010 to 16 in 2017 ( Figure S1 ).7 Novel modalities, such as cell therapy, gene therapy, and RNA therapeutics have also begun to receive US Food and Drug Administration (FDA) approval. For example, one gene therapy (Amgen's talimogene laherparepvec (Imlygic)) was approved in 2015 and three were approved in 2017 (counting chimeric antigen receptor T (CAR‐T) and viral vector gene therapy). Further, two RNA therapeutics were approved in 2016 and, although data for 2018 are not yet complete, the FDA granted its first approval of an RNA interference therapy to Alnylam's patisiran (Onpattro) in August 2018.8 With more complex therapeutics, novel delivery systems are also being explored, including lipid‐based nanoparticles, microneedles, functionalized quantum dots, and nanogels,9 adding to the complexity of regulatory approval. This complexity affects all phases of development: preclinical development to characterize the novel agent, clinical trial design and execution, and preparation for regulatory submissions.
Rapidly Evolving Clinical Trial Designs
The need to seize opportunities in the areas of greatest unmet need—including domains lacking an established pathway to regulatory approval—has pressed pharmaceutical companies to modify their established clinical trial designs and to experiment with entirely novel trial designs. Innovative trial designs have been previously categorized10, 11 and fall into three broad categories: biomarker‐led design, adaptive design, and cohort‐led design (Table 1).5, 6, 11, 12, 13, 14, 15, 16, 17 Biomarker‐led designs incorporate a genetic or other biomarker to either stratify patients or to select the best treatment for the patient based on their genetic profiling (e.g., umbrella, basket trials). In particular, these designs can enable the predictive and prognostic enrichment of trials with high‐value patients. Traditional biomarkers, such as glycosylated hemoglobin (HbA1c), are excluded from our definition as they are well‐established and validated end points with a long history of acceptance by regulators. Adaptive‐design trials incorporate modifications to the trial strategy during the trial based either on predefined rules or interim results. Cohort‐led designs use novel approaches for trial enrollment, monitoring, and recruitment, often focused on understanding the efficacy of a drug in real‐world settings or addressing the challenges of recruiting patients with rare diseases. Not all of these trial types have been used for primary regulatory approval, but some may be used to fulfill postmarketing commitments to regulators or to extend drug developers’ understanding of efficacy and safety post launch.
Table 1.
Types of novel clinical trial designs
| Type of clinical trial design | Description | Example trials | ||
|---|---|---|---|---|
| Trial | Indication | |||
| Biomarker‐led design | Marker strategy trial | Randomizes patients to two treatment strategies: marker‐based and nonmarker‐based | ERCC1 trial | Non‐small cell lung cancer |
| Umbrella trial | Forms patient subgroups based on biomarkers for different genetic mutations in a single type of disease to test the impact of different drugs | FOCUS4 | Colorectal cancers | |
| Basket trial | Tests the effect of single drug or drug combination on a variety of disease or disease subtypes | SIGNATURE | Solid tumors | |
| Biomarker‐driven adaptive enrichment trial | Changes patient randomization based on interim Bayesian predictive analysis of the marker | BATTLE‐2 | Non‐small cell lung cancer | |
| Adaptive design | Platform adaptive trial | Tests multiple interventions, with interventions changing throughout the trial, on the basis of predefined rules and dose adjustments | DIAN‐TU | Alzheimer's |
| Sample‐size re‐estimation trial | Changes the size of the trial population based on interim results | CHAMPION PHOENIX | Percutaneous coronary interventiona | |
| Changing end point trial | Changes the end point throughout the study based on interim results | EXAMINE | Type 2 diabetesa | |
| Seamless phase II–III trial | Combines treatment selection and confirmation into one trial based on interim results | INHANCE | Chronic obstructive pulmonary diseasea | |
| Cohort‐led design | N‐of‐1‐trial | Assesses the impact of test drug(s) and/or placebo on the same patient with washout period in between the different treatment regimens; several different designs possible | Australian Health Ministers Advisory Council trial | Chronic neuropathic pain |
| Single‐arm vs. natural history cohort | Places patients on the treatment regime and compares efficacy/safety against an independent natural history cohort | Brineura phase I/II | Neuronal ceroid lipofuscinosis type 2 (CLN2) (Batten disease)a | |
| Prospective cohort trial | Places patients in cohorts based on pre‐existing patient databases in order to monitor patients over time to determine how factors/exposure affect a given outcome | SABLE | Systemic lupus erythematosusa | |
| Pragmatic trial | Uses real‐world data from health system (electronic health records) to produce evidence and demonstrate clinical benefit | Salford lung study | Chronic obstructive pulmonary disease | |
Drug approved by the US Food and Drug Administration using the novel clinical trial design.
Biomarker‐led trials have become particularly important in oncology, as drug development has increasingly focused on developing treatments for tumors harboring specific genetic markers. These trials blur clinical research and practice. Umbrella trials, such as the FOCUS trial, in colorectal cancer assess multiple biomarker‐based approaches to treating the same disease or tumor type in parallel. The FOCUS trial, for example, stratified patients into five molecular cohorts at the outset of treatment to test five treatments in parallel.18, 19 Basket trials test the effect of a single drug or drug combination on a variety of diseases or disease subtypes. The most common basket trials to date enroll patients with a broad variety of tumor types (e.g., non‐small cell lung cancer, ovarian, and colorectal, for example) in a single study. In basket trials, enrollment of patients with a specific genetic marker linked to a response to therapy allows the trial to demonstrate a statistically significant response to therapy with a relatively small number of enrolled patients and a variety of affected tissues.20 This design allows investigators to explore the effect of a therapy targeting a specific molecular pathway that may be shared across a variety of tumor types but which may be relatively uncommon in any single tumor type. Thus, investigators can benefit from pooling patients with a variety of different tumors who may nevertheless be likely to respond to a single therapy targeting the specific mutation associated with those tumors. The earliest basket trials enrolled patients with a variety of tumor types that shared a common genetic marker and investigated their response to a single agent.21 The Novartis SIGNATURE program expanded on this approach and consisted of eight phase II single‐agent protocols running in parallel22 testing eight agents (buparlisib, dovitinib, binimetinib, encorafenib, sonidegib, BGJ398, ceritinib, and ribociclib23) in biomarker‐defined populations. The program enrolled patients with a broad variety of malignancies, including both solid tumors (e.g., non‐small cell lung cancer, breast cancer, colorectal cancer, and melanoma) and hematological cancers (chronic myeloid leukemia, acute lymphoblastic leukemia, and acute myeloid leukemia). The program did not depend on specific study sites; any physicians aware of the trial and with access to adequate research capabilities could identify patients for one of the eight trial protocols. This approach provides two opportunities: to minimize research costs by eliminating unproductive sites and comparing several agents at the same time and opening access to enrollment to patients treated at a broader variety of locations than a conventional trial.
Adaptive trials are used across a broad variety of therapeutic areas and can address a variety of challenges in trial design for both acute and chronic conditions. The CHAMPION PHOENIX trial, for example, compared the rate of percutaneous coronary intervention–related ischemic complications in patients treated with cangrelor and those treated with clopidogrel. The study design allowed for an interim analysis to assess whether to increase the sample size of the trial based on whether results thus far were deemed sufficiently promising by the data and safety monitoring committee.11 Although the study did not require an increase in the sample size, the study's design allowed for the possibility of expanding enrollment to achieve adequate statistical power. The EXAMINE trial took a different approach that allowed investigators to consider whether to change the primary end point from noninferiority to superiority based on an interim analysis.11 EXAMINE compared the novel antihyperglycemic drug alogliptin to standard of care in reducing cardiovascular outcomes in patients with type 2 diabetes. At the trial's interim analysis, investigators assessed the probability of demonstrating superiority rather than inferiority; because the planned full trial enrollment was unlikely to achieve this goal and noninferiority had already been achieved, the trial was not continued beyond that point. In both of these examples, the inclusion of an interim analysis allowed investigators to avoid unnecessarily expanding or continuing trials—saving significant time and cost, but also ultimately benefiting patients, too.
Cohort‐led design encompasses a variety of study designs and goals. Pragmatic trials, for example, aim to demonstrate clinical benefit in the real‐world setting. GlaxoSmithKline's Salford lung study provides one example of how pharmaceutical companies are trying to use pragmatic trials. The Salford lung study was an open‐label, randomized, real‐world trial comparing a once‐daily inhaler containing the long‐acting beta2 agonist vilanterol and the inhaled corticosteroid fluticasone furoate against existing therapy.16 The trial design included relatively broad inclusion criteria and compared the fluticasone furoate/vilanterol combination inhaler against patients’ existing therapy, with the goal of producing results that can be more confidently generalized to real‐world medical practice and patient populations. The study showed a statistically significant reduction in exacerbations of chronic obstructive pulmonary disease compared with usual care and showed improved disease control in asthma,24 providing proof‐of‐concept for the approach.25 However, to date, based on publicly available information, data from the Salford lung study has only been used in a regulatory context to fulfill a postapproval commitment on safety from the European Medicines Agency (EMA), rather than for efficacy.
N‐of‐1 trials also use a nontraditional approach to measuring efficacy by comparing the response of a single patient to different interventions, typically introducing an intervention after charting the patient's progress without an intervention. Researchers can address the challenges associated with wide variation in individual responses to treatment by assessing individual responses in a cohort of patients and also in disease areas, such as rare diseases where there is a limited pool of patients. One example of an n‐of‐1 trial is the Australian Health Ministers’ Advisory Council trial, which generated results suggesting lower efficacy for gabapentin in treating neuropathic pain than had been estimated based on traditional randomized controlled trials.15 For the purposes of this trial, the n‐of‐1 design presented an advantage over conventional trial design because individual responses to pain medications vary widely, and investigators wanted to better understand responses at the individual level. The trial enrolled a total of 73 patients, each of whom was treated with three cycles of gabapentin and placebo assigned in random order, and patients were assessed individually for responses to gabapentin and to placebo to arrive at the conclusion that gabapentin may not be as effective as previously believed.
Regulators Welcome Innovative Clinical Trial Designs
Regulators are responding to the increasing complexity of the new therapeutic drugs in the pipeline by encouraging innovative approaches to clinical trials while demanding that new treatments demonstrate meaningful patient outcomes, often with a drug comparator (not placebo). As shown in Figure 3 a, the number of innovative trials included in approved FDA applications was greater in 2017 than in 2010 across all therapeutic areas. For example, in oncology, avelumab (Bavencio), a drug approved by the FDA in 2017, used a novel design in early stage clinical trials. The phase I Javelin solid tumor study, a novel dose‐expansion design, was used to test safety and efficacy in multiple solid tumor types in parallel.26 The study enrolled a total of 1,700 patients comprising 12 solid tumor types and took place in two parts. The first part of the study used a conventional dose‐escalation design, whereas the second part divided patients into cohorts for a dose‐expansion study. The goal of the latter was to further understand toxicity and to explore efficacy in specific tumor types identified as having high unmet need, programmed cell death‐ligand 1 overexpression, and likelihood of responsiveness to immunotherapy based on prior evidence and data gathered in the initial part of the Javelin trial. Trials using this type of design are becoming increasingly common in oncology because, although they prolong the duration of phase I, they can provide more information at an earlier stage of development than conventional designs.27 As oncology moves increasingly toward precision medicine, clinical trial design in oncology will likely continue to evolve.28
Figure 3.

Use of novel clinical trial designs. (a) Number of trials where a novel clinical trial design was used in an approved US Food and Drug Administration (FDA) submission. (b) Number of approved submissions that used surrogate end points. (c) Number of approved submissions that included studies with patient‐reported outcomes and/or real‐world data. Source data: ClinicalTrials.gov,6 FDA,7 Bhatt et al.,11 Zang et al.,12 TrialTrove,13 Bateman et al.,14 Yelland et al.,15 Albertson et al.,16 and Batten Disease News.17
Oncology is not the only therapeutic area in which regulators are signaling an openness to novel trial designs. For the central nervous system product for enzyme replacement therapy in Batten disease, cerliponase alfa (Brineura), one single‐arm trial was used to support the FDA approval, and it was also continued post approval, demonstrating continued reduction.8 Patients on treatment were compared against a similar natural history cohort. Opting for a single‐arm design meant that a smaller pool of patients was sufficient to run the trial, which was critical given the limited available patient pediatric population.
However, regulators are not yet ready to grant approval based on real‐world data studies; these remain limited to postapproval safety and efficacy studies rather than pre approval studies.29 This is not surprising given the risks of failing to show significance in a heterogeneous population pre approval. Pharmaceutical companies are reluctant to jeopardize approval by collecting data from a broad population with little exclusion. Nevertheless, both pharmaceutical companies and regulators are moving toward trial designs that more closely reflect real‐world clinical practice than traditional randomized controlled trials. Pfizer's inotuzumab ozogamicin (Besponsa) received FDA approval in 2016 based on the results of the phase III INO‐VATE ALL study, an open‐label study that compared the activity of inotuzumab ozogamicin to investigator's choice of chemotherapy regimen8, 30 rather than to a single comparator. Pragmatic trials have also been suggested as a potentially valuable way to provide expanded insights about drugs approved through accelerated pathways or that demonstrate exceptional activity during pivotal trials, leading to early termination and approval.31 Post approval, Tesaro is conducting a pragmatic trial of its drug niraparib (Zejula), which received FDA approval in 2017 for ovarian cancer. The BRAVO trial is comparing progression‐free survival with niraparib or with investigator's choice from four standard of care therapies in patients with advanced/metastatic breast cancer who have a breast cancer gene change.32 Unfortunately, physicians opted for other poly ADP ribose polymerase therapies rather than the standard of care chemotherapies and, therefore, enrollment in the trial has been low. This is a downside of pragmatic trials in which there is less control of how patients are managed and where exclusion criteria may still not be sufficiently wide to capture real‐life clinical practice. Others have described how often trials coined as “pragmatic” may still have characteristics of a tightly controlled randomized clinical trial.33
Companies have other postapproval uses of real‐world data, to support payer negotiations and pricing as well as to strengthen commercial claims.34 For example, Boehringer Ingelheim's dabigatran (Pradaxa) has been examined in several real‐world trials to assess both postapproval efficacy and safety including the FDA Mini‐Sentinel Trial, the FDA Medicare Assessment Trial, and the Department of Defense trial.35 This has served the company well in its claims for safety and efficacy.
It is interesting to note that our research did not identify any innovative trial designs for many indications that were in the spotlight over the past decade, such as psoriasis, rheumatoid arthritis, type 2 diabetes, and others. This underscores the extent to which innovation in trial design is currently driven heavily by the characteristics of diseases with significant unmet medical need, where few precedents exist, patient populations are small, and well‐validated clinical end points are difficult to identify.
Surrogate End points on the Up
In addition to the focus on outcomes by regulators more broadly,2 regulators are open to the use of surrogate end points in conditions with particular unmet need. Figure 3 b shows a fourfold increase in the use of surrogate end points from 2010−2017. The most marked increase in surrogate end points is in oncology, driven by the use of biomarkers that provide confidence in relying on the surrogate end point objective response rate (ORR) rather than requiring the traditional direct end point overall survival. The FDA's accelerated approval of copanlisib (Aliqopa), for example, was based on a single‐arm phase II trial as the pivotal trial with ORR as the primary end point in relapsed follicular lymphoma.7 Surrogate end points have become particularly important in oncology trials because drug developers are pursuing rarer cancers with narrowly defined subpopulations for which achieving sufficient enrollment to measure traditional end points would substantially slow or limit development. A recent analysis of the acceptance of ORR in regulatory submissions concluded that it was an appropriate end point for assessing efficacy in regulatory submissions, underscoring regulators’ openness to new approaches to trial design in oncology.36 The increase in surrogate end points in 2017 in anti‐infectives is also driven by the approval of two hepatitis C treatments on the basis of sustained viral response after 12 weeks of treatment levels; other anti‐infective surrogate end points for vaccines have been used in the past.
Patient‐Reported Outcomes complementing the clinical trial execution toolkit
Both regulators and pharmaceutical companies have also shown interest in collecting PROs. To date, PROs have been included in trials as secondary end points. In 2010, only one product approved by the FDA included PROs as part of the regulatory submission, but by 2017, this number had increased to 12, as shown in Figure 3 c. To date, PROs have already been gathered across a wide variety of therapeutic areas. Again, oncology has the greatest number of regulatory submissions, including PROs as secondary end points. For 2017 FDA approvals, five drugs included PROs (Bayer's copanlisib, Takeda's brigatinib (Alunbrig), Novartis’ midostaurin (Rydapt), Eli Lilly's abemaciclib (Verzenio), and Tesaro's niraparib). In addition, they have also been included in regulatory submissions for the rare blood disorder hemophilia A (Roche's emicizumab‐kxwh (Hemlibra)), asthma (AstraZeneca's benralizumab (Fasenra)), psoriasis (Janssen's guselkumab (Tremfya) and Bausch Health's brodalumab (Siliq)), and type 2 diabetes (Novo Nordisk's semaglutide (Ozempic)). PROs have increased because they offer unique opportunities to pharmaceutical companies: for example, to more effectively assess whether the drug is delivering effective patient‐centered care and the ability to test companion digital applications for postapproval use in the clinical trial. Although regulators have not yet reached the stage in which they would rely on PROs over traditional end points, they are supportive of their inclusion in pivotal trials. PROs can provide useful validation that objective measurements of efficacy correlate with meaningful improvements in quality of life for patients.
One notable example of the value of including PROs in regulatory submissions is Incyte's ruxolitinib (Jakafi). In 2011, ruxolitinib became the first drug to receive FDA approval for the treatment of myelofibrosis, a chronic neoplastic myeloproliferative disorder with debilitating symptoms.37 The end points that were included in the COMFORT‐1 trial (one of two pivotal trials) were a primary end point measuring reduction in spleen size and a secondary end point designed to measure the impact of therapy on patients’ symptoms that used a PRO tool called the modified Myelofibrosis Symptom Assessment Form.37, 38 The study not only supported regulatory approval for the drug but also underscored the value of assessing symptoms as well as objective measures of spleen size reduction in myelofibrosis.
In contrast with other innovative trial designs and end points, PROs are also being included in clinical trials of long‐established conditions with well‐defined clinical trial designs and regulatory pathways, such as asthma, psoriasis, and type 2 diabetes. This suggests companies perceive additional value from PROs that they cannot capture from traditional direct end points, surrogate end points, and investigators’ assessments of disease. For example, psoriasis trials have historically relied heavily on the Psoriasis Area and Severity Index, an investigator assessment of patients’ psoriasis symptoms. However, psoriasis has a substantial impact on patients’ quality of life, and trials have traditionally included quality of life assessments, such as the Dermatology Life Quality Index, but the Dermatology Life Quality Index and similar instruments were not developed specifically for psoriasis.39 Investigators see additional value in psoriasis‐specific PROs developed to conform to the FDA PRO guidance as the landscape for psoriasis treatment becomes more crowded with an array of novel therapies.39 It is not surprising then that Janssen and Bausch Health included PROs in the regulatory submissions for guselkumab and brodalumab.
Novel Pathways for Faster Drug Access
Linked with the increase in innovative clinical trial designs, multiple stakeholders, including regulators, payers, Health Technology Assessment (HTA) bodies, patient groups, and drug developers across the globe have recognized the need to develop pathways for accelerated access to innovative drugs.40, 41, 42 Regulators have developed programs to expedite drug development to bring innovative medicines to patients more quickly. Although each program/scheme has slightly different eligibility criteria and definitions of unmet need, the principles and intentions of these top regulators are similar: provide guidance to drug developers regarding key issues in trial design and analysis, and shorten development and review timelines. In Japan, the development was part of a broader initiative by the Pharmaceuticals and Medical Devices Agency (PMDA), which put the SAKIGAKE program in place in 2014 ( Table S1 ) to reduce the well‐documented lag in approvals of innovative medicines in Japan following European Union and FDA approvals.43 As of 2016, Japan seemed to be closing the gap in approval timing; 41 drugs with new active ingredients were approved in Japan compared with 27 in the European Union, and the Japan review timelines were approximately half those of the European Union.44
Table S1 summarizes different initiatives introduced by regulators in major markets, all with the aim of accelerating access to novel therapies.45, 46, 47, 48 The FDA provides five specific pathways to allow therapies in areas of high unmet need to reach the market more rapidly. Notably, both fast track and accelerated approval have clear implications for companies developing clinical trial programs in terms of both benefits and requirements. Drugs receiving fast track designation may be approved based on phase IIa trials, and drugs considered under accelerated approval are able to use surrogate end points that can be measured in trials of shorter duration than may be necessary to measure direct clinical outcomes. The outcome of both schemes is earlier market entry with a commitment to validate findings with real outcomes in longer term, postapproval studies, often in a real‐world setting.
Similarly, the EMA has several programs in place to accelerate drug development for areas of high unmet medical need. These include Accelerated Review, Conditional Approval, and two relatively new programs, Adaptive Pathways and PRIME. The Adaptive Pathways program was introduced in 2014 with the goal of providing a pathway to approval for areas of high unmet need where collecting data through traditional randomized controlled trials is particularly challenging. The randomized clinical trial design can be particularly challenging to correlate with real‐world outcomes in rare diseases; Fabry's disease has been cited as an example of a rare disease where a regulatory program like Adaptive Pathways would have yielded more meaningful, applicable trial results than a traditional program of randomized controlled trials.49 The EMA concluded a pilot assessment of the program in 2016 and decided to continue to launch the program going forward.50, 51 Some stakeholders—especially HTAs and payers—have expressed concerns that their evidentiary requirements differ from those of the Adaptive Pathways program, and that determining how to assess these agents will pose significant challenges, especially as they will need to reassess initial evaluations as postmarketing data become available.52, 53 However, an EMA official cited the case of alemtuzumab (Lemtrada), a high‐cost multiple sclerosis drug, as a case study in how even under conventional regulatory procedures, the argument for cost‐effectiveness of a drug can evolve over time.54 As the EMA seems committed to the Adaptive Pathways program, these issues will need to be addressed by HTAs and payers.55 It also noted that the Adaptive Pathways program and PRIME involve discussions with payers in which the payer evidentiary needs can be highlighted.
Within and in addition to the above programs, regulators are also putting effort behind developing guidance for industry on how and where to utilize genetic biomarkers and real‐world data. Enacted in the United States in 2016, the 21st Century Cures Act mandated that the FDA provide clear guidance to industry on where and how real‐world data can be used to supplement randomized controlled trials. The FDA expects to issue guidance on real‐world evidence in December 2018 that will provide a framework for assessing data sources and will establish standards and methodologies for the use of real‐world data in clinical trials.56 Notably, the 21st Century Cures Act only requires the FDA to consider real‐world evidence for extension of indications to currently approved drugs. This is not surprising, as the risk to regulator and company are less with existing products, whereas the methodological aspects and limitations are worked out.
Innovation in accelerated pathways continues. The FDA has as recently as this year created two new pilot programs to facilitate more efficient drug development specifically in oncology. The Real‐Time Oncology Review (RTOR) program allows for FDA review of clinical trial data prior to formal submission to provide feedback to the trial sponsor regarding data analysis.57 The Assessment Aid program provides a structured template to applicants to help streamline the submission and review process.57 In July 2018, the agency announced that Novartis’ breast cancer drug ribociclib (Kisquali) was the first drug to receive approval after using the new programs,8 and it also benefited from priority review and breakthrough designation.8 The RTOR scheme is similar to the EMA's adaptive pathways scheme, which also enables companies to submit data as it becomes available.
Implications for Pharma sponsor regulatory approaches
With an array of regulatory flexibilities and new tools at their disposal (e.g., real‐world data, biomarkers, and digital tools to collect PROs), it is now up to pharmaceutical companies to take advantage of these capabilities to bring innovative products to market earlier. The trend in the use of regulatory flexibilities has been an overall increase since 2010 in the United States (Figure 4 a,b). The most commonly used flexibility as of 2017 in the United States was Priority Review, but the use of Breakthrough Therapy designation has increased markedly in recent years.
Figure 4.
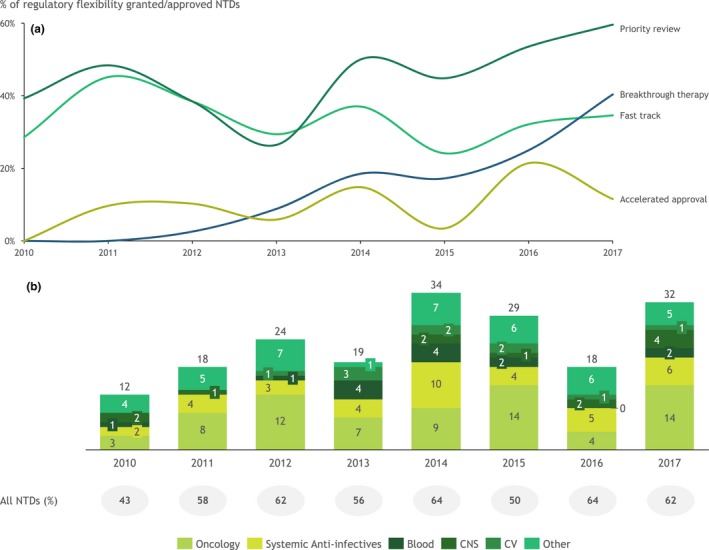
Approvals using regulatory flexibilities. (a) Percentage of new therapeutic drugs (NTD)s approved using regulatory flexibilities in the US Food and Drug Administration (FDA) by type of regulatory flexibility, 2010–2017. Regulatory flexibilities include Breakthrough Therapy, Fast Track, Accelerated Approval, and Priority Review. Note: some NTDs used more than one regulatory flexibility. (b) Number of NTDs using at least one regulatory flexibility and percentage of all NTDs, 2010–2017. Other includes musculoskeletal, systemic anti‐infectives, blood, and various. CNS, central nervous system; CV, cardiovascular. Source data: Evaluate Pharma,5 FDA,45 EMA,46 PMDA,47 and Biomedtracker.48
Although EMA's Adaptive Pathways scheme has not yet been widely used and the full impact of the 21st Century Cures Act is still years away, there is a clear intent of regulators to encourage the use of real‐world data, especially in the postapproval setting. However, real‐world data can also be used to inform drug development programs, even when the data will not feed into a regulatory submission. The information can be used to chart natural disease progression and be used to inform baseline improvements that could be made with interventions as well as for collecting safety information and refining the populations that would benefit most for the product post approval. Pharmaceutical companies are, therefore, increasing their efforts to identify how they can use advanced analytics to mine existing regulatory submissions and assessment reports from regulators to increase the accuracy with which they predict regulatory success. Pharmaceutical companies have already successfully mined data to identify safety signals and inform risk management plans, and companies have also attempted to mine clinical data to identify repositioning and new indication opportunities.58
Several published studies have demonstrated factors that correlate with a higher likelihood of regulatory success. Factors that correlated with higher frequency of regulatory approval included orphan drug status, high unmet need, clear evidence on efficacy, scientific advice, and prior company experience in the relevant therapeutic area. However, companies have not yet leveraged artificial intelligence or undertaken internal initiatives to understand which factors within their own portfolios serve as predictors of regulatory success.59 Ultimately, better predictive analytics could help identify likely regulatory success based on prior experience internally with a type of trial, type of comparator, or number of trials submitted, thereby improving development and avoiding costly delays and failures in late‐phase development.60 Such information could also inform discussions with regulators on trial design.
Finally, implementing true live licensing would represent a significant milestone. Pharmaceutical companies could provide data updates to regulators as data becomes available, allowing timely feedback between companies and regulators. Both the EMA's Adaptive Pathways and the FDA's RTOR pilot program are clear initial steps in this direction. The recent approval of Novartis’ ribociclib is an early success story in developing a live licensing pathway, as it provides proof‐of‐concept that the FDA can conduct a rolling review for a product in development.
Emerging Markets: Rapidly Gaining Ground
Although much of the focus on both trial design and digital innovation has been trained on developed markets such as the United States, Europe, and Japan, the emerging markets cannot be overlooked. Moreover, some of these regulators may be well‐positioned to respond rapidly to digital and perhaps “leapfrog” mature markets.
With emerging markets growing in importance, the pharmaceutical industry can partner with these markets to strengthen regulatory systems and support harmonization. Regulators in BRIC (Brazil, Russia, India, and China), Africa, the ASEAN region, and the Middle East are adapting to the changing environment in the pharmaceutical industry, trying to keep pace with their peers in the United States, Europe, and Japan. Mexico's COFEPRIS recently underwent a major modernization exercise and has updated its generic drugs licensing pathway and reduced its backlogs.61 Saudi Arabia's FDA released its 5‐year strategy in May 2018 highlighting the desire to proactively monitor use and safety of products in a real‐life setting by specific registries in addition to the already ongoing pack serialization scheme “track and trace.”62
Many organizations, including the World Health Organization, and individual regional harmonization initiatives have been encouraging emerging market regulators to rely on prior approvals and Good Manufacturing Practice inspections to accelerate access to products in their markets.63 Several not‐for‐profit organizations have provided significant support for these efforts, recognizing that reliance, harmonization, and coordination encourages innovators to bring products intended for diseases that otherwise would be neglected. However, as noted in a study examining adoption of technical documents developed to harmonize regulatory standards in the Pan‐American region, progress toward harmonization remains dependent on countries having adequate capabilities to perform the regulatory functions recommended in harmonization efforts.64 As a result, progress toward harmonization varies considerably by region, and some regions have relatively ambitious harmonization initiatives underway, whereas others remain in relatively early stages of development. The level of ambition of regulatory harmonization initiatives across the globe is summarized in Figure 5.64, 65, 66, 67, 68, 69 The ZAZIBONA collaboration of countries from the South African Development Community and the Gulf region's Gulf Cooperation Council (GCC)‐DR are both relatively ambitious initiatives that created collaborative or centralized review and approval of drugs that have already been used extensively and resulted in hundreds of approvals.65 In contrast, the ASEAN initiative has comparatively modest ambitions, with its goals focused on strengthening regulatory functions and developing guidelines but not implementing a centralized review process. The PANDRH initiative in Latin America is similarly focused on improving existing processes rather than developing a centralized process for review and approval.
Figure 5.
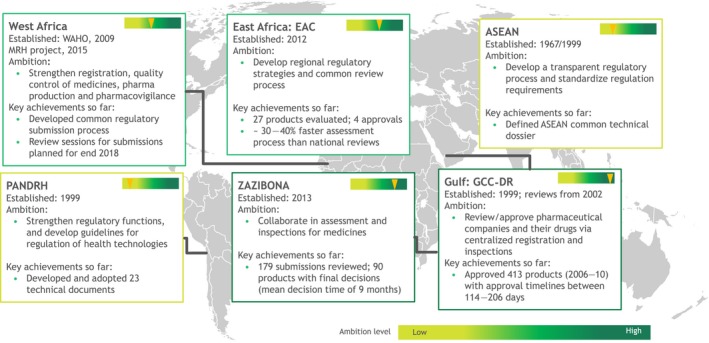
Major emerging markets regional harmonization initiatives. Source data: Primary research—PANDRH,64 GCC‐DR,65 ASEAN,66 WAHO,67 EAC,68 and ZAZIBONA.69
Leaps made with digital products
The technological advances with digital products are also shaping regulatory policy. Regulators see opportunities with digital therapeutics and are encouraging digital applications and predictive analytics to support clinical development. As the personalization of health care expands, the expectation is that there will be more digital products marketed as companion products for specific drugs to support dosing and treatment duration. Clear guidelines regarding what is required for approval will be important to support the industry and ensure sufficient safeguards are in place when tools can alter the course of diagnosis or treatment. This is because there are significant risks for patients with these products where the predictive algorithm may negatively impact patient treatment decisions.
As a result, the landscape of digital oversight by regulators is rapidly evolving. Major milestones in the FDA's oversight of digital products and applications are outlined in Figure 6.8, 70, 71 The FDA published its first guidance regarding mobile medical applications in 2013 and updated that guidance in 2015. Since then, the pace of the FDA's actions in the digital sphere has accelerated, and, in 2017, the agency approved the first digital drug‐device, aripiprazole tablets with sensor (Abilify MyCite), which tracks patients’ adherence to dosing schedules.
Figure 6.
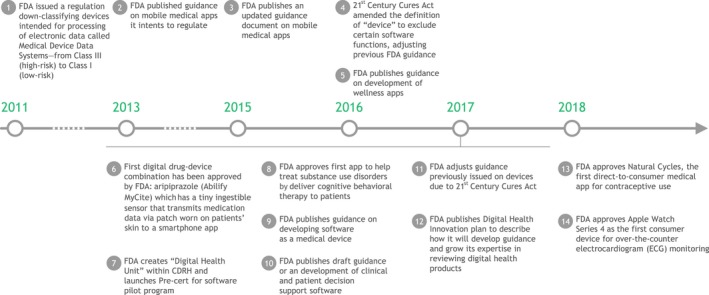
Major milestones in regulation of digital health products by the US Food and Drug Administration (FDA). Source data: FDA.8, 70, 71 CDRH, FDA Center for Devices and Radiological Health.
In 2017, the FDA published its Digital Health Action Plan,72 outlining steps the agency has already taken to clarify its role in oversight of digital products and to set forth the agency's next steps in developing guidance. These steps include issuing draft guidance regarding the medical software provisions in the 21st Century Cures Act, as well as to continue to contribute to international efforts to develop regulatory principles for Software as a Medical Device. Notably, the 21st Century Cures Act reclassified some digital applications to no longer be considered medical devices, including software that “supports administrative functions, encourages a healthy lifestyle, assists in displaying or storing data, or provides limited clinical decision support.” The Digital Health Action Plan also outlines a plan to develop a precertification program for some digital health products, with the goal of streamlining and accelerating submission and review. To date, however, the agency has provided little clarity on the regulatory requirements for approval of digital solutions that may have predictive capabilities, and this limits companies from applying for these approvals.
Change imperative for pharma regulatory teams
The rapidly evolving regulatory landscape demands that pharmaceutical companies take stock of how they position internal regulatory teams and how they leverage the power of digital applications and analytics to improve productivity and increase efficiency. One of the fundamental changes that will impact in the pharmaceutical industry in the coming years is the gradual breakdown of silos along the drug development value chain. Clinical practice could end up evolving much faster than clinical science as opposed to how it used to be—in part driving a larger need for interconnectedness across regulatory teams and other functions.
In order for internal regulatory teams to be ready for the challenges ahead, we advocate that they need to be more business‐minded and empowered to guide development and support commercial lifecycle strategies. To also be effective in supporting other teams they need to work more cross‐functionally than they currently do, having a seat at the table when key product decisions are made, whether prelaunch or postlaunch. They also need to think of how regulatory strategies can accelerate early access, minimize duration and size of clinical trials, and support payer interactions and submissions. More and more, the reimbursement/formulary placement step is proving to be the most difficult. Therefore, increasingly, market access, health economics, and real‐world data teams with the support of medical teams will need to develop integrated plans to include real‐world data in development programs preapproval as well as post approval.73
Existing structures are not optimized to allow regulatory to interact seamlessly as part of an integrated team with commercial, market access, medical, clinical operations, and supply chain, as regulatory issues touch all of those teams. Resource management and prioritization across regulatory priorities are also not optimal, as often resources are allocated by product rather than by business need and a consolidated list of priorities across the portfolio. Therefore, any opportunities to reduce manual and repetitive effort as well as to connect and increase the accuracy and consistency of disparate regulatory and allied systems can bring significant cost and efficiency gains.
Regulatory teams’ evolving role will also need to include providing input into interactions with academic centers and academic investigators who participate in clinical trials. For example, regulatory and clinical teams will need to work proactively with investigators to design clinical trials that better reflect real‐world clinical practice. Pharma companies may need to provide support with how to develop the relevant tools and platforms necessary for real‐world studies, leveraging current electronic health records, where possible. This is likely an attainable goal, as academic centers are already exploring new approaches to gathering and analyzing clinical data to support personalized medicine, in many cases working with pharmaceutical companies.74, 75
We see digital technologies as a key enabler to unlock the potential for more efficient drug development and regulatory activities. There are many potential digital use cases across regulatory from the very simple automation to sophisticated predictive and prescriptive analytics using past data to provide recommendations on future studies and likelihood of regulatory success (Figure 7):
Figure 7.
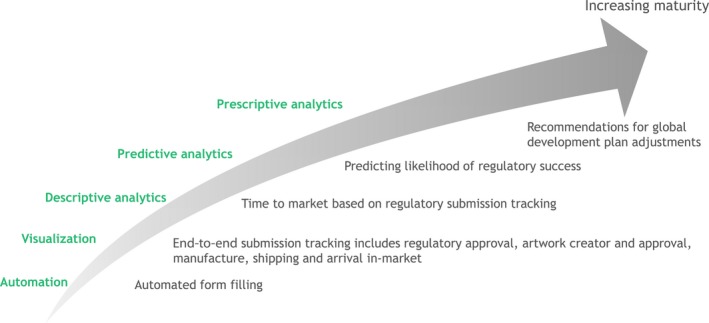
Exemplary digital use cases for regulatory teams.
Automatic form filling for submissions using structured data and structured authoring
Collection and use of past information and regulatory intelligence to define requirements for future submissions and reduce time taken to develop regulatory strategies
End‐to‐end visibility of impact of regulatory strategy on supply chain and allied systems around the critical time of new product launches or postapproval changes
Predicting regulatory success using artificial intelligence, specifically machine learning
Maintaining the label and creating virtual, digital patient information and artwork
Although each function is considering how to adopt digital technologies, regulatory teams have so far only initiated small pilots but struggle to scale the digital solutions. Together with each new digital application should also come a new, streamlined way of working; one without the other cannot be successful. Pilots and rapid sprints are a good way of testing and validating new ideas for potential digital solutions that address crucial bottlenecks in core regulatory processes.
Conclusion
The pharmaceutical industry is poised to establish a trend toward greater productivity by focusing on therapeutic areas with high unmet need, expanding its use of innovative trial designs and accelerated pathways to approval, and by reaping the tangible benefits of digitization. Pathways put in place by regulators and availability of new digital tools provide pharmaceutical companies with opportunities to increase the likelihood of success of development programs and to create commercialization opportunities for established products while containing costs. To fully realize these benefits, however, pharmaceutical companies will need to uplift capabilities of regulatory teams, repositioning and empowering them to shape development and support lifecycle strategies, as critical members of integrated teams throughout the product lifecycle.
Funding
The authors of this article are employees of the Boston Consulting Group (BCG), a management consultancy that works with biopharmaceutical companies, payers, providers, and other healthcare organizations.
Conflict of Interest
The authors of this article are employees of the Boston Consulting Group (BCG), a management consultancy that works with biopharmaceutical companies, payers, providers, and other healthcare organizations.
Supporting information
Table S1. Summary of initiatives across the United States, European Union, and Japanese regulators to accelerate approvals of innovative medicines. Source data: Evaluate Pharma,5 FDA,45 EMA,46 PMDA,47 Biomedtracker.48
Acknowledgments
Cynthia Mundy contributed intellectual input and editing of this article.
References
- 1. Soderlund, N. , Kent, J. , Lawyer, P. & Larsson, S. Progress Toward Value‐Based Health Care (Boston Consulting Group, Boston, MA, 2012). [Google Scholar]
- 2. Marwaha, S. , Ruhl, M. & Shorkey, P. Doubling Pharma Value with Data Science (Boston Consulting Group, Boston, MA, 2018). [Google Scholar]
- 3. McCallister, E. , Usdin, S. , Fishburn, C.S. & Flores, D. A Pathway to Biopharma 3.0 (BioCentury; <https://www.biocentury.com/biocentury/politics-policy-law/2018-08-31/back-school-2018-how-external-disrupters-will-drive-?kwh=pathway<|>biopharma<|>3.0<|>pathways>. Published August 31, 2018). [Google Scholar]
- 4. Ringel, M. et al Occlusion in the flow of new drugs for cardiovascular disease. Clin. Pharmacol. Ther. 102, 246–253 (2017). [DOI] [PubMed] [Google Scholar]
- 5. Evaluate . EvaluatePharma (2018). http://www.evaluate.com/about. Accessed September 27, 2018.
- 6. US National Library of Medicine . ClinicalTrials.gov <https://www.clinicaltrials.gov/>. August 15, 2018.
- 7. US Food and Drug Administration . Drugs@FDA: FDA approved drug products <https://www.accessdata.fda.gov/scripts/cder/daf/> (2018).
- 8. US Food and Drug Administration. Press announcements <https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/default.htm>. Accessed September 15, 2018.
- 9. Mühlebach, S. Regulatory challenges of nanomedicines and their follow‐on versions: a generic or similar approach? Adv. Drug Deliv. Rev. 131, 122–131 (2018). [DOI] [PubMed] [Google Scholar]
- 10. Mahajan, R. & Gupta, K. Adaptive design clinical trials: methodology, challenges and prospect. Indian J. Pharmacol. 42, 201–207 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhatt, D.L. & Mehta, C. Adaptive designs for clinical trials. N. Engl. J. Med. 375, 65–74 (2016). [DOI] [PubMed] [Google Scholar]
- 12. Zang, Y. , Liu, S. & Yuan, Y. Optimal marker‐strategy clinical trial design to detect predictive markers for targeted therapy. Biostatistics 17, 549–560 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pharma Intelligence . Trialtrove (Informa, London, 2018). [Google Scholar]
- 14. Bateman, R.J. et al The DIAN‐TU Next Generation Alzheimer's prevention trial: adaptive design and disease progression model. Alzheimers Dement. 13, 8–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yelland, M.J. et al N‐of‐1 randomized trials to assess the efficacy of gabapentin for chronic neuropathic pain. Pain Med. 10, 754–761 (2009). [DOI] [PubMed] [Google Scholar]
- 16. Albertson, T. , Murin, S. , Sutter, M. & Chenoweth, J. The Salford Lung Study: a pioneering comparative effectiveness approach to COPD and asthma in clinical trials. Pragmatic Obs. Res. 8, 175–181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brineura approved as batten treatment in both Europe and US as clinical studies continue. Batten Disease News <https://battendiseasenews.com/2017/06/02/brineura-approved-as-batten-treatment-in-europe-us-as-clinical-trials-continue/> (2017).
- 18. Schmoll, H.J. FOCUS4: a new trial design for evaluation of targeted drugs in colorectal cancer? Lancet Gastroenterol. Hepatol. 3, 143–145 (2018). [DOI] [PubMed] [Google Scholar]
- 19. Adams, R. et al Inhibition of EGFR, HER2, and HER3 signalling in patients with colorectal cancer wild‐type for BRAF, PIK3CA, KRAS, and NRAS (FOCUS4‐D): a phase 2–3 randomised trial. Lancet Gastroenterol. Hepatol. 3, 162–171 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redig, A.J. & Jänne, P.A. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J. Clin. Oncol. 33, 975–977 (2015). [DOI] [PubMed] [Google Scholar]
- 21. Cunanan, K.M. et al Basket trials in oncology: a trade‐off between complexity and efficiency. J. Clin. Oncol. 35, 271–273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang, B.P. et al The signature program: bringing the protocol to the patient. Clin. Pharmacol. Ther. 98, 124–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slosberg, E.D. et al Signature program: a platform of basket trials. Oncotarget 9, 21383–21395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. GSK . Relvar Ellipta significantly improved asthma control in Salford Lung Study patients compared with their usual care <https://www.gsk.com/en-gb/media/press-releases/relvar-ellipta-significantly-improved-asthma-control-in-salford-lung-study-patients-compared-with-their-usual-care/>. Accessed September 27, 2018.
- 25. Vestbo, J. et al Effectiveness of fluticasone furoate–vilanterol for COPD in clinical practice. N. Engl. J. Med. 375, 1253–1260 (2016). [DOI] [PubMed] [Google Scholar]
- 26. Chin, K. , Chand, V.K. & Nuyten, D.S.A. Avelumab: clinical trial innovation and collaboration to advance anti‐PD‐L1 immunotherapy. Ann. Oncol. 28, 1658–1666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iasonos, A. & O'Quigley, J. Dose expansion cohorts in phase I trials. Stat. Biopharm. Res. 8, 161–170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Brien, C. , Carter, L. , Cook, N. & Dean, E. Novel early phase clinical trial design in oncology. Pharmaceut. Med. 31, 297–307 (2017). [Google Scholar]
- 29. Wechsler, J. FDA moves to broaden acceptance of real‐world evidence in clinical research. Appl. Clin. Trials 7, 26 <http://www.appliedclinicaltrialsonline.com/fda-moves-broaden-acceptance-real-world-evidence-clinical-research> October 1, 2017. [Google Scholar]
- 30. Pfizer . Pfizer receives U.S. FDA approval for BESPONSA® (inotuzumab ozogamicin) <https://www.pfizer.com/news/press-release/press-release-detail/pfizer_receives_u_s_fda_approval_for_besponsa_inotuzumab_ozogamicin> (August 17, 2017).
- 31. Koehler, M. , Donnelly, E.T. , Kalanovic, D. , Dagher, R. & Rothenberg, M.L. Pragmatic randomized clinical trials: a proposal to enhance evaluation of new cancer therapies with early signs of exceptional activity. Ann. Oncol. 27, 1342–1348 (2016). [DOI] [PubMed] [Google Scholar]
- 32. Tryfonidis, K. et al A phase III trial of niraparib versus physician's choice in HER2 negative, germline BRCA Mutation‐positive Breast Cancer Patients (BRAVO). J. Clin. Oncol. 32 (2014). 10.1200/jco.2014.32.15_suppl.tps659 [DOI] [Google Scholar]
- 33. Dal‐ré, R. , Janiaud, P. & Ioannidis, J.P.A. Real‐world evidence: how pragmatic are randomized controlled trials labeled as pragmatic? BMC Med. 16, 1–6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faulkner, S.D. et al Pricing and reimbursement experiences and insights in the European Union and the United States: lessons learned to approach adaptive payer pathways. Clin. Pharmacol. Ther. 100, 730–742 (2016). [DOI] [PubMed] [Google Scholar]
- 35. Pradaxa . <https://www.pradaxa.com/study-data>. Accessed September 19, 2018.
- 36. Oxnard, G.R. et al Response rate as a regulatory end point in single‐arm studies of advanced solid tumors. JAMA Oncol. 2, 772–779 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Acquadro, C. & Regnault, A. Patient‐reported outcomes in drug development for hematology. Hematology 2015, 496–500 (2015). [DOI] [PubMed] [Google Scholar]
- 38. Miller, C.B. et al Practical measures of clinical benefit with ruxolitinib therapy: an exploratory analysis of COMFORT‐I. Clin. Lymphoma Myeloma Leuk. 17, 479–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feldman, S.R. et al Development of a patient‐reported outcome questionnaire for use in adults with moderate‐to‐severe plaque psoriasis: the psoriasis symptoms and signs diary. J. Dermatol. Dermatol. Surg. 20, 19–26 (2016). [Google Scholar]
- 40. Liberti, L. et al FDA facilitated regulatory pathways: visualizing their characteristics, development, and authorization timelines. Front. Pharmacol. 8, 1–6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kakkis, E.D. et al Recommendations for the development of rare disease drugs using the accelerated approval pathway and for qualifying biomarkers as primary endpoints. Orphanet J. Rare Dis. 10, 16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baird, L.G. et al Accelerated access to innovative medicines for patients in need. Clin. Pharmacol. Ther. 96, 559–571 (2014). [DOI] [PubMed] [Google Scholar]
- 43. Tsuji, K. & Tsutani, K. Approval of new drugs 1999‐2007: comparison of the US, the EU and Japan situations. J. Clin. Pharm. Ther. 35, 289–301 (2010). [DOI] [PubMed] [Google Scholar]
- 44. Kondo, H. , Saint‐Raymond, A. & Yasuda, N. What to know about medicines with new active ingredients approved in FY 2016/2016 in Japan and EU. Ther. Innov. Regul. Sci. 52, 214–219 (2018). [DOI] [PubMed] [Google Scholar]
- 45. US Food and Drug Administration . Fast track, breakthrough therapy, accelerated approval, priority review <https://www.fda.gov/forpatients/approvals/fast/default.htm>. Accessed September 15, 2018.
- 46. European Medicines Agency. Human medicines: regulatory information <https://www.ema.europa.eu/en/human-medicines-regulatory-information>. Accessed September 15, 2018.
- 47. Pharmaceuticals and Medical Devices Agency . Strategy of SAKIGAKE by MHLW <https://www.pmda.go.jp/english/review-services/reviews/advanced-efforts/0001.html>. Accessed September 15, 2018.
- 48. Pharma Intelligence . Biomedtracker <https://www.biomedtracker.com/> 2018. Accessed September 27, 2018.
- 49. Schuller, Y. , Arends, M. , Körver, S. , Langeveld, M. & Hollak, C.E.M. Adaptive pathway development for Fabry disease: a clinical approach. Drug Discov. Today 23, 1251–1257 (2018). [DOI] [PubMed] [Google Scholar]
- 50. European Medicines Agency . Final Report on the Adaptive Pathways Pilot (European Medicines Agency, London, UK, 2016). [Google Scholar]
- 51. Bouvy, J.C. , Sapede, C. & Garner, S. Managed entry agreements for pharmaceuticals in the context of adaptive pathways in Europe. Front. Pharmacol. 9, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ermisch, M. et al Payers’ views of the changes arising through the possible adoption of adaptive pathways. Front. Pharmacol. 7, 1–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bonanno, P.V. et al Adaptive pathways: possible next steps for payers in preparation for their potential implementation. Front. Pharmacol. 8, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Donnell, P. Adaptive pathways—pilot or plot. App. Clin. Trials. (2016). <http://www.appliedclinicaltrialsonline.com/adaptive-pathways-pilot-or-plot> January 8, 2016. Accessed September 25, 2018. [Google Scholar]
- 55. Eichler, H. et al Medicines adaptive pathways to patients: why, when, and how to engage? Clin. Pharmacol. Ther. 10.1002/cpt.1121. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. US Food and Drug Administration . 21st Century Cures Act deliverables <https://www.fda.gov/regulatoryinformation/lawsenforcedbyfda/significantamendmentstothefdcact/21stcenturycuresact/ucm562475.htm>. Accessed September 28, 2018.
- 57. US Food and Drug Administration . Oncology Center of Excellence <https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/OCE/default.htm>. Accessed September 23, 2018.
- 58. The Economist Intelligence Unit. The innovation imperative: the future of drug development Part II: barriers, enablers and calls to action <http://www.eiu.com/public/marketing/ContactUs.aspx> (2018). Accessed September 27, 2018.
- 59. Liberti, L. et al Factors related to drug approvals: predictors of outcome? Drug Discov. Today 22, 937–946 (2017). [DOI] [PubMed] [Google Scholar]
- 60. Belleli, R. , Fisch, R. & Szucs, T.D. Efficiency indicators for new drugs approved by the FDA from 2003 to 2013. Nat. Rev. Drug Discov. 14, 156 (2015). [DOI] [PubMed] [Google Scholar]
- 61. Arriola Peñalosa, M.A. , Cavazos Cepeda, R. , Alanis Garza, M. & Lumpkin, M.M. Optimized medical product regulation in Mexico: a win‐win for public and economic health. Ther. Innov. Regul. Sci. 51, 744–750 (2017). [DOI] [PubMed] [Google Scholar]
- 62. Saudi Food and Drug Authority (SFDA) . Saudi Food & Drug Authority Strategic Plan 2018‐2022 Current State Assessment Report <https://www.sfda.gov.sa/en/about/Documents/SFDA-StrategicPlan.pdf> (2018). Accessed September 27, 2018.
- 63. World Health Organization . Annual Report 2013 Prequalification of Medicines (World Health Organization, Geneva, Switzerland, 2014). [Google Scholar]
- 64. Pombo, M.L. , Porrás, A. , Saidon, P.C. & Cascio, S.M. Regulatory convergence and harmonization: barriers to effective use and adoption of common standards. Rev. Panam. Salud Publica 39, 217–225 (2016). [PubMed] [Google Scholar]
- 65. Al‐Rubaie, M.H. Evaluation of the Regulatory Review Process of the GCC Centralised Procedure: Development of a Model for Improving the Approval Process (Cardiff University, Cardiff, 2013). [Google Scholar]
- 66. Association of South East Asian Nations. ACCSQ Pharmaceutical Product Working Group. <https://asean.org/?static_post=accsq-pharmaceutical-product-working-group>. Accessed September 15, 2018.
- 67. West African Health Organization . About WAHO <http://wahooas.org/spip.php?page=rubriqueS&id_rubrique=24&lang=en>. Accessed September 15, 2018.
- 68. Tanzania Food & Drugs Authority . East Africcan Community Medicines Regulatory (EAC– MRH) programme <https://www.tfda.go.tz/?q=node/188>. Accessed September 15, 2018.
- 69. Medicines Control Authority of Zimbabwe . ZAZIBONA collaborative medicines registration process <https://www.mcaz.co.zw/index.php/latest-news/16-zazibona-collaborative-medicines-registration-process. Accessed September 15, 2018.
- 70. US Food and Drug Administration . Mobile medical applications <https://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm>. Accessed September 15, 2018.
- 71. US Food and Drug Administration . Medical device data systems <https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/GeneralHospitalDevicesandSupplies/MedicalDeviceDataSystems/default.htm>. Accessed September 15, 2018.
- 72. US Food and Drug Administration . Digital health innovation action plan <https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/UCM568735.pdf> (2017).
- 73. Lamberti, M.J. et al The use of real‐world evidence and data in clinical research and postapproval safety studies. Ther. Innov. Regul. Sci. 52, 778–783 (2018). [DOI] [PubMed] [Google Scholar]
- 74. Brustugun, O.T. , Sprauten, M. & Helland, A. Real‐world data on nivolumab treatment of non‐small cell lung cancer. Acta Oncol. (Madr.) 56, 438–440 (2017). [DOI] [PubMed] [Google Scholar]
- 75. Ginsburg, G.S. , Staples, J. & Abernethy, A.P. Academic medical centers: ripe for rapid‐learning personalized health care. Sci. Transl. Med. 3, 101 cm27 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of initiatives across the United States, European Union, and Japanese regulators to accelerate approvals of innovative medicines. Source data: Evaluate Pharma,5 FDA,45 EMA,46 PMDA,47 Biomedtracker.48


