Key Points
Question
Is P2Y12 inhibitor monotherapy for 9 months after 3 months of dual antiplatelet therapy (DAPT) noninferior to 12 months of DAPT in patients undergoing percutaneous coronary intervention?
Findings
In this randomized clinical trial including 2993 patients, the rate of all-cause death, myocardial infarction, or stroke at 12 months was 2.9% with P2Y12 inhibitor monotherapy and 2.5% for patients receiving DAPT. The 1-sided confidence limit of this difference was within the noninferiority margin of 1.8%.
Meaning
Although in this study P2Y12 inhibitor monotherapy after a short duration of DAPT resulted in a noninferior rate of major cardiovascular events compared with prolonged DAPT, further research is needed in other populations.
Abstract
Importance
Data on P2Y12 inhibitor monotherapy after short-duration dual antiplatelet therapy (DAPT) in patients undergoing percutaneous coronary intervention are limited.
Objective
To determine whether P2Y12 inhibitor monotherapy after 3 months of DAPT is noninferior to 12 months of DAPT in patients undergoing PCI.
Design, Setting, and Participants
The SMART-CHOICE trial was an open-label, noninferiority, randomized study that was conducted in 33 hospitals in Korea and included 2993 patients undergoing PCI with drug-eluting stents. Enrollment began March 18, 2014, and follow-up was completed July 19, 2018.
Interventions
Patients were randomly assigned to receive aspirin plus a P2Y12 inhibitor for 3 months and thereafter P2Y12 inhibitor alone (n = 1495) or DAPT for 12 months (n = 1498).
Main Outcomes and Measures
The primary end point was major adverse cardiac and cerebrovascular events (a composite of all-cause death, myocardial infarction, or stroke) at 12 months after the index procedure. Secondary end points included the components of the primary end point and bleeding defined as Bleeding Academic Research Consortium type 2 to 5. The noninferiority margin was 1.8%.
Results
Among 2993 patients who were randomized (mean age, 64 years; 795 women [26.6%]), 2912 (97.3%) completed the trial. Adherence to the study protocol was 79.3% of the P2Y12 inhibitor monotherapy group and 95.2% of the DAPT group. At 12 months, major adverse cardiac and cerebrovascular events occurred in 42 patients in the P2Y12 inhibitor monotherapy group and in 36 patients in the DAPT group (2.9% vs 2.5%; difference, 0.4% [1-sided 95% CI, –∞% to 1.3%]; P = .007 for noninferiority). There were no significant differences in all-cause death (21 [1.4%] vs 18 [1.2%]; hazard ratio [HR], 1.18; 95% CI, 0.63-2.21; P = .61), myocardial infarction (11 [0.8%] vs 17 [1.2%]; HR, 0.66; 95% CI, 0.31-1.40; P = .28), or stroke (11 [0.8%] vs 5 [0.3%]; HR, 2.23; 95% CI, 0.78-6.43; P = .14) between the 2 groups. The rate of bleeding was significantly lower in the P2Y12 inhibitor monotherapy group than in the DAPT group (2.0% vs 3.4%; HR, 0.58; 95% CI, 0.36-0.92; P = .02).
Conclusions and Relevance
Among patients undergoing percutaneous coronary intervention, P2Y12 inhibitor monotherapy after 3 months of DAPT compared with prolonged DAPT resulted in noninferior rates of major adverse cardiac and cerebrovascular events. Because of limitations in the study population and adherence, further research is needed in other populations.
Trial Registration
ClinicalTrials.gov Identifier: NCT02079194
This randomized noninferiority trial compares the effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy (DAPT) on cardiac and cerebrovascular events in patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents.
Introduction
Current standard antiplatelet therapy after percutaneous coronary intervention (PCI) with drug-eluting stents is dual antiplatelet therapy (DAPT) with aspirin plus a P2Y12 inhibitor followed by aspirin monotherapy.1,2 However, the optimal duration of DAPT after PCI remains controversial. Although 3 or 6 months of DAPT followed by aspirin monotherapy was comparable to 12 months of DAPT in several randomized studies, such as the OPTIMIZE and EXCELLENT trials,3,4 a short duration of DAPT was associated with an increased risk of myocardial infarction and stent thrombosis in meta-analyses.5,6 Conversely, prolonged DAPT increases the risk of bleeding, which offsets the benefit from reducing recurrent ischemic events.5,7,8 Therefore, neither prolonged DAPT nor short-duration DAPT followed by aspirin monotherapy is fully satisfactory. To develop novel antiplatelet strategies that maintain efficacy for ischemic events while reducing the bleeding risk after PCI is of paramount importance.
P2Y12 inhibitor monotherapy has recently been suggested as a new alternative antiplatelet strategy in patients with atherosclerotic cardiovascular disease.9 Clopidogrel reduced the risk of subsequent ischemic events with a similar risk of bleeding compared with aspirin in patients with atherosclerotic cardiovascular disease or those undergoing PCI.10,11 Compared with DAPT, clopidogrel monotherapy was not associated with an increased thrombotic risk in high-risk patients with recent ischemic stroke or transient ischemic attack.12 Moreover, several studies reported that clopidogrel monotherapy conferred a lower risk of bleeding than DAPT.12,13 These results suggest that P2Y12 inhibitor monotherapy may be comparable to DAPT for the prevention of recurrent ischemic events, with a lower risk of bleeding in patients undergoing PCI. Therefore, the Smart Angioplasty Research Team: Comparison Between P2Y12 Antagonist Monotherapy vs Dual Antiplatelet Therapy in Patients Undergoing Implantation of Coronary Drug-Eluting Stents (SMART-CHOICE) trial sought to compare P2Y12 inhibitor monotherapy after 3 months of DAPT with 12 months of DAPT in patients receiving current-generation drug-eluting stents.
Methods
Study Design
This trial was an investigator-initiated, multicenter, open-label, noninferiority, randomized study performed at 33 sites in Korea. The trial was designed by the steering committee and was coordinated by the Academic Clinical Research Organization of Samsung Medical Center (Seoul, Korea). The open-label design was selected because of limited funding; therefore, we took several precautions to minimize the possibility of bias. First, to prevent crossovers from the allocated treatment to the alternative treatment, we emphasized the importance of adherence to the protocol to the investigators during trial preparation and throughout the duration of the study via newsletters and telephone communications. Second, we regularly monitored adherence to the protocol of study participants and investigators. Third, an independent clinical event adjudication committee, whose members were unaware of the study-group assignments, adjudicated all the clinical outcomes. The institutional review board at each participating center approved the trial protocol. All participants provided written informed consent. The independent data and safety monitoring board oversaw the safety of the trial. The full protocol and statistical analysis plan for this trial are available in Supplement 1. The rationale and design of this study have been previously published.14
Eligible patients were aged 20 years or older and had 1 or more coronary artery stenoses of 50% or greater in a native coronary artery with visually estimated diameter of 2.25 mm or greater and 4.25 mm or smaller amenable to stent implantation, and underwent PCI. Patients with known hypersensitivity or contraindication to aspirin, clopidogrel, prasugrel, ticagrelor, everolimus, or sirolimus were excluded. Additional exclusion criteria included hemodynamic instability or cardiogenic shock; active pathologic bleeding, including gastrointestinal or genitourinary bleeding; drug-eluting stent implantation within 12 months before the index procedure; women of childbearing potential; noncardiac comorbid conditions with a life expectancy less than 2 years; or conditions that may result in protocol nonadherence. A complete list of the inclusion and exclusion criteria is provided in eTable 1 in Supplement 2.
Randomization and Study Procedures
Patients were randomly assigned to the P2Y12 inhibitor monotherapy group (aspirin plus a P2Y12 inhibitor for 3 months and thereafter a P2Y12 inhibitor alone) or to the DAPT group (aspirin plus a P2Y12 inhibitor for at least 12 months) in a 1:1 ratio. Enrollment and random assignment were conducted at the index procedure or at a follow-up visit within 3 months after the index procedure. Randomization was performed with a web-based response system (http://www.ecrf.kr/smartchoice) in blocks of 4 and was stratified by clinical presentation (stable ischemic heart disease or acute coronary syndrome), enrolling center, type of P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor), and type of stent used. To minimize the bias from different stent devices, the stents used were limited to cobalt-chromium everolimus-eluting stents (Xience Prime, Xience Expedition, or Xience Alpine; Abbott Vascular), platinum-chromium everolimus-eluting stents (Promus Element, Promus Premier, or SYNERGY; Boston Scientific), or sirolimus-eluting stents with biodegradable polymer (Orsiro; Biotronik). For each patient, all lesions had to be treated with the identical type of stent; however, other stents were allowed in case of device failure or situations in which the operators decided otherwise, considering the best interests of the patient.
Percutaneous coronary intervention was conducted according to standard techniques. The length and diameter of the stent were not restricted. Intravascular imaging or fractional flow reserve was also conducted according to the operators’ discretion. All patients received 300 mg of aspirin and a 300- or 600-mg clopidogrel loading dose orally at least 12 hours before PCI, unless they had previously received these antiplatelet medications. However, if administration of a loading dose was not possible 12 hours in advance, a 600-mg loading dose of clopidogrel was given as early as possible before intervention. For patients with acute coronary syndrome, 60 mg prasugrel or 180 mg ticagrelor as well as clopidogrel was used. After the procedure, patients received DAPT with aspirin 100 mg once daily plus clopidogrel 75 mg once daily or prasugrel 10 mg once daily or ticagrelor 90 mg twice daily for 3 months in both groups. The administration of aspirin was stopped at 3 months after the index procedure in the P2Y12 inhibitor monotherapy group but was continued indefinitely in the DAPT group. A P2Y12 inhibitor was prescribed continuously in both groups.
It was recommended that all patients receive optimal pharmacologic therapy, including statins, β-blockers, or renin-angiotensin system blockade, if indicated, following clinical guidelines.15,16 Clinical follow-up was performed at 3, 6, and 12 months after index PCI. At follow-up, data about patients’ clinical status, all interventions received, outcome events, and adverse events were recorded. In particular, information on the use of aspirin or a P2Y12 inhibitor was assessed at each follow-up. Patients who discontinued antiplatelet therapy as a result of clinically significant active bleeding or for other procedures were monitored carefully for cardiac events, and, once they were stabilized, their allocated antiplatelet therapy was restarted as soon as possible.
Study End Points
The primary end point was major adverse cardiac and cerebrovascular events, defined as a composite of all-cause death, myocardial infarction, or stroke at 12 months after the index procedure. Secondary end points included the components of the primary end point, cardiac death, target lesion revascularization, target vessel revascularization, any revascularization, stent thrombosis, Bleeding Academic Research Consortium bleeding type of at least 2 or 3, and a composite of death, myocardial infarction, cerebrovascular event, or any revascularization at 12 months after the index procedure, and each component of primary and secondary end points at 2 and 3 years.
All deaths were considered cardiac unless a definite noncardiac cause could be established. Myocardial infarction was defined as elevated cardiac enzyme levels (cardiac troponin or myocardial band fraction of creatine kinase) above the upper reference limit with ischemic symptoms or electrocardiographic findings indicative of ischemia. However, periprocedural enzyme-level elevation within 48 hours after the index procedure without concomitant ischemic symptoms or electrocardiographic findings indicative of ischemia was excluded in the assessment of end points.17 Stroke was defined as any nonconvulsive focal or global neurologic deficit of abrupt onset lasting for more than 24 hours or leading to death, which was caused by ischemia or hemorrhage within the brain. Stent thrombosis was defined as definite or probable stent thrombosis according to the Academic Research Consortium classification.17 Bleeding was defined as Bleeding Academic Research Consortium type 2 to 5 bleeding.18 Major bleeding was defined as Bleeding Academic Research Consortium type 3, 4, and 5 bleeding. For a post hoc analysis, net adverse clinical and cerebral events were defined as major adverse cardiac and cerebrovascular events plus Bleeding Academic Research Consortium type 2 to 5 bleeding.
Statistical Analysis
The sample size was calculated for noninferiority comparison between P2Y12 inhibitor monotherapy and DAPT in regard to the primary outcome of major adverse cardiac and cerebrovascular events (statistical analysis plan in Supplement 1). According to data from previous trials, the event rate of major adverse cardiac and cerebrovascular events in the DAPT group at 12 months after the index procedure was estimated to be 4.0%.3,19 Selection of the noninferiority margin (ie, the limit below which the upper limit of a 1-sided 95% CI would consider monotherapy to be noninferior to DAPT) was complicated by the limited information available from existing trials at study design. We chose the noninferiority margin in accordance with clinical judgment and other relevant studies with a noninferiority design. There was consensus among the steering committee that the noninferiority margin should be less than a 50% increase compared with the expected event rate of the standard treatment group, and the noninferiority margin of 2 trials that were available up to that time was equivalent to a 40% increase in the expected event rate.3,20 It is generally desirable to choose a smaller value for the noninferiority margin, but the feasibility of study recruitment was another important consideration. Therefore, we chose the noninferiority margin of 1.8% that was equivalent to a 45% increase in the expected event rate. With a sampling ratio of 1:1 allowing for 2% attrition in each group during 12 months, a total of 3000 patients (1500 per group) would provide 80% power at a 1-sided type I error of 5%. If the upper limit of the 1-sided 95% CI of the difference were less than the prespecified noninferiority margin, P2Y12 inhibitor monotherapy would be considered noninferior to DAPT.
The analysis of the primary end point followed the intention-to-treat principle, with inclusion of all randomized patients according to original group allocation. A per-protocol analysis excluded patients who did not receive the assigned treatment, according to regular assessments of study participants every 3 months with time-to-event methods. Cumulative event rates were estimated with the Kaplan-Meier method and compared with log-rank tests. Analyses of secondary end points and subgroup analyses were performed with 2-tailed superiority hypothesis testing, with α = .05 and with 2-sided 95% CIs. A post hoc landmark analysis was performed with a landmark of aspirin discontinuation at 3 months. As a post hoc sensitivity analysis, repeated analysis using adherence as a time-varying exposure and a frailty term for site differences was performed. The proportion of patients with at least 1 missing datum was 1.7%, and no imputation methods were used to infer missing data of baseline variables. Patients who were lost to follow-up were censored at the time of the last known contact. Prespecified subgroups included acute coronary syndrome; diabetes mellitus; implanted stent type; type of P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor); chronic kidney disease, defined as estimated glomerular filtration rate less than 60 mL/min/m2; and multivessel PCI. Prespecified subgroup analyses of the primary end point were performed to evaluate the consistency of treatment effects of P2Y12 inhibitor monotherapy compared with DAPT, using Cox regression models with tests for interaction. Because of the potential for type I error caused by multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Categoric variables are presented as numbers and percentages and compared with the χ2 test or Fisher exact test. Continuous variables are presented as mean (SD) and compared with the t test. P values and CIs were 2-tailed except those for noninferiority testing of the primary end point. All analyses were performed with SAS version 9.2.
Results
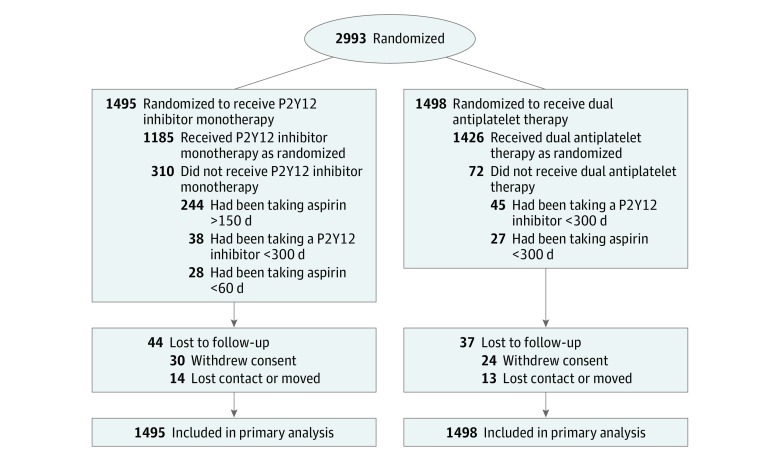
From March 18, 2014, to July 7, 2017, a total of 2993 patients were enrolled. Of these, 1495 patients were randomly assigned to receive P2Y12 inhibitor monotherapy and 1498 were randomly assigned to receive 12-month DAPT (Figure 1). Study participants were followed until the development of an event, death, or July 19, 2018, whichever came first. The median time from the index event to randomization was 1 day (interquartile range, 0 to 13) in the P2Y12 inhibitor monotherapy group and 1 day (interquartile range, 0 to 11) in the DAPT group. All baseline demographic, clinical, angiographic, and procedural characteristics were well balanced in the 2 groups (Table 1). The mean age was 64 years, 26.6% of all patients were women, 37.5% had diabetes mellitus, 58.2% presented with acute coronary syndrome, and 49.5% had multivessel disease. Medications at discharge from the index PCI were similar in both groups (eTable 2 in Supplement 2).
Figure 1. Randomization and Patient Flow in the Study Comparing P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy in Patients Undergoing Percutaneous Coronary Intervention.
Sites were not required to provide screening logs during the recruitment phase. Thus, the number of patients approached for participation is not available. Outcomes of patients who were lost to follow-up were included to the point of final contact. Their time-to-event measure was censored at the last contact date. There was no imputation of outcome events.
Table 1. Characteristics of Patients at Baseline.
| Characteristic | No. (%) | |
|---|---|---|
| P2Y12 Inhibitor Monotherapy (n = 1495) | Dual Antiplatelet Therapy (n = 1498) | |
| Age, mean (SD), y | 64.6 (10.7) | 64.4 (10.7) |
| Men | 1087 (72.7) | 1111 (74.2) |
| Women | 408 (27.3) | 387 (25.8) |
| Body mass index, mean (SD)a | 24.5 (3.1) | 24.7 (3.2) |
| Comorbidities | ||
| Hypertension | 921 (61.6) | 919 (61.3) |
| Dyslipidemia | 673 (45.1) | 679 (45.5) |
| Diabetes mellitus | 570 (38.2) | 552 (36.8) |
| Current smoking | 424 (28.4) | 367 (24.5) |
| Previous revascularization | 172 (11.5) | 177 (11.8) |
| Previous stroke | 99 (6.6) | 102 (6.8) |
| Previous myocardial infarction | 62 (4.1) | 65 (4.3) |
| Chronic renal failure | 44 (2.9) | 53 (3.5) |
| Left ventricular ejection fraction, mean (SD), % | 60.0 (10.9) | 59.9 (10.7) |
| Clinical presentation | ||
| Stable angina | 625 (41.8) | 625 (41.8) |
| Unstable angina | 467 (31.2) | 491 (32.8) |
| Non–ST-segment elevation myocardial infarction | 239 (16.0) | 230 (15.4) |
| ST-segment elevation myocardial infarction | 164 (11.0) | 150 (10.0) |
| Transradial approach | 1091 (73.0) | 1091 (72.8) |
| Multiple vessels disease | 749 (50.1) | 734 (49.0) |
| No. of lesion treated | 1849 | 1885 |
| Location of lesions | ||
| Left main | 23 (1.2) | 35 (1.9) |
| Left anterior descending artery | 903 (48.8) | 950 (50.4) |
| Left circumflex | 399 (21.6) | 376 (19.9) |
| Right coronary artery | 524 (28.3) | 524 (27.8) |
| Lesion complexity | ||
| Calcified | 235 (15.7) | 229 (15.3) |
| Bifurcation | 199 (13.3) | 181 (12.1) |
| Thrombotic | 110 (7.4) | 112 (7.5) |
| Use of intravascular ultrasonography | 372 (25.0) | 406 (27.2) |
| Treated lesions per patient | ||
| 1 | 1065 (71.2) | 1041 (69.5) |
| 2 | 329 (22.0) | 351 (23.4) |
| 3 | 86 (5.8) | 91 (6.1) |
| ≥4 | 15 (1.0) | 15 (1.0) |
| Multilesion intervention | 430 (28.8) | 457 (30.5) |
| Multivessel intervention | 337 (22.5) | 368 (24.6) |
| No. of stents per patient | ||
| 1 | 975 (65.2) | 978 (65.3) |
| 2 | 380 (25.4) | 374 (25.0) |
| 3 | 102 (6.8) | 115 (7.7) |
| ≥4 | 38 (2.6) | 31 (2.0) |
| Stent length per patient, mean (SD), mm | 38.0 (22.5) | 37.8 (22.9) |
| Type of drug-eluting stents | ||
| Cobalt-chromium everolimus eluting | 525 (35.1) | 526 (35.1) |
| Platinum-chromium everolimus eluting | 489 (32.7) | 478 (31.9) |
| Sirolimus-eluting with biodegradable polymer | 481 (32.2) | 491 (32.8) |
| Zotarolimus eluting | 0 | 1 (0.1) |
| Paclitaxel-cilostazol eluting | 0 | 1 (0.1) |
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Antiplatelet Therapy
Overall adherence to the study protocol was 79.3% in the P2Y12 inhibitor monotherapy group and 95.2% in the DAPT group. The rates of P2Y12 inhibitor use were similar in both groups: 96.4% at 6 months and 95.0% at 12 months in the P2Y12 inhibitor monotherapy group and 98.1% at 6 months and 96.6% at 12 months in the DAPT group. The median duration of aspirin was 96 days (interquartile range, 88-118 days) in the P2Y12 inhibitor monotherapy group and 365 days (interquartile range, 363-365) in the DAPT group. The proportion of patients receiving aspirin beyond 3 months in the P2Y12 inhibitor monotherapy group was 14.4% at 6 months and 8.9% at 12 months. Clopidogrel was used as the P2Y12 inhibitor in 1149 patients (76.9%) in the P2Y12 inhibitor monotherapy group and 1163 (77.6%) in the DAPT group. Potent P2Y12 inhibitors, prasugrel or ticagrelor, were used in 346 patients (23.1%) in the P2Y12 inhibitor monotherapy group and 335 (22.4%) in the DAPT group.
End Points
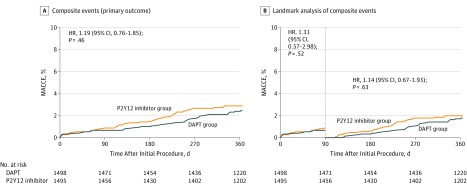
Follow-up for the primary end point was complete for 1451 (97.1%) of patients in the P2Y12 inhibitor monotherapy group and 1461 (97.5%) in the DAPT group. At 12 months, the primary end point of major adverse cardiac and cerebrovascular events occurred in 42 patients in the P2Y12 inhibitor monotherapy group and 36 in the DAPT group. Cumulative rates of major adverse cardiac and cerebrovascular events at 12 months were 2.9% for the P2Y12 inhibitor monotherapy group and 2.5% for the DAPT group (difference, 0.4% [1-sided 95% CI, –∞% to 1.3%]; P = .007 for noninferiority), meeting criteria for noninferiority of P2Y12 inhibitor monotherapy to DAPT (Table 2, Figure 2A; eFigure 1 in Supplement 2). In the per-protocol analysis, major adverse cardiac and cerebrovascular events occurred in 36 of 1185 patients in the P2Y12 inhibitor monotherapy group and 35 of 1426 in the DAPT group. Cumulative rates of major adverse cardiac and cerebrovascular events at 12 months were 3.1% for the P2Y12 inhibitor monotherapy group and 2.5% for the DAPT group (difference, 0.6% [1-sided 95% CI, –∞% to 1.5%]; P = .02 for noninferiority) (eTable 3 and eFigure 2 in Supplement 2), meeting criteria for noninferiority P2Y12 inhibitor monotherapy to DAPT in the per-protocol analysis.
Table 2. Outcomes at 12 Months.
| Outcome | No. (%) | Estimate of Difference, % (95% 1-Sided CI) | P Value | |
|---|---|---|---|---|
| P2Y12 Inhibitor Monotherapy (n = 1495)a | Dual Antiplatelet Therapy (n = 1498)a | |||
| Primary End Point | ||||
| MACCEb | 42 (2.9) | 36 (2.5) | 0.4 (−∞ to 1.3) | .007 (noninferiority) |
| Secondary End Points | Hazard Ratio (95% CI) | |||
| All-cause death | 21 (1.4) | 18 (1.2) | 1.18 (0.63 to 2.21) | .61 |
| Myocardial infarction | 11 (0.8) | 17 (1.2) | 0.66 (0.31 to 1.40) | .28 |
| Stroke | 11 (0.8) | 5 (0.3) | 2.23 (0.78 to 6.43) | .14 |
| Cardiac death | 11 (0.8) | 13 (0.9) | 0.86 (0.38 to 1.91) | .70 |
| Stent thrombosis | 3 (0.2) | 2 (0.1) | 1.51 (0.25 to 9.02) | .65 |
| Bleeding BARC type 2-5 | 28 (2.0) | 49 (3.4) | 0.58 (0.36 to 0.92) | .02 |
| Major bleedingc | 12 (0.8) | 14 (1.0) | 0.87 (0.40 to 1.88) | .72 |
| Post Hoc Analysis | ||||
| Death or myocardial infarction | 31 (2.1) | 32 (2.2) | 0.98 (0.60 to 1.61) | .94 |
| Cardiac death or myocardial infarction | 21 (1.5) | 27 (1.9) | 0.79 (0.45 to 1.39) | .50 |
| Net adverse clinical and cerebral eventsd | 65 (4.5) | 81 (5.6) | 0.81 (0.58 to 1.12) | .20 |
Abbreviations: BARC, Bleeding Academic Research Consortium; MACCE, major adverse cardiac and cerebrovascular events.
Data are presented for the intention-to-treat population. The percentages are Kaplan-Meier estimates.
A composite of all-cause mortality, myocardial infarction, or stroke.
BARC type 3 to 5 bleeding.
MACCE plus BARC type 2 to 5 bleeding.
Figure 2. Time-to-Event Curves for the Major Adverse Cardiovascular and Cerebrovascular Events and Landmark Analysis at 3 Months.
A, Results of the analysis of the primary end point of major adverse cardiovascular and cerebrovascular events (a composite of death, myocardial infarction, or stroke) at 12 months. B, Results of the landmark analysis at 3 months (the point after which one group received P2Y12 inhibitor only and the other received DAPT) for the primary end point. Event rates were based on Kaplan-Meier estimates in time-to-first-event analyses. Hazard ratios are for the patients in the P2Y12 inhibitor monotherapy group. DAPT indicates dual antiplatelet therapy; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular events. MACCE was defined as a composite of all-cause death, myocardial infarction, or stroke. The median length of patient follow-up was 365 days (25th and 75th percentile, 365 and 365) in the P2Y12 inhibitor monotherapy group and 365 days (25th and 75th percentile, 365 and 365) in the DAPT group.
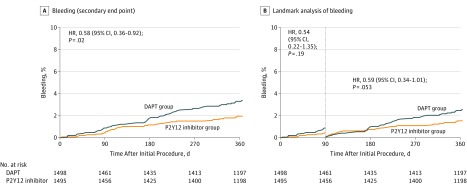
There were no significant differences in the cumulative rates of the components of the primary end point at 12 months for all-cause death, myocardial infarction, and stroke (Table 2; eFigures 3-5 in Supplement 2). The risk of stent thrombosis was not significantly different between the 2 groups (Table 2). The results from the per-protocol analysis were similar to those from the intention-to-treat analysis (eTable 3 in Supplement 2). A post hoc landmark analysis showed that the risk of major adverse cardiac and cerebrovascular events between 3 and 12 months was not significantly different between the groups (hazard ratio, 1.14; 95% CI, 0.67-1.93; P = .63) (Figure 2B). A post hoc analysis using adherence as a time-varying exposure and a frailty term for site differences gave similar results (hazard ratio, 1.26; 95% CI, 0.79-2.00; P = .33). The rate of bleeding was significantly lower in the P2Y12 inhibitor monotherapy group than in the DAPT group (2.0% vs 3.4%; hazard ratio, 0.58; 95% CI, 0.36-0.92; P = .02) (Table 2 and Figure 3A). There was no significant difference in the risk of bleeding between the groups in the post hoc 3-month landmark analysis (hazard ratio, 0.59; 95% CI, 0.34-1.01; P = .053) (Figure 3B). The rate of major bleeding did not differ significantly between the 2 groups (Table 2). In the per-protocol analysis, the risk of bleeding was significantly lower in the P2Y12 inhibitor monotherapy group than in the DAPT group (1.8% vs 3.1%; hazard ratio, 0.58; 95% CI, 0.34-0.97; P = .04) (eTable 3 and eFigure 2 in Supplement 2).
Figure 3. Time-to-Event Curves for the Bleeding and Landmark Analysis at 3 Months.
A, Results of the analysis of the bleeding at 12 months. B, Results of the landmark analysis at 3 months (the point after which one group received P2Y12 inhibitor only and the other received DAPT) for bleeding. Event rates were based on Kaplan-Meier estimates in time-to-first-event analyses. Hazard ratios are for the patients in the P2Y12 inhibitor monotherapy group. DAPT indicates dual antiplatelet therapy; HR, hazard ratio.
The treatment effects of the P2Y12 inhibitor monotherapy compared with DAPT were consistent across various subgroups for the major adverse cardiac and cerebrovascular events, including the subgroups according to clinical presentation (acute coronary syndrome vs stable ischemic heart disease) and type of P2Y12 inhibitors (eFigure 6 in Supplement 2). In post hoc subgroup analyses in regard to the risk of bleeding, the results were consistent across various subgroups (eFigure 7 in Supplement 2).
Discussion
In this randomized trial, P2Y12 inhibitor monotherapy after 3 months of DAPT was noninferior to 12-month DAPT for the primary end point of major adverse cardiac and cerebrovascular events at 12 months after the index procedure, and was associated with a lower rate of bleeding.
There are several plausible explanations for these results. First, aspirin might provide little additional inhibition of platelet aggregation in the presence of a P2Y12 inhibitor. P2Y12 receptor activation is important to platelet thromboxane A2 production, and P2Y12 inhibition can result in a substantial degree of inhibition of thromboxane A2.21,22 It was also reported that P2Y12 inhibitor monotherapy inhibited hemostatic system activation to a similar extent compared with DAPT.23 Second, the risk of bleeding was significantly lower with P2Y12 inhibitor monotherapy than with DAPT in the present study. Reduction of bleeding after PCI is of great importance because it has a strong relationship with subsequent all-cause mortality and major adverse cardiovascular events.24 In addition to bleeding-related death, interruption of antiplatelet therapy because of bleeding may be associated with an increase in thrombotic events. Third, second-generation drug-eluting stents with documented safety that significantly reduced stent thrombosis and myocardial infarction compared with the first-generation drug-eluting stents were exclusively used.6 In the present study, the rate of stent thrombosis was low and similar in both groups. Three-month DAPT in the P2Y12 inhibitor monotherapy group might be adequate to prevent stent thrombosis after implantation of current-generation drug-eluting stents.
In the GLOBAL LEADERS trial, the researchers compared ticagrelor plus aspirin for 1 month followed by ticagrelor alone for 23 months vs 12 months of standard DAPT followed by 12 months of aspirin alone in patients undergoing PCI.25 Although the trial was large and well conducted, generalizability is limited by the exclusive use of ticagrelor even in patients with stable ischemic heart disease. On the contrary, in this trial, there was no restriction on type of P2Y12 inhibitors and clinical presentation. As a result, clopidogrel was the predominant P2Y12 inhibitor in the patients in the trial. Response to clopidogrel shows wide individual variability, and there might be concerns that clopidogrel monotherapy is inadequate to prevent recurrent ischemic events in the substantial portion of patients with high platelet reactivity with clopidogrel.26,27 However, there was no significant interaction between type of P2Y12 inhibitors and the treatment effects of the 2 antiplatelet regimens on the major adverse cardiac and cerebrovascular events in the present study.
Limitations
This study has several limitations. First, the noninferiority margin of 1.8% (corresponding to a 45% increase with respect to the expected event rate and a 72% increase with respect to the observed event rate) was relatively wide and this study might be underpowered. However, the noninferiority margin of major trials on duration of DAPT was equivalent to a 20% to 67% increase in the expected event rate and a 47% to 133% increase in the observed event rate of the standard treatment group.3,4,20,28,29,30,31,32 Therefore, the noninferiority margin of this trial was not exceptionally wide, but was typical compared with that of the previous trials on DAPT duration. Second, the present study was an open-label trial, not placebo controlled, and was conducted in a low risk-population. Although several precautions were taken to maximize adherence and minimize treatment crossovers, the possibility of biases toward noninferiority originating from an open-label intervention or from low adherence in the monotherapy group could not be excluded. However, intention-to-treat and per-protocol analyses showed similar conclusions, suggesting that any potential biases caused by differential adherence and treatment crossover are likely small. Third, not all consecutive patients were considered for enrollment and the number of patients screened was not available. Selection bias for enrollment of patients with relatively low risk may limit application of the results of this study in other populations. In the SMART-DATE trial that compared 6-month DAPT with the conventional 12-month or longer duration of DAPT in patients with acute coronary syndrome, myocardial infarction significantly increased with 6-month DAPT.31 Although more than half of patients presented with acute coronary syndrome in this trial, the safety of P2Y12 inhibitor monotherapy after a short duration of DAPT in patients with a high-risk profile needs to be confirmed in a future study, such as the TWILIGHT trial.33
Conclusions
Among patients undergoing percutaneous coronary intervention, P2Y12 inhibitor monotherapy after 3 months of DAPT compared with prolonged DAPT resulted in noninferior rates of major adverse cardiac and cerebrovascular events. Because of limitations in the study population and adherence, further research is needed in other populations.
Trial protocol and SAP
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Discharge medication
eTable 3. Clinical outcomes by per-protocol analysis
eFigure 1. Treatment Difference for the Major Adverse Cardiovascular and Cerebrovascular Events
eFigure 2. Time-to-event curves for the end points in the per-protocol population
eFigure 3. Time-to-event curves and landmark analysis for all-cause death in the intention-to-treat population
eFigure 4. Time-to-event curves and landmark analysis for myocardial infarction in the intention-to-treat population
eFigure 5. Time-to-event curves and landmark analysis for stroke in the intention-to-treat population
eFigure 6. Subgroup Analyses of the Major Adverse Cardiovascular and Cerebrovascular Events (Primary End Point) at 12 Months
eFigure 7. Subgroup Analyses of BARC type 2-5 Bleeding at 12 Months
Data sharing statement
References
- 1.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115. doi: 10.1016/j.jacc.2016.03.513 [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 3.Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125(3):505-513. doi: 10.1161/CIRCULATIONAHA.111.059022 [DOI] [PubMed] [Google Scholar]
- 4.Feres F, Costa RA, Abizaid A, et al. ; OPTIMIZE Trial Investigators . Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310(23):2510-2522. doi: 10.1001/jama.2013.282183 [DOI] [PubMed] [Google Scholar]
- 5.Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385(9985):2371-2382. doi: 10.1016/S0140-6736(15)60263-X [DOI] [PubMed] [Google Scholar]
- 6.Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2015;65(23):2496-2507. doi: 10.1016/j.jacc.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 7.Giustino G, Baber U, Sartori S, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015;65(13):1298-1310. doi: 10.1016/j.jacc.2015.01.039 [DOI] [PubMed] [Google Scholar]
- 8.Palmerini T, Bacchi Reggiani L, Della Riva D, et al. Bleeding-related deaths in relation to the duration of dual-antiplatelet therapy after coronary stenting. J Am Coll Cardiol. 2017;69(16):2011-2022. doi: 10.1016/j.jacc.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 9.Capodanno D, Mehran R, Valgimigli M, et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018;15(8):480-496. doi: 10.1038/s41569-018-0049-1 [DOI] [PubMed] [Google Scholar]
- 10.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348(9038):1329-1339. doi: 10.1016/S0140-6736(96)09457-3 [DOI] [PubMed] [Google Scholar]
- 11.Park TK, Song YB, Ahn J, et al. Clopidogrel versus aspirin as an antiplatelet monotherapy after 12-month dual-antiplatelet therapy in the era of drug-eluting stents. Circ Cardiovasc Interv. 2016;9(1):e002816. doi: 10.1161/CIRCINTERVENTIONS.115.002816 [DOI] [PubMed] [Google Scholar]
- 12.Diener HC, Bogousslavsky J, Brass LM, et al. ; MATCH Investigators . Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331-337. doi: 10.1016/S0140-6736(04)16721-4 [DOI] [PubMed] [Google Scholar]
- 13.Hallas J, Dall M, Andries A, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. BMJ. 2006;333(7571):726. doi: 10.1136/bmj.38947.697558.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song YB, Oh SK, Oh JH, et al. Rationale and design of the comparison between a P2Y12 inhibitor monotherapy versus dual antiplatelet therapy in patients undergoing implantation of coronary drug-eluting stents (SMART-CHOICE): a prospective multicenter randomized trial. Am Heart J. 2018;197:77-84. doi: 10.1016/j.ahj.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 15.Valgimigli M, Frigoli E, Leonardi S, et al. ; MATRIX Investigators . Bivalirudin or unfractionated heparin in acute coronary syndromes. N Engl J Med. 2015;373(11):997-1009. doi: 10.1056/NEJMoa1507854 [DOI] [PubMed] [Google Scholar]
- 16.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 17.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 18.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 19.Valgimigli M, Campo G, Monti M, et al. ; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators . Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125(16):2015-2026. doi: 10.1161/CIRCULATIONAHA.111.071589 [DOI] [PubMed] [Google Scholar]
- 20.Kim BK, Hong MK, Shin DH, et al. ; RESET Investigators . A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor Zotarolimus-Eluting Stent Implantation). J Am Coll Cardiol. 2012;60(15):1340-1348. doi: 10.1016/j.jacc.2012.06.043 [DOI] [PubMed] [Google Scholar]
- 21.Armstrong PC, Dhanji AR, Tucker AT, Mitchell JA, Warner TD. Reduction of platelet thromboxane A2 production ex vivo and in vivo by clopidogrel therapy. J Thromb Haemost. 2010;8(3):613-615. doi: 10.1111/j.1538-7836.2009.03714.x [DOI] [PubMed] [Google Scholar]
- 22.Armstrong PC, Leadbeater PD, Chan MV, et al. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011;9(3):552-561. doi: 10.1111/j.1538-7836.2010.04160.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traby L, Kollars M, Kaider A, Eichinger S, Wolzt M, Kyrle PA. Effects of P2Y12 receptor inhibition with or without aspirin on hemostatic system activation: a randomized trial in healthy subjects. J Thromb Haemost. 2016;14(2):273-281. doi: 10.1111/jth.13216 [DOI] [PubMed] [Google Scholar]
- 24.Généreux P, Giustino G, Witzenbichler B, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66(9):1036-1045. doi: 10.1016/j.jacc.2015.06.1323 [DOI] [PubMed] [Google Scholar]
- 25.Vranckx P, Valgimigli M, Jüni P, et al. ; GLOBAL LEADERS Investigators . Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940-949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 26.Park KW, Jeon KH, Kang SH, et al. Clinical outcomes of high on-treatment platelet reactivity in Koreans receiving elective percutaneous coronary intervention (from results of the CROSS VERIFY study). Am J Cardiol. 2011;108(11):1556-1563. doi: 10.1016/j.amjcard.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 27.Tantry US, Bonello L, Aradi D, et al. ; Working Group on On-Treatment Platelet Reactivity . Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261-2273. doi: 10.1016/j.jacc.2013.07.101 [DOI] [PubMed] [Google Scholar]
- 28.Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64(20):2086-2097. doi: 10.1016/j.jacc.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 29.Gilard M, Barragan P, Noryani AAL, et al. 6- Versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol. 2015;65(8):777-786. doi: 10.1016/j.jacc.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 30.Schulz-Schüpke S, Byrne RA, Ten Berg JM, et al. ; Intracoronary Stenting and Antithrombotic Regimen: Safety and Efficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators . ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 2015;36(20):1252-1263. doi: 10.1093/eurheartj/ehu523 [DOI] [PubMed] [Google Scholar]
- 31.Hahn JY, Song YB, Oh JH, et al. ; SMART-DATE Investigators . 6-Month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018;391(10127):1274-1284. doi: 10.1016/S0140-6736(18)30493-8 [DOI] [PubMed] [Google Scholar]
- 32.Kedhi E, Fabris E, van der Ent M, et al. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): randomised, multicentre, non-inferiority trial. BMJ. 2018;363:k3793. doi: 10.1136/bmj.k3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baber U, Dangas G, Cohen DJ, et al. Ticagrelor with aspirin or alone in high-risk patients after coronary intervention: rationale and design of the TWILIGHT study. Am Heart J. 2016;182:125-134. doi: 10.1016/j.ahj.2016.09.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and SAP
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Discharge medication
eTable 3. Clinical outcomes by per-protocol analysis
eFigure 1. Treatment Difference for the Major Adverse Cardiovascular and Cerebrovascular Events
eFigure 2. Time-to-event curves for the end points in the per-protocol population
eFigure 3. Time-to-event curves and landmark analysis for all-cause death in the intention-to-treat population
eFigure 4. Time-to-event curves and landmark analysis for myocardial infarction in the intention-to-treat population
eFigure 5. Time-to-event curves and landmark analysis for stroke in the intention-to-treat population
eFigure 6. Subgroup Analyses of the Major Adverse Cardiovascular and Cerebrovascular Events (Primary End Point) at 12 Months
eFigure 7. Subgroup Analyses of BARC type 2-5 Bleeding at 12 Months
Data sharing statement