Key Points
Question
Does a 6-week course of intensive home-based telehealth targeting arm movements after stroke provide rehabilitation benefits that are comparable with those derived from dose-matched traditional in-clinic rehabilitation therapy?
Findings
In this randomized, assessor-blinded, noninferiority clinical trial of 124 adults following stroke, telerehabilitation showed comparable efficacy to traditional in-clinic rehabilitation for improving motor status (Fugl-Meyer arm motor scale) and for improving patient knowledge about stroke.
Meaning
Telehealth is an effective means to provide rehabilitation therapy and improve patient outcomes after stroke and may be useful for improving access to rehabilitation therapy.
Abstract
Importance
Many patients receive suboptimal rehabilitation therapy doses after stroke owing to limited access to therapists and difficulty with transportation, and their knowledge about stroke is often limited. Telehealth can potentially address these issues.
Objectives
To determine whether treatment targeting arm movement delivered via a home-based telerehabilitation (TR) system has comparable efficacy with dose-matched, intensity-matched therapy delivered in a traditional in-clinic (IC) setting, and to examine whether this system has comparable efficacy for providing stroke education.
Design, Setting, and Participants
In this randomized, assessor-blinded, noninferiority trial across 11 US sites, 124 patients who had experienced stroke 4 to 36 weeks prior and had arm motor deficits (Fugl-Meyer [FM] score, 22-56 of 66) were enrolled between September 18, 2015, and December 28, 2017, to receive telerehabilitation therapy in the home (TR group) or therapy at an outpatient rehabilitation therapy clinic (IC group). Primary efficacy analysis used the intent-to-treat population.
Interventions
Participants received 36 sessions (70 minutes each) of arm motor therapy plus stroke education, with therapy intensity, duration, and frequency matched across groups.
Main Outcomes and Measures
Change in FM score from baseline to 4 weeks after end of therapy and change in stroke knowledge from baseline to end of therapy.
Results
A total of 124 participants (34 women and 90 men) had a mean (SD) age of 61 (14) years, a mean (SD) baseline FM score of 43 (8) points, and were enrolled a mean (SD) of 18.7 (8.9) weeks after experiencing a stroke. Among those treated, patients in the IC group were adherent to 33.6 of the 36 therapy sessions (93.3%) and patients in the TR group were adherent to 35.4 of the 36 assigned therapy sessions (98.3%). Patients in the IC group had a mean (SD) FM score change of 8.36 (7.04) points from baseline to 30 days after therapy (P < .001), while those in the TR group had a mean (SD) change of 7.86 (6.68) points (P < .001). The covariate-adjusted mean FM score change was 0.06 (95% CI, –2.14 to 2.26) points higher in the TR group (P = .96). The noninferiority margin was 2.47 and fell outside the 95% CI, indicating that TR is not inferior to IC therapy. Motor gains remained significant when patients enrolled early (<90 days) or late (≥90 days) after stroke were examined separately.
Conclusions and Relevance
Activity-based training produced substantial gains in arm motor function regardless of whether it was provided via home-based telerehabilitation or traditional in-clinic rehabilitation. The findings of this study suggest that telerehabilitation has the potential to substantially increase access to rehabilitation therapy on a large scale.
Trial Registration
ClinicalTrials.gov identifier: NCT02360488
This randomized clinical trial examines whether a treatment targeting arm movement delivered via a home-based telerehabilitation system has comparable efficacy with dose-matched, intensity-matched therapy delivered in a traditional in-clinic setting for adults following stroke.
Introduction
The most common deficits after stroke are in the motor system, affecting more than 80% of patients.1 Few patients fully recover from arm weakness after a stroke. The remainder demonstrate persistent arm impairments that are directly linked to activity limitations, participation restrictions, reduced quality of life, and decreased well-being.2,3,4 Some rehabilitation therapies can improve these deficits, with higher doses associated with better outcomes.5
However, many patients do not receive high doses of rehabilitation therapy, for reasons that include cost, difficulty traveling to the location where therapy is provided, shortage of regional rehabilitation care, and poor adherence with assignments. Furthermore, even when patients can access stroke rehabilitation therapy, the amount of therapy provided is limited, averaging 32 arm movements per session.6 Qualitative aspects of telerehabilitation (TR) are also important and can increase the extent to which clinical neuroplasticity is harnessed,7 for instance, by using games to modulate therapy complexity, feedback, and enjoyment.8
Telerehabilitation is the delivery of rehabilitation services via communication technologies9 and can address these issues. In a pilot study of home-based daily TR targeting arm motor function after chronic stroke, 4 weeks of daily therapy was associated with excellent (97.9%) adherence and significant clinical improvement, and was not dependent on computer skill level.10 Because patients with stroke often have fundamental gaps in stroke knowledge and secondary stroke prevention, a stroke education module was included and associated with significant increases in stroke knowledge.
The present study built on these findings, comparing home-based TR with an active comparator using a noninferiority, randomized clinical trial design. The current target was arm movements after stroke, given their high prevalence and effect.1,2,3,4 The hypothesis was that activity-based training targeting arm movement after stroke delivered via home-based TR would have efficacy comparable with that of dose-matched, intensity-matched activity-based training delivered in a traditional clinic setting. Secondary hypotheses examined these 2 treatment approaches in relation to stroke education and participant motivation.
Methods
Overview
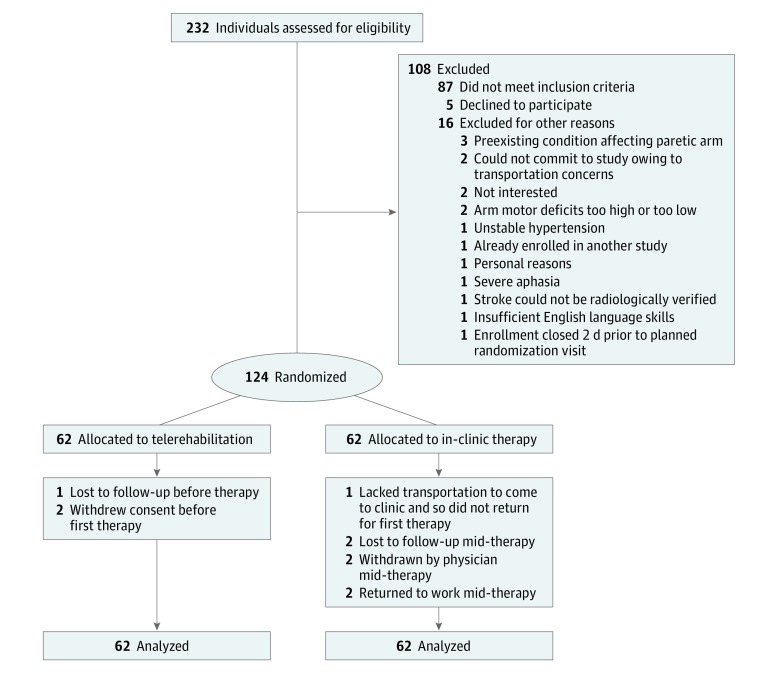
At 11 US sites in the National Institutes of Health StrokeNet clinical trials network, 124 patients with arm motor deficits (Fugl-Meyer [FM] score, 22-56 of 66) 4 to 36 weeks after stroke onset were enrolled between September 18, 2015, and December 28, 2017, and randomized (1:1) to receive intensive arm motor therapy in the clinic (IC), or in the patient’s home using TR to deliver services via an internet-connected computer (Figure 1 and trial protocol in Supplement 1). Therapy intensity (amount of activity per therapy session), duration (number of weeks), and frequency (sessions per week) were matched across the 2 treatment groups: all patients received 36 treatment sessions (70 minutes plus a 10-minute break; 18 supervised and 18 unsupervised) during a 6- to 8-week period. This study used an assessor-blinded, randomized, controlled, noninferiority design to test whether efficacy is comparable between treatment groups. The primary end point was within-patient change in arm motor FM score11 from baseline to 30 days after treatment. This study was approved by the StrokeNet Central Institutional Review Board and determined by the US Food and Drug Administration to be a nonsignificant risk device study. Patients provided written informed consent. Race/ethnicity data were collected to characterize the population and were classified by investigators at each site.
Figure 1. CONSORT Diagram of Study Enrollment.
Eligible patients (eTable 1 in Supplement 2) were 18 years or older, experienced ischemic stroke or intracerebral hemorrhage 4 to 36 weeks prior, had mild to severe arm motor deficits, and had no major deficits in mood or cognition. Each patient underwent baseline testing and was randomized using a web-based central system. Each site had 2 or more assessment therapists and 2 or more treatment therapists, each a licensed occupational or physical therapist. Treatment therapists oversaw therapy for both treatment groups. Assessment therapists performed study testing and underwent training and formal certification on the FM scale, National Institutes of Health Stroke Scale, modified Rankin Scale, and Montreal Cognitive Assessment; plus FM scale11 recertification every 4 months.
Assessments
Testing (eTable 2 in Supplement 2) occurred during 4 study visits to the research center. Additional assessments were made during in-person visits for the IC group and via the TR system for the TR group. Secondary motor outcomes were scores on the Box and Blocks Test and Stroke Impact Scale–hand motor domain (version 3.0). A stroke knowledge examination was scored before and after treatment.
Adherence with therapy was calculated as percentage of 36 therapy sessions for which the patient completed 40 or more of the assigned 70 minutes. For the TR group, the number of minutes of therapy completed at each session was determined via patient report; for the IC group, patient report was used for unsupervised sessions and therapists recorded treatment duration for supervised sessions.
Two measures of motivation were assessed, each scored from 1 to 7 (where 1 indicated low motivation and 7 indicated high motivation). Activity-inherent motivation, reflecting how much patients liked their rehabilitation therapy, was measured as the change over time in the Physical Activity Enjoyment Scale12 score. Consequence-related motivation, reflecting the patient’s dedication to treatment goals, was measured using the Optimization in Primary and Secondary Control13 scale.
Treatment
For Both Groups
The goal was to provide all patients with 18 supervised and 18 unsupervised 70-minute sessions. The content of therapy was carefully matched across groups. All patients signed a behavioral contract14 that included a personal treatment goal, after which treatment therapists explained the assigned rehabilitation therapy. The treatment approach was based on an upper-extremity task-specific training manual15 and Accelerated Skill Acquisition Program.16 Therapists could revise the treatment plan as often as desired; revision was required at least every 2 weeks. Provision of feedback to patients was a core feature. Individuals in the IC group received therapist feedback on supervised days based on the therapist’s observations. Individuals in the TR group also received therapist feedback on supervised days, based on the therapist’s videoconference observations plus the therapist’s review of electronic data (prior days’ use, scores, and photographs during game play) and also received feedback on all days during game play.
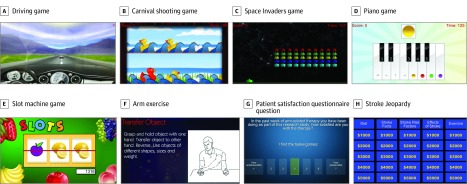
To match treatment across groups, all sessions for both groups included (1) at least 15 minutes per day of arm exercises. The same 88 exercises were used for both groups (Figure 2F; eTable 3 in Supplement 2). These exercises used standard exercise equipment (eg, resistance tubing [TheraBand] or putty) provided to all patients and incorporated standard therapy approaches (eg, stretching, strengthening, and active range of motion). For patients in the IC group, exercises were demonstrated by the therapist on supervised days or via printed homework on unsupervised days. For patients in the TR group, exercises were presented on the computer screen on supervised and unsupervised days and could be demonstrated by the therapist during videoconferences on supervised days. All sessions for both groups also included (2) at least 15 minutes per day of functional training. The strategy for functional training was the same across groups, provided using functional tasks for the IC group and functional games for the TR group (Figure 2A-E; eTable 4 in Supplement 2). Standard exercise hardware was used during functional tasks for the IC group; 12 input devices (eg, PlayStation Move controller [Sony] or trackpad) were used during functional games for the TR group. All sessions for both groups also included (3) 5 minutes per day of stroke education. The education content was the same for both groups, targeted stroke prevention and risk factors, and corresponded to the stroke knowledge examination. At the beginning of unsupervised sessions, patients answered multiple-choice questions, delivered via paper booklets for the IC group and a video Jeopardy17 game format for the TR group, and then received feedback (Figure 2H).
Figure 2. Examples of Telerehabilitation Therapy Content.
For the IC Group
The 18 supervised treatment sessions took place at the research center, during which treatment therapists provided 70 minutes of continuous supervision. The 18 unsupervised treatment sessions were at home, guided by an individualized booklet created and printed by treatment therapists and containing diagrams and instructions for functional tasks plus exercises from the same list available during supervised days.
For the TR Group
The TR system (eMethods in Supplement 2) consisted of an internet-enabled computer with table, chair, and 12 gaming input devices, but no keyboard, as no computer operation was required by patients. System software supported videoconferencing and organized the 70 minutes of therapy, which consisted of exercises, functional games, and stroke education as described in “For Both Groups.” Patients were trained to use the TR system at the baseline visit. A study team member delivered the TR system to the home, where all 36 sessions took place.
During the 30 minutes prior to each session, the computer alerted the patient to the start time. Patients pressed a tabletop button to begin the session, and to start subsequent games and exercises. Supervised sessions began with a 30-minute patient-therapist videoconference, during which therapists supervised therapy, answered questions, reviewed treatment plans, and performed study assessments. Unsupervised sessions had the same treatment content as supervised sessions but without therapist contact. Exercises and stroke education in the TR group were strictly matched to IC group content, being presented via the TR system. Functional training in TR (25 functional games) was designed to match functional training in IC (functional tasks), and feedback was provided during game play (ie, success or not plus the final score). Games emphasized various motor control features (eg, varying movement speed, range of motion, target size, or level of cognitive demand) as adjusted by therapists (eTable 4 in Supplement 2). For example, during the whack-a-mole game, higher difficulty level meant a broader area where targets could appear and a shorter duration of time to hit the target. Therapists also selected which input device patients would use to play each game. For example, the flappy bird game could be played using grip force cylinder, pinch force cube, trackpad, or other devices.
Statistical Analysis
Minimal sufficient balance randomization18 was used to prevent serious imbalances across the 2 treatment groups in (1) number of days after stroke at the time of randomization, (2) FM score at baseline, and (3) number of patients, within site. The difference in mean change in FM score between treatment groups was estimated using linear regression, with change in FM from baseline to 30 days after therapy as the dependent variable and treatment group as the independent variable. The model was adjusted for study site, age, time after stroke, stroke subtype, and baseline FM score. To accommodate FM change scores that were negative or zero, the noninferiority margin was additionally assessed using a modified linear regression model estimating the difference between mean FM change among patients in the TR group and 70% of the mean FM change among patients in the IC group. Linear regression models also examined treatment group in relation to secondary end points, while a linear mixed-effects model was used to assess whether groups differed in change in stroke knowledge examination scores from screening to the end of therapy. Primary efficacy analysis used the intent-to-treat population (all randomized patients) and multiple imputation for missing data. Secondary analyses were: (1) intent-to-treat population with substitution of “worst-best-case” missing outcomes19 (eMethods in Supplement 2), (2) complete case intent-to-treat population, restricted to randomized patients for whom complete data were available, and (3) per-protocol population, defined as all patients with complete data who completed 40 minutes or more of assigned activities on 15 or more of the 18 supervised therapy sessions during no more than 8 weeks. A linear mixed-effects regression model was used to examine whether adherence (session length, in minutes) changed across the weeks of therapy, with a random intercept for patient. All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Sample Size
The trial aimed to establish comparable efficacy based on a noninferiority margin of 30% of the change in FM score in the IC group. Under these assumptions at α = .05 and assuming a SD of 3.8 points,10 124 patients would need to be enrolled to provide 85% power; this sample was pursued independent of patient withdrawal.
Results
Patients and Procedures
Key characteristics appear in Table 1. Ten patients (3 in the TR group and 7 in the IC group) were not available for follow-up (Figure 1); on average, the patients not available for follow-up were younger than patients who completed the study (mean [SD] age, 53.6 [10.2] vs 62.1 [13.3] years), with milder deficits (mean [SD] FM score, 47.7 [8.8] vs 42.3 [8.1]). Among patients who initiated at least 1 treatment session, those in the TR group were adherent with 35.4 of the 36 assigned therapy sessions (98.3%), while those in the IC group were adherent with 33.6 of the 36 assigned therapy sessions (93.3%); this value did not differ significantly between study groups, whether examining all sessions together (P = .91), or supervised (P = .89) and unsupervised sessions (P = .73) separately. The median number of supervised and unsupervised sessions with which patients were adherent was 18 (interquartile range, 18-18) in both groups. Session length did not significantly change across the weeks of therapy, increasing by 0.04 minutes per week for both supervised (95% CI, −0.10 to 0.18; P = .55) and unsupervised sessions (95% CI, −0.14 to 0.23; P = .64). Technical issues occurred with decreasing frequency over time. University of California Irvine received 1 call per week for assistance from sites during the first 2 months, with topics ranging from program navigation to wireless connectivity. This number decreased to 1 call every 2 weeks during the last 2 months as sites became more familiar with operations. Game and device use appear in eTables 5 and 6 in Supplement 2. Serious adverse events occurred in 1 patient in the TR group and 6 patients in the IC group, all unrelated to study procedures. Nonserious adverse events considered reasonably or definitely related to study procedures occurred in 6 patients in the TR group (arm and shoulder pain) and 5 patients in the IC group (fatigue and arm and shoulder pain). Most patients (81 of 112 [72.3%]) received rehabilitation therapy outside of study procedures (eTable 7 in Supplement 2); however, doses were small (median, 2 hours of physical therapy, 1 hour of occupational therapy, and 0 hours of speech therapy) and did not differ between groups.
Table 1. Characteristics of Patients in Both Groups.
| Characteristic | TR Group (n = 62)a | IC Group (n = 62)a |
|---|---|---|
| Age, mean (SD), y | 62 (14) | 60 (13) |
| Baseline arm motor Fugl-Meyer score, mean (SD) | 42.8 (7.8) | 42.7 (8.7) |
| Box and Blocks score, mean (SD) | 21.3 (13.3) | 23.8 (12.7 |
| Stroke Impact Scale hand domain score, mean (SD) | 38.8 (26.3) | 42.6 (24.1) |
| Handedness, No. | ||
| Right | 56 | 54 |
| Ambidextrous | 3 | 4 |
| Left | 3 | 4 |
| Time after stroke | ||
| No. of days, mean (SD) | 132 (65) | 129 (59) |
| Patients enrolled <90 d after stroke | 16 (25.8) | 22 (35.5) |
| Stroke subtype | ||
| Ischemic | 54 (87.1) | 52 (83.9) |
| Intracerebral hemorrhage | 8 (12.9) | 10 (16.1) |
| Female sex | 14 (22.6) | 20 (32.3) |
| Race | ||
| Asian | 6 (9.7) | 4 (6.5) |
| Black | 15 (24.2) | 18 (29.0) |
| White | 41 (66.1) | 39 (62.9) |
| Unknown | 0 | 1 (1.6) |
| Ethnicity, Hispanic | 3 (4.8) | 0 |
| Geriatric Depression Scale score, mean (SD) | 3.4 (3.1) | 3.6 (2.7) |
| Montreal Cognitive Assessment score, mean (SD) | 24.9 (4.1) | 24.4 (5.0) |
| Nottingham Sensory score, mean (SD) | 9.5 (2.5) | 9.9 (2.7) |
| Modified Ashworth Spasticity scale score, median (IQR) | 0 (0-1) | 1 (0-2) |
| Paretic side, right | 27 (43.5) | 36 (58.1) |
| Baseline NIHSS score, median (IQR) | 3 (2-5) | 3 (2-4) |
| Baseline Modified Rankin scale score, median (IQR) | 2 (2-3) | 2 (2-3) |
| Hypertension | 50 (80.6) | 53 (85.5) |
| Diabetes mellitus | 14 (22.6) | 17 (27.4) |
| Atrial fibrillation | 10 (16.1) | 4 (6.5) |
| Hypercholesterolemia | 40 (64.5) | 39 (62.9) |
Abbreviations: IC, in-clinic; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; TR, telerehabilitation.
Data are presented as number (percentage) of patients unless otherwise indicated.
Treatment-Related Arm Motor Gains
Motor status at enrollment was stable, with median FM score change of 0 points (interquartile range, −1 to 2 points) from screening to baseline visits, which were 8 days apart (interquartile range, 5-13 days); for the 38 patients enrolled less than 90 days after stroke, the change in median FM score between these 2 visits was also 0 points (interquartile range, −1 to 2 points). Both groups showed significant treatment-related motor gains, with a mean (SD) unadjusted FM score change from baseline to 30 days after therapy of 8.36 (7.04) points in the IC group (P < .001) and 7.86 (6.68) points in the TR group (P < .001). The adjusted mean change in FM score was 0.06 points larger in the TR group (95% CI, −2.14 to 2.26; P = .96). The noninferiority margin (30% of the mean FM score change in the IC group) was 2.47, which fell outside of this 95% CI, indicating that TR was not inferior to IC therapy on the primary end point; results did not differ in secondary analyses (Table 2).
Table 2. Treatment-Related Change in FM Motor Scorea.
| Model | Patients, No. | FM Score for IC Group, Mean Change | FM Change (TR-IC), Difference Between Groups (95% CI)b | ||
|---|---|---|---|---|---|
| TR | IC | Total | |||
| Primary analysis | |||||
| ITT with multiple imputation of missing outcomes | 62 | 62 | 124 | 8.23 | 0.06 (−2.14 to 2.26) |
| Secondary analyses | |||||
| ITT with substitution of “worst-best-case” missing outcomes | 62 | 62 | 124 | 8.58 | −0.19 (−2.29 to 1.92) |
| Complete case ITT | 59 | 55 | 114 | 8.36 | 0.00 (−2.27 to 2.27) |
| Complete case PP | 58 | 55 | 113 | 8.36 | −0.15 (−2.41 to 2.10) |
Abbreviations: FM, Fugl-Meyer; IC, in-clinic; ITT, intent to treat; PP, per protocol; TR, telerehabilitation.
Noninferiority margin is 30% of mean change in FM score for the IC group. Data are from baseline to 30 days after therapy.
Covariate adjusted.
In an additional assessment of the noninferiority margin, the estimated difference between the mean FM score changes for patients in the TR group and the mean of 70% of FM score changes for patients in the IC group was 2.38 points (95% CI, 0.50-4.26; P = .01). That is, the mean FM score change for patients in the TR group exceeded a 30% reduction in the mean FM score change for patients in the IC group, again indicating that TR was not inferior to IC therapy on the primary end point (eTable 10 in Supplement 2). Findings with secondary outcomes were largely concordant: Box and Blocks Test scores increased by 9.5 (P < .001) in the TR group and by 8.8 (P < .001) in the IC group and indicated noninferiority of TR therapy. Stroke Impact Scale hand motor domain scores increased by 23.7 (P < .001) in the TR group and by 29.2 (P < .001) in the IC group, although noninferiority was not demonstrated with this outcome (eResults in Supplement 2). Fugl-Meyer arm motor score gains did not differ among patients with aphasia (n = 39) vs without aphasia (n = 75) (unadjusted mean [SD] FM score change, 9.46 [7.06] vs 7.40 [6.65]; P = .13).
When examining only the 86 patients enrolled 90 days or more after stroke, the unadjusted mean (SD) change in FM score from baseline to 30-day follow-up was 7.39 (6.15) points for the TR group and 6.63 (7.22) points for the IC group. Change for patients enrolled less than 90 days after stroke was higher (mean [SD], 9.28 [8.12] points for the TR group and 11.40 [5.69] points for the IC group), which is not surprising given that some degree of spontaneous recovery is common during this time interval. For both time intervals, FM gains were significant in both groups (P < .001), and group differences were not significant.
The number of arm movement repetitions during 36 TR treatment sessions was calculated for a convenience sample of 4 patients, using actual use counts for games where these were available, otherwise using conservative estimates. Values ranged from 26 452 to 47 253, for a mean of 37 125 or 1031 per day.
Stroke Education
A stroke knowledge examination score was available at follow-up for 112 patients. At screening, patients in the TR group answered a mean of 22.4 of 30 (74.7%) questions on the stroke knowledge examination correctly and patients in the IC group answered a mean of 22.8 of 30 (76.0%) questions correctly. When retested at the posttherapy visit, both groups showed significant improvement, correctly answering a mean additional 3.3 of 30 questions (11.0%) in the TR group and 2.5 of 30 questions (8.3%) in the IC group (P < .001; P = .20 for estimated difference in covariate-adjusted group mean change).
Motivation
Consequence-related motivation at baseline did not differ between groups (mean [SD] Optimization in Primary and Secondary Control scale score, 4.78 [0.58] in the TR group vs 4.88 [0.57] in the IC group; P = .35), reflecting similar dedication to treatment goals. Activity-inherent motivation from baseline to end of therapy (eTable 8 in Supplement 2) was 0.47 points higher in the IC group compared with the TR group (P = .008), indicating larger boosts in enjoyment in the IC group. Activity-inherent motivation was related to treatment-related arm motor gains, with adjusted mean change in FM score being 0.97 points larger for each additional point increase in the Physical Activity Enjoyment Scale score from baseline to end of therapy (95% CI, 0.02-1.92; P = .046), a finding that did not differ across treatment groups.
Patients rated the experience of trial participation favorably, providing high scores on the 70-point Patient Satisfaction Questionnaire (eTable 9 in Supplement 2), with those in the IC group reporting slightly higher satisfaction at the end of therapy than those in the TR group (mean [SD], 55.2 [7.7] vs 58.5 [8.0]; P = .02).
Discussion
Most patients have arm deficits after stroke and do not fully recover. Our study found that a 6-week course of daily home-based TR is safe, is rated favorably by patients, is associated with excellent treatment adherence, and produces substantial gains in arm function that were not inferior to dose-matched interventions delivered in the clinic.
Both groups improved with treatment, with unadjusted mean FM score gains of 7.86 to 8.36 points, values that substantially exceed the 5.25-point minimal clinically important difference for FM in chronic stroke.20 More important, benefits were substantial whether treatment was initiated early (<90 days) or late (≥90 days) after stroke. Mean values for FM score gains among patients 90 days or more after stroke were 6.6 to 7.4 points, well above the minimal clinically important difference, indicating that behavioral gains are likely attributable to the intervention rather than spontaneous recovery, as motor recovery plateaus before 90 days after stroke.21 In an era when prescribed doses of poststroke rehabilitation therapy are declining,22 adversely affecting patient outcomes,23 these and prior24,25 findings suggest that outcomes could be improved for many patients who have experienced a stroke if larger doses of rehabilitation therapy were prescribed.
In-clinic therapy was comparably efficacious with TR therapy as provided in this study for improving arm function. Six weeks of therapy produced a 7.86-point FM score gain in patients in the TR group 90 days or more after stroke, larger than the 4.8-point increase found after 4 weeks of therapy in a prior study of TR in patients with chronic stroke.10 The observation that larger behavioral gains are achieved with a higher dose of TR is consistent with a meta-analysis that found higher rehabilitation therapy doses to be associated with better behavioral outcomes.5
Preclinical studies indicate that hundreds of limb movements per day are needed to achieve optimal motor cortex plasticity after stroke.26 A convenience sample of individuals in the TR group was found to have performed 1031 arm movement repetitions per day, suggesting that TR may have value for maximizing useful brain plasticity after stroke,7 especially given that the number of arm movement repetitions during standard of care therapy is a mean of 32 per session.6
Knowledge about stroke27 and secondary stroke prevention28 are often deficient among patients who have experienced stroke. Optimizing medical status can improve functional status and prevent secondary stroke, making patient education a key strategy toward effective stroke rehabilitation. Here, daily stroke education significantly improved stroke knowledge, and results were comparable between groups. Telerehabilitation is ideally suited to integrate education with activity-based goals.29
Effectiveness of rehabilitation therapy after stroke is linked to high patient motivation.30 Maintaining motivation with rehabilitation is challenging, however, with rates of nonadherence up to 70%, especially for unsupervised traditional home rehabilitation activities.31 Current enrollees had high dedication to treatment goals (Optimization in Primary and Secondary Control scale scores) and enjoyed therapy (positive change in Physical Activity Enjoyment Scale score). In the TR group, this finding might be attributable to design features including convenience, ease of use, frequent interaction with clinicians, multiple means of providing patient feedback,32,33 using a behavioral contract14 and games to drive adherence,34,35 using several input devices to practice movement,36 and using the TR system to generate appointment reminders.10 Patients in the IC group had slightly higher activity-inherent motivation and satisfaction with therapy, findings that might suggest a preference for in-person human contact or for longer patient-therapist interactions, which were 30 minutes for the TR group vs 70 minutes for the IC group during supervised sessions. This same preference might have contributed to the finding that gains in Stroke Impact Scale hand motor domain scores, a patient-reported subjective measure of participation, while substantial and largely comparable across groups, did not demonstrate noninferiority of TR therapy. Adherence was high in both groups, ranging from 93.4% (IC group) to 98.3% (TR group); the high IC group P values exceed usual estimates,31 making the extent to which current results are generalizable uncertain.
Limitations
This study has some limitations. It was focused on arm motor deficits, and while these are common after stroke, there are many other deficits that can also benefit from high-dose rehabilitation therapy, such as deficits in leg motor function or language. Telehealth methods of care delivery can save time and money, but no economic analysis was performed in the current trial. Performances on the stroke education quiz were high in both groups at baseline, blunting our ability to detect a difference between groups in gains in stroke knowledge over time.
Conclusions
Our results support the study hypothesis that TR is not inferior to IC therapy for improving arm motor function and stroke knowledge. The extent to which current findings are generalizable requires further study (eg, by evaluating improvements with other types of TR or other dosing schedules). Higher activity-inherent motivation and satisfaction in the IC group suggest areas for improving TR therapy, possibly by increasing time spent interacting with a therapist. A TR approach may be useful for studying effects of prolonged, intensive rehabilitation interventions, although an economic analysis is needed to understand comparative costs of TR vs IC therapy. Other behaviors affected by stroke, such as language and memory, could be targets for future TR therapies, separately or in combination with motor therapy. Current results underscore the importance of maintaining a licensed therapist’s involvement during TR (eg, patients in the TR group reported arm and shoulder pain as often as those in the IC group did). The current telehealth platform contained 12 forms of input device, enabling digital phenotyping37 through measurement of many poststroke behaviors, including movement, communication, and mood. The US Bipartisan Budget Act of 2018 expanded telehealth benefits; eventually, home-based TR may play an ascendant role for improving patient outcomes.
Trial Protocol
eTable 1. Complete Eligibility Criteria
eTable 2. Data Collection Schedule
eTable 3. The 88 Exercises for IC and TR Groups
eTable 4. Games and Their Adjustable Features
eTable 5. Usage Statistics for Each of the 25 Games in the TR Group
eTable 6. Usage Statistics for Each of the Input Devices in the TR Group
eTable 7. Rehabilitation Therapy Received Outside of Study Procedures
eTable 8. Activity-Inherent Motivation (Change in PACES Scores)
eTable 9. Patient Satisfaction Questionnaire Scores
eTable 10. Additional Evaluation Method for Assessing Non-Inferiority
eMethods.
eResults.
Data Sharing Statement
References
- 1.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: findings from the Atherosclerosis Risk in Communities Study. Stroke. 2002;33(11):2718-2721. doi: 10.1161/01.STR.0000035286.87503.31 [DOI] [PubMed] [Google Scholar]
- 2.Winstein CJ, Stein J, Arena R, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 3.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44(4):1111-1116. doi: 10.1161/STROKEAHA.111.674671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyller TB, Sveen U, Sødring KM, Pettersen AM, Bautz-Holter E. Subjective well-being one year after stroke. Clin Rehabil. 1997;11(2):139-145. doi: 10.1177/026921559701100207 [DOI] [PubMed] [Google Scholar]
- 5.Lohse KR, Lang CE, Boyd LA. Is more better? using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45(7):2053-2058. doi: 10.1161/STROKEAHA.114.004695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang CE, Macdonald JR, Reisman DS, et al. . Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692-1698. doi: 10.1016/j.apmr.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer SC, Sur M, Dobkin BH, et al. . Harnessing neuroplasticity for clinical applications. Brain. 2011;134(Pt 6):1591-1609. doi: 10.1093/brain/awr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis GN, Rosie JA. Virtual reality games for movement rehabilitation in neurological conditions: how do we meet the needs and expectations of the users? Disabil Rehabil. 2012;34(22):1880-1886. doi: 10.3109/09638288.2012.670036 [DOI] [PubMed] [Google Scholar]
- 9.Richmond T, Peterson C, Cason J, et al. . American Telemedicine Association’s principles for delivering telerehabilitation services. Int J Telerehabil. 2017;9(2):63-68. doi: 10.5195/IJT.2017.6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodakian L, McKenzie AL, Le V, et al. . A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. 2017;31(10-11):923-933. doi: 10.1177/1545968317733818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.See J, Dodakian L, Chou C, et al. . A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27(8):732-741. doi: 10.1177/1545968313491000 [DOI] [PubMed] [Google Scholar]
- 12.Kendzierski D, Morganstein MS. Test, revision, and cross-validation of the Physical Activity Self-Definition Model. J Sport Exerc Psychol. 2009;31(4):484-504. doi: 10.1123/jsep.31.4.484 [DOI] [PubMed] [Google Scholar]
- 13.Heckhausen J, Schulz R, Wrosch C. Developmental regulation in adulthood: Optimization in Primary and Secondary Control–a multiscale questionnaire (OPS-Scales) In: Heckhausen J, Dweck C, eds. Motivation and Self-Regulation Across the Life Span. New York, NY: Cambridge University; 1998:50-77. doi: 10.1017/CBO9780511527869.004 [DOI] [Google Scholar]
- 14.Wolf SL, Winstein CJ, Miller JP, et al. ; EXCITE Investigators . Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095-2104. doi: 10.1001/jama.296.17.2095 [DOI] [PubMed] [Google Scholar]
- 15.Lang C, Birkenmeier R. Upper-Extremity Task-Specific Training After Stroke or Disability. Bethesda, MD: AOTA Press; 2013. [Google Scholar]
- 16.Winstein CJ, Wolf SL, Dromerick AW, et al. ; Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE) Investigative Team . Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. JAMA. 2016;315(6):571-581. doi: 10.1001/jama.2016.0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirth LA, Breiner J. Jeopardy: using a familiar game to teach health. J Sch Health. 1997;67(2):71-74. doi: 10.1111/j.1746-1561.1997.tb06304.x [DOI] [PubMed] [Google Scholar]
- 18.Zhao W, Hill MD, Palesch Y. Minimal sufficient balance—a new strategy to balance baseline covariates and preserve randomness of treatment allocation. Stat Methods Med Res. 2015;24(6):989-1002. doi: 10.1177/0962280212436447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791-798. doi: 10.2522/ptj.20110009 [DOI] [PubMed] [Google Scholar]
- 21.Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75(4):394-398. doi: 10.1016/0003-9993(94)90161-9 [DOI] [PubMed] [Google Scholar]
- 22.Ottenbacher KJ, Smith PM, Illig SB, Linn RT, Ostir GV, Granger CV. Trends in length of stay, living setting, functional outcome, and mortality following medical rehabilitation. JAMA. 2004;292(14):1687-1695. doi: 10.1001/jama.292.14.1687 [DOI] [PubMed] [Google Scholar]
- 23.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741-754. doi: 10.1016/S1474-4422(09)70150-4 [DOI] [PubMed] [Google Scholar]
- 24.Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354(9174):191-196. doi: 10.1016/S0140-6736(98)09477-X [DOI] [PubMed] [Google Scholar]
- 25.Duncan PW, Sullivan KJ, Behrman AL, et al. ; LEAPS Investigative Team . Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026-2036. doi: 10.1056/NEJMoa1010790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffers MS, Karthikeyan S, Gomez-Smith M, et al. . Does stroke rehabilitation really matter? part b: an algorithm for prescribing an effective intensity of rehabilitation. Neurorehabil Neural Repair. 2018;32(1):73-83. doi: 10.1177/1545968317753074 [DOI] [PubMed] [Google Scholar]
- 27.Zerwic J, Hwang SY, Tucco L. Interpretation of symptoms and delay in seeking treatment by patients who have had a stroke: exploratory study. Heart Lung. 2007;36(1):25-34. doi: 10.1016/j.hrtlng.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 28.Qureshi AI, Suri MF, Guterman LR, Hopkins LN. Ineffective secondary prevention in survivors of cardiovascular events in the US population: report from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2001;161(13):1621-1628. doi: 10.1001/archinte.161.13.1621 [DOI] [PubMed] [Google Scholar]
- 29.Demiris G, Shigaki CL, Schopp LH. An evaluation framework for a rural home-based telerehabilitation network. J Med Syst. 2005;29(6):595-603. doi: 10.1007/s10916-005-6127-z [DOI] [PubMed] [Google Scholar]
- 30.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693-1702. doi: 10.1016/S0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- 31.McLean SM, Burton M, Bradley L, Littlewood C. Interventions for enhancing adherence with physiotherapy: a systematic review. Man Ther. 2010;15(6):514-521. doi: 10.1016/j.math.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 32.Jimison H, Gorman P, Woods S, et al. . Barriers and Drivers of Health Information Technology Use for the Elderly, Chronically Ill, and Underserved. AHRQ Publication No. 09-E004. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PMC free article] [PubMed] [Google Scholar]
- 33.Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25(9):788-798. doi: 10.1177/1545968311425908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brox E, Fernandez-Luque L, Tøllefsen T. Healthy gaming—video game design to promote health. Appl Clin Inform. 2011;2(2):128-142. doi: 10.4338/ACI-2010-10-R-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieberman D. Designing serious games for learning and health in informal and formal settings In: Ritterfeld M, Vorderer P, eds. Serious Games: Mechanisms and Effects. New York, NY: Routledge; 2009:117-130. [Google Scholar]
- 36.Miller EL, Murray L, Richards L, et al. ; American Heart Association Council on Cardiovascular Nursing and the Stroke Council . Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke. 2010;41(10):2402-2448. doi: 10.1161/STR.0b013e3181e7512b [DOI] [PubMed] [Google Scholar]
- 37.Insel TR. Digital phenotyping: technology for a new science of behavior. JAMA. 2017;318(13):1215-1216. doi: 10.1001/jama.2017.11295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Complete Eligibility Criteria
eTable 2. Data Collection Schedule
eTable 3. The 88 Exercises for IC and TR Groups
eTable 4. Games and Their Adjustable Features
eTable 5. Usage Statistics for Each of the 25 Games in the TR Group
eTable 6. Usage Statistics for Each of the Input Devices in the TR Group
eTable 7. Rehabilitation Therapy Received Outside of Study Procedures
eTable 8. Activity-Inherent Motivation (Change in PACES Scores)
eTable 9. Patient Satisfaction Questionnaire Scores
eTable 10. Additional Evaluation Method for Assessing Non-Inferiority
eMethods.
eResults.
Data Sharing Statement