Abstract
A new material concept of soft crystals is proposed. Soft crystals respond to gentle stimuli such as vapor exposure and rubbing but maintain their structural order and exhibit remarkable visual changes in their shape, color, and luminescence. Various interesting examples of soft crystals are introduced in the article. By exploring the interesting formation and phase‐transition phenomena of soft crystals through interdisciplinary collaboration, new materials having both the characteristics of ordered hard crystals and those of flexible soft matter are expected.
Keywords: crystal structures, phase transition, photofunction, soft crystals, stimulus response
Introduction
Generally, the term crystal evokes thoughts of hard and stable materials like diamond and quartz that are distinct from liquids and polymers. This hardness is mainly because the atoms are arranged regularly, forming stable structures. In 1992, crystals were redefined as solid materials that exhibit discrete diffraction patterns, thus including quasicrystals in addition to normal periodic crystals.1 In any case, crystals have regular and highly ordered structures. However, these ordered structures do not always guarantee the hardness and stability of crystals. In fact, there are increasing reports of crystals that exhibit remarkable changes in their appearance on the application of external stimuli, for example, crystals that exhibit luminescence color changes in response to small molecule vapor2 or mechanical stimuli such as manual grinding.3 In contrast to conventional hard crystals, these new materials have some flexibility and can respond to macroscopic stimuli such as vapor exposure, rubbing, and rotation at around room temperature, exhibiting visually remarkable changes in luminescence and optical properties (Figure 1). These crystals can be organic crystals, inorganic crystals, or inorganic–organic hybrid crystals. We term these materials “soft crystals”.
Figure 1.
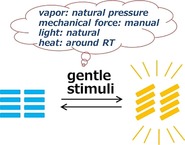
Soft crystals: flexible and ordered systems that respond to gentle stimuli.
In this article, the concept of “soft crystals” is proposed and several interesting examples are discussed. We also discuss the aspects of these materials requiring clarification and the prospects of this new area.
Our Definition of Soft Crystals
As mentioned in the Introduction, we have named this new crystal group “soft crystals.” These materials exhibit changes in luminescence and optical properties in response to gentle stimuli. Here, we would like to scientifically define soft crystals. Figure 2 shows a schematic of the structural order of different types of condensed matter and the activation energies of their structural transformations (ΔG ≠).
Figure 2.
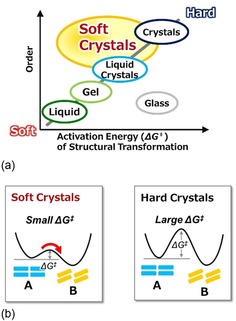
Schematic drawings showing (a) the structural order in condensed matter and the activation energy of structural transformations (ΔG ≠), and (b) the energetic characteristics of soft crystals compared to those of conventional hard crystals.
The structural order of atoms and molecules increases from liquids to gels, liquid crystals, and, finally, crystals, and the degree of order can be roughly related to the activation energy of structural transformation. Glasses or amorphous solids are positioned in the area of high ΔG ≠ but low order (Figure 2 a). Thus, we noticed that there is a new category of materials located in the area of high structural order but relatively low ΔG ≠. These materials are what we call soft crystals. Soft crystals possess highly ordered structures, but they can easily change their structures because of the much lower activation energies compared with those of conventional hard crystals (Figure 2 b). Therefore, soft crystals respond to gentle stimuli while maintaining their structural order. Furthermore, the changes can be detected visually as changes in color or luminescence.
The term “soft crystals” can include various crystalline materials. Molecular crystals composed of organic compounds and metal complexes are expected to be the main targets for soft crystals because these are constructed of weak intermolecular interactions such as such as hydrogen bonds, π–π and CH–π interactions, charge‐transfer interactions, and van der Waals interactions. The lattice energies for molecular crystals are mostly much less than 100 kJ mol−1 in contrast to those for ionic crystals (several hundred kJ mol−1 and more).4 However, metal‐organic frameworks (MOFs) and inorganic compounds could also be included in the category of soft crystals if they transform to different crystal phases in response to gentle external stimuli and exhibit visible changes.5, 6 So, how do we estimate the activation energy (ΔG ≠) for “soft crystals” and what is a clear definition of gentle stimuli? Fundamentally, the structural transformation should occur at around room temperature, although this does not necessarily mean the phase‐transition temperature (T c) is at room temperature. Various stimuli can act as triggers for the structural transformation. We define gentile stimuli here as those available in the natural environment (Figure 1), for example, vapor at less‐than‐saturated vapor pressure at room temperature and atmospheric pressure, mechanical stimuli such as manual forces (grinding), and natural light.
The term “soft crystal” can be found in various papers to date. Soft metals, such as gold, and ionic crystals, such as sodium chloride, have sometimes been called soft crystals in comparison with those of hard metals or inorganic oxides.7 This term has also been used for organic molecular crystals with low melting points or those with plasticity.8 In other papers, the crystalline state near the melting point is called the soft crystal state.9 In these examples, the term is used for a mechanically “soft” state of crystals. On the other hand, a soft‐crystal phase is known as one of the liquid crystal phases: the smectic phase (SmE), which has a layered structure and restricted molecular motion.10 This is very similar to our soft crystals in principle and important as a boundary state connecting crystals and liquid crystals. Since 2000, many papers regarding the soft‐crystal phase of liquid crystals have been published.11 More recently, Kitagawa and co‐workers used the same term for crystals of a metal‐organic framework (MOF), {[Ce(tci)]}n (H3tci=tris(2‐carboxyethyl)isocyanurate), which transformed its crystal structure from two‐ to three‐dimensional polymers reversibly by desorbing and absorbing water molecules (Figure 3).12 Although it was not reported that the crystal transformation was accompanied by any particular change in the optical properties, this phenomenon viewed from the stimulus‐induced structural change is essentially the same as that proposed for soft crystals. Kitagawa and co‐workers have further developed various types of porous coordination polymers or MOFs that possess both a highly ordered network and structural transformability, the so‐called “soft porous crystals”.13 Soft porous crystals have flexible frameworks that depend on the guest molecules.
Figure 3.
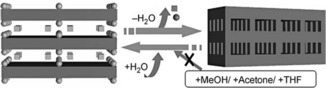
Schematic representation of a single‐crystal‐to‐single‐crystal transformation from a 2D to a 3D nonporous framework triggered by a specific guest. The connections between the plates represent the formation of new hydrogen bonds. Reprinted with permission from reference 12.
Examples of Soft Crystals
Vapochromic crystals
It is well known that square‐planar PtII complexes exhibit characteristic colors and luminescence when they are stacked to form an assembled structure with Pt⋅⋅⋅Pt electronic interactions.14 Such stacked structures formed by weak intermolecular interactions are sensitive to external stimuli such as temperature, pressure, and other environmental perturbations. Vapor exposure is the most characteristic stimulus for PtII complexes because vapor molecules can induce selective and drastic structural transformations, leading to remarkable changes in color and luminescence.2 In fact, the term vapochromism was first used for the vapor‐induced color changes of the double salts of PtII complexes, [Pt(p‐CN‐C6H4CnH2n+1)4][M(CN)4] (n=6, 10, 12, and 14; M=Pt and Pd), reported by Nagle,15 and the vapochromism of this type has been further investigated by Mann and co‐workers.16 Since then, many PtII complexes have been developed as sensing materials for volatile organic compounds (VOCs) and humidity. For example, Kato et al. reported the vapochromic luminescence of the dinuclear PtII complex syn‐[Pt2(bpy)2(pyt)2][PF6]2 (bpy=2,2′‐bipyridine, pyt=pyridine‐2‐thiolate, Figure 4 a) and its structural transformation.17 The crystalline samples of this complex exhibits remarkable luminescence changes in the presence of organic vapors such as acetonitrile or ethanol, as shown in Figure 4 b. The dark‐red form includes vapor molecules, whereas the light‐red form has lost the guest vapor molecules. The conversion from the light‐red form exhibiting red luminescence to the dark‐red form occurs immediately on exposure to acetonitrile vapor. The mechanism of the distinctive color change has been elucidated by X‐ray analysis to be a single‐crystal‐to‐single‐crystal (SCSC) transformation. The SCSC transformation was observed on keeping the crystal at 0 °C under a nitrogen atmosphere. The dinuclear complexes adopt a dimer‐of‐dimer arrangement in the dark‐red form with a short Pt⋅⋅⋅Pt contact of 3.434(2) Å, suggesting that electronic interactions could occur between the dinuclear complexes. For the light‐red form, on the other hand, two dinuclear units are arranged with a π⋅⋅⋅π contact between bipyridine ligands and a long intermolecular Pt⋅⋅⋅Pt distance (>5 Å). The two structures have the same space group (Pbcn) but a different arrangement of the two dinuclear units (Figure 4 c).
Figure 4.
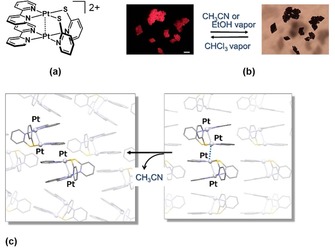
(a) Structural formula of syn‐[Pt2(bpy)2(pyt)2]2+, (b) vapochromic behavior of the crystals (the white scale bar in the left photo is 100 μm in length), and (c) vapor‐induced structural transformation of the dimer‐of‐dimer.17
In addition to PtII complexes, vapochromic compounds have been developed for other metal complex systems18 and pure organic crystals.19 In particular, much attention has focused on new systems that exhibit additional functions coupled with color changes accompanied by the structural transformation.20 For example, Kato and co‐workers recently found that a NiII‐quinonoid complex exhibits selective spin‐state switching in response to methanol vapor (Figure 5).20a The selective response is based on the hydrogen‐bonding network constructed between the nickel complexes and methanol molecules.
Figure 5.
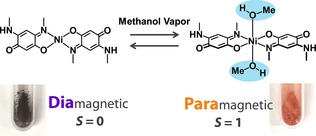
Vapochromic behavior coupled with spin switching of a nickel(II)‐quinonoid complex, which exhibits a reversible structural transformation including a coordination geometrical change between the square planar and octahedral structures on the selective uptake and removal of methanol vapor.
Mechanochromic crystals
Mechanochromism is the phenomenon of color change induced by mechanical stimuli such as the grinding, rubbing, or scratching of solid materials and has been observed for polymers and powdery materials for many years.3, 21 A soft crystal showing mechanochromic luminescence was reported by Ito et al. in 2008.22 As shown in Figure 6, the crystalline powder of a dinuclear isocyanide AuI complex exhibits blue luminescence under UV irradiation; however, on scratching the powder with a spatula, the luminescence color immediately changes from blue to yellow. The luminescence color change is based on the formation of Au⋅⋅⋅Au interactions by the crystal to amorphous transformation. The crystalline state exhibiting blue luminescence was recovered immediately on adding a drop of solvent.
Figure 6.
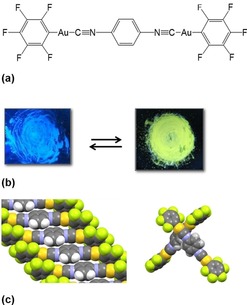
Mechanochromic luminescence of an AuI complex. (a) Structural formula of the AuI‐isocyanide complex and (b) luminescence color change accompanied by (c) the crystal‐to‐amorphous transformation on grinding.
To date, various organic and inorganic crystalline systems have also been reported to show mechanochromic luminescence and related phenomena. Araki and co‐workers found organic crystals that exhibit piezochromic luminescence.23 In the crystals of a tetraphenylpyrene derivative, the luminescence color remarkably changes from blue to green on pressing or grinding, and the color reverts on heating (Figure 7). The color change has been ascribed to the transformation of the crystal packing structures built with hydrogen bonds. The piezochromism here is the same as the above‐mentioned mechanochromism in the sense that a very mild stimulus, such as manual pressing with a spatula, is sufficient to induce a color change.
Figure 7.
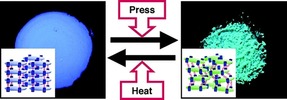
Piezochromic luminescence of a tetraphenylpyrene derivative. Reprinted with permission from reference 23. Copyright 2007, American Chemical Society.
Ito et al. also reported an interesting luminescence color change triggered by a gentle mechanical stimulus, the contact of crystals of a AuI complex, [phenyl(phenylisocyanide)]gold(I).24 The AuI complex has two polymorphs, blue and yellow luminescent forms. Interestingly, if the yellow‐emissive crystal is placed in contact with the blue‐emissive crystal, a phase transition from the blue‐emissive form to the yellow‐emissive form occurs in the blue‐emissive crystal, propagating over the whole crystal from the point of contact (Figure 8). The yellow luminescence is due to the formation of Au−Au interactions.
Figure 8.
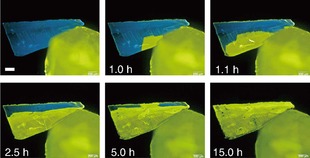
Mechanical‐stimulus‐triggered phase transformation from the blue‐emissive form to the yellow‐emissive form.24 The white scale bar in the upper‐left photo is 200 μm in length. Reprinted with permission from reference 24.
Organic crystals that exhibit superelasticity and ferroelasticity
In 2014, Takamizawa et al. found very interesting organic crystals that exhibit superelasticity.25 Although the phenomenon is well‐known for several metal alloys such as Ni−Ti alloys, this is the first example of superelastic organic crystals. For crystals of terephthalamide, as shown in Figure 9 a,26 the crystal phase transformation occurs on the application of gentle shear forces to the crystal (in the upper right of the Figure), and it reverts back to the original phase on release. On the other hand, a single crystal of tetrabutyl‐n‐phosphonium tetraphenylborate deforms plastically, and the bent crystal cannot revert automatically, as shown in Figure 9 b.26 However, the bent crystal returned to the original form on heating at around 150 °C. Thus, the crystal can be said to exhibit a shape‐memory property like shape‐memory alloys. Takamizawa also found several organic crystals that exhibit ferroelasticity.27 Soft crystals with properties such as superelasticity and ferroelasticity are expected to be useful for the creation of finely designed materials with interesting optical properties and photofunctions.
Figure 9.
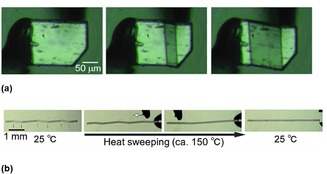
(a) Structural transformation followed by phase transition on the application of slight shear stress for a single crystal of telephthalamide, and (b) shape recovery via heat‐sweeping over the deformed zigzag tetrabutyl‐n‐phosphonium tetraphenylborate crystal. Reprinted with permission from references 25, 26.
Other attractive phenomena associated with soft crystals
Concerning the concept of soft crystals, molecular assemblies with high structural order may be included in the group of soft crystals. For example, the aggregation of porphyrins, macrocyclic aromatic compounds, has been extensively investigated to control and expand their optical properties in relation to biosystems, such as photosynthetic systems.28 From the viewpoint of the initial stages soft crystal growth, mechanical stimuli have a significant effect on the molecular assemblies. Ribó et al. showed that the chiral J‐aggregates of porphyrins, which have been extensively investigated in term of their optical and photofunctional properties, can be enantioselectively prepared by controlling the rotational direction of a rotary evaporator, and this has been supported by further experiments and analyses.29 The crystal structural changes in polymorphs of phthalocyanines are known to influence the crystalline functions, such as photoconducting and conducting properties. For instance, in the case of oxotitanium(IV) phthalocyanine, which is used in GaAsAl laser printers as a near‐IR photoconductor, only the photoactive phase II is used.30 Ozaki and co‐workers reported the growth of single‐crystal films of a polymorphic phthalocyanine derivative by solvent vapor exposure.31 This is a very attractive phenomenon connecting liquid crystals and soft crystals, and this simple method is promising for the fabrication of highly ordered organic semiconductors.
Reddy, Naumov, and co‐workers have been actively investigating mechanically flexible single crystals of organic compounds including hydrogen bonds or halogen⋅⋅⋅halogen interactions.32, 33 These single crystals can easily bend like polymers while maintaining their crystallinity. In addition, Ramamurty, Desiraju, and co‐workers have been investigating the elastic modulus and hardness of molecular crystals from the viewpoint of crystal engineering.34
Challenges in Soft Crystal Science
As shown in the previous section, various examples of soft crystals have been found and research has accelerated recently. For example, Figure 10 shows the remarkable increase in the number of papers concerning “mechanochromism” and “vapochromism”. It is clear that the number of papers is increasing year‐on‐year. However, the total number of papers (ca. 770 for mechanochromism and ca. 350 for vapochromism) is not significant compared to the more established chromic phenomena, such as photochromism, which is discussed in over 25 000 published papers.35 Overall, there are many unknowns in the phenomena of soft crystals, such as their mechanochromism and vapochromism, and the area of soft crystals is a frontier and a mine of new materials.
Figure 10.
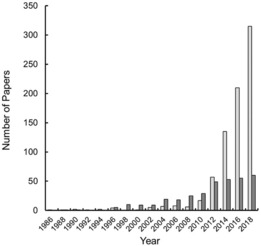
Increase in the number of publications concerning “mechanochromism” (light grey bars) and “vapochromism” (dark grey bars), as obtained from SciFinder.
To date, however, most examples of soft crystals have been found by chance or the screening of related crystals. In addition, the mechanism behind the formation of soft crystals has not elucidated yet because this is influenced by many complex factors. Even in cases where the initial and final structures of the crystal have been determined, the dynamic behavior remains unknown. Consequently, it is essential to understand the various factors that govern the formation and phase‐transition phenomena of molecular crystals formed of multiple intermolecular interactions. For the rational design of soft crystals, we must consider the intermolecular interactions in the crystal as a whole. As mentioned in the previous section regarding the definition of soft crystals, molecular crystals composed of organic compounds and metal complexes include diverse intermolecular interactions such as hydrogen bonds and metal‐metal, π–π, CH–π, and interhalogen interactions, as well as van der Waals interactions. Chemists can design molecules for soft crystals considering the introduction of multiple points for these intermolecular interactions on the basis of their intuition and experience. Hydrogen bonds could be the most powerful interaction for the control of the structures of soft crystals, as in the case of proteins and DNA. The void space in crystals would make molecules and crystal structures flexible. It is also well known that the introduction of long alkyl chains is effective in improving both the flexibility and order of crystals. In addition, the theoretical prediction of crystal structures has attracted many researchers’ interests and has developed remarkably recently.4a, 36 Computer simulations exploring the dynamics of the self‐organization of crystals and doped crystalline materials have also been reported recently.9, 37 These theoretical approaches are expected to be important tools for the understanding and design of sophisticated soft crystals.
Soft crystals can be further characterized considering the findings discussed above. Soft crystals have various polymorphs with small transition energies, as shown in Figure 2 b, so structural transformations occur easily on the application of a gentle external stimulus, such as exposure to vapor or mechanical stimuli. In some cases, these stimuli can change the potential energy curves themselves. Therefore, versatile structural transformations are possible for soft crystals on the addition of a new parameter, that is, the stimulus (Figure 11). At the same time, fine structural control is possible for soft crystals. In other words, soft crystals are promising materials that have the characteristics of stimulus‐sensitive soft matter and finely controlled crystals.
Figure 11.
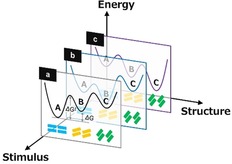
Schematic potential energy diagram of soft crystals, demonstrating their versatility and flexibility depending on the stimulus as a parameter. Potential curves a, b, and c can be expected under different stimuli.
Bearing these points in mind, we must promote research to establish the science of soft crystals. Key steps and challenges in soft crystal science include (1) the establishment of the science of soft crystals based on in‐depth investigation of the phenomenon; (2) the development of new materials based on soft crystal design principles; and (3) technical innovations using soft crystals. One of the scientifically most important challenges is to clarify the formation and phase transitions of soft crystals. Through further research, a new research area providing new materials beyond conventional hard crystals and soft matter could be created.
Conclusions and Future Prospects
In this article, we have proposed our concept of soft crystals; flexible responsive systems with high structural order. Soft crystals are regular solids with stable and highly ordered structures. However, they undergo characteristic facile structural transformations and phase transitions in response to weak but specific stimuli. Related to soft crystals, active research has been carried out in the field of organic crystals such as plastic crystals38 and amphidynamic crystals,39 and the world of crystals is full of wonders and possibilities.
Toward the scientific goals mentioned above, close interdisciplinary collaborations are necessary, not only in the various sub‐disciplines of chemistry such as inorganic, organic, polymer, and physical chemistry, but also physics, mathematics, materials science, engineering, and informatics. Through collaboration, material creation, structural control, physical measurements, and theoretical studies, the discipline of soft crystals will emerge as a new area of science. A deep understanding of such complex systems would lead the development of novel materials such as sophisticated responsive materials and materials with specialized functions.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by JSPS KAKENHI for Scientific Research on Innovative Areas “Soft Crystals” (Area No. 2903, No. JP17H06366), Japan.
M. Kato, H. Ito, M. Hasegawa, K. Ishii, Chem. Eur. J. 2019, 25, 5105.
References
- 1.
- 1a. Acta Crystallogr 1992, A48, 922–946;
- 1b. Authier A., Chapuis G., A Little Dictionary of Crystallography, International Union of Crystallography, Chester, 2014. [Google Scholar]
- 2.
- 2a. Kato M., Bull. Chem. Soc. Jpn. 2007, 80, 287–294; [Google Scholar]
- 2b. Zhang X., Li B., Chen Z.-H., Chen Z.-N., J. Mater. Chem. 2012, 22, 11427–11441; [Google Scholar]
- 2c. Wenger O. S., Chem. Rev. 2013, 113, 3686–3733. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Zhang X., Chi Z., Zhang Y., Liu S., Xu J., J. Mater. Chem. C 2013, 1, 3376–3390; [Google Scholar]
- 3b. Seki T., Ito H., Chem. Eur. J. 2016, 22, 4322–4329; [DOI] [PubMed] [Google Scholar]
- 3c. Kobayashi A., Kato M., Chem. Lett. 2017, 46, 154–162. [Google Scholar]
- 4.
- 4a. Beran G. J., Chem. Rev. 2016, 116, 5567–5613; [DOI] [PubMed] [Google Scholar]
- 4b. Liu D., Zhang S., Wu Z., Inorg. Chem. 2003, 42, 2465–2469. [DOI] [PubMed] [Google Scholar]
- 5. Férey G., Serre C., Chem. Soc. Rev. 2009, 38, 1380–1399. [DOI] [PubMed] [Google Scholar]
- 6. Han Y., Sun J., Ye S., Zhang Q., J. Mater. Chem. C 2018, 6, 11184–11192. [Google Scholar]
- 7.
- 7a.Z. Hurych, K. Vindis, Czech., 1966, Pat. No. CS118839;
- 7b. Kosevich Y. A., Phys. Rev. B 1995, 52, 1017–1024; [DOI] [PubMed] [Google Scholar]
- 7c. Mukerji S., Kar T., Cryst. Res. Technol. 1999, 34, 1323–1328. [Google Scholar]
- 8. Koide T., Oda T., Nitta I., Bull. Chem. Soc. Jpn. 1956, 29, 738–740. [Google Scholar]
- 9. Taubera J., Higlera R., Sprakela J., Proc. Natl. Acad. Sci. USA 2016, 113, 13660–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Dierking I., Textures of Liquid Crystals, Wiley-VCH, Weinheim, 2003; [Google Scholar]
- 10b. Ishimaru S., Saito K., Ikeuchi S., Massalska-Arodz M., Witko W., J. Phys. Chem. B 2005, 109, 10020–10024. [DOI] [PubMed] [Google Scholar]
- 11. Ren Y., Kan W. H., Henderson M. A., Bomben P. G., Berlinguette C. P., Thangadurai V., Baumgartner T., J. Am. Chem. Soc. 2011, 133, 17014–17026. [DOI] [PubMed] [Google Scholar]
- 12. Ghosh S. K., Zhang J. P., Kitagawa S., Angew. Chem. Int. Ed. 2007, 46, 7965–7968; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 8111–8114. [Google Scholar]
- 13. Horike S., Shimomura S., Kitagawa S., Nat. Chem. 2009, 1, 695–704. [DOI] [PubMed] [Google Scholar]
- 14. Gliemann G., Yersin H., Struct. Bonding 1985, 62, 87–153. [Google Scholar]
- 15. Nagle C. C., Patent U. S., 1989, No. 4826774.
- 16.
- 16a. Exstrom C. L., J. R. Sowa, Jr. , Daws C. A., Janzen D., Mann K. R., Chem. Mater. 1995, 7, 15; [Google Scholar]
- 16b. Buss C. E., Anderson C. E., Pomije M. K., Lutz C. M., Britton D., Mann K. R., J. Am. Chem. Soc. 1998, 120, 7783; [Google Scholar]
- 16c. Buss C. E., Mann K. R., J. Am. Chem. Soc. 2002, 124, 1031. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Kato M., Omura A., Toshikawa A., Kishi S., Sugimoto Y., Angew. Chem. Int. Ed. 2002, 41, 3183–3185; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2002, 114, 3315–3317; [Google Scholar]
- 17b. Ohba T., Kobayashi A., Chang H.-C., Kato M., Dalton Trans. 2013, 42, 5514–5523. [DOI] [PubMed] [Google Scholar]
- 18.
- 18a. Yam V. W., Au V. K., Leung S. Y., Chem. Rev. 2015, 115, 7589–7728; [DOI] [PubMed] [Google Scholar]
- 18b. Katz M. J., Sakai K., Leznoff D. B., Chem. Soc. Rev. 2008, 37, 1884–1895; [DOI] [PubMed] [Google Scholar]
- 18c. Kojima M., Taguchi H., Tsuchimoto M., Nakajima K., Coord. Chem. Rev. 2003, 237, 183–196; [Google Scholar]
- 18d. Huang R. W., Wei Y. S., Dong X. Y., Wu X. H., Du C. X., Zang S. Q., Mak T. C. W., Nat. Chem. 2017, 9, 689–697; [DOI] [PubMed] [Google Scholar]
- 18e. Varju B. R., Ovens J. S., Leznoff D. B., Chem. Commun. 2017, 53, 6500–6503; [DOI] [PubMed] [Google Scholar]
- 18f. Yang N. N., Sun W., Xi F. G., Sui Q., Chen L. J., Gao E. Q., Chem. Commun. 2017, 53, 1747–1750. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a. Ogoshi T., Shimada Y., Sakata Y., Akine S., Yamagishi T. A., J. Am. Chem. Soc. 2017, 139, 5664–5667; [DOI] [PubMed] [Google Scholar]
- 19b. Panda T., Maiti D. K., Panda M. K., ACS Appl. Mater. Interfaces 2018, 10, 29100–29106; [DOI] [PubMed] [Google Scholar]
- 19c. Iasilli G., Martini F., Minei P., Ruggeri G., Pucci A., Faraday Discuss. 2017, 196, 113–129. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a. Kar P., Yoshida M., Shigeta Y., Usui A., Kobayashi A., Minamidate T., Matsunaga N., Kato M., Angew. Chem. Int. Ed. 2017, 56, 2345–2349; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 2385–2389; [Google Scholar]
- 20b. Ohkoshi S., Arai K., Sato Y., Hashimoto K., Nat. Mater. 2004, 3, 857–861; [DOI] [PubMed] [Google Scholar]
- 20c. Mastuzaki H., Kishida H., Okamoto H., Takizawa K., Matsunaga S., Takaishi S., Miyasaka H., Sugiura K., Yamashita M., Angew. Chem. Int. Ed. 2005, 44, 3240–3243; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 3304–3307. [Google Scholar]
- 21.
- 21a. Chi Z., Zhang X., Xu B., Zhou X., Ma C., Zhang Y., Liu S., Xu J., Chem. Soc. Rev. 2012, 41, 3878–3896; [DOI] [PubMed] [Google Scholar]
- 21b. Sagara Y., Kato T., Nat. Chem. 2009, 1, 605–610. [DOI] [PubMed] [Google Scholar]
- 22. Ito H., Saito T., Oshima N., Kitamura N., Ishizaka S., Hinatsu Y., Wakeshima M., Kato M., Tsuge K., Sawamura M., J. Am. Chem. Soc. 2008, 130, 10044–10045. [DOI] [PubMed] [Google Scholar]
- 23. Sagara Y., Mutai T., Yoshikawa I., Araki K., J. Am. Chem. Soc. 2007, 129, 1520–1521. [DOI] [PubMed] [Google Scholar]
- 24. Ito H., Muromoto M., Kurenuma S., Ishizaka S., Kitamura N., Sato H., Seki T., Nat. Commun. 2013, 4, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Takamizawa S., Miyamoto Y., Angew. Chem. Int. Ed. 2014, 53, 6970–6973; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 7090–7093. [Google Scholar]
- 26. Takamizawa S., Takasaki Y., Chem. Sci. 2016, 7, 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mir S. H., Takasaki Y., Engel E. R., Takamizawa S., Angew. Chem. Int. Ed. 2017, 56, 15882–15885; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16098–16101. [Google Scholar]
- 28. Miyatake T., Tamiaki H., J. Photochem. Photobiol. C 2005, 6, 89–107. [DOI] [PubMed] [Google Scholar]
- 29.
- 29a. Ribó J. M., Crusats J., Sagués F., Claret J., Rubires R., Science 2001, 292, 2063–2066; [DOI] [PubMed] [Google Scholar]
- 29b. Kitagawa Y., Segawa H., Ishii K., Angew. Chem. Int. Ed. 2011, 50, 9133–9136; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 9299–9302; [Google Scholar]
- 29c. Hamba F., Niimura K., Kitagawa Y., Ishii K., Phys. Fluids 2014, 26, 017101. [Google Scholar]
- 30. Nakai K., Ishii K., Kobayashi N., Yonehara H., Pac C., J. Phys. Chem. B 2003, 107, 9749–9755. [Google Scholar]
- 31. Higashi T., Ohmori M., Ramananarivo M. F., Fujii A., Ozaki M., APL Mater. 2015, 3, 126107. [Google Scholar]
- 32. Panda M. K., Ghosh S., Yasuda N., Moriwaki T., Mukherjee G. D., Reddy C. M., Naumov P., Nat. Chem. 2015, 7, 65–72. [DOI] [PubMed] [Google Scholar]
- 33. Krishna G. R., Devarapalli R., Lal G., Reddy C. M., J. Am. Chem. Soc. 2016, 138, 13561–13567. [DOI] [PubMed] [Google Scholar]
- 34. Varughese S., Kiran M. S., Ramamurty U., Desiraju G. R., Angew. Chem. Int. Ed. 2013, 52, 2701–2712; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 2765–2777. [Google Scholar]
- 35. Irie M., Fukaminato T., Matsuda K., Kobatake S., Chem. Rev. 2014, 114, 12174–12277. [DOI] [PubMed] [Google Scholar]
- 36.
- 36a. Gavezzotti A., Acc. Chem. Res. 1994, 27, 309–314; [Google Scholar]
- 36b. Reilly A. M., Cooper R. I., Adjiman C. S., Bhattacharya S., Boese A. D., Brandenburg J. G., Bygrave P. J., Bylsma R., Campbell J. E., Car R., Case D. H., Chadha R., Cole J. C., Cosburn K., Cuppen H. M., Curtis F., Day G. M., R. A. DiStasio, Jr. , Dzyabchenko A., van Eijck B. P., Elking D. M., van den Ende J. A., Facelli J. C., Ferraro M. B., Fusti-Molnar L., Gatsiou C. A., Gee T. S., de Gelder R., Ghiringhelli L. M., Goto H., Grimme S., Guo R., Hofmann D. W., Hoja J., Hylton R. K., Iuzzolino L., Jankiewicz W., de Jong D. T., Kendrick J., de Klerk N. J., Ko H. Y., Kuleshova L. N., Li X., Lohani S., Leusen F. J., Lund A. M., Lv J., Ma Y., Marom N., Masunov A. E., McCabe P., McMahon D. P., Meekes H., Metz M. P., Misquitta A. J., Mohamed S., Monserrat B., Needs R. J., Neumann M. A., Nyman J., Obata S., Oberhofer H., Oganov A. R., Orendt A. M., Pagola G. I., Pantelides C. C., Pickard C. J., Podeszwa R., Price L. S., Price S. L., Pulido A., Read M. G., Reuter K., Schneider E., Schober C., Shields G. P., Singh P., Sugden I. J., Szalewicz K., Taylor C. R., Tkatchenko A., Tuckerman M. E., Vacarro F., Vasileiadis M., Vazquez-Mayagoitia A., Vogt L., Wang Y., Watson R. E., de Wijs G. A., Yang J., Zhu Q., Groom C. R., Acta Crystallogr. B 2016, 72, 439–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.
- 37a. Bousquet D., Coudert F. X., Fossati A. G., Neimark A. V., Fuchs A. H., Boutin A., J. Chem. Phys. 2013, 138, 174706; [DOI] [PubMed] [Google Scholar]
- 37b. Takae K., Tanaka H., Proc. Natl. Acad. Sci. USA 2018, 115, 9917–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harada J., Shimojo T., Oyamaguchi H., Hasegawa H., Takahashi Y., Satomi K., Suzuki Y., Kawamata J., Inabe T., Nat. Chem. 2016, 8, 946–952. [DOI] [PubMed] [Google Scholar]
- 39.
- 39a. Vogelsberg C. S., Garcia-Garibay M. A., Chem. Soc. Rev. 2012, 41, 1892–1910; [DOI] [PubMed] [Google Scholar]
- 39b. Jin M., Chung T. S., Seki T., Ito H., Garcia-Garibay M. A., J. Am. Chem. Soc. 2017, 139, 18115–18121. [DOI] [PubMed] [Google Scholar]


