Abstract
Objective
The diagnosis of fetal akinesia deformation sequence (FADS) is a challenge. Motor assessment is of additional value to advanced ultrasound examinations (AUE) for in utero FADS diagnosis before 24 weeks of gestation.
Methods
All consecutive fetuses with greater than or equal to two contractures on the 20 week structural anomaly scan (2007–2016) were included. Findings at AUE, including motor assessment were analysed and related to outcome.
Results
Sixty‐six fetuses fulfilled the inclusion criteria. On the basis of the first AUE, FADS was suspected in 13 of 66, arthrogryposis multiplex congenita (AMC) in 12 of 66, bilateral pes equinovares (BPEV) in 40 of 66, and Holt‐Oram syndrome in one of 66. On the basis of the first motor assessment, the suspected diagnosis changed in 19 of 66, in 13 of 66 worsening to FADS, six of 66 amelioration from FADS, and confirmed FADS in seven of 13. The result was 20 FADS, seven AMC, and 38 BPEV. Second AUE in 44 fetuses showed additional contractures in two of eight FADS, and one intrauterine fetal death (IUFD). The second motor assessment changed the diagnosis in three of 43, one worsening from BPEV into FADS, two ameliorations from FADS, and confirmed FADS in seven by deterioration of motility. The result was nine FADS, six AMC, and 29 BPEV.
Conclusion
The results suggest that motor assessment has additional value to distinguish between FADS, AMC, and BPEV.
Short abstract
Systematic motor assessment for differentiation, quality, and quantity of the movements has been performed in a high risk population for FADS.1
This analysis revealed abnormal quality in all fetuses with deterioration in a 2‐week period, reduced differentiation in the majority and only half showed abnormal quantity.
This study evaluates whether motor assessment in addition to advanced ultrasound examination in case of multiple contractures, supports the timely diagnosis of FADS.
What's known:
Systematic motor assessment for differentiation, quality, and quantity of the movements has been performed in a high‐risk population for FADS.1 Families with recurrent FADS were examined every 2 weeks from 12 to 24 weeks of gestation. The fetuses suspected of FADS were compared with a normal population and abnormalities in motor assessment were used to detect recurrent disease. This analysis revealed abnormal quality in all fetuses with deterioration in a 2 week period, reduced differentiation in the majority and only half showed abnormal quantity.
What's new:
This study evaluates whether motor assessment, in addition to advanced ultrasound examination in case of multiple contractures, supports the timely diagnosis of FADS. All consecutive fetuses with more than two contractures on the 20‐week structural anomaly scan were included; findings at AUE, including motor assessment, were analysed and related to outcome. It is the first study that uses motor assessment to make a distinction in the diagnosis between BPEV, AMC, and FADS.
1. INTRODUCTION
Prenatal detection of the phenotype fetal akinesia deformation sequence (FADS) through ultrasound examination has been reported as early as the first trimester of pregnancy, but also during the third trimester with varied expressions, making diagnosis challenging.1, 2, 3 Arthrogryposis multiplex congenita (multiple contractures in various regions, AMC) including FADS was missed in 73.8% during prenatal ultrasound examination in 107 cases with neonatal confirmed arthrogryosis.4
Sonographically prenatal diagnosis of FADS is based on multiple contractures, reduced motility, flattening of facial profile and—with increasing gestational age—growth restriction, reduced cardiothoracic ratio, and polyhydramnios.1, 4, 5, 6 Although FADS is considered to be an expression of various autosomal recessive disorders, DNA‐diagnostic possibilities during pregnancy are still limited.4 Single clubfeet to AMC can be seen at the onset of FADS. Clubfeet, multiple contractures, and FADS have a prevalence of 1 to 3:1000,7 1:3000 to 5000, and 1:13 000 pregnancies, respectively.5 The prognosis of FADS is dependent on its cause. Approximately 30% are stillborn, the majority of live‐born infants die of pulmonary hypoplasia.8
Abnormal motility has been reported when FADS was suspected.1, 9, 10, 11, 12, 13 Systematic motor assessment for differentiation into specific movement patterns, quality of general movements, isolated arm/leg movements, and quantity of general movements have been performed in a high‐risk population for FADS, irrespective of gestational age in our multidisciplinary expert centre: prenatal centre for diagnosing neuromuscular disorders, in particular FADS.1 This analysis revealed abnormal quality in all fetuses with deterioration in a 2‐week period, reduced differentiation in the majority, and only half showed abnormal quantity.
The present study evaluates whether motor assessment, in addition to advanced ultrasound examination (AUE) in case of multiple contractures, supports the timely prenatal diagnosis of FADS before 24 weeks of gestation. All consecutive fetuses with multiple contractures will be presented together with their outcome and follow‐up examinations since the introduction of the 20‐week standard anomaly (2007) from one tertiary centre in the Netherlands. We expected that additional single and/or repeated sonographic motor assessment would support the distinction between the phenotypes AMC and FADS before 24 weeks of gestation, the utmost gestational age at which termination of pregnancy (TOP) is permitted in the Netherlands.
2. METHODS
2.1. Population
Women who underwent an AUE for multiple contractures in the tertiary hospital VU University Medical Centre during the period from 2007 to 2016 were extracted from the digitalized ultrasound database, ASTRAIA Software GmbH, Copyright 2000‐2016©, version 1.24.8 P1 54095.3245. The database was searched for the following terms: contracture(s), FADS, Pena‐Shokeir (prior nomenclature of FADS), limb deformity, AMC, and hypokinesia. The inclusion criteria were; more than one contracture at the 20‐week standard anomaly scan and/or AUE and availability of a motor assessment. Data were stored in an anonymized digital database containing the results of the AUE, the postural and motor assessment together with the outcome measures, and if known, the underlying disease. Approval of the local research ethics committee was obtained from the Amsterdam UMC, location VUmc. Ethical approval was waived by the ethics committee of the Amsterdam UMC, location VUmc since it is a retrospective study without patient identifiers.
2.2. Definitions of FADS, AMC, and bilateral clubfeet
At the first and second structural assessment, bilateral pes equinovares (BPEV) was defined as bilateral clubfeet without contractures in other regions or structural abnormalities related to FADS, eg, polyhydramnios, IUGR, flattened face profile, and/or increased cardiothoracic ratio. AMC was defined as multiple contractures in more than one region, eg, arms and legs, without other structural abnormalities related to FADS. FADS was defined as contractures in combination with other structural abnormalities related to FADS.
At the first and second motor assessment, the information of abnormal motility in addition to the structural assessment was used to confirm the diagnosis of FADS based on the structural assessment or change the diagnosis of AMC/BPEV into FADS. Suspect motility at the motor assessment did not change the diagnosis based on the structural assessment. For classification of normal, suspect, and abnormal motility, see Table 1.
Table 1.
The motor assessment adapted from Donker et al.1
| Description | Normal | Abnormal | FADS suspected | |
|---|---|---|---|---|
| Differentiation into specific movement patterns (SMPs) | SMPs are:‐ general movement (GM)‐ isolated arm movement (IAM)‐ isolated leg movement (ILM),‐ breathing movement‐ hand–face contact, startle ‐ hiccup, jaw opening, sucking and swallowing, stratching, isolated retroflexion head, isolated anteflexion head, isolated rotation head, yawning, twitch spine | ≥8 SMPs in a 15‐minute observation |
<8 SMPs in 15‐minute observation (FADS fetuses had 7+/−2 SMPs) |
+/− |
| Quality |
Quality of GM, IAM, and ILM The six aspects:1. Apmlitude 2. Speed 3. Participanting body parts 4. Complexity in the direction 5. Fluent movements 6. Waxing and waning |
Variation in all aspects. If the IAMs/ILMs are normal, except for the affected joints, the quality is also considered normal. |
Suspect:No variation in one or two aspects. GM abnormal: No variation in three or four aspects for GM. ILM/IAM abnormal: No variation in two aspects for ILM/IAM. |
+ |
| Quantity | Quantity of the movements | GMs above the 10th percentile from a normal population examined throughout gestation 8 to 40 weeks14 | GMs below the 10th percentile of the normal population14 | +/− |
+: yes, FADS suspected +/−: only for the severity of FADS.
2.3. Motor assessment
The systematic motor assessment is performed during a 15‐minute ultrasound examination. The observation time was doubled in case of absence of motility during 13 minutes or more, since this is the maximal pause between consecutive movements in a normal population at this gestational age.13 During the examination, the investigator holds the probe still, visualizing the head/jaw, trunk, and part of the arm and leg of the fetus. The evaluation consists of three aspects: (1) observation of the differentiation into specific movement patterns, (2) quality of general movements, isolated arm and leg movements, and (3) quantity of movements. The evaluation is adapted from Donker et al1 (Table 1).
The phenotype FADS was diagnosed if the quality of the motility was abnormal, with or without reduced differentiation into specific movement patterns or quantity. From the 10‐year cohort of Donker et al,1 we learned that in FADS cases, the quality was abnormal in all, however, not the quantity (only in half) and differentiation. Still, in case of severe deteriorated FADS, examination of the differentiation and quantity supported to diagnose FADS. In two cases, the quality could not be evaluated since general movements were absent.
The motor assessment was recorded digitally to facilitate offline examination. After 2 weeks, the motor assessment was repeated until 24 weeks of gestation to detect changes over time (deterioration, amelioration, and stability). The recordings and assessments were performed by dedicated advanced ultrasound MD's; van der Knoop, Adriaanse, Burger, or Schreurs, and all recordings were assessed and consented to by de Vries. We have reported good inter‐observer agreement in pregnancies and fetuses affected with FADS concerning the three motor aspects, differentiation into specific movement patterns Cohen's kappa 0.95, movement quality Cohen's kappa 0.83, and on quantity of general movements Pearson correlation r = 0.88.1
2.4. Postural and other ultrasound findings related to FADS
The following findings were stored from all fetuses; contractures in the upper and/or lower limbs, facial anomalies, increased cardiothoracic circumference ratio (CT‐ratio), and presence of polyhydramnios. The face was examined for micro‐ and/or retrognathia, flattened nose, and reduced prominence of the lips.
2.5. Outcome measures
The structural and motor assessment of all cases were discussed by a multidisciplinary team consisting of the prenatal staff of obstetricians, neonatologist, sonographers, clinical geneticist, and child neurologist. The parents were offered consultations with the clinical geneticist, child neurologist, or orthopaedic surgeon if applicable, and were counselled by the obstetrician with the obtained knowledge on the prognosis, and decided for continuation or TOP and location of delivery. Outcome parameters were: live born infant, results of paediatric, orthopaedic, and if available, neurologic evaluation. In case of a TOP or perinatal death, post‐mortem examination was offered. The results of the consultation with the clinical geneticist together with the chromosome and DNA‐investigations were, if available, recorded. The outcome of the pregnancy and outcome of the child was derived from the medical chart of the women giving birth in our hospital. In case of delivery elsewhere, information about the pregnancy outcome was sent to our clinic by the referring hospital and recorded in Astraia.
2.6. Confirmation of diagnosis
The prenatal diagnosis was examined postnatally by the different disciplines. The living neonates were seen by the paediatrician and orthopaedic surgeon for physical examination of the contractures. In case of TOP or perinatal death, the diagnosis was investigated by the pathologist and clinical geneticist when permitted by the parents. The evaluation of the contractures of the joints in combination with the dysmorphologic features together with the histologic and genetic evaluations led to the diagnosis FADS, AMC, or BPEV.
2.7. Descriptive statistics
To examine whether motor assessment had additional value in the distinction between BPEV, AMC, and FADS, the diagnoses are presented in four steps. They were based on the: (1) the first structural AUE, (2) additional first motor assessment, (3) 2 weeks later second structural AUE, and (4) the second motor assessment, also 2 weeks later. Confirmation of the prenatal diagnosis was based on the various outcome parameters described above.
3. RESULTS
3.1. Study population
A total of 260 ultrasound recordings matched the search containing 140 fetuses from 128 women. Of the 140 cases, 74 were excluded because they did not meet the criteria. The prenatal diagnosis of these 74 fetuses, together with their outcome, are presented in Table 2. In 16 of 74 cases, no motor assessment had been performed, no other anomalies were suspected, and one developed FADS, a stillborn boy was delivered at 30 weeks. In the same time period, another fetus suspected of FADS presented itself at 35 weeks. The fetus, however, did not fulfil the inclusion criteria of the present study, since no contractures were observed at the 20 weeks structural anomaly scan. The case was referred to our hospital at 35 weeks for polyhydramnios, limited stomach filling, and reduced motility. The mother had experienced reduced fetal movements from 24 weeks onwards. Motor assessment at 35 weeks was suspect for FADS. At 35 weeks and 3 days a stillborn boy was born. FADS was confirmed at post‐mortem examination.
Table 2.
Prenatal diagnoses and outcome of the excluded fetuses with AUE before 24 weeks for contractures
| Reason of Exclusion | Suspected Diagnosis Prenatally | Number | Confirmation | Outcome |
|---|---|---|---|---|
| Single contractures | Single contractures | 27 | 27 | 27 walking |
| No motor assessment | Bilateral pes equinovares | 14 | 13 | 13 walking |
| 1 FADS | 30 weeks stillborn | |||
| Motor assessment after 24 weeks |
Arthrogryposis multiplex congenita Bilateral pes equinovares |
2 2 |
2 2 |
one congenital myotonic dystrophia with mental retardation and delay in motor development, able to walk (with buggy) one intrauterine fetal death two walking |
| Other suspected diagnosis | Trisomy 18 | 11 | 11 | Termination of pregnancy (TOP) |
| Skeletal dysplasia | 5 | 5 | TOP | |
| Omphalocele‐exstrophy imperforate anus‐spinal defects | 3 | 3 | TOP | |
| Trisomy 13 | 2 | 2 | TOP | |
| Spina bifida, ventriculomegaly and bilateral pes equinovares | 1 | 1 | TOP | |
| Spina bifida with contractures in hands and pes equinovares bilaterally | 1 | 1 | TOP | |
| Hydrancephaly | 1 | 1 | TOP | |
| Exencephaly | 1 | 1 | TOP | |
| Potter sequence | 1 | 1 | TOP | |
| Abdominal cyst with contractures of the legs | 1 | 1 | TOP | |
| Mucopolysaccharidosis type 7 | 1 | 1 | TOP | |
| Unbalanced translocation 9 and 12 | 1 | 1 | TOP |
The final study population consisted of 66 fetuses from 65 women (median age 32.2 years; range 21.1‐43.8 years). Consanguinity was present in four couples (three BPEV and one FADS), absent in 50, and unknown in 11. The mean gestational age at the first assessment was 20 weeks, the mean gestational age at the second assessment was 22 weeks and 1 day.
3.2. Advanced ultrasound examination and motor assessment
The distribution of the phenotypes BPEV, FADS, and AMC are presented per examination, together with the changes over time (see Figure 1). First step, based on the structural AUE, with observation of the contractures (44 BPEV, one BPEV together with hip or knee, 15 in upper and lower limbs, four in wrist and elbows/shoulders, two in wrists), flattening of the facial profile (n = 9), polyhydramnios (n = 2), fetal hydrops (n = 3), and increased CT ratio (n = 2). FADS was suspected in 13 of 66 cases, AMC in 12 of 66, BPEV in 40 of 66, and Holt‐Oram Syndrome in one of 66. The latter was confirmed genetically and is not included in Figure 1.
Figure 1.
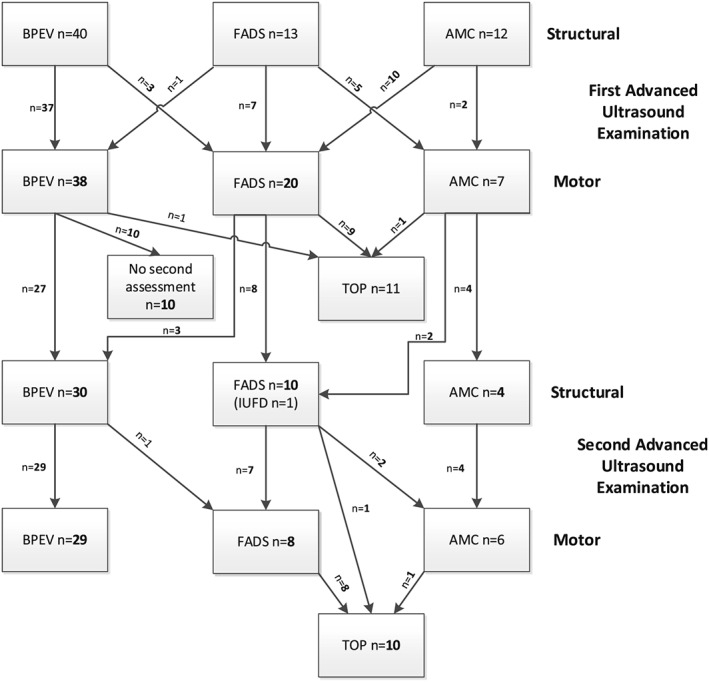
Suspected diagnosis based on the advanced ultrasound examination and changes in diagnosis based on the motor assessments, with the decision on termination or continuation of the pregnancy. AMC, arthrogryposis multiplex congenita; FADS, fetal akinesia deformation sequence; BPEV, bilateral pes equinovares; TOP, termination of pregnancy; IUFD, intrauterine fetal death
The second structural AUE twice demonstrated progression in contractures, one from contractures in wrist, elbow/shoulder and one from BPEV and knee/hip to contractures in upper and lower limbs. The AUE of the fetus are provided for in Table A1.
3.3. Motor assessment
At the first motor assessment, nine of 66 showed reduced differentiation, 18 of 66 reduced quality, and nine of 66 reduced quantity. The second motor assessment showed reduced differentiation in seven of 43, reduced quality in eight of 43, and reduced quantity in three of 43. Individual motor assessments are presented in Appendix 1.
In three of 40, BPEV was changed to FADS after the first motor assessment because of abnormal quality in all. FADS was changed to AMC in five of 13 because of normal quality, differentiation, and quantity in all. AMC was changed to FADS in 10 of 13 because of abnormal quality in all, abnormalities in all aspects in one of 10, and abnormal quality and differentiation in one of 10; besides this, reduced quantity was seen in two of 10.
At the second motor assessment, the quality was progressively abnormal in eight cases. Reduction of amplitude, speed, and participation was seen in all, reduction in waxing and waning of the general movement was seen in five of eight fetuses, and reduced fluency in one of eight. However, in one of these FADS cases, the hypokinetic movements alternated with hyperkinetic movements with increased twitches and abrupt general movements. In four cases, quality and differentiation could not be assessed because the quantity of the movements was too low.
At the second motor assessment, BPEV was changed to FADS in one of 30 because of abnormal quality (see Figure 1). FADS was changed to AMC in two of 10 because of reduced variability of the quality but not in the pattern known for FADS, but the majority of general movements, isolated arm and leg movements were performed without fluency.
In two cases three motor assessments could be performed before 24 weeks gestational age, the results of the third examination were in line with the second motor assessment, and did not change the diagnoses.
Late referral gave no time before 24 weeks for repeated motor assessment after 2 weeks in 10 cases. Because of a reassuring first assessment and no other signs of FADS, the pregnancies were continued. None in this group developed FADS, and BPEV was confirmed in all after birth.
One TOP was performed in a fetus with BPEV after the first structural and motor assessment; motor assessment demonstrated normal motility. The diagnosis, BPEV was confirmed by autopsy after TOP.
The pregnancy outcome was available for all fetuses and the prenatal diagnosis of FADS, AMC, and BPEV or other contractures at the latest examination were confirmed by postnatal examination in all fetuses. In four cases, an underlying diagnosis was found in addition to BPEV (spinal muscular atrophy, Down's syndrome, Zellweger syndrome, and lissencephaly). A detailed description with the phenotype suspected prenatally, decision concerning the pregnancy, diagnosis postnatally, the investigation, and consultations after birth are depicted for all the cases in Table 3. In six of 18 FADS, genetic examination showed an underlying cause; MUSK mutation, SCN4A mutation (n = 2), RyR1 mutation (n = 2), and diastrophic dysplasia. In three of seven fetuses with AMC, an underlying genetic cause was found; nemaline myopathy, a TGFBR1 gene mutation, and a PIEZO2‐gene mutation. Intra uterine fetal death (IUFD) occurred in one fetus with FADS, one with lissencephaly and BPEV, and one with trisomy 21 and BPEV. The follow‐up of the living children is described in Table 4.
Table 3.
Outcome of the living infants with bilateral pes equinovares (BPEV) and arthrogryposis multiplex congenita (AMC)
| Living | Confirmation After Birth | Orthopaedic Correction | Walking | Other diagnoses |
|---|---|---|---|---|
| BPEV n = 44 |
43 BPEV one single PEV |
41 1 |
41 1 |
1 spinal muscular atrophy type 1, died 3 months after birth 1 Zellweger syndrome, not walking at 3 years, dependent on care for all daily life activities, treated by rehabilitation team, paediatrician and child neurologist, disease progresses 1 down syndrome 1 lissencephaly, intrauterine fetal death None |
| AMC n = 5 | All |
1. No 2. Yes 3. No 4. No 5. In the future knee operations |
With braces Yes, braces for arms With braces Yes Unknown yet |
None None Nemaline myopathy. Trachea canule required, recurrent lung infections, delayed motor development at 6 years, special school. De novo mutation in TGFBR1, possible coherent with Shprintzen‐Goldberg syndrome, walks and cycles, attends regular school, independent for daily life activities. PIEZO2‐gene mutations, tube feeding, delayed growth, |
Table 4.
Outcome after birth based on orthopaedic, post‐mortem and genetic examination and evaluation
| Number | Diagnosis After Birth | Orthopaedic Surgeon | Pathologist (Findings in Addition to Ultrasound) | Clinical Geneticist | Birthweight in Gram | Apgar After 5 min |
|---|---|---|---|---|---|---|
| 1 | BPEV | Ponseti method | ‐ | ‐ | 2255 | 9 |
| 2 | BPEV | Ponseti method | ‐ | ‐ | 2935 | 10 |
| 3 | BPEV | Ponseti method | ‐ | ‐ | 3295 | 10 |
| 4 | BPEV | Ponseti method | ‐ | ‐ | 2818 | 10 |
| 5 | BPEV | Ponseti method | ‐ | ‐ | 3375 | 10 |
| 6 | BPEV | Ponseti method with surgery | ‐ | ‐ | 3690 | 10 |
| 7 | BPEV | Ponseti method | ‐ | ‐ | 2960 | 10 |
| 8 | BPEV | Ponseti method with surgery | ‐ | ‐ | 3490 | 10 |
| 9 | FADS | ‐ | Low implant of the ears, short and deep implant of the nose bridge, micro‐, retrognathia, webbing and contractures of all joints, both hands and feet arachnodactyly, syndactyly, palatal schisms, and small thorax;Normal internal organs, lung hypoplasiaX‐ray: No signs of dysplasiaBrain dissection: Normal | No gene mutations found | 303 | 0 |
| 10 | FADS | ‐ | Contractures elbows, hips and left knee, small pointy chin, and dystrophic musclesNormal internal organsBrain: Possibly micro gyri | No gene mutations found | 642 | 0 |
| 11 | FADS | ‐ | Relatively small hart | No gene mutations found | 605 | 0 |
| 12 | FADS | ‐ | No post‐mortem examinationX‐ray: No signs of dysplasia | No gene mutations found | 248 | 0 |
| 13 | AMC | ‐ | Horseshoe kidney, palatal schisms | No gene mutations found | 570 | 0 |
| 14 | FADS, underlying diagnosis diastrophic dysplasia | ‐ | Short limbs, elbows dislocated, hitchhiker‐thumbs, ulnar deviation fingers, scoliosis;Normal internal organsX‐ray: Short limbs |
Diastrophic dysplasia, Compound heterozygous mutation in SLC26A2‐gene: c.931 T > C and c.1957 T > A |
655 | 0 |
| 15 | FADS | ‐ | Retrognathia, unibrow, small pelvis.Normal internal organsmuscle biopsy: Increased centrally localised nuclei, possibly tubular myopathy Brain dissection: Normal | No gene mutations found | 404 | 0 |
| 16 | FADS | ‐ | Low implant of the ears and rotated backwards, nose bridge broadened;two lobes in right lung, with rudimentary middle lobe, oval shaped kidney with hilum dorsally rotated, ectopic adrenal gland in thymus, haemorrhage in both hemispheresX‐ray: No signs of dysplasiaBrain dissection: NormalMuscle biopsy: myopathic pattern with reduced type 1 fibres | No gene mutations found | 860 | 0 |
| 17 | FADS | ‐ | Low implant of the ears, hypertelorism, contractures in elbow on left sided with webbing, knees;Unicornuate uterus, right lung incomplete septationX‐ray: No signs of dysplasiabrain dissection: Normal | Homozygous missense variant in MUSK gene, of unknown significance | 275 | 0 |
| 18 | FADS | ‐ | Micro‐, retrognathia, low implant ears, flat nose, webbing, growth restriction | Heterozygous mutation in NEB‐gene, probably non‐pathogenic | 105 | 0 |
| 19 | FADS | ‐ | Hypertelorism, retrognathia, short eyelids, high nose bridge, broad nose tip, thin upper lip, low implant of the ears and rotated backwards, atrophic muscles; Normal internal organsX‐ ray: Mirror polydactyly right foot, tibia aplasia Brain dissection: Normal | No gene mutations found | 495 | 0 |
| 20 | FADS | ‐ | Normal internal organsX‐ray: No signs of dysplasiaBrain dissection: Normal | No gene mutations found | 150 | 0 |
| 21 | AMC | Ponseti method, wrist extension bars, eventually also elbow bars | ‐ | No gene mutations found | 2840 | 1 |
| 22 | AMC | Ponseti method with surgery, physiotherapy | ‐ | PIEZO2‐gene mutation | 2505 | 10 |
| 23 | FADS | ‐ | Contractures lower limbs, including webbing; Lung hypoplasia;Normal internal organs and nervous system | Homozygous pathogenic mutation in RyR1 gene | 3000 | 0 |
| 24 | FADS | ‐ | ‐ | Homozygous pathogenic mutation in RyR1 gene | 11 | 0 |
| 25 | FADS | ‐ | Broad fingertips, anteversion nostrils, micrognathia, long philtrum, low implant ears, broad neck, contractures ankles, fingers, hypotrophic musclesGrowth restrictionX‐ray: No signs of dysplasiaMuscle biopsy: myopathy suspected with type 2 atrophia | No gene mutations found | ‐ | 0 |
| 26 | AMC | ‐ | ‐ | Phenotypically fitting Amyoplasia | 85 | 0 |
| 27 | AMC | Physiotherapy | ‐ | No gene mutations found | 78 | 0 |
| 28 | BPEV | Ponseti method | ‐ | ‐ | 489 | 0 |
| 29 | BPEV | Ponseti method | ‐ | 2693 | 10 | |
| 30 | BPEV | Ponseti method | ‐ | ‐ | 3375 | 10 |
| 31 | BPEV | Ponseti method | ‐ | ‐ | 3830 | 10 |
| 32 | BPEV | Ponseti method with surgery | ‐ | ‐ | 3740 | 10 |
| 33 | BPEV | Ponseti method | ‐ | ‐ | 3595 | 1 |
| 34 | BPEV | Ponseti method | ‐ | ‐ | 2690 | 9 |
| 35 | BPEV | Ponseti method | ‐ | ‐ | 940 | 10 |
| 36 | BPEV | No treatment needed | ‐ | ‐ | 3861 | 10 |
| 37 | BPEV | Ponseti method | ‐ | ‐ | 3340 | 10 |
| 38 | BPEV | Ponseti method | ‐ | ‐ | 3202 | 10 |
| 39 | BPEV | Ponseti method with surgery | ‐ | ‐ | 2556 | 10 |
| 40 | BPEV | Ponseti method | ‐ | ‐ | 3290 | 10 |
| 41 | BPEV | Ponseti method | ‐ | 2930 | 9 | |
| 42 | BPEV | Ponseti method | ‐ | ‐ | 3333 | 10 |
| 43 | BPEV | Ponseti method with surgery | ‐ | ‐ | 3045 | 10 |
| 44 | Down syndrome, BPEV | ‐ | ‐ | Trisomy 21 | 2430 | 10 |
| 45 | BPEV | Ponseti method | ‐ | ‐ | 4169 | 10 |
| 46 | BPEV | Ponseti method | ‐ | ‐ | 2260 | 9 |
| 47 | BPEV | Ponseti method | ‐ | ‐ | 3340 | 10 |
| 48 | BPEV | Ponseti method | ‐ | ‐ | 3200 | 10 |
| 49 | BPEV | Ponseti method with surgery | ‐ | ‐ | 3320 | 10 |
| 50 | BPEV | Ponseti method | ‐ | ‐ | 2470 | 9 |
| 51 | Zellweger,BPEV | No treatment needed | ‐ | Compound heterozygote mutation in PEX1 gene | 2705 | 7 |
| 52 | BPEV | ‐ | ‐ | ‐ | 710 | 0 |
| 53 | Spinal muscular atrophy type 1, BPEV | ‐ | ‐ |
SMA type I, Homozygous SMN1 gene mutation |
3424 | 9 |
| 54 | FADS | ‐ | Low implant of the ears, whipped up noseNormal internal organs with malrotation of the intestinesMuscle biopsy: myopathic pattern X‐ray: 11 ribs, hypoplasia first pair, no ossification pubis | Homozygous pathogenic mutations in SCN4A | 570 | 0 |
| 55 | Lissencephaly,BPEV | ‐ | ‐ | ‐ | 2546 | 0 |
| 56 | FADS | ‐ | Small thymus, no spleen | No gene mutations found | ‐ | 0 |
| 57 | Holt‐Oram syndrome | ‐ | ‐ | Deletion TBX5 gene | 629 | 1 |
| 58 | AMC | Ponseti method with surgery | ‐ | Nemaline myopathy congenital intermediate form | 2110 | 6 |
| 59 | AMC | Ponseti method | ‐ | Sphrintzen‐Goldberg syndrome, TGFBR1 mutation confirmed | 3400 | 10 |
| 60 | BPEV | Ponseti method with surgery | ‐ | ‐ | 3160 | 10 |
| 61 | BPEV | Ponseti method with surgery | ‐ | Normal array | 3415 | 10 |
| 62 | BPEV | No treatment needed | ‐ | ‐ | 4120 | 10 |
| 63 | BPEV | Ponseti method with surgery | ‐ | ‐ | 3106 | 10 |
| 64 | BPEV | Ponseti method with surgery | ‐ | Normal array | 4036 | 10 |
| 65 | FADS | ‐ | ‐ | No gene mutations found | ‐ | 0 |
| 66 | Skeletal dysplasia, presenting as FADS | ‐ | ‐ | ‐ | ‐ | 0 |
Abbreviations: AMC, arthrogryposis multiplex congenital; BPEV, bilateral pes equinovares; FADS, fetal akinesia deformation sequence.
3.4. Follow‐up pregnancies
From the 18 women diagnosed with FADS (17 cases, less than 24 weeks gestation, and one late referral), nine got pregnant again. Motor assessments were performed biweekly from 12 to 24 weeks gestation because of prior FADS, following the same protocol according to Donker et al.1 In seven of nine pregnancies, normal motility was seen, healthy children were born. In the two of nine cases, recurrent FADS was suspected, however, no motor assessments were made. In the first case, fetal hydrops was seen at 11 weeks, and TOP was performed at 12 weeks gestation. In the second case, IUFD with signs of fetal hydrops at 13 weeks of gestation was found, TOP was performed. In both cases, the diagnosis of FADS was confirmed by the clinical geneticist and child pathologist by post‐mortem examination.
4. DISCUSSION
The first 10 years of FADS expertise centre demonstrated the different motor activity of fetuses suspected of FADS in comparison with a normal population and was used to detect recurrent disease. With this knowledge, we expected that systematic motor assessment would be of additional value for the diagnosis of FADS in fetuses showing greater than or equal to two contractures at 20 weeks. During the consecutive 10 years, the descriptive analysis of the AUE over time, with a 100% follow‐up, supported that the extra parameter motor assessment assisted the distinction between the phenotypes of AMC, FADS, and BPEV.
The motor assessment resulted in change of the suspected phenotype based on first structural AUE in 19 of 65, and the second, in three of 43. In the remaining examinations, the motor assessments confirmed the already suspected diagnosis.
Systematic fetal motor assessment, however, is still limitedly applied and not yet regularly performed in AUE. From the three motor aspects studied here, the quality assessment and reduced differentiation into specific movement patterns were most informative about the presence of FADS, whereas quantity was only affected in half. This is in line with Donker et al.1 The quality of the movement of the fetuses with FADS in our study was abnormal, showing reduced variability: small amplitude, slow movements, movements in one direction only, and reduced participation of the body parts (especially trunk and head). Moreover, the finding that hypokinetic movement patterns can be alternated by hyperkinetic movements in the fetuses suspected for FADS is in agreement with other studies.1, 10 Even fetal seizures have been described prior to the emergence of contractures.10 Decreased fetal movements, independent of its cause, eg, central nervous system abnormalities or restrictive dermopathy, are widely accepted to be the cause of multiple contractures and other sequence anomalies. When severe, it can result in FADS.15, 16, 17, 18, 19, 20, 21 However, the limited serial motor assessments demonstrated which reduced activity, is a late expression of affected motor activity and possibly not initiating the contractures.8, 9, 10 Though different authors advise evaluation of the fetal motility, until now, the main focus has been on diagnosing the different structural anomalies known to occur with FADS.2, 3, 22
The varied expressions of the structural anomalies in FADS are illustrated by the following case series. Helmund et al evaluated ultrasound findings, post‐mortem reports, and paediatric charts of 79 cases with FADS.2 They found that FADS presents with various ultrasound findings, mostly affecting the profile, elbow, knee, ankle joint, wrist, and fingers. Fetal hydrops and nuchal oedema were earlier signs, whereas pulmonary hypoplasia, polyhydramnios, and IUGR were found later in pregnancy.2 Most of our cases did not show these latter features since the pregnancy was often terminated before 24 weeks of gestation. The second case series on antenatal findings in 21 cases with AMC and FADS examined between 12–30 weeks gestational age suggested that pulmonary hypoplasia is obligatory in FADS and cannot be found in AMC.3 When pulmonary hypoplasia was found, the pregnancy was terminated. However, this makes counselling before 24 weeks gestational age difficult, since this often emerges thereafter.2
The strength of our study is that we prospectively studied all fetuses with more than one contracture as examined at the 20‐week standard anomaly scan, comparing the value of advanced anomaly scan and the additional value of the motor assessment, both repeated after 2 weeks, with a 100% multidisciplinary follow‐up.
A limitation of our study is that the second assessment to detect progression of the motility was not performed in all cases. This was because: (1) the combination of the structural abnormalities, together with severe abnormal motility at first examination, already led to TOP; and (2) late referral allowed no time to timely repeat the ultrasound examination before 24 weeks. Another limitation is that in 16 cases with multiple contractures, 14 with bilateral talipes equinovares and two with AMC, no motor assessment was made. They were missed because of unfamiliarity with the protocol. One of these 16 fetuses developed FADS. Our evaluation emphasises that clinicians need to be aware that the onset of FADS varies, illustrated by the case with FADS at 35 weeks where there were no contractures at 20 weeks, as well as possible underlying disorders in cases of BPEV or AMC. Motor assessment did not reveal the severity of the later developing or ongoing diseases.
We suggest that in cases where multiple contractures are observed on the 20‐week structural anomaly scan, the pregnant woman and partner should be informed about the rare disease resulting in the phenotype FADS and the possibilities of repeated ultrasound examination including the present knowledge on motor assessment before the 24‐week gestational age. Counselling has to be performed with the knowledge of the low prevalence of FADS and higher rate of AMC and BPEV. Moreover, parents have to be made aware that motility is an expression of the central nervous system and neuromuscular system at that very moment. The fetal motility is used as an extra parameter when multiple contractures are seen at the structural anomaly scan. Since the motility is a functional expression of the central nervous system, the motor assessment can be used to counsel the parents about the extensiveness of the findings and can help the parents to make an informed choice to continue or terminate the pregnancy. In this cohort, motor assessment, in addition to the structural ultrasound assessment, resulted in a high‐predictive value nearing 100%, but we are aware that later onset of FADS can be missed before 24 weeks gestation. Hence, late‐onset progressive neuromuscular diseases cannot be excluded. The application of motor assessments should be seen within the scope of AUE.
This manuscript is a second step in creating the awareness of systematic motor assessment supporting the prenatal diagnosis of FADS. The first step assessing in a cohort irrespective of the gestational age (Donker et al. 2009). Second step is the present study accessing a cohort at 20 weeks gestation after the introduction of the 20‐week standard anomaly scan. Our next step is focused on the distinction between normal and abnormal fetal motility by other centres. Presently, we are working on learning strategies recognizing abnormal motility and will apply this on an e‐learning. The hope is that we reach caregivers with interest in this field of neuromuscular disorders. This may help to lower the number of missed FADS. This study tried to demonstrate the additional value of motor assessments. Future studies have to pave the way whether the use of systematic motor assessment can be supported in larger populations and should be implemented in the AUE to detect FADS when contractures are seen. Moreover, finding an underlying genetic cause through next genome sequencing may obtain a more exact diagnosis than prenatal ultrasound examination and autopsy.
5. CONCLUSION
This is a retrospective study on the additional value of motor assessment to AUE to distinguish between stable AMC or BPEV versus deteriorating AMC into FADS. The results suggest that the expectation that motor assessment is of additional value to distinguish between FADS and AMC/BPEV is plausible.
Multidisciplinary approach during and after pregnancy will enhance counselling, follow‐up treatment, and finding underlying diseases.
CONFLICT OF INTEREST
The authors of this article declare that there is no conflict of interest.
ACKNOWLEDGEMENTS
Adriaanse B., MD, PhD, Burger N., MD, PhD, and Schreurs C., MD, for their dedicated motor assessments.
APPENDIX 1.
Table A1.
Individual diagnosis after advanced ultrasonographic examination (AUE) and the motor assessment(s) with decision concerning the pregnancy
| Gesta‐tional | AUE Structural | AUE Motor | ||||||
|---|---|---|---|---|---|---|---|---|
| Number | Age | Additional findings (F/P/CT) | Diagnoses | Differentiation | Quality | Quantity | Diagnoses | Continuation/Terminationof Pregnancy |
| 1 | 22 + 3 | ‐ | BPEV | + | ‐ | + | FADS | Continuation |
| 22 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 23 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 2 | 22 + 5 | ‐ | BPEV | ++ | +/‐ | + | BPEV | Continuation |
| 26 + 6 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation | |
| 3 | 21 + 5 | ‐ | BPEV | + | ‐ | + | FADS | Continuation |
| 23 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 4 | 21 + 3 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation |
| 23 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 27 + 4 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 5 | 24 + 0 | F | FADS | + | ‐ | ‐ | FADS | Continuation |
| 24 + 1 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 28 + 0 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation | |
| 6 | 19 + 4 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation |
| 21 + 4 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 7 | 21 + 6 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation |
| 23 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 8 | 22 + 2 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation |
| 9 | 16 + 1 | CT, fetal hydrops | FADS | NA | NA | ‐ (0) | FADS | Continuation |
| 19 + 5 | CT, fetal hydrops | FADS | NA | NA | ‐ (0) | FADS | TOP | |
| 10 | 23 + 1 | ‐ | BPEV | + | ‐ | + | FADS | TOP |
| 11 | 19 + 2 | ‐ | AMC | + | ‐ | + | FADS | TOP |
| 12 | 19 + 3 | Fetal hydrops | FADS | ‐ | ‐ | ‐ | FADS | TOP |
| 13 | 19 + 5 | Increased CT‐ratio | FADS | + | ‐ | + | FADS | Continuation |
| 21 + 5 | Increased CT‐ratio | FADS | + | +/‐ | + | AMC | TOP | |
| 14 | 23 + 1 | ‐ | AMC | ‐ | ‐ | ‐ | FADS | TOP |
| 15 | 21 + 2 | F | FADS | ‐ | ‐ | ‐ | FADS | TOP |
| 16 | 22 + 2 | ‐ | AMC | + | ‐ | ‐ | FADS | Continuation |
| 23 + 3 | ‐ | AMC | ‐ | ‐ ‐ | ‐ | FADS | TOP | |
| 17 | 17 + 0 | ‐ | AMC | + | ‐ | + | FADS | Continuation |
| 19 + 2 | ‐ | AMC | + | ‐ ‐ | + | FADS | TOP | |
| 18 | 14 + 3 | ‐ | AMC | + | ‐ | + | FADS | Continuation |
| 16 + 3 | Increased contractures | FADS | + | ‐ ‐ | + | FADS | TOP | |
| 19 | 17 + 2 | Mouth constantly open | FADS | + | ‐ | + | FADS | Continuation |
| 19 + 1 | Mouth constantly open | FADS | ‐ | ‐ | + | FADS | Continuation | |
| 21 + 2 | Mouth constantly open | FADS | + | ‐ ‐ | ‐ | FADS | TOP | |
| 20 | 15 + 0 | Fetal hydrops | FADS | NA | NA | ‐ (0) | FADS | Continuation |
| 17 + 0 | Fetal hydrops | FADS | NA | NA | ‐ (0) | FADS | TOP | |
| 21 | 22 + 1 | ‐ | AMC | + | +/‐d | + | AMC | Continuation |
| 24 + 2 | ‐ | AMC | + | +/‐ | + | AMC | Continuation | |
| 28 + 1 | ‐ | AMC | + | +/‐ | + | AMC | Continuation | |
| 22 | 19 + 3 | F | FADS | + | ‐a | + | AMC/syndrome | Continuation |
| 21 + 3 | F | FADS | + | ‐b | + | AMC/syndrome | Continuation | |
| 23 | 12 + 2 | ‐ | AMC | NA | NA | ‐ (0) | FADS | TOP |
| 24 | 10 + 5 | ‐ | AMC | NA | NA | ‐ (0) | FADS | TOP |
| 25 | 13 + 1 | ‐ | AMC | + | ‐a , b | + | FADS | Continuation |
| 14 + 6 | ‐ | AMC | ‐ | ‐ ‐b, b | + | FADS | TOP | |
| 26 | 22 + 2 | ‐ | AMC | + | ‐a | + | AMC | TOP |
| 27 | 20 + 3 | ‐ | AMC | + | + | + | AMC | Continuation |
| 22 + 4 | F | FADS | + | + | + | AMC | Continuation | |
| 28 | 20 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 29 | 14 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 16 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 30 | 18 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 20 + 3 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation | |
| 31 | 23 + 4 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 32 | 22 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 33 | 20 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 34 | 21 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 35 | 23 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 36 | 21 + 5 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 23 + 5 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 37 | 21 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 38 | 21 + 5 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 39 | 21 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 23 + 1 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 40 | 20 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 23 + 5 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 41 | 22 + 2 | P | FADS | + | + | + | BPEV | Continuation |
| 42 | 22 + 1 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 28 + 5 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation | |
| 43 | 20 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 44 | 23 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 45 | 21 + 1 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 46 | 22 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 47 | 20 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 48 | 20 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 21 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 49 | 21 + 0 | ‐ | BPEV | ‐c | + | + | BPEV | Continuation |
| 22 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 50 | 20 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 51 | 20 + 1 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 52 | 22 + 0 | ‐ | BPEV | + | + | + | BPEV | TOP |
| 53 | 20 + 1 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 54 | 20 + 5 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 23 + 4 | ‐ | BPEV | + | ‐ | + | FADS | TOP | |
| 55 | 21 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 23 + 4 | ‐ | BPEV | ‐ | + | + | BPEV | Continuation | |
| 56 | 12 + 5 | Prominent head | FADS | +/‐ | + | + | AMC | Continuation |
| 14 + 5 | ‐ | FADS | ‐ | ‐ | ‐ | FADS | IUFD, TOP | |
| 57 | 20 + 2 | Radius aplasia | AMC/syndrome | + | +/‐ | + | AMC/Holt‐Oram syndrome | TOP |
| 58 | 21 + 4 | F | FADS | + | + | + | AMC | Continuation |
| 23 + 4 | ‐ | FADS | + | + | + | AMC | Continuation | |
| 59 | 21 + 2 | F | FADS | + | + | + | AMC | Continuation |
| 23 + 2 | ‐ | FADS | + | + | + | AMC | Continuation | |
| 60 | 18 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 20 + 3 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 61 | 20 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 0 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 62 | 19 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 21 + 6 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 63 | 20 + 4 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 22 + 4 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 64 | 21 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation |
| 23 + 2 | ‐ | BPEV | + | + | + | BPEV | Continuation | |
| 65 | 21 + 0 | F/P/CT | FADS | ‐ | ‐ | + | FADS | TOP |
| 66 | 11 + 5 | ‐ | AMC | ‐ | ‐ | + | FADS | TOP |
Abbreviations: (0), No movements seen; AMC, Arthrogryposis multiplex congenital; BPEV, bilateral pes equinovares; BPEV+K/H, bilateral pes equinovares and contractures knees/hips; Continuation, continuation of pregnancy; CT, increased cardio‐thorax ratio, longhypoplasia; F, flattening of the face; FADS, fetal akinesia deformation sequence; NA, not able to access; P, polyhydramnios; TOP, termination of pregnancy; U + L, contractures upper and lower limbs; W, contractures wrists; W + E/S, contractures wrists and elbows/shoulders. Motor assessment: +, Normal; ‐, Abnormal; +/‐, slightly abnormal; ‐ ‐, progressively abnormal; ++, increased.
Wrists and elbows are not participating in movements.
Reduced variability, mainly movements with large amplitude and fast movements, direction of movement does change, mainly participation of all body parts, not all fluently.
Hypo‐ and hyperkinetic movement patterns seen.
Included stepping, movements, so slightly reduced differentiation was interpreted as normal.
Tjon JK, Tan‐Sindhunata GM, Bugiani M, et al. Fetal akinesia deformation sequence, arthrogryposis multiplex congenita, and bilateral clubfeet: Is motor assessment of additional value for in utero diagnosis? A 10‐year cohort study. Prenatal Diagnosis. 2019;39:219–231. 10.1002/pd.5411
All participants are member of the Expertise Centre on rare disease: prenatal diagnosis of neuromuscular disorders especially FADS.
REFERENCES
- 1. Donker ME, Eijckelhof BHW, Tan MB, et al. Serial postural and motor assessment of fetal akinesia deformation sequence (FADS). Early Hum Dev. 2009;85(12):785‐790. [DOI] [PubMed] [Google Scholar]
- 2. Hellmund A, Berg C, Geipel A, Müller A, Gembruch U. Prenatal diagnosis of fetal akinesia deformation sequence (FADS): a study of 79 consecutive cases. Arch Gynecol Obstet. 2016. Oct;294(4):697‐707. [DOI] [PubMed] [Google Scholar]
- 3. Hoellen F, Schröer A, Kelling K, et al. Arthrogryposis multiplex congenita and Pena‐Shokeir phenotype: challenge of prenatal diagnosis–report of 21 cases, antenatal findings and review. Fetal Diagn Ther. 2011;30(4):289‐298. [DOI] [PubMed] [Google Scholar]
- 4. Filges I, Hall JG. Failure to identify antenatal multiple congenital contractures and fetal akinesia–proposal of guidelines to improve diagnosis. Prenat Diagn. 2013. Jan;33(1):61‐74. [DOI] [PubMed] [Google Scholar]
- 5. Lowry RB, Sibbald B, Bedard T, Hall JG. Prevalence of multiple congenital contractures including arthrogryposis multiplex congenita in Alberta, Canada, and a strategy for classification and coding. Birth Defects Res A Clin Mol Teratol. 2010. Dec;88(12):1057‐1061. [DOI] [PubMed] [Google Scholar]
- 6. Shenker L, Reed K, Anderson C, Hauck L, Spark R. Syndrome of camptodactyly, ankyloses facial anomalies, and pulmonary hypoplasia (Pena‐Shokeir syndrome): obstetric and ultrasound aspects. Am J Obstet Gynecol. 1985. June;152(3):303‐307. [DOI] [PubMed] [Google Scholar]
- 7. Wynne‐Davies R. Genetic and environmental factors in the etiology of talipes equinovarus. Clin Orthop Relat Res. 1972;84:9‐13. [DOI] [PubMed] [Google Scholar]
- 8. Jones KL, Graham PA, Sanchez‐Lara. Fetal Akinesia Deformation Sequence in Smith's Recognizable patterns in human malformation. Philadelphia: Elsevier; 2013:318‐324. [Google Scholar]
- 9. Yfantis H, Nonaka D, Castellani R, Harman C, Sun CC. Heterogeneity in fetal akinesia deformation sequence (FADS): autopsy confirmation in three 20‐21‐week fetuses. Prenat Diagn. 2002. Jan;22(1):42‐47. [DOI] [PubMed] [Google Scholar]
- 10. Sheizaf B, Mazor M, Landau D, Burstein E, Bashiri A, Hershkovitz R. Early sonographic prenatal diagnosis of seizures. Ultrasound Obstet Gynecol. 2007;30(7):1007‐1009. [DOI] [PubMed] [Google Scholar]
- 11. Tongsong T, Chanprapaph P, Khunamornpong S. Prenatal ultrasound of regional akinesia with Pena‐Shokier phenotype. Prenat Diagn. 2000. May;20(5):422‐425. [DOI] [PubMed] [Google Scholar]
- 12. Makrydimas G, Sotiriadis A, Papapanagiotou G, Tsopelas A, Lolis D. Fetal akinesia deformation sequence presenting with increased nuchal translucency in the first trimester of pregnancy. Fetal Diagn Ther. 2004. Jul‐Aug;19(4):332‐335. [DOI] [PubMed] [Google Scholar]
- 13. De Vries JI, Fong BF. Changes in fetal motility as a result of congenital disorders: an overview. Ultrasound Obstet Gynecol. 2007;29(5):590‐599. [DOI] [PubMed] [Google Scholar]
- 14. De Vries JIP, Visser GHA, Prechtl HFR. The emergence of fetal behaviour III. Individual differences and consistencies. Early Hum Dev. 1988;16(1):85‐130. [DOI] [PubMed] [Google Scholar]
- 15. Hall JG. Arthrogryposis multiplex congenita: etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B. 1997. Jul;6(3):156‐159. [PubMed] [Google Scholar]
- 16. Hall JG. Pena‐Shokeir phenotype (fetal akinesia deformation sequence) revisited. Birth Defects Res A Clin Mol Teratol. 2009. Aug;85(8):677‐694. [DOI] [PubMed] [Google Scholar]
- 17. Hall JG. Arthrogryposis (multiple congenital contractures): diagnostic approach to etiology, classification, genetics, and general principles. Eur J Med Genet. 2014. Aug;57(8):464‐472. [DOI] [PubMed] [Google Scholar]
- 18. Chih‐Ping C. Prenatal diagnosis and genetic analysis of fetal akinesia deformation sequence and multiple pterygium syndrome associated with neuromuscular junction disorders: a review. Taiwan J Obstet Gynecol. 2012. March;51:12‐17. [DOI] [PubMed] [Google Scholar]
- 19. Haliloglu G, Topaloglu H. Arthrogryposis and fetal hypomobility syndrome. Handb Clin Neurol. 2013;113:1309‐1311. [DOI] [PubMed] [Google Scholar]
- 20. Ferguson J, Wainwright A. Arthrogryposis. Orthopaedics and Trauma. 2013. June;27(3):171‐180. [Google Scholar]
- 21. Kalampokas E, Kalampokas T, Sofoudis C, Deligeoroglou E, Botsis D. Diagnosing arthrogryposis multiplex congenita: a review. ISRN Obstetrics and Gynecology. 2012;2012:1‐6. 10.5402/2012/264918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Witters I, Moerman P, Fryns JP. Fetal akinesia deformation sequence: a study of 30 consecutive in utero diagnoses. Am J Med Genet. 2002. Nov;113(1):23‐28. [DOI] [PubMed] [Google Scholar]


