Abbreviations
- pBA
plasma bile acid
- PFIC
progressive familial intrahepatic cholestasis
- UDCA
ursodeoxycholic acid
Cholestasis during pregnancy can lead to severe maternal pruritus and even suicidal ideation. In addition, plasma bile acid (pBA) levels >40 μmol/L are associated with an increased risk of fetal complications.1 Intrahepatic cholestasis of pregnancy is the most studied underlying cause and resolves spontaneously after delivery. Other causes are preexisting liver diseases associated with cholestasis that worsen during pregnancy. Pharmacological options such as ursodeoxycholic acid (UDCA), cholestyramine, rifampicin, dexamethasone, and S‐adenosyl‐methionine are not always sufficient in lowering pruritus and pBA levels.2
Nasobiliary drainage is an alternative treatment but is associated with an increased risk of pancreatitis in nonpregnant patients.3 Other experimental approaches are molecular adsorbent recirculation system (MARS) therapy and plasmapheresis (Table 1).4 Here, we present a report on plasmapheresis for cholestasis in a pregnant woman with Alagille‐like syndrome, with a detailed profiling of its effect on pBA levels and serum autotaxin activity, a biomarker and potential mediator of cholestatic pruritus.4
Table 1.
Case Reports on Plasmapheresis for Cholestatic Pruritus During Pregnancy
| Reference | Liver Disease | Start PPh (Weeks of Pregnancy) | Number of Sessions | Effect on pBA | Effect on Pruritus | Fetal Complications | Maternal Complications |
|---|---|---|---|---|---|---|---|
| 6 | PFIC | 19 | 15 | ↓* | ↓ | Absent | Absent |
| 7 | PFIC | 13 | 4 | ↓* | ↓ | Absent | Absent |
| 8 | PFIC | 31 | 4 | Immediate decrease | ↓ | Absent | Not reported |
| 9 | PBC, Gilbert’s syndrome | 22 | 4 | Not reported | ↓ | Absent | Absent |
| 9 | PBC | 31 | 6 | Not reported | ↓ | Absent | Absent |
| 10 | ICP | 32 | 5 | No decrease* | ↓ | Absent | Not reported |
| 11 | ICP, hepatitis C | 27 | 7 | No decrease* | ↓ | Absent | Not reported |
Measured at start of next plasmapheresis session; no immediate effect reported.
Abbreviations: ICP, intrahepatic cholestasis of pregnancy; PBC, primary biliary cholangitis; PPh, plasmapheresis.
Case Report
A 35‐year‐old pregnant woman with Alagille‐like syndrome (chronic cholestasis, characteristic facial phenotype, and NOTCH2 c.14A>C mutation) was referred to our hospital because of severe pruritus at 16 weeks of pregnancy.5 No mutations of the ATPase phospholipid transporting 8B1 gene (benign recurrent intrahepatic cholestasis [BRIC] 1/progressive familial intrahepatic cholestasis [PFIC] 1), the ATP binding cassette subfamily B member 11 (ABCB11) gene (BRIC2/PFIC2), and the ABCB4 gene (PFIC3) were found. She used UDCA 1,000 mg/day and rifampicin 300 mg/day. The transient elastography result was 7.7 kPa, ruling out cirrhosis. In her first pregnancy 4 years before, she developed suicidal ideation because of drug‐resistant pruritus that never disappeared completely after that delivery. Because of the recurrent severe pruritus and the history of suicidal ideation, we started plasmapheresis at 17+5 weeks of pregnancy. UDCA and rifampicin were continued in unaltered dose throughout the pregnancy. A total of 30 plasmapheresis sessions were performed, each consisting of the replacement of 2.5 L 4% albumin dissolved in a Ringer solution (Fig. 1).
Figure 1.
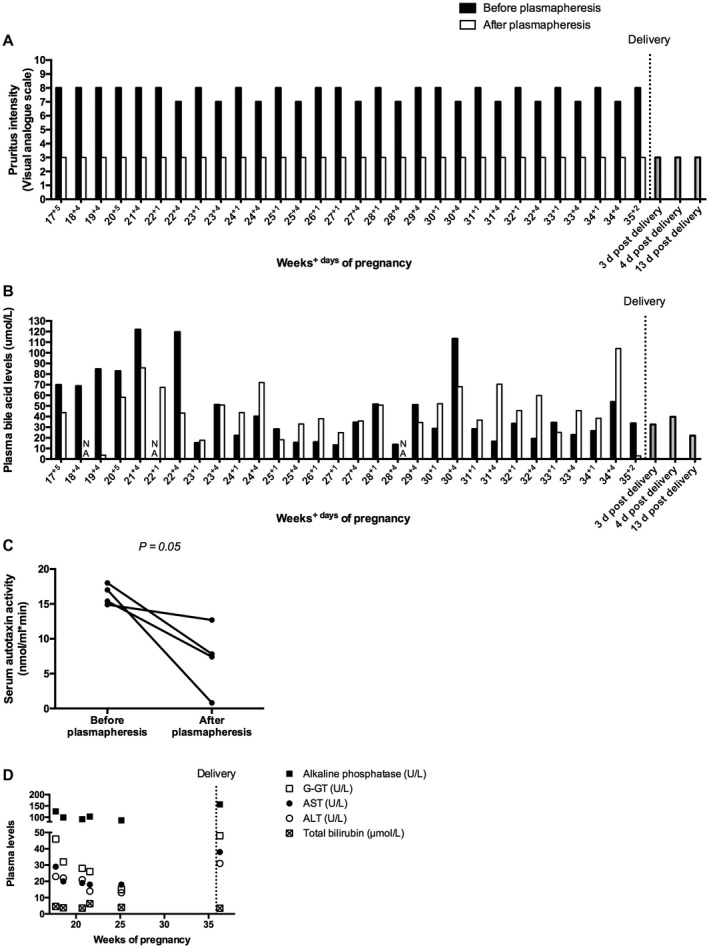
Direct effect of plasmapheresis on (A) pruritus intensity scores, (B) plasma bile acid levels, and (C) autotaxin activity (measured at 17+5, 19+4, 20+5, and 21+4 weeks of pregnancy). (D) Evolution of liver tests during the course of pregnancy. Liver tests were measured directly before the start of plasmapheresis at the indicated time points of pregnancy. Lab reference values are pBA levels <12 μmol/L, alkaline phosphatase <98 U/L, gamma‐glutamyl transpeptidase <38 U/L, aspartate aminotransferase <31 U/L, alanine aminotransferase <34 U/L, and total bilirubin <20 μmol/L. pBA levels and serum autotaxin activity were measured directly before and after plasmapheresis. One plasmapheresis session took approximately 90 minutes. The first three sessions were performed through a peripheral line. The remaining sessions were performed through a permanent double‐lumen central venous line that did not lead to adverse events and was removed after pregnancy. Statistical analysis was performed with a paired t test. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; G‐GT, gamma‐glutamyl transpeptidase; NA, not available.
The patient reported instant relief of pruritus after every session (Fig. 1A), while the immediate effect of plasmapheresis on pBA levels was variable (Fig. 1B). Additionally, serum autotaxin activity was measured directly before and after plasmapheresis in the first month of treatment and decreased significantly after each session (Fig. 1C). The evolution of the serum liver tests during the course of pregnancy is shown in Figure 1D. The patient reported no side effects of the treatment. Delivery was induced at 35+6 weeks because of deflecting fetal growth and reduced fetal movement. Through vaginal delivery a girl of 2,084 g was born. She had a cleft lip (visualized on ultrasound already before the start of plasmapheresis), an atrial septum defect, and a pulmonary artery stenosis. After delivery, plasmapheresis was discontinued and pruritus was only moderately present.
Discussion
Plasmapheresis is effective and safe to treat drug‐resistant pruritus in cholestasis during pregnancy, and its effect is not related to pBA levels. The mechanism whereby plasmapheresis decreases pruritus is unknown. In this report, we show that plasmapheresis decreases autotaxin activity, a potential mediator of cholestatic pruritus. Although our case report consists of only one patient, the observed effect is in line with previous findings after MARS therapy and nasobiliary drainage.4 Further research is needed to elucidate how these therapies affect autotaxin activity and how this relates to pruritus, in order to develop pharmacological mimetics to treat cholestatic pruritus in pregnant and nonpregnant patients. For the time being, plasmapheresis is a valuable alternative, but it remains an invasive and expensive procedure and thus should be restricted to severe therapy‐resistant cases.
Potential conflict of interest: Nothing to report.
References
- 1. Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology 2004;40:467‐474. [DOI] [PubMed] [Google Scholar]
- 2. Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder J, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta‐analysis. Gastroenterology 2012;143:1492‐1501. [DOI] [PubMed] [Google Scholar]
- 3. Hegade VS, Krawczyk M, Kremer AE, Kuczka J, Gaouar F, Kuiper EM, et al. The safety and efficacy of nasobiliary drainage in the treatment of refractory cholestatic pruritus: a multicentre European study. Aliment Pharmacol Ther 2016;43:294‐302. [DOI] [PubMed] [Google Scholar]
- 4. Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology 2012;56:1391‐1400. [DOI] [PubMed] [Google Scholar]
- 5. Kamath BM, Bauer RC, Loomes KM, Chao G, Gerfen J, Hutchinson A, et al. NOTCH2 mutations in Alagille syndrome. J Med Genet 2012;49:138‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Branger B, Ribard D, Tailland ML, Zabadani B. Apheresis for Byler syndrome in pregnancy: tolerance and effectiveness. [in French] Ann Med Interne (Paris) 1999;150:70. [PubMed] [Google Scholar]
- 7. Lemoine M, Revaux A, Francoz C, Ducarne G, Brechignac S, Jacquemin E, et al. Albumin liver dialysis as pregnancy‐saving procedure in cholestatic liver disease and intractable pruritis. World J Gastroenterol 2008;14:6572‐6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathias A, Wax JR, Pinette MG, Cartin A, Blackstone J. Progressive familial intrahepatic cholestasis complicating pregnancy. J Matern Fetal Neonatal Med 2009;22:816‐818. [DOI] [PubMed] [Google Scholar]
- 9. Alallam A, Barth D, Heathcote EJ. Role of plasmapheresis in the treatment of severe pruritis in pregnant patients with primary biliary cirrhosis: case reports. Can J Gastroenterol 2008;22:505‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warren JE, Blaylock RC, Silver RM. Plasmapheresis for the treatment of intrahepatic cholestasis of pregnancy refractory to medical treatment. Am J Obstet Gynecol 2005;192:2088‐2089. [DOI] [PubMed] [Google Scholar]
- 11. Covach AJ, Rose WN. Intrahepatic cholestasis of pregnancy refractory to multiple medical therapies and plasmapheresis. AJP Rep 2017;7:e223‐e225. [DOI] [PMC free article] [PubMed] [Google Scholar]


