Abstract
This multicentre retrospective cohort study included 447 patients with Hinchey Ib and II diverticular abscesses, who were treated with antibiotics, with or without percutaneous drainage. Abscesses of 3 and 5 cm in size were at higher risk of short‐term treatment failure and emergency surgery respectively. Initial non‐surgical treatment of Hinchey Ib and II diverticular abscesses was comparable between patients treated with antibiotics only and those who underwent percutaneous drainage in combination with antibiotics, with regard to short‐ and long‐term outcomes.
Most do not need drainage
Abstract
Background
Treatment strategies for diverticulitis with abscess formation have shifted from (emergency) surgical treatment to non‐surgical management (antibiotics with or without percutaneous drainage (PCD)). The aim was to assess outcomes of non‐surgical treatment and to identify risk factors for adverse outcomes.
Methods
Patients with a first episode of CT‐diagnosed diverticular abscess (modified Hinchey Ib or II) between January 2008 and January 2015 were included retrospectively, if initially treated non‐surgically. Baseline characteristics, short‐term (within 30 days) and long‐term treatment outcomes were recorded. Treatment failure was a composite outcome of complications (perforation, colonic obstruction and fistula formation), readmissions, persistent diverticulitis, emergency surgery, death, or need for PCD in the no‐PCD group. Regression analyses were used to analyse risk factors for treatment failure, recurrences and surgery.
Results
Overall, 447 patients from ten hospitals were included (Hinchey Ib 215; Hinchey II 232), with a median follow‐up of 72 (i.q.r. 55–93) months. Most patients were treated without PCD (332 of 447, 74·3 per cent). Univariable analyses, stratified by Hinchey grade, showed no differences between no PCD and PCD in short‐term treatment failure (Hinchey I: 22·3 versus 33 per cent, P = 0·359; Hinchey II: 25·9 versus 36 per cent, P = 0·149) or emergency surgery (Hinchey I: 5·1 versus 6 per cent, P = 0·693; Hinchey II: 10·4 versus 15 per cent, P = 0·117), but significantly more complications were found in patients with Hinchey II disease undergoing PCD (12 versus 3·7 per cent; P = 0·032). Multivariable analyses showed that treatment strategy (PCD versus no PCD) was not independently associated with short‐term treatment failure (odds ratio (OR) 1·47, 95 per cent c.i. 0·81 to 2·68), emergency surgery (OR 1·29, 0·56 to 2·99) or long‐term surgery (hazard ratio 1·08, 95 per cent c.i. 0·69 to 1·69). Abscesses of at least 3 cm in diameter were associated with short‐term treatment failure (OR 2·05, 1·09 to 3·86), and abscesses of 5 cm or larger with the need for surgery during short‐term follow‐up (OR 2·96, 1·03 to 8·13).
Conclusion
The choice between PCD with antibiotics or antibiotics alone as initial non‐surgical treatment of Hinchey Ib and II diverticulitis does not seem to influence outcomes.
Introduction
Diverticulosis is common in the Western world and is estimated to affect more than half of the population over the age of 65 years1. Diverticulosis might lead to diverticulitis in approximately 4·3–7 per cent of patients2, 3, of which 25 per cent present with acute complicated diverticulitis; this can consist of severe complications, such as abscess, perforation, stenosis or fistula4. Abscess formation occurs in approximately 15 per cent of patients with acute complicated diverticulitis5, 6, 7. It can be classified according to the modified Hinchey classification as type Ib (confined pericolic abscess smaller than 5 cm) or Hinchey II (pelvic, distant intra‐abdominal or retroperitoneal abscess at least 5 cm in size)8, 9.
Over the years, treatment strategies for diverticulitis with abscess formation have gradually shifted from (emergency) surgical treatment to non‐surgical management comprising antibiotics with or without percutaneous drainage (PCD)10. Currently, guidelines11, 12, 13 advise that small pericolic abscesses can be treated with antibiotics, whereas distant (pelvic) or larger abscesses, usually defined as those with a diameter of 3–5 cm or larger, should be treated with PCD, if possible. As patients undergoing non‐surgical treatment are at risk of adverse outcomes such as emergency surgery, disease recurrence, readmission and even death (both in the short and long term)10, 14, adequate patient selection for the optimal choice of treatment has come to play an important role in the management of these patients.
However, the clinical course of complicated diverticulitis with abscess formation after non‐surgical treatment, as well as the risk factors for adverse outcomes, have not been analysed adequately10, 12. Most of the existing studies15, 16, 17, 18, 19, 20, 21, 22 addressing these topics are limited by a short follow‐up, small and single‐institution study populations and a lack of time‐to‐event analysis.
Therefore, the primary aim of this multicentre retrospective study was to assess both the short‐ and long‐term outcomes of initial non‐surgical treatment strategies for acute complicated diverticulitis with abscess formation (Hinchey Ib and II) in a large number of patients. The second aim was to identify risk factors associated with adverse outcomes, to help facilitate adequate patient selection and assess the optimal treatment strategy.
Methods
This multicentre retrospective study was conducted in two academic and eight teaching hospitals in the Netherlands. The study was approved by the institutional review boards of all participating hospitals. This article was written in accordance with the STROBE statement and checklist23. All patients aged 18 years and older, who had a first episode of CT‐diagnosed complicated diverticulitis with abscess formation (modified Hinchey Ib or II8), and who had initial non‐surgical treatment, being either antibiotic treatment (no PCD) or antibiotic treatment with PCD, were eligible for inclusion in the cohort. Patients with perforated diverticulitis with peritonitis (Hinchey III or IV) and those with signs of sepsis or concurrent fistula formation were excluded. Potentially eligible patients who presented between 1 January 2008 and 31 January 2015 were sought by using a diagnosis‐specific code (Diagnose Behandeling Combinatie or Diagnosis Related Group), ICD‐9 or ICD‐10 codes in all hospital databases. In Gelre Hospital, patients could only be identified between 1 January 2012 and 31 January 2015. Subsequently, patients' medical records were screened for inclusion and exclusion criteria before definitive inclusion in the study cohort.
Data collection
All medical records were reviewed retrospectively. Baseline patient characteristics were collected, such as age, BMI, co‐morbidities, medical and surgical history, previous episodes of uncomplicated diverticulitis, medication, smoking, alcohol consumption and ASA fitness grades. Radiological details of the number, location and size of abscesses were recorded, as well as clinical signs and symptoms (nausea, vomiting, bowel complaints, rectal blood loss), and laboratory parameters (C‐reactive protein (CRP) and white blood cell count (WBC)). The largest reported size of the abscess was used as the measure of abscess size. Details of treatment were recorded, including type and duration of antibiotic treatment, PCD (approach, type of drain and duration of drainage) and surgical procedures (for example, elective or emergency resection or stoma reversal surgery).
Outcomes
Short‐term outcomes were: treatment failure, complications (colonic obstruction, perforation and fistula formation), clinical deterioration/progression of disease, emergency surgery (all unscheduled operations), readmissions, persistent diverticulitis (complaints lasting more than 30 days) and death. Long‐term outcomes were: recurrent (un)complicated diverticulitis episodes, sigmoid resection and death. Short term was defined as the first 30 days after diagnosis of abscess, or during the primary admission if a patient was still in hospital after 30 days, whereas long term was defined as the period thereafter. Treatment failure was defined as the composite outcome of complications, readmissions, persistent diverticulitis, emergency surgery, death or need for PCD in the no‐PCD group. Recurrent diverticulitis was registered as complicated in the presence of a phlegmon, abscess, fistula, stenosis or perforation, whereas uncomplicated diverticulitis was registered if it was mentioned in the medical record as recurrent disease, in the absence of the abovementioned complications.
Statistical analysis
Multiple imputation techniques were used to impute missing data to avoid selection bias. Data were assumed to be missing at random. All reported results are based on the imputed data, where the estimates of interests at the final computational step were combined across the imputed data sets using Rubin's rules24. Continuous variables are presented as mean(s.d.) or median (i.q.r.), depending on the normality of data distribution, and compared using the independent t test or Mann–Whitney U test, as appropriate. Categorical variables are presented as numbers with percentages, and were analysed using Pearson's χ2 test and Fisher's exact test.
Differences in patient and disease characteristics between patients with and without treatment failure and emergency surgery were assessed to identify risk factors for these outcomes. Univariable logistic regression analyses were used to calculate crude odds ratios (ORs) with 95 per cent confidence intervals. Inclusion of relevant diagnostic items in the multivariable model, to identify independent predictors, was based on clinical knowledge and P values (P < 0·200 or P < 0·050, depending on the event rate). Recurrence and sigmoid resection in the long term were assessed by means of Kaplan–Meier estimates, stratified by Hinchey classification and treatment (no PCD versus PCD), with censoring at the end of study follow‐up or death. The effect of Hinchey classification and treatment on the outcome was assessed by means of the Mantel–Cox log rank test. Cox proportional hazards regression was used to analyse risk factors for recurrence and sigmoid resection in the long term. Hazard ratios (HRs) with 95 per cent confidence intervals are presented for co‐variables associated with recurrence or sigmoid resection during long‐term follow‐up. Differences between hospitals could have an effect on treatment outcomes; to test for this bias by clustering of data, the short‐ and long‐term analyses were also adjusted for hospital. Short‐term outcomes were adjusted by fitting a generalized linear mixed model for each outcome, using a logistic regression mixed model. Hinchey classification and PCD were entered separately as fixed effects and hospital as a random effect. For the short‐term multivariable logistic regression analyses, hospital was entered as a co‐variable in each multivariable model. Long‐term Cox regression analyses were adjusted by entering hospital as a co‐variable in each multivariable model. Finally, sensitivity analyses of the non‐imputed data set were undertaken to test whether the imputation technique had any influence on the outcomes of interest. All analyses were done using SPSS® version 24.0 (IBM, Armonk, New York, USA).
Results
Patient and disease characteristics are shown in Table 1. A total of 447 patients with CT‐proven Hinchey type Ib (215 patients) or II (232) diverticulitis were included. The Academic Medical Centre contributed 20 patients (4·5 per cent), Erasmus University Medical Centre 11 (2·5 per cent), Meander Medical Centre 69 (15·4 per cent), Havenziekenhuis 4 (0·9 per cent), IJsselland Hospital 24 (5·4 per cent), Amphia Hospital 84 (18·8 per cent), Reinier de Graaf Gasthuis 32 (7·2 per cent), Onze Lieve Vrouwe Gasthuis 99 (22·1 per cent), Gelre Hospital 51 (11·4 per cent) and Catharina Hospital 53 (11·9 per cent).
Table 1.
Baseline characteristics
| Total cohort (n = 447) | No PCD (n = 332) | PCD (n = 115) | P § | |
|---|---|---|---|---|
| Patient demographics | ||||
| Age (years)* | 61 (13) | 60 (13) | 63 (13) | 0·140¶ |
| Sex ratio (M : F) | 182 : 265 | 139 : 193 | 43 : 72 | 0·400 |
| BMI (kg/m2)* | 27·8 (5·7) | 27·6 (5·5) | 28·4 (6·3) | 0·404¶ |
| Smoking | 228 (51·0) | 167 (50·3) | 61 (53·0) | 0·603 |
| Alcohol consumption | 232 (51·9) | 178 (53·6) | 54 (47·0) | 0·274 |
| Co‐morbidities | ||||
| ASA fitness grade > II | 123 (27·5) | 84 (25·3) | 39 (33·9) | 0·075 |
| Patients with registered co‐morbidity | 271 (60·6) | 201 (60·5) | 70 (60·9) | 0·952 |
| Medical history | ||||
| History of diverticulitis | 137 (30·6) | 93 (28·0) | 44 (38·3) | 0·035 |
| History of abdominal surgery | 151 (33·8) | 119 (35·8) | 32 (27·8) | 0·130 |
| Medication | ||||
| NSAIDs | 182 (40·7) | 133 (40·1) | 49 (42·6) | 0·573 |
| Steroids | 43 (9·4) | 35 (10·5) | 8 (7·0) | 0·239 |
| Clinical symptoms | ||||
| Duration of symptoms (days)† | 7 (3–14) | 7 (3–12) | 8 (4–14) | 0·062# |
| Nausea | 236 (52·8) | 163 (49·1) | 73 (63·5) | 0·018 |
| Vomiting | 108 (24·2) | 65 (19·6) | 43 (37·4) | 0·001 |
| Diffuse abdominal pain | 61 (13·6) | 44 (13·3) | 16 (13·9) | 0·704** |
| Change in bowel habit | 294 (65·8) | 221 (66·6) | 73 (63·5) | 0·548 |
| Rectal blood loss | 70 (15·7) | 44 (13·3) | 26 (22·6) | 0·044 |
| Clinical signs | ||||
| Rebound tenderness | 141 (31·5) | 104 (31·3) | 37 (32·1) | 0·602 |
| Local muscular guarding/resistance | 115 (25·7) | 81 (24·4) | 34 (29·6) | 0·259 |
| Diffuse muscular guarding | 50 (11·2) | 32 (9·6) | 18 (15·7) | 0·255** |
| Temperature (°C)* | 37·7 (0·9) | 37·7 (0·9) | 37·8 (1·0) | 0·319¶ |
| Laboratory parameters* | ||||
| C‐reactive protein (mg/l) | 168 (106) | 149 (96) | 222 (114) | < 0·001¶ |
| White blood cell count (× 109/l) | 14·8 (5·2) | 14·3 (4·9) | 16·3 (5·6) | 0·001¶ |
| Radiological parameters | ||||
| Hinchey II‡ | 232 (51·9) | 135 (40·7) | 97 (84·3) | < 0·001 |
| Largest abscess diameter (cm)† | 4·2 (2·7–6·1) | 3·6 (2·5–5·1) | 6·4 (5·0–8·5) | < 0·001# |
| Distant location of abscess | 106 (23·7) | 71 (21·4) | 35 (30·4) | 0·046 |
| No. of patients with multiple abscesses | 63 (14·1) | 40 (12·0) | 23 (20·0) | 0·035 |
| Free peridiverticular air | 143 (32·0) | 106 (31·9) | 37 (32·2) | 0·925 |
| Free air in abdomen | 56 (12·5) | 36 (10·8) | 20 (17·4) | 0·252 |
| Free fluid | 90 (20·1) | 61 (18·4) | 29 (25·2) | 0·187 |
| Duration of hospital stay (days)† | 7 (5–13) | 7 (4–10) | 10 (7–18) | < 0·001# |
Values in parentheses are percentages, unless indicated otherwise; values are
mean(s.d.) and
median (i.q.r.).
Abscess 5 cm or larger in diameter and/or distant abscess. PCD, percutaneous drainage; NSAID, non‐steroidal anti‐inflammatory drug;
Pearson χ2 test, except
independent t test,
Mann–Whitney U test and
Fisher's exact test.
The mean(s.d.) age of the patients was 61(13) years and 40·7 per cent were men. The mean BMI of the total cohort was 27·8(5·7) kg/m2. Some 271 patients (60·6 per cent) had co‐morbidities and 123 (27·5 per cent) had an ASA fitness grade above II. The mean CRP level was 168(106) mg/l for the total cohort and mean WBC was 14·8(5·2) × 109/l.
Most patients were treated with amoxicillin–clavulanic acid (90 of 289, 31·1 per cent), cefuroxime and metronidazole (88 of 289, 30·4 per cent), ceftriaxone and metronidazole (41 of 289, 14·2 per cent), or other antibiotics (70 of 289, 24·2 per cent) such as clindamycin, co‐trimoxazole or piperacillin tazobactam; median duration of antibiotic treatment was 7 (i.q.r. 5–12) days. Information on route of antibiotic administration was available for 174 of 332 patients in the no‐PCD group and 67 of 115 in the PCD group; 36 (20·6 per cent) and six (9 per cent) patients respectively received oral antibiotics. Most patients (332, 74·3 per cent) were initially treated without PCD; the remaining 115 patients (26·7 per cent) underwent PCD for a median of 6 (3–16) days. The PCD approach was mainly transabdominal (86 of 115, 74·8 per cent), guided by either ultrasound imaging (49 of 115, 42·6 per cent) or CT (63 of 115, 54·8 per cent). Median duration of hospital stay was 7 (5–13) days and median follow‐up was 72 (55–93) months.
Levels of inflammatory parameters were higher in the PCD group, with a mean CRP concentration of 222(114) mg/l compared with 149(96) mg/l in the no‐PCD group, and mean WBC of 16·3(5·6) versus 14·3(4·9) × 109/l respectively. A larger proportion of patients in the PCD group were classified as having Hinchey II disease (84·3 versus 40·7 per cent), and with multiple abscesses (20·0 versus 12·0 per cent). Of 63 patients with multiple abscesses, four were known to use corticosteroids, one to use mycophenolic acid, and one patient had undergone renal transplantation, whereas none of these patients received chemotherapy around the time of presentation. Median abscess diameter was 6·4 (5·0–8·5) cm in the PCD group compared with 3·6 (2·5–5·1) cm in the group treated without PCD. Median duration of hospital stay was longer in the PCD group: 10 (7–18) versus 7 (4–10) days.
Missing data
All candidate predictors had missing data, except age, sex and ASA classification. Most variables had between 1 and 20 per cent missing data. Three variables had a large amount of missing data: BMI (47·9 per cent), smoking (60·6 per cent) and alcohol consumption (64·2 per cent). For abscess size, 31·5 per cent of data were missing. In total, 2140 data items (14·9 per cent) were imputed.
Short‐ and long‐term outcomes
Short‐and long‐term outcomes are summarized in Table 2. Of the total cohort, 120 patients (26·8 per cent) experienced treatment failure and 40 (8·9 per cent) required emergency surgery within 30 days after first presentation. One patient had operative drainage and a stoma was constructed in three patients, two of whom also underwent sigmoid resection in a second stage. Seventy‐one patients (15·9 per cent) were readmitted to hospital within 30 days after first presentation and 63 (14·1 per cent) had persistent diverticulitis. Overall, 16 patients in the no‐PCD group (4·8 per cent) underwent PCD during short‐term follow‐up and two in the PCD group (1·7 per cent) had a second PCD procedure. Five patients (1·1 per cent) died from severe sepsis caused by perforated diverticulitis. Three of these patients died after undergoing emergency surgery, whereas two did not have surgery or receive further treatment owing to co‐morbidity. In all, 122 patients (27·3 per cent) experienced one or more episodes of recurrent diverticulitis. In total, 166 episodes of recurrent diverticulitis were recorded, of which 94 (56·6 per cent) were uncomplicated and 72 (43·4 per cent) were complicated. Median time to recurrence was 8 (3–24) months. Eighteen patients (14·8 per cent) had a first recurrence within 1 month after the end of short‐term follow‐up.
Table 2.
Short‐ and long‐term outcomes
| Total cohort (n = 447) | Hinchey Ib (n = 215) | Hinchey II* (n = 232) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 447) | No PCD (n = 332) | PCD (n = 115) | P ‡ | No PCD (n = 197) | PCD (n = 18) | P | No PCD (n = 135) | PCD (n = 97) | P ‡ | |
| Short‐term outcomes | ||||||||||
| Treatment failure | 120 (26·8) | 79 (23·8) | 41 (35·7) | 0·013 | 44 (22·3) | 6 (33) | 0·359§ | 35 (25·9) | 35 (36) | 0·149 |
| Complications† | 25 (5·6) | 13 (3·9) | 12 (10·4) | 0·009 | 8 (4·2) | 0 (0) | 0·908§ | 5 (3·7) | 12 (12) | 0·032§ |
| Clinical deterioration/disease progression | 95 (21·3) | 59 (17·8) | 36 (31·3) | 0·002 | 30 (15·2) | 6 (33) | 0·091§ | 29 (21·5) | 30 (31) | 0·147 |
| Readmission | 71 (15·9) | 49 (14·8) | 22 (19·1) | 0·253 | 27 (13·7) | 5 (28) | 0·178§ | 22 (16·3) | 17 (18) | 0·714 |
| Persistent diverticulitis | 63 (14·1) | 42 (12·7) | 21 (18·3) | 0·130 | 23 (11·7) | 5 (28) | 0·100§ | 19 (14·1) | 16 (16) | 0·583 |
| Emergency surgery (sigmoid resection) | 40 (8·9) | 24 (7·2) | 16 (13·9) | 0·030 | 10 (5·1) | 1 (6) | 0·693§ | 14 (10·4) | 15 (15) | 0·117 |
| Death | 5 (1·1) | 3 (0·9) | 2 (1·7) | 0·607§ | 3 (1·5) | 0 (0) | 1·000§ | 0 (0) | 2 (2) | 0·332§ |
| Long‐term outcomes | ||||||||||
| Complications† | 74 (16·6) | 46 (13·9) | 28 (24·3) | 0·009§ | 25 (12·7) | 7 (39) | 0·016§ | 21 (15·6) | 21 (22) | 0·245 |
| Overall recurrence | 122 (27·3) | 93 (28·0) | 29 (25·2) | 0·474¶ | 54 (27·4) | 7 (39) | 0·623¶ | 39 (28·9) | 22 (23) | 0·349¶ |
| Sigmoid resection | 124 (27·7) | 87 (26·2) | 37 (32·2) | 0·07¶ | 57 (28·9) | 6 (33) | 0·474¶ | 30 (22·2) | 31 (32) | 0·046¶ |
| Death | 28 (6·3) | 16 (4·8) | 12 (10·4) | 0·048¶ | 8 (4·1) | 2 (11) | 0·263¶ | 8 (5·9) | 10 (10) | 0·270¶ |
Values in parentheses are percentages.
Abscess 5 cm or larger in diameter and/or distant abscess.
Colonic obstruction/ileus, fistula or perforation. PCD, percutaneous drainage.
Pearson χ2 test, except
Fisher's exact test and
Mantel–Cox log rank test.
During long‐term follow‐up, 13 patients (2·9 per cent) underwent PCD, seven in the no‐PCD and six in the PCD group. A total of 124 patients (27·7 per cent) required sigmoid resection, 14 in an emergency setting. Median time to operation was 5 (3–13) months. Twenty‐eight patients died (6·3 per cent) during long‐term follow‐up, two from diverticulitis‐related causes. One of these patients died from severe sepsis caused by anastomotic leakage after Hartmann reversal surgery, and one from severe sepsis owing to intestinal ischaemia after sigmoid resection for diverticular stenosis. Overall, data on colonic evaluation was available for 394 patients, of whom 239 (PCD 58, no PCD 181) underwent colonoscopy during follow‐up after a median of 10·9 (7·0–21·6) weeks. A malignancy was found in 12 of these patients, including nine in the no‐PCD group (P = 1·000).
During short‐term follow‐up, patients in the PCD group had significantly more emergency resections (13·9 versus 7·2 per cent; P = 0·030), as well as complications, treatment failure and clinical deterioration/disease progression. In analyses stratified by Hinchey grade, among patients with Hinchey II disease, significantly more complications were found in the PCD group (12 versus 3·7 per cent; P = 0·032). Figs 1 and 2 show the time‐to‐event analyses of recurrence and surgery during long‐term follow‐up; there were no significant differences in recurrence (P = 0·544) or surgery (P = 0·088). Overall, patients in the PCD group had significantly more complications during long‐term follow‐up (24·3 versus 13·9 per cent; P = 0·009), which was also evident in the Hinchey Ib subgroup (39 versus 12·7 per cent; P = 0·016). The mortality rate was higher in the PCD group (10·4 versus 4·8 per cent; P = 0·048). In the subgroup with Hinchey II disease, there were more sigmoid resections among patients who underwent PCD (32 versus 22·2 per cent; P = 0·046). No other differences between treatment groups were found in short‐ and long‐term outcomes.
Figure 1.
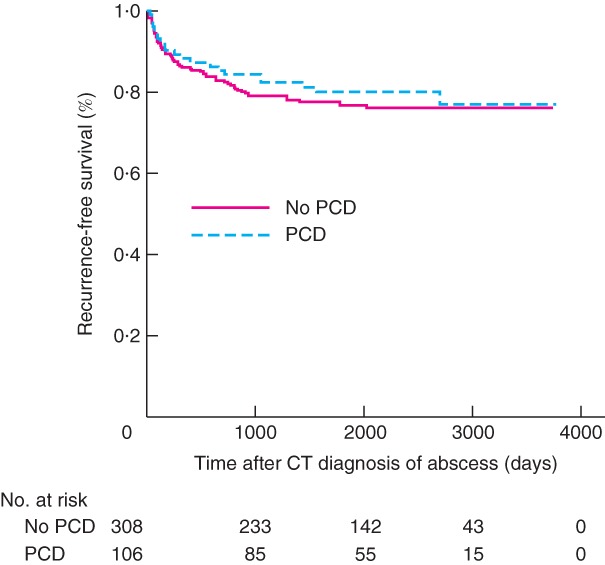
Kaplan–Meier analysis of recurrence‐free survival according to whether the patient underwent percutaneous abscess drainage
PCD, percutaneous drainage. P = 0·544 (log rank test).
Figure 2.
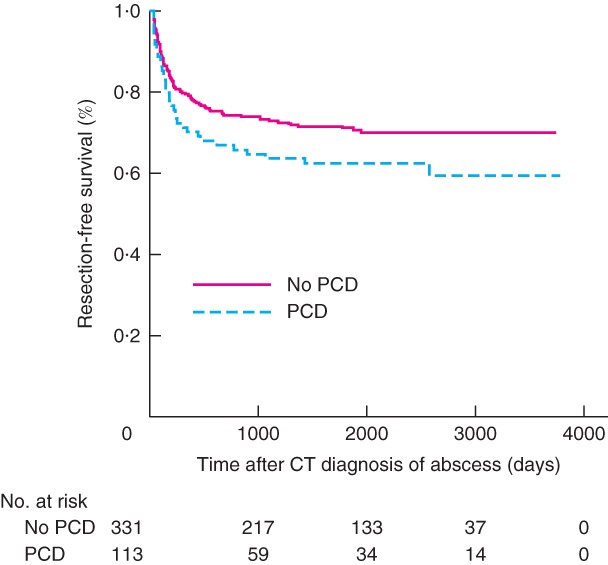
Kaplan–Meier analysis of resection‐free survival according to whether the patient underwent percutaneous abscess drainage
PCD, percutaneous drainage. P = 0·088 (log rank test).
Risk factors for treatment failure and emergency surgery during short‐term follow‐up
Univariable analyses of all possible predictors for treatment failure and emergency surgery are shown in Table S1 (supporting information). Different cut‐off sizes for abscess diameter were reviewed univariably to analyse which could best predict outcome. A cut‐off size of 3 cm seemed to be the best predictor of treatment failure (univariable OR 2·33, 95 per cent c.i. 1·32 to 4·11), and a cut‐off size of 5 cm the best predictor of emergency surgery (univariable OR 2·97, 1·28 to 6·85). The results of multivariable analysis are shown in Table 3. A higher BMI slightly decreased the risk of treatment failure (OR 0·94, 0·89 to 0·997), whereas an abscess size of at least 3 cm increased the risk (OR 2·05, 1·09 to 3·86). With regard to emergency surgery, history of abdominal surgery increased the risk (OR 2·05, 1·04 to 4·05), as did an abscess size of 5 cm or larger (OR 2·96, 1·08 to 8·13). No other variable had an effect on the risk of treatment failure or emergency surgery.
Table 3.
Multivariable logistic regression analysis of risk factors for short‐term treatment failure and emergency surgery
| Odds ratio | P | |
|---|---|---|
| Treatment failure | ||
| Age (per year) | 1·001 (0·98, 1·02) | 0·955 |
| BMI (per kg/m2) | 0·94 (0·89, 0·997) | 0·041 |
| Alcohol consumption | 0·63 (0·36, 1·10) | 0·099 |
| Co‐morbidity | 1·40 (0·85, 2·29) | 0·183 |
| NSAID prescription | 0·58 (0·21, 1·57) | 0·242 |
| Nausea | 1·32 (0·83, 2·12) | 0·245 |
| C‐reactive protein (mg/l) | 1·001 (0·998, 1·003) | 0·656 |
| Abscess ≥ 3 cm | 2·05 (1·09, 3·86) | 0·027 |
| Percutaneous drainage | 1·47 (0·81, 2·68) | 0·185 |
| Emergency surgery | ||
| History of abdominal surgery | 2·05 (1·04, 4·05) | 0·038 |
| Rebound tenderness | 2·03 (0·98, 4·21) | 0·058 |
| Abscess ≥ 5 cm | 2·96 (1·08, 8·13) | 0·036 |
| Percutaneous drainage | 1·29 (0·56, 2·99) | 0·554 |
Values in parentheses are 95 per cent confidence intervals. NSAID, non‐steroidal anti‐inflammatory drug.
Two separate subgroup analyses were performed to assess the effect of PCD on the outcome for different abscess sizes (at least 3 cm and at least 5 cm). The first included only the 324 patients with an abscess of 3 cm or larger. In this subgroup, there were no differences in rate of treatment failure between patients treated with (109) or without (215) PCD (35·7 versus 28·4 per cent respectively; P = 0·200), or in rate of emergency surgery (14·3 versus 9·3 per cent; P = 0·198). The second subgroup analysis included only the 185 patients with an abscess size of at least 5 cm. In this subgroup, there were also no differences in rate of treatment failure between patients treated with (94) and without (91) PCD (35 versus 28 per cent respectively; P = 0·409), or in rate of emergency surgery (16 versus 12 per cent; P = 0·416).
Risk factors for recurrence and sigmoid resection during long‐term follow‐up
Univariable analyses of all possible predictors for treatment failure and emergency surgery during long‐term follow‐up are shown in Table S2 (supporting information) and results of the subsequent multivariable analysis in Table 4. A history of diverticulitis increased the risk of recurrence (HR 1·71, 95 per cent c.i. 1·17 to 2·48). A history of abdominal surgery (HR 0·63, 0·42 to 0·98) and sigmoid resection (HR 0·15, 0·05 to 0·48) decreased the risk of recurrence. Older patients seemed to be at slightly higher risk of sigmoid resection during long‐term follow‐up (HR 1·02, 1·001 to 1·03) and the symptoms vomiting (HR 1·82, 1·13 to 2·93) and nausea (HR 1·72, 1·03 to 2·85) also increased this risk. No other variable had an effect on the occurrence of sigmoid resection during long‐term follow‐up.
Table 4.
Multivariable Cox regression analysis of risk factors for recurrence and surgery during long‐term follow‐up
| Hazard ratio | P | |
|---|---|---|
| Recurrence | ||
| Age (per year) | 0·995 (0·98, 1·01) | 0·481 |
| History of diverticulitis | 1·71 (1·17, 2·48) | 0·005 |
| History of abdominal surgery | 0·63 (0·42, 0·98) | 0·040 |
| Rebound tenderness | 0·72 (0·46, 1·13) | 0·152 |
| Sigmoid resection during short‐term follow‐up | 0·15 (0·05, 0·48) | 0·001 |
| Surgery | ||
| Age (per year) | 1·02 (1·001, 1·03) | 0·042 |
| Alcohol consumption | 0·64 (0·29, 1·39) | 0·218 |
| Co‐morbidity | 1·49 (0·96, 2·31) | 0·078 |
| History of diverticulitis | 1·30 (0·88, 1·93) | 0·190 |
| Duration of symptoms (per day) | 1·01 (0·996, 1·03) | 0·136 |
| Nausea | 1·72 (1·03, 2·85) | 0·037 |
| Vomiting | 1·82 (1·13, 2·93) | 0·014 |
| Diffuse abdominal pain | 0·60 (0·29, 1·25) | 0·161 |
| Distant abscess | 0·72 (0·42, 1·23) | 0·221 |
| Free peridiverticular air | 1·39 (0·91, 2·12) | 0·129 |
| Percutaneous drainage | 1·08 (0·69, 1·69) | 0·736 |
Values in parentheses are 95 per cent confidence intervals.
Sensitivity analyses
Overall, sensitivity analyses of the non‐imputed data set showed similar results for short‐ and long‐term outcomes, and short‐term complications and emergency resection were not significantly different in hospital‐adjusted analyses (Table S3, supporting information). Stratified analyses by Hinchey grade showed significant differences in the non‐imputed data for short‐term readmission and persistent diverticulitis (Table S4, supporting information). In addition, sensitivity analyses of the non‐imputed data set and hospital‐adjusted analyses were undertaken for multivariable logistic regression and Cox regression analyses (Tables S5 and S6, supporting information).
Discussion
In the present study, multivariable analysis showed that initial non‐surgical treatment of Hinchey Ib and II diverticular abscesses (antibiotics alone versus PCD in combination with antibiotics) had no independent effect on short‐ and long‐term outcomes. Abscess size of at least 3 cm was identified as an independent risk factor for short‐term treatment failure, and 5 cm or more as an independent risk factor for short‐term emergency surgery. Previous studies of treatment outcomes of diverticular abscesses have been limited by factors such as small and single‐institution study populations, a lack of time‐to‐event analysis and short follow‐up10, 12. The cohort study of 3148 patients with Hinchey stage Ib and II disease investigated by Gregersen and colleagues25, 26 remains the largest reporting on both short‐ and long‐term treatment outcomes. However, an important limitation of that study was the absence of data on the clinical condition of the patients, as well as data on abscess size and location. This complicates comparison of treatment modalities because, owing to the introduction of selection bias and confounding, differences in outcomes may primarily reflect disease and clinical severity. The present study, with a total of 447 patients, took these patient and disease characteristics into account, and also assessed long‐term outcomes.
The comparison of PCD and no PCD in this cohort showed that patients who underwent PCD seemed to have worse outcomes, in terms of a greater likelihood of short‐term emergency resections, complications, disease progression and treatment failure, as well as more long‐term complications. However, confounding by indication cannot be excluded from this analysis and differences may primarily reflect disease and clinical severity. Indeed, patients undergoing PCD had more advanced disease than those in the no‐PCD group, with the majority of patients having Hinchey stage II disease (84·3 versus 40·7 per cent; P < 0·001). Patients in the PCD group had larger abscesses, as well as significantly more distant abscesses or multiple abscesses. Hence, when the patients were stratified by Hinchey grade, most short‐ and long‐term outcomes did not differ between the PCD and no‐PCD groups, with the exception of short‐term complications and long‐term resections among patients with Hinchey II disease, and long‐term complications in those with Hinchey Ib diverticulitis. More importantly, in the multivariable analyses, the initial treatment strategy did not seem to be a predictor with regard to treatment failure, emergency surgery, or sigmoid resection in long‐term follow‐up, strengthening the conclusion that treatment strategy has no effect on the outcome.
The short‐term mortality rate in the present study ranged between 0 and 2 per cent across the groups analysed, which is comparable to pooled average mortality rates derived from previous studies of treatment with antibiotics (0·6 per cent) and PCD (1·6 per cent)14. Short‐term emergency surgery rates ranged from 5·1 to 15 per cent, which is also largely in accordance with the pooled average of 12·1 per cent14. Reported rates of diverticulitis recurrence vary from 3 to 68 per cent, with an average of 28 per cent; recurrent disease consists mostly of uncomplicated or locally complicated diverticulitis10. Although rates may vary between studies because of differences in median follow‐up or in definitions, the recurrence rates reported in the present study seem to be in line with earlier reports27, 28. In light of the long‐term surgery and recurrence rates in the present study, which were relatively low (and the recurrences mostly uncomplicated), it can be questioned whether elective surgery is indicated in conservatively managed patients, as surgery comes with an inherent risk of complication and most patients seem to fare well with conservative management alone.
The rates of adverse and unwanted outcomes in patients with diverticular abscess remain high and present a major burden to the patient, as well the healthcare system. Therefore, an aim of the present study was to identify potential risk factors related to these adverse outcomes in order to help improve individual patient management. Abscess size was shown to be an independent predictor of adverse outcome; an abscess diameter of at least 3 cm increased the risk of short‐term treatment failure, whereas an abscess of 5 cm or larger increased the risk of emergency surgery. These results indicate that, for abscesses larger than 3 cm, and particularly those larger than 5 cm, it can still be debated which treatment strategy is most appropriate, as the results show no definite advantage of one strategy over the other in short‐term outcomes. Treatment should not be based solely on abscess size, but other patient and disease characteristics should also be considered. However, no other significant predictors were found in the multivariable analyses for treatment failure or emergency surgery, making it difficult to select a subpopulation of patients who would benefit from PCD. The findings do seem to acknowledge that a cut‐off value of 3 cm is appropriate for differentiating between small and large abscesses11, 13, 17, 29, 30.
An important limitation of this study is its retrospective design, which introduces the potential for selection bias and confounding by indication. However, registration of a wide range of baseline patient and disease characteristics allowed correction for potential known confounders in multivariable logistic and Cox regression analyses. Another inevitable consequence of retrospective observational research is the potential risk of missing data, as the availability of baseline and outcome data is largely dependent on the completeness of medical records. It was hypothesized that re‐evaluation of CT images by one or more radiologists could have led to the introduction of (hindsight) bias, as a result of the radiologists' foreknowledge of the reasons for and outcomes of reviewing the images. To prevent selection bias introduced by missing data, multiple imputation methods were used to handle the missing data. Sensitivity analyses of the non‐imputed data set did not significantly change the results. With regard to outcome data, it is possible that patients might have received care at a general practitioner or in other hospitals during follow‐up, creating the potential for an underestimation of disease recurrences and readmissions. Finally, the multicentre setting of this study could have introduced heterogeneity through between‐hospital differences in treatment, such as reasons for choosing PCD or criteria for drain removal. However, these differences were considered small, because all hospitals base their practice on the national guideline for treatment of acute diverticulitis and hospital‐adjusted analyses showed comparable outcomes. In addition, the multicentre setting had beneficial effects by increasing both the study's generalizability and sample size.
This study of a large cohort of patients with Hinchey stage Ib and II abscesses has provided evidence that patients with abscesses of at least 3 or 5 cm are at a higher risk of short‐term treatment failure or emergency surgery respectively, regardless of the choice of non‐surgical treatment strategy. As no clear difference between the two treatment strategies was found, it remains debatable how to treat these patients appropriately. Nevertheless, these data help facilitate informed and shared decision‐making, as well as providing valuable information for future prospective studies regarding PCD treatment in patients with abscess formation.
Supporting information
Table S1. Univariable logistic regression analysis of risk factors for short‐term treatment failure and emergency surgery
Table S2. Univariable Cox regression analysis of risk factors for recurrence and surgery in long‐term follow‐up
Table S3. Sensitivity analyses of short‐ and long‐term outcomes
Table S4. Sensitivity analyses of Short‐ and long‐term outcomes in Hinchey IB and II patients
Table S5. Sensitivity analysis multivariable logistic regression (short‐term)
Table S6. Sensitivity analysis Cox regression (long‐term)
Acknowledgements
D.P.V.L and H.E.B. are joint first authors of this article.
Disclosure: The authors declare no conflict of interest.
Presented to a meeting of the Dutch Surgical Society, Veldhoven, the Netherlands, May 2015
References
- 1. Commane DM, Arasaradnam RP, Mills S, Mathers JC, Bradburn M. Diet, ageing and genetic factors in the pathogenesis of diverticular disease. World J Gastroenterol 2009; 15: 2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loffeld RJ. Long‐term follow‐up and development of diverticulitis in patients diagnosed with diverticulosis of the colon. Int J Colorectal Dis 2016; 31: 15–17. [DOI] [PubMed] [Google Scholar]
- 3. Shahedi K, Fuller G, Bolus R, Cohen E, Vu M, Shah R et al. Long‐term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol 2013; 11: 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andeweg CS, Mulder IM, Felt‐Bersma RJ, Verbon A, van der Wilt GJ, van Goor H et al.; Netherlands Society of Surgery; Working group from Netherlands Societies of Internal Medicine, Gastroenterologists, Radiology, Health Technology Assessment and Dieticians. Guidelines of diagnostics and treatment of acute left‐sided colonic diverticulitis. Dig Surg 2013; 30: 278–292. [DOI] [PubMed] [Google Scholar]
- 5. Ambrosetti P. Value of CT for acute left‐colonic diverticulitis: the surgeon's view. Dig Dis 2012; 30: 51–55. [DOI] [PubMed] [Google Scholar]
- 6. Eglinton T, Nguyen T, Raniga S, Dixon L, Dobbs B, Frizelle FA. Patterns of recurrence in patients with acute diverticulitis. Br J Surg 2010; 97: 952–957. [DOI] [PubMed] [Google Scholar]
- 7. Trenti L, Kreisler E, Galvez A, Golda T, Frago R, Biondo S. Long‐term evolution of acute colonic diverticulitis after successful medical treatment. World J Surg 2015; 39: 266–274. [DOI] [PubMed] [Google Scholar]
- 8. Wasvary H, Turfah F, Kadro O, Beauregard W. Same hospitalization resection for acute diverticulitis. Am Surg 1999; 65: 632–635. [PubMed] [Google Scholar]
- 9. Klarenbeek BR, de Korte N, van der Peet DL, Cuesta MA. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis 2012; 27: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamb MN, Kaiser AM. Elective resection versus observation after nonoperative management of complicated diverticulitis with abscess: a systematic review and meta‐analysis. Dis Colon Rectum 2014; 57: 1430–1440. [DOI] [PubMed] [Google Scholar]
- 11. Feingold D, Steele SR, Lee S, Kaiser A, Boushey R, Buie WD et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum 2014; 57: 284–294. [DOI] [PubMed] [Google Scholar]
- 12. Sartelli M, Catena F, Ansaloni L, Coccolini F, Griffiths EA, Abu‐Zidan FM et al. WSES Guidelines for the management of acute left sided colonic diverticulitis in the emergency setting. World J Emerg Surg 2016; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galetin T, Galetin A, Vestweber KH, Rink AD. Systematic review and comparison of national and international guidelines on diverticular disease. Int J Colorectal Dis 2018; 33: 261–272. [DOI] [PubMed] [Google Scholar]
- 14. Gregersen R, Mortensen LQ, Burcharth J, Pommergaard HC, Rosenberg J. Treatment of patients with acute colonic diverticulitis complicated by abscess formation: a systematic review. Int J Surg 2016; 35: 201–208. [DOI] [PubMed] [Google Scholar]
- 15. Ambrosetti P, Chautems R, Soravia C, Peiris‐Waser N, Terrier F. Long‐term outcome of mesocolic and pelvic diverticular abscesses of the left colon: a prospective study of 73 cases. Dis Colon Rectum 2005; 48: 787–791. [DOI] [PubMed] [Google Scholar]
- 16. Kaiser AM, Jiang JK, Lake JP, Ault G, Artinyan A, Gonzalez‐Ruiz C et al. The management of complicated diverticulitis and the role of computed tomography. Am J Gastroenterol 2005; 100: 910–917. [DOI] [PubMed] [Google Scholar]
- 17. Brandt D, Gervaz P, Durmishi Y, Platon A, Morel P, Poletti PA. Percutaneous CT scan‐guided drainage vs. antibiotherapy alone for Hinchey II diverticulitis: a case–control study. Dis Colon Rectum 2006; 49: 1533–1538. [DOI] [PubMed] [Google Scholar]
- 18. Elagili F, Stocchi L, Ozuner G, Dietz DW, Kiran RP. Outcomes of percutaneous drainage without surgery for patients with diverticular abscess. Dis Colon Rectum 2014; 57: 331–336. [DOI] [PubMed] [Google Scholar]
- 19. Gaertner WB, Willis DJ, Madoff RD, Rothenberger DA, Kwaan MR, Belzer GE et al. Percutaneous drainage of colonic diverticular abscess: is colon resection necessary? Dis Colon Rectum 2013; 56: 622–626. [DOI] [PubMed] [Google Scholar]
- 20. Hall J. Should elective colectomy be routine following percutaneous drainage of a diverticular abscess? Dis Colon Rectum 2013; 56: 533–534. [DOI] [PubMed] [Google Scholar]
- 21. Neff CC, vanSonnenberg E, Casola G, Wittich GR, Hoyt DB, Halasz NA et al. Diverticular abscesses: percutaneous drainage. Radiology 1987; 163: 15–18. [DOI] [PubMed] [Google Scholar]
- 22. Singh B, May K, Coltart I, Moore NR, Cunningham C. The long‐term results of percutaneous drainage of diverticular abscess. Ann R Coll Surg Engl 2008; 90: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ et al.; STROBE initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007; 147: W163–W194. [DOI] [PubMed] [Google Scholar]
- 24. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley: Hoboken, 2004. [Google Scholar]
- 25. Gregersen R, Andresen K, Burcharth J, Pommergaard HC, Rosenberg J. Short‐term mortality, readmission, and recurrence in treatment of acute diverticulitis with abscess formation: a nationwide register‐based cohort study. Int J Colorectal Dis 2016; 31: 983–990. [DOI] [PubMed] [Google Scholar]
- 26. Gregersen R, Andresen K, Burcharth J, Pommergaard HC, Rosenberg J. Long‐term mortality and recurrence in patients treated for colonic diverticulitis with abscess formation: a nationwide register‐based cohort study. Int J Colorectal Dis 2018; 33: 431–440. [DOI] [PubMed] [Google Scholar]
- 27. Buchwald P, Dixon L, Wakeman CJ, Eglinton TW, Frizelle FA. Hinchey I and II diverticular abscesses: long‐term outcome of conservative treatment. ANZ J Surg 2017; 87: 1011–1014. [DOI] [PubMed] [Google Scholar]
- 28. Jalouta T, Jrebi N, Luchtefeld M, Ogilvie JW Jr. Diverticulitis recurrence after percutaneous abscess drainage. Int J Colorectal Dis 2017; 32: 1367–1373. [DOI] [PubMed] [Google Scholar]
- 29. Dharmarajan S, Hunt SR, Birnbaum EH, Fleshman JW, Mutch MG. The efficacy of nonoperative management of acute complicated diverticulitis. Dis Colon Rectum 2011; 54: 663–671. [DOI] [PubMed] [Google Scholar]
- 30. Siewert B, Tye G, Kruskal J, Sosna J, Opelka F, Raptopoulos V et al. Impact of CT‐guided drainage in the treatment of diverticular abscesses: size matters. AJR Am J Roentgenol 2006; 186: 680–686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariable logistic regression analysis of risk factors for short‐term treatment failure and emergency surgery
Table S2. Univariable Cox regression analysis of risk factors for recurrence and surgery in long‐term follow‐up
Table S3. Sensitivity analyses of short‐ and long‐term outcomes
Table S4. Sensitivity analyses of Short‐ and long‐term outcomes in Hinchey IB and II patients
Table S5. Sensitivity analysis multivariable logistic regression (short‐term)
Table S6. Sensitivity analysis Cox regression (long‐term)


