Abstract
Purpose: Diabetic foot osteomyelitis (DFO) is the most frequent infection associated with diabetic foot ulcers, occurs in >20% of moderate infections and 50%–60% of severe infections, and is associated with high rates of amputation. DFO represents a challenge in both diagnosis and therapy, and many consequences of its condition are related to late diagnosis, delayed referral, or ill-indicated treatment. This review aimed to analyze the current evidence on DFO management and to discuss advantages and disadvantages of different treatment options.
Methods: A narrative review of the evidence was begun by searching Medline and PubMed databases for studies using the keywords “management”, “diabetic foot”, “osteomyelitis”, and “diabetic foot osteomyelitis” from 2008 to 2018.
Results: We found a great variety of studies focusing on both medical and surgical therapies showing a similar rate of effectiveness and outcomes; however, the main factors in choosing one over the other seem to be associated with the presence of soft-tissue infection or ischemia and the clinical presentation of DFO.
Conclusion: Further randomized controlled trials with large samples and long-term follow-up are necessary to demonstrate secondary outcomes, such as recurrence, recurrent ulceration, and reinfection associated with both medical and surgical options.
Keywords: diabetic foot, diabetic foot infection, bone infection, diabetic foot ulcers
Introduction
Diabetic foot ulcers (DFUs) are a complication of diabetes mellitus caused by external or internal trauma associated with different stages of diabetic neuropathy and peripheral vascular disease.1
The most serious consequence of DFUs is major or minor amputation.2 Major amputation has been related to a dramatic loss in the life expectancy of these patients, which places them at risk of higher mortality rates than colon, prostate, and breast cancers or Hodgkin's disease.3
The most frequent causes of amputation in patients with DFUs are ischemia and infection.4 Diabetic foot infection (DFI) remains the most frequent diabetic complication, affecting 60% of DFUs, sometimes requires hospitalization, and is the most common precipitating event leading to amputations.5–7
Managing infection requires careful attention to have a proper and early diagnosis of the condition, obtain appropriate specimens for culture, thoughtfully select empirical and then definitive antimicrobial therapy, quickly determine when surgical interventions are needed, and provide all other necessary types of wound care.4
Osteomyelitis (OM) is the most frequent infection of DFUs, occurs in >20% of moderate infections and 50%–60% of severe infections, and is associated with high rates of amputation.8
Diabetic foot OM (DFO) typically involves the forefoot (the most common location of DFUs) and develops by contiguous spread from overlying soft tissue and penetration through cortical bone and into the medullary cavity.9
Traditionally, DFO has been considered a complex and difficult-to-treat infection, with a high rate of relapse,10 and is one of the most controversial issues when dealing with diabetic foot syndrome.11
Despite the seriousness of this complication, unfortunately there are no agreed-upon guidelines for the management of DFO, and this is one of the most controversial and challenging problems in the field. The International Working Group on the Diabetic Foot recognized that DFO was an area in which guidelines for diagnosis and treatment (which could be modified according to the availability of local services and resources in different centers and communities) were needed.11,12
DFO represents a challenge in both diagnostic and therapeutic aspects, and many consequences of its condition are related to late diagnosis, delayed referral, or ill-indicated treatment.
This review aimed to analyze the evidence on the management of DFO and to discuss different options, challenges, and needs regarding this issue.
Methods
A narrative revision of the evidence was performed, focusing on treatment options of medical therapy (type, route, and duration of antibiotics), surgical therapy, and coadjuvant therapy for DFO.
Search strategy
PubMed, Cochrane Library, and Web of Science were searched in December 2018 for retrospective and prospective studies and randomized controlled trials (RCTs) published from January 2008 to December 2018. Databases were searched using the keywords “management”, “diabetic foot”, “osteomyelitis”, and “diabetic foot osteomyelitis”. Searches were filtered for studies published in English.
Selection of studies
Two independent reviewers screened all titles and abstracts for eligibility based on predefined inclusion criteria (EGM and YGA). If the eligibility criteria were unclear based on this first screening, the full text was obtained for further evaluation. A third reviewer resolved disagreements (JLM).
We included studies published in English and Spanish. The studied population of the studies was defined as subjects with diagnoses of DFO. We limited our review from interventions to therapeutic modalities, excluding diagnostic, preventive, or educational interventions. We did not limit the care setting of the included studies. Reference lists of all retrieved studies were cross-checked for additional reports. Abstracts of all studies were reviewed to exclude articles meeting our exclusion criteria. Full-text reviews were performed to determine whether the remaining studies met the inclusion criteria.
Exclusion criteria were unoriginal articles, including letters or comments, case series, and studies without available data for analysis. Additionally, references of narrative and systematic reviews were scrutinized for additional articles.
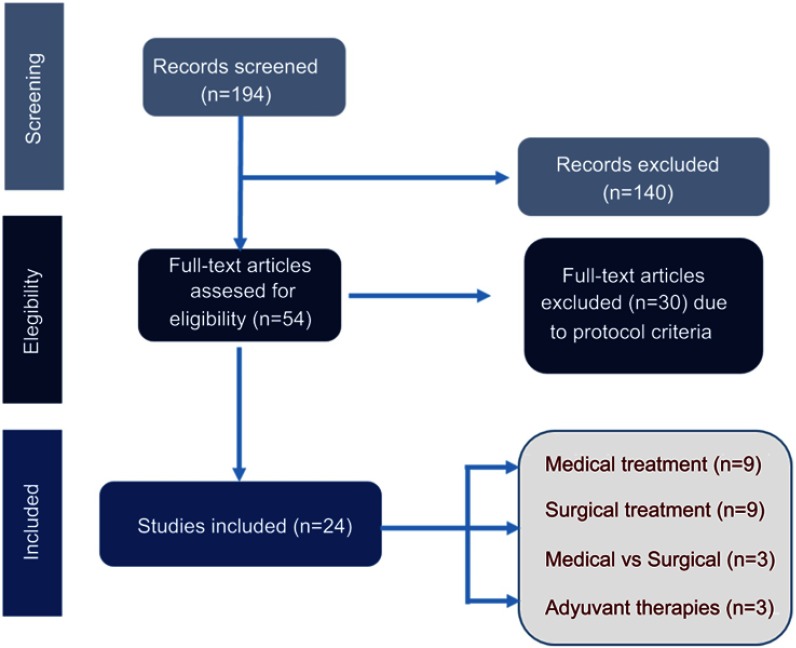
A total of 194 records were initially identified by the literature search. At the end of the screening process, 24 studies met the inclusion criteria. The distribution of studies was medical treatment (n=9), surgical treatment (n=9), medical and surgical treatment (n=3), and adjuvant therapies (n=3; Figure 1).
Figure 1.
Flow of studies through the review.
Diabetic foot osteomyelitis–treatment options: evidence analysis
Medical treatment
The literature shows that the traditional treatment of DFO has been the resection of necrotic and infected bone. However, there were some studies that demonstrated highest remission rates when patients with DFO were treated exclusively with antibiotics. Probably, the main limitation for supporting this therapeutic option is that these studies13,14 were retrospective and did not include sufficient posttreatment follow-up (at least 12 months) to detect episodes of new DFO and/or recurrent ulceration. Nowadays, there is an increasing tendency toward nonsurgical therapy for DFO.15
According to the most accepted guidelines,4,16 there is a consensus about when nonsurgical treatment can be tried firstly. These criteria are:
There is no persisting sepsis associated with DFO.
Patient can receive and tolerate appropriate antibiotic therapy.
The degree of bone destruction has not caused irretrievable compromise to foot mechanics.
The patient prefers to avoid surgery.
The patient’s comorbidities confer high risk to surgery.
There are no contraindications to prolonged antibiotic therapy.
Surgery is not otherwise required in adjacent soft-tissue infection or necrosis.
Infection is confined to small forefoot lesions that are easily off-loaded.
Patients have good vascular status that allows drug spreading and tissue availability.
No adequately skilled surgeon is available.
Operating room and other surgical facilities are not available.
Surgery cost prohibits the patient from undergoing the surgery.
The main advantages of medically treating DFO are absence of biomechanical changes that increase recurrent ulceration rates by pressure transfer to other foot locations that may occur after surgical procedures,17 the absence of available expert surgeons or surgical facilities needed,16 and a better cost-effective profile by reducing the risk and hospitalization associated with the surgical procedures. However, it has limitations, which include the risk of recurrent infections due to remaining infected bone, the risk of recurrent ulceration due to the persistence of the bone deformity at the origin of the FU, and toxicity and adverse effects related to prolonged administration of the antibiotic, eg, the development of bacterial resistance or the risk of Clostridium difficile disease.16,18–21
Recent literature corroborates antibiotics as the first-line treatment, especially from small forefoot lesions that are easy to off-load and in cases where surgery leads to destabilization of foot mechanics;16,22 however, some forefoot locations, such as the metatarsal area, have shown higher risks of complications than other forefoot locations.23
Based on studies that analyzed medical treatment,18,22–30 good remission rates have been demonstrated —>63.5%–82.3%23,25 — that can be assumed to be a positive response to treatment. However, there are problems when it comes to transferal to clinical practice, due to the lack of consensus on the duration, route of administration, and diagnostic criteria for bone infection.
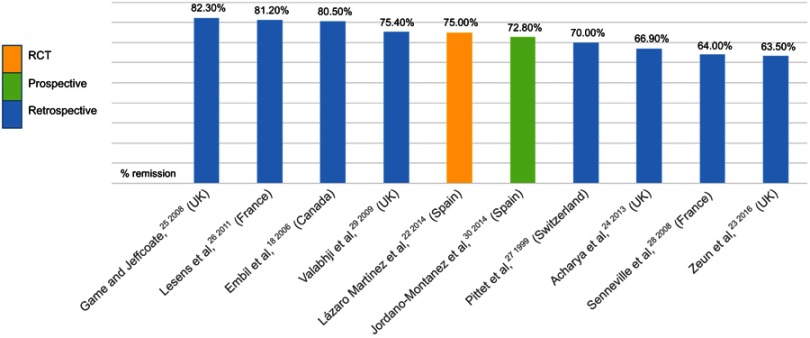
Almost 80% of studies based on medical treatment of DFO have been retrospective, and there has only been one RCT, which demonstrated that for appropriately selected patients, antibiotic therapy without surgery was effective22 (Figure 2). Game and Jeffcoate25 provided the highest remission rates of patients with DFO treated with a broad-spectrum antibiotic regimen chosen empirically. The criteria used to define remission was survival of the patient with an intact limb at 12 months after the physician considered that bone infection had been eradicated, but without any imaging tests performed for confirmation. Studies that performed microbiological bone culture to establish antibiotic regimens using antibiograms presented DFO-remission rates of 64%,28 72.8%,30 and 81.2%.26 The difference between remission rates could be associated with the methods used in obtaining bone samples, ie, by means of percutaneous biopsy in the first study and then bone debridement of the ulcer in the other two studies. In general, they reported successful treatment without surgical treatment with remission in approximately two-thirds of cases.
Figure 2.
Distribution of rates of remission of diabetic foot osteomyelitis with antibiotic treatment.
Additionally, some studies have investigated other parameters associated with good DFO outcomes, which include a decrease in inflammatory biomarkers, such as erythrocyte-sedimentation rate (ESR) and CRP,31–33 bone remineralization on plain radiography, and complete healing of any overlying soft-tissue wounds.9 However, comparison of these studies is difficult, due to the variability in protocols in antibiotic prescription and the lack of inflammatory markers or radiological evidence for confirmation.
How to choose antibiotics and route of administration
For many years, antibiotic therapy for DFO was administered intravenously for prolonged periods.34
However, in the last few years, two reviews of the literature did not find any statistically significant difference between oral and parenteral administration of antibiotics for the treatment of OM if bacteria were sensitive to the antibiotic administered.35,36
On the other hand, interesting pharmacokinetic data have shown that that antibiotics that reach the highest bone:serum concentration ratios (ie, fluoroquinolones, sulfonamides, cyclins, macrolides, rifampin, fusidic acid, and oxazolidinones) are also those with the highest bioavailability during oral administration of these agents.36
In a review conducted in 2017,37 Senneville et al said that it is logical that preference be given to antibiotics that exhibit high diffusion into the bone (ie, a bone:blood ratio >0.3) and have good oral bioavailability (ie, >90%), due to the prolonged duration of treatment that is usually recommended in these settings and the chronic nature of bone infection that is encountered in patients with DFO.
The selection of an antibiotic agent to treat DFO should begin with the selection of agents that cover the presumed pathogens tested. Bone culture provides the most accurate microbiological information, and surgical or percutaneous bone biopsy is the optimal method of obtaining a sample of uncontaminated bone.37,38
Combinations of two agents with high oral availability and bone diffusion have been shown to treat DFO. Rifampicin, fluoroquinolone (ofloxacin, ciprofloxacin, levofloxacin, or moxifloxacin), and β-lactam–fluoroquinolone combinations seem appropriate for the treatment of Staphylococcus-induced and Gram-negative DFO.28,39,40
However, this may be limited, due to the risk of occurrence of adverse events, with antibiotics being hepatotoxic and nephrotoxic in patients who are likely to have comorbidities and who receive multiple treatments. Therefore, we must take into account daily doses and potential adverse events of antibiotics with satisfactory oral bioavailability and bone diffusion for the treatment of patients with DFO.36,41
Duration of antibiotic regimen
Lengthy antibiotic treatment has been usual in retrospective studies and case series published on the medical treatment of DFO. Embil et al18 reported a mean duration of oral antimicrobial therapy of 40±30 weeks. Valabhji et al29 reported a medianduration of antibiotics of 24 (12–48) weeks.
However, in the last decade, studies with better methodological designs began to show shorter antibiotic treatments for DFO. In a retrospective study, Senneville et al described a mean duration of antibiotic treatment of 11.5±4.21 weeks. Game and Jeffcoate25 reported a mean duration of initial empirical treatment with oral and intravenous antibiotics of 61 days (range 3–349 days) and 16 days (range 1–44 days). In a study of 77 patients with microbiological results from bone biopsy, Lesens et al26 reported that 34% received treatment for 6 weeks, 36% for 9 weeks, and 30% for 12 weeks or more. Even so, the methodological variability among these series does not allow easy comparison, and conclusions cannot be drawn about the period of antibiotic treatment.
For this reason, in 2012 an Infectious Diseases Society of America guideline4 provided recommended durations of antibiotic treatment: short duration (2–5 days) when a radical resection does not leave a residual infected tissue and prolonged treatment (≥4 weeks) when there remains an infected and/or necrotic bone.
As such, after prolongation of antibiotic therapy after debridement for >6 weeks and administration of an intravenous treatment for more than a week, it seems likely that the residual bone in the sea is vital; therefore, it can be treated more quickly than bones that are infected with necrosis.
A short time later, Tone et al42 published the first trial comparing 6 weeks versus 12 weeks of treating DFO medically. The uthors performed microbiological analysis of bacterial isolates and chose a specific antibiotic regimen. There were no significant differences in remission rates between groups (60% versus 70%, P=0.50), but significantly fewer adverse events with the shorter treatment. It has been shown that antibiotic treatment can lead to kidney failure in more than a quarter of patients.43
Because the concept of DFO remission is subjective, recently Vouillarmet et al44 examined the utility of white blood cell (WBC) single-photon-emission computed tomography (SPECT)/CT as a predictive marker of DFO remission after 6 weeks of medical treatment of patients with DFO. Among the 45 patients, 51.1% had a negative WBC SPECT/CT after 6 weeks of antibiotic therapy. During 12 months of follow-up, there was no relapse in any patient with a negative WBC SPECT/CT. In the total sample, the remission rate of DFO was 84.4%, and the sensitivity of WBC SPECT/CT at 12 weeks to predict remission was 100%.
In conclusion, more trials are urgently needed to extend the results of the first and only RCT published,42 in which a 6-week course of antibiotics was not inferior to a longer course and to determine duration of antibiotics in the management of OM when it is associated with soft-tissue infection. Given the high rate of recurrences observed in patients with DFOs, it seems more appropriate to consider treatment success as a remission of all signs of infection, including imaging assessment by the year following the end of treatment.4,9
Key points: medical treatment
The duration of antibiotic treatment should not exceed 6 weeks.
Oral administration has shown more successful results than parenteral.
Medical treatment performed must be based on the bacteria identified during bone sampling whenever possible (percutaneous biopsy is the safest method, but requires professional training).
Prolonged treatment with antibiotics could be a limitation in patients with infection caused by complicated, anticoagulated, or multiresistant bacteria.
The worldwide increase in the prevalence of multiresistant bacteria could affect the choice of medical treatment. This concern may favor preference for bone-infected resection for the safety of the patient and decreased complications in the near future.
Surgical treatment
Despite the published studies on the effectiveness of surgery in OM, the International Working Group on the Diabetic Foot guidelines recommended that surgical intervention in cases of OM accompanied by spreading soft-tissue infection, destroyed soft-tissue envelope, progressive bone destruction on X-ray, or bone protruding through the ulcer should be considered.9
In a consensus statement for the initial diagnosis and selection of patients for the surgical management of diabetic forefoot OM, some criteria were defined with a high rate of agreement among the authors, who concluded that surgical treatment of DFO should be performed primarily in certain circumstances:
DFO with systemic toxicity associated with soft-tissue infection
substantial cortical destruction, osteolysis, macroscopic bone fragmentation (sequestration), or necrotic bone seen on X-ray
visible, chronically exposed trabecular bone identified within a forefoot ulcer
open or infected joint space
prosthetic heart valves45
Surgery is essential in patients with DFI to drain pus, economically resect all necrotic tissues, and drastically reduce biofilm and thus bacteria included inside. However, while surgery may be required urgently for the treatment of soft-tissue infections, OM of the diabetic foot in itself is not a reason for urgent surgery oramputation. The most severe and acute complications related to DFIs, such as gangrene, septicemia, and septic shock, are secondary to soft-tissue infections and/or necrosis of ischemic tissues, rather than to osteoarticular infections.37
Recently, surgical management of DFO has been based on conservative surgery (CS) with the aim of avoiding minor and major amputations.46,47 The advantages of surgery as a treatment have long been considered essential in the treatment of DFO, in order to ease the action of the antibiotics and even to replace them when we get poor results by the administration of antibiotics alone. Other factors to consider are micro- and macrovascular complications that compromise blood supply to infected tissue of the foot and characteristics of infected bone that affects principally the cortical part of the bone. All these processes could result in decreased efficacy of antibiotics in these areas. Moreover, intolerance to some antibiotics due to renal or hepatic diseases and the presence of resistant bacteria have been described as potential indications for a surgical approach to DFO. Another advantage of surgical treatment based on CS may be a reduction in duration of antibiotic therapy.46 Advantages described by previous studies have been lower amputation rate, high percentage of limb salvage, low risk of recurrence through surgical off-loading, and taking samples for microbiological and histological analysis.48,49 Because of these advantages, surgical therapy has been considered a primary option for some authors.20,39,46,49–51
The main disadvantages of surgical procedures are possible occurrence of a transfer syndrome where new ulcers can lead to another complication, including new bone infection, higher cost, increased operative comorbidity, and occurrence of an unstable foot.17,46
Most studies that have analyzed surgical treatment of OM established different outcomes regarding remission, recurrent ulceration, new episodes of OM, major or minor amputation, and death, but there have been few studies with long-term follow-up and fewer still that compared both treatments prospectively. It has been shown that CS outcomes are largely related to the presence of ischemia or soft-tissue infections.52
There have been several studies and different results on recurrence rate and recurrent ulceration. Aragón-Sánchez et al published a prospective study to determine these and obtained rates of 4.6% and 43%, respectively, for outcomes described. Regarding amputation and mortality rates, the same study obtainedrates of 39.5% for minor amputations, 1.2% for major amputation, and 13% during follow-up.52 Another prospective study achieved a recurrent-ulceration rate of 41%, and showed that the first metatarsal bone has the highest risk of recurrent ulceration.17
One of the complications that can occur after surgery is residual OM, and its outcome has been analyzed in several studies. Atway et al53 obtained a 40.7% rate of residual OM; however, in another study this was lower — 16.9%.52
Other studies have found that an aggressive surgical approach against FI, including OM, in hospitalized diabetic patients was associated with a 13% rate of above-ankle amputation.54
One of the prognostic factors for surgical management of DFO is the presence of ischemia, necrosis, or soft-tissue infection.50 Fuji et al proposed an appropriate surgical treatment for diabetic forefoot OM involving ischemia or moderate–severe soft-tissue infection, and the healing rate of patients with ischemic involvement was 86.6%, with OM recurrence not observed.55
Choosing the proper surgical technique for DFO
Some studies have concluded that CS without local or high-level amputation is successful in almost half the cases of DFO.50
Choosing different surgical options depends sometimes on the surgeon's skills when working in multidisciplinary teams. A sample of surgical techniques has been provided in resecting bone infection from the forefoot while avoiding amputations. The definitive role of such procedures must be evaluated in prospective trials addressed by experienced diabetic foot teams.47 Many studies have concluded that limited surgery (resection of infected and necrotic bone without amputation) combined with antibiotic therapy may be the most appropriate treatment.49,56,57
Another study that determined the incidence of complications associated with primary closure in surgical procedures performed for DFO compared to those healed by secondary intention concluded that primary surgical closure was not associated with more complications.58
Regarding surgical techniques, another recent study has evaluated the recovery time and the development of complications in the dorsal and plantar approach to metatarsal head resections in patients with DFUs complicated by OM. Both approaches rendered similar healing times; however, patients undergoing a dorsal approach developed more postsurgical complications than those undergoing a plantar approach.59
Key points: surgical treatment
Guidelines recommend that surgical intervention should be recommended in cases of OM accompanied by spreading soft-tissue infection, destroyed soft-tissue envelope, progressive bone destruction on X-ray, or bone protruding through the ulcer.
Surgical treatment has long been considered essential in the treatment of DFO to ease the action of antibiotics.
CS in the management of DFO is indicated to avoid minor and major amputations.
The main disadvantages of surgical procedures are recurrent ulceration, higher costs, increased operative comorbidity, and occurrence of an unstable foot.
Choosing different surgical options depends sometimes on the surgeon's skills when working in multidisciplinary teams.
Surgical versus medical treatment for DFO
There have been few studies to analyze medical treatment versus surgical treatment, receiving little attention.
Van et al compared outcomes of patients with DFO treated medically or surgically. Surgical patients underwent CS associated with antibiotics, whereas medical patients only received antibiotics.46 CS contributed to an increase in healing rate of FUs with OM compared with medical treatment alone.
In a study of patients from four centers in France and Spain, Lesens et al compared outcomes of those with bone culture–proven Staphylococcus aureus DFO who were treated medically (just antibiotic therapy, other than soft-tissue debridement at the bedside) or surgically (operative treatment combined with prolonged antibiotic therapy). Outcomes were similar for the two groups: favorable in 80% in the surgical group and 87% in the medical group.60
In another retrospective study with 147 DFO patients, Game and Jeffcoate found that 113 patients treated with antibiotic therapy alone underwent limb amputation (major amputation in six patients and minor amputation in 28), and remission rates were similar in both the surgical and medical groups (78.6% and 82.3%).61
Tan et al reported a lower rate of above-ankle amputation in patients who underwent debridement or local limited amputation than in patients treated with antibiotic therapy alone, with shorter hospital stay.54
The first randomized clinical study prospectively to compare outcomes of patients treated with medical versus surgical approaches for DFO was in 2014, reporting the results of a prospective study that aimed to compare outcomes of patients with DFO treated with antibiotics alone versus patients who underwent CS. At the end of a 12-week posttreatment follow-up, 18 patients (75%) achieved primary healing in the medical group versus 19 patients (86.3%) in the surgical group (P=0.33). No difference was found between the two groups regarding time to healing (7 versus 6 weeks) or minor amputations (P=0.336). The authors concluded that antibiotics and surgical treatment have similar outcomes in terms of healing rates, time to healing, and short-term complications in patients with neuropathic forefoot ulcers complicated by OM without ischemia or necrotizing soft-tissue infections.22
In Table 1, major decision criteria for medical versus surgical approaches have been summarized.
Table 1.
Criteria for selecting primarily antibiotic or surgical approaches for diabetic foot osteomyelitis
| Medical | Surgical |
|---|---|
|
|
A prospective cohort study of efficacy of combined surgical and medical treatment in patients with OM mainly involving the forefoot concluded that combined surgical and medical treatment for DFO can achieve acceptable limb-salvage rates and reduce time to healing, duration of antibiotic treatment, and wound-recurrence rate.62
In conclusion, expert opinion and retrospective studies with low-level evidence often determine how patients are treated, not allowing for a clear and standardized consensus on therapy.63
Despite the existence of published guidelines, approaches to management may vary widely,64,65 and different professionals have different views on the choice of antibiotics, route and duration of administration, and place of surgery.66
Adjuvant therapies
To date, there are insufficient data to demonstrate the efficacy of different adjuvant therapeutic practices, such as granulocyte growth factors, hyperbaric oxygen therapy, and local antibiotic-delivery systems, in the treatment of DFO.67–72
The serious problem of resistance to positive pathogens and the lack of new antimicrobial agents are the major challenges in the management of these patients. As a solution to this problem, many have chosen local antibiotic-delivery systems.73–76
In theory, the main advantages of local antibiotic-delivery systems are higher levels of antibiotic concentration in the affected area, pharmacokinetic advantages, ability to overcome the possibility of resistant pathogens, and in cases of biodegradable material, avoidance of additional surgical procedures. However, experience in DFO has been limited to case reports and case series, and there are no data that can be used to compare this therapy with standard medical therapy. Therefore, we cannot currently make any specific recommendations on indications or application times for this treatment.77
The most recent review on local antibiotic-delivery systems78 concluded that they represent a promising pharmaceutical option in the treatment of DFIs. Well-designed randomized clinical trials are required to establish their efficacy and define the framework for their usage. Currently, the role of local antibiotic-delivery systems in treating DFIs is limited and outside routine practice.
Discussion
Both medical and surgical options have been shown to be effective in the treatment of OM.21,22,28,37,60,66 However, there are also some criteria where there is consensus on which would be the best initial treatment depending on the characteristics of the patient. In such a way, when OM is associated with soft-tissue infection or ischemia,9 both presentation and clinical characteristics are different, and thus also management. Therefore, the first conclusion could be that there is not a single treatment for DFO because it is not a single disease, and its association with soft-tissue infection, ischemia, location, and patient characteristics determines the outcome, regardless of the treatment options.
After analysis of the literature, we can say that there is consensus on when surgical or medical treatment would be the first option in treating DFO.9,16,22,37
However, when we are treating DFO medically, it is necessary to ensure a good antibiotic choice with good bioavailability and proper duration of therapy depending on the characteristics of the patient and the infection,37 assessing dosage,36 since in the absence of a bone-resection therapies, antibiotic treatment should be at least 6 weeks,4,42 which is what the literature indicates, and in that case duration and posology will be driven by the characteristics and comorbidities of the patient. In patients with kidney disease, the dosage of antibiotics must be adjusted from bactericidal to bacteriostatic affecting the effectiveness of the antibiotic.
Another barrier to the medical treatment of OM is the exact time as to when antibiotic therapy should be discontinued. The 6-week reference margin collected in the literature is based on a single study.42 It does not seem strong enough to support a universal recommendation. There may be patients in whom antibiotic therapy would have to be extended, but we still do not have the answer about its duration.
Another important limitation regarding the medical treatment of DFO is the difficulty of obtaining a bone sample for microbiological culture, since there are limitations in obtaining culture samples. There are discrepancies with respect to the best way to obtain the sample. Recommendations in the literature for percutaneous bone biopsy are also based on the experience of a single group.79 Note that these procedures are difficult to perform, due to training limitations of the professional, the instruments, and the facilities (such resources as an operating room).26,80 In such a way, sometimes — especially in primary care, where obtaining bone cultures and even more, percutaneous cultures would be limited — antibiotic treatment is guided blindly, limiting also the beginning of this management. It is important to highlight that better results of DFO with antibiotics have been obtained in studies in which antibiotic selection has been done based on bone culture; therefore, these results cannot be extrapolated to daily practice when patients are treated medically, but without bone-culture confirmation.
Another concern is that the type of DFO that should be treated primarily with antibiotics has a chronic character profile, and could be treated in ambulatory settings (primary care and community), where patients have no access to an expert in infectious diseases, such as an infectious disease specialist or internal medicine specialist.
Being sure that DFO is ruled out is another issue when treating patients exclusively with antibiotics. One study has showed that even when a surgical bone resection is performed, >40% of patients remain infected at bone margins;17 therefore, after a regimen of medical treatment, we cannot be sure what percentage of residual infections we would have after 6 weeks of treatment recommended by the literature. Probably, the question is: Do we have any inflammatory marker that could indicate DFO remission? Reduction in inflammatory clinical signs cannot be related to resolution of DFO, especially in chronic OM, which is one of the main indications for medical treatment. Additionally, some inflammatory markers, such as CRP, ESR, and procalcitonine may be related to inflammatory responses and not really with bone healing. Only normalization of ESR has demonstrated a potential association with remission of DFO.22
It is important to take into consideration that when we are treating patients with DFO only with antibiotics, patients should be closely monitored with follow-up to identify early complications that they may develop during the course of treatment and have a clear picture as to when and where to refer the patient to a skilled surgeon when there are complications in infection. This means that the exclusive management of these patients in a setting where there is no specialized surgeon who can address or resolve complications is logically a limitation, since these patients must be treated by a multidisciplinary team with availability of a skilled surgeon at any given time to solve complications with medical treatment.81
There has been no any study so far that has analyzed patient compliance when we are treating DFO with antibiotics. We could probably find patients with low adherence to antibiotic regimens, especially in mid- or long-term antibiotic therapy, which means that implementation based on the results of these studies in daily practice could have different outcomes.
Regarding surgical treatment, many authors have defended it as a practically unique and most efficient option for the treatment of OM. However, treating these patients with surgery does not exempt them from major postsurgical complications, the most important of all being recurrent ulceration.17 It has been demonstrated that the level of recurrent ulceration after resection of a metatarsal head is high, depending on the head that is resected. The choice of technique is also important, as the amount of bone resected is important when it comes to the occurrence of recurrent ulceration.82
On the other hand, the increasing prevalence of lesions with neuroischemic etiology will probably lead to greater surgical contraindications in future, since the patient’s vascular status would avoid surgery, especially those that require large debridements.9,48 Therefore, another possible barrier to this treatment will be reconciliation of the vascular state with performance of the surgical technique, especially for patients who undergorevascularization, with deficient levels of vascular status to support extensive surgical debridement or extensive articular resection.
Additionally, performing surgical procedures requires specialization of the surgeon to reduce the risk of postsurgical complications,83,84 especially recurrent ulceration.85 CS has been described as an efficient and safe alternative for these patients,47 but requires knowledge of specific techniques of the foot, which makes it difficult to transfer this treatment to other surgical specialties, such as general surgery, plastic surgery, vascular surgery, or other types that lack experimentation with surgical techniques in the foot.
Moreover, cost is another limitation, especially in certain settings, where the patient must assume costs related to treatment, or certain countries, where there are few resources and access to surgical management, increasing the cost in a substantial way and thus reducing the chances of these patients, who generally have a low socioeconomic level, and access to it in those countries where there is no universal management coverage is complicated.
Tips, challenges, and solutions
Any professional who manages DFO must bear in mind that it is a disease that has several clinical presentations and therapy will be influenced mainly by the presence of both soft-tissue and necrotizing infections, vascular disease, and ulcer location. When associated soft-tissue infection spreads quickly and is located at the mid- or rearfoot, surgery is mandatory.
DFO with a chronic course confined at the forefoot, associated with small DFUs, with good vascular status patient-compliance profile, and that is easy to off-load could be treated primarily by antibiotics. However, close follow-up and limitation of antibiotic duration therapy be considered during treatment. If medical therapy does not resolve bone infection or if a complication appears, a surgical option should be offered to the patient.
In cases where ulcers expose joint cartilage or there is clear visualization of the bone, most publications have shown that the possibility of the bone getting covered by new tissue is very low; therefore, in these cases, it would be more advisable to manage these patients through bone resection.
More RCTs are needed, though probably the main limitation would be related to the selection of patients as homogeneous as possible, which would suffer infections with similar bacteria, since, depending on the type of bacteria, the response to antibiotics would be different, and conditioning management of patients based on the microbiological variability of DFO.
Further RCTs with large samples and long-term follow-up are needed. Much research has been done on postoperative complications where surgical treatment has been applied, but medical treatment studies have had brief follow-up. It would also be interesting to know what happens in this group of patients in long-term follow-up to evaluate all possible complications they may have, such as recurrence, recurrent ulceration, or the development of new infections.
With medical or surgical treatment, in both options, being ready appears more than reasonable when we are treating patients with DFO, which means a multidisciplinary approach is necessary in this kind of patient to avoid complications.
Acknowledgments
We want to acknowledge to Angellie D Fern for the professional english editing and the proofreading of the manuscript. This research received no grants from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217 [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi: 10.1016/S0140-6736(05)67698-2 [DOI] [PubMed] [Google Scholar]
- 3.Schofield CJ, Libby G, Brennan GM, et al. Mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care. 2006;29(10):2252–2256. doi: 10.2337/dc06-0926 [DOI] [PubMed] [Google Scholar]
- 4.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132–e173. doi: 10.1093/cid/cis346 [DOI] [PubMed] [Google Scholar]
- 5.Pecoraro RE, Ahroni JH, Boyko EJ, Stensel VL. Chronology and determinants of tissue repair in diabetic lower-extremity ulcers. Diabetes. 1991;40(10):1305–1313. [DOI] [PubMed] [Google Scholar]
- 6.Reiber GE, Pecoraro RE, Koepsell TD. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann Intern Med. 1992;117(2):97–105. [DOI] [PubMed] [Google Scholar]
- 7.Lavery LA, Armstrong DG, Murdoch DP, Peters EJ, Lipsky BA. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis. 2007;44(4):562–565. doi: 10.1086/511036 [DOI] [PubMed] [Google Scholar]
- 8.Lipsky BA. Bone of contention: diagnosing diabetic foot osteomyelitis. Clin Infect Dis. 2008;47(4):528–530. doi: 10.1086/590012 [DOI] [PubMed] [Google Scholar]
- 9.Lipsky BA, Aragon-Sanchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):45–74. doi: 10.1002/dmrr.2699 [DOI] [PubMed] [Google Scholar]
- 10.Berendt AR, Peters EJ, Bakker K, et al. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev. 2008;24(Suppl 1):S145–S161. doi: 10.1002/dmrr.836 [DOI] [PubMed] [Google Scholar]
- 11.Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39(7):885–910. doi: 10.1086/424846 [DOI] [PubMed] [Google Scholar]
- 12.Lipsky BA. International consensus group on d, treating the infected diabetic f. A report from the international consensus on diagnosing and treating the infected diabetic foot. Diabetes Metab Res Rev. 2004;20(Suppl 1):S68–S77. doi: 10.1002/dmrr.453 [DOI] [PubMed] [Google Scholar]
- 13.Yadlapalli N, Vaishnav A, Sheehan P. Conservative management ofdiabetic foot ulcers complicated by osteomyelitis. Wounds. 2002;14:31–35. [Google Scholar]
- 14.Embil J. The management of diabetic foot osteomyelitis. Diabetic Foot. 2000;3:76–84. [Google Scholar]
- 15.Mutluoglu M, Lipsky BA. Non-surgical treatment of diabetic foot osteomyelitis. Lancet Diabetes Endocrinol. 2017;5(8):668. doi: 10.1016/S2213-8587(16)30141-3 [DOI] [PubMed] [Google Scholar]
- 16.Lipsky BA. Treating diabetic foot osteomyelitis primarily with surgery or antibiotics: have we answered the question? Diabetes Care. 2014;37(3):593–595. doi: 10.2337/dc13-2510 [DOI] [PubMed] [Google Scholar]
- 17.Molines-Barroso RJ, Lazaro-Martinez JL, Aragon-Sanchez J, Garcia-Morales E, Beneit-Montesinos JV, Alvaro-Afonso FJ. Analysis of transfer lesions in patients who underwent surgery for diabetic foot ulcers located on the plantar aspect of the metatarsal heads. Diabet Med. 2013;30(8):973–976. doi: 10.1111/dme.12202 [DOI] [PubMed] [Google Scholar]
- 18.Embil JM, Rose G, Trepman E, et al. Oral antimicrobial therapy for diabetic foot osteomyelitis. Foot Ankle Int. 2006;27(10):771–779. doi: 10.1177/107110070602701003 [DOI] [PubMed] [Google Scholar]
- 19.Hartemann-Heurtier A, Senneville E. Diabetic foot osteomyelitis. Diabetes Metab. 2008;34(2):87–95. doi: 10.1016/j.diabet.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 20.Henke PK, Blackburn SA, Wainess RW, et al. Osteomyelitis of the foot and toe in adults is a surgical disease: conservative management worsens lower extremity salvage. Ann Surg. 2005;241(6):885–92; discussion 92–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragon-Sanchez J, Lipsky BA. Modern management of diabetic foot osteomyelitis. The when, how and why of conservative approaches. Expert Rev Anti Infect Ther. 2018;16(1):35–50. doi: 10.1080/14787210.2018.1417037 [DOI] [PubMed] [Google Scholar]
- 22.Lazaro-Martinez JL, Aragon-Sanchez J, Garcia-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes Care. 2014;37(3):789–795. doi: 10.2337/dc13-1526 [DOI] [PubMed] [Google Scholar]
- 23.Zeun P, Gooday C, Nunney I, Dhatariya K. Predictors of outcomes in diabetic foot osteomyelitis treated initially with conservative (nonsurgical) medical management: a retrospective study. Int J Low Extrem Wounds. 2016;15(1):19–25. doi: 10.1177/1534734615596892 [DOI] [PubMed] [Google Scholar]
- 24.Acharya S, Soliman M, Egun A, Rajbhandari SM. Conservative management of diabetic foot osteomyelitis. Diabetes Res Clin Pract. 2013;101(3):e18–e20. doi: 10.1016/j.diabres.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 25.Game FL, Jeffcoate WJ. Primarily non-surgical management of osteomyelitis of the foot in diabetes. Diabetologia. 2008;51(6):962–967. doi: 10.1007/s00125-008-0976-1 [DOI] [PubMed] [Google Scholar]
- 26.Lesens O, Desbiez F, Vidal M, et al. Culture of per-wound bone specimens: a simplified approach for the medical management of diabetic foot osteomyelitis. Clin Microbiol Infect. 2011;17(2):285–291. doi: 10.1111/j.1469-0691.2010.03194.x [DOI] [PubMed] [Google Scholar]
- 27.Pittet D, Wyssa B, Herter-Clavel C, Kursteiner K, Vaucher J, Lew PD. Outcome of diabetic foot infections treated conservatively: a retrospective cohort study with long-term follow-up. Arch Intern Med. 1999;159(8):851–856. [DOI] [PubMed] [Google Scholar]
- 28.Senneville E, Lombart A, Beltrand E, et al. Outcome of diabetic foot osteomyelitis treated nonsurgically: a retrospective cohort study. Diabetes Care. 2008;31(4):637–642. doi: 10.2337/dc07-1744 [DOI] [PubMed] [Google Scholar]
- 29.Valabhji J, Oliver N, Samarasinghe D, Mali T, Gibbs RG, Gedroyc WM. Conservative management of diabetic forefoot ulceration complicated by underlying osteomyelitis: the benefits of magnetic resonance imaging. Diabet Med. 2009;26(11):1127–1134. doi: 10.1111/j.1464-5491.2009.02828.x [DOI] [PubMed] [Google Scholar]
- 30.Jordano-Montanez Q, Muniz-Tatay M, Viade-Julia J, et al. [Diabetic foot osteomyelitis: is conservative treatment possible?]. Enferm Infecc Microbiol Clin. 2014;32(9):555–559. doi: 10.1016/j.eimc.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 31.Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism–prognostic factors for outcome. Saudi Med J. 2007;28(2):225–230. [PubMed] [Google Scholar]
- 32.Malabu UH, Al-Rubeaan KA, Al-Derewish M. Diabetic foot osteomyelitis: usefulness of erythrocyte sedimentation rate in its diagnosis. West Afr J Med. 2007;26(2):113–116. [PubMed] [Google Scholar]
- 33.Ertugrul BM, Savk O, Ozturk B, Cobanoglu M, Oncu S, Sakarya S. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15(6):CR307–CR312. [PubMed] [Google Scholar]
- 34.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med. 1970;282(6):316–322. doi: 10.1056/NEJM197002052820606 [DOI] [PubMed] [Google Scholar]
- 35.Conterno LO, Da Silva Filho CR. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev. 2009;3:CD004439. [DOI] [PubMed] [Google Scholar]
- 36.Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393–407. doi: 10.1093/cid/cir842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senneville E, Robineau O. Treatment options for diabetic foot osteomyelitis. Expert Opin Pharmacother. 2017;18(8):759–765. doi: 10.1080/14656566.2017.1316375 [DOI] [PubMed] [Google Scholar]
- 38.Lavigne JP, Sotto A. Microbial management of diabetic foot osteomyelitis. Future Microbiol. 2017;12:1243–1246. doi: 10.2217/fmb-2017-0174 [DOI] [PubMed] [Google Scholar]
- 39.Venkatesan P, Lawn S, Macfarlane RM, Fletcher EM, Finch RG, Jeffcoate WJ. Conservative management of osteomyelitis in the feet of diabetic patients. Diabet Med. 1997;14(6):487–490. doi: [DOI] [PubMed] [Google Scholar]
- 40.Senneville E, Yazdanpanah Y, Cazaubiel M, et al. Rifampicin-ofloxacin oral regimen for the treatment of mild to moderate diabetic foot osteomyelitis. J Antimicrob Chemother. 2001;48(6):927–930. [DOI] [PubMed] [Google Scholar]
- 41.Boselli E, Allaouchiche B. [Diffusion in bone tissue of antibiotics]. Presse Med. 1999;28(40):2265–2276. [PubMed] [Google Scholar]
- 42.Tone A, Nguyen S, Devemy F, et al. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: a multicenter open-label controlled randomized study. Diabetes Care. 2015;38(2):302–307. doi: 10.2337/dc14-1514 [DOI] [PubMed] [Google Scholar]
- 43.van Asten SAV, Mithani M, Peters EJG, La Fontaine J, Kim PJ, Lavery LA. Complications during the treatment of diabetic foot osteomyelitis. Diabetes Res Clin Pract. 2018;135:58–64. doi: 10.1016/j.diabres.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 44.Vouillarmet J, Moret M, Morelec I, Michon P, Dubreuil J. Application of white blood cell SPECT/CT to predict remission after a 6 or 12 week course of antibiotic treatment for diabetic foot osteomyelitis. Diabetologia. 2017;60(12):2486–2494. doi: 10.1007/s00125-017-4417-x [DOI] [PubMed] [Google Scholar]
- 45.Allahabadi S, Haroun KB, Musher DM, Lipsky BA, Barshes NR. Consensus on surgical aspects of managing osteomyelitis in the diabetic foot. Diabet Foot Ankle. 2016;7:30079. doi: 10.3402/dfa.v7.30079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van GH, Siney H, Danan JP, Sachon C, Grimaldi A. Treatment of osteomyelitis in the diabetic foot: contribution of conservative surgery. Diabetes Care. 1996;19(11):1257–1260. [DOI] [PubMed] [Google Scholar]
- 47.Aragon-Sanchez J, Lazaro-Martinez JL, Alvaro-Afonso FJ, Molines-Barroso R. Conservative surgery of diabetic forefoot osteomyelitis: how can i operate on this patient without amputation? Int J Low Extrem Wounds. 2015;14(2):108–131. doi: 10.1177/1534734614550686 [DOI] [PubMed] [Google Scholar]
- 48.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. J Am Podiatr Med Assoc. 2013;103(1):2–7. [DOI] [PubMed] [Google Scholar]
- 49.Aragon-Sanchez J. Treatment of diabetic foot osteomyelitis: a surgical critique. Int J Low Extrem Wounds. 2010;9(1):37–59. doi: 10.1177/1534734610361949 [DOI] [PubMed] [Google Scholar]
- 50.Aragon-Sanchez FJ, Cabrera-Galvan JJ, Quintana-Marrero Y, et al. Outcomes of surgical treatment of diabetic foot osteomyelitis: a series of 185 patients with histopathological confirmation of bone involvement. Diabetologia. 2008;51(11):1962–1970. doi: 10.1007/s00125-008-1131-8 [DOI] [PubMed] [Google Scholar]
- 51.Piaggesi A, Schipani E, Campi F, et al. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabet Med. 1998;15(5):412–417. doi: [DOI] [PubMed] [Google Scholar]
- 52.Aragon-Sanchez J, Lazaro-Martinez JL, Hernandez-Herrero C, et al. Does osteomyelitis in the feet of patients with diabetes really recur after surgical treatment? Natural history of a surgical series. Diabet Med. 2012;29(6):813–818. doi: 10.1111/j.1464-5491.2011.03528.x [DOI] [PubMed] [Google Scholar]
- 53.Atway S, Nerone VS, Springer KD, Woodruff DM. Rate of residual osteomyelitis after partial foot amputation in diabetic patients: a standardized method for evaluating bone margins with intraoperative culture. J Foot Ankle Surg. 2012;51(6):749–752. doi: 10.1053/j.jfas.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 54.Tan JS, Friedman NM, Hazelton-Miller C, Flanagan JP, File TM Jr. Can aggressive treatment of diabetic foot infections reduce the need for above-ankle amputation? Clin Infect Dis. 1996;23(2):286–291. [DOI] [PubMed] [Google Scholar]
- 55.Fujii M, Terashi H, Yokono K. Surgical treatment strategy for diabetic forefoot osteomyelitis. Wound Repair Regen. 2016;24(2):447–453. doi: 10.1111/wrr.12418 [DOI] [PubMed] [Google Scholar]
- 56.Shaikh N, Vaughan P, Varty K, Coll AP, Robinson AH. Outcome of limited forefoot amputation with primary closure in patients with diabetes. Bone Joint J. 2013;95-B(8):1083–1087. doi: 10.1302/0301-620X.95B8.31280 [DOI] [PubMed] [Google Scholar]
- 57.Widatalla AH, Mahadi SE, Shawer MA, Mahmoud SM, Abdelmageed AE, Ahmed ME. Diabetic foot infections with osteomyelitis: efficacy of combined surgical and medical treatment. Diabet Foot Ankle 2012;3:1. doi: 10.3402/dfa.v3i0.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Morales E, Lazaro-Martinez JL, Aragon-Sanchez J, Cecilia-Matilla A, Garcia-Alvarez Y, Beneit-Montesinos JV. Surgical complications associated with primary closure in patients with diabetic foot osteomyelitis. Diabet Foot Ankle. 2012;3:1. doi: 10.3402/dfa.v3i0.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tardaguila-Garcia A, Sanz-Corbalan I, Molines-Barroso RJ, Alvaro-Afonso FJ, Garcia-Alvarez Y, Lazaro-Martinez JL. Complications associated with the approach to metatarsal head resection in diabetic foot osteomyelitis. Int Wound J. 2019;16(2):467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lesens O, Desbiez F, Theis C, et al. Staphylococcus aureus-related diabetic osteomyelitis: medical or surgical management? A French and Spanish retrospective cohort. Int J Low Extrem Wounds. 2015;14(3):284–290. doi: 10.1177/1534734614559931 [DOI] [PubMed] [Google Scholar]
- 61.Game F, Jeffcoate W. MRSA and osteomyelitis of the foot in diabetes. Diabet Med. 2004;21(Suppl 4):16–19. doi: 10.1111/j.1464-5491.2004.1424-8.x [DOI] [PubMed] [Google Scholar]
- 62.Widatalla AH, Mahadi SE, Shawer MA, Mahmoud SM, Abdelmageed AE, Ahmed ME. Diabetic foot infections with osteomyelitis: efficacy of combined surgical and medical treatment. Diabetic Foot Ankle. 2012;3:18809. doi: 10.3402/dfa.v3i0.18809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sagray BA, Malhotra S, Steinberg JS. Current therapies for diabetic foot infections and osteomyelitis. Clin Podiatr Med Surg. 2014;31(1):57–70. doi: 10.1016/j.cpm.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 64.Richard JL, Lavigne JP, Sotto A. Diabetes and foot infection: more than double trouble. Diabetes Metab Res Rev. 2012;28(Suppl 1):46–53. doi: 10.1002/dmrr.2234 [DOI] [PubMed] [Google Scholar]
- 65.Bakker K, Schaper NC; International Working Group on Diabetic Foot Editorial B. The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28(Suppl 1):116–118. doi: 10.1002/dmrr.2254 [DOI] [PubMed] [Google Scholar]
- 66.Game FL. Osteomyelitis in the diabetic foot: diagnosis and management. Med Clin North Am. 2013;97(5):947–956. doi: 10.1016/j.mcna.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 67.Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev. 2013;8:CD006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dumville JC, Hinchliffe RJ, Cullum N, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD010318. [DOI] [PubMed] [Google Scholar]
- 69.Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev. 2009;3:CD006810. [DOI] [PubMed] [Google Scholar]
- 70.Margolin L, Gialanella P. Assessment of the antimicrobial properties of maggots. Int Wound J. 2010;7(3):202–204. doi: 10.1111/j.1742-481X.2010.00234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun X, Jiang K, Chen J, et al. A systematic review of maggot debridement therapy for chronically infected wounds and ulcers. Int J Infect Dis. 2014;25:32–37. doi: 10.1016/j.ijid.2014.03.1397 [DOI] [PubMed] [Google Scholar]
- 72.Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010;1:CD003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barth RE, Vogely HC, Hoepelman AI, Peters EJ. ‘To bead or not to bead?’ Treatment of osteomyelitis and prosthetic joint-associated infections with gentamicin bead chains. Int J Antimicrob Agents. 2011;38(5):371–375. doi: 10.1016/j.ijantimicag.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 74.Roeder B, Van Gils CC, Maling S. Antibiotic beads in the treatment of diabetic pedal osteomyelitis. J Foot Ankle Surg. 2000;39(2):124–130. [DOI] [PubMed] [Google Scholar]
- 75.Kumari S, Harjai K, Chhibber S. Efficacy of bacteriophage treatment in murine burn wound infection induced by klebsiella pneumoniae. J Microbiol Biotechnol. 2009;19(6):622–628. [DOI] [PubMed] [Google Scholar]
- 76.Mendes JJ, Leandro C, Mottola C, et al. In vitro design of a novel lytic bacteriophage cocktail with therapeutic potential against organisms causing diabetic foot infections. J Med Microbiol. 2014;63(Pt 8):1055–1065. doi: 10.1099/jmm.0.071753-0 [DOI] [PubMed] [Google Scholar]
- 77.Panagopoulos P, Drosos G, Maltezos E, Papanas N. Local antibiotic delivery systems in diabetic foot osteomyelitis: time for one step beyond? Int J Low Extrem Wounds. 2015;14(1):87–91. doi: 10.1177/1534734614566937 [DOI] [PubMed] [Google Scholar]
- 78.Markakis K, Faris AR, Sharaf H, Faris B, Rees S, Bowling FL. Local antibiotic delivery systems: current and future applications for diabetic foot infections. Int J Low Extrem Wounds. 2018;17(1):14–21. doi: 10.1177/1534734618757532 [DOI] [PubMed] [Google Scholar]
- 79.Senneville E, Melliez H, Beltrand E, et al. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: concordance with ulcer swab cultures. Clin Infect Dis. 2006;42(1):57–62. doi: 10.1086/498112 [DOI] [PubMed] [Google Scholar]
- 80.Richard JL, Lavigne JP, Got I, et al. Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study. Diabetes Metab. 2011;37(3):208–215. doi: 10.1016/j.diabet.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 81.Schaper NC, Apelqvist J, Bakker K. Reducing lower leg amputations in diabetes: a challenge for patients, healthcare providers and the healthcare system. Diabetologia. 2012;55(7):1869–1872. doi: 10.1007/s00125-012-2588-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molines-Barroso RJ, Lazaro-Martinez JL, Alvaro-Afonso FJ, Sanz-Corbalan I, Garcia-Klepzig JL, Aragon-Sanchez J. Validation of an algorithm to predict reulceration in amputation patients with diabetes. Int Wound J. 2017;14(3):523–528. doi: 10.1111/iwj.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aragon-Sanchez J. Seminar review: a review of the basis of surgical treatment of diabetic foot infections. Int J Low Extrem Wounds. 2011;10(1):33–65. doi: 10.1177/1534734611400259 [DOI] [PubMed] [Google Scholar]
- 84.Armstrong DG, Lipsky BA. Diabetic foot infections: stepwise medical and surgical management. Int Wound J. 2004;1(2):123–132. doi: 10.1111/j.1742-4801.2004.00035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazaro-Martinez JL, Aragon-Sanchez J, Alvaro-Afonso FJ, Garcia-Morales E, Garcia-Alvarez Y, Molines-Barroso RJ. The best way to reduce reulcerations: if you understand biomechanics of the diabetic foot, you can do it. Int J Low Extrem Wounds. 2014;13(4):294–319. doi: 10.1177/1534734614549417 [DOI] [PubMed] [Google Scholar]