Key Points
Question
Do plasma levels of β-amyloid 42, β-amyloid 40, and tau detect cerebral β-amyloid status when measured using fully automated immunoassays?
Findings
In 2 cross-sectional studies, plasma β-amyloid 42 to β-amyloid 40 ratio, measured using immunoassay, accurately predicted cerebral β-amyloid status in all stages of Alzheimer disease in the BioFINDER cohort (n = 842) and in an independent validation cohort (n = 237). The diagnostic accuracy was further increased by analyzing APOE genotype.
Meaning
Blood-based β-amyloid 42 and β-amyloid 40 ratio together with APOE genotype may be used as prescreening tests in primary care and in clinical Alzheimer disease trials to lower the costs and number of positron emission tomography scans and lumbar punctures.
This corss-sectional diagnostic study evaluates the accuracy of fully automated plasma assays in measuring plasma β-amyloid and tau in patients with and without cognitive impairment in the Swedish BioFINDER study and an independent validation cohort.
Abstract
Importance
Accurate blood-based biomarkers for Alzheimer disease (AD) might improve the diagnostic accuracy in primary care, referrals to memory clinics, and screenings for AD trials.
Objective
To examine the accuracy of plasma β-amyloid (Aβ) and tau measured using fully automated assays together with other blood-based biomarkers to detect cerebral Aβ.
Design, Setting, and Participants
Two prospective, cross-sectional, multicenter studies. Study participants were consecutively enrolled between July 6, 2009, and February 11, 2015 (cohort 1), and between January 29, 2000, and October 11, 2006 (cohort 2). Data were analyzed in 2018. The first cohort comprised 842 participants (513 cognitively unimpaired [CU], 265 with mild cognitive impairment [MCI], and 64 with AD dementia) from the Swedish BioFINDER study. The validation cohort comprised 237 participants (34 CU, 109 MCI, and 94 AD dementia) from a German biomarker study.
Main Outcome and Measures
The cerebrospinal fluid (CSF) Aβ42/Aβ40 ratio was used as the reference standard for brain Aβ status. Plasma Aβ42, Aβ40 and tau were measured using Elecsys immunoassays (Roche Diagnostics) and examined as predictors of Aβ status in logistic regression models in cohort 1 and replicated in cohort 2. Plasma neurofilament light chain (NFL) and heavy chain (NFH) and APOE genotype were also examined in cohort 1.
Results
The mean (SD) age of the 842 participants in cohort 1 was 72 (5.6) years, with a range of 59 to 88 years, and 446 (52.5%) were female. For the 237 in cohort 2, mean (SD) age was 66 (10) years with a range of 23 to 85 years, and 120 (50.6%) were female. In cohort 1, plasma Aβ42 and Aβ40 predicted Aβ status with an area under the receiver operating characteristic curve (AUC) of 0.80 (95% CI, 0.77-0.83). When adding APOE, the AUC increased significantly to 0.85 (95% CI, 0.82-0.88). Slight improvements were seen when adding plasma tau (AUC, 0.86; 95% CI, 0.83-0.88) or tau and NFL (AUC, 0.87; 95% CI, 0.84-0.89) to Aβ42, Aβ40 and APOE. The results were similar in CU and cognitively impaired participants, and in younger and older participants. Applying the plasma Aβ42 and Aβ40 model from cohort 1 in cohort 2 resulted in slightly higher AUC (0.86; 95% CI, 0.81-0.91), but plasma tau did not contribute. Using plasma Aβ42, Aβ40, and APOE in an AD trial screening scenario reduced positron emission tomography costs up to 30% to 50% depending on cutoff.
Conclusions and Relevance
Plasma Aβ42 and Aβ40 measured using Elecsys immunoassays predict Aβ status in all stages of AD with similar accuracy in a validation cohort. Their accuracy can be further increased by analyzing APOE genotype. Potential future applications of these blood tests include prescreening of Aβ positivity in clinical AD trials to lower the costs and number of positron emission tomography scans or lumbar punctures.
Introduction
A key hallmark of Alzheimer disease (AD) is the gradual accumulation of β-amyloid (Aβ) in the brain, which starts decades before the onset of cognitive symptoms. Detection of abnormal Aβ accumulation (Aβ positivity) may support the clinical diagnosis of AD1,2 and is essential for including participants in clinical AD trials targeting Aβ.3 β-Amyloid can be detected in vivo using positron emission tomography (PET) with ligands that bind to Aβ fibrils or by measuring the levels of the peptide Aβ1-42 (Aβ42) in cerebrospinal fluid (CSF).4 Alzheimer disease affects 1 in 10 persons aged 65 years and older and is expected to affect more than 100 million people by 2050.5,6 The costs and limited access to PET or CSF analysis may restrict their use to a minority of cases. There is thus a great need for readily available methods that can detect brain Aβ, and perhaps the most desirable goal has been to establish blood-based biomarkers of Aβ. Many candidate blood biomarkers have failed in replication studies,7,8 but somewhat promising results have been seen for plasma tau, neurofilament light chain (NFL), and combinations of Aβ42 and Aβ40.9,10,11,12,13,14,15,16,17 Although there are diagnostic inconsistencies regarding the plasma Aβ42/Aβ40 ratio in older studies,18 more recent studies have demonstrated that it correlates with brain Aβ and can differentiate patients with AD from healthy control participants.12,13 Most recently, 2 independent groups demonstrated improved accuracy for plasma Aβ42/Aβ40 using immunoprecipitation–mass spectrometry assays.19,20 Although these studies are promising and show the potential of plasma Aβ as a true AD biomarker, they are costly and need extensive development before they can be implemented in primary care or in large screenings where cost-effective, fully automated, high-throughput, and highly reliable analysis methods are needed.
Measuring plasma Aβ presents the same challenges as measuring CSF Aβ in that several analysis methods exist and unified cutoffs have been difficult to establish, even using the same assay, owing to high variability between laboratories and assay batches.21,22 Recently, fully automated immunoassays have been developed by several different vendors with improved reliability and precision for CSF Aβ and tau species.23,24,25 For example, for the Elecsys immunoassays (Roche Diagnostics), it has been shown that CSF cutoffs established in one European cohort could be applied to another independent cohort in the United States to determine amyloid PET status with high accuracy.24
Using these newly developed Elecsys assays for detection of Aβ42, Aβ40, and tau, our aims were to examine the accuracy of plasma Aβ42, Aβ40, and tau to estimate Aβ positivity, whether the accuracy could be improved by adding plasma neurofilament (light and heavy chain) and APOE genotype to the models, and how the Elecsys assays perform in an independent validation cohort.
Methods
Participants
The study population was included from the prospective Swedish BioFINDER Study, which enrolled participants between July 6, 2009, to February 11, 2015, from the southern part of Sweden. Of all 892 participants in BioFINDER’s control, mild cognitive symptoms, and AD cohorts, plasma samples were hemolyzed or not available in sufficient amount for 50 individuals. Thus, 842 participants could be included in the present study. They were classified as cognitively unimpaired26 (CU; 513 participants, of whom 195 had subjective cognitive decline)27; mild cognitive impairment28 (MCI; 265 participants); or AD dementia2 (64 participants). In subsample analyses, we grouped the population into CU and cognitively impaired (MCI + AD), because all participants with AD were Aβ positive and therefore could not be examined separately using Aβ status as outcome. Study design and specific inclusion and exclusion criteria are described elsewhere29 (eMethods in the Supplement). The study was approved by the Regional Ethics Committee in Lund, Sweden, and all participants gave their written informed consent to participate in the study. For the independent validation cohort, the study was approved by the ethical committee of the Medizinische Hochschule in Hannover and the ethical committee of the University of Ulm, and all participants gave written informed consent.
Plasma and CSF Procedures
Blood samples were collected at the same time as CSF samples, and the collection was performed in the morning with participants not fasting. Blood samples were collected and analyzed according to a standardized protocol. For each study participant, blood was collected in 6 EDTA-plasma tubes (Vacutainer K2EDTA tube; BD Diagnostics) and centrifuged (2000 g, 4°C) for 10 minutes. After centrifugation, plasma from all 6 tubes was transferred into one 50-mL tube (62.547.254, Sarstedt), mixed, and 1 mL was aliquoted into polypropylene tubes (72.694.100; Sarstedt) and stored at –80°C within 30 to 60 minutes of collection. All plasma samples went through 1 freeze-thaw cycle before the analysis, when 300 μL was further aliquoted into Lobind tubes (72.704.600; Sarstedt). The current standardized protocol is consistent with recent findings that blood must be centrifuged within 1 hour and frozen shortly thereafter; however, up to 3 freeze-thaw cycles and 5 tube transfers do not affect plasma Aβ and tau values.30 Lumbar puncture and CSF handling followed a structured protocol.31 Plasma and CSF Aβ42, Aβ40, total tau (tau), and phosphorylated tau (P-tau; only in CSF) were analyzed using the Elecsys immunoassays on a cobas e 601 analyzer (Roche Diagnostics) at the Clinical Neurochemistry Laboratory, University of Gothenburg, Sweden. Additional assay data (also including NFL and neurofilament heavy chain [NFH] analyses) can be found in the eMethods and eTables 1 and 2 in the Supplement.
Reference Standard for Aβ Status
β-Amyloid status was determined using the Elecsys CSF Aβ42/Aβ40 ratio, which is a ratio that has been validated against amyloid PET status with more than 90% agreement.32,33,34 An unbiased cutoff of less than 0.059 was used to define Aβ positivity based on mixture modeling statistics, which previously has proved to provide robust and accurate thresholds.35,36 In a secondary analysis (eFigure 3 and eFigure 4 in the Supplement), we used the Elecsys CSF P-tau/Aβ42 ratio to define Aβ positivity, using the predefined cutoff of 0.022 or greater.24
Independent Validation Cohort
All 237 participants of this study were enrolled between January 29, 2000, and October 11, 2006, at 2 clinical sites in Germany, Ulm and Hannover, as part of a prospective validation study of new biomarkers for the early diagnosis of AD. The participants were classified as having CU (n = 34), MCI (n = 109),37 or AD mild dementia38 (Mini-Mental State Examination score >22; n = 94). Specific inclusion/exclusion criteria and CSF and blood collection procedures30 are described in the eMethods in the Supplement. The cutoff of CSF Aβ42/Aβ40 of less than 0.059 established in BioFINDER to define Aβ positivity was also used in the validation cohort after a thorough assessment of the CSF Aβ42/Aβ40 distribution. As in BioFINDER, the previously published cutoff of CSF P-tau/Aβ42 0.022 or greater24 was used as a secondary reference standard for Aβ status also in the validation cohort.
Statistical Analysis
According to previous publications39,40 and present analyses (eResults in the Supplement), APOE (OMIM:107741) genotype analyzed from blood was grouped into (A) ε2/ε2 or ε2/ε3; (B) ε3/ε3; (C) ε2/ε4 or ε3/ε4; and (D) ε4/ε4. APOE ε3/ε3 was the reference category in the statistical models. β-Amyloid status was predicted in logistic regression models to produce estimates of the predictors, probabilities for Aβ positivity, and resulting area under the receiver operating characteristic curve (AUC). The examined predictors in BioFINDER were the plasma biomarkers Aβ42, Aβ40, tau, NFH, and NFL and APOE genotype. The models were built using the Akaike information criterion (AIC) to evaluate the model fit. A predictor was kept in the model if AIC improved significantly (a decrease in AIC of at least 2, noted as “ΔAIC -2”).41 Differences in AUCs were compared using DeLong statistics.42 In the replication analysis, the models (intercepts and estimates) established in BioFINDER were applied to the validation cohort. The resulting probabilities from the validation cohort were used to calculate the AUCs (only plasma Aβ42, Aβ40, and tau were available in this cohort). Additional statistical methods are described in the eMethods in the Supplement. SPSS version 24 (IBM) and R version 3.4 (R Foundation for Statistical Computing) were used for the statistical analyses. Two-sided P < .05 indicated statistical significance.
Results
Among the 842 study participants in BioFINDER, mean (SD) age was 72.0 (5.6) years, and 446 (52.5%) were female. Demographic and clinical data for the study participants in BioFINDER are shown in Table 1. In the total BioFINDER population of 842, 368 were positive for Aβ (prevalence, 44%); 147 of 513 with CU (29%) were positive; 157 of 265 (60%) with MCI; and, by definition, all 64 (100%) with AD dementia.
Table 1. Demographic and Clinical Dataa.
| Characteristic | CU Aβ− (n = 366) | CU Aβ+ (n = 147) | MCI Aβ− (n = 108) | MCI Aβ+ (n = 157) | AD Aβ+ (n = 64) |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 152 | 69 | 76b | 79 | 25 |
| Female | 214 | 78 | 32 | 78 | 39 |
| Age, y | 72 (5) | 73 (5) | 69 (6)b | 72 (5) | 76 (5)b |
| APOE genotype, % | |||||
| 1 or 2 ε4 alleles, | 19 | 63b | 24 | 70b | 69b |
| MMSE | 28.9 (1.1) | 28.6 (1.3) | 27.5 (1.8)b | 26.7 (1.8)b | 21.8 (3.7)b |
| Delayed recall (ADAS-cog; errors)c | 2.2 (1.9) | 3.2 (2.3)b | 5.7 (2.4)b | 7.0 (2.1)b | 8.6 (1.6)b |
| CSF | |||||
| Aβ42, pg/mL | 1665 (596) | 819 (303)b | 1572 (605) | 706 (256)b | 671 (315)b |
| Aβ40, ng/mL | 18.2 (5.2) | 19.5 (5.9)d | 17.3 (5.7) | 17.8 (5.0) | 17.9 (6.2) |
| Aβ42/Aβ40 | 0.091 (0.016) | 0.042 (0.009)b | 0.090 (0.014) | 0.040 (0.098)b | 0.037 (0.009)b |
| T-tau, pg/mL | 209 (62) | 309 (112)b | 209 (76) | 341 (136)b | 384 (143)b |
| P-tau, pg/mL | 17.5 (5.3) | 28.5 (12.0)b | 16.9 (6.4) | 32.2 (14.5) b | 36.3 (16.3)e |
| NFL, pg/mL | 918 (490) | 1216 (842)b | 1648 (1517)b | 1531 (1195)b | 2002 (1835)b |
| NFH, pg/mL | 504 (190) | 584 (241)b | 641 (463)b | 637 (303) b | 821 (687)b |
| Plasma | |||||
| Aβ42, pg/mL | 32.8 (4.9) | 29.6 (4.3)b | 33.1 (5.2) | 30.3 (4.5)b | 23.3 (8.2)b |
| Aβ40, pg/mL | 482 (63.3) | 479 (67.5) | 495 (83.2) | 492 (75.4) | 380 (131.7)e |
| T-tau, pg/mL | 16.6 (4.7) | 17.9 (5.4)e | 18.7 (6.1)b | 19.1 (5.2)b | 16.7 (6.0) |
| Aβ42/Aβ40 | 0.068 (0.007) | 0.062 (0.007)b | 0.067 (0.007) | 0.062 (0.006)b | 0.062 (0.010)b |
| NFL, pg/mL | 21.0 (11.8) | 29.1 (59.6)e | 28.3 (28.4)b | 29.0 (17.9)b | 43.8 (28.7)b |
| NFH, pg/mLf | 51.4 (68.2) | 53.7 (48.7) | 59.7 (55.1) | 65.9 (56.6)b | 79.8.4 (77.0)b |
Abbreviations: Aβ, β-amyloid; Aβ+, Aβ positive; Aβ−, Aβ negative; CSF, cerebrospinal fluid; MMSE, Mini-Mental State Examination; NHL, neurofilament heavy chain; NFL, neurofilament light chain.
β-Amyloid status was defined based on a CSF Aβ42/Aβ40 cutoff of ≤0.059. Data are shown as mean (SD) unless otherwise specified. Demographic factors, clinical characteristics, and biomarkers levels were compared using χ2 test and 1-way analysis of variance (not adjusted for multiple comparisons). Neurofilament light chain and NFH values were ln-transformed before the analysis. In the receiver operating characteristic subanalyses, the mild cognitive impairment and Alzheimer disease cohorts are combined as cognitively impaired (Figure 3A and B; eFigure 3C and D; eTable 4 in the Supplement). When calculating the Aβ42/Aβ40 ratio, picomolar per milliliter was used for both peptides.
P < .001 compared with CU Aβ−.
Data were missing for 1 CU Aβ−, 1 MCI Aβ−, 8 MCI Aβ+ and 5 AD Aβ+ individuals.
P < .05.
P < .01.
Data were missing for 6 CU Aβ−, 3 CU Aβ+, 2 MCI Aβ−, 5 MCI Aβ+ and 5 AD Aβ+ individuals.
Correlations Between Plasma and CSF Biomarkers
In the whole BioFINDER population, there were statistically significant positive correlations between all plasma and corresponding CSF biomarkers (eTable 3 in the Supplement). The correlations were similar within diagnostic subgroups (eFigure 1 and eTable 3 in the Supplement).
Plasma Aβ and tau Levels in Diagnostic Groups
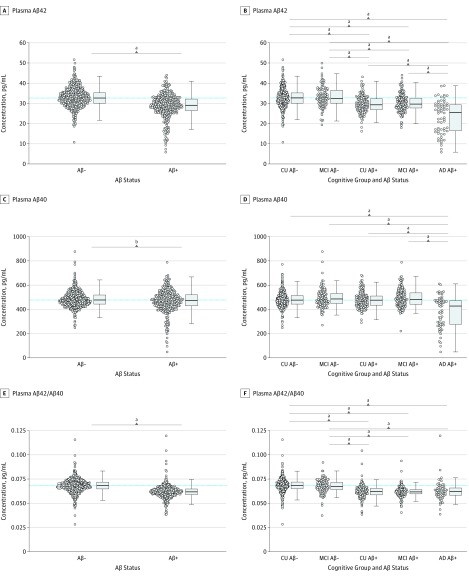
In BioFINDER, plasma levels of Aβ42, Aβ40, and Aβ42/Aβ40 were decreased in Aβ-positive (CSF Aβ42/Aβ40 ≤ 0.059) compared with Aβ-negative (CSF Aβ42/Aβ40 > 0.059) participants (Aβ42, P < .001; Aβ40 P = .003, Aβ42/Aβ42, P < .001; Figure 1A-C). When comparing Aβ groups stratified by diagnostic subgroup, plasma levels of Aβ42 were lower in the CU Aβ-positive, MCI Aβ-positive and AD Aβ-positive dementia groups compared with the CU Aβ-negative and MCI Aβ-negative groups (P < .001 for all; Figure 1D). The decrease in plasma Aβ42 was more pronounced in AD Aβ-positive dementia compared with CU Aβ-positive and MCI Aβ positive groups. Plasma Aβ40 levels were lower in the AD Aβ-positive dementia group compared with all other groups (P < .001 for all), but there were no differences between CU Aβ-negative, CU Aβ-positive, and MCI Aβ-positive participants (Figure 1E). The plasma Aβ42/Aβ40 ratio was lower in the CU Aβ-positive, MCI Aβ-positive, and AD Aβ-positive dementia groups than in the CU Aβ-negative and MCI Aβ-negative groups with no differences across the Aβ-positive groups (Figure 1F). The significant findings were very similar when adjusting for age and sex (data not shown). Comparisons of plasma tau, NFL, and NFH are shown in eFigure 2 in the Supplement.
Figure 1. Levels of Plasma β-Amyloid (Aβ) Biomarkers.
Plasma Aβ42 (A), Aβ40 (C), and the plasma Aβ42/Aβ40 ratio (E) in the Aβ-positive (Aβ+) (CSF Aβ42/Aβ40 ≤ 0.059) and Aβ-negative (Aβ−) (CSF Aβ42/Aβ40 > 0.059) groups. Plasma Aβ42 (B), Aβ40 (D), and the plasma Aβ42/Aβ40 ratio (F) in the CU, MCI, and AD participant groups stratified by Aβ status. The dotted lines indicate median levels in the CU Aβ-negative group. P values are calculated from t test (A, C, E) or 1-way analysis of variance and post hoc tests with the statistical significance set to P < .005 (.05/10.00) to account for the Bonferroni correction (B, D, F). The significant findings were similar when adjusting for age and sex (data not shown). Group comparisons of plasma tau, NFH, and NFL are shown in eFigure 2 in the Supplement. AD, Alzheimer disease; CSF, cerebrospinal fluid; CU, cognitively unimpaired; MCI, mild cognitive impairment; NFH, neurofilament heavy chain; and NFL, neurofilament light chain.
Accuracy of Plasma Aβ42 and Aβ40 for Predicting Brain Aβ Positivity
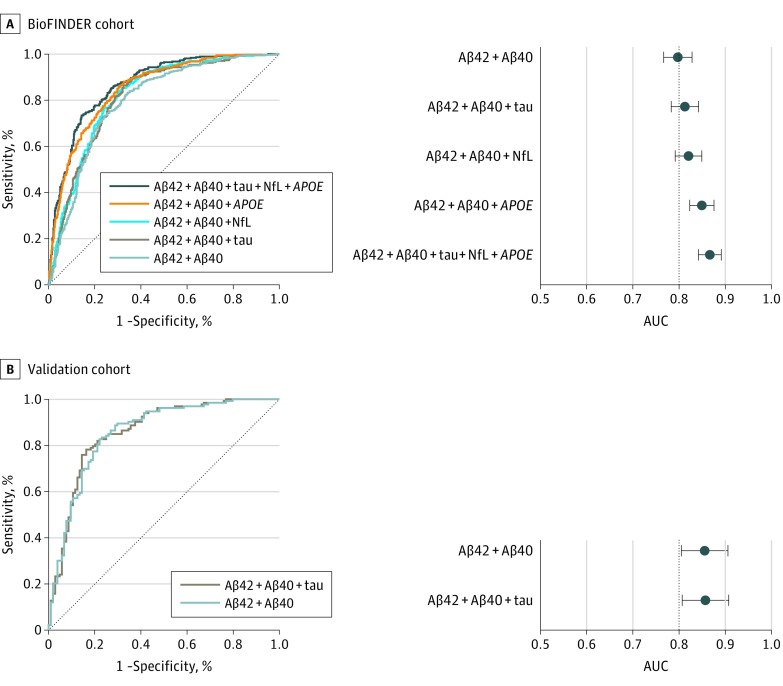
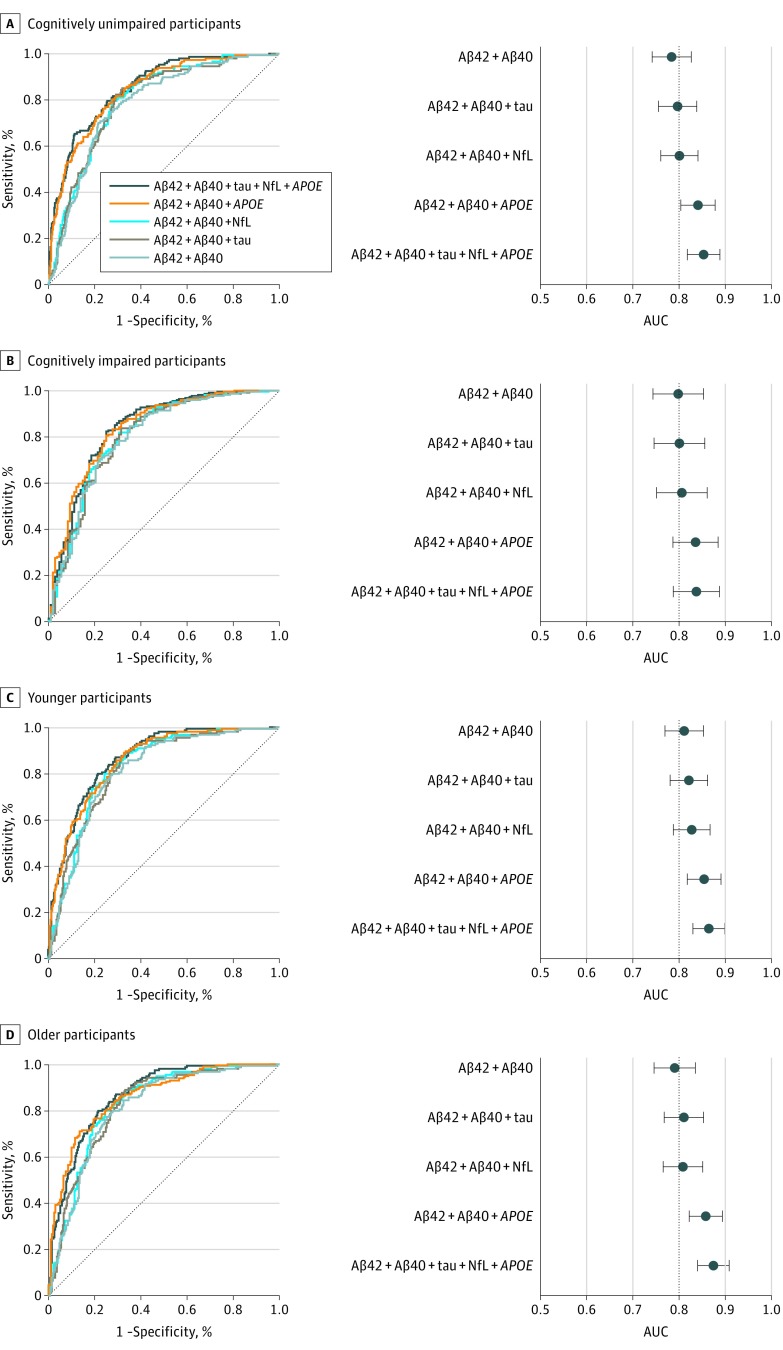
The results from the logistic regression models in BioFINDER of all tested single and combined biomarkers are shown in eTables 4, 5, and 6 in the Supplement (including AUC and AIC values). The receiver operating characteristic curves and AUCs of selected biomarkers for predicting Aβ positivity are shown in Figure 2A and B (sensitivity, specificity, and cutoffs are shown in Table 2). The plasma Aβ42/Aβ40 ratio predicted Aβ positivity with an AUC of 0.77 (95% CI, 0.74-0.81) in the whole BioFINDER population. Using plasma Aβ42 and Aβ40 as separate predictors in a logistic regression resulted in a slightly but significantly better AUC (0.80; 95% CI, 0.77-0.83; P = .01) and a better model fit (ΔAIC, –66). We also tested the accuracy of the biomarkers in different age groups and in those with and without cognitive impairment (Figure 3; eTable 4 and eTable 6 in the Supplement), with similar results (AUC ±0.02 compared with the total population).
Figure 2. Receiver Operating Characteristic (ROC) Analysis of Plasma Biomarkers in the BioFINDER and Validation Cohorts.
Optimized ROC curves and corresponding areas under the curve (AUCs) for plasma Aβ together with the additional predictors, APOE, plasma tau, and neurofilament light chain (NFL) to assess accuracy when predicting Aβ positivity (crebrospinal fluid Aβ42/Aβ40 ≤ 0.059) in the BioFINDER (A and B, n = 842); and the replication of these models (C and D, n = 237) in the validation cohort using the estimates and intercepts established in BioFINDER. APOE genotype and NFL were not available in the validation cohort. Error bars indicate 95% CIs. ROC analyses in subpopulations can be found in Figure 3 and eTable 4 and 6 in the Supplement. Sensitivities and specificities are shown in Table 2. ROC analyses using the alternative reference standard for Aβ positivity (CSF P-tau/Aβ42 ≥ 0.022) are shown in eFigures 3 and 4 in the Supplement.
Table 2. Sensitivity and Specificity for Aβ Status in the BioFINDER Cohort and the Validation Cohort.
| Plasma Biomarkers | Cutoffa | % (95% CI) | |
|---|---|---|---|
| Sensitivity | Specificity | ||
| BioFINDER cohort | |||
| Aβ42/Aβ40 ratio | 0.065 | 75 (68-80) | 72 (65-77) |
| Aβ42, Aβ40 | 0.45 | 73 (65-78) | 76 (68-80) |
| Aβ42, Aβ40, tau | 0.36 | 86 (78-90) | 68 (61-72) |
| Aβ42, Aβ40, NFL | 0.38 | 84 (76-88) | 70 (62-74) |
| Aβ42, Aβ40, APOE | 0.29 | 88 (82-92) | 68 (58-72) |
| Aβ42, Aβ40, tau, NFL, APOE | 0.52 | 73 (64-78) | 86 (77-89) |
| Validation cohort | |||
| Aβ42/Aβ40 ratio | 0.065 | 70 (61-80) | 73 (61-81) |
| Aβ42, Aβ40 | 0.45 | 89 (80-95) | 69 (54-81) |
| Aβ42, Aβ40, tau | 0.36 | 89 (74-94) | 64 (49-74) |
Abbreviations: Aβ, β-amyloid; NFL, neurofilament light chain.
Cutoffs were determined based on the highest Youden index (sensitivity + specificity – 1) for Aβ positivity in the BioFINDER cohort. The cutoffs were then replicated in the validation cohort. Cutoffs are from the probabilities from the corresponding logistic regression models, except for Aβ42/Aβ40 where the actual ratio of the biomarker levels constitute the cutoff. Aβ status (reference standard) was determined using the cerebrospinal fluid Aβ42/40 ratio (<0.059). The 95% CIs were computed using 2000 stratified bootstrap replicates. Neurofilament light chain and APOE genotype were not available in the validation cohort.
Figure 3. Receiver Operating Characteristic (ROC) Analysis of Plasma Biomarkers in Subpopulations in BioFINDER.
ROC curves and corresponding areas under the curve (AUCs) from logistic regression models for plasma Aβ together with the additional predictors APOE, plasma tau, and neurofilament light chain (NFL), to assess accuracy when detecting Aβ positivity (cerebrospinal fluid Aβ42/Aβ40 ≤ 0.059) in cognitively unimpaired participants (A and B, n = 513), cognitively impaired participants (C and D, n = 329), the younger half of the cohort (E and F, n = 428; 60-72 y), and the older half of the cohort (G and H, n = 414; 73-88 y). Cognitively unimpaired comprised of cognitively healthy controls and participants with subjective cognitive decline. Cognitively impaired comprised of participants with mild cognitive impairment and Alzheimer disease dementia. AUC indicates area under the curve; and NFL, neurofilament light chain.
Aβ Detection With Additional Predictors
The accuracy of predicting Aβ status was further examined by adding APOE genotype, and plasma levels of tau, NFL, and NFH to plasma Aβ42 and Aβ40 in logistic regression models (Figure 2A-B). When adding plasma tau, AUC increased nonsignificantly to 0.81 (95% CI, 0.78-0.84) and further improved the model fit (ΔAIC, –27). However, when instead adding APOE genotype to plasma Aβ42 and Aβ40, AUC increased significantly from 0.80 to 0.85 (95% CI, 0.82-0.88; P < .001; Figure 2A-B; eTable 4 in the Supplement). Adding plasma tau to plasma Aβ42, Aβ40, and APOE increased the AUC slightly to 0.86 (95% CI, 0.83-0.88; ΔAIC, –20). A further slight increase was seen when adding plasma NFL to plasma Aβ42, Aβ40, tau, and APOE (AUC, 0.87; 95% CI, 0.84-0.89; ΔAIC –16; Figure 2A-B). The results were similar in CU and cognitively impaired participants, respectively, except that plasma tau and NFL were not a significant predictor in the cognitively impaired group (eTable 4 in the Supplement). The results were also similar when the CSF P-tau/Aβ42 ratio was used to define Aβ positivity (eFigure 3 in the Supplement). Plasma NFH did not contribute to Aβ prediction in addition to plasma Aβ42 and Aβ40 (eTable 5 in the Supplement).
Independent Validation Cohort
Among the 237 study participants in the independent validation cohort, mean (SD) age was 66 (10) years with a range of 23 to 85 years, and 120 (50.6%) were female. The demographic characteristics are shown in eTable 7 in the Supplement and the accuracy of the plasma assays in Figure 2C and D. The AUC for plasma Aβ42 and Aβ40 to predict Aβ positivity was 0.86 (95% CI, 0.81-0.91) when applying the estimates from the model established in BioFINDER (compared with an AUC of 0.80, 95% CI 0.77-0.83 in BioFINDER). When applying the BioFINDER model that included plasma Aβ42, Aβ40, and tau in the validation cohort, the AUC was slightly lower than when using only plasma Aβ42 and Aβ40 (AUC, 0.84; 95% CI, 79-89). With the alternative reference standard for Aβ status (CSF P-tau/Aβ42 ≥ 0.022; eFigure 4 in the Supplement), the accuracy was slightly lower for plasma Aβ42 and Aβ40 (AUC, 0.83; 95% CI, 0.78-0.89) but still better than the corresponding results in the BioFINDER cohort (AUC, 0.79; 95% CI, 0.76-0.82; eFigure 3 in the Supplement). Sensitivities and specificities using the cutoffs established in BioFINDER are shown in Table 2. Plasma NFH, NFL, and APOE genotype were not available in the validation cohort.
Cost-Benefit Analysis
Finally, we performed a cost-benefit analysis (eFigure 5 in the Supplement) where we show a scenario in which 1000 Aβ-positive participants are included in a trial where the screening cost for Aβ PET is $4000 per participant.43 For example, using the highest Youden index cutoff (Table 2) for plasma Aβ42, Aβ40, and APOE reduces the number of PET scans by approximately 800 and lowers the PET costs by approximately $3.2 million (from a total cost of approximately $9.2 million).
Discussion
In this study of 842 participants, we found that plasma Aβ42 and Aβ40 using the fully automated Elecsys platform detected abnormal levels of Aβ in the brain with an AUC of 0.80 (Figure 2A and B). The addition of APOE genotype increased the AUC significantly to 0.85 (Figure 2A and B). Plasma tau and NFL had a slight effect on the accuracy (AUC, +0.01 to 0.02; Figure 2A and B). The results were similar in cognitively impaired and unimpaired and older and younger participants (Figure 3), with the exception that plasma tau and NFL generally did not improve accuracy in addition to plasma Aβ and APOE genotype in cognitively impaired participants (eTable 4 in the Supplement). When applying the plasma Aβ42 and Aβ40 model from BioFINDER to the independent validation cohort (n = 237), the AUC was greater compared with BioFINDER (AUC, 0.86; 95% CI, 0.81-0.91), but no improvement was seen when adding plasma tau (Figure 2C and D).
Although previous studies have found associations between CSF and PET Aβ and plasma Aβ using different immunoassays,7,12,13,14,44,45 the present Elecsys assays produced among the best accuracies and they are the first fully automated assays to have these greater accuracies. In mass spectrometry–based techniques, 2 recent studies have provided overall better accuracies for plasma Aβ42/Aβ40 (AUC, 0.84-0.97 depending on population and reference standard).19,20 However, these are labor-intensive, time-consuming, low-throughput methods that currently are not feasible to implement in clinical practice on a large scale. Fully automated Elecsys assays, on the other hand, are already implemented in many clinical chemistry laboratories worldwide that provide analyses (eg, for primary care).
Historically, the ratio of plasma Aβ42 to Aβ40 has been used to optimize the concordance with CSF or PET Aβ. Here, Aβ40 acts as a reference peptide that accounts for interindividual variability in the overall Aβ production and CSF turnover. We found that instead of using the fixed ratio of Aβ42/Aβ40, both the model fit (AIC) and accuracy (AUC) were slightly but significantly improved when the model was adjusted for Aβ40 concentrations independent of Aβ42 (ie, used as a separate predictor in the logistic regression models) (AUC 0.77 vs 0.80; P = .01; ΔAIC –66; eTable 4 in the Supplement). As a single additional biomarker to Aβ42 and Aβ40, APOE genotype increased the accuracy most markedly, from AUC 0.80 to 0.85 (P < .001) (Figure 2A and B; eTable 4 in the Supplement). Plasma tau increased the AUC slightly, and provided a better model fit (ΔAIC –27), but clinically this is not comparable with the contribution CSF tau has combined with CSF Aβ42,24 and improved plasma tau assays are probably needed in the future, such as measurement of specifically phosphorylated tau,46 to increase the added value of plasma tau to plasma Aβ42 and Aβ40.
Despite the present and previous results showing relatively high correlations between plasma and CSF NFL (eTable 3 in the Supplement and the study by Hansson et al47), we saw a modest increase in accuracy in addition to plasma Aβ42 and Aβ40 (Figure 2; eTable 4 in the Supplement). Because NFL generally is late biomarker in the disease process and a non–AD specific biomarker for axonal degeneration,15 the poor result could be because most of the participants (513 of 842) were cognitively unimpaired and only 64 had AD dementia. Compared with plasma tau and NFL, plasma NFH had a poorer performance and did not improve accuracy (eTable 5 in the Supplement). However, 101 plasma NFH measurements were below the detection limit of the assays, and development of more sensitive plasma NFH assays is thus warranted to establish whether this biomarker could further improve the diagnostic performance of plasma Aβ.
Overall, the accuracies of the Aβ42 and Aβ40 assays are not sufficient to be used on their own as a clinical test of Aβ positivity; additional assay development is needed before this can be recommended, possibly together with other blood biomarkers and screening tools in diagnostic algorithms. In the present study, we showed that the Aβ assays perform similarly in CU populations with lower prevalence of Aβ positivity (Figure 3A and B; Aβ-positive prevalence, 29%). Nonetheless, further studies would be valuable in populations with lower prevalence of Aβ positivity, such as primary care settings, as well as more heterogeneous dementia cohorts with different neurodegenerative disorders. To some extent, the generalizability of the BioFINDER results has already been shown in the present study where the plasma Aβ42 and Aβ40 model established in BioFINDER could be applied in the independent validation cohort with better accuracy (AUC, 0.86 vs 0.80; Figure 2). This robust result is similar to what has been shown when using the Elecsys assays for CSF to establish a cutoff in one cohort and replicating it in a second cohort.24
Limitations
Limitations of the present validation analysis include the lack of APOE data, the lack of improvement when replicating the model that included plasma tau, and the smaller population size, resulting in a lack of analyses in subpopulations. The latter was, however, tested in BioFINDER and the accuracies were similar in different subsamples including CU (Figure 3A and B) and younger participants (Figure 3E and F) where Aβ positivity might be more difficult to identify using alternative methods such as cognitive testing and age stratification.40
Conclusions
From a practical perspective, we believe that the most advantageous future use of optimized blood Aβ assays is as a screening tool for identifying subjects at a higher risk of being Aβ positive. They could, for example, be applied as an initial test together with other noninvasive, cost-efficient tools that aid the decision about whom a general practitioner should refer for further investigation at memory clinics where CSF or PET and more extensive clinical assessment could be used to support the AD diagnosis. Another useful setting for the blood biomarkers are clinical AD trials enrolling Aβ-positive participants, where they can be used for prescreening to minimize the number of unnecessary (Aβ-negative) lumbar punctures and Aβ PET scans, as well as lowering the costs for the examinations up to 30% to 50% depending on the cutoff (eFigure 5 in the Supplement).48 Although further validation studies are needed, this illustrates the potential usefulness blood assays might have, especially considering the ongoing great need to recruit large cohorts for AD drug trials in preclinical and prodromal stages.
eMethods.
eResults.
eTable 1. Performance Characteristics of the Plasma Aβ42, Aβ40 and Tau Elecsys Assays
eTable 2. Performance Characteristics of CSF and Plasma NFH Assays
eTable 3. Associations Between Plasma and CSF Biomarkers.
eTable 4. Area Under the Curves From Logistic Regression Models for Prediction of Aβ Positivity
eTable 5. Plasma NfH as Additional Predictor for Aβ Positivity.
eTable 6. Area Under the Curves From Logistic Regression Models for Prediction of Aβ Positivity in the Younger and Older Half of the BioFINDER Cohort.
eTable 7. Demographic and Clinical Data of the German Validation Cohort
eFigure 1. Correlations Between Plasma and CSF Biomarkers.
eFigure 2. Plasma Biomarkers in Diagnostic Groups.
eFigure 3. ROC Analysis of Plasma Biomarkers Using the Ratio of CSF P-tau/Aβ42 as Reference Standard in BioFINDER.
eFigure 4. ROC Analysis of Plasma Biomarkers Using the Ratio of CSF P-tau/Aβ42 as Reference Standard in the Independent Validation Cohort.
eFigure 5. Implementation of Plasma Aβ42, Aβ40 and APOE Genotype in an AD Trial Screening Scenario.
eReferences.
References
- 1.Albert MS, DeKosky ST, Dickson D, et al. . The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKhann GM, Knopman DS, Chertkow H, et al. . The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattsson N, Carrillo MC, Dean RA, et al. . Revolutionizing Alzheimer’s disease and clinical trials through biomarkers. Alzheimers Dement (Amst). 2015;1(4):412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297-309. doi: 10.1016/j.tips.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017;13 (4):325-273. doi: 10.1016/j.jalz.2017.02.001 [DOI] [Google Scholar]
- 6.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186-191. doi: 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 7.Keshavan A, Heslegrave A, Zetterberg H, Schott JM. Blood biomarkers for Alzheimer’s disease: much promise, cautious progress. Mol Diagn Ther. 2017;21(1):13-22. doi: 10.1007/s40291-016-0241-0 [DOI] [PubMed] [Google Scholar]
- 8.Voyle N, Baker D, Burnham SC, et al. ; AIBL research group . Blood protein markers of neocortical amyloid-β burden: a candidate study using SOMAscan technology. J Alzheimers Dis. 2015;46(4):947-961. doi: 10.3233/JAD-150020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee P, Goozee K, Sohrabi HR, et al. . Association of plasma neurofilament light chain with neocortical amyloid-β load and cognitive performance in cognitively normal elderly participants. J Alzheimers Dis. 2018;63(2):479-487. doi: 10.3233/JAD-180025 [DOI] [PubMed] [Google Scholar]
- 10.Dage JL, Wennberg AMV, Airey DC, et al. . Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12(12):1226-1234. doi: 10.1016/j.jalz.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deters KD, Risacher SL, Kim S, et al. ; Alzheimer Disease Neuroimaging Initiative . Plasma tau association with brain atrophy in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2017;58(4):1245-1254. doi: 10.3233/JAD-161114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fandos N, Pérez-Grijalba V, Pesini P, et al. ; AIBL Research Group . Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement (Amst). 2017;8:179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janelidze S, Stomrud E, Palmqvist S, et al. . Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6:26801. doi: 10.1038/srep26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lue LF, Sabbagh MN, Chiu MJ, et al. . Plasma levels of Aβ42 and tau identified probable Alzheimer’s dementia: findings in two cohorts. Front Aging Neurosci. 2017;9:226. doi: 10.3389/fnagi.2017.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattsson N, Andreasson U, Zetterberg H, Blennow K; Alzheimer’s Disease Neuroimaging Initiative . Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557-566. doi: 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson N, Zetterberg H, Janelidze S, et al. ; ADNI Investigators . Plasma tau in Alzheimer disease. Neurology. 2016;87(17):1827-1835. doi: 10.1212/WNL.0000000000003246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W, Zhang J, Ye F, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Plasma neurofilament light chain levels in Alzheimer’s disease. Neurosci Lett. 2017;650:60-64. doi: 10.1016/j.neulet.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 18.Olsson B, Lautner R, Andreasson U, et al. . CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673-684. doi: 10.1016/S1474-4422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura A, Kaneko N, Villemagne VL, et al. . High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249-254. doi: 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 20.Ovod V, Ramsey KN, Mawuenyega KG, et al. . Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841-849. doi: 10.1016/j.jalz.2017.06.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattsson N, Andreasson U, Persson S, et al. ; Alzheimer’s Association QC Program Work Group . CSF biomarker variability in the Alzheimer’s Association quality control program [published correction appears in Alzheimers Dement. 2015;11(2):237]. Alzheimers Dement. 2013;9(3):251-261. doi: 10.1016/j.jalz.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vos SJ, Visser PJ, Verhey F, et al. . Variability of CSF Alzheimer’s disease biomarkers: implications for clinical practice. PLoS One. 2014;9(6):e100784. doi: 10.1371/journal.pone.0100784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bittner T, Zetterberg H, Teunissen CE, et al. . Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12(5):517-526. doi: 10.1016/j.jalz.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Hansson O, Seibyl J, Stomrud E, et al. ; Swedish BioFINDER study group; Alzheimer’s Disease Neuroimaging Initiative . CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470-1481. doi: 10.1016/j.jalz.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janelidze S, Pannee J, Mikulskis A, et al. . Concordance between different amyloid immunoassays and visual amyloid positron emission tomographic assessment. JAMA Neurol. 2017;74(12):1492-1501. doi: 10.1001/jamaneurol.2017.2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Bennett DA, Blennow K, et al. ; Contributors . NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattsson N, Insel PS, Palmqvist S, et al. . Increased amyloidogenic APP processing in APOE ɛ4-negative individuals with cerebral β-amyloidosis. Nat Commun. 2016;7:10918. doi: 10.1038/ncomms10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen RC. Mild cognitive impairment: current research and clinical implications. Semin Neurol. 2007;27(1):22-31. doi: 10.1055/s-2006-956752 [DOI] [PubMed] [Google Scholar]
- 29.The Swedish BIOFINDER Study. http://biofinder.se/. Accessed May 24, 2019.
- 30.Rózga M, Bittner T, Batrla R, Karl J. Preanalytical sample handling recommendations for Alzheimer’s disease plasma biomarkers. Alzheimers Dement (Amst). 2019;11:291-300. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmqvist S, Zetterberg H, Blennow K, et al. . Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid β-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71(10):1282-1289. doi: 10.1001/jamaneurol.2014.1358 [DOI] [PubMed] [Google Scholar]
- 32.Janelidze S, Zetterberg H, Mattsson N, et al. ; Swedish BioFINDER study group . CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol. 2016;3(3):154-165. doi: 10.1002/acn3.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leuzy A, Chiotis K, Hasselbalch SG, et al. . Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain. 2016;139(Pt 9):2540-2553. doi: 10.1093/brain/aww160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewczuk P, Matzen A, Blennow K, et al. . Cerebrospinal fluid Aβ42/40 corresponds better than Aβ42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis. 2017;55(2):813-822. doi: 10.3233/JAD-160722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertens D, Tijms BM, Scheltens P, Teunissen CE, Visser PJ. Unbiased estimates of cerebrospinal fluid β-amyloid 1-42 cutoffs in a large memory clinic population. Alzheimers Res Ther. 2017;9(1):8. doi: 10.1186/s13195-016-0233-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmqvist S, Schöll M, Strandberg O, et al. . Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun. 2017;8(1):1214. doi: 10.1038/s41467-017-01150-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303-308. doi: 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 38.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 39.Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924-1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmqvist S, Insel PS, Zetterberg H, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Swedish BioFINDER study . Accurate risk estimation of beta-amyloid positivity to identify prodromal Alzheimer’s disease: cross-validation study of practical algorithms. Alzheimers Dement. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olofsen E, Dahan A. Using Akaike’s information theoretic criterion in mixed-effects modeling of pharmacokinetic data: a simulation study. F1000Res. 2013;2:71. doi: 10.12688/f1000research.2-71.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 43.Insel PS, Palmqvist S, Mackin RS, et al. . Assessing risk for preclinical β-amyloid pathology with APOE, cognitive, and demographic information. Alzheimers Dement (Amst). 2016;4:76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HJ, Park KW, Kim TE, et al. . Elevation of the plasma Aβ40/Aβ42 ratio as a diagnostic marker of sporadic early-onset Alzheimer’s disease. J Alzheimers Dis. 2015;48(4):1043-1050. doi: 10.3233/JAD-143018 [DOI] [PubMed] [Google Scholar]
- 45.Mayeux R, Honig LS, Tang MX, et al. . Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61(9):1185-1190. doi: 10.1212/01.WNL.0000091890.32140.8F [DOI] [PubMed] [Google Scholar]
- 46.Mielke MM, Hagen CE, Xu J, et al. . Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14(8):989-997. doi: 10.1016/j.jalz.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansson O, Janelidze S, Hall S, et al. ; Swedish BioFINDER study . Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930-937. doi: 10.1212/WNL.0000000000003680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmqvist S, Insel PS, Zetterberg H, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Swedish BioFINDER study . Accurate risk estimation of β-amyloid positivity to identify prodromal Alzheimer’s disease: cross-validation study of practical algorithms. Alzheimers Dement. 2019;15(2):194-204. doi: 10.1016/j.jalz.2018.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eTable 1. Performance Characteristics of the Plasma Aβ42, Aβ40 and Tau Elecsys Assays
eTable 2. Performance Characteristics of CSF and Plasma NFH Assays
eTable 3. Associations Between Plasma and CSF Biomarkers.
eTable 4. Area Under the Curves From Logistic Regression Models for Prediction of Aβ Positivity
eTable 5. Plasma NfH as Additional Predictor for Aβ Positivity.
eTable 6. Area Under the Curves From Logistic Regression Models for Prediction of Aβ Positivity in the Younger and Older Half of the BioFINDER Cohort.
eTable 7. Demographic and Clinical Data of the German Validation Cohort
eFigure 1. Correlations Between Plasma and CSF Biomarkers.
eFigure 2. Plasma Biomarkers in Diagnostic Groups.
eFigure 3. ROC Analysis of Plasma Biomarkers Using the Ratio of CSF P-tau/Aβ42 as Reference Standard in BioFINDER.
eFigure 4. ROC Analysis of Plasma Biomarkers Using the Ratio of CSF P-tau/Aβ42 as Reference Standard in the Independent Validation Cohort.
eFigure 5. Implementation of Plasma Aβ42, Aβ40 and APOE Genotype in an AD Trial Screening Scenario.
eReferences.