Key Points
Question
Is use of mineralocorticoid receptor antagonist at discharge associated with better outcomes in patients hospitalized for acute decompensated heart failure?
Findings
In this cohort study of 2068 propensity score–matched Japanese patients hospitalized for acute decompensated heart failure, mineralocorticoid receptor antagonist administered at discharge was statistically significantly associated with a lower risk for the primary composite outcome of mortality or heart failure readmission, although no difference in all-cause death was observed.
Meaning
Use of mineralocorticoid receptor antagonist at discharge from acute decompensated heart failure hospitalization may be associated with heart failure hospitalization but not with lower mortality.
Abstract
Importance
Scarce data are available on the association of mineralocorticoid receptor antagonist (MRA) use with outcomes in acute decompensated heart failure (ADHF).
Objective
To investigate the association of MRA use with all-cause mortality and hospital readmission in patients with ADHF.
Design, Setting, and Participants
This cohort study examines participants enrolled in the Kyoto Congestive Heart Failure (KCHF) registry, a physician-initiated, prospective, multicenter cohort study of consecutive patients admitted for ADHF, between October 1, 2014, and March 31, 2016, into 1 of 19 secondary and tertiary hospitals throughout Japan. To balance the baseline characteristics associated with the selection of MRA use, a propensity score–matched cohort design was used, yielding 2068 patients. Data analysis was conducted from April to August 2018.
Exposures
Prescription of MRA at discharge from the index hospitalization.
Main Outcomes and Measures
Composite of all-cause death or heart failure hospitalization after discharge.
Results
Among 3717 patients hospitalized for ADHF, 1678 patients (45.1%) had received MRA at discharge and 2039 (54.9%) did not. After propensity score matching, 2068 patients (with a median [interquartile range] age of 80 [72-86] years, and of whom 937 [45.3%] were women) were included. In the matched cohort (n = 1034 in each group), the cumulative 1-year incidence of the primary outcome was statistically significantly lower in the MRA use group than in the no MRA use group (28.4% vs 33.9%; hazard ratio [HR], 0.81; 95% CI, 0.70-0.93; P = .003). Of the components of the primary outcome, the cumulative 1-year incidence of heart failure hospitalization was significantly lower in the MRA use group than in the no MRA use group (18.7% vs 24.8%; HR, 0.70; 95% CI, 0.60-0.86; P < .001), whereas no difference in mortality was found between the 2 groups (15.6% vs 15.8%; HR, 0.98; 95% CI, 0.82-1.18; P = .85). No difference in all-cause hospitalization was observed between the 2 groups (35.3% vs 38.2%; HR, 0.88; 95% CI, 0.77-1.01; P = .07). In additional analyses that stratified by left ventricular ejection fraction, the association of MRA use with the primary outcome was statistically significant in patients with left ventricular ejection fraction of 40% or greater.
Conclusions and Relevance
Use of MRA at discharge from ADHF hospitalization did not appear to be associated with lower mortality but was associated with a lower risk of heart failure readmission. This finding suggests that MRA treatment at discharge may have minimal, if any, clinical advantages.
This cohort study of Japanese participants with acute decompensated heart failure in the Kyoto Congestive Heart Failure study evaluates the association of mineralocorticoid receptor antagonist use with all-cause mortality and hospital readmission.
Introduction
Mineralocorticoid receptor antagonists (MRAs), such as spironolactone and eplerenone, have been associated with reductions in mortality in patients with stable chronic heart failure with reduced ejection fraction (HFrEF).1,2 In patients with stable heart failure with preserved ejection fraction (HFpEF), a randomized clinical trial (RCT) has suggested that MRA is associated with reductions in heart failure hospitalization, although the study did not meet the primary composite end point of death from cardiovascular causes, aborted cardiac arrest, or heart failure hospitalization.3,4
In contrast, scarce data are available on the long-term outcomes of MRA use after discharge of patients hospitalized for acute decompensated heart failure (ADHF).5 Because these patients had experienced the acute exaggeration of heart failure and hospital admission, they had a high risk for cardiac mortality and rehospitalization owing to worsening of heart failure.6 In addition, the contemporary patient population hospitalized for ADHF might be substantially different from stable patients with heart failure who are enrolled in RCTs. The role of MRA in postdischarge management needs to be investigated in patients with ADHF and multiple comorbidities. Therefore, we sought to explore the association between MRA administered at discharge from ADHF hospitalization and clinical outcomes using the registry of a large contemporary all-comer study (Kyoto Congestive Heart Failure [KCHF]) in Japan of patients with ADHF hospitalization.
Methods
Study Design, Setting, and Population
The KCHF is a physician-initiated, prospective, multicenter cohort study that enrolled consecutive patients who were hospitalized for ADHF for the first time between October 1, 2014, and March 31, 2016. These patients were admitted into 19 secondary and tertiary hospitals, including rural and urban as well as large and small institutions, throughout Japan. The study protocol was approved by the institutional review board of each participating hospital. A waiver of written informed consent from each patient was granted by the institutional review boards of Kyoto University and each participating center because the study met the conditions of the Japanese ethical guidelines for epidemiological study and the US policy for protecting human research participants.7,8 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The details of the KCHF study design and patient enrollment are described elsewhere.9,10 Briefly, we enrolled all patients with ADHF, as defined by the modified Framingham criteria, who were admitted to the participating hospitals and patients who underwent heart failure–specific treatment involving intravenous drugs within 24 hours after hospital presentation. Patient records were anonymized before analysis. Data analysis was conducted from April 2018 to August 2018.
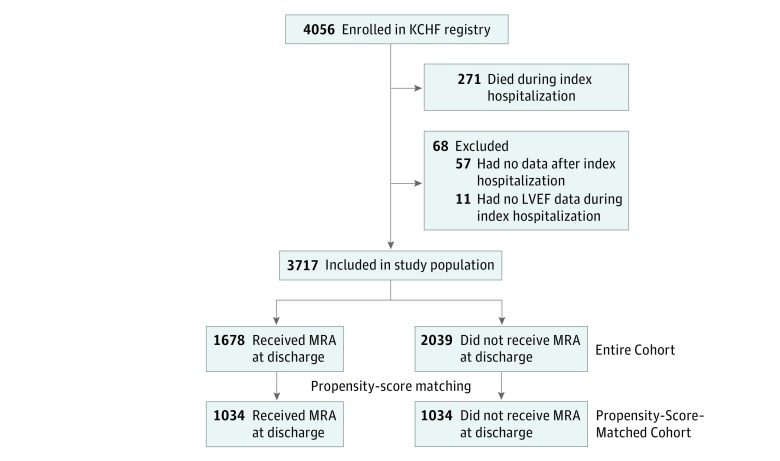
Among the 4056 enrolled patients in the KCHF registry, 3785 patients (93.3%) were discharged alive after hospitalization for ADHF. Clinical follow-up data were collected in October 2017. The attending physicians or research assistants at each participating facility collected clinical events data after the index hospitalization from hospital medical records or from patients, their relatives, or their referring physicians (with patient consent).
In the present study, we compared the clinical outcomes between patients who received MRA at discharge and those who did not receive it. We excluded 11 patients for missing data on left ventricular ejection fraction (LVEF) during the index hospitalization to stratify the analysis according to LVEF. After further exclusions of 57 patients without any follow-up data after discharge, the study population consisted of 3717 patients with known LVEF and postdischarge clinical follow-up data (Figure 1).
Figure 1. Study Flowchart.
KCHF indicates Kyoto Congestive Heart Failure; LVEF, left ventricular ejection fraction; and MRA, mineralocorticoid receptor antagonist.
Definitions
We defined the use of MRA (MRA use group) as any new or continued prescription of spironolactone or eplerenone at discharge from the index hospitalization. The detailed definitions of baseline clinical characteristics have been described previously.10
The primary outcome measure was a composite of all-cause death or heart failure hospitalization after discharge from the index hospitalization. Other outcome measures included heart failure hospitalization, all-cause death, cardiovascular death, sudden death, and any-cause hospitalization. Death was regarded as cardiovascular in origin unless obvious noncardiovascular causes could be identified. Cardiovascular death included death related to heart failure, sudden death, death related to stroke, and death from other cardiovascular causes. Sudden death was the unexplained death in a previously stable patient. Stroke was either ischemic or hemorrhagic that required either acute or prolonged hospitalization and had symptoms that lasted more than 24 hours. Heart failure hospitalization was due to worsening of heart failure, requiring intravenous drug therapy.9 Heart failure was classified according to baseline LVEF as with reduced LVEF (<40%) or with preserved LVEF (≥40%).
Laboratory tests were performed on patient admission. Missing laboratory values are presented in eTable 1 in the Supplement.
Statistical Analysis
Categorical variables were presented as numbers with percentages and were compared with the χ2 test. Continuous variables were expressed as means with SDs or medians with interquartile range and were compared with an unpaired, 2-tailed t test when normally distributed or with Wilcoxon rank sum test when not normally distributed. Cumulative incidences were estimated by the Kaplan-Meier method and compared using the log-rank test. To account for the competing risk of all-cause death, we also calculated cumulative incidence functions of heart failure hospitalization and compared the differences between the 2 groups by Gray test in the matched cohort.11,12
We regarded the date of discharge as time 0 for clinical follow-up. We compared baseline characteristics with clinical outcomes on the basis of the presence or absence of the use of MRA at discharge from the index hospitalization. To balance the baseline characteristics associated with the selection of MRA use, we used a propensity score–matched cohort design as the main analysis. We also performed analysis in the entire cohort as the sensitivity analysis to explore the robustness of the findings. We compared groups by intention-to-treat analysis, regardless of the discontinuation of MRA during follow-up.
A logistic regression model was developed to make the propensity score for the choice of MRA with 16 baseline variables that were clinically relevant to the choice of MRA treatment (Table 1). Based on the estimated propensity score, patients in the group who did not receive MRA treatment (no MRA use) were matched with those in the group who received MRA treatment (MRA use) by using a 1:1 greedy matching technique.13 We compared the baseline characteristics and evaluated the cumulative incidences using the propensity score–matched cohort. We estimated the hazard ratios (HRs) and 95% CIs with Cox proportional hazards regression model. We conducted subgroup analyses stratified by LVEF (<40% or ≥40%) and other clinically relevant factors in the Cox models. We assessed the interactions between the subgroup factors and the associations of MRA use in the Cox models. For the sensitivity analysis using the entire cohort, MRA and the 25 clinically relevant risk-adjusting variables were simultaneously included in the Cox models (Table 1). The continuous variables were dichotomized by clinically meaningful reference values or median values. We expressed the association of the MRA use group with the no MRA use group with all of the outcome measures as HRs with 95% CIs.
Table 1. Patient Characteristics of the Study Population in the Matched and Entire Cohort.
| Variable | No. (%) | |||||
|---|---|---|---|---|---|---|
| Propensity Score-Matched Cohort | Entire Cohort | |||||
| MRA Use (n = 1034) | No MRA Use (n = 1034) | P Value | MRA Use (n = 1678) | No MRA Use (n = 2039) | P Value | |
| Clinical Characteristic | ||||||
| Age, median (IQR), y | 80 (72-86) | 80 (73-87) | .37 | 79 (70-85) | 81 (73-87) | <.001 |
| Age ≥80 ya,b | 536 (52) | 534 (52) | .93 | 793 (47) | 1135 (56) | <.001 |
| Female sexa,b | 469 (45) | 468 (45) | .96 | 763 (45) | 905 (44) | .51 |
| BMI, mean (SD) | 23.0 (4.7) | 22.8 (4.3) | .26 | 23.0 (4.8) | 22.8 (4.2) | .20 |
| BMI ≤22b | 449 (46) | 458 (46) | .92 | 744 (47) | 893 (46) | .57 |
| Origin | ||||||
| Ischemic heart disease | 335 (32) | 323 (31) | .57 | 530 (32) | 675 (33) | .31 |
| ACSb | 61 (5.9) | 58 (5.6) | .78 | 86 (5.1) | 119 (5.8) | .34 |
| Hypertensive heart disease | 260 (25) | 269 (26) | .65 | 368 (22) | 559 (27) | <.001 |
| Cardiomyopathy | 159 (15) | 144 (14) | .35 | 318 (19) | 238 (12) | <.001 |
| Valvular heart disease | 200 (19) | 210 (20) | .58 | 338 (20) | 397 (19) | .61 |
| Other heart disease | 80 (7.7) | 88 (8.5) | .52 | 124 (7.4) | 170 (8.3) | .29 |
| Medical history | ||||||
| Previous HF hospitalizationa,b | 314 (30) | 299 (29) | .47 | 549 (33) | 768 (39) | <.001 |
| Atrial fibrillation or flutterb | 418 (40) | 447 (43) | .20 | 714 (43) | 836 (41) | .34 |
| Hypertensiona,b | 758 (73) | 761 (74) | .88 | 1135 (68) | 1555 (76) | <.001 |
| Diabetesa,b | 378 (37) | 348 (34) | .17 | 595 (35) | 797 (39) | .02 |
| Dyslipidemia | 380 (37) | 413 (40) | .14 | 612 (36) | 840 (41) | .003 |
| Previous myocardial infarctiona,b | 225 (22) | 223 (22) | .92 | 366 (22) | 470 (23) | .37 |
| Previous strokeb | 161 (16) | 139 (13) | .17 | 248 (15) | 342 (17) | .10 |
| Previous PCI or CABG | 248 (24) | 250 (24) | .92 | 392 (23) | 561 (28) | .004 |
| Current smokingb | 131 (13) | 129 (13) | .89 | 222 (13) | 230 (12) | .08 |
| VT or VF | 44 (4.3) | 41 (4.0) | .74 | 86 (5.1) | 68 (3.3) | .007 |
| Chronic kidney disease | 397 (38) | 418 (40) | .35 | 604 (36) | 1033 (51) | <.001 |
| Chronic lung diseaseb | 122 (12) | 138 (13) | .29 | 203 (12) | 285 (14) | .09 |
| Malignant neoplasm | 153 (15) | 143 (14) | .56 | 234 (14) | 301 (15) | .47 |
| Dementia | 187 (18) | 177 (17) | .56 | 281 (17) | 373 (18) | .21 |
| Social background | ||||||
| Poor medical adherence | 183 (18) | 162 (16) | .22 | 293 (17) | 335 (16) | .40 |
| With occupation | 146 (14) | 128 (12) | .24 | 261 (16) | 233 (11) | <.001 |
| Daily life activities | ||||||
| Ambulatoryb | 833 (81) | 818 (80) | .57 | 1352 (81) | 1589 (79) | .04 |
| Use of wheelchair, outdoor only | 66 (6.4) | 82 (8.0) | .16 | 109 (6.6) | 165 (8.2) | .06 |
| Use of wheelchair, outdoor and indoor | 92 (8.9) | 91 (8.9) | .97 | 141 (8.5) | 195 (9.7) | .22 |
| Bedridden | 39 (3.8) | 33 (3.2) | .49 | 59 (3.6) | 70 (3.4) | .89 |
| Vital Signs at Presentation | ||||||
| BP, mm Hg | ||||||
| Systolic, mean (SD) | 149 (34) | 149 (34) | .91 | 145 (34) | 151 (36) | <.001 |
| <90a,b | 19 (1.8) | 25 (2.4) | .36 | 48 (2.9) | 47 (2.3) | .29 |
| Diastolic, mean (SD) | 87 (24) | 85 (24) | .25 | 85 (24) | 85 (24) | .53 |
| Heart rate, mean (SD), bpm | 98 (27) | 97 (29) | .93 | 97 (27) | 95 (28) | .005 |
| <60 bpmb | 51 (4.9) | 72 (6.9) | .051 | 87 (5.2) | 163 (8.1) | <.001 |
| Rhythms at presentation | ||||||
| Sinus rhythm | 582 (56) | 583 (56) | .96 | 897 (53) | 1173 (58) | .01 |
| Atrial fibrillation or flutter | 376 (36) | 386 (37) | .65 | 652 (39) | 703 (34) | .006 |
| NYHA class III or IVa,b | 904 (87) | 913 (88) | .54 | 1456 (87) | 1766 (87) | .64 |
| Tests at admission | ||||||
| LVEF, mean (SD), % | 46 (16) | 47 (16) | .11 | 44 (16) | 48 (16) | <.001 |
| HFrEF (EF <40%)a,b | 368 (36) | 370 (36) | .93 | 722 (43) | 661 (32) | <.001 |
| BNP, median (IQR), pg/mL | 699 (381-1228) | 699 (402-1218) | .71 | 700 (381-1216) | 721 (403-1287) | .20 |
| NT-proBNP, median (IQR), pg/mL | 4640 (2189-9690) | 5530 (2947-9692) | .14 | 4810 (2427-10 773) | 6405 (3008-14 109) | .003 |
| Serum creatinine, median (IQR), mg/dL | 1.0 (0.7-1.3) | 1.1 (0.8-1.4) | <.001 | 1.0 (0.8-1.3) | 1.3 (0.9-1.9) | <.001 |
| eGFR, mean (SD), mL/min/1.73m2 | 50 (35-67) | 45 (33-59) | <.001 | 51 (37-67) | 38 (24-55) | <.001 |
| <30 mL/min/1.73m2a,b | 189 (18) | 186 (18) | .86 | 253 (15) | 725 (36) | <.001 |
| Blood urea nitrogen, median (IQR), mg/dL | 22 (16-30) | 23 (18-32) | .001 | 21 (16-29) | 26 (19-39) | <.001 |
| Albumin, mean (SD), g/dL | 3.5 (0.5) | 3.5 (0.5) | .74 | 3.5 (0.5) | 3.5 (0.5) | .04 |
| <3.0 g/dLb | 130 (13) | 122 (12) | .54 | 210 (13) | 270 (14) | .52 |
| Sodium, mean (SD), mEq/L | 139 (4.3) | 139 (4.1) | .34 | 139 (4.3) | 139 (4.1) | .16 |
| <135 mEq/Lb | 131 (13) | 101 (9.8) | .04 | 222 (13) | 211 (10) | .007 |
| Potassium, mean (SD), mEq/L | 4.1 (0.6) | 4.2 (0.6) | .003 | 4.1 (0.6) | 4.3 (0.7) | <.001 |
| ≥5.0 mEq/La | 96 (9.3) | 108 (10) | .38 | 135 (8.1) | 297 (15) | <.001 |
| Hemoglobin, mean (SD), g/dL | 11.8 (2.3) | 11.6 (2.3) | .18 | 11.9 (2.4) | 11.2 (2.3) | <.001 |
| Anemia, No. (%)a,b | 665 (64) | 670 (65) | .82 | 995 (59) | 1462 (72) | <.001 |
| MRA before the index admissiona,b | 117 (11) | 112 (11) | .73 | 522 (31) | 129 (6.3) | <.001 |
| Medications at discharge | ||||||
| ACEI/ARB and β-blocker | 464 (45) | 463 (45) | .96 | 819 (49) | 742 (36) | <.001 |
| ACEI or ARBa,b | 625 (60) | 627 (61) | .93 | 1051 (63) | 1086 (53) | <.001 |
| β-Blockera,b | 702 (68) | 703 (68) | .96 | 1203 (72) | 1266 (62) | <.001 |
| Loop diureticsa,b | 911 (88) | 908 (88) | .84 | 1541 (92) | 1474 (72) | <.001 |
| Thiazide | 40 (3.9) | 63 (6.1) | .02 | 73 (4.4) | 145 (7.1) | <.001 |
| Tolvaptan | 97 (9.4) | 92 (8.9) | .70 | 176 (10) | 214 (11) | .99 |
| Digoxin | 59 (5.7) | 58 (5.6) | .92 | 127 (7.6) | 84 (4.1) | <.001 |
| Warfarin sodium | 236 (23) | 242 (23) | .75 | 420 (25) | 504 (25) | .83 |
| DOAC | 230 (22) | 244 (24) | .46 | 379 (23) | 381 (19) | .003 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin-receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, brain-type natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass graft; DOAC, direct oral anticoagulant; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; IQR, interquartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal-proBNP; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; VF, ventricular fibrillation; VT, ventricular tachycardia.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; blood urea nitrogen level to millimoles per liter, multiply by 0.357; BNP to nanograms per liter, multiply by 1.0; eGFR to milliliters per second per meters squared, multiply by 0.0167; hemoglobin level to grams per liter, multiply by 10.0; potassium level to millimoles per liter, multiply by 1.0; serum creatinine to micromoles per liter, multiply by 88.4; and sodium to millimoles per liter, multiply by 1.0.
Variables relevant to the choice of MRA were selected for logistic regression model for developing a propensity score for the choice of MRA.
Risk-adjusting variables were selected for Cox proportional hazard models in the unmatched cohort.
To focus the association of LVEF, we performed post hoc analyses. First, we stratified the entire cohort into the 2 strata by LVEF (<40% or ≥40%) and calculated the propensity score for the choice of MRA use in each LVEF stratum. Then, we generated the propensity score–matched cohort in the same fashion as the main analysis and compared the differences between the 2 groups in each LVEF stratum.
All statistical analyses were conducted by 2 of our physicians (H.Y. and Y.Y.) and our statistician (T. Morimoto) using JMP, version 13.0, and SAS, version 9.4 (SAS Institute Inc) or R (R Project for Statistical Computing). Two-tailed P < .05 was considered statistically significant.
Results
Patient Characteristics
In the study population of 3717 patients, 1678 (45.1%) patients had received MRA treatment at discharge and 2039 (54.9%) did not. The MRA treatment included spironolactone (median dose, 25 mg) in 1570 patients (93.6%) and eplerenone (median dose, 50 mg) in 108 patients (6.4%). Regarding the baseline clinical characteristics before matching, the patients in the MRA use group were younger and had a lower prevalence of previous heart failure hospitalization, hypertension, diabetes, dyslipidemia, previous percutaneous coronary intervention or coronary artery bypass graft, renal dysfunction, and anemia (Table 1). No significant differences in body mass index, atrial fibrillation (AF) or atrial flutter (AFL), previous myocardial infarction, previous stroke, chronic lung disease, malignant neoplasm, and dementia were observed between the 2 groups (Table 1). The MRA use group was more likely to have a hypertensive origin and AF or AFL at presentation, higher heart rate, lower blood pressure, lower levels of blood urea nitrogen and potassium, and a reduced LVEF (Table 1). Regarding medical treatment at discharge, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, β-blocker, and loop diuretics were more often prescribed in the MRA use group (Table 1).
The propensity score matching yielded a total of 2068 patients: 1034 patients in the MRA use group were matched to 1034 reference patients in the no MRA use group (main cohort: median [IQR] age, 80 [72-86] years; 937 [45.3%] women; mean [SD] LVEF, 46.7% [16.0%]). In the matched cohort, baseline characteristics were well balanced between the 2 groups, except for the slightly but statistically significantly better renal function in the MRA use group than in the no MRA use group (Table 1).
Clinical Outcomes in the Matched and Entire Cohort
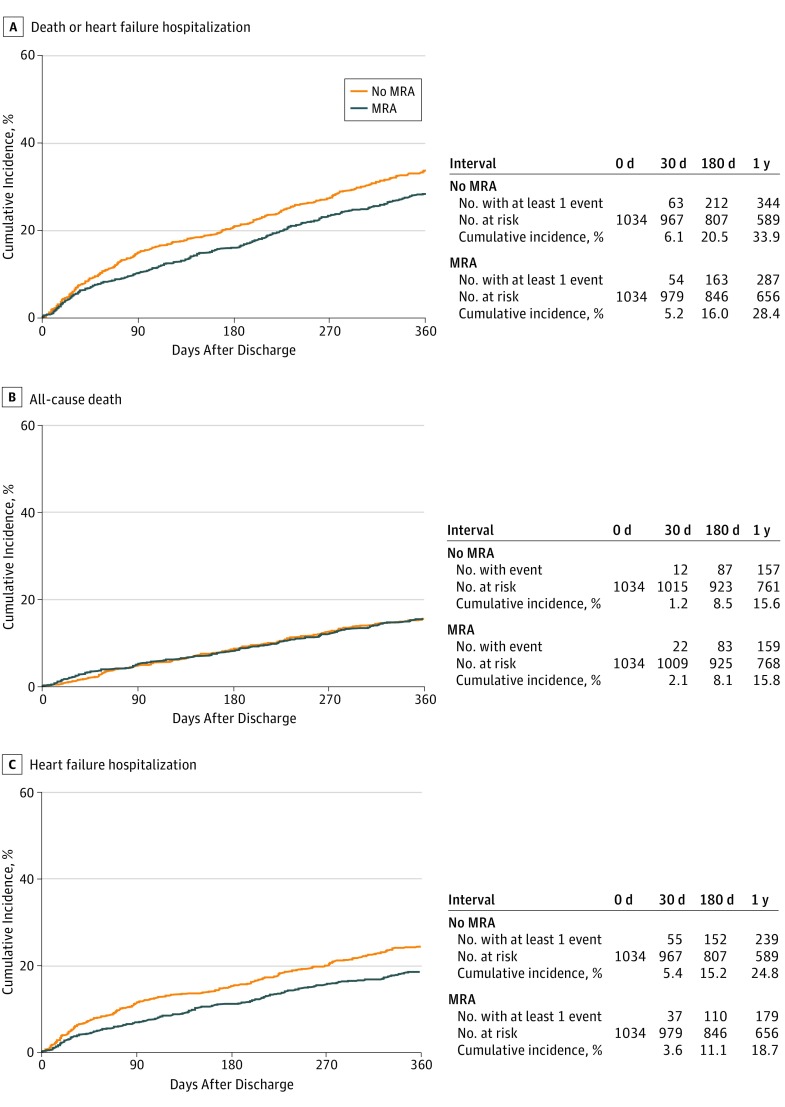
The median (interquartile range [IQR]) length of follow-up was 470 days (357-649 days), with a 96% follow-up rate at 1 year. In the propensity score–matched cohort, the cumulative 1-year incidence of the primary outcome measure (a composite of all-cause death or heart failure hospitalization) in the MRA use group was statistically significantly lower compared with the no MRA use group (28.4% vs 33.9%; HR, 0.81; 95% CI, 0.70-0.93; P = .003) (Figure 2A). The cumulative 1-year incidence of all-cause death was not statistically significantly different between the 2 groups (15.6% vs 15.8%; HR, 0.98; 95% CI, 0.82-1.18; P = .85) (Figure 2B and Table 2), whereas the cumulative 1-year incidence of heart failure hospitalization in the MRA use group was statistically significantly lower than that in the no MRA use group (18.7% vs 24.8%; HR, 0.70; 95% CI, 0.60-0.86; P < .001) (Figure 2C and Table 2). The result was consistent with the result derived from the cumulative incidence function curves accounting for the competing risk of death by Gray test (eFigure 1 in the Supplement).
Figure 2. Cumulative Incidence Rates of the Primary Outcome Measure in the Propensity Score–Matched Cohort.
Log-rank P = .003 (A), P = .85 (B), and P < .001 (C). MRA indicates mineralocorticoid receptor antagonist.
Table 2. Clinical Outcomes in the Matched and Entire Cohort.
| Outcome | Propensity Score–Matched Cohort | Entire Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%)a | HR (95% CI) | P Value | No. (%)a | HR (95% CI) | P Value | Adjusted HR (95% CI)b | P Value | |||
| MRA Use (n = 1034) | No MRA Use (n = 1034) | MRA Use (n = 1678) | No MRA Use (n = 2039) | |||||||
| Composite of all-cause death or HF hospitalization | 287 (28.4) | 344 (33.9) | 0.81 (0.70-0.93) | .003 | 503 (30.6) | 739 (36.8) | 0.79 (0.71-0.88) | <.001 | 0.81 (0.71-0.92) | .001 |
| HF hospitalization | 179 (18.7) | 239 (24.8) | 0.70 (0.60-0.86) | <.001 | 335 (21.5) | 494 (26.2) | 0.77 (0.68-0.88) | <.001 | 0.74 (0.63-0.87) | <.001 |
| All-cause death | 157 (15.6) | 159 (15.8) | 0.98 (0.82-1.18) | .85 | 257 (15.7) | 367 (18.4) | 0.83 (0.72-0.95) | .007 | 0.93 (0.78-1.11) | .42 |
| Cardiovascular death | 96 (9.8) | 93 (9.4) | 1.07 (0.84-1.36) | .59 | 155 (9.8) | 217 (11.3) | 0.88 (0.74-1.05) | .16 | 1.03 (0.82-1.29) | .80 |
| Sudden death | 23 (2.5) | 18 (1.9) | 1.59 (0.93-2.78) | .09 | 33 (2.2) | 43 (2.3) | 1.09 (0.73-1.61) | .68 | 1.51 (0.92-2.45) | .10 |
| Any hospitalization | 344 (35.3) | 375 (38.2) | 0.88 (0.77-1.01) | .07 | 580 (36.6) | 779 (40.8) | 0.85 (0.77-0.94) | .002 | 0.84 (0.74-0.96) | .007 |
Abbreviations: HF, heart failure; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist.
Number of patients with at least 1 event reported as cumulative 1-year incidence, counted through the entire follow-up period.
Adjusted for the clinically relevant variables described in Table 1.
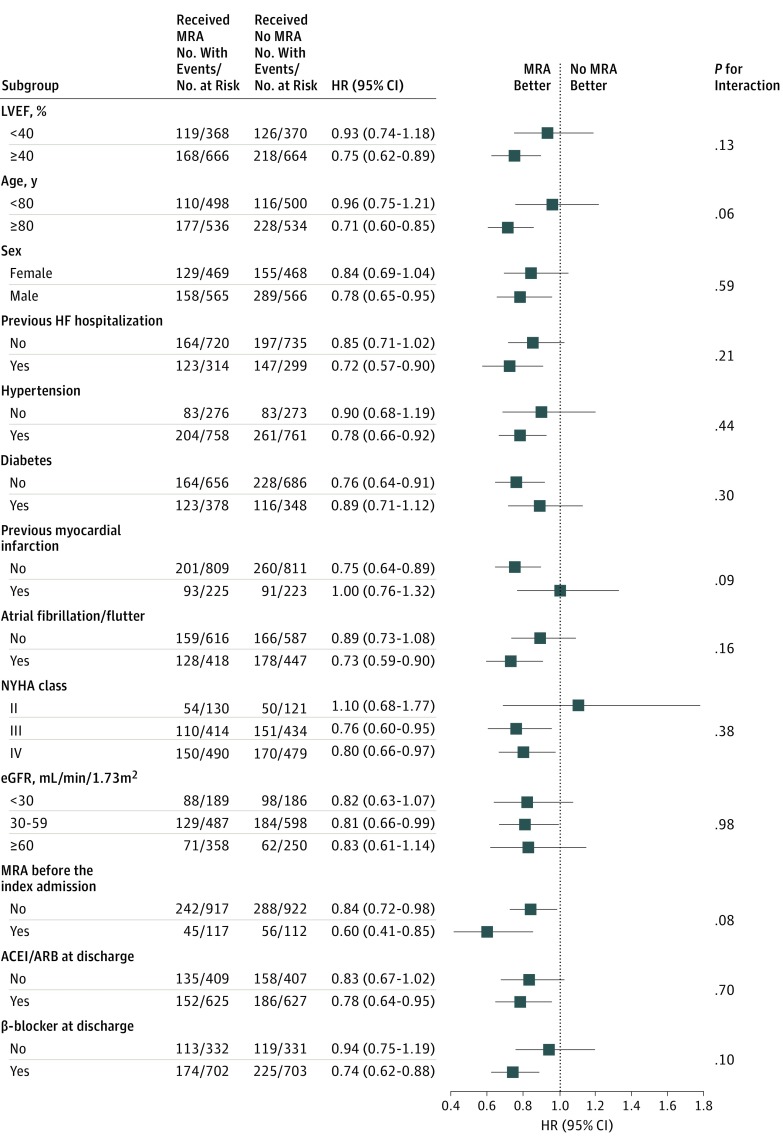
In the subgroup analysis (Figure 3), no statistically significant interaction was observed between the HRs for the primary outcome measure associated with the use of MRA and the clinically relevant subgroup factors such as LVEF, age, sex, previous heart failure hospitalization, diabetes, myocardial infarction, AF or AFL, New York Heart Association class, estimated glomerular filtration rate, and use of the antagonists of the renin-angiotensin system and β-blocker (eTable 2 and eTable 3 in the Supplement). Nevertheless, the association of MRA use with the primary outcome measure was greater in patients with HFpEF (25.9% vs 33.4%; HR, 0.75; 95% CI, 0.62-0.89; P = .001) than in those with HFrEF (33.1% vs 34.7%; HR, 0.93; 95% CI, 0.74-1.18; P = .56) (Figure 3; eFigure 2 in the Supplement).
Figure 3. Subgroup Analysis for the Primary Outcome Measure in the Propensity Score–Matched Cohort.
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; and NYHA, New York Heart Association.
In the entire cohort, the cumulative 1-year incidence of the primary outcome measure in the MRA use group was statistically significantly lower than that in the no MRA use group (30.6% vs 36.8%; P < .001) (eFigure 3 in the Supplement). After adjusting for baseline characteristics, the favorable association of the MRA use group compared with the no MRA use group remained statistically significant (adjusted HR, 0.81; 95% CI, 0.71-0.92; P = .001) (Table 2). The results in the entire cohort for the secondary outcome measures, including all-cause death and heart failure hospitalization, were consistent with those in the propensity score–matched cohort (Table 2; eFigure 3 in the Supplement). No difference in any unexpected hospitalization was observed between the 2 groups in the propensity score–matched cohort (35.3% vs 38.2%; HR, 0.88; 95% CI, 0.77-1.01; P = .07), although the cumulative 1-year incidence of any unexpected hospitalization in the MRA use group was statistically significantly lower than that in the no MRA use group in the entire cohort (36.6% vs 40.8%; adjusted HR, 0.84; 95% CI, 0.74-0.96; P = .007) (Table 2). The results in the entire cohort for the subgroup analysis stratified by LVEF (eFigure 4 and eTable 4 in the Supplement) were consistent with those in the propensity score–matched cohort.
Post Hoc Analyses
Of the population of 3717 patients, 1383 (37.2%) showed reduced LVEF, and 2334 (62.8%) showed preserved LVEF (eFigure 5 in the Supplement). Propensity score matching resulted in a total of 385 patients with HFrEF and 690 patients with HFpEF for the MRA use group or no MRA use group (eFigure 5, eTable 5, and eTable 6 in the Supplement). In the matched cohort of patients with HFrEF, the cumulative 1-year incidence of the primary outcome measure was not statistically significantly different between the 2 groups (34.7% vs 33.7%; HR, 0.87; 95% CI, 0.78-1.21; P = .78) (eFigure 6 in the Supplement). In the matched cohort of patients with HFpEF, the cumulative 1-year incidence of the primary outcome measure in the MRA use group was statistically significantly lower than that in the no MRA use group (26.8% vs 33.7%; HR, 0.78; 95% CI, 0.66-0.93; P = .005) (eFigure 6 in the Supplement). The results were consistent with the main results (eFigure 2 in the Supplement) in the propensity score–matched cohort, including patients with HFrEF or HFpEF.
Discussion
The main findings of the present study were as follows. First, the use of MRA at hospital discharge was associated with a lower risk for the primary outcome measure (a composite of all-cause death or heart failure hospitalization) in patients hospitalized for ADHF; however, MRA use did not seem to be associated with lower mortality but was associated with heart failure hospitalization. Second, when patients were stratified by LVEF at first, some advantages appeared to have potentially accrued to patients with HFpEF.
The clinical utility of MRA in stable patients with heart failure and reduced LVEF was first demonstrated in the Randomized Aldactone Evaluation Study (RALES) in 1991.1 Subsequently, a line of evidence emerged that MRA treatment (spironolactone or eplerenone) was associated with reduced morbidity and mortality in patients with HFrEF.2,14 The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial15 in patients with stable HFpEF suggested the reduction of heart failure hospitalization with MRA treatment, although the study failed to show the evidence for the mortality advantage of MRA treatment during the median follow-up of 3.3 years. However, to our knowledge, no previous RCT investigated the association of MRA use in patients hospitalized for ADHF with the postdischarge outcomes. Acute decompensated heart failure has the potential risk for decline and eventually in-hospital death. Even during the recovery phase, a certain proportion of patients hospitalized for ADHF are hemodynamically unstable, often with worsening renal function. Therefore, the optimal management of patients hospitalized for ADHF would be defined specifically in this patient population. In the case of β-blocker treatment, the heart failure guidelines recommend its early induction in patients with ADHF and reduced LVEF, although the recommendation was based on small studies showing the association between withdrawal of β-blocker therapy at admission for ADHF and an increase in mortality.16,17,18,19
In the present study, MRA treatment was not associated with a lower risk for all-cause death but instead with heart failure hospitalization only in contemporary patients with ADHF who were discharged alive. To our knowledge, the baseline patient characteristics were substantially different from those in previous RCTs. A large proportion of patients with advanced age (median age of 80 years) and a substantial proportion of patients with comorbidities, such as chronic kidney disease and AF or AFL, were represented in this study. Despite these demographic differences, the favorable association of MRA with heart failure hospitalization but not with mortality in patients with HFpEF was consistent with findings reported in previous RCTs.
The mechanisms by which MRA may become potentially advantageous to patients with HFpEF from the point of heart failure readmission rate are uncertain. However, the neurohormonal pathways were excessively activated in a large proportion of patients with ADHF through sympathetic nerve systems, worsening renal function, and the aggressive use of loop diuretics.20,21,22 The use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker was less common in HFpEF than in HFrEF. Therefore, the blockade of the renin-angiotensin system by MRA might have promoted the favorable association with heart failure hospitalization in patients with HFpEF. Another possible mechanism was the diuretic nature of MRA. In this study, the combination of MRA and loop diuretics was more frequently observed in patients with HFpEF. Long-term use of loop diuretics is associated with diuretic resistance through tubule-glomerular feedback mechanisms enhanced by renin-angiotensin-aldosterone system activation.20,21,22 The blockade of aldosterone statistically significantly enhances the blockade of sodium reabsorption in the distal tubules and collecting duct. These speculated mechanisms were consistent with the observed favorable associations of MRA use with the heart failure readmission in patients with HFpEF.
However, no difference in all-cause mortality or any unexpected hospitalization was observed regardless of MRA use. One reason for this finding might be that many patients, particularly those with HFpEF, had many comorbidities owing to their advanced age. Thus, the outcomes of MRA may be counterbalanced by electrolyte imbalance, worsening renal function, and noncardiovascular mortality or hospitalization in patients who received MRA. Another consideration is that patients treated with MRA in the entire population were hypotensive, more likely to have reduced LVEF, and less likely to have ADHF from hypertensive heart disease. Therefore, the adverse effects of MRA use may have been avoided because the heart failure stage in the MRA use group was more progressive than the no MRA use group, although we performed the propensity score matching using 16 variables.
The lack of mortality advantage from MRA use despite a reduction in heart failure hospitalization was consistent with findings of an observational study in patients with ADHF and the TOPCAT trial.3,5,15 The therapy to reduce heart failure hospital readmissions has an important role in daily practice in a rapidly aging society because repeated heart failure hospitalizations are associated with high mortality rates, diminished quality of life and functional status, inadequate recovery after heart failure deterioration, and the escalation of the medical costs.23 In contrast, the rate of any unexpected hospitalization did not differ between the MRA use group and no MRA use group in the propensity score–matched cohort. Considering no differences in mortality or overall rate of hospitalization, MRA use may be associated with minimal, if any, clinical net advantages. Our additional analyses suggested the potential value of MRA use for patients with HFpEF. Exploratory studies to identify the patient groups that find MRA use advantageous are needed and should be confirmed by RCTs in patients hospitalized for ADHF.
Limitations
This study has several limitations. First, the observational study design is subject to selection bias and residual confounding. The KCHF registry had comprehensive data on patient demographics, medical history, underlying heart disease, prehospital activities, socioeconomic status, signs, symptoms, medications, laboratory tests, electrocardiogram, and echocardiography results, acute management in the emergency department, status at discharge, and clinical events during the index hospitalization. By adjusting for 25 variables, we accounted for most conceivable confounders. Nevertheless, residual unmeasured confounding could affect the results. Second, we had no prescription data after discharge from the index hospitalization; therefore, we could not deny the possibility of substantial crossover in MRA use. Third, this observational study used propensity score matching. Thus, we did not perform a confidence set or power analysis in advance. Further studies, including those on quality of life and cost, may clarify the clinical net advantage of MRA use in this cohort. Fourth, data on the reasons for MRA use or no MRA use by individual patients were not available.
Conclusions
Use of MRA at discharge from ADHF hospitalization appeared to be associated with a lower risk of heart failure hospitalization but not with lower all-cause mortality or overall rate of hospitalization. These findings suggest that MRA use might be associated with minimal, if any, clinical advantage. Further studies appear to be needed to identify the patient groups that may find value in MRA treatment and for findings to be confirmed by RCTs in patients hospitalized for ADHF.
eTable 1. Number of Missing Values in the Matched and Entire Cohort
eTable 2. The Prescription of ACEI/ARB and Beta-blocker at Discharge in Patients With Reduced LVEF in the Matched and Entire Cohort
eTable 3. The Prescription of ACEI/ARB and Beta-blocker at Discharge in Patients With Preserved LVEF in the Matched and Entire Cohort
eTable 4. Association Between the Prescription of MRA at Discharge and Clinical Outcomes by the LVEF Categories in the Entire Cohort
eTable 5. Patient Characteristics Before and After Propensity Score Matching in Reduced LVEF
eTable 6. Patient Characteristics Before and After Propensity Score Matching in Preserved LVEF
eFigure 1. Cumulative Incidence Function Curves of the MRA and No MRA Groups for HF Hospitalization
eFigure 2. Cumulative Incidences of the Primary Outcome Measure (Death or HF Hospitalization) by LVEF in the Propensity Score-Matched Cohort
eFigure 3. Cumulative Incidences of the Primary Outcome Measure (Death or HF Hospitalization) (A) All-Cause Death (B) and HF Hospitalization (C) in the Entire Cohort
eFigure 4. Cumulative Incidences of the Primary Outcome Measure (Death or HF Hospitalization) (A) All-Cause Death (B) and HF Hospitalization (C) by LVEF in the Entire Cohort
eFigure 5. Flowchart of the Propensity Score-Matched Cohort in Each LVEF Strata; <40% and ≥40%
eFigure 6. Cumulative Incidences of the Primary Outcome Measure (Death or HF Hospitalization) in the Propensity Score-Matched Cohort in Each LVEF Strata
References
- 1.Pitt B, Zannad F, Remme WJ, et al. ; Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):-. doi: 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 2.Pitt B, Remme W, Zannad F, et al. ; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309-1321. doi: 10.1056/NEJMoa030207 [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Pfeffer MA, Assmann SF, et al. ; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJV, O’Connor C. Lessons from the TOPCAT trial. N Engl J Med. 2014;370(15):1453-1454. doi: 10.1056/NEJMe1401231 [DOI] [PubMed] [Google Scholar]
- 5.Gayat E, Arrigo M, Littnerova S, et al. ; GREAT Network . Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity-score matched study. Eur J Heart Fail. 2018;20(2):345-354. doi: 10.1002/ejhf.932 [DOI] [PubMed] [Google Scholar]
- 6.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501-506. doi: 10.1161/CIRCULATIONAHA.112.125435 [DOI] [PubMed] [Google Scholar]
- 7.Ministry of Education, Culture, Sports, Science and Technology Ministry of Health, Labour and Welfare. Ethical guidelines for epidemiologic research. http://www.lifescience.mext.go.jp/files/pdf/n796_01.pdf. Accessed May 15, 2018.
- 8.US Department of Health and Human Services. 45 CFR 46.116(d). https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html#46.116. Published 2009. Accessed May 15, 2018.
- 9.Yamamoto E, Kato T, Ozasa N, et al. ; KCHF study investigators . Kyoto Congestive Heart Failure (KCHF) study: rationale and design. ESC Heart Fail. 2017;4(3):216-223. doi: 10.1002/ehf2.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaku H, Ozasa N, Morimoto T, et al. ; KCHF Study Investigators . Demographics, management, and in-hospital outcome of hospitalized acute heart failure syndrome patients in contemporary real clinical practice in Japan—observations from the prospective, multicenter Kyoto Congestive Heart Failure (KCHF) registry. Circ J. 2018;82(11):2811-2819. doi: 10.1253/circj.CJ-17-1386 [DOI] [PubMed] [Google Scholar]
- 11.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154. doi: 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 12.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gran JM, Wasmuth L, Amundsen EJ, Lindqvist BH, Aalen OO. Growth rates in epidemic models: application to a model for HIV/AIDS progression. Stat Med. 2008;27(23):4817-4834. doi: 10.1002/sim.3219 [DOI] [PubMed] [Google Scholar]
- 14.Zannad F, McMurray JJV, Krum H, et al. ; EMPHASIS-HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11-21. doi: 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. 2015;131(1):34-42. doi: 10.1161/CIRCULATIONAHA.114.013255 [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJV, Adamopoulos S, Anker SD, et al. ; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847. doi: 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines; Heart Failure Society of America . 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America [published online September 27, 2016]. Circulation. doi: 10.1161/CIR.0000000000000435 [DOI] [PubMed]
- 19.Mebazaa A, Yilmaz MB, Levy P, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17(6):544-558. doi: 10.1002/ejhf.289 [DOI] [PubMed] [Google Scholar]
- 20.Alla F, Zannad F, Filippatos G. Epidemiology of acute heart failure syndromes. Heart Fail Rev. 2007;12(2):91-95. doi: 10.1007/s10741-007-9009-2 [DOI] [PubMed] [Google Scholar]
- 21.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53(7):557-573. doi: 10.1016/j.jacc.2008.10.041 [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Jones D, Adams R, Carnethon M, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480-486. doi: 10.1161/CIRCULATIONAHA.108.191259 [DOI] [PubMed] [Google Scholar]
- 23.Lewis EF, Lamas GA, O’Meara E, et al. ; CHARM Investigators . Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9(1):83-91. doi: 10.1016/j.ejheart.2006.10.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Number of Missing Values in the Matched and Entire Cohort
eTable 2. The Prescription of ACEI/ARB and Beta-blocker at Discharge in Patients With Reduced LVEF in the Matched and Entire Cohort
eTable 3. The Prescription of ACEI/ARB and Beta-blocker at Discharge in Patients With Preserved LVEF in the Matched and Entire Cohort
eTable 4. Association Between the Prescription of MRA at Discharge and Clinical Outcomes by the LVEF Categories in the Entire Cohort
eTable 5. Patient Characteristics Before and After Propensity Score Matching in Reduced LVEF
eTable 6. Patient Characteristics Before and After Propensity Score Matching in Preserved LVEF
eFigure 1. Cumulative Incidence Function Curves of the MRA and No MRA Groups for HF Hospitalization
eFigure 2. Cumulative Incidences of the Primary Outcome Measure (Death or HF Hospitalization) by LVEF in the Propensity Score-Matched Cohort
eFigure 3. Cumulative Incidences of the Primary Outcome Measure (Death or HF Hospitalization) (A) All-Cause Death (B) and HF Hospitalization (C) in the Entire Cohort
eFigure 4. Cumulative Incidences of the Primary Outcome Measure (Death or HF Hospitalization) (A) All-Cause Death (B) and HF Hospitalization (C) by LVEF in the Entire Cohort
eFigure 5. Flowchart of the Propensity Score-Matched Cohort in Each LVEF Strata; <40% and ≥40%
eFigure 6. Cumulative Incidences of the Primary Outcome Measure (Death or HF Hospitalization) in the Propensity Score-Matched Cohort in Each LVEF Strata