Abstract
To liberate society from its dependence on fossil‐based fuels and materials it is pivotal to explore components of renewable plant biomass in applications that benefit from their intrinsic biodegradability, safety, and sustainability. Lignin, a byproduct of the pulp and paper industry, is a plausible material for carrying various types of cargo in small‐ and large‐scale applications. Herein, possibilities and constraints regarding the physical–chemical properties of the lignin source as well as modifications and processing required to render lignins suitable for the loading and release of pesticides, pharmaceuticals, and biological macromolecules is reviewed. In addition, the technical challenges, regulatory and toxicological aspects, and future research needed to realize some of the promises that nano‐ and microscaled lignin materials hold for a sustainable future are critically discussed.
Keywords: drug delivery, immobilization, lignin, nanoparticles, sustainable chemistry
Introduction
Lignin is a fascinating biopolymer with a high valorization potential. Besides being the most abundant aromatic renewable biopolymer, its complex structure is responsible for its antioxidant1, 2, 3, 4, 5 and antimicrobial activity.6, 7, 8, 9, 10, 11, 12 Lignins arise from the plants’ secondary metabolism of 4‐hydroxyphenylpropanoid precursors that differ in the number of methoxy groups at the 3‐ and 5‐positions of the phenolic ring.13 The structure of a specific lignin material strongly depends on the botanical origin and the isolation process. Especially the harsh thermochemical processes used to separate cellulose from other cell‐wall components cause fragmentation, functional group eliminations, and process‐typical chemical functionalization of lignin.14, 15, 16, 17, 18, 19, 20, 21 In recent years, apart from its combustion for energy, isolation of lignin originating from industrial kraft pulping processes as well as biorefinery plants producing ethanol as biofuel has seen a steady increase. Parallel to the surge in the availability of lignin, nano‐ and microscaled lignin‐based materials22, 23, 24, 25 and state‐of‐the‐art analytical techniques that provide a more detailed understanding of the structural differences of the various lignins were developed.26, 27, 28, 29, 30
One of the emerging utilization areas of lignin particles and capsules is their use as carriers for biologically active substances. This Review focuses on three applications of lignin‐based carrier systems, plant protection, nanomedicine, and biocatalysis, which share some common drivers. Efficient binding/encapsulation of active substances in lignin‐based structures is important for all of the above applications whereas controlled release is emphasized in biomedicine and plant protection.
Current plant protection applications require a vast amount of binders and other materials that facilitate dispersing, shielding from photodegradation, and reducing uncontrolled leaching of agricultural chemicals. According to the Agri‐Environmental Indicators in the FAOSTAT database provided by the Food and Agriculture organization of the United Nations (FAO),31 global pesticide use was, on average, 4.2 kg ha−1 arable land in 2012, with a total annual consumption of more than 4 Mt. Alongside with the expensive search of new active substances, development of new formulations that improve performance of currently approved substances presents many possibilities.32
Compared to the vast tonnage of pesticides, the material flows in biomedical applications are far less yet with potential for higher added value. Arguably, the currently available amount of lignins suffices to meet the need of biomedical materials. Instead of focusing on the quantity, more attention is required here regarding the assessment of suitability of industrially produced lignins in emerging applications such as in nanomedicine. A shortage of literature in this respect concerns the purity and chemical properties of lignin and also the associated processes that need to be compatible with the application. A comprehensive and critical review on this subject is therefore of pivotal importance. This Review takes an in‐depth view on lignin‐based carriers, including synthesis and chemical functionalization used. We begin with an outlook on the properties of technical lignins and their suitability in various fields and continue with the methods used to realize the various carrier structures, to load the active cargo, and to trigger its release. Based on a critical review of materials that have been used as carriers for pharmaceuticals, biological macromolecules, and pesticides, we identify challenges and opportunities in current and future applications before closing with regulatory issues encountered when aiming for nanoscaled materials in marketed applications in the various fields touched in here.
1. Overview of Material Properties of Lignin
The abundance and relatively low cost of lignin in combination with its antioxidant activity1, 2, 3, 4, 5 and UV‐shielding properties2, 3, 33, 34 attract renewed interest in this natural polyphenol. The main lignin types available in relevant quantities are lignosulfonates (LSs), kraft lignin (KL), organosolv lignins (OSLs), and biorefinery lignins that differ notably in their chemical structure, molecular weight, and purity, which in turn determine their solubility and organoleptic qualities. These are important factors influencing the selection of suitable applications in active delivery. For instance, solubility of lignin during and after fabrication of lignin‐based carrier materials is of central importance. Depending on their purity, KLs are soluble in aqueous alkali and in some organic solvent mixtures such as aqueous acetone or tetrahydrofuran (THF). LSs are soluble in aqueous media over a broad pH range whereas solubility of OLSs varies largely depending on the pulping process. Biorefineries hold considerable potential as important sources of industrial lignins,35 but the solubility properties of the lignins are still unpredictable due to the unsettled process conditions and/or variability in feedstock materials. Table 1 gives an overview of lignins most commonly used as raw materials when fabricating carriers for a variety of active ingredients. The selection of lignin type depends on the application category, that is, purified or fractionated lignins should be considered for biomedicine whereas less pure preparations are deemed more suitable for technical applications such as controlled release of pesticides. Many physical and chemical routes are available to modify existing functionalities or to insert new groups into lignins.36, 37 However, little attention has been paid to the quality–safety aspect of lignin, especially in the context of chemical modifications that introduce new covalently linked groups to lignins.
Table 1.
Molecular weight and solubility characteristics of common lignins used in fabrication of various carrier materials for active substances.
| Lignin source | Type[a] | Isolation process | Typical impurities |
|---|---|---|---|
| softwood, hardwood | KL | kraft pulping | carbohydrates <5 % |
| softwood, hardwood | LS | sulfite pulping | salts |
| softwood, hardwood | OS | ethanol–water organosolv pulping | carbohydrates, extractives |
| annual plants | SL[a] | soda pulping | carbohydrates <10 %, silica |
| annual plants | HTL | autohydrolysis or acid‐catalyzed pretreatment and extraction | carbohydrates <10 % |
| various types of biomass | EHL | solid fraction recovered after saccharification of pretreated biomass | carbohydrates >10 % |
[a] Type of lignin: SL—soda lignin, HTL—hydrothermal lignin, EHL—enzymatic hydrolysis lignin.
2. Lignin‐Based Materials and Methods for Active‐Substance Loading
Lignin‐based materials can be ordered according to descending particle size in hydrogels, granules, microcapsules, microparticles, nanocapsules, and nanoparticles. Research output in these categories has increased within the span of the last six years, with the exception of lignin granules (Figure 1). The most extensive growth in the number of publications dealing with lignin nanoparticles (LNPs) reflects strong anticipation for their potential applications. Some authors use the term “colloidal lignin particle” (CLP) interchangeably with LNP; the total number of non‐duplicate publications in these two categories is shown in Figure 1.
Figure 1.
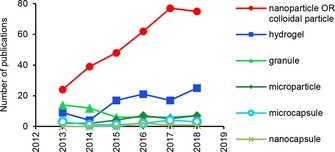
Number of publications returned to the search string “Lignin AND keyword” in the Scopus database (search limited to title, abstract, keywords, duplicates removed).
Fabrication of lignin particles on nano‐ and microscales and some of their general applications were reviewed recently,22, 23, 24, 38 and we therefore limit our efforts to a brief overview of the methods frequently used (Figure 2). The most common method for the preparation of LNPs involves solvent exchange by adding a non‐solvent into lignin solution, or vice versa, causing formation of spherical particles due to the minimization of surface energy (Figure 2 a–c).39 Aqueous and non‐aqueous solvents (THF,40, 41 dioxane,42 dimethyl sulfoxide,33, 43 acetone,2, 33, 44 and ethanol45, 46) have been used to dissolve lignin, with water being the most commonly used non‐solvent to produce spherical particles. In the case of ethanol–water solvent mixture, the particle diameter has been reported to controllably increase as the dilution rate decreases (Figure 2 c).45, 46 The resulting spherical and spheroidal particles exhibit surface charges comparable to those formed using the regular solvent‐exchange method. Stable CLP dispersions have been reported at concentrations up to 3 % (w/w) using ethanol as co‐solvent with THF.47 CLPs exhibit lignin‐typical antioxidant and UV‐protective properties3 that are useful for instance in sunscreens.48, 49, 50, 51, 52 Aerosol technology is another approach to prepare lignin nano‐ and microparticles (Figure 2 d).53 In this process, solvent is vaporized from a solution of lignin, forming particles at the hydrophobic solvent–air interface. Emulsion templates represent a common approach to the synthesis of lignin nano‐ and microcapsuIes (Figure 2 f).54, 55, 56, 57, 58 In addition, synthesis of lignin nanotubes was conducted using sacrificial aluminum templates.60
Figure 2.
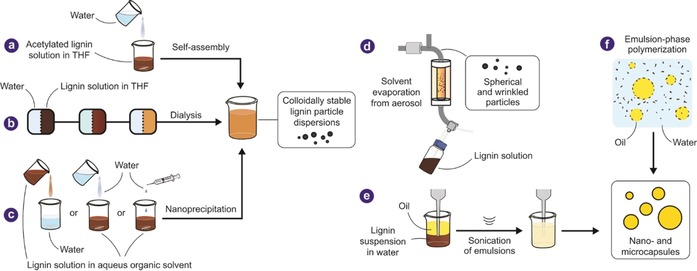
Methods used in fabrication of nano‐ and microscale lignin materials. Colloidally stable lignin nanoparticle (LNP) dispersions prepared by (a) adding water to a THF solution of acetylated wheat AL; (b) dialyzing non‐acetylated softwood KL in THF solution against deionized water (adapted from Ref. 40 with permission from The Royal Society of Chemistry); (c) nanoprecipitation by adding water to a lignin solution, or vice versa. Common solvents used include ethanol,1, 46 THF,41 and acetone.2 (d) Formation of micro‐ and nanoparticles in an aerosol flow reactor (adapted with permission from Ref. 53 Copyright (2016) American Chemical Society). (e) Microcapsules formed by ultrasonication of KL containing a cross‐linker (adapted with permission from Ref. 54 Copyright (2014) American Chemical Society). (f) Synthesis of nanoparticles, nanocapsules, and porous microparticles by emulsion‐phase polymerization and cross‐linking (adapted with permission from Refs. 55, 59 Copyright (2016, 2017) American Chemical Society).
Although essentially not present in the final product, it is worth mentioning that lignin was used as a precursor for carbon nanodots61 and as a sacrificial template during synthesis of the target material. Tardy et al. used KL and alkali lignins (ALs) as sacrificial cores in the generation of capsules comprised of “phenol–metal” complexes.62 In this case, the phenols forming the shell of the capsule after dissolution of the sacrificial lignin core were tannins. The advantage of using lignin as a sacrificial template is that problems usually encountered in the dissolution of sacrificial polystyrene cores using non‐green and/or non‐benign solvents, are avoided. Later, Piccinino et al. constructed micro‐ and nanocapsules/nanoparticles using a sacrificial core made of microsized beads from manganese carbonate or OSL‐based nanoparticles and tannic acid and LS for coating.63 The products showed antioxidant activity, UV‐shielding properties, and electrochemical responsiveness through synergistic effects.
Entrapment, encapsulation, adsorption, and covalent binding are common methods for loading active substances into lignin materials (Figure 3). The loading of the cargo may take place during or after the formation of nanoparticles, nanocapsules, among others. Simultaneous loading was achieved by entrapment in LNPs1, 44, 64, 65, 66, 67 and encapsulation in emulsion polymerization.56, 68, 69 Moreover, layer‐by‐layer (LbL) adsorption can be used to assembly active substances within polymer multilayers,70 for instance to increase the amount of loaded active, while reducing its release rate.
Figure 3.
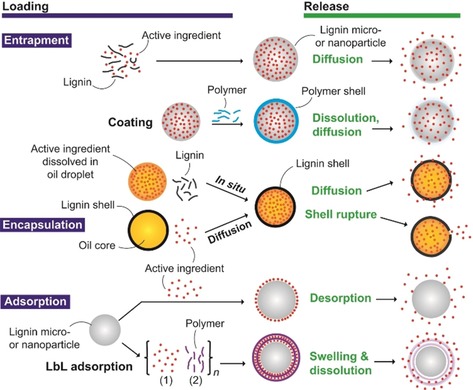
General methods for loading and releasing active substances from various lignin‐based particulate and capsule carriers.
3. Entrapment, Encapsulation, and Adsorption of Active Substances in/on Lignin Carriers
3.1. Entrapment
Entrapment is by far the most common method to load active cargo in lignin‐based materials. The current view is that LNPs form by supramolecular assembly of poorly water‐soluble molecule domains through electric interactions with aromatic rings.1, 39, 40 As could be expected from this mechanism, most of the substances entrapped in LNPs are low‐molecular‐weight compounds with low water solubility (Figure 4). Pesticides (herbicides, insecticides, microbicides)44, 71, 72, 73, 74, 75, 76, 77, 78, 79 constitute the largest class of compounds that were entrapped. Commercial interest in lignin‐based carriers is at least partly due to the well‐suited intrinsic properties of lignin as a polyphenol. Lignins can (i) provide carriers capable of triggered slow or fast release of pesticides; (ii) shield UV‐sensitive or toxic substances; (iii) facilitate dispersion of actives in liquid and solid matrices; (iv) prevent unwanted erosion of volatile, eventually toxic active substances; and (v) substitute currently used synthetic polymers in these applications.76, 80, 81 Additionally, due to the slow rate of biodegradation, lignin can contribute to maintaining the soil carbon balance.82 Besides pesticides, there are a few studies that used lignin‐based materials for enzyme immobilization83, 84 and delivery of active pharmaceutical ingredients.1, 64, 65, 85
Figure 4.
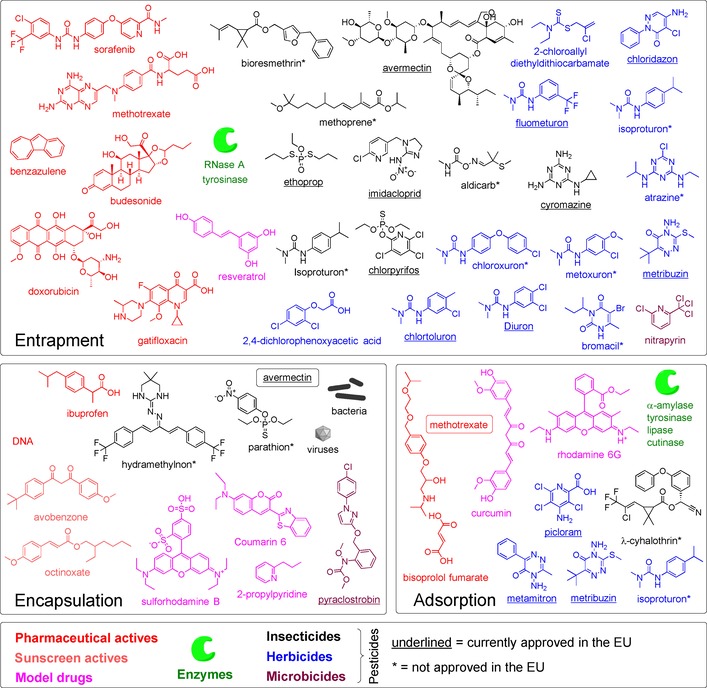
Organic substances and corresponding loading methods onto/into lignin materials. Current approval status of the pesticides was retrieved from Ref. 86.
Entrapment efficiencies (EE [%]) were reported to vary broadly from 4 % to >95 % depending on the carrier formation process, the lignin carrier morphology, and the type of entrapped active ingredient (Table 2). Typical loading capacities (LC) were less than 20 wt %, with a few exceptions of higher concentrations.66, 75, 83, 85 However, methods used to analyze the LC and EE have not been standardized for lignin particles. A particular challenge needing attention relates to the isolation of the loaded LNPs from the (aqueous) medium that contains the free, soluble active substances. Centrifugation using ultrafiltration membranes was used to isolate LNPs from non‐entrapped active substances in aqueous phase.1, 83 However, such a procedure may not be suitable for all types of active substances that may be released rapidly during the purification procedure. Further work is required to establish purification methods that remove selectively the non‐entrapped active substances while leaving the entrapped molecules intact in the solid particles. Solvent extraction with water‐immiscible solvents that do not disrupt the LNPs may be one possible analytical route towards a more reliable, standardized methodology.
Table 2.
Lignin‐based materials used for the entrapment of active substances.
| Carrier material[a] | Active substance[b] | EE[c] [%] | LC[c] [wt %] | Ref. |
|---|---|---|---|---|
| LNPs (WS soda lignin) | budesonide | 35 | 3.5 | 1 |
| LNPs (SKL) | BZL, SFN | 77 BZL, 68 SFN | 8 BZL, 7 SFN | 64 |
| LNPs (AL, Sigma–Aldrich) | DOX, GFLX | 90 DOX, 5–37 GFLX | 47 % DOX, 5–27 % GFLX[d] | 65 |
| LNPs (AL from HT‐pretreated corn cobs) | resveratrol | 71–95 | 19–26 | 66 |
| LNPs (dioxane lignin from subabul stems) | Diuron | 74 | 5.2 | 44 |
| LNPs (OSL) | tyrosinase | 69 | 12 | 84 |
| LNPs (succinylated SKL) | BZL | 50–57 | 9–11 | 67 |
| lignin‐based complex micelles (AL) | ibuprofen | 74 | 46 | 85 |
| chitosan–LNPs (calcium LS) | RNase A | 61–40 | 6.6–43 | 83 |
| lignin–PVA microparticles (spruce ionic lignin, sugarcane bagasse OSL) |
atrazine | 39–78 | 4–15 | 71 |
| lignin microcapsules (azo‐modified poplar AL) | avermectin | 61 | 17 | 72 |
| SLS–CTAB microspheres | avermectin | 71 | 63 | 87 |
| lignin microcapsules (SKL, SLS) | nitrapyrin, chlorpyrifos | >69 | 6.6 | 73 |
| cross‐linked xanthan/lignin hydrogel (ALS) | bisoprolol fumarate | n.a. | 14–19 | 74 |
| self‐assembled alkyl‐modified lignosulfonates | avermectin | 50 | 57 | 75 |
| lignin hydrogel (SKL, sulfonated SKL, lignosulfonate) | ethoprop, methoprene,bioresmethrin | 97–100[e] | n.a. | 76 |
| Lignin/silica hydrogel (sugarcane bagasse soda lignin) | methotrexate | n.a. | n.a. | 77 |
| dried lignin hydrogel (SKL) | 2‐chloroallyl diethyldithiocarbamate | n.a. | n.a. | 79 |
| lignin–CMC hydrogel (SKL) | aldicarb | 4–13 | ≤3.0 | 78 |
| functionalized lignin‐based nanocomposite hydrogels | resveratrol | n.a. | n.a. | 88 |
[a] WS—wheat straw; SKL—softwood kraft lignin; PVA—poly(vinyl alcohol); CTAB—cetyltrimethylammonium bromide; SLS—sodium lignosulfonate; ALS—ammonium lignosulfonate; CMC—carboxymethyl cellulose. [b] BZL—benzazulene, SFN—sorafenib; DOX—Doxorubicin; GFLX—gatifloxacin; the active substances are classified in Figure 4 into pesticides, pharmaceuticals, and enzymes. [c] n.a.—not available. [d] Calculated from the reported EE and initial mass ratio of lignin and active substances. [e] Based on mass balance.
3.1.1. Pesticides
Many pesticides are potent groundwater pollutants, and hence their leaching from soil should be avoided. We note that the regulatory status of pesticides has changed in recent years, and almost half of the pesticides shown in Figure 4 are now banned in the European Union. Here, we visit a few examples of studies involving currently non‐banned pesticides in nano‐ and microscaled lignin‐based materials. Among pesticides still approved for use is avermectin, a cyclic lactone excreted by the soil bacterium Streptomyces avermitilis. In addition to its insecticidal activity, avermectin is used medicinally as an antiparasitic substance.89 Deng et al. demethylated poplar AL and derivatized it by azo‐coupling with diazonium salt of aniline.72 Co‐precipitation of these lignins with avermectin from THF solution in water formed loaded LNPs that protected avermectin against photodegradation under UV irradiation compared to non‐entrapped avermectin. However, the authors did not show photostability of avermectin in regular LNPs. Two recent publications developed alternative lignin‐based carriers for avermectin. Entrapment in alkylated LS microcapsules protected avermectin from UV irradiation and additionally retarded its release in aqueous ethanol.75
Li et al.87 generated lignin‐based microspheres through self‐assembly. LS and CTAB self‐assembled into spherical particles by dropwise addition of water into their ethanol solution. Exhibiting a reversible aggregation behavior, the material was used for preparing microspheres to encapsulate water‐soluble avermectin. Highlightable features of this work comprise a tunable release profile adjustable by varying the ratio between LS and CTAB. The half‐life of UV‐sensitive avermectin under UV irradiation could be increased more than seven times once encapsulated. On the other hand, concentration of the avermectin‐containing microsphere suspension was only 0.06 wt % and optimization of this important parameter was not shown. Furthermore, although the work by Li et al.87 avoided using covalent modification, all of the above procedures with avermectin used accessory synthetic chemicals that should be abandoned to realize more environmentally benign approaches.
Herbicides are chemical substances to control weed growth. Chloridazon is a volatile herbicide that was loaded from methanol solution into a priori formed microcapsules in lignin gel.73 After nine days of storage at 55 °C, only 40 % of chloridazon was lost due to volatilization, and the remaining amount remained stable for five consecutive days, whereas exposure to water triggered a faster release. Lignin isolated using the acidic dioxane method from subabul stems was used to entrap the herbicide Diuron by nanoprecipitatation from a acetone–water (9:1 v/v) mixture containing 1 % PVA.44 Release of Diuron from the LNPs immersed in aqueous buffer solutions increased with increasing pH value from 5 to 9 and was significantly slower compared to the dissolution of bulk Diuron or commercial Diuron formulation.
3.1.2. Pharmaceuticals
Pharmaceuticals and other substances used in biomedical applications represent a growing group of active cargo. Figueiredo et al.64 entrapped the poorly water‐soluble drugs SFN and BZL as a cytotoxic agent in LNPs prepared from softwood kraft lignin (SKL) (Table 2). Loading capacities of 7–8 % were obtained at 68–77 % EE. SFN and BZL are essentially water insoluble, differing from the water‐soluble drug capecitabine that could not be entrapped. The same group reported a 50 % EE of BZL in LNPs prepared from succinylated lignin that allowed conjugation of the particles with amine‐functionalized polyethylene glycol and cell‐penetrating peptides.67 Sipponen et al.1 entrapped 3.5 % budesonide in LNPs prepared from wheat straw soda lignin, which means that less than 300 mg of the nanoformulation would fulfil the daily dosage of this anti‐inflammatory corticosteroid drug. Although the EE was only 35 %, successful recovery and reuse of soluble budesonide was demonstrated. The cytotoxic agent DOX and antibiotic GFLX were dissolved in aqueous p‐toluenesulfonate solution with AL and precipitated by adding water, entrapping the active substances in the LNPs.65 The authors reported a high EE of 90 % for DOX, which was higher compared to the maximum of 37 % obtained with GFLX. Likely due to its low water‐solubility, DOX was released incompletely when the loaded LNPs were immersed in saline solution. Likewise, to entrap the active substances in LNPs, pH‐responsive lignin‐based complex micelles in green solvents were realized by Li et al.85 They used purified AL, which was cationized and then self‐assembled into lignin‐based complex micelles by using sodium dodecyl benzenesulfonate in an ethanol/water mixture. The micelles were pH sensitive. The entrapment/encapsulation efficiently protected ibuprofen in simulated intestinal fluid and pH triggering caused a controllable release.
3.1.3. Enzymes
Enzymes are so far the only macromolecules entrapped in lignin‐based nano‐ and microscaled materials. The motivation for enzyme immobilization arises from the need to reuse water‐soluble catalysts, improve their activity, separate products from the catalyst, and application of flow‐through reactions.90 The enzyme supports must be stable under the reaction conditions, that is, be inert towards solvents and reagents, and resist shear and thermal stress. The use of low‐cost lignin‐based materials for immobilization enables disposal of the inactivated biocatalyst by environmentally benign ways such as composting or combustion. Entrapment of RNase A in chitosan–LS NPs was achieved by ultrasonication of the components at an oil–water interface.83 The maximum EE and LC of RNase A were 61 % and 43 wt %, respectively. Different release profiles observed with low and high amounts of protein cargo indicated its entrapment in the bulk matrix besides adsorption on the surfaces. Another type of enzyme entrapped in an LNP matrix is tyrosinase, which catalyzes oxidation of tyrosine and a number of other phenolic substances.84 The activity‐based immobilization yield at a 12 wt % LC was 69 %, which is roughly similar as the values reported for RNase A at comparable LCs.83 As opposed to the physically entrapped enzyme inside LNPs, systematically higher catalytic activity in oxidative conversion of 4‐methylphenol was achieved with tyrosinase adsorbed on LNPs.84
3.2. Encapsulation
Encapsulation is a strategy to deliver lipophilic drugs that are soluble in the water‐immiscible cores of capsules dispersed in aqueous media, and vice versa. The sphere volume‐to‐surface area ratio equals diameter/6; therefore, microcapsules appear to be more efficient for encapsulation than nanocapsules when compared solely based on the volume available for loading of the active substance. However, LNPs with diameters of around 200 nm exhibited benefits over larger particles in drug delivery to cancer cells.64, 91, 92 In contrast to problems encountered elsewhere (e.g., analysis, colloids), the (self‐)aggregation tendency of lignins can be exploited in the formation of capsules, that is, structures comprised per definition of a core–shell construct; shells can be of varying rigidity, cores can be solid or liquid. It is needless to emphasize that systems are preferred that can be obtained without the need of sacrificial elements and/ or materials.
3.2.1. Pesticides and miscellaneous active substances
Relying on self‐aggregation of lignins alone during the sonication step, recent studies describe lignin microcapsules able to encapsulate hydrophobic active substances.54, 93 Starting from an emulsion comprised of a lignin‐containing aqueous phase and an active substance‐containing oil phase, amphiphilic lignin oligomers and polymers arranged at the water–oil interface upon ultrasonication and aggregated under these conditions.54 Addition of organic cross‐linkers such as diglycidyl‐terminated poly(ethylene glycol) (PEG) can strengthen the capsule structure. In addition, the use of ferric chloride as inorganic cross‐linker, relying on the complexation capabilities of the phenolic OH groups towards metal ions, strengthen the shell with respect to regular capsules.93 Hydrophobic active substances mixed into the oil prior to capsule generation remain in the internal oil phase. Lignin microcapsules obtained by sonication showed a good shelf life and a general non‐cytotoxicity.54 These features, together with the ability to only slowly disintegrate when chemical triggers such as higher hydronium ion concentrations are used, make them potentially versatile candidates for drug‐release applications.
Yiamsawas et al. applied the opposite strategy for the preparation of their lignin‐based nanocontainers.69 The aqueous lignin solution was emulsified in an organic phase of cyclohexane containing toluene diisocyanate and a surfactant. The polymerization of lignin–polyurethane takes place at the water–cyclohexane interface, yielding hollow nanocapsules of lignin‐based polyurethane. Because of the reaction of toluene diisocyanate with water, urea linkages are formed together with the target urethane linkages. The loading capacity (LC) and stability of the capsules were evaluated using the hydrophilic fluorescent dye sulforhodamine 101 (Table 3). Long‐term stability was observed over several months in both aqueous and organic phases. The release of the dye from the capsules could be triggered using laccase as biocatalyst. Consequently, such enzyme‐responsive nanocarriers were proposed for agricultural applications. The same group later described solid, porous, and core–shell microparticulate systems prepared from methacrylated KL by radical‐initiated emulsion polymerization.55 The morphology of the capsules depended on the proportion of dispersed hydrophobic phase in the emulsion. Time‐dependent release of 2‐propylpyridine as a model hydrophobic molecule was also studied from the microparticles. As with nanocapsules,69 laccase activity increased the release rate of the encapsulated cargo, but resulting changes in the lignin structure were not reported.
Table 3.
Lignin‐based materials used for the encapsulation of pesticides and other active substances.
| Carrier material | Active substance[b] | EE [%] | LC [wt %] | Ref. |
|---|---|---|---|---|
| LNCs (AL)[c] | Coumarin 6 | 70–90 | n.a. | 93 |
| LNCs (SLS) | Coumarin 6 | n.a. | n.a. | 56 |
| LNCs (SLS) | sulforhodamine 101 | n.a. | n.a. | 69 |
| LNPs and LNCs (KL) | 2‐propylpyridine | n.a. | n.a. | 55 |
| hollow LNPs (AL)[c] | pyraclostrobin | 64–100 | 2.1–15 | 68 |
| LNC (EHL) | avobenzone, octinoxate | 98 | 53 | 51 |
| LMC (AL)[c] | Coumarin 6 | n.a. | n.a. | 54 |
| lignin–gelatin–formaldehyde resin (azo‐modified LS) | parathion | n.a. | n.a. | 80 |
| LMC (SKL) | hydramethylnon, Bacillus thuringiensis, viruses | n.a. | n.a. | 81 |
[a] LNC—lignin nanocapsule; LMC—lignin microcapsule; LS—lignosulfonate. [b] The active substances are classified in Figure 4 into pesticides, pharmaceuticals, and enzymes. [c] AL from Sigma–Aldrich.
The emulsion approach was also used in a system including methacrylated KL, the fungicide pyraclostrobin, and hexadecane or plant oil, which were dispersed by adding chloroform into an aqueous solution containing a low concentration of sodium dodecyl sulfate, resulting in an oil‐in‐water (O/W) emulsion.68 Cross‐linking of lignin was initiated by the addition of a diamine soluble in both phases, and the product was recovered by evaporating chloroform from the emulsion. LCs from 4 % to 15 % were reported at EEs exceeding 94 %.
Chen et al.56 used sonication to create very stable nanocapsules based on chemically modified lignin, more precisely, allylated LS and trimethylolpropane tris(3‐mercaptopropionate) as cross‐linker. Cross‐linking was achieved by a radical polymerization using azobisisobutyronitrile as initiator that was activated upon sonication of the O/W emulsion. Using Coumarin 6 as water‐insoluble hydrophobic cargo, the authors showed pH‐dependent release characteristics of the capsules.
In 2014, Zou et al. synthesized multicore capsules through O/W Pickering emulsion polymerization using lignin, styrene, divinylbenzene, and hexadecane.94 Later, Yi et al. loaded isophorone diisocyanate as a healing reagent in multilayer composite microcapsules based on LNP‐stabilized O/W Pickering emulsion templates.95 Size control of the microcapsules was achieved by varying the lignin content and the oil/water volume ratios of the Pickering emulsions. The capsules exhibited unique characteristics of 81 wt % core ratio, excellent thermal stability, and high durability in aqueous solution–submersion as well as >90 % mass retention in solution–submersion and air‐exposure tests. One of the merits of their approach is that the internal phase of the capsule solely consisted of the active agents in contrast to many aforementioned approaches in which the oil core functioned as a solvent phase.
Within patent literature, several examples are found in which the use of lignins, most often LSs and ALs, is claimed for the generation of micro‐ and nanocapsules for controlled‐release and storage applications. Only a few patents, however, list lignin‐based capsules in more specific examples, and only these will be discussed here. Wurm et al.68 patented the synthesis of lignin‐based capsules from an O/W emulsion. The oil contained the hydrophobic active substance, and capsule stability was realized by cross‐linking processes, including click chemistry. Aiming for agricultural applications, the patent lists as an example encapsulation and release of pyraclostrobin. Already as early as 1970, LS was used together with gelatin to generate capsules by coacervation; capsules were shown to be pH responsive, as one might expect.96 In 1992, microcapsules based on various lignins and cross‐linking agents or co‐polymers were reported in which an insecticide, a hormone, or a fertilizer for protection against UV light prior to application to vegetation were encapsulated.97 Microcapsules were obtained by combining an emulsion of lignin‐covered droplets with a “hardening” solution, which fixes the droplets that contained up to 30 % (w/w) of lignin. In 1996, LS microcapsules were patented for the protective encapsulation of parathion, an organophosphate insecticide, in high‐bloom gelatin.80 The patented invention made use of the improved UV protection achieved by the use of LS or its azo‐derivative.
3.2.2. Sunscreen active substances
Many recent studies used lignin‐based micro‐ and nanocapsules for sunscreen applications, relying on the natural UV absorbance of lignin and making use of synergistic effects between conventional, normally very hydrophobic sunscreen active substances and lignin.48, 49, 50, 51, 88, 98 Qiu et al. synthesized by ultrasonication LNCs from EHL that were washed with water several times before its use in encapsulating chemical sunscreens, namely avobenzone (UV A blocker) and octinoxate (UV B blocker).51 The authors showed that these capsules, dispersed in suncreens, combine the previously observed beneficial synergistic effect48 with a long‐lasting performance because of the gradual release of the active substances from the lignin capsules.
3.3. Adsorption of enzymes and other active substances
Adsorption is a common method for the immobilization of enzymes, and especially lipases were immobilized in lignin‐based materials (Table 4). Lipase B from Candida antarctica was adsorbed on conjugate material of oxidized KL and amino‐derivatized chitin with a Brunauer–Emmett–Teller surface area of 194 m2 g−1.99 In another study lipase was adsorbed from Candida rugose on composite beads prepared by co‐precipitation of lignin and cellulose from the ionic liquid 1‐ethyl‐3‐methylimidazolium acetate.100 Compared to the hydrolytic activity of beads containing only cellulose, the supplementation of KL into the beads increased lipase activity by a factor of 2.6. A higher activity increase by a factor of 3.2 was reported when using lipase from Mucor javanicus, which was first adsorbed on cationic lignin nanospheres and subsequently entrapped in calcium alginate beads at an EE of 96 %.101 The same authors showed that, in addition to lipases, immobilized cutinase from Humicola insolens catalyzed the synthesis of butyl butyrate in a biphasic solvent mixture (water/hexane ratio 9:1 v/v).
Table 4.
Lignin‐based materials used for the adsorption of active substances.
| Carrier material | Active substance[a] | EE [%] | LC [wt %] | Ref. |
|---|---|---|---|---|
| cellulose/AL hydrogel beads | lipase | 52 | 1.4 | 100 |
| chitin–KL composite | lipase | 1.0–2.0 | 33–11 | 102 |
| silica–lignin composite (AL)[b] | lipase | 1.7 | 42 | 99 |
| cationic LNPs (SKL) entrapped in calcium alginate | lipase, cutinase | 96 | 5.5 | 101 |
| acetic acid lignin from bamboo shoot shells | α‐amylase | n.a. | 1.9 | 103 |
| lignin NPs (OSL) | tyrosinase | 71–90 | 12–15 | 84 |
| lignin nanotubes (various lignins) | Plasmid DNA | n.a. | n.a. | 104 |
| sugarcane bagasse soda lignin | methotrexate | n.a. | n.a. | 77 |
| lignin microspheres (EHL) | λ‐cyhalothrin | n.a. | 5.7 | 57 |
| lignin–alginate hydrogel beads (hydrolytic lignin, Aldrich) | isoproturon | ≈40[c] | 0.002–0.005[d] | 105 |
| aspen rot wood lignin | metamitron, metribuzin | n.a. | n.a. | 106 |
| lignosulfonate‐coated microcapsules (SLS) | picloram | 50–88 | 93–97 | 70 |
| lignin‐based carbon nanodots (LS[e]) | curcumin | 67 | 11 | 61 |
[a] The active substances are classified in Figure 4 into pesticides, pharmaceuticals, and enzymes. [b] AL from Sigma–Aldrich. [c] Calculated from the adsorption data. [d] Relative to the moist lignin–alginate beads. [e] LS not specified.
Activation of lipases occurs because of the amphiphilic properties of lignins,41, 101, 107 which are similar to those of synthetic surfactants.108, 109, 110 Lipase from Aspergillus niger was adsorbed on a composite material consisting of chitin and oxidized KL.102 The immobilization increased thermal and pH stability compared to that of native lipase. Besides lipases and cutinases, immobilization of porcine pancreatic α‐amylase was reported by adsorption on acetic acid lignin isolated from bamboo shoot shells.103 The entrapment efficiency (EE) was not determined, but the weight fraction of enzyme adsorbed on lignin was 1.9 %. Activation of the enzyme similar to that reported for microbial lipases, but with unknown mechanism, was observed.
Lignins were also used as precursors for the fabrication of nanotubes for affinity‐binding60 and adsorption applications.104 Ten et al. synthesized lignin nanotubes (LNTs) on a sacrificial alumina membrane template.104 LNTs were used to transfer deoxyribonucleic acid (DNA) into HeLa cells; they appeared to be better tolerated than carbon nanotubes (CNTs). Wahba et al. reported the adsorption of methotrexate on sugarcane bagasse soda lignin for the treatment of rats induced with rheumatoid arthritis.77 The release rate of this zwitterionic aromatic active ingredient was slower from lignin than from mesoporous silica or a magnetite–silica nanocomposite, which was attributed to noncovalent attraction forces and smaller average pore radius for lignin (4.9 nm) compared to the other adsorbents.
Due to the low cost and high availability, many lignins, including various crude lignin preparations, were tested as adsorbents to carry pesticides for agricultural applications.57, 70, 105, 106 The UV‐protective properties of lignin were utilized to increase the stability against UV light of crystalline picloram, a systemic herbicide. LbL coating of the crystals with a polymeric shell of alternating chitosan and SLS layers additionally contributed towards its slow release in dilute aqueous ethanol solution.70
4. Covalent and Noncovalent Functionalization
Modification and grafting of new chemical functionality are generally aimed at improving targeting and controlled release properties of lignin‐based carriers. Compared to the abundant literature on the preparation of lignin‐based nano‐ and microstructures as such, reports regarding their surface modification is relatively scarce. Caicedo et al. functionalized LNTs with avidin for their specific immobilization onto desthiobiotin‐grafted glass surfaces.60 They also demonstrated binding of LNTs carrying anti‐concanavalin A onto glass functionalized with concanavalin A. Figueiredo et al. succinylated SKL and used the product to generate carboxylated lignin particles (CLNPs) that were further modified by grafting on them a block copolymer made of PEG, poly(histidine), and a cell‐penetrating peptide.67 The functionalized CLNPs showed spherical shape, cationic net charge, good stability in physiological media, and low cytotoxicity in all the tested cell lines. Benzazulene, a poorly water‐soluble cytotoxic agent, was successfully loaded into the CLNPs, leading to a pH‐sensitive release profile and an enhanced antiproliferative effect in the different cancer cells compared with a normal endothelial cell line.
In addition to covalent grafting reactions, anionic LNPs were modified by adsorption of cationic polymers such as poly(diallyldimethylammonium chloride), (PDADMAC) on their surfaces.40, 111 These monolayer‐coated particles were stable even under strongly alkaline conditions and are thus usable in an extended range of applications. To reduce the carbon footprint of the product, Sipponen et al. adsorbed water‐soluble cationized SKL on colloidal lignin particles, achieving a pH‐dependent cationic net charge.41 Such cationic LNPs were used for emulsion stabilization41 and enzyme immobilization,101 but their cytocompatibility has not been assessed. Cationic polymers carrying quaternary amine headgroups are well known for their antimicrobial properties,112, 113 but cationic lignin particles have not been systematically studied for their possible cytoxicity for mammalian cells. Instead of cationic polymers, LNPs coated with proteins114 are expected to improve biocompatibility and reduce clearance rate; however, these important aspects are yet to be demonstrated under physiological conditions.
Ag ions and Ag NPs were used to incorporate antibacterial functionality into lignin materials. In 2015, Zhong et al. reported antibacterial Ag NP composites in a matrix of PVA and lignin isolated from spent pulping liquor.115 Klapiszewski et al. prepared silica/lignin hybrid particles grafted with Ag NPs.116 Commercial silica material was modified with 2‐aminoethyl‐3‐aminopropyltrimethoxysilane to increase affinity to KL oxidized with sodium periodate. Ag NPs grafted onto the resulting silica/lignin hybrids were stable and active against Pseudomonas aeruginosa. Still in 2015, Richter et al. synthesized Ag‐ion‐infused LNPs as an environmentally benign substitute for pure Ag NPs in antimicrobial applications. In contrast to the Ag NPs, the Ag‐infused LNPs may have a lower eco‐toxicity due to a reduced durability of LNPs.117
5. Effects of Nano‐ and Microsized Lignins on Living Organisms
The antibacterial activity and cytotoxicity of carbon nanomaterials depends, for example, on particle size, shape, surface chemistry, charge, and the type of cells incubated with them.118, 119 This interdependence has made it difficult to generalize the various mechanisms of the cytotoxic effects. Still, the phenomena were studied quite extensively for silica NPs,120, 121, 122 Ag NPs,123, 124, 125, 126 fullerenes,127 CNTs,128, 129 and carbon nanodots.130 Lignin nanomaterials, being a relatively new research subject, have not yet been subjected to thorough safety assessment, but this should be done in the light of their recently demonstrated potential as drug and gene vectors and antimicrobial agents. Table 5 presents a comparison between nano‐ and microsized lignin along with inorganic and carbon nanomaterials and studies that assessed their interactions with living organisms both in vitro and/or in vivo.
Table 5.
Comparison of testing of lignin‐, silica‐, and carbon‐based nano‐ and microscaled materials on living organisms.
| Test type | Lignin‐based materials | Inorganic NPs | Carbon‐based nanomaterials[a] |
|---|---|---|---|
| In vitro | |||
| antiviral | no published studies | Ag NPs131 | functionalized fullerenes132, 133, 134 |
| antibacterial | Ag‐infused NPs (SKL),117 lignin‐capped Ag NPs (AL),135, 136 silica/lignin–Ag NPs (KL),116 acid‐precipitated lignin particles (AL)6 | Ag NPs,137 silica NPs (4 nm),138 nitric oxide‐ releasing silica NPs139 |
SWCNTs140
fullerenes141 |
| antifungal | lignin‐capped Ag NPs (AL)135 | Ag NPs,142 amphotericin B‐conjugated silica NPs143 | functionalized MWCNTs144 |
| anticancer | lignin NPs with active substances (AL[b]),66 lignin‐derived carbon dots (LS),61 emulsified trans‐resveratrol (SLS)145 | silica NPs146
silica NPs loaded with active substances147 |
fullerenes148
crystalline fullerenes127 |
| cell viability | lignin microcapsules (KL),54 LNPs (SKL),64 nanoemulsion encapsulating trans‐resveratrol (SLS),147 lignin‐derived carbon nanodots61 | silica NPs,149, 150 polyamidoamine dendrimer‐capped silica NPs151 | C NPs,129 MWCNTs,129 crystalline fullerenes127 |
| In vivo | |||
| resveratrol‐entrapped LNPs (AL[a]),66 doxorubicin‐entrapped LNPs,65 methotrexate adsorbed on sugarcane bagasse soda lignin.77 | silica NPs,120, 121, 122 Ag NPs123, 124, 125, 126 | C nanodots,130 SWCNTs,128 crystalline fullerenes127 |
[a] SWCNTs—single‐walled carbon nanotubes; MWCNTs—multi‐walled carbon nanotubes. [b] Isolated from hydrothermally treated corn cobs.
Ag NPs131 and fullerene derivatives132, 133, 134 have been used for antiviral studies. In sharp contrast, no studies on nano‐ or microscaled antiviral lignin materials have been published, although antiviral properties have been reported for different types of lignins.152, 153, 154, 155, 156 Ag NPs are well known for their antibacterial effects.135, 157, 158 Recent studies also reported silica particles,138, 139 SWCNTs,140 and fullerene141 for antibacterial activity. The majority of antibacterial lignin materials make use of Ag ions117 or Ag NPs116, 135 136, 137 as active payload. Nevertheless, also metal‐free acid‐precipitated LNPs were recently shown to possess antibacterial activity against Gram‐negative plant pathogen strains.6 The antimicrobial activity was related to the penetration of LNPs into the bacteria, under concomitant disruption of cellular functions. The authors did not study intracellular disintegration of LNPs, but leaching of low‐molecular‐weight lignin fragments is a plausible reason behind the antibacterial activity. Similar to pristine Ag NPs,142 lignin‐capped Ag NPs135 possessed antifungal activity.
The important findings from investigations of effects of lignin‐based materials on living organisms are summarized in Table 6. Studies with fibroblast cells showed that the commercially available AL is biocompatible both as such136 and in form of AL–PHB composite nanofibers.159 Moreover, the presence of residual lignin in cellulose hydrogel films increased cell viability compared to those observed on polystyrene or bleached cellulose hydrogel surfaces.160 Evidence accumulated from studies using LNPs as carriers for anticancer drugs reveals that the transformation to the nanoscale does not drastically alter biocompatibility of lignin in vitro. LNPs are not antiproliferative towards cancerous cells in the absence of active payload nor do they cause notable cytotoxic effects.54, 61, 64, 66 Endocytosis of Coumarin 6‐loaded lignin microcapsules in Chinese hamster ovary cells showed no interference of cell functions.54 Although interactions of nanoscaled lignins with cells in vitro attracted increasing attention in recent years,6, 54, 64, 66, 117, 135, 161 there are very few in vivo studies on nano‐ and microscaled lignin materials. Dai et al. studied effects of resveratrol‐loaded LNPs on cancer cells and red blood cells in vitro and on tumor‐transplanted mice in vivo.66 Non‐loaded particles did not exhibit adverse haemolysis effects or reduction of cell viability whereas resveratrol‐loaded particles showed significant inhibition of tumor growth in vivo.
Table 6.
Findings from studies involving lignin‐based nano‐ and microscaled materials in vitro and in vivo.
| Type of lignin material | Type of organism(s) | Main findings | Ref. |
|---|---|---|---|
| LMCs (AL)[a] | Chinese hamster ovary cells | non‐cytotoxic without encapsulated drug | 54 |
| Ag‐infused LNPs (SKL) | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus epidermidis, Ralstonia sp. | Ag provides antibacterial effect whereas antibacterial activity of empty particles was due to PDADMAC coating | 117 |
| lignin‐capped Ag NPs (AL)[a] | Escherichia coli, Staphylococcus aureus, Aspergillus niger | antifungal activity>antibacterial activity | 135 |
| lignin‐capped Ag and Au NPs (various lignins) | Escherichia coli, Staphylococcus aureus, 3T3 fibroblasts | lignin‐capped Ag NPs and AL as such were non‐cytotoxic to the fibroblasts | 136 |
| resveratrol‐loaded LNPs (lignin extracted from hydrothermally pretreated corn cobs with alkali) | human lung cancer cells, murine Lewis lung carcinoma cells, mice | LNPs were non‐cytotoxic; entrapped resveratrol reduced tumor volume | 66 |
| LNPs (SKL) | various cancerous and non‐cancerous human cell lines | no significant cytotoxic effects | 64 |
| Fe‐containing LNPs (iron isopropoxide‐modified SKL) | various cancerous and non‐cancerous human cell lines | some cytotoxic effects | 64 |
| magnetite NP‐loaded LNPs (SKL) | Various cancerous and non‐cancerous human cell‐lines | some cytotoxic effects | 64 |
| LNPs (AL)[a] | NIH/3T3 fibroblast, mice implanted with B16F10 tumors | some cytotoxic effects at high concentrations; LNPs from p‐toluenesulfonic acid were more biocompatible than LNPs from THF/ethanol/water solvent mixture; histology did not show cytotoxicity of LNPs in mice, doxorubicin‐loaded LNPs, but not bare LNPs, reduced tumor volume | 65 |
| acid‐precipitated LNPs (AL)[a] | Pseudomonas syringae, Xanthomonas axonopodis, Xanthomonas arboricola | antimicrobial activity | 6 |
| soda lignin | albino rats induced with rheumatoid arthritis | intraperitoneal administration of methotrexate‐loaded lignin alleviated symptoms and increased animal growth rates compared to control groups. | 77 |
| lignin‐PHB composite nanofibers (AL)[a,b] | NIH/3T3 fibroblast | no cytotoxicity, good biocompatibility | 159 |
| lignin‐PLA/PLLA nanofibers (AL)[c] | PC12 cell line[d], human mesenchymal stem cells, human dermal fibroblasts | reduced ROS[e] generation and cytotoxicity | 161 |
[a] AL from Sigma–Aldrich. [b] PHB—poly(3‐hydroxybutyrate); PLA—poly(lactic acid); PLLA—poly(L‐lactic acid). [c] AL from TCI. [d] PC12 is a rat adrenal gland‐derived cell line with neuronal characteristics. [e] ROS—reactive oxygen species
A comparison of lignin to other organic nanomaterials reveals some intriguing differences. Hydroxylation of fullerenes mitigates the generation of ROS that cause cytotoxicity of fullerene‐based nanomaterials.162 Although the number of available publications is still quite low, it can be speculated that various oxygen‐containing functional groups make lignin generally more biocompatible than oxygen‐depleted carbon materials due to an increased hydrophilicity and number of sites at which the metabolic system can attack. Stemming from their intrinsic polyphenol functionality, LNPs were found to alleviate oxidative stress exerted by PLA161 and to provide beneficial radical scavenging activity in nanocomposites.2 In nanomedicine, LNPs did not cause notable generation of ROS while particles of ≈200 nm diameter exhibited selective uptake by cancerous cells.64 It is important to note that excessive covalent modification may have detrimental effects on biocompatibility of lignin materials. Liu et al.163 found that 2‐(dimethylamino)ethyl (DMAEMA)‐grafted lignin copolymers with 40–65 nm hydrodynamic radii enabled plasmid DNA binding for gene‐delivery purposes. The in vitro cytotoxicity of the copolymers increased with increasing copolymer length; however, 5–6 DMAEMA units enabled transfection with sufficient biocompatibility.
6. Technical Challenges
Nano‐ and microscale lignin particles and capsules exhibit several promising features as carriers of bioactive molecules. The fact that lignin is a natural antioxidative compound, possesses antimicrobial activity, and that LNPs are, so far, considered nontoxic encourage work in this area. However, there are also challenges related to the solvents used in the fabrication process that may restrain applicability of the formed lignin nanomaterials. Especially medically administered materials are very strictly regulated, and traces of harmful solvents will not be accepted. To this end, the use of safe and green solvents is preferable. However, there should not be major setbacks in yield or performance of materials fabricated from biocompatible solvents. Recently, aqueous acetone was used in the preparation of LNPs at a mass yield of 88 %.2 Li et al. described precipitation of ethanolic KL solutions by the addition of water, which induced formation of nanocapsule structures.46 However, in that case only a fraction of KL was soluble in ethanol and could be used in the process. Sipponen et al. showed that aqueous ethanol solubilized 87 % of WS soda lignin that can be used for the entrapment of active substances such as budesonide in LNPs.1 The drawback of aqueous ethanol is that the concentration of colloidally stable LNPs that can be prepared by direct non‐solvent precipitation is lower than that achieved with THF or acetone. New solvent systems such as aqueous p‐toluenesulfonic acid show potential to increase concentration of LNPs,65 but more work is needed to assess their performance and overall production cost. Overall, there is an obvious need for engineered nano‐ and micromaterial processes that minimize use of harmful solvents without compromising the dynamic range of nano‐ and microcarrier fabrication.
Another type of technical challenge arises from the structural features of lignin as the starting material itself in connection with the regulatory issues discussed in Section 8. Regardless of the type of lignin chosen for the application, it usually comes in form of a structurally and physicochemical heterogeneous mixture of substances.19, 164 Improved understanding of the starting materials and their reactivity is a prerequisite to control the cytotoxicity and eco‐toxicity profiles of lignin‐based micro‐ and nanostructures. The use of fractionated lignins will here only reduce the problem but not fully resolve it. A more promising route might be the genetic engineering of plants in a way that they produce a “more uniform” lignin, which then can be isolated without drastic structural changes.165, 166 This, however, causes different regulatory and legal issues in other, related fields.
7. Regulatory and Toxicological Aspects
Although they are composed of materials that are well known since decades, evaluation of engineered nanomaterials poses a particular regulatory challenge.167, 168 The European Commission (EC) and the Food and Drug Administration (FDA) are directing efforts towards more unified testing and assessment criteria.169, 170, 171 In the past, membership to the family of nanomaterials was granted on the basis of physical dimensions; however, modern regulation and guidelines by the FDA and the EC are going away from this strict size dependence and extend the family also to microsized agglomerates. Recent research pursued standardized toxicological assessment of nanomaterials,168, 172, 173, 174 and proposed undertaking several prerequisite steps during nanoparticle generation and optimization stages to improve the eco‐toxicity analysis of nanomaterials.175 This is all the more noteworthy since nanomaterials are penetrating all sectors of modern life: Biomedical materials, home and personal care, agricultural products, among others. Moreover, engineered nanomaterials, also those composed of otherwise innocuous natural substances, show yet not fully understood short‐ and long‐term effects.
Being a natural substance, basic eco‐toxicity of lignin is not a problem as such; problems are more likely to arise from chemical alteration during or for the fabrication process. Materials in which the nanostructure is due only to macromolecular interactions of lignin pose intrinsically less problems compared to those containing chemically modified lignins. For example, cross‐linking of lignin can drastically reduce biodegradability by blocking the phenolic OH groups, which are primary sites of attack within the enzymatic degradation of lignins in nature. One problem remains, however, independent of whether a lignin was modified or not: A micro‐ or nanoparticle represents always an augmented local concentration in compact form in the environment that as such is not natural. This fact can lead to an “unexpected” eco‐toxicity such as increased occurrence of Fenton‐like oxidation products.176 It is obvious that more research is needed to shed light on the nanosafety and environmental impacts of lignin‐based nanomaterials in various emerging applications.
8. Summary and Outlook
Utilization of lignin in materials science yet has mainly exploited its “passive” functionality, that is, adsorption and entrapment of active substances, antioxidant, antimicrobial, UV‐scavenging activity, or pH‐triggered release. We are now approaching the watershed at which efforts will be focused on the design of lignin‐based “active” functional materials. Biopolymer engineering with lignin will likely take inspiration from advanced materials developed from other polymers and colloidal materials. There is also a profound strive to extend the toolbox of different well‐defined nanoscale building blocks. We envision that standardization of the production of spherical lignin nanoparticles, lignin capsules, and lignin nanofibers will lead to consistent material properties and foster the development of the next‐generation materials. To facilitate assessment of various lignin‐based carrier materials, we urge the researchers to adopt unambiguous reporting of encapsulation efficiency and loading capacity values along other meaningful results.
Many of the reports focus on finding use for lignin, but not so much about taking advantage of its specific properties. The future is to more actively utilize the special chemistry of lignins such as pH‐responsive solubility and tailorable thermal properties for added value. Specific functionality is important for many applications in biomedicine, biosensors, isomeric purification, and other high value‐added applications. Materials carrying more than one active substance,177 with synergistic simultaneous action or potential for controlled sequential release, will be the next generation systems. Typical controlled release materials are composed of an enzyme‐sensitive substrate linked to another component that leads to changes in macroscopic conformation.178 Many of the systems developed thus far involve polymer hydrogels, including nanoparticle–polymer hydrogels,177 which can also be formed from lignin.2, 101
In addition to the first exploratory activities reviewed herein, it is imperative, to develop efficient and sustainable new soft materials, to focus on clear‐cut approaches using green‐chemistry techniques. More specifically, the possible fields of applications of active delivery require the use of nontoxic solvents that would possibly remain in traces in the final product. In addition, for the same issues of human toxicity or environmental hazard, the new technologies should clearly avoid use of covalent cross‐linking agents.
Advanced lignin‐based materials will consist of engineered signaling chains that lead to the formation of the active form of the active substance only upon response of specific external stimuli. Such systems are already established in polymer science and typically involve changes in solubility or macromolecular conformation.179 The stimuli can be based on changes in chemical triggers like pH, electrolyte concentration, properties of the external fluid, moisture, and binding of a ligand,180 as well as physical triggers such as temperature, light, mechanical forces, electric, magnetic, and sonic fields. For instance, ultrasonic treatment is known to assemble lignin into shells of microcapsules in emulsions.54 Targeted and nonexcessive dosing of active substances is an objective in medicinal applications because of the systemic toxicity and harmful side effects of many drugs. In addition to nanomedicine, a prominent large‐scale application potential exists in agricultural and industrial applications such as stimuli‐responsive release of plant‐protection agents, sensory/protective environmental materials, and immobilization of enzymes or cells for processing and upgrading of organic substances. Development of standardized purification procedures and safety demonstration of various lignin grades remain of outmost importance for future applications in food, nanomedicine, and healthcare materials.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Mika H. Sipponen is postdoctoral researcher of the Academy of Finland. He obtained his MSc (2010) and DSc (2015) degrees in chemical technology (applied biochemistry) from Aalto University in Finland. He was a visiting doctorate (2012) at Institut Jean‐Pierre Bourgin (IJPB) in Versailles, France with Prof. S. Baumberger and a visiting postdoctoral researcher (2017–2018) at the University of Rome Tor Vergata with Prof. C. Crestini. He was also a research scientist (2016, eight months) at VTT Technical Research Centre of Finland. His research interests in the group of Prof. Österberg included production and functionalization of colloidal lignin particles and development of lignin‐based functional materials for sustainable applications according to green chemistry principles.

Biographical Information
Monika Österberg is Associate Professor in bioproducts chemistry at the Department of Bioproducts and Biosystems at the School of Chemical Engineering, Aalto University. She received her PhD in surface chemistry in 2000 from the Royal Institute of Technology (KTH), Stockholm, Sweden. In 2012 she joined the faculty of Aalto University as Associate Professor and was tenured in 2016. She is teaching pharmaceutical nanotechnology at Helsinki University, Faculty of Pharmacy. The aim of her research is to develop a more sustainable use of natural resources. Her research interests are fundamental interfacial phenomena of forest biomaterials, such as lignin, cellulose and hemicelluloses, and the development of new materials from these polymers.

Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
M.H.S. acknowledges Academy of Finland for funding (Grant 296547).
M. H. Sipponen, H. Lange, C. Crestini, A. Henn, M. Österberg, ChemSusChem 2019, 12, 2039.
Contributor Information
Dr. Mika Henrikki Sipponen, Email: mika.sipponen@aalto.fi.
Prof. Dr. Monika Österberg, Email: monika.osterberg@aalto.fi.
References
- 1. Sipponen M. H., Lange H., Ago M., Crestini C., ACS Sustainable Chem. Eng. 2018, 6, 9342–9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farooq M., Zou T., Riviere G., Sipponen M. H., Österberg M., Biomacromolecules 2019, 20, 693–704. [DOI] [PubMed] [Google Scholar]
- 3. Yearla S. R., Padmasree K., J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar]
- 4. Vinardell M. P., Ugartondo V., Mitjans M., Ind. Crops Prod. 2008, 27, 220–223. [Google Scholar]
- 5. Catignani G. L., Carter M. E., J. Food Sci. 1982, 47, 1745. [Google Scholar]
- 6. Yang W., Fortunati E., Gao D., Balestra G. M., Giovanale G., He X., Torre L., Kenny J. M., Puglia D., ACS Sustainable Chem. Eng. 2018, 6, 3502–3514. [Google Scholar]
- 7. Nada A. M. A., El-Diwany A. I., Elshafei A. M., Acta Biotechnol. 1989, 9, 295–298. [Google Scholar]
- 8. Dong X., Dong M., Lu Y., Turley A., Jin T., Wu C., Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar]
- 9. Gordobil O., Herrera R., Yahyaoui M., Ilk S., Kaya M., Labidi J., RSC Adv. 2018, 8, 24525–24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larrañeta E., Imízcoz M., Toh J. X., Irwin N. J., Ripolin A., Perminova A., Domínguez-Robles J., Rodríguez A., Donnelly R. F., ACS Sustainable Chem. Eng. 2018, 6, 9037–9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang G., Xia Y., Liang B., Sui W., Si C., J. Chem. Technol. Biotechnol. 2018, 93, 2977–2987. [Google Scholar]
- 12. Oh-Hara T., Kawazoe Y., Sakagami H., Chem. Pharm. Bull. 1990, 38, 3031–3034. [DOI] [PubMed] [Google Scholar]
- 13. Vanholme R., Demedts B., Morreel K., Ralph J., Boerjan W., Plant Physiol. 2010, 153, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sipponen M. H., Rahikainen J., Leskinen T., Pihlajaniemi V., Mattinen M.-L., Lange H., Crestini C., Österberg M., Nord. Pulp Pap. Res. J. 2017, 32, 550–571. [Google Scholar]
- 15. Lange H., Schiffels P., Sette M., Sevastyanova O., Crestini C., ACS Sustainable Chem. Eng. 2016, 4, 5136–5151. [Google Scholar]
- 16. Lange H., Decina S., Crestini C., Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar]
- 17. Crestini C., Crucianelli M., Orlandi M., Saladino R., Catal. Today 2010, 156, 8–22. [Google Scholar]
- 18. Gellerstedt G., Ind. Crops Prod. 2015, 77, 845–854. [Google Scholar]
- 19. Lignin and Lignans Advances in Chemistry (Eds.: C. Heitner, D. R. Dimmel, J. A. Schmidt), CRC Press, New York, 2010. [Google Scholar]
- 20. Biorefineries, An Introduction (Eds.: M. Aresta, A. Dibenedetto, F. Dumeignil), De Gruyter, Berlin, 2015. [Google Scholar]
- 21. Lignin Valorization, Emerging Approaches (Ed.: G. T. Beckham), The Royal Society of Chemistry, Cambridge, 2018. [Google Scholar]
- 22. Beisl S., Miltner A., Friedl A., Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar]
- 23. Ago M., Tardy B. L., Wang L., Guo J., Khakalo A., Rojas O. J., MRS Bull. 2017, 42, 371–378. [Google Scholar]
- 24. Beisl S., Friedl A., Miltner A., Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar]
- 25. Grossman A., Wilfred V., Curr. Opin. Biotechnol. 2019, 56, 112–120. [DOI] [PubMed] [Google Scholar]
- 26. Crestini C., Lange H., Sette M., Argyropoulos D. S., Green Chem. 2017, 19, 4104–4121. [Google Scholar]
- 27. Lancefield C. S., Wienk H. J., Boelens R., Weckhuysen B. M., Bruijnincx P. C. A., Chem. Sci. 2018, 9, 6348–6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crestini C., Melone F., Sette M., Saladino R., Biomacromolecules 2011, 12, 3928–3935. [DOI] [PubMed] [Google Scholar]
- 29. Sette M., Wechselberger R., Crestini C., Chem. Eur. J. 2011, 17, 9529–9535. [DOI] [PubMed] [Google Scholar]
- 30. Lancefield C. S., Constant S., De Peinder P., Bruijnincx P. C. A., ChemSusChem 2019, 12, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FAO, “FAOSTAT Database on Production,” can be found under http://www.fao.org/faostat/en/#data, 2017.
- 32. Horn D., Rieger J., Angew. Chem. Int. Ed. 2001, 40, 4330–4361; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 4460–4492. [Google Scholar]
- 33. Tian D., Hu J., Bao J., Chandra R. P., Saddler J. N., Lu C., Biotechnol. Biofuels 2017, 10, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sadeghifar H., Venditti R., Jur J., Gorga R. E., Pawlak J. J., ACS Sustainable Chem. Eng. 2017, 5, 625–631. [Google Scholar]
- 35. Ragauskas A. J., Beckham G. T., Biddy M. J., Chandra R., Chen F., Davis M. F., Davison B. H., Dixon R. A., Gilna P., Keller M., Langan P., Naskar A. K., Saddler J. N., Tschaplinski T. J., Tuskan G. A., Wyman C. E., Science 2014, 344, 1246843. [DOI] [PubMed] [Google Scholar]
- 36. Laurichesse S., Avérous L., Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar]
- 37. Figueiredo P., Lintinen K., Hirvonen J. T., Kostiainen M. A., Santos H. A., Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar]
- 38. Zhao W., Simmons B., Singh S., Ragauskas A., Cheng G., Green Chem. 2016, 18, 5693–5700. [Google Scholar]
- 39. Xiong F., Han Y., Wang S., Li G., Qin T., Chen Y., Chu F., Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar]
- 40. Lievonen M., Valle-Delgado J. J., Mattinen M.-L., Hult E.-L., Lintinen K., Kostiainen M. A., Paananen A., Szilvay G. R., Setälä H., Österberg M., Green Chem. 2016, 18, 1416–1422. [Google Scholar]
- 41. Sipponen M. H., Smyth M., Leskinen T., Johansson L.-S., Österberg M., Green Chem. 2017, 19, 5831–5840. [Google Scholar]
- 42. Li H., Deng Y., Wu H., Ren Y., Qiu X., Zheng D., Li C., Holzforschung 2016, 70, 725–731. [Google Scholar]
- 43. Tian D., Hu J., Chandra R. P., Saddler J. N., Lu C., ACS Sustainable Chem. Eng. 2017, 5, 2702–2710. [Google Scholar]
- 44. Yearla S. R., Padmasree K., Environ. Sci. Pollut. Res. 2016, 23, 18085–18098. [DOI] [PubMed] [Google Scholar]
- 45. Leskinen T., Smyth M., Xiao Y., Lintinen K., Mattinen M., Kostiainen M. A., Oinas P., Nord. Pulp Pap. Res. J. 2017, 32, 586–596. [Google Scholar]
- 46. Li H., Deng Y., Liu B., Ren Y., Liang J., Qian Y., Qiu X., Li C., Zheng D., ACS Sustainable Chem. Eng. 2016, 4, 1946–1953. [Google Scholar]
- 47. Lintinen K., Xiao Y., Bangalore Ashok R. P., Leskinen T., Sakarinen E., Sipponen M. H., Farooq M., Oinas P., Österberg M., Kostiainen M. A., Green Chem. 2018, 20, 843–850. [Google Scholar]
- 48. Qian Y., Qiu X., Zhu S., Green Chem. 2015, 17, 320–324. [Google Scholar]
- 49. Qian Y., Zhong X., Li Y., Qiu X., Ind. Crops Prod. 2017, 101, 54–60. [Google Scholar]
- 50. Qian Y., Qiu X., Zhu S., ACS Sustainable Chem. Eng. 2016, 4, 4029–4035. [Google Scholar]
- 51. Qiu X., Li Y., Qian Y., Wang J., Zhu S., ACS Appl. Biol. Mater. 2018, 1, 1276–1285. [DOI] [PubMed] [Google Scholar]
- 52. Wang B., Sun D., Wang H.-M., Yuan T.-Q., Sun R.-C., ACS Sustainable Chem. Eng. 2019, 7, 2658–2666. [Google Scholar]
- 53. Ago M., Huan S., Borghei M., Raula J., Kauppinen E. I., Rojas O. J., ACS Appl. Mater. Interfaces 2016, 8, 23302–23310. [DOI] [PubMed] [Google Scholar]
- 54. Tortora M., Cavalieri F., Mosesso P., Ciaffardini F., Melone F., Crestini C., Biomacromolecules 2014, 15, 1634–1643. [DOI] [PubMed] [Google Scholar]
- 55. Yiamsawas D., Beckers S., Lu H., Landfester K., Wurm F. R., ACS Biomater. Sci. Eng. 2017, 3, 2375–2383. [DOI] [PubMed] [Google Scholar]
- 56. Chen N., Dempere L. A., Tong Z., ACS Sustainable Chem. Eng. 2016, 4, 5204–5211. [Google Scholar]
- 57. Pan J., Yin Y., Gan M., Meng M., Dai X., Wu R., Shi W., Yan Y., Chem. Eng. J. 2015, 266, 299–308. [Google Scholar]
- 58. Yang Y., Wei Z., Wang C., Tong Z., Chem. Commun. 2013, 49, 7144–7146. [DOI] [PubMed] [Google Scholar]
- 59. Jin W., Chen L., Hu M., Sun D., Li A., Li Y., Hu Z., Zhou S., Tu Y., Xia T., Wang Y., Xie G., Li Y., Bai B., Peng L., Appl. Energy 2016, 175, 82–90. [Google Scholar]
- 60. Caicedo H. M., Dempere L. A., Vermerris W., Nanotechnology 2012, 23, 105605. [DOI] [PubMed] [Google Scholar]
- 61. Rai S., Singh B. K., Bhartiya P., Singh A., Kumar H., Dutta P. K., Mehrotra G. K., J. Lumin. 2017, 190, 492–503. [Google Scholar]
- 62. Tardy B. L., Richardson J. J., Guo J., Lehtonen J., Ago M., Rojas O. J., Green Chem. 2018, 20, 1335–1344. [Google Scholar]
- 63. Piccinino D., Capecchi E., Botta L., Bizzarri B. M., Bollella P., Antiochia R., Saladino R., Biomacromolecules 2018, 19, 3883–3893. [DOI] [PubMed] [Google Scholar]
- 64. Figueiredo P., Lintinen K., Kiriazis A., Hynninen V., Liu Z., Bauleth-Ramos T., Rahikkala A., Correia A., Kohout T., Sarmento B., Yli-Kauhaluoma J., Hirvonen J., Ikkala O., Kostiainen M. A., Santos H. A., Biomaterials 2017, 121, 97–108. [DOI] [PubMed] [Google Scholar]
- 65. Chen L., Zhou X., Shi Y., Gao B., Wu J., Kirk T. B., Xu J., Xue W., Chem. Eng. J. 2018, 346, 217–225. [Google Scholar]
- 66. Dai L., Liu R., Hu L., Zou Z., Si C., ACS Sustainable Chem. Eng. 2017, 5, 8241–8249. [Google Scholar]
- 67. Figueiredo P., Ferro C., Kemell M., Liu Z., Kiriazis A., Lintinen K., Florindo H. F., Yli-Kauhaluoma J., Hirvonen J., Kostiainen M. A., Santos H. A., Nanomedicine 2017, 12, 2581. [DOI] [PubMed] [Google Scholar]
- 68. Wurm F., Landfester K., Yiamsawas D., Thines E., Fischer J., (Max-Planck Gesellschaft, Institut für Biotechnologie und Wirkstoff-Forschung GGMBH), WO2017134308A1, 2017.
- 69. Yiamsawas D., Baier G., Thines E., Landfester K., Wurm F. R., RSC Adv. 2014, 4, 11661. [Google Scholar]
- 70. Wang X., Zhao J., J. Agric. Food Chem. 2013, 61, 3789–3796. [DOI] [PubMed] [Google Scholar]
- 71. Taverna M. E., Busatto C. A., Lescano M. R., Nicolau V. V., Zalazar C. S., Meira G. R., Estenoz D. A., J. Hazard. Mater. 2018, 359, 139–147. [DOI] [PubMed] [Google Scholar]
- 72. Deng Y., Zhao H., Qian Y., Lü L., Wang B., Qiu X., Ind. Crops Prod. 2016, 87, 191–197. [Google Scholar]
- 73. Smith G. W., (Dow Chemical Co.), US4746513, 1988.
- 74. Răschip I. E., Panainte A. D., Pamfil D., Profire L., Vasile C., Rev. Med. Chir. Soc. Med. Nat. Iasi 2015, 119, 1189–1194. [PubMed] [Google Scholar]
- 75. Liu Z., Qie R., Li W., Hong N., Li Y., Li C., Wang R., Shi Y., Guo X., Jia X., New J. Chem. 2017, 41, 3190–3195. [Google Scholar]
- 76. DelliColli H. T., Dilling P., (Westvaco Corp.), US4244729, 1981.
- 77. Wahba S. M. R., Darwish A. S., Shehata I. H., Abd Elhalem S. S., Mater. Sci. Eng. C 2015, 48, 599–610. [DOI] [PubMed] [Google Scholar]
- 78. Kök F. N., Wilkins R. M., Cain R. B., Arica M. Y., Alaeddinoglu G., Hasirci V., J. Microencapsulation 1999, 16, 613–623. [DOI] [PubMed] [Google Scholar]
- 79. Dimitri M. S., Falkehag S. I. (Westvaco Corp., New York, NY), US3929453, 1977.
- 80. S. E. Lebo, Jr. , Detroit W. J., (Lignotech Inc.), US5552149A, 1996.
- 81. Moss R. D., (Lim Lab Inc.), WO1992019102A1, 1992.
- 82. Lignin Biodegradation: Microbiology, Chemistry and Potential Applications (Eds.: T. K. Kirk, T. Higuchi, H. Chang), CRC Press, Boca Raton, FL, 1980. [Google Scholar]
- 83. Kim S., Fernandes M. M., Matamá T., Loureiro A., Gomes A. C., Cavaco-Paulo A., Colloids Surf. B 2013, 103, 1–8. [DOI] [PubMed] [Google Scholar]
- 84. Capecchi E., Piccinino D., Delfino I., Bollella P., Antiochia R., Saladino R., Nanomaterials 2018, 8, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li Y., Qiu X., Qian Y., Xiong W., Yang D., Chem. Eng. J. 2017, 327, 1176–1183. [Google Scholar]
- 86.“EU Pesticides database,” can be found under https://ec.europa.eu/food/plant/pesticides/, accessed February 17, 2019.
- 87. Li Y., Zhou M., Pang Y., Qiu X., ACS Sustainable Chem. Eng. 2017, 5, 3321–3328. [Google Scholar]
- 88. Zhu W., Lu J., Dai L., Part. Part. Syst. Charact. 2018, 35, 1800145. [Google Scholar]
- 89. Campbell W. C., Curr. Pharm. Biotechnol. 2012, 13, 853–865. [DOI] [PubMed] [Google Scholar]
- 90. Datta S., Christena L. R., Rajaram Y. R. S., 3 Biotech 2013, 3, 10.1007/s13205-012-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wei Y., Quan L., Zhou C., Zhan Q., Nanomedicine 2018, 13, 1495–1512. [DOI] [PubMed] [Google Scholar]
- 92. Alexis F., Pridgen E., Molnar L. K., Farokhzad O. C., Mol. Pharm. 2008, 5, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bartzoka E. D., Lange H., Thiel K., Crestini C., ACS Sustainable Chem. Eng. 2016, 4, 5194–5203. [Google Scholar]
- 94. Zou S., Hu Y., Wang C., Macromol. Rapid Commun. 2014, 35, 1414–1418. [DOI] [PubMed] [Google Scholar]
- 95. Yi H., Yang Y., Gu X., Huang J., Wang C., J. Mater. Chem. A 2015, 3, 13749–13757. [Google Scholar]
- 96. Maierson T., V. A. Crainich, Jr. , (NCR Co.), US3494872, 1970.
- 97. Moss R. D., Lim F., (Lim Lab Inc.), WO1992019102A1, 1992.
- 98. Zhang H., Chen F., Liu X., Fu S., ACS Sustainable Chem. Eng. 2018, 6, 12532–12540 . [Google Scholar]
- 99. Zdarta J., Klapiszewski L., Jedrzak A., Nowicki M., Moszynski D., Jesionowski T., Catalysts 2017, 7, 14. [Google Scholar]
- 100. Park S., Kim S. H., Kim J. H., Yu H., Kim H. J., Yang Y. H., Kim H., Kim Y. H., Ha S. H., Lee S. H., J. Mol. Catal. B 2015, 119, 33–39. [Google Scholar]
- 101. Sipponen M. H., Farooq M., Koivisto J., Pellis A., Seitsonen J., Österberg M., Nat. Commun. 2018, 9, 2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zdarta J., Klapiszewski Ł., Wysokowski M., Norman M., Kołodziejczak-Radzimska A., Moszyński D., Ehrlich H., Maciejewski H., Stelling A. L., Jesionowski T., Mar. Drugs 2015, 13, 2424–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gong W., Ran Z., Ye F., Zhao G., Food Chem. 2017, 228, 455–462. [DOI] [PubMed] [Google Scholar]
- 104. Ten E., Ling C., Wang Y., Srivastava A., Dempere L. A., Vermerris W., Biomacromolecules 2014, 15, 327–338. [DOI] [PubMed] [Google Scholar]
- 105. Van Beinum W., Beulke S., Brown C. D., Environ. Sci. Technol. 2006, 40, 494–500. [DOI] [PubMed] [Google Scholar]
- 106. Ludvík J., Zuman P., Microchem. J. 2000, 64, 15–20. [Google Scholar]
- 107. Rojas O. J., Bullón J., Ysambertt F., Forgiarini A., Salager D. S. Argyropoulos in J.-L. in Materials, Chemicals, and Energy from Forest Biomass (Ed.: D. S. Argyropoulos), American Chemical Society, Washington, DC, 2007, pp. 182–199. [Google Scholar]
- 108. Goto M., Goto M., Kamiya N., Nakashio F., Biotechnol. Bioeng. 1995, 45, 27–32. [DOI] [PubMed] [Google Scholar]
- 109. Huang S. Y., Chang H. L., Goto M., Enzyme Microb. Technol. 1998, 22, 552–557. [Google Scholar]
- 110. Yamada Y., Kuboi R., Komasawa I., Biotechnol. Prog. 1993, 9, 468–472. [DOI] [PubMed] [Google Scholar]
- 111. Richter A. P., Bharti B., Armstrong H. B., Brown J. S., Plemmons D., Paunov V. N., Stoyanov S. D., Velev O. D., Langmuir 2016, 32, 6468–6477. [DOI] [PubMed] [Google Scholar]
- 112. Carmona-Ribeiro A. M., de Melo Carrasco L. D., Int. J. Mol. Sci. 2013, 14, 9906–9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Murata H., Koepsel R. R., Matyjaszewski K., Russell A. J., Biomaterials 2007, 28, 4870–4879. [DOI] [PubMed] [Google Scholar]
- 114. Leskinen T., Witos J., Valle Delgado J. J., Lintinen K. S., Kostiainen M. A., Wiedmer S. K., Österberg M., Mattinen M.-L., Biomacromolecules 2017, 18, 2767–2776. [DOI] [PubMed] [Google Scholar]
- 115. Zhong J. F., Xu L., Qin X. L., J. Compos. Mater. 2015, 49, 2329–2335. [Google Scholar]
- 116. Klapiszewski Ł., Rzemieniecki T., Krawczyk M., Malina D., Norman M., Zdarta J., Majchrzak I., Dobrowolska A., Czaczyk K., Jesionowski T., Colloids Surf. B 2015, 134, 220–228. [DOI] [PubMed] [Google Scholar]
- 117. Richter A. P., Brown J. S., Bharti B., Wang A., Gangwal S., Houck K., Cohen Hubal E. A., Paunov V. N., Stoyanov S. D., Velev O. D., Nat. Nanotechnol. 2015, 10, 817–823. [DOI] [PubMed] [Google Scholar]
- 118. Kang S., Herzberg M., Rodrigues D. F., Elimelech M., Langmuir 2008, 24, 6409–6413. [DOI] [PubMed] [Google Scholar]
- 119. Lewinski N., Colvin V., Drezek R., Small 2008, 4, 26–49. [DOI] [PubMed] [Google Scholar]
- 120. Kaewamatawong T., Shimada A., Okajima M., Inoue H., Morita T., Inoue K., Takano H., Toxicol. Pathol. 2006, 34, 958–965. [DOI] [PubMed] [Google Scholar]
- 121. Liu T., Li L., Teng X., Huang X., Liu H., Chen D., Ren J., He J., Tang F., Biomaterials 2011, 32, 1657–1668. [DOI] [PubMed] [Google Scholar]
- 122. Fu C., Liu T., Li L., Liu H., Chen D., Tang F., Biomaterials 2013, 34, 2565–2575. [DOI] [PubMed] [Google Scholar]
- 123. Tiwari D. K., Jin T., Behari J., Toxicol. Mech. Methods 2011, 21, 13–24. [DOI] [PubMed] [Google Scholar]
- 124. Kim Y. S., Kim J. S., Cho H. S., Rha D. S., Kim J. M., Park J. D., Choi B. S., Lim R., Chang H. K., Chung Y. H., Kwon I. H., Jeong J., Han B. S., Yu I. J., Inhalation Toxicol. 2008, 20, 575–583. [DOI] [PubMed] [Google Scholar]
- 125. Asharani P. V., Lian Wu Y., Gong Z., Valiyaveettil S., Nanotechnology 2008, 19, 255102. [DOI] [PubMed] [Google Scholar]
- 126. Samberg M. E., Oldenburg S. J., Monteiro-Riviere N. A., Environ. Health Perspect. 2010, 118, 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zogovic N. S., Nikolic N. S., Vranjes-Djuric S. D., Harhaji L. M., Vucicevic L. M., Janjetovic K. D., Misirkic M. S., Todorovic-Markovic B. M., Markovic Z. M., Milonjic S. K., Trajkovic V. S., Biomaterials 2009, 30, 6940–6946. [DOI] [PubMed] [Google Scholar]
- 128. Yang S. T., Guo W., Lin Y., Deng X. Y., Wang H. F., Sun H. F., Liu Y. F., Wang X., Wang W., Chen M., Huang Y.-p., Sun Y.-P., J. Phys. Chem. C 2007, 111, 17761–17764. [Google Scholar]
- 129. Zhu Y., Li W., Li Q., Li Y., Li Y., Zhang X., Huang Q., Carbon 2009, 47, 1351–1358. [Google Scholar]
- 130. Yang S.-T., Cao L., Luo P. G., Lu F., Wang X., Wang H., Meziani M. J., Liu Y., Qi G., Sun Y.-P., J. Am. Chem. Soc. 2009, 131, 11308–11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Galdiero S., Falanga A., Vitiello M., Cantisani M., Marra V., Galdiero M., Molecules 2011, 16, 8894–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bakry R., Vallant R. M., Najam-ul-Haq M., Rainer M., Szabo Z., Huck C. W., Bonn G. K., Int. J. Nanomed. 2007, 2, 639–649. [PMC free article] [PubMed] [Google Scholar]
- 133. Piotrovsky L. B., Kiselev O. I., Fullerenes Nanotubes Carbon Nanostruct. 2005, 12, 397–403. [Google Scholar]
- 134. Marchesan S., Da Ros T., Spalluto G., Balzarini J., Prato M., Bioorg. Med. Chem. Lett. 2005, 15, 3615–3618. [DOI] [PubMed] [Google Scholar]
- 135. Marulasiddeshwara M. B., Dakshayani S. S., Sharath Kumar M. N., Chethana R., Raghavendra Kumar P., Devaraja S., Mater. Sci. Eng. C 2017, 81, 182–190. [DOI] [PubMed] [Google Scholar]
- 136. Rocca D. M., Vanegas J. P., Fournier K., Becerra M. C., Scaiano J. C., Lanterna A. E., RSC Adv. 2018, 8, 40454–40463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Panáček A., Kvitek L., Prucek R., Kolář M., Večeřová R., Pizúrová N., Sharma V. K., Nevecná T., Zbořil R., J. Phys. Chem. B 2006, 110, 16248–16253. [DOI] [PubMed] [Google Scholar]
- 138. Mathelié-Guinlet M., Grauby-Heywang C., Martin A., Février H., Moroté F., Vilquin A., Béven L., Delville M. H., Cohen-Bouhacina T., J. Colloid Interface Sci. 2018, 529, 53–64. [DOI] [PubMed] [Google Scholar]
- 139. Hetrick E. M., Shin J. H., Paul H. S., Schoenfisch M. H., Biomaterials 2009, 30, 2782–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kang S., Pinault M., Pfefferle L. D., Elimelech M., Langmuir 2007, 23, 8670–8673. [DOI] [PubMed] [Google Scholar]
- 141. Lyon D. Y., Fortner J. D., Sayes C. M., Colvin V. L., Hughes J. B., Environ. Toxicol. Chem. 2005, 24, 2757–2762. [DOI] [PubMed] [Google Scholar]
- 142. Panáček A., Kolář M., Večeřová R., Prucek R., Soukupová J., Kryštof V., Hamal P., Zbořil R., Kvítek L., Biomaterials 2009, 30, 6333–6340. [DOI] [PubMed] [Google Scholar]
- 143. Paulo C. S. O., Vidal M., Ferreira L. S., Biomacromolecules 2010, 11, 2810–2817. [DOI] [PubMed] [Google Scholar]
- 144. Zare-Zardini H., Amiri A., Shanbedi M., Memarpoor-Yazdi M., Asoodeh A., Surf. Interface Anal. 2013, 45, 751–755. [Google Scholar]
- 145. Dai L., Zhu W., Liu R., Si C., Part. Part. Syst. Charact. 2018, 35, 1700447. [Google Scholar]
- 146. Lin W., Huang Y. W., Zhou X. D., Ma Y., Toxicol. Appl. Pharmacol. 2006, 217, 252–259. [DOI] [PubMed] [Google Scholar]
- 147. Kim S., Ohulchanskyy T. Y., Pudavar H. E., Pandey R. K., Prasad P. N., J. Am. Chem. Soc. 2007, 129, 2669–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang Q., Yang W., Man N., Zheng F., Shen Y., Sun K., Li Y., Wen L. P., Autophagy 2009, 5, 1107–1117. [DOI] [PubMed] [Google Scholar]
- 149. Park E. J., Park K., Toxicol. Lett. 2009, 184, 18–25. [DOI] [PubMed] [Google Scholar]
- 150. Vallhov H., Gabrielsson S., Strømme M., Scheynius A., Garcia-Bennett A. E., Nano Lett. 2007, 7, 3576–3582. [DOI] [PubMed] [Google Scholar]
- 151. Radu D. R., Lai C. Y., Jeftinija K., Rowe E. W., Jeftinija S., Lin V. S. Y., J. Am. Chem. Soc. 2004, 126, 13216–13217. [DOI] [PubMed] [Google Scholar]
- 152. Suzuki H., Tochikura T. S., Iiyama K., Yamazaki S., Yamamoto N., Toda S., Agric. Biol. Chem. 1989, 53, 3369–3372. [Google Scholar]
- 153. Thakkar J. N., Tiwari V., Desai U. R., Biomacromolecules 2010, 11, 1412–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Gordts S. C., Férir G., D′Huys T., Petrova M. I., Lebeer S., Snoeck R., Andrei G., Schols D., PLoS One 2015, 10, e0131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Srisapoome P., Hamano K., Tsutsui I., Iiyama K., Fish Shellfish Immunol. 2018, 72, 494–501. [DOI] [PubMed] [Google Scholar]
- 156. Harada H., Sakagami H., Nagata K., Oh-hara T., Kawazoe Y., Ishihama A., Hata N., Misawa Y., Terada H., Konno K., Antiviral Res. 1991, 15, 41–49. [DOI] [PubMed] [Google Scholar]
- 157. Rai M., Yadav A., Gade A., Biotechnol. Adv. 2009, 27, 76–83. [DOI] [PubMed] [Google Scholar]
- 158. Kim J. S., Kuk E., Yu K. N., Kim J.-H., Park S. J., Lee H. J., Kim S. H., Park Y. K., Park Y. H., Hwang C.-Y., Kim Y.-K., Lee Y.-S., Jeong D. H., Cho M.-H., Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar]
- 159. Kai D., Chong H. M., Chow L. P., Jiang L., Lin Q., Zhang K., Zhang H., Zhang Z., Loh X. J., Compos. Sci. Technol. 2018, 158, 26–33. [Google Scholar]
- 160. Nakasone K., Kobayashi T., Mater. Sci. Eng. C 2016, 64, 269–277. [DOI] [PubMed] [Google Scholar]
- 161. Kai D., Ren W., Tian L., Chee P. L., Liu Y., Ramakrishna S., Loh X. J., ACS Sustainable Chem. Eng. 2016, 4, 5268–5276. [Google Scholar]
- 162. Isakovic A., Markovic Z., Todorovic-Marcovic B., Nikolic N., Vranjes-Djuric S., Mirkovic M., Dramicanin M., Harhaji L., Raicevic N., Nikolic Z., Trajkovic V., Toxicol. Sci. 2006, 91, 173–183. [DOI] [PubMed] [Google Scholar]
- 163. Liu X., Yin H., Zhang Z., Diao B., Li J., Colloids Surf. B 2015, 125, 230–237. [DOI] [PubMed] [Google Scholar]
- 164. Hu T. Q., Ed., Chemical Modification, Properties, and Usage of Lignin, Kluwer Academic/Plenum, New York, 2002. [Google Scholar]
- 165. Mottiar Y., Vanholme R., Boerjan W., Ralph J., Mansfield S. D., Curr. Opin. Biotechnol. 2016, 37, 190–200. [DOI] [PubMed] [Google Scholar]
- 166. Baucher M., Halpin C., Petit-Conil M., Boerjan W., Crit. Rev. Biochem. Mol. Biol. 2003, 38, 305–350. [DOI] [PubMed] [Google Scholar]
- 167. Reich E. S., Nature 2012, 480, 160–161. [Google Scholar]
- 168. Warheit D. B., Borm P. J. A., Hennes C., Lademann J., Inhalation Toxicol. 2007, 19, 631–643. [DOI] [PubMed] [Google Scholar]
- 169.“Safety of manufactured nanomaterials,” can be found under http://www.oecd.org/env/ehs/nanosafety/, accessed February 17, 2019.
- 170.“FDA′s Approach to Regulation of Nanotechnology Products,” can be found under https://www.fda.gov/scienceresearch/specialtopics/nanotechnology/ucm301114.htm, accessed February 17, 2019.
- 171. Rauscher H., Rasmussen K., Sokull-Klüttgen B., Chem. Ing. Tech. 2017, 89, 224–231. [Google Scholar]
- 172. Nel A. E., J. Int. Med. 2013, 274, 561–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Gavankar S., Suh S., Keller A. F., Int. J. Life Cycle Assess. 2012, 17, 295–303. [Google Scholar]
- 174. Hristozov D. R., Gottardo S., Critto A., Marcomini A., Nanotoxicology 2012, 6, 880–898. [DOI] [PubMed] [Google Scholar]
- 175. Petersen E. J., Henry T. B., Zhao J., MacCuspie R. I., Kirschling T. L., Dobrovolskaia M. A., Hackley V., Xing B., White J. C., Environ. Sci. Technol. 2014, 48, 4226–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zazo J. A., Casas J. A., Molina C. B., Quintanilla A., Rodriguez J. J., Environ. Sci. Technol. 2007, 41, 7164–7170. [DOI] [PubMed] [Google Scholar]
- 177. Appel E. A., Tibbitt M. W., Webber M. J., Mattix B. A., Veiseh O., Langer R., Nat. Commun. 2015, 6, 6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ulijn R. V., J. Mater. Chem. 2006, 16, 2217. [Google Scholar]
- 179. Theato P., Sumerlin B. S., O'Reilly R. K., T. H. Epps, III , Chem. Soc. Rev. 2013, 42, 7055. [DOI] [PubMed] [Google Scholar]
- 180. Crestini L. Zongo H. Lange C., ACS Omega 2019, 4, 6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


