Abstract
Objective
To report long‐term health‐related quality of life (HRQoL) and fatigue outcomes in patients with systemic lupus erythematosus (SLE) receiving belimumab.
Methods
Patients with SLE who completed the Study of Belimumab in Subjects with SLE 76‐week trial (BLISS‐76) were enrolled in this continuation study (BEL112233 [ClinicalTrials.gov identifier: NCT00724867]). The belimumab groups continued to receive the same dose (1 mg/kg or 10 mg/kg) intravenously. After March 2011, all patients received belimumab 10 mg/kg every 28 days plus standard therapy. The placebo group switched to belimumab 10 mg/kg. HRQoL and fatigue assessments included the Short Form 36 (SF‐36) health survey and the Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue subscale. Post hoc subgroup analyses (BEL206350) assessed clinical characteristics associated with improved HRQoL and fatigue.
Results
Of the 268 patients enrolled, 140 completed the study. Patients receiving long‐term belimumab treatment reported continued improvements in HRQoL and fatigue. At study year 6, the mean ± SD SF‐36 physical component summary (PCS) score and the mental component summary (MCS) score increased from 37.0 ± 9.9 at baseline to 41.7 ± 10.0 (mean ± SD change 4.8 ± 9.4) and from 44.3 ± 11.3 to 47.0 ± 11.6 (mean ± SD change 2.7 ± 11.3) for the PCS and MCS, respectively, exceeding the minimum clinically important difference (MCID) for improvement (2.5 units). The mean ± SD FACIT–Fatigue score exceeded the MCID of 4 at study years 1–5; at study year 6, the mean ± SD change was 3.7 ± 11.8. Statistically significant associations were observed between parent trial treatment groups and change from baseline in PCS, MCS, and FACIT–Fatigue scores (P < 0.01).
Conclusion
Long‐term control of SLE disease activity with belimumab plus standard therapy translates into meaningful improvements in patient‐reported fatigue and HRQoL.
Introduction
Systemic lupus erythematosus (SLE) is a relapsing, chronic, inflammatory autoimmune disease characterized by periods of disease flare, with multisystem manifestations that lead to organ damage over time 1, 2, 3. SLE causes a pronounced impairment in patients’ health‐related quality of life (HRQoL), resulting in significantly reduced work productivity and increased absenteeism, which can lead to permanent work disability 4. Physical, psychological, and social aspects of HRQoL are affected, and the extent of these effects is specific to each patient 2, 5, 6. Fatigue is a frequently reported symptom in SLE, which affects HRQoL and is associated with behavioral and psychosocial factors and disease activity 7, 8, 9. Fifty percent of patients regard fatigue as the most disabling symptom of SLE 10, 11. Baseline HRQoL scores across several randomized controlled trials (RCTs) in SLE are comparable or worse than those recorded for patients with chronic congestive heart failure or following myocardial infarction 8, 12.
SIGNIFICANCE & INNOVATIONS.
This is the first report of health‐related quality of life (HRQoL) and fatigue outcomes in patients with active systemic lupus erythematosus (SLE) with long‐term exposure to belimumab.
The study provides evidence that long‐term control of SLE disease activity with belimumab plus standard therapy translates into meaningful and sustained benefits in patients’ fatigue and HRQoL.
These benefits are consistent with results from the parent trial Study of Belimumab in Subjects with SLE 76‐week trial (BLISS‐76).
Although there have been some improvements in treatment options, there is still a significant unmet need for effective and well‐tolerated options. Patients with SLE characteristically have elevated levels of circulating B lymphocyte stimulator, a key cytokine that promotes B cell survival 13, 14, 15. Belimumab is a human IgG1λ monoclonal antibody that binds to and inhibits the activity of B lymphocyte stimulator protein 16. It is licensed in the US and Europe for the treatment of adult patients with active, autoantibody‐positive SLE who are receiving standard treatment 16, 17, 18.
Pooled post hoc analyses 19 from the 2 phase III trials, the Study of Belimumab in Subjects with SLE 52‐week study (BLISS‐52) (ClinicalTrials.gov identifier: NCT00424476) 20 and the BLISS 76‐week study (BLISS‐76) (ClinicalTrials.gov identifier: NCT00410384) 16 showed improvements in patients’ HRQoL outcomes, including significantly greater improvements at week 52 in Short Form 36 version 2 (SF‐36) health survey physical component summary (PCS) scores with belimumab 1 mg/kg and 10 mg/kg and improvements in the mental component summary (MCS) scores with belimumab 1 mg/kg compared with placebo 19. Greater mean changes from baseline at week 52 were also reported in the belimumab groups compared with placebo for individual domains of the SF‐36 including physical functioning, bodily pain, general health, and vitality 19. In addition, improvements in fatigue scores (as assessed using the Functional Assessment of Chronic Illness Therapy [FACIT]–Fatigue subscale) were significantly greater for both belimumab doses at week 52 compared with placebo 16, 19, 20. Post hoc analyses of the BLISS trials showed greater mean changes in PCS, MCS, all 8 SF‐36 domains, and FACIT–Fatigue for SLE responder index 4 (SRI4) responders compared with nonresponders 21. To date, the long‐term effects of belimumab treatment on HRQoL and fatigue have not been assessed.
The objectives of this long‐term continuation study of the phase III RCT BLISS‐76 trial in the US 16 were to evaluate the long‐term safety and tolerability, impact on HRQoL and fatigue, and efficacy of belimumab plus standard therapy. Efficacy and safety data have been reported elsewhere 22. Here, we present the longer‐term HRQoL and fatigue outcomes and the impact of clinical characteristics on these outcomes.
Patients and Methods
The study was conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines for Good Clinical Practice, all applicable patient privacy requirements, and the ethics principles outlined in the Declaration of Helsinki 2008. The study was monitored in accordance with ICH E6, Section 5.18. Country‐specific approvals were obtained from individual national, regional, or investigational center ethics committees or institutional review boards. Written informed consent was obtained from all patients prior to enrollment in the study.
Study design
This was a multicenter continuation study (BEL112233; ClinicalTrials.gov identifier: NCT00724867) in patients with SLE who completed the 76‐week parent trial BLISS‐76 (BEL110751; ClinicalTrials.gov identifier: NCT00410384) in the US 16, 22. In BLISS‐76, patients were randomized to receive belimumab 1 mg/kg intravenously (IV), 10 mg/kg IV, or placebo every 28 days and continued with standard therapy. The inclusion and exclusion criteria for BLISS‐76 have been reported previously 16. Briefly, adult patients with a diagnosis of SLE according to the American College of Rheumatology revised criteria 23 who were positive for antinuclear antibodies or anti–double‐stranded DNA antibodies (anti‐dsDNA) and had a Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) version of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (SELENA–SLEDAI) score of ≥6 were included. All patients who completed BLISS‐76 in the US had the option to enroll in this continuation study 22, which was conducted from August 5, 2008 to March 26, 2015.
Patients who received belimumab in the parent trial continued to receive the same dose of belimumab (belimumab/belimumab group), and those who had previously received placebo then received belimumab 10 mg/kg (placebo/belimumab group); following a protocol amendment, all patients received belimumab 10 mg/kg after March 9, 2011. The data presented here are for the pooled patient population; post hoc subgroup analyses were also conducted to provide results based on treatment group in the parent trial. In total, up to 8 calendar years of data were collected, including the parent trial (maximum exposure 2,908 days). However, in the continuation study, assessments were made in accordance with a 48‐week study year; therefore, study years do not align with calendar years. Due to the low number of patients remaining at study year 7 (n = 65), the data exported here are up to study year 6.
Assessments
The primary efficacy assessment (reported elsewhere [22]) was the SRI4 response 24, defined as a ≥4‐point reduction in the SELENA–SLEDAI score, no worsening (<0.3 increase) in the physician's global assessment, and no new British Isles Lupus Assessment Group (BILAG) A or ≤1 new BILAG B organ domain scores, all versus the baseline SRI response. HRQoL was assessed using the SF‐36 25, and fatigue was assessed with the FACIT–Fatigue scale 26, 27, 28, 29. The SF‐36 measures 8 HRQoL domains that are the weighted sums of the questions for each. Raw domain scores are converted to a 0–100 scale, with higher scores indicating better health. These scores are Z‐transformed and weighted to yield values used to calculate PCS and MCS scores, which are norm based with a mean of 50 and SD of 10. The generally accepted minimum clinically important difference (MCID) for improvement of 2.5 units was applied for assessment of the SF‐36 PCS and MCS scores, and an MCID of 5.0 was applied for domain scores 30.
The FACIT–Fatigue scale is a 13‐item scale that measures physical and mental fatigue and their effects on functioning and daily living. Scores range from 0 to 52, with lower scores indicating more fatigue. An MCID of 4 units was used for assessment of the FACIT–Fatigue scores. This was previously confirmed in a sample of 271 patients with rheumatoid arthritis enrolled in a double‐blind RCT of adalimumab versus placebo 27 and supported by data from an RCT in patients with SLE (Exploratory Phase II/III SLE Evaluation of Rituximab [EXPLORER] study), in which it was concluded that a range of 3–4 is the MCID in SLE 31. HRQoL and fatigue assessments were exploratory and were administered at week 48 in each calendar year and at the exit visit. All assessments were carried out in the modified intent‐to‐treat (ITT) population, defined as all patients who were enrolled in the continuation study and were treated with at least 1 dose of belimumab. Patients were not followed up after treatment discontinuation.
Post hoc analyses (BEL206350) were conducted to examine clinical characteristics associated with improvements in HRQoL and fatigue. These analyses included several subgroups of interest: 1) parent trial treatment group (placebo versus belimumab), 2) SRI responders versus nonresponders (SRI4 response in this continuation study, i.e., study year 1), 3) patients with high anti‐dsDNA and/or low serum complement 3 or 4 (C3 or C4) levels versus those without, 4) disease flare at baseline versus no flare at baseline (SLE Flare Index [SFI]) 32 (the observation period included time since the last flare assessment prior to baseline [for placebo, the last SFI assessment made in the parent trial; for belimumab, the SFI assessment made prior to belimumab exposure in the parent trial]), 5) glucocorticoid dose (or prednisone equivalent) at baseline (none, <7.5 or ≥7.5 mg/day), 6) organ damage at baseline (Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index [SDI] score 33, 34; 0, 1, or ≥2).
Statistical analysis
No formal statistical hypothesis testing was performed, and all analyses were descriptive and exploratory. For all patients, baseline was defined as the assessment prior to the first dose of belimumab. Therefore, baseline for patients already receiving belimumab at enrollment into the continuation study was the latest assessment prior to commencing the parent trial BLISS‐76; for patients who had received placebo during the parent trial, baseline was the last assessment prior to the first dose of belimumab in this long‐term continuation study. Mixed‐effects models were used to obtain the mean ± SD values for change from baseline over time, with adjustments for disease duration and activity, baseline HRQoL scores, age, sex, and race.
Results
Patient population
The modified ITT population included 268 patients (91 in the placebo/belimumab group and 177 in the belimumab/belimumab group); 140 patients completed the study, and 128 discontinued. Reasons for discontinuation included patient request (n = 31), adverse events (n = 25), other (n = 22), physician decision (n = 17), lack of efficacy (n = 14), lost to follow‐up (n = 12), noncompliance with study drug (n = 6), and protocol deviation (n = 1). The majority of discontinuations due to lack of efficacy (n = 11) and adverse events (n = 22) occurred in the first 4 years of the study. All adverse events that led to belimumab discontinuation were different, with the exception of an intraductal proliferative breast lesion, which was reported by 2 patients. SLE flares (n = 5) were reported as a common reason for belimumab discontinuation due to lack of efficacy. The rate of discontinuations reported each year remained consistent throughout the study. In addition, a decline in the number of patients starting each yearly interval was similar between patients initially treated with belimumab in the parent trial and those treated with placebo. Further data on discontinuation have been reported elsewhere 35. The baseline characteristics of this patient population are shown in Table 1. At baseline (modified ITT population), the mean ± SD SF‐36 PCS score (37.0 ± 9.9) and MCS score (44.3 ± 11.3) were below the normative mean score of 50 for the general US population 25. Approximately one‐third (36.2%) of patients had a FACIT–Fatigue score of <20 (indicating that fatigue was experienced), and the mean ± SD baseline FACIT–Fatigue score was 26.5 ± 12.4. The majority of patients (70.1%) had a SELENA–SLEDAI score of ≤9, and the mean ± SD score was 7.8 ± 3.9. The mean ± SD SDI score was 1.2 ± 1.5.
Table 1.
Characteristics of the patients at baselinea
| Characteristic | Placebo/ belimumab (n = 91) | Belimumab/ belimumab (n = 177) | Total (n = 268) |
|---|---|---|---|
| Female sex | 84 (92.3) | 166 (93.8) | 250 (93.3) |
| Age, mean ± SD years | 43.4 ± 12.4 | 42.5 ± 10.8 | 42.8 ± 11.3 |
| Race | |||
| White | 61 (67.0) | 125 (70.6) | 186 (69.4) |
| Black or African American/African heritage | 20 (22.0) | 37 (20.9) | 57 (21.3) |
| Other | 10 (11.0) | 15 (8.5) | 25 (9.3) |
| SLE disease duration, mean ± SD yearsb | 8.2 (6.0) | 7.4 (7.2) | 7.7 (6.8) |
| SRI4 responderc | 17 (18.9) | 109 (61.6) | 126 (47.2) |
| SELENA–SLEDAI score, mean ± SD | 5.6 ± 3.6 | 8.9 ± 3.5 | 7.8 ± 3.9 |
| Anti‐dsDNA positive | 49 (53.8) | 86 (48.6) | 135 (50.4) |
| Low C3 and/or low C4 levels | 41 (45.1) | 78 (44.1) | 119 (44.4) |
| SDI score, mean ± SD | 1.2 ± 1.7 | 1.1 ± 1.4 | 1.2 ± 1.5 |
| SFI (≥1 flare) | 18 (19.8) | 47 (26.6) | 65 (24.3) |
| Daily glucocorticoid dose, mean ± SD mg/day | 5.3 ± 6.0 | 6.8 ± 7.4 | 6.3 ± 7.0 |
| Glucocorticoid dosage >7.5 mg/day | 25 (27.5) | 61 (34.5) | 86 (32.1) |
| SF‐36 PCS, mean ± SD | 42.0 ± 9.8 | 34.5 ± 9.0 | 37.0 ± 9.9 |
| SF‐36 MCS, mean ± SD | 47.9 ± 10.3 | 42.4 ± 11.4 | 44.3 ± 11.3 |
| SF‐36 domain scores, mean ± SD | |||
| Bodily pain | 58.0 ± 22.6 | 41.7 ± 20.0 | 47.2 ± 22.3 |
| General health | 45.3 ± 20.8 | 38.2 ± 19.3 | 40.6 ± 20.1 |
| Mental health | 71.0 ± 19.5 | 64.9 ± 20.2 | 67.0 ± 20.1 |
| Physical functioning | 63.2 ± 27.4 | 51.0 ± 26.8 | 55.2 ± 27.5 |
| Role emotional | 73.1 ± 25.2 | 63.9 ± 28.6 | 67.0 ± 27.8 |
| Role physical | 60.9 ± 28.0 | 46.0 ± 26.6 | 51.1 ± 27.9 |
| Social functioning | 69.1 ± 25.8 | 54.0 ± 26.5 | 59.1 ± 27.2 |
| Vitality | 48.7 ± 24.4 | 34.2 ± 20.3 | 39.1 ± 22.8 |
| FACIT–Fatigue score, mean ± SD | 31.2 ± 13.3 | 24.1 ± 11.2 | 26.5 ± 12.4 |
| FACIT–Fatigue scale score | |||
| 20–34 | 28 (30.8) | 67 (37.9) | 95 (35.4) |
| <20 | 23 (25.3) | 74 (41.8) | 97 (36.2) |
| ≥35 | 40 (44.0) | 35 (19.8) | 75 (28.0) |
Data for the Short Form 36 (SF‐36) and Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue were available for only 176 patients in the belimumab/belimumab group. Except where indicated otherwise, values are the number (%). SRI4 = FACIT–Fatigue for Systemic Lupus Erythematosus (SLE) responder index 4; SELENA–SLEDAI = Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) version of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI); anti‐dsDNA = anti–double‐stranded DNA; SDI = Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SFI = SLE Flare Index; PCS = physical component summary; MCS = mental component summary.
Duration is defined as the time from the screening date to the time of SLE diagnosis date plus 1 day.
Responders 1 year (48 weeks in study terms) after long‐term extension baseline; 1 patient in the belimumab/placebo group was lost to follow‐up; the percentages were adjusted accordingly.
SF‐36 scores
In the overall population, the mean ± SD PCS score increased overall from 37.0 ± 9.9 (n = 267) at baseline to 40.3 ± 10.4 (n = 260) at study year 1 (mean ± SD change from baseline 3.4 ± 8.6 [n = 259]; the sample size difference was due to missing data for 1 patient]) (Figure 1A). This increase was maintained during the long‐term continuation study to 41.7 ± 10.0 (n = 185) at study year 6 (mean ± SD change from baseline 4.8 ± 9.4 [n = 185] and exceeded the MCID for improvement of 2.5 units 27, 30. The mean ± SD MCS score increased overall from 44.3 ± 11.3 (n = 267) at baseline to 46.6 ± 11.7 (n = 260) at study year 1 (mean ± SD change from baseline 2.5 ± 10.1 [n = 259]; the sample size difference was due to missing data for 1 patient) (Figure 1A). At study year 6, the mean ± SD MCS score reached 47.0 ± 11.6 (n = 185); the mean ± SD change from baseline was 2.7 ± 11.3 (n = 185), exceeding the MCID. The number of patients who reported improvements of at least the MCID (5.0) across all domains for study years 1–6 are shown in Table 2.
Figure 1.
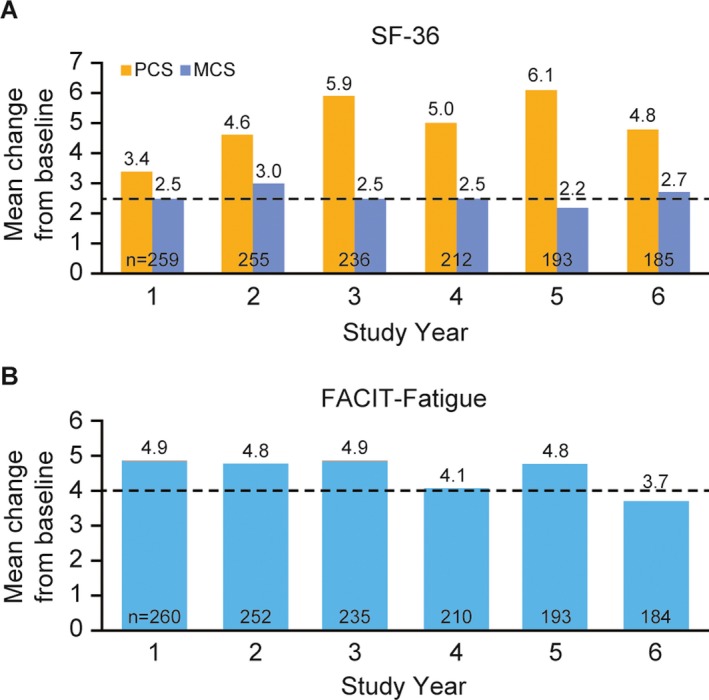
Changes in A, Short Form 36 (SF‐36) physical component summary (PCS) and mental component summary (MCS) scores and B, Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scores between baseline and year 6. The timing of baseline assessments differed across the population, according to parent trial treatment. Broken lines represent the minimum clinically important difference 27, 30. Values beneath each pair of columns represent the number of patients assessed at that time point.
Table 2.
Patients who reported improvements ≥MCID for each SF‐36 domain and the FACIT–Fatigue score, by study yeara
| Study yearsb | SF‐36 Domains | FACIT–Fatigue | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bodily pain | General health | Mental health | Physical functioning | Role emotional | Role physical | Social functioning | Vitality | ||
| 1 (n = 268) | 148 (55.2) | 151 (56.3) | 139 (51.9) | 141 (52.6) | 111 (41.4) | 142 (53.0) | 127 (47.4) | 152 (56.7) | 135 (50.4) |
| 2 (n = 259) | 137 (52.9) | 148 (57.1) | 133 (51.4) | 129 (49.8) | 114 (44.0) | 139 (53.7) | 125 (48.3) | 144 (55.6) | 123 (47.5) |
| 3 (n = 244) | 135 (55.3) | 143 (58.6) | 116 (47.5) | 124 (50.8) | 98 (40.2) | 131 (53.7) | 124 (50.8) | 134 (54.9) | 119 (48.8) |
| 4 (n = 219) | 117 (53.4) | 127 (58.0) | 102 (46.6) | 112 (51.1) | 91 (41.6) | 113 (51.6) | 103 (47.0) | 122 (55.7) | 97 (44.3) |
| 5 (n = 202) | 109 (54.0) | 126 (62.4) | 100 (49.5) | 105 (52.0) | 82 (40.6) | 109 (54.0) | 93 (46.0) | 112 (55.4) | 108 (53.5) |
| 6 (n = 192) | 100 (52.1) | 100 (52.1) | 97 (50.5) | 100 (52.1) | 72 (37.5) | 96 (50.0) | 88 (45.8) | 98 (51.0) | 89 (46.4) |
For Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue improvements, the minimum clinically important difference (MCID) was ≥4 units. For Short Form 36 (SF‐36) improvements, the MCID was ≥5 units. Values are the number (%).
The n values in parentheses represent the number of non‐missing patients at each study interval.
Scores varied across individual SF‐36 domains (Figure 2). At study year 1, mean changes from baseline in the scores for 7 of 8 SF‐36 domains (physical functioning, role physical, bodily pain, general health, vitality, role emotional, and social functioning but not mental health) met or exceeded the MCID of 5.0, representing clinically meaningful improvements in these domains 30, 31. Improvements were maintained, and at study year 6, the mean changes from baseline exceeded the MCID in 6 of 8 SF‐36 domains (bodily pain, general health, physical functioning, role physical, social functioning, vitality) but not role emotional and mental health.
Figure 2.
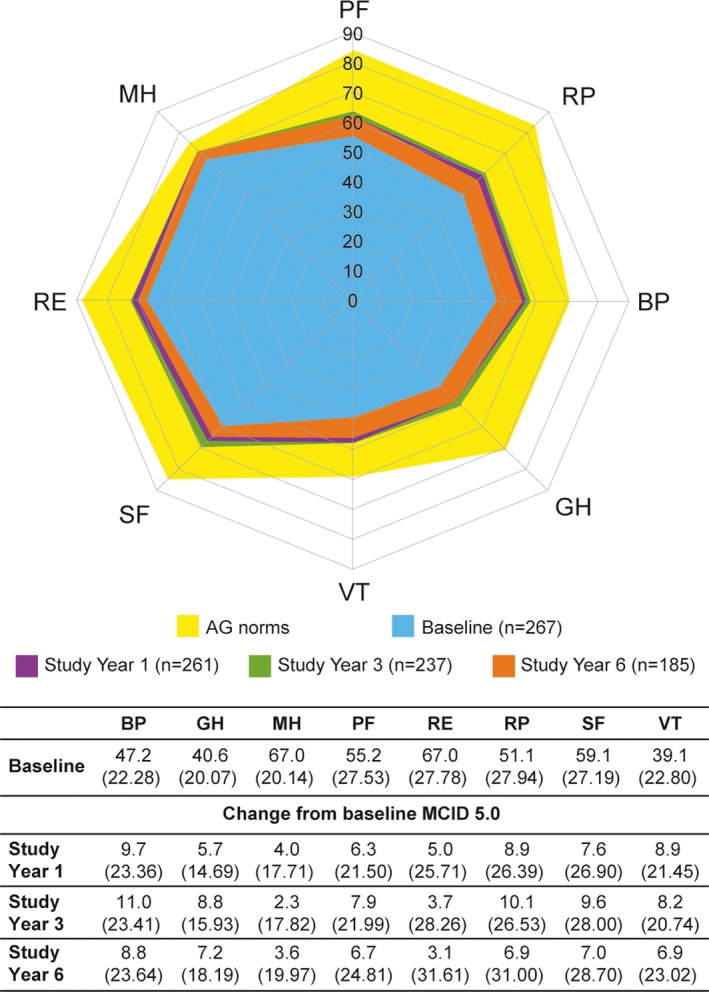
Short Form 36 domain scores at study years 1, 3, and 6. PF = physical functioning; RP = role physical; BP = bodily pain; GH = general health; VT = vitality; SF = social functioning; RE = role emotional; MH = mental heath; AG = age‐ and sex‐matched. MCID = minimum clinically important difference.
FACIT–Fatigue
The mean ± SD FACIT–Fatigue score increased from 26.5 ± 12.4 (n = 267) at baseline to 31.3 ± 13.3 (n = 261) at study year 1 (mean ± SD change from baseline 4.9 ± 10.8 [n = 260]; the sample size difference was due to missing data for 1 patient) and 30.2 ± 13.5 (n = 184) at study year 6 (mean ± SD change from baseline, 3.7 ± 11.8 [n = 184]) (Figure 1B). These mean changes from baseline exceeded the MCID of 4 up to study year 5 and approached the MCID at study year 6. Almost half of the patients (46.4% [89 of 192]) experienced an improvement in the FACIT–Fatigue score that exceeded the MCID (≥4) at the study year 6 visit, with the highest percentage (53.5% [108 of 202]) exceeding the MCID at the study year 5 visit. The number of patients who reported improvements of ≥MCID (≥4 units) for FACIT–Fatigue scores for study years 1–6 is shown in Table 2.
Clinical characteristics associated with improved HRQoL and fatigue (post hoc analyses)
Patients who received belimumab treatment in both the parent study and the continuation study showed statistically significantly larger HRQoL improvements from baseline compared with those who received placebo in the parent trial and switched to belimumab treatment at entry into the continuation study. For patients already receiving belimumab at enrollment into the continuation study, baseline was defined as the latest assessment prior to commencing the parent trial BLISS‐76; for patients who had received placebo during the parent trial, baseline was defined as the last assessment prior to the first dose of belimumab in this long‐term continuation study. Post hoc analyses showed that at study years 2 through 6, statistically significant associations were observed between the parent trial treatment groups and changes from baseline in SF‐36 PCS and MCS scores and FACIT‐Fatigue scores (all P < 0.01) (Figures 3A and B) 27, 30.
Figure 3.
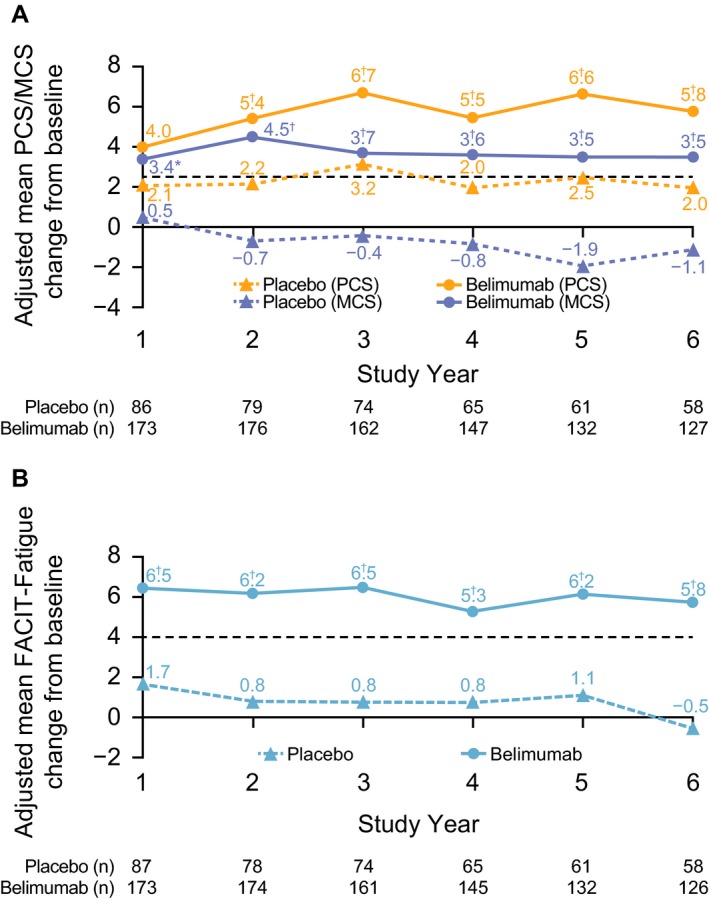
Adjusted mean changes from baseline over time for A, SF‐36 PCS and MCS scores and B, FACIT–Fatigue scores. The timing of baseline assessments differed across the population, according to parent trial treatment. Broken lines represent the minimum clinically important difference 27, 30. Values were means‐adjusted for baseline SF‐36 MCS and PCS, FACIT–Fatigue, age, disease duration, Safety of Estrogens in Lupus Erythematosus National Assessment Systemic Lupus Erythematosus Disease Activity Index score, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index score, sex, and race. MCID = minimum clinically important difference (see Figure 1 for other definitions). * = P < 0.05; † = P < 0.01 versus placebo.
SRI4 responders at study year 1 reported numerically larger improvements from baseline in PCS and MCS scores up to study year 6 compared with nonresponders (Figure 4A) 27, 30. Changes from baseline in FACIT–Fatigue scores were also greater for SRI4 responders versus nonresponders and reached statistical significance at study years 2 and 3 (Figure 4B) 27, 30. At study year 6, reported PCS, MCS, and FACIT–Fatigue score improvements from baseline were numerically higher for patients with elevated anti–dsDNA and/or low C3/C4 levels at baseline compared with patients with normal levels at baseline (Figures 4C and D). In addition, improvements in PCS scores were statistically significantly greater at study years 1–4. At study year 6, mean changes from baseline in the PCS, MCS, and FACIT–Fatigue scores were not significantly different from those in the other subgroup comparisons (data not shown for flare at baseline, glucocorticoid dose, and SDI score).
Figure 4.
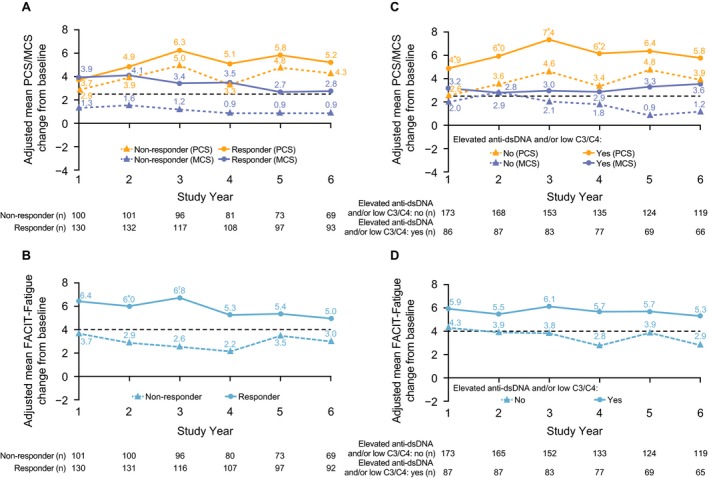
Adjusted mean changes from baseline over time. Values were means‐adjusted for baseline SF‐36 MCS and PCS, FACIT–Fatigue, age, disease duration, Safety of Estrogens in Lupus Erythematosus National Assessment Systemic Lupus Erythematosus Disease Activity Index (SELENA‐SLEDAI) score, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index score, sex, and race. A and C, SF‐36 PCS and MCS scores. B and D, FACIT–Fatigue scores. In A and B, data were stratified according to responder status. In C and D, data were stratified according to elevated anti–double‐stranded DNA (anti‐dsDNA) and/or low C3/C4 status. Patients with a baseline SELENA‐SLEDAI score of <4 were excluded from the subgroup analysis. The timing of baseline assessments differed across the population, according to parent trial treatment. Broken lines represent the minimum clinically important difference 27, 30. See Figure 1 for other definitions. * = P < 0.05; † = P < 0.01 versus nonresponders (A and B) and versus no elevated anti‐dsDNA and/or low C3/C4 (C and D).
Discussion
Diminished HRQoL and elevated levels of fatigue are common symptoms in patients with SLE and patients with other chronic illnesses, such as cancer 36 and rheumatoid arthritis 37. Mood disturbances, pain, sleep disturbances, and functional limitations, including inability to work, are frequently associated with decreased HRQoL and fatigue 27, 38, 39. Improvements in HRQoL in patients with SLE who received belimumab have been reported for up to 76 weeks 19. Here, we present the first patient‐reported HRQoL and fatigue data in patients with active SLE with long‐term exposure to belimumab.
Patients receiving belimumab reported long‐term improvements in HRQoL and fatigue, based on SF‐36 PCS, MCS, and domains as well as FACIT–Fatigue scores. These benefits are consistent with results from the parent trial BLISS‐76 16 and pooled results from both phase III studies of belimumab in SLE, BLISS‐52, and BLISS‐76 19. The largest improvements in HRQoL and fatigue were reported in the first study year of treatment and were maintained or further increased during long‐term exposure to belimumab through study year 6. Previously reported efficacy and safety data from this long‐term continuation study demonstrated an overall decrease in disease activity, along with a decrease in prednisone use, low rates of flare, and organ damage accrual and an acceptable safety profile 22. Taken together, these results suggest that long‐term control of disease activity with belimumab plus standard therapy results in significant benefits in both HRQoL and fatigue.
In post hoc analyses, belimumab treatment (versus placebo) was associated with statistically significant changes in HRQoL scores at all time points, with the exception of the study year 1 PCS score. SRI4 response at study year 1 was also identified as a characteristic associated with improved HRQoL and fatigue, although differences were not statistically significant at all time points. The definition of responders, which was based on clinician assessments, may not have fully captured the patient experience and could partly explain the lack of significant associations based on response status. These post hoc analyses of long‐term data are consistent with previous post hoc pooled analyses of the BLISS studies, which demonstrated global benefits in patients who were SRI responders, including improvements in HRQoL 21.
The results of other post hoc analyses of the BLISS studies have suggested that belimumab may have greater therapeutic benefits in patients with elevated anti‐dsDNA or low C3/C4 levels or in those receiving glucocorticoid treatment compared with patients without those characteristics 40. Thus, it may be expected that in the subgroup analyses presented here, these characteristics may also be associated with improved HRQoL. Indeed, elevated anti‐dsDNA and/or low C3/C4 levels were identified as characteristics associated with improvement, but this was not statistically significant at all time points. A strong trend between baseline glucocorticoid dosage categories (none, <7.5, or ≥7.5 mg/day) and each yearly time point was not identified.
It is important to consider the study design when interpreting these post hoc analyses. The reported improvements in SF‐36 and FACIT–Fatigue scores were based on combined data. However, it is notable that within this total population, baseline in the placebo/belimumab group was prior to the first dose of belimumab in the continuation study. These patients had already completed the parent trial, during which their standard therapy and care would have been optimized, and their health likely improved as they continued in the trial. For example, the mean baseline SELENA–SLEDAI score was lower in the placebo/belimumab group compared with the pre‐treatment belimumab/belimumab group (5.6 versus 8.9), and the percentage of patients with ≥1 SFI flare was also lower (19.8% versus 26.6%). In addition, baseline HRQoL scores were higher in the placebo/belimumab group compared with the belimumab/belimumab group, for whom baseline scores were prior to treatment in the parent trial. Future analyses using baseline scores from the same time point (at parent trial entry) for all treatment groups could be carried out to more accurately assess changes from baseline. However, this approach would present a new challenge for capturing the effects of belimumab exposure, because this would include a period of time during which some patients received placebo.
A potential confounder of interpretation is the small sample size for some subgroup analyses, particularly at later time points. In addition, the continuation phase of this study had an open‐label design with no placebo control group, which presents a challenge in determining the true effect of treatment. Furthermore, not all patients received the same belimumab dose for the full study, because after March 2011, patients who received 1 mg/kg at the time of enrollment into this continuation study had their dose increased to 10 mg/kg. Possible selection bias may be present among patients who elected to enroll in this continuation study and among those who remained in the study, because patients who responded to and tolerated belimumab treatment may be more likely to continue long‐term treatment. In addition, ~30% of patients in the extension study were treated with placebo (plus standard therapy) for 76 weeks in the parent trial and thus may represent a population with benign SLE. However, the rate of decline in the number of patients starting each yearly interval in the extension study was similar between the belimumab and placebo groups in the parent trial.
Although there were numerous withdrawals during the study, as anticipated, more than half of the patients (140 of 268) remained in the study until completion. In our analyses, we used the MCID for FACIT–Fatigue of 4 units, as established by Cella et al 27 based on RCT data from patients with rheumatoid arthritis 27. This estimate is supported by an analysis of results from the EXPLORER RCT, which showed that the MCID in SLE is 3–4 units 31. The FACIT–Fatigue results reported here exceeded the lower MCID estimate of 3 units at all time points. A longitudinal observational study used an alternative approach to estimate the MCID in SLE as 5.9 9. However, as the methodology used for this estimate differed because it was not linked to initiation of therapy, as in an RCT, we believe that the MCID of 4.0 is more appropriate 19, 21, 41.
Overall, the data reported here indicate that early and long‐term control of disease activity with belimumab plus standard therapy translates into meaningful and sustained benefits in patient‐reported fatigue and HRQoL.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Ramachandran had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Strand, Berry, Lin, Asukai, Ramachandran.
Acquisition of data
Berry, Lin, Asukai, Punwaney, Ramachandran.
Analysis and interpretation of data
Strand, Berry, Lin, Asukai, Punwaney, Ramachandran.
Role of Study Sponsor
GlaxoSmithKline was involved in designing the study, contributed to the collection, analysis, and interpretation of the data, supported the authors in the development of the manuscript, and funded the medical writing assistance provided by Nicole Cash, MRes, PhD, and Louisa Pettinger, PhD (Fishawack Indicia Ltd). All authors, including those employed by GlaxoSmithKline, approved the content of the submitted manuscript and were involved in the decision and to submit the manuscript for publication.
ClinicalTrials.gov identifier: NCT00724867.
Previously published in abstract form: Strand V, Berry P, Lin X, Asukai Y, Fettiplace J, Ramachandran S. Long‐term impact of belimumab on health‐related quality of life and fatigue in patients with systemic lupus erythematosus following 7 years of treatment exposure: impact of clinical characteristics over time [abstract]. Arthritis Rheumatol 2016;68 Suppl:S10.
Supported by GlaxoSmithKline and Human Genome Sciences.
Dr. Strand has received consulting fees from AbbVie, Amgen, AstraZeneca, Bristol‐Myers Squibb, Celgene, EMD Serono, Genentech/Roche, GlaxoSmithKline, Janssen, Kypha, Eli Lilly, Novartis, Pfizer, Regeneron, Sanofi, and UCB (all less than $10,000). Ms Berry, Punwaney, and Ramachandran, own stock or stock options in GlaxoSmithKline.
References
- 1. Gilboe IM, Kvien TK, Husby G. Disease course in systemic lupus erythematosus: changes in health status, disease activity, and organ damage after 2 years. J Rheumatol 2001;28:266–74. [PubMed] [Google Scholar]
- 2. Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. Relationship between flare and health‐related quality of life in patients with systemic lupus erythematosus. J Rheumatol 2010;37:568–73. [DOI] [PubMed] [Google Scholar]
- 3. Lopez R, Davidson JE, Beeby MD, Egger PJ, Isenberg DA. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology 2012;51:491–8. [DOI] [PubMed] [Google Scholar]
- 4. Baker K, Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology 2009;48:281–4. [DOI] [PubMed] [Google Scholar]
- 5. Strand V, Chu AD. Generic versus disease‐specific measures of health‐related quality of life in systemic lupus erythematosus. J Rheumatol 2011;38:1821–3. [DOI] [PubMed] [Google Scholar]
- 6. Strand CV, Russell AS. WHO/ILAR Taskforce on quality of life. J Rheumatol 1997;24:1630–3. [PubMed] [Google Scholar]
- 7. Bruce IN, Mak VC, Hallett DC, Gladman DD, Urowitz MB. Factors associated with fatigue in patients with systemic lupus erythematosus. Ann Rheum Dis 1999;58:379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thumboo J, Strand V. Health‐related quality of life in patients with systemic lupus erythematosus: an update. Ann Acad Med Singapore 2007;36:115–22. [PubMed] [Google Scholar]
- 9. Goligher EC, Pouchot J, Brant R, Kherani RB, Avina‐Zubieta JA, Lacaille D, et al. Minimal clinically important difference for 7 measures of fatigue in patients with systemic lupus erythematosus. J Rheumatol 2008;35:635–42. [PubMed] [Google Scholar]
- 10. Kent T, Davidson A, Newman D, Buck G, D'Cruz D. Burden of illness in systemic lupus erythematosus: results from a UK patient and carer online survey. Lupus 2017;26:1095–100. [DOI] [PubMed] [Google Scholar]
- 11. Kier AO, Midtgaard J, Hougaard KS, Berggreen A, Bukh G, Hansen RB, et al. How do women with lupus manage fatigue? A focus group study. Clin Rheumatol 2016;35:1957–65. [DOI] [PubMed] [Google Scholar]
- 12. Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005;32:1706–8. [PubMed] [Google Scholar]
- 13. Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune‐based rheumatic diseases. Arthritis Rheum 2001;44:1313–9. [DOI] [PubMed] [Google Scholar]
- 14. Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum 2008;58:2453–9. [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Roschke V, Baker KP, Wang Z, Alarcón GS, Fessler BJ, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001;166:6–10. [DOI] [PubMed] [Google Scholar]
- 16. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo‐controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. GlaxoSmithKline . Benlysta Prescribing Information. URL: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Benlysta/pdf/BENLYSTA-PI-MG-IFU-COMBINED.PDF.
- 18. EMA . Benlysta summary of product characteristics (SmPC). URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002015/WC500110150.pdf.
- 19. Strand V, Levy RA, Cervera R, Petri MA, Birch H, Freimuth WW, et al. Improvements in health‐related quality of life with belimumab, a B‐lymphocyte stimulator‐specific inhibitor, in patients with autoantibody‐positive systemic lupus erythematosus from the randomised controlled BLISS trials. Ann Rheum Dis 2014;73:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo‐controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 21. Furie R, Petri MA, Strand V, Gladman DD, Zhong ZJ, Freimuth WW, et al. Clinical, laboratory and health‐related quality of life correlates of Systemic Lupus Erythematosus Responder Index response: a post hoc analysis of the phase 3 belimumab trials. Lupus Sci Med 2014;1:e000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furie R, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, et al. 7‐year safety and efficacy of belimumab in patients with systemic lupus erythematosus [poster]. Presented at the Annual American College of Rheumatology/Association of Rheumatology Health Professionals; 2016 November 11–16; Washington DC.
- 23. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 24. Furie RA, Petri MA, Wallace DJ, Ginzler EM, Merrill JT, Stohl W, et al. Novel evidence‐based systemic lupus erythematosus responder index. Arthritis Rheum 2009;61:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ware J. User's manual for the SF‐36v2® health survey. Lincoln (RI): QualityMetric Incorporated; 2007. [Google Scholar]
- 26. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag 1997;13:63–74. [DOI] [PubMed] [Google Scholar]
- 27. Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811–9. [PubMed] [Google Scholar]
- 28. Kosinski M, Gajria K, Fernandes A, Cella D. Qualitative validation of the FACIT‐Fatigue scale in systemic lupus erythematosus. Lupus 2013;22:422–30. [DOI] [PubMed] [Google Scholar]
- 29. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strand V, Crawford B. Improvement in health‐related quality of life in patients with SLE following sustained reductions in anti‐dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res 2005;5:317–26. [DOI] [PubMed] [Google Scholar]
- 31. Lai JS, Beaumont JL, Ogale S, Brunetta P, Cella D. Validation of the functional assessment of chronic illness therapy‐fatigue scale in patients with moderately to severely active systemic lupus erythematosus, participating in a clinical trial. J Rheumatol 2011;38:672–9. [DOI] [PubMed] [Google Scholar]
- 32. Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus 1999;8:685–91. [DOI] [PubMed] [Google Scholar]
- 33. Gladman DD, Goldsmith CH, Urowitz MB, Bacon P, Fortin P, Ginzler E , et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol 2000;27:373–6. [PubMed] [Google Scholar]
- 34. Gladman DD, Urowitz MB, Goldsmith CH, Fortin P, Ginzler E, Gordon C, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 1997;40:809–13. [DOI] [PubMed] [Google Scholar]
- 35. Furie RA, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, et al. Long‐term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a seventy‐six–week phase III parent study in the United States. Arthritis Rheumatol 2018;70:868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002;94:528–38. [DOI] [PubMed] [Google Scholar]
- 37. Huyser BA, Parker JC, Thoreson R, Smarr KL, Johnson JC, Hoffman R. Predictors of subjective fatigue among individuals with rheumatoid arthritis. Arthritis Rheum 1998;41:2230–7. [DOI] [PubMed] [Google Scholar]
- 38. Baker K, Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology 2009;48:281–4. [DOI] [PubMed] [Google Scholar]
- 39. Utset TO, Fink J, Doninger NA. Prevalence of neurocognitive dysfunction and other clinical manifestations in disabled patients with systemic lupus erythematosus. J Rheumatol 2006;33:531–8. [PubMed] [Google Scholar]
- 40. Van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis 2012;71:1343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nantes SG, Strand V, Su J, Touma Z. Comparison of the sensitivity to change of the 36‐item Short Form Health Survey and the LupusQoL using various definitions of minimal clinically important differences in patients with active systemic lupus erythematosus. Arthritis Care Res 2018;70:125–33. [DOI] [PubMed] [Google Scholar]


