Abstract
This study assesses whether the US Food and Drug Administration regulation to limit the sale of flavored tobacco products to age-restricted locations is adequate based on a 1-year review of violations rates in age-restricted shops.
In 2018, the US Centers for Disease Control and Prevention announced a 78% increase in vaping from 2017 to 2018 among high school students, an epidemic characterized by increased use of flavored tobacco products.1 With a goal to reverse this trend, the US Food and Drug Administration (FDA) announced its intent to limit sales of flavored (excluding menthol) tobacco products to age-restricted (adult-only) locations, such as tobacco and vape shops.2
However, the 2017 California tobacco purchase survey3 reported that tobacco and vape shops had the highest rate of underage sales compared with other types of tobacco retailers. We investigated whether disparate violations persisted in 2018 and whether the FDA’s intention to limit the sale of flavored tobacco products to age-restricted locations is adequate.
Methods
This study used data from the 2018 sample (n = 1746) of the California Tobacco Control Program’s Young Adult Tobacco Purchase Survey that was drawn from the statewide tobacco retail license list. The data were collected by the California State University, Sacramento. Their institutional review board did not consider this study to involve human subjects’ research.
From March through June 2018, decoys (aged 18-19 years) were randomly assigned to purchase either cigarettes (n = 1123) or vape products (n = 498), such as e-liquids and e-cigarettes. The sample also included stores that were considered noncompletes (n = 98) and stores where decoys asked for other tobacco products (eg, little cigars or cigars) (n = 27). According to the standard protocol, decoys did not carry identification (ID) and told the truth about their age. A trained chaperone observed whether ID was requested from the decoy and whether a sale occurred. Tobacco and vape shops were defined as retailers that primarily sell tobacco products. Data were weighted to account for sampling design. Rao-Scott χ2 tests (2-sided with significance set at P < .05) were performed to examine the association between retailer type and outcomes using SAS, version 9.4 (SAS Institute Inc).
Results
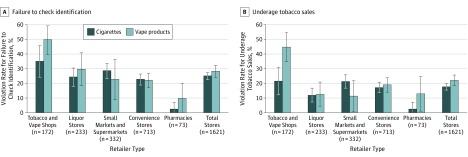
Although FDA regulation requires retailers to check ID for all persons under 27 years, 49.8% of tobacco and vape shops failed to check ID for underaged decoys when decoys attempted to purchase vape products. The violation rate in tobacco and vape shops was significantly higher than for other types of retailers (P < .05) (Figure, A). Furthermore, 44.7% of tobacco and vape shops sold vape products to underage decoys also at a higher rate compared with other tobacco retailers (P < .05) (Figure, B). Overall sales violations were higher for vape products compared with cigarettes (χ2 = 4.3938; P < .05) (Figure, B).
Figure. Violation Rates for Failing to Check Identification and for Underage Tobacco Sales by Retailer Type in California, 2018.
Whiskers indicate 95% CI.
Discussion
Tobacco and vape shops had a worse record for checking ID and preventing underage sales, which may undermine the FDA’s plan to restrict youth access to flavored tobacco products. This concern is not unique to California. Other states, including North Carolina and Oklahoma, reported underage sales rates of 20% or higher in tobacco and vape shops in federal fiscal year 2019.4
The FDA’s 2009 ban on the sale of flavored cigarettes was associated with reduced smoking among youth; however, research suggests the association was lessened because of the availability of menthol cigarettes and other flavored tobacco products.5 Evidence is needed to show that limiting the sale of these tobacco products to age-restricted locations will prevent sales to minors. Although this study did not record whether retailers posted age-restricted entry signs at their shops, the study results suggest a higher rate of sales violations by retailers whose primary business is the sale of an age-restricted product.
Presumably tobacco and vape shops would be the most compliant with age-of-sale laws, particularly in states where license suspension or revocation would jeopardize the business. However, these results suggest that the FDA’s proposal to relegate sales of flavored tobacco products to adult-only facilities are not likely to be effective without significant age-verification requirements and increases in the number and frequency of compliance checks that the FDA conducts.6 An effective plan to limit sales of flavored tobacco products to youth may include accountability throughout the tobacco distribution chain (including manufacturers and distributors), retailer education, and enforcement. States can further limit the availability and affordability of flavored tobacco by increasing the minimum legal sales age to 21 years, restricting sales of flavored tobacco (including menthol), prohibiting self-service displays, and pursuing tax and nontax mechanisms to increase price.
References
- 1.Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011-2018. MMWR Morb Mortal Wkly Rep. 2018;67(45):1276-1277. doi: 10.15585/mmwr.mm6745a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, MD, on proposed new steps to protect youth by preventing access to flavored tobacco products and banning menthol in cigarettes [press release]. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-proposed-new-steps-protect-youth-preventing-access. Published November 15, 2018.Accessed May 9, 2019.
- 3.Zhang X, Vuong TD, Andersen-Rodgers E, Roeseler A. Evaluation of California’s ‘Tobacco 21’ law. Tob Control. 2018;27(6):656-662. doi: 10.1136/tobaccocontrol-2017-054088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration Web Block Grant Application System (WebBGAS). https://bgas.samhsa.gov/Module/BGAS/Users Accessed February 18, 2019.
- 5.Courtemanche CJ, Palmer MK, Pesko MF. Influence of the flavored cigarette ban on adolescent tobacco use. Am J Prev Med. 2017;52(5):e139-e146. doi: 10.1016/j.amepre.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levinson AH, Ma M, Jason LA, et al. . Assessment of the US federal retailer violation rate as an estimate of the proportion of retailers that illegally sell tobacco to adolescents. JAMA Pediatr. 2018;172(10):966-972. doi: 10.1001/jamapediatrics.2018.2038 [DOI] [PMC free article] [PubMed] [Google Scholar]