Key Points
Question
In patients undergoing percutaneous coronary intervention, is 1 month of dual antiplatelet therapy (DAPT) followed by clopidogrel monotherapy noninferior to 12 months of DAPT with aspirin and clopidogrel for adverse cardiovascular and bleeding events?
Findings
In this randomized clinical trial that included 3045 patients, the 1-year cumulative incidence of a composite end point consisting of cardiovascular death, myocardial infarction, ischemic or hemorrhagic stroke, definite stent thrombosis, and major bleeding was 2.4% in the 1-month DAPT group and 3.7% in the 12-month DAPT group, a difference that met the noninferiority margin of a hazard ratio of 0.5, as well as superiority.
Meaning
These findings suggest that 1 month of DAPT followed by clopidogrel monotherapy provided benefit compared with 12 months of DAPT, although additional research is needed in other populations.
Abstract
Importance
Very short mandatory dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) with a drug-eluting stent may be an attractive option.
Objective
To test the hypothesis of noninferiority of 1 month of DAPT compared with standard 12 months of DAPT for a composite end point of cardiovascular and bleeding events.
Design, Setting, and Participants
Multicenter, open-label, randomized clinical trial enrolling 3045 patients who underwent PCI at 90 hospitals in Japan from December 2015 through December 2017. Final 1-year clinical follow-up was completed in January 2019.
Interventions
Patients were randomized either to 1 month of DAPT followed by clopidogrel monotherapy (n=1523) or to 12 months of DAPT with aspirin and clopidogrel (n=1522).
Main Outcomes and Measures
The primary end point was a composite of cardiovascular death, myocardial infarction (MI), ischemic or hemorrhagic stroke, definite stent thrombosis, or major or minor bleeding at 12 months, with a relative noninferiority margin of 50%. The major secondary cardiovascular end point was a composite of cardiovascular death, MI, ischemic or hemorrhagic stroke, or definite stent thrombosis and the major secondary bleeding end point was major or minor bleeding.
Results
Among 3045 patients randomized, 36 withdrew consent; of 3009 remaining, 2974 (99%) completed the trial. One-month DAPT was both noninferior and superior to 12-month DAPT for the primary end point, occurring in 2.36% with 1-month DAPT and 3.70% with 12-month DAPT (absolute difference, −1.34% [95% CI, −2.57% to −0.11%]; hazard ratio [HR], 0.64 [95% CI, 0.42-0.98]), meeting criteria for noninferiority (P < .001) and for superiority (P = .04). The major secondary cardiovascular end point occurred in 1.96% with 1-month DAPT and 2.51% with 12-month DAPT (absolute difference, −0.55% [95% CI, −1.62% to 0.52%]; HR, 0.79 [95% CI, 0.49-1.29]), meeting criteria for noninferiority (P = .005) but not for superiority (P = .34). The major secondary bleeding end point occurred in 0.41% with 1-month DAPT and 1.54% with 12-month DAPT (absolute difference, −1.13% [95% CI, −1.84% to −0.42%]; HR, 0.26 [95% CI, 0.11-0.64]; P = .004 for superiority).
Conclusions and Relevance
Among patients undergoing PCI, 1 month of DAPT followed by clopidogrel monotherapy, compared with 12 months of DAPT with aspirin and clopidogrel, resulted in a significantly lower rate of a composite of cardiovascular and bleeding events, meeting criteria for both noninferiority and superiority. These findings suggest that a shorter duration of DAPT may provide benefit, although given study limitations, additional research is needed in other populations.
Trial Registration
ClinicalTrials.gov Identifier: NCT02619760
This randomized trial compares the effects of 1 month of dual antiplatelet therapy (DAPT) followed by clopidogrel monotherapy vs 12 months of DAPT with aspirin and clopidogrel on cardiovascular and bleeding events in patients in Japan who underwent percutaneous coronary intervention (PCI).
Introduction
The optimal duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) using drug-eluting stents is still under debate. The current US and European guidelines recommend DAPT for at least 12 months in acute coronary syndrome and for at least 6 months in stable coronary artery disease without high bleeding risk.1,2 In a meta-analyses of randomized trials comparing short (≤6 months) vs prolonged (≥12 months) DAPT duration, short DAPT was associated with lower bleeding risk without a significant increase in ischemic risk.3,4 Furthermore, there is lingering concern about data suggesting increased mortality with prolonged DAPT.4,5 The introduction of second-generation and newer drug-eluting stents has markedly decreased the incidence of stent thrombosis, and widespread acceptance of optimal medical therapy, statins in particular, has reduced the incidence of myocardial infarction (MI) unrelated to the stent.6,7,8,9 Therefore, it is becoming increasingly important to avoid bleeding events because the mortality associated with a bleeding event has been reported to be comparable with that of MI.10,11 In this context, very short DAPT duration after drug-eluting stent implantation may be an attractive option if it is not associated with an increase in cardiovascular events disproportionate to the reduction in bleeding events. In the STOPDAPT (Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent) trial, the incidence of adverse events associated with 3 months of DAPT followed by aspirin monotherapy after implantation of a polymer-based drug-eluting stent with small late lumen loss was acceptable compared with the performance goal based on a historical control.12 Given the very low rate of stent thrombosis with new-generation drug-eluting stents, the hypothesis of this study was that further shortening of the mandatory DAPT duration could be possible without increasing cardiovascular events. Therefore, the present study sought to explore the efficacy of 1 month of DAPT compared with the standard of 12 months of DAPT after cobalt-chromium everolimus-eluting stent (CoCr-EES) implantation.
Methods
Study Design and Population
The STOPDAPT-2 trial was a multicenter, open-label, adjudicator-blinded randomized clinical trial in Japan designed to compare 1 month of DAPT with 12 months of DAPT after CoCr-EES implantation. This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan.13 The ethical committee in each participating center approved the study protocol. The protocol and statistical analysis plan are available in Supplement 2. We screened patients who underwent successful PCI with CoCr-EES (Xience Series, Abbott Vascular) without concomitant use of other types of drug-eluting stent or in-hospital major complications other than periprocedural MI. We chose CoCr-EES as the drug-eluting stent type in the present study because of its thromboresistance demonstrated in the experimental model and the consistently low rates of stent thrombosis in previous studies.12,14,15
Exclusion criteria were need for oral anticoagulation or antiplatelet therapy other than aspirin and P2Y12 receptor blockers, history of intracranial bleeding, and known intolerance to clopidogrel. Patients with scheduled staged PCI were to be enrolled after completion of all procedures. Before hospital discharge after the index PCI, eligible patients who provided written informed consent were randomly assigned in a 1-to-1 ratio either to the experimental group of 1 month of DAPT followed by clopidogrel monotherapy or to the control group of 12 months of DAPT with aspirin and clopidogrel, which was the standard antiplatelet regimen after drug-eluting stent implantation in patients with both stable coronary artery disease and acute coronary syndrome in Japan.16 Randomization was performed centrally through the electronic data capture system with a stochastic minimization algorithm to balance treatment assignment within centers. Twenty percent of patients were randomly selected for angiographic analysis in the core laboratory (Cardio Core Japan, Tokyo). The angiographic core laboratory calculated a SYNTAX (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) score, which indicated the degree of coronary anatomic complexity, ranged from 0 to greater than 50 for very complex lesions, and categorized patients as having low (≤22), intermediate (23-32), and high (≥33) coronary anatomic complexity.17 The statistician, independent clinical event committee, steering committee, and sponsor (Abbott Vascular) were blinded to study group assignments. A complete list of the study organization, participating centers, and investigators is available in eAppendix 1 in Supplement 1.
Antiplatelet Regimen
One-month DAPT regimens (given between 30 and 59 days after PCI) were either aspirin, 81 to 200 mg/d, and clopidogrel, 75 mg/d, or aspirin, 81 to 200 mg/d, and prasugrel, 3.75 mg/d, at the discretion of the attending physician. After hospital discharge, antiplatelet agents were to be prescribed not by referring practitioners but by the physicians at the participating centers. At 1 month, patients in the experimental group were to stop aspirin and receive clopidogrel monotherapy for up to 5 years, while patients in the control group were to receive DAPT with aspirin and clopidogrel for up to 12 months. For patients who had received prasugrel, prasugrel was switched to clopidogrel at 1 month in both groups. At 12 months (between 335 and 394 days), patients in the control group were to stop clopidogrel and receive aspirin monotherapy for up to 5 years. Clopidogrel was chosen as monotherapy after stopping DAPT at 1 month in the 1-month DAPT group because in the planning stage of this study in 2015, many investigators were concerned about a possible increase of stent thrombosis with very short DAPT duration, and use of a P2Y12 receptor blocker, which was demonstrated to be the key drug for prevention of stent thrombosis, might ameliorate any increase in stent thrombosis.18,19 Furthermore, stopping aspirin might be associated with lower risks of gastrointestinal and intracranial bleeding, and use of a more potent antiplatelet agent might make use of aspirin no longer necessary.20,21 Persistent DAPT discontinuation was defined as discontinuation of either aspirin or P2Y12 receptor blockers according to the study protocol or discontinuation lasting more than 60 days.
End Points
The primary end point was a composite of cardiovascular and bleeding events (cardiovascular death, MI, definite stent thrombosis, ischemic or hemorrhagic stroke, or Thrombolysis in Myocardial Infarction [TIMI] major or minor bleeding).22 The major secondary end points included the cardiovascular end point (a composite of cardiovascular death, MI, definite stent thrombosis, or ischemic or hemorrhagic stroke) and the bleeding end point (TIMI major or minor bleeding). Myocardial infarction and stent thrombosis were defined by Academic Research Consortium criteria.23 TIMI major bleeding included intracranial bleeding, a decrease in hemoglobin concentration of at least 5 g/dL, or an absolute decrease in hematocrit of at least 15%. TIMI minor bleeding included a decrease in hemoglobin concentration of at least 3 g/dL or an absolute decrease in hematocrit of at least 10% when blood loss was observed and a decrease in hemoglobin concentration of at least 4 g/dL or an absolute decrease in hematocrit of at least 12% when no blood loss was observed.22 Other prespecified secondary end points included all-cause death, death due to cardiovascular cause, MI, definite stent thrombosis, definite or probable stent thrombosis, ischemic or hemorrhagic stroke, TIMI major bleeding, TIMI minor bleeding, Bleeding Academic Research Consortium (BARC)24 type 3 or 5 bleeding, BARC type 5 bleeding, BARC type 3 bleeding, Global Use of Strategies to Open Occluded Arteries (GUSTO)25 moderate or severe bleeding, GUSTO severe bleeding, GUSTO moderate bleeding, gastrointestinal bleeding, any coronary revascularization, target lesion revascularization (TLR), clinically driven TLR, non-TLR coronary revascularization, coronary artery bypass graft surgery, a composite of death or MI, a composite of cardiovascular death or MI, and major adverse cardiac events (a composite of cardiac death, MI, and clinically driven TLR). In addition, post hoc secondary end points included death due to a cardiac cause, death due to a noncardiovascular cause, large MI (creatine kinase MB ≥10 times the upper limit of normal), small MI (creatine kinase MB <10 times the upper limit of normal), MI without creatine kinase MB elevation (troponin positive), MI without measurement of creatine kinase MB, ischemic stroke, hemorrhagic stroke, and intracranial bleeding. The definitions of clinical end points are described in eAppendix 2 in Supplement 1. Follow-up was commenced at randomization, with time interval indicated by date of index PCI. All end points were assessed at 12 months (between 335 and 394 days), with censoring on day 366. All clinical events comprising the primary end points were adjudicated based on source documents by the independent clinical event committee blinded to randomized treatment group.
Statistical Analysis
The primary hypothesis of this study was that the experimental group (1-month DAPT) was noninferior to the control group (12-month DAPT) in terms of the primary end point at 1 year. In the original protocol (June 25, 2015), a sample size of 2730 patients was calculated assuming a 4.4% estimated event rate (the 80% upper limit of the confidence interval of 4.0% event rate in the RESET trial),16 setting a noninferiority margin of 2.2% (50% of the estimated event rate) with a power of 80% and a 1-sided α = .025. In a discussion among the investigators for institutional review board review, the estimated event rate was set at 4.6% (the 90% upper limit of the confidence interval of 4.0% in the RESET trial), and the noninferiority margin was set at 2.3% (50% of the estimated event rate). As a result, the recalculated sample size was 2980 with a power of 85% and a 1-sided α = .025 (October 11, 2015). On May 30, 2017, the noninferiority margin was changed again, from an absolute noninferiority margin of 2.3% to a relative margin of 50% on the hazard ratio (HR) scale, to avoid making the margin too large in case of a lower-than-expected actual event rate. The relative noninferiority margin of 50% was chosen considering the feasibility of patient enrollment and the margins adopted in previous major trials.26,27
If noninferiority was demonstrated, superiority analysis for the primary end point was to follow. Patients were analyzed according to their randomization group after excluding patients who withdrew consent during follow-up (the intention-to-treat population). Patients with missing outcome data were censored at the time of loss to follow-up. We also performed analyses in the per-protocol and as-treated populations, which were defined as patients continuing the randomized antiplatelet regimen on day 60 excluding and including, respectively, those with protocol violations for inclusion criteria. In addition, we performed a sensitivity analysis assuming that patients lost to follow-up in the 1-month DAPT group had a primary end point event, while those in the 12-month DAPT group did not have an event. We performed subgroup analyses with interaction tests in the prespecified clinically relevant subgroups, including age 75 years or older, acute coronary syndrome, ST-segment elevation myocardial infarction, severe chronic kidney disease, diabetes, total stent length of 28 mm or longer, and 2 or more target vessels, and in post hoc subgroups based on the Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients (PARIS) thrombotic/bleeding risk scores and Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) thrombotic/bleeding risk scores.28,29 The PARIS thrombotic risk score incorporates 6 factors including diabetes, acute coronary syndrome, current smoking, creatinine clearance less than 60 mL/min, prior PCI, and prior coronary artery bypass graft surgery. The scores range from 0 to 10, and patients are grouped according to low (0-2), intermediate (3 or 4), or high (≥5) thrombotic risk. The PARIS bleeding risk score incorporates 6 factors including age, body mass index, current smoking, anemia, creatinine clearance less than 60mL/min, and triple antithrombotic therapy at discharge. The scores range from 0 to 14, with patients categorized as having low (0-3), intermediate (4-7), or high (≥8) bleeding risk. The CREDO-Kyoto thrombotic risk score incorporates 8 factors including severe chronic kidney disease, atrial fibrillation, peripheral vascular disease, anemia, age, heart failure, diabetes, and chronic total occlusion. The scores range from 0 to 12, with patients categorized as having low (0-1), intermediate (2-3), or high (≥4) thrombotic risk. The CREDO-Kyoto bleeding risk score incorporates 7 factors including thrombocytopenia, severe chronic kidney disease, peripheral vascular disease, heart failure, prior MI, malignancy, and atrial fibrillation. The scores range from 0 to 11, with patients categorized as having low (0), intermediate (1-2), or high (≥3) bleeding risk.
We conducted a noninferiority analysis (followed by a superiority analysis if noninferiority was met) for the major secondary cardiovascular end point and superiority analyses for the other 35 secondary end points. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Landmark analyses at 30 days and 60 days after the index PCI were also performed for all end points. As a post hoc analysis, a mixed-effects model was also constructed with site as a random effect for the primary end point.
Categorical variables were expressed as frequency and percentage, and continuous variables were expressed as mean with standard deviation or median with interquartile range depending on the distribution. Patients with missing values for clinical characteristics other than left ventricular ejection fraction less than 40% were regarded as not having these characteristics. We did not perform imputation for missing values for left ventricular ejection fraction. The proportion of patients with persistent DAPT discontinuation and cumulative incidences of end points were estimated by the Kaplan-Meier method, and the differences were assessed by the log-rank test. Hazard ratios and 95% confidence intervals were estimated with the Cox proportional hazards model. We used the same Cox proportional hazards model to estimate P values for interaction in the subgroup analysis. Proportional hazards assumptions were assessed on the plots of log(time) vs log(−log[survival]) and were verified as acceptable. A physician (H.W.) and a statistician (T.M.) performed all statistical analyses using JMP version 14.0 and SAS version 9.4 (SAS Institute Inc). All reported P values were 2-sided and P<.05 was regarded as statistically significant, except for noninferiority testing, in which a 1-sided P<.025 was considered statistically significant.
Results
Patient Recruitment and Randomization
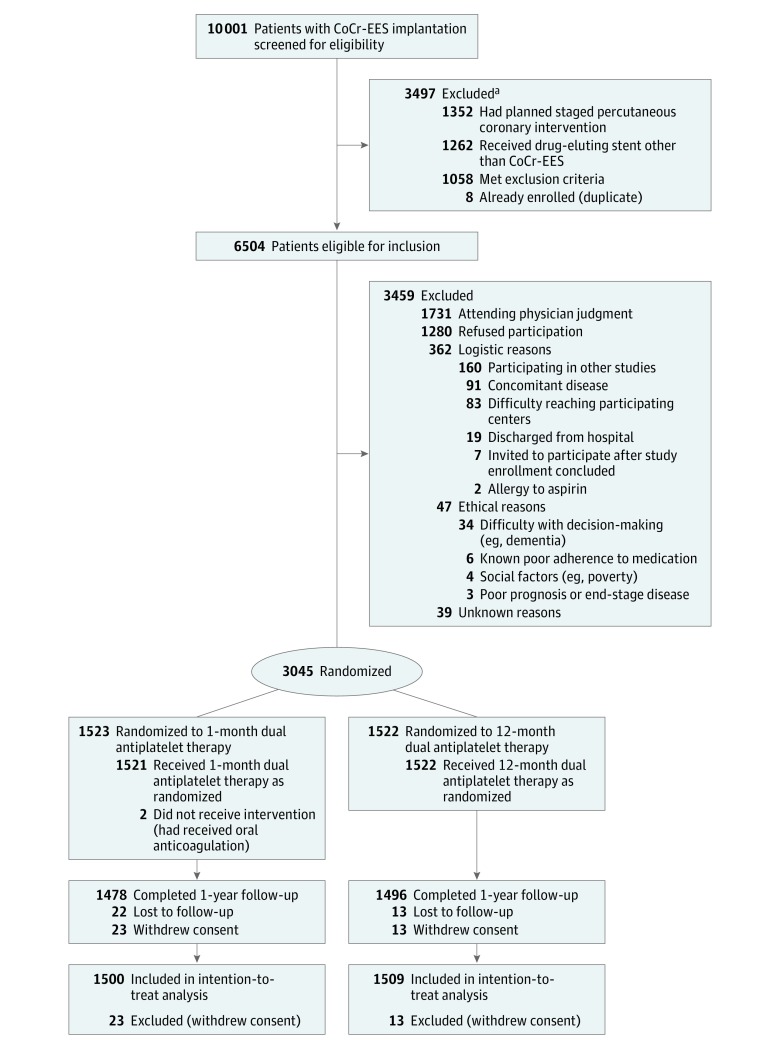
From December 25, 2015, to December 8, 2017, among 6504 patients eligible for the study, 3045 patients were randomized at 90 centers in Japan; 3459 eligible patients were not enrolled in the study, mainly because of judgment of attending physician or patient refusal. Excluding 36 patients who withdrew consent, 3009 patients were included in the main analysis: 1500 patients in the 1-month DAPT group and 1509 patients in the 12-month DAPT group (Figure 1). Randomization was performed a median of 1 day (interquartile range, 0-4 days) after the index PCI.
Figure 1. Participant Flow in the STOPDAPT-2 Randomized Clinical Trial.
CoCr-EES indicates cobalt-chromium everolimus-eluting stent.
aA total of 183 patients had planned staged procedure and received other drug-eluting stents, so numbers are not mutually exclusive.
Among patients who were eligible for the study, baseline characteristics were significantly different in several respects between patients who were or were not enrolled in the trial. The 3287 nonenrolled patients with baseline information were older (mean, 70.0 [SD, 11.7] vs 68.6 [SD, 10.7] years; P < .001) and had more ST-segment elevation MI (21.7% vs 18.6%; P = .003), more prior MI (22.7% vs 13.5%; P < .001), more prior ischemic or hemorrhagic stroke (7.7% vs 6.2%; P = .018), more prior PCI (38.1% vs 34.3%; P = .002), higher serum creatinine (mean, 1.27 [SD, 1.69] mg/dL vs 1.12 [SD, 1.36 mg/dL]; P < .001), more dialysis (5.2% vs 3.4%; P < .001), a greater number of target vessels (mean, 1.2 [SD, 0.5] vs 1.1 [SD, 0.4]; P < .001), and more often a left main coronary artery target (4.7% vs 2.7%; P < .001) than the enrolled patients (eTable 1 in Supplement 1).
Baseline Characteristics and Medications
The study population reflected a typical Japanese PCI population, including patients with advanced age (mean, 68.6 years), male sex (78%), diabetes (39%), stable coronary artery disease (62%), and acute coronary syndrome (38%). The majority of patients had low or intermediate thrombotic and bleeding risks based on both the CREDO-Kyoto risk score (92% and 93%, respectively) and the PARIS risk score (86% and 80%, respectively).28,29 Angiographic and procedural characteristics also reflected typical Japanese PCI practice, with predominance of the radial approach and intracoronary imaging guidance. The median SYNTAX score was 9 (categorized as low for coronary anatomic complexity) among 589 patients randomly selected for core laboratory assessment. Regarding medications at discharge, statins were prescribed in 88% of patients and β-blockers in 44%. Proton pump inhibitors were prescribed in 79% of patients. Baseline characteristics and medications were well balanced between the 2 groups (Table 1; eTables 2 and 3 in Supplement 1). Data were missing for prior first-generation drug-eluting stents in 2 patients, for prior MI in 1 patient, for anemia in 6 patients, for severe chronic kidney disease in 10 patients, for thrombocytopenia in 11 patients, and for left ventricular ejection fraction in 246 patients.
Table 1. Patient, Lesion, and Procedural Characteristics and Medications.
| Characteristics | 1-Month DAPT (n = 1500) | 12-Month DAPT (n = 1509) |
|---|---|---|
| Age, mean (SD), y | 68.1 (10.9) | 69.1 (10.4) |
| ≥75, No. (%) | 448 (29.9) | 499 (33.1) |
| Men, No. (%) | 1183 (78.9) | 1154 (76.5) |
| Women, No. (%) | 317 (21.1) | 355 (23.5) |
| BMI, mean (SD) | 24.4 (3.5) | 24.2 (3.5) |
| <25, No. (%) | 879 (58.6) | 936 (62.0) |
| Acute coronary syndrome, No. (%)a | 565 (37.7) | 583 (38.6) |
| ST-segment elevation myocardial infarction | 291 (19.4) | 270 (17.9) |
| Non–ST-segment elevation myocardial infarction | 81 (5.4) | 99 (6.6) |
| Unstable anginab | 193 (12.9) | 214 (14.2) |
| Stable coronary artery disease, No. (%) | 935 (62.3) | 926 (61.4) |
| Prior percutaneous coronary intervention, No. (%) | 503 (33.5) | 529 (35.1) |
| Prior first-generation drug-eluting stents, No. (%) | 65 (4.3) | 47 (3.1) |
| Prior coronary artery bypass graft surgery, No. (%) | 17 (1.1) | 42 (2.8) |
| Prior myocardial infarction, No. (%) | 207 (13.8) | 199 (13.2) |
| Prior ischemic or hemorrhagic stroke, No. (%) | 81 (5.4) | 105 (7.0) |
| Comorbidities, No. (%) | ||
| Hypertension | 1105 (73.7) | 1116 (74.0) |
| Hyperlipidemia | 1116 (74.4) | 1128 (74.8) |
| Diabetes | 585 (39.0) | 574 (38.0) |
| Requiring insulin | 104 (6.9) | 98 (6.5) |
| Current smoker | 399 (26.6) | 311 (20.6) |
| Anemiac | 121 (8.1) | 142 (9.4) |
| Heart failure | 115 (7.7) | 107 (7.1) |
| Cancer | 114 (7.6) | 142 (9.4) |
| Peripheral artery disease | 96 (6.4) | 100 (6.6) |
| Severe chronic kidney diseased | 82 (5.5) | 84 (5.6) |
| Estimated glomerular filtration rate <30 mL/min/1.73 m2 without dialysis | 30 (2.0) | 34 (2.3) |
| Dialysis | 52 (3.5) | 50 (3.3) |
| Chronic obstructive pulmonary disease | 40 (2.7) | 44 (2.9) |
| Atrial fibrillation | 35 (2.3) | 22 (1.5) |
| Prior bleeding events | 19 (1.3) | 28 (1.9) |
| Thrombocytopeniae | 15 (1.0) | 16 (1.1) |
| Cirrhosis | 6 (0.4) | 4 (0.3) |
| Left ventricular ejection fraction, mean (SD), % | 59.8 (10.2) | 59.7 (10.6) |
| <40, No. (%) | 59/1368 (4.3) | 56/1395 (4.0) |
| PARIS thrombotic risk score, median (IQR)f | 3 (1-4) | 2 (2-4) |
| High (≥5), No. (%) | 211 (14.1) | 215 (14.3) |
| Intermediate (3-4), No. (%) | 560 (37.3) | 536 (35.5) |
| Low (0-2), No. (%) | 729 (48.6) | 758 (50.2) |
| PARIS bleeding risk score, median (IQR)f | 5 (3-7) | 5 (3-7) |
| High (≥8), No. (%) | 302 (20.1) | 291 (19.3) |
| Intermediate (4-7), No. (%) | 757 (50.5) | 801 (53.1) |
| Low (0-3), No. (%) | 441 (29.4) | 417 (27.6) |
| CREDO-Kyoto thrombotic risk score, median (IQR)g | 1 (0-2) | 1 (0-2) |
| High (≥4), No. (%) | 113 (7.5) | 122 (8.1) |
| Intermediate (2-3), No. (%) | 318 (21.2) | 358 (23.7) |
| Low (0-1), No. (%) | 1069 (71.3) | 1029 (68.2) |
| CREDO-Kyoto bleeding risk score, median (IQR)g | 0 (0-1) | 0 (0-1) |
| High (≥3), No. (%) | 106 (7.1) | 112 (7.4) |
| Intermediate (1-2), No. (%) | 398 (26.5) | 401 (26.6) |
| Low (0), No. (%) | 996 (66.4) | 996 (66.0) |
| Procedural characteristics | ||
| Radial approach, No. (%) | 1232 (82.1) | 1264 (83.8) |
| Femoral approach, No. (%) | 202 (13.5) | 180 (11.9) |
| Invasive fractional flow reserve, No. (%)h | 213 (14.2) | 202 (13.4) |
| No. of target lesions, mean (SD) | 1.1 (0.4) | 1.1 (0.4) |
| Target lesion location, No. (%) | ||
| Left main coronary artery | 43 (2.9) | 37 (2.5) |
| Left anterior descending artery | 828 (55.2) | 854 (56.6) |
| Left circumflex coronary artery | 268 (17.9) | 305 (20.2) |
| Right coronary artery | 436 (29.1) | 410 (27.2) |
| Bypass graft | 3 (0.2) | 3 (0.2) |
| Chronic total occlusion, No. (%) | 55 (3.7) | 67 (4.4) |
| Bifurcation lesion, No. (%) | 376 (25.1) | 393 (26.0) |
| ≥2 Target vessels, No. (%) | 100 (6.7) | 116 (7.7) |
| Use of intravascular ultrasound, No. (%) | 1276 (85.1) | 1280 (84.8) |
| Use of optical coherence tomography, No. (%) | 210 (14.0) | 233 (15.4) |
| No. of implanted stents, mean (SD) | 1.3 (0.5) | 1.3 (0.6) |
| Minimal stent diameter, mean (SD), mm | 2.98 (0.49) | 2.96 (0.48) |
| <3.0, No. (%) | 610 (40.7) | 627 (41.6) |
| Total stent length, mean (SD), mm | 30.3 (16.7) | 30.5 (16.8) |
| ≥28, No. (%) | 742 (49.5) | 787 (52.2) |
| Medications at discharge, No. (%) | ||
| Aspirin | 1497 (99.8) | 1509 (100) |
| P2Y12 receptor blockers | 1499 (99.9) | 1508 (99.9) |
| Clopidogrel | 903 (60.2) | 949 (62.9) |
| Prasugrel | 594 (39.6) | 557 (37.0) |
| Anticoagulants | 7 (0.5) | 6 (0.4) |
| Angiotensin converting enzyme inhibitors/angiotensin II receptor blockers | 934 (62.3) | 939 (62.2) |
| β-blockers | 672 (44.8) | 643 (42.6) |
| Statins | 1318 (87.9) | 1318 (87.3) |
| Proton pump inhibitors | 1190 (79.3) | 1193 (79.1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CREDO-Kyoto, Coronary Revascularization Demonstrating Outcome Study in Kyoto; DAPT, dual antiplatelet therapy; IQR, interquartile range; PARIS, Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients.
Acute coronary syndrome was defined as myocardial infarction within 7 days or unstable angina.
Unstable angina was defined as Braunwald classification I to III, without confirmation of any biomarker elevation.
Anemia was defined as a preprocedural hemoglobin level less than 11 g/dL in both men and women.
Severe chronic kidney disease was defined as a preprocedural estimated glomerular filtration rate less than 30 mL/min/1.73 m2 or receipt of maintenance dialysis. Preprocedural creatinine data were missing for 10 patients. Two of these patients who were undergoing dialysis were included in severe chronic kidney disease, while the other 8 patients were regarded as not having severe chronic kidney disease.
Thrombocytopenia was defined as a preprocedural platelet count less than 100×109/L.
The PARIS thrombotic risk score ranges from 0 to 12 and is categorized as low (0-2), intermediate (3-4), and high (≥5) thrombotic risk. The PARIS bleeding risk score ranges from 0 to 15 and is categorized as low (0-3), intermediate (4-7), and high (≥8) bleeding risk.
The CREDO-Kyoto thrombotic risk score ranges from 0 to 12 and is categorized as low (0-1), intermediate (2-3), and high (≥4) thrombotic risk. The CREDO-Kyoto bleeding risk score ranges from 0 to 11 and is categorized as low (0), intermediate (1 or 2), and high (≥3) bleeding risk.
Invasive fractional flow reserve by intracoronary flow wire, not by computed tomography.
Antiplatelet Therapy
For DAPT treatment during month 1, the selected P2Y12 receptor blocker was clopidogrel in 62% of patients and prasugrel in 38% of patients. In the 1-month DAPT group, DAPT was stopped in 150 patients (10.0%) during the first 30 days, in 752 patients (50.1%) during the first 37 days, in 1090 patients (72.7%) during the first 44 days, in 1286 patients (85.7%) during the first 51 days, and in 1428 patients (95.2%) during the first 60 days, while in the 12-month DAPT group, DAPT was maintained in 1331 patients (88.2%) for 335 days and in 848 patients (56.2%) for 365 days (eFigure 1 in Supplement 1).
1-Year Clinical Outcomes
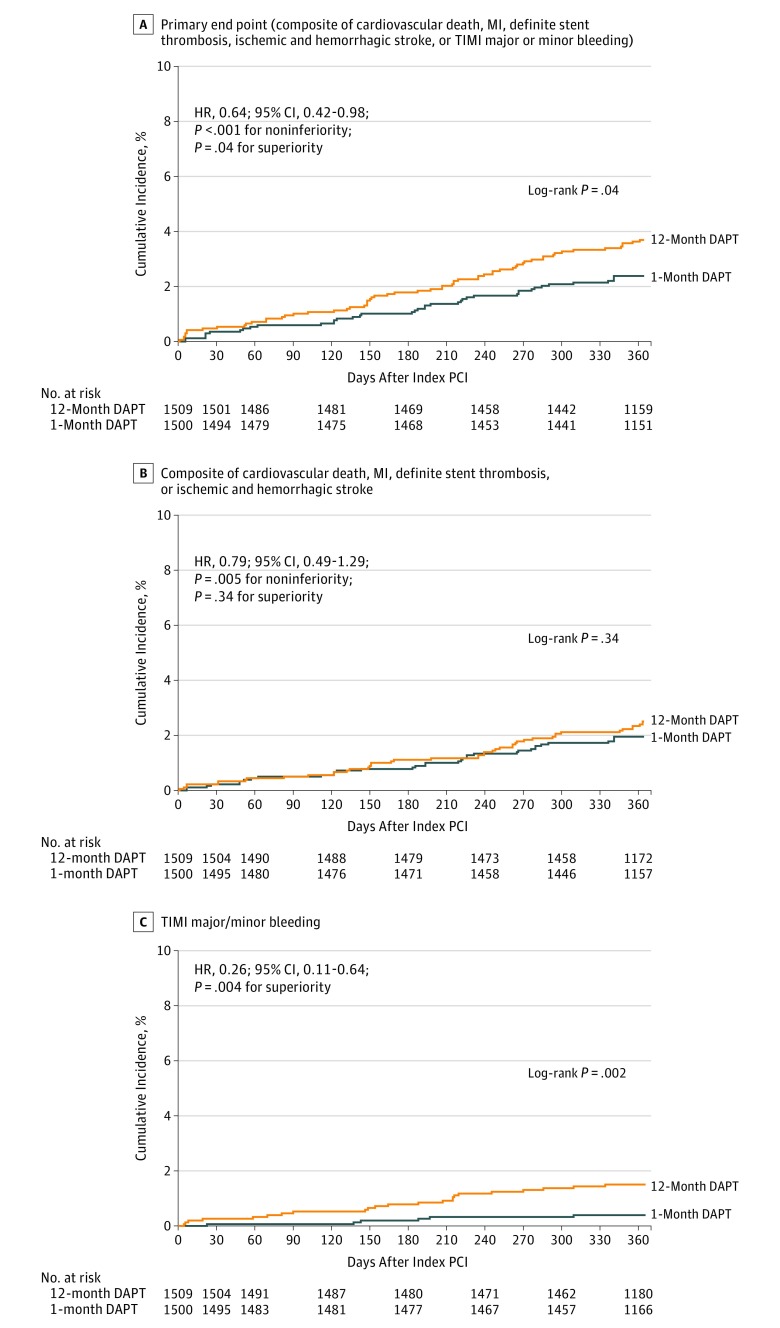
Final 1-year clinical follow-up was completed in January 2019. Complete 1-year clinical follow-up was achieved in 2974 patients (98.8%) (Figure 1). The primary end point occurred in 35 patients (2.36%) in the 1-month DAPT group and in 55 patients (3.70%) in the 12-month DAPT group. One month of DAPT met criteria for noninferiority and also met criteria for superiority to 12 months of DAPT for the primary end point (absolute difference, −1.34% [95% CI, −2.57% to −0.11%]; HR, 0.64 [95% CI, 0.42-0.98]; P < .001 for noninferiority; P = .04 for superiority) (Figure 2A and Table 2). From the post hoc analysis model with site as a random effect, the effects of multiple sites were nonsignificant (eTable 4 in Supplement 1). Noninferiority of 1 month of DAPT compared with 12 months of DAPT was also confirmed for the primary end point in the per-protocol population (absolute difference, −0.62% [95% CI, −1.77% to 0.53%]; HR, 0.77 [95% CI, 0.47-1.26]; P = .004 for noninferiority) and in the as-treated population (absolute difference −0.30% [95% CI, −1.45% to .85%]; HR, 0.89 [95% CI, 0.56-1.42]; P = .015 for noninferiority) as well as in the sensitivity analysis (absolute difference, −0.02% [95% CI, −1.38% to 1.34%]; HR, 1.00 [95% CI, 0.69-1.46]; P = .02 for noninferiority) (eTable 5 and eFigures 2-4 in Supplement 1).
Figure 2. One-Year Time to Events for the Primary and Major Secondary End Points.
HR indicates hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction. The median observation periods in the last data set were 400 (interquartile range, 368-732) days in the 1-month dual antiplatelet therapy (DAPT) group and 414 (interquartile range, 369-733) days in the 12-month DAPT group. The last day of data collection was day 365; patients with follow-up beyond 1 year were censored on day 366.
Table 2. Clinical Outcomes at 1 Year.
| Outcomes | No. of Patients With Event (Cumulative Incidence, %)a | Hazard Ratio (95% CI) | P Valueb | ||
|---|---|---|---|---|---|
| 1-Month DAPT (n = 1500) | 12-Month DAPT (n = 1509) | Noninferiority | Superiority | ||
| Primary End Point | |||||
| Composite of cardiovascular death, myocardial infarction, definite stent thrombosis, ischemic or hemorrhagic stroke, or TIMI major or minor bleeding | 35 (2.36) | 55 (3.70) | 0.64 (0.42-0.98) | <.001 | .04 |
| Major Secondary End Points | |||||
| Cardiovascular end point: composite of cardiovascular death, myocardial infarction, definite stent thrombosis, or ischemic or hemorrhagic stroke | 29 (1.96) | 37 (2.51) | 0.79 (0.49-1.29) | .005 | .34 |
| Bleeding end point: TIMI major or minor bleeding | 6 (0.41) | 23 (1.54) | 0.26 (0.11-0.64) | .004 | |
| Other Secondary End Points | |||||
| Death | 21 (1.42) | 18 (1.21) | 1.18 (0.63-2.21) | .61 | |
| Death due to cardiac cause (post hoc) | 8 (0.54) | 8 (0.54) | 1.01 (0.38-2.69) | .98 | |
| Death due to cardiovascular cause | 9 (0.61) | 11 (0.74) | 0.83 (0.34-1.99) | .67 | |
| Death due to noncardiovascular cause (post hoc) | 12 (0.82) | 7 (0.47) | 1.73 (0.68-4.40) | .25 | |
| Myocardial infarction | 13 (0.88) | 11 (0.75) | 1.19 (0.54-2.67) | .66 | |
| Large myocardial infarction (CK-MB ≥10 × ULN) (post hoc) | 5 (0.34) | 2 (0.13) | 2.52 (0.49-13.01) | .27 | |
| Small myocardial infarction (CK-MB <10 × ULN) (post hoc) | 7 (0.48) | 5 (0.34) | 1.42 (0.45-4.46) | .55 | |
| Myocardial infarction without CK-MB elevation (post hoc) | 1 (0.07) | 2 (0.14) | 0.51 (0.05-5.59) | .58 | |
| Myocardial infarction without measurement of CK-MB (post hoc) | 0 | 2 (0.13) | |||
| Definite stent thrombosis | 2 (0.13) | 1 (0.07) | 2.02 (0.18-22.26) | .57 | |
| Definite or probable stent thrombosis | 4 (0.27) | 1 (0.07) | 4.03 (0.45-36.08) | .21 | |
| Stroke (ischemic or hemorrhagic) | 8 (0.54) | 16 (1.09) | 0.50 (0.22-1.18) | .11 | |
| Ischemic (post hoc) | 8 (0.54) | 15 (1.03) | 0.54 (0.23-1.27) | .16 | |
| Hemorrhagic (post hoc) | 0 | 1 (0.07) | |||
| Bleedingc | |||||
| TIMI major | 3 (0.20) | 16 (1.07) | 0.19 (0.05-0.65) | .01 | |
| TIMI minor | 3 (0.20) | 7 (0.47) | 0.43 (0.11-1.67) | .22 | |
| BARC type 3 or 5 | 8 (0.54) | 27 (1.81) | 0.30 (0.13-0.65) | .003 | |
| BARC type 5 | 1 (0.07) | 3 (0.20) | 0.34 (0.03-3.23) | .34 | |
| BARC type 3 | 7 (0.47) | 24 (1.61) | 0.29 (0.13-0.68) | .004 | |
| GUSTO moderate or severe | 6 (0.40) | 23 (1.54) | 0.26 (0.11-0.64) | .004 | |
| GUSTO severe | 4 (0.27) | 11 (0.74) | 0.37 (0.12-1.15) | .09 | |
| GUSTO moderate | 2 (0.14) | 12 (0.80) | 0.17 (0.04-0.75) | .02 | |
| Intracranial (post hoc) | 2 (0.14) | 5 (0.34) | 0.40 (0.08-2.08) | .29 | |
| Gastrointestinal | 6 (0.40) | 19 (1.27) | 0.32 (0.13-0.79) | .01 | |
| Death or myocardial infarction | 32 (2.17) | 29 (1.95) | 1.11 (0.67-1.84) | .67 | |
| Cardiovascular death or myocardial infarction | 21 (1.42) | 22 (1.48) | 0.96 (0.53-1.75) | .90 | |
| Major adverse cardiac eventsd | 38 (2.57) | 32 (2.19) | 1.20 (0.75-1.93) | .44 | |
| Any coronary revascularizatione | 98 (6.77) | 76 (5.26) | 1.31 (0.97-1.77) | .08 | |
| TLR | 35 (2.38) | 23 (1.60) | 1.55 (0.91-2.62) | .10 | |
| Clinically driven | 26 (1.77) | 19 (1.32) | 1.39 (0.77-2.51) | .28 | |
| Non-TLR | 71 (4.93) | 60 (4.13) | 1.20 (0.85-1.69) | .30 | |
| Coronary artery bypass graft surgery | 6 (0.42) | 5 (0.34) | 1.21 (0.37-3.98) | .75 | |
Abbreviations: BARC, Bleeding Academic Research Consortium; CK-MB, creatine kinase MB; DAPT, dual antiplatelet therapy; GUSTO, Global Use of Strategies to Open Occluded Arteries; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction; TLR, target lesion revascularization; ULN, upper limit of normal.
Percentages are Kaplan-Meier estimates at day 365.
P values are derived from Cox proportional hazards model.
For details of the TIMI, BARC, and GUSTO bleeding criteria, see eAppendix 3 in Supplement 1.
Major adverse cardiac events are defined as a composite of cardiac death, myocardial infarction, and clinically TLR.
Clinical events after randomization.
For the major secondary cardiovascular end point, 1 month of DAPT also met criteria for noninferiority to 12 months of DAPT (1.96% vs 2.51%; absolute difference, −0.55% [95% CI, −1.62% to 0.52%]; HR, 0.79 [95% CI, 0.49-1.29]; P = .005 for noninferiority; P = .34 for superiority). For the major secondary bleeding end point, 1 month of DAPT was superior to 12 months of DAPT (0.41% vs 1.54%; absolute difference, −1.13% [95% CI, −1.84% to −0.42%]; HR, 0.26 [95% CI, 0.11-0.64]; P = .004) (Figure 2, B and C, and Table 2). The incidence of bleeding was consistently lower in the 1-month DAPT group than in the 12-month DAPT group by BARC type 3 or 5 criteria (0.54% vs 1.81%; absolute difference, −1.27% [95% CI, −2.03% to −0.51%]; HR, 0.30 [95% CI, 0.13-0.65]; P = .003) and by GUSTO moderate or severe criteria (0.40% vs 1.54%; absolute difference, −1.14% [95% CI, −1.84% to −0.44%]; HR, 0.26 [95% CI, 0.11-0.64]; P = .004) (Table 2). Five additional secondary bleeding end points were statistically significantly more frequent in the 12-month DAPT group than in the 1-month DAPT group (Table 2). The incidence of definite or probable stent thrombosis was very low: 4 patients (0.27%) in the 1-month DAPT group and 1 patient (0.07%) in the 12-month DAPT group. Two probable stent thrombosis events in the 1-month DAPT group occurred within 1 month before stopping aspirin (eTable 6 in Supplement 1).
The results from the 30-day and 60-day landmark analyses were consistent with the main analyses for the primary end point (30 days: 2.04% vs 3.25%; absolute difference, −1.21% [95% CI, −2.37% to −0.05%]; HR, 0.63 [95% CI, 0.40-0.99]; P < .001 for noninferiority; P = .045 for superiority; 60 days: 1.84% vs 2.99%; absolute difference, −1.15% [95% CI, −2.25% to −0.05%]; HR, 0.62 [95% CI, 0.38-0.99]; P < .001 for noninferiority; P = .047 for superiority) (eTables 7 and 8 in Supplement 1).
Subgroup Analysis
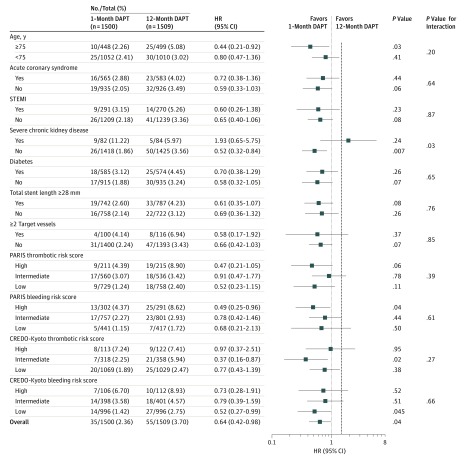
In the subgroup analysis, the lower risk of 1 month of DAPT compared with 12 months of DAPT for the primary end point was consistently seen across subgroups except for the small subgroup of patients with severe chronic kidney disease (P = .03 for interaction). Patients with high PARIS and CREDO-Kyoto thrombotic risk scores had numerically higher incidences of the primary end point than patients with intermediate or low scores (PARIS: high, 8.90%; intermediate, 3.42%; and low, 2.40% in the 12-month DAPT group and high, 4.39%; intermediate, 3.07%; and low, 1.24% in the 1-month DAPT group; CREDO-Kyoto: high, 7.41%; intermediate, 5.94%; and low, 2.47% in the 12-month DAPT group and high, 7.24%; intermediate, 2.25%; and low, 1.89% in the 1-month DAPT group). However, there was no significant interaction between the subgroup factors of PARIS and CREDO-Kyoto thrombotic risk scores and the effects of 1 month of DAPT compared with 12 months of DAPT on the primary end point (P = .39 [PARIS] and P = .27 [CREDO-Kyoto] for interaction) (Figure 3).
Figure 3. Subgroup Analyses for the Effect of 1-Month DAPT on the Primary End Point.
CREDO-Kyoto indicates Coronary Revascularization Demonstrating Outcome Study in Kyoto; HR, hazard ratio; PARIS, Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients; STEMI, ST-segment elevation myocardial infarction. The vertical dashed line indicates the prespecified relative 50% noninferiority margin. Numbers and percentages shown are number of patients with event/number of patients at risk and incidences at 1 year. Acute coronary syndrome was the clinical presentation for the index percutaneous coronary intervention. Severe chronic kidney disease is defined as preprocedural estimated glomerular filtration rate less than 30 mL/min/1.73 m2 or undergoing maintenance dialysis. See Table 1 for definitions of PARIS and CREDO-Kyoto risk scores.
Discussion
In this randomized clinical trial, 1 month of DAPT followed by clopidogrel monotherapy met criteria for noninferiority and also was associated with a net clinical benefit for the primary end point, a composite of cardiovascular and bleeding events, compared with 12 months of DAPT with aspirin and clopidogrel after CoCr-EES implantation. In addition, 1 month of DAPT was noninferior for the cardiovascular composite secondary end point and superior for the major secondary bleeding end point compared with 12 months of DAPT.
In previous studies, attempts to deescalate the intensity of DAPT were initiated mainly in patients at high bleeding risk. The LEADERS FREE trial compared drug-coated stents with bare-metal stents under the protocol of 1 month of DAPT in patients at high bleeding risk.30 Despite a very short duration of DAPT, the 1-year incidence of major bleeding (BARC type 3, 4, or 5) was as high as 7%. Therefore, the standard DAPT regimen would not be appropriate, and further de-escalation of antiplatelet therapy might be preferable in these patients at high risk of bleeding. The GLOBAL LEADERS trial explored an experimental regimen of 1 month of DAPT followed by ticagrelor monotherapy for up to 24 months compared with the standard 12 months of DAPT followed by aspirin monotherapy for up to 24 months regardless of patients’ bleeding risk. The post hoc analysis within 12 months demonstrated significant reduction of the primary end point of all-cause death and new Q-wave MI in the experimental group, suggesting a possible benefit of stopping aspirin at 1 month followed by ticagrelor monotherapy, although the overall trial result was negative at 2 years.31
The present study also explored 1 month of DAPT after CoCr-EES implantation. One month of DAPT followed by clopidogrel monotherapy provided a net clinical benefit for a composite of cardiovascular and bleeding events compared with 12 months of DAPT with aspirin and clopidogrel. The benefit was driven by a significant reduction of bleeding events without an increase in cardiovascular events. Therefore, the very short DAPT duration of 1 month would be a potential option even in patients without high bleeding risk. Given the very low rates of stent thrombosis in studies using contemporary drug-eluting stents, avoiding bleeding with de-escalation of antiplatelet therapy may be more important than attempting further reduction of stent thrombosis with intensive antiplatelet therapy.12,15,16 There may be some patients with very high ischemic risk, who might benefit from more intensive antithrombotic therapy. Nevertheless, in the subgroup analysis of the present study, there was no interaction between thrombotic risk scores and the effect of 1 month of DAPT compared with 12 months of DAPT for the primary end point. Furthermore, in general, patients with very high ischemic risk also have high bleeding risk, making the choice of intensive antithrombotic therapy difficult.28 Further studies would be needed to reconcile the concept of very short mandatory DAPT duration with the demonstrated ischemic benefit with more intensive antithrombotic regimens such as DAPT using ticagrelor or low-dose rivaroxaban with aspirin in patients with very high ischemic risk.32,33 This study suggested that 1 month of DAPT may be sufficient after PCI using CoCr-EES in a population at low ischemic risk such as was enrolled in the present study. Very short DAPT in a population at high bleeding risk may be a viable option but needs further study because of the high ischemic risk of this population.
Limitations
This study has several limitations. First, a composite end point assessing both cardiovascular and bleeding events was used as the primary end point to evaluate the net clinical benefit. However, a consensus has not been yet reached on the definition and validity of the end point evaluating net clinical benefit, although it would be relevant for comparing a given antithrombotic regimen with another.34 Second, the present study was not powered for noninferiority for the major secondary cardiovascular composite end point. Third, the present study could not assess the risk of stent thrombosis with very short DAPT. Fourth, the lower-than-expected actual event rate for the primary end point reduced the statistical power of this noninferiority study. In addition, patients were randomized not at 1 month but shortly after PCI. Therefore, the noninferiority analysis included patients who had an event before 1 month, making the difference between the 1-month and 12-month DAPT groups smaller. However, the results from the 30-day landmark analysis excluding patients who had an event before 1 month were fully consistent with the main results. Fifth, the majority of enrolled patients had low or intermediate ischemic risk. Many eligible patients were not enrolled in the study by the judgment of the attending physicians, suggesting the possibility of selective enrollment of patients with low ischemic risk. Indeed, among the patients who were eligible for the study, the baseline characteristics were different in several aspects between patients who did vs did not enroll in the trial. However, the majority of patients in the derivation cohort of the CREDO-Kyoto risk score, in which patients with first coronary revascularization were consecutively enrolled, also had low or intermediate ischemic risk, suggesting that patients with high ischemic risk may not be dominant in the Japanese PCI population.28 Regarding the generalizability of the present study results, further research would be warranted in patients with high ischemic risk. Sixth, we chose clopidogrel rather than the more commonly used aspirin as the antiplatelet agent for monotherapy in the 1-month DAPT group. Therefore, we could not assess the role of aspirin monotherapy shortly after the very short DAPT period, although the incidence of adverse events with aspirin monotherapy beyond 3 months was acceptable in the STOPDAPT study.12 In terms of long-term therapy, clopidogrel monotherapy will continue to be compared with aspirin monotherapy beyond 12 months and up to 5 years in the present study. Seventh, it is well known that Japanese patients with coronary artery disease have lower ischemic risk compared with US and European patients.9,35,36 Furthermore, potent P2Y12 receptor blockers such as ticagrelor or standard-dose prasugrel were not available in Japan. In addition, the vast majority of patents in this study underwent PCI guided by intracoronary imaging devices, which are rarely used in the United States and Europe. Therefore, caution is warranted in extrapolating the current study results outside of Japan. Eighth, this study was conducted exclusively in patients who received CoCr-EES, and therefore, it is unknown whether the present study results may be extrapolated to other currently used drug-eluting stents. Ninth, the open-label trial design has inherent limitations. However, the majority of patients followed the assigned antiplatelet regimen appropriately, and the components of the primary composite end point in this study were less likely to be affected by the open-label trial design.
Conclusions
Among patients undergoing PCI using CoCr-EES, 1 month of DAPT followed by clopidogrel monotherapy, compared with 12 months of DAPT with aspirin and clopidogrel, resulted in a significantly lower rate of a composite of cardiovascular and bleeding events, meeting criteria for both noninferiority and superiority. These findings suggest that a shorter duration of DAPT may provide benefit, although given the study limitations, additional research is needed in other populations.
eAppendix 1. Study Organization and Participating Centers
eAppendix 2. Primary and Secondary Endpoints and the Time Point to Assessment
eAppendix 3. Definition of Endpoints
eTable 1. Clinical and Procedural Characteristics Compared Between the Enrolled Patients and the Eligible But Non-enrolled Patients
eTable 2. Complete Baseline Characteristics
eTable 3. Baseline Angiographic Data Analyzed in the Angiographic Core Laboratory
eTable 4. Post Hoc Analysis of the Mixed Effect Model With Site as a Random Effect for the Primary Endpoint
eTable 5. Per-Protocol and As-Treated Population According to the Mode of Antithrombotic Therapy at 60 Days
eTable 6. Details of Cases With Definite or Probable Stent Thrombosis
eTable 7. Clinical Outcomes With Landmark Analysis at 30 Days and up to 1 Year
eTable 8. Clinical Outcomes With Landmark Analysis at 60 Days and up to 1 Year
eFigure 1. DAPT Discontinuation Rate
eFigure 2. Per-Protocol Analysis for the Primary Endpoint
eFigure 3. As-Treated Analysis for the Primary Endpoint
eFigure 4. Sensitivity Analysis
Study Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123-e155. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines; ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for Dual Antiplatelet Therapy in Coronary Artery Disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 3.Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385(9985):2371-2382. doi: 10.1016/S0140-6736(15)60263-X [DOI] [PubMed] [Google Scholar]
- 4.Toyota T, Shiomi H, Morimoto T, Natsuaki M, Kimura T. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12(9):e0174502. doi: 10.1371/journal.pone.0174502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauri L, Kereiakes DJ, Yeh RW, et al. ; DAPT Study Investigators . Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155-2166. doi: 10.1056/NEJMoa1409312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Räber L, Magro M, Stefanini GG, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125(9):1110-1121. doi: 10.1161/CIRCULATIONAHA.111.058560 [DOI] [PubMed] [Google Scholar]
- 7.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389. [PubMed] [Google Scholar]
- 8.Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-1504. doi: 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 9.Taguchi I, Iimuro S, Iwata H, et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation. 2018;137(19):1997-2009. doi: 10.1161/CIRCULATIONAHA.117.032615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Généreux P, Giustino G, Witzenbichler B, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66(9):1036-1045. doi: 10.1016/j.jacc.2015.06.1323 [DOI] [PubMed] [Google Scholar]
- 11.Valgimigli M, Costa F, Lokhnygina Y, et al. Trade-off of myocardial infarction vs bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. 2017;38(11):804-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natsuaki M, Morimoto T, Yamamoto E, et al. One-year outcome of a prospective trial stopping dual antiplatelet therapy at 3 months after everolimus-eluting cobalt-chromium stent implantation: Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent (STOPDAPT) trial. Cardiovasc Interv Ther. 2016;31(3):196-209. doi: 10.1007/s12928-015-0366-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Otsuka F, Cheng Q, Yahagi K, et al. Acute thrombogenicity of a durable polymer everolimus-eluting stent relative to contemporary drug-eluting stents with biodegradable polymer coatings assessed ex vivo in a swine shunt model. JACC Cardiovasc Interv. 2015;8(9):1248-1260. doi: 10.1016/j.jcin.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 15.Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2015;65(23):2496-2507. doi: 10.1016/j.jacc.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 16.Kimura T, Morimoto T, Natsuaki M, et al. ; RESET Investigators . Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-Eluting Versus Everolimus-Eluting Stent Trial (RESET). Circulation. 2012;126(10):1225-1236. doi: 10.1161/CIRCULATIONAHA.112.104059 [DOI] [PubMed] [Google Scholar]
- 17.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219-227. [PubMed] [Google Scholar]
- 18.Leon MB, Baim DS, Popma JJ, et al. ; Stent Anticoagulation Restenosis Study Investigators . A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med. 1998;339(23):1665-1671. doi: 10.1056/NEJM199812033392303 [DOI] [PubMed] [Google Scholar]
- 19.Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334(17):1084-1089. doi: 10.1056/NEJM199604253341702 [DOI] [PubMed] [Google Scholar]
- 20.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348(9038):1329-1339. doi: 10.1016/S0140-6736(96)09457-3 [DOI] [PubMed] [Google Scholar]
- 21.Capodanno D, Mehran R, Valgimigli M, et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018;15(8):480-496. doi: 10.1038/s41569-018-0049-1 [DOI] [PubMed] [Google Scholar]
- 22.Rao AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) trial—phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11(1):1-11. doi: 10.1016/0735-1097(88)90158-1 [DOI] [PubMed] [Google Scholar]
- 23.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 24.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 25.GUSTO investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329(10):673-682. doi: 10.1056/NEJM199309023291001 [DOI] [PubMed] [Google Scholar]
- 26.Serruys PW, Morice MC, Kappetein AP, et al. ; SYNTAX Investigators . Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972. doi: 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 27.Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364(18):1718-1727. doi: 10.1056/NEJMoa1100452 [DOI] [PubMed] [Google Scholar]
- 28.Natsuaki M, Morimoto T, Yamaji K, et al. ; CREDO‐Kyoto PCI/CABG Registry Cohort 2, RESET, and NEXT Trial Investigators . Prediction of thrombotic and bleeding events after percutaneous coronary intervention: CREDO-Kyoto thrombotic and bleeding risk scores. J Am Heart Assoc. 2018;7(11):e008708. doi: 10.1161/JAHA.118.008708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baber U, Mehran R, Giustino G, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67(19):2224-2234. doi: 10.1016/j.jacc.2016.02.064 [DOI] [PubMed] [Google Scholar]
- 30.Urban P, Meredith IT, Abizaid A, et al. ; LEADERS FREE Investigators . Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373(21):2038-2047. doi: 10.1056/NEJMoa1503943 [DOI] [PubMed] [Google Scholar]
- 31.Vranckx P, Valgimigli M, Jüni P, et al. ; GLOBAL LEADERS Investigators . Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940-949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 32.Bonaca MP, Bhatt DL, Cohen M, et al. ; PEGASUS-TIMI 54 Steering Committee and Investigators . Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791-1800. doi: 10.1056/NEJMoa1500857 [DOI] [PubMed] [Google Scholar]
- 33.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Garcia HM, McFadden EP, Farb A, et al. ; Academic Research Consortium . Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 consensus document. Circulation. 2018;137(24):2635-2650. doi: 10.1161/CIRCULATIONAHA.117.029289 [DOI] [PubMed] [Google Scholar]
- 35.LaRosa JC, Grundy SM, Waters DD, et al. ; Treating to New Targets Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425-1435. doi: 10.1056/NEJMoa050461 [DOI] [PubMed] [Google Scholar]
- 36.Onuma Y, Kimura T, Räber L, et al. ; Bern-Rotterdam and j-Cypher Registries . Differences in coronary risk factors, procedural characteristics, mortality and stent thrombosis between two all-comers percutaneous coronary intervention registries from Europe and Japan: a patient-level data analysis of the Bern-Rotterdam and j-Cypher registries. EuroIntervention. 2015;11(5):533-540. doi: 10.4244/EIJY14M06_09 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Study Organization and Participating Centers
eAppendix 2. Primary and Secondary Endpoints and the Time Point to Assessment
eAppendix 3. Definition of Endpoints
eTable 1. Clinical and Procedural Characteristics Compared Between the Enrolled Patients and the Eligible But Non-enrolled Patients
eTable 2. Complete Baseline Characteristics
eTable 3. Baseline Angiographic Data Analyzed in the Angiographic Core Laboratory
eTable 4. Post Hoc Analysis of the Mixed Effect Model With Site as a Random Effect for the Primary Endpoint
eTable 5. Per-Protocol and As-Treated Population According to the Mode of Antithrombotic Therapy at 60 Days
eTable 6. Details of Cases With Definite or Probable Stent Thrombosis
eTable 7. Clinical Outcomes With Landmark Analysis at 30 Days and up to 1 Year
eTable 8. Clinical Outcomes With Landmark Analysis at 60 Days and up to 1 Year
eFigure 1. DAPT Discontinuation Rate
eFigure 2. Per-Protocol Analysis for the Primary Endpoint
eFigure 3. As-Treated Analysis for the Primary Endpoint
eFigure 4. Sensitivity Analysis
Study Protocol and Statistical Analysis Plan
Data Sharing Statement