Abstract
Aim
The aim of the present study was to investigate the patterns of platelet reactivity and discriminators of therapeutic response to dual antiplatelet therapy (DAPT) with aspirin and ticagrelor or prasugrel in patients with acute coronary syndrome (ACS).
Design
In this multicentre prospective observational study, 492 patients with ACS were enrolled. Platelet aggregation was determined by multiple electrode aggregometry after stimulation with adenosine diphosphate (ADP) or arachidonic acid (AA) as agonists in the maintenance phase of treatment with prasugrel or ticagrelor.
Results
Age emerged as the strongest variable influencing aspirin response status: The mean AA‐induced platelet aggregation in patients <49 years of age was 49% higher than in those >49 years (13.1 U vs 8.8 U; P = 0.011). The second strongest discriminator of aspirin response was sex: Male patients had a 40% higher AA‐induced platelet aggregation values than female patients (9.5 U vs 6.8 U; P = 0.026). Platelet count emerged as the only variable influencing ADP antagonists response status showing that patients with platelet count >320 g/L displayed higher ADP‐induced platelet aggregation. About 12% of patients had high on‐treatment platelet reactivity (HTPR) to aspirin, 3% and 4% a HTPR to prasugrel and ticagrelor, respectively, and only 2% displayed HTPR to dual antiplatelet therapy.
Conclusion
When potent platelet inhibitors as prasugrel and ticagrelor are administered with aspirin, HTPR to DAPT plays only a marginal role.
Keywords: ACS, HTPR, LTPR, MEA, platelets, prasugrel, ticagrelor
1. INTRODUCTION
Dual antiplatelet therapy (DAPT) combining aspirin and a P2Y12 inhibitor is a widespread therapy with proven benefit to reduce recurrent adverse ischaemic events in patients after acute coronary syndrome (ACS).1, 2 Current guidelines of ACS primarily recommend ticagrelor and prasugrel combined with aspirin, leaving clopidogrel solely recommended in patients with contraindications for the novel P2Y12 inhibitors.3 Both ticagrelor and prasugrel have been shown to provide a significant mortality reduction, including prevention of major adverse cardiac events (MACE).4, 5 These benefits go in hand with increased procedural and nonprocedural bleeding risk.6 Novel P2Y12 inhibitors harbour superior pharmacodynamic characteristics with a faster and more potent pharmacological response as compared to clopidogrel.7 Although both substances were designed to overcome individual treatment responses, a significant proportion of ACS patients still exhibit high or low on‐treatment reactivity with subsequent ischaemic or bleeding events.8, 9 To date, clinical benefits of platelet reactivity testing remain controversial, but it is a valuable tool to identify those patients at risk for adverse outcomes even in patients treated with novel P2Y12 inhibitors whose treatment responses are more predictable. In contrast, clinical role of high on‐treatment reactivity (HTPR) to aspirin remains an issue of debate.10
For this purpose, we aimed to characterize platelet reactivity in a large prospectively enrolled registry of ACS patients treated with novel antiplatelet agents and aspirin to better depict those factors associated with individual response to mono and dual antiplatelet therapy.
2. METHODS
2.1. Study design
The study was a prospective observational cohort study performed at two Austrian University Hospitals: the Medical University of Vienna and the Medical University of Graz. The Ethics Committee of the Medical University of Vienna and the Medical University of Graz approved the study protocol in accordance with the Declaration of Helsinki. Participants were included in the study starting in July 2012 until December 2016. Inclusion criteria were a written informed consent obtained before the study entry, treatment with prasugrel or ticagrelor for ACS and age >18 years. The only exclusion criterion was participation in interventional trials. Patients were followed up by a telephone interview at 1, 6 and 12 months. Four hundred and ninety‐two consecutive patients fulfilling the inclusion and exclusion criteria were enrolled. All patients received a loading dose of prasugrel (60 mg) or ticagrelor (180 mg) followed by a once daily dose of 10 mg prasugrel or two daily dose of ticagrelor (90 mg), respectively. The type of the P2Y12 blocker used was at the discretion of the treating interventional cardiologist according to the current guidelines and the intern standard operating procedures (SOP) for the antiplatelet treatment of ACS patients in the both study centres. According to the intern standard operating procedure (SOP) of the Department, which are based on the current guidelines11, 12 the following treatment algorithm was used: Prasugrel was the P2Y12 receptor inhibitor of the first choice in patients presenting with STEMI or patients with NSTE‐ACS and diabetes, if the coronary angiography was already available (unless not contraindicated). Ticagrelor was the P2Y12 receptor inhibitor of the first choice in patients with NSTE‐ACS (unless not contraindicated). Blood samples from patients were obtained in the in‐hospital maintenance phase of treatment, at least 24 hour after loading dose of prasugrel/ticagrelor and after PCI, at 8 am after the morning maintenance dose administration of prasugrel/ticagrelor. The study is reported according to the STROBE (strengthening the reporting of observational studies in epidemiology) standards.
2.2. Impedance aggregometry
Whole blood aggregation was determined using multiple electrode aggregometry (MEA) on a new generation impedance aggregometer (Multiplate Analyzer, Verum Diagnostica GmbH, Munich, Germany).13, 14 The system detects the electrical impedance change due to the adhesion and aggregation of platelets on two independent electrode‐set surfaces in the test cuvette. We used hirudin as anticoagulant, which is recommended by the manufacturer. We used adenosine diphosphate (ADP) and or arachidonic acid (AA) as agonists.15 A 1:2 dilution of whole blood anticoagulated with hirudin and 0.9% NaCl was stirred at 37°C for 3 minute in the test cuvettes, ADP: 6.4 µmol/L or AA: 0.5 mmol/L were added, and the increase in electrical impedance was recorded continuously for 6 minute.16 The mean values of the 2 independent determinations are expressed as the area under the curve (AUC) of the aggregation tracing. We reported AUC in units (U).17 Platelet aggregometry was performed directly after blood sampling at the Department of Cardiology at the Medical University of Vienna. The tests were performed in each participant. According to the position document, ADP values up to 19 U were classified as low on‐treatment platelet reactivity (LTPR), 20‐45 U as moderate on‐treatment platelet reactivity (MTPR) and values >46 as HTPR to prasugrel or ticagrelor.18 AA values > 20 U were classified as HTPR to aspirin, according to the previous study.10 For platelet aggregation analyses comparing ticagrelor and prasugrel, patients receiving abciximab were excluded.
2.3. Study endpoints
The primary endpoint was ADP‐ and AA‐induced platelet aggregation.
2.4. Statistical analysis
We estimated an exploratory sample size of 492 patients to characterize patients treated with prasugrel or ticagrelor and aspirin with regard to platelet aggregation under real‐life clinical conditions. Normal distribution was tested with the Kolmogorov‐Smirnov test. Data are expressed as mean, standard deviation (SD), 95% confidence intervals (CI) median or interquartile range (IQR), as appropriate. Statistical comparisons were performed with the Mann‐Whitney U test and the chi‐squared test when applicable. Classification tree analysis (chi‐squared automatic interaction detection: CHAID) was used to detect discriminators of platelet reactivity to ADP and AA, as described previously.19, 20 The analysis included common risk factors for coronary artery disease (cigarette smoking, diabetes mellitus, hypertension, family history of coronary artery disease and hyperlipidemia), past medical history (stroke, previous PCI and previous myocardial infarction), co‐morbidities (renal failure, periphery or cerebral vascular disease), age, status at hospitalization (STEMI or NSTE‐ACS), laboratory values at the day of hospitalization and sex. All statistical calculations were performed using commercially available statistical software (IBM SPSS Version 22.0; Chicago, USA).
3. RESULTS
3.1. Patient demographics
Patient flow through the study is shown in Figure 1. Of 1386 ACS patients screened, 492 fulfilled the inclusion criteria and were included in the study. Due to the standard operating procedures (SOP) for the antiplatelet treatment of ACS patients in the both study centres, prasugrel was mainly administered to patients with STEMI (92%), whereas patients with NSTE‐ACS were more frequently treated with ticagrelor (68%; P < 001; Table 1). The majority of further demographic differences between the both agents is most probably a consequence of this skew distribution of P2Y12 receptor blocker use between STEMI and NSTE‐ACS patients. Prasugrel‐treated patients were younger (57 vs 63 years of age; P < 0.001), more frequently male (86% vs 72%; P < 0.001), smoker (78% vs 58%; P < 0.001), with a family history of CAD (44% vs 35%; P = 0.035) and a higher procedural GpIIb/IIIa blocker use compared with ticagrelor‐treated individuals, respectively (Table 1). Patients treated with ticagrelor had more frequently a history of hypertension (75% vs 64%; P = 0.011) and a longer median total stent length (33.8 mm vs 30.1 mm; P = 0.004) compared with prasugrel‐treated individuals, respectively (Table 1).
Figure 1.
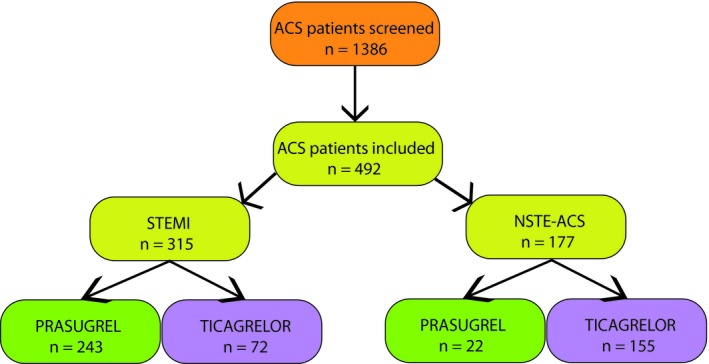
Patient flow
Table 1.
Patient demographics
| Patient demographics | Overall N = 492 | Prasugrel N = 265 | Ticagrelor N = 227 | P |
|---|---|---|---|---|
| Age (y) | 59 ± 12 | 57 ± 11 | 63 ± 13 | <0.001 |
| Gender (male) n (%) | 390 (79) | 227 (86) | 163 (72) | <0.001 |
| Risk factors/past medical history n (%) | ||||
| Body mass index (BMI; mean ± SD) | 27.7 ± 4.8 | 27.9 ± 4.7 | 27.5 ± 4.9 | Ns |
| Hypertension | 337 (69) | 168 (64) | 169 (75) | 0.011 |
| Hyperlipidemia | 273 (56) | 139 (53) | 134 (59) | Ns |
| Smoking | 336 (67) | 205 (78) | 131 (58) | <0.001 |
| Family history of CAD | 196 (40) | 117 (44) | 79 (35) | 0.035 |
| Diabetes mellitus | 97 (20) | 47 (18) | 50 (22) | Ns |
| Prior PCI | 60 (12) | 31 (12) | 29 (13) | Ns |
| Prior myocardial infarction | 89 (18) | 47 (18) | 42 (19) | Ns |
| Peripheral arterial occlusive disease | 25 (5) | 16 (6) | 9 (4) | Ns |
| Cerebrovascular disease | 196 (40) | 117 (44) | 79 (35) | Ns |
| Laboratory data (mean ± SD) | ||||
| Platelets (x109/L) | 233 ± 69 | 265 ± 66 | 235 ± 72 | Ns |
| C reactive protein (mg/dL) | 4.6 ± 7.2 | 3.4 ± 7.6 | 6.6 ± 6.4 | Ns |
| White blood cell count (WBC; x109/L) | 10.1 ± 3.8 | 11.5 ± 3.6 | 10.3 ± 3.9 | <0.001 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.4 | Ns |
| Haemoglobin (g/dL) | 14.2 ± 1.8 | 14.6 ± 1.7 | 13.8 ± 1.9 | <0.001 |
| Fibrinogen (mg/dL) | 387 ± 94 | 377 ± 88 | 399 ± 100 | 0.039 |
| Concomitant medications n (%) | ||||
| GpIIb/IIIa blocker during the PCI | 146 (30) | 105 (40) | 41 (18) | <0.001 |
| Aspirin | 492 (100) | 265 (100) | 227 (100) | Ns |
| Proton pump Inhibitors (PPI) | 397 (83) | 212 (82) | 185 (83) | Ns |
| ß blockers | 429 (89) | 231 (89) | 197 (89) | Ns |
| Statins | 456 (95) | 249 (96) | 207 (93) | Ns |
| Angiotensin converting enzyme (ACE) inhibitors | 426 (88) | 233 (90) | 193 (87) | Ns |
| Calcium channel blockers | 34 (7) | 14 (5) | 20 (9) | Ns |
| ACS data | ||||
| NSTE‐ACS | 177 (36) | 22(8) | 155(68) | <0.001 |
| STEMI | 315 (64) | 243 (92) | 72 (32) | <0.001 |
| PCI | 452 (93) | 250 (94) | 202 (92) | Ns |
| Number of stents per patient | 1.5 ± 1.0 | 1.5 ± 0.9 | 1.6 ± 1.2 | Ns |
| Total stent length | 32.1 ± 22.0 | 30.1 ± 19.3 | 33.8 ± 24.8 | 0.004 |
CAD, coronary artery disease; MEA, multiple electrode aggregometry; NSTE‐ACS, none ST‐elevation acute coronary syndrome; PCI, percutaneous coronary intervention; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
Data are reported as Mean ± standard deviation (SD), n (number of patients) or percentages.
3.2. ADP‐ and AA‐induced platelet aggregation
ADP‐induced platelet aggregation was almost normally distributed in both ticagrelor‐ and prasugrel‐treated patients (Figure 2A). In contrast, AA‐induced platelet aggregation was skewed distributed in patients treated with ticagrelor and prasugrel (Figure 2B).
Figure 2.
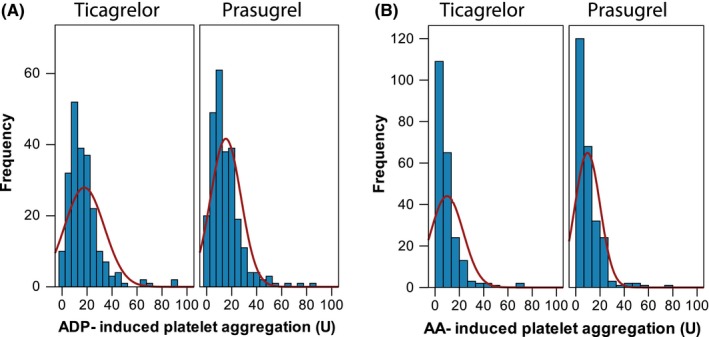
Adenosine diphosphate (ADP)‐ and arachidonic acid (AA)‐induced platelet aggregation assessed by multiple electrode aggregometry (MEA) in patients treated with ticagrelor or prasugrel and aspirin
3.3. ADP‐ and AA‐induced platelet aggregation according to clinical presentation
In patients presenting with NSTE‐ACS, the median level of ADP‐induced platelet aggregation did not differ between prasugrel and ticagrelor (12 U, interquartile range IQR 8‐22 U vs 12 U, IQR 7‐21 U; respectively, P = 0.605; Figure 3A). In line, in STEMI patients, there was no difference in ADP‐induced platelet aggregation between prasugrel and ticagrelor (11 U, interquartile range IQR 5‐22 U vs 15 U, IQR 9‐22 U; respectively, P = 0.115; Figure 3A).
Figure 3.
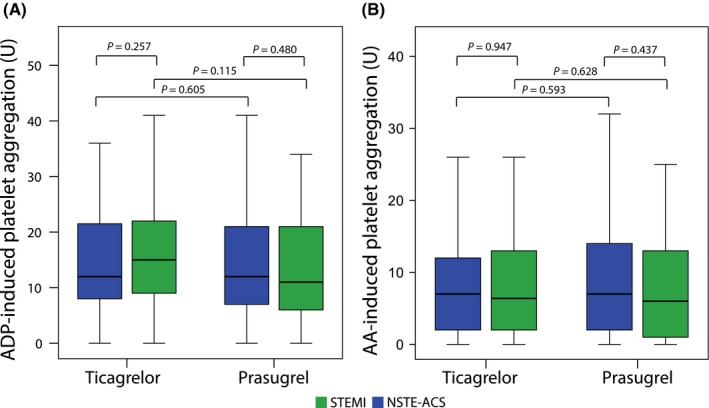
A, Adenosine diphosphate (ADP)‐ and (B): arachidonic acid (AA) ‐ induced platelet aggregation assessed by multiple electrode aggregometry (MEA) in patients treated with ticagrelor and prasugrel according to the clinical presentation: ST‐elevation myocardial infarction (STEMI) vs. none ST‐elevation acute coronary syndrome (ACS) during the hospital stay after the maintenance dose administration
In patients presenting with NSTE‐ACS, the median level of AA‐induced platelet aggregation did not differ between prasugrel and ticagrelor (6.4 U, interquartile range IQR 2‐13 U vs 6 U, IQR 1‐14 U; respectively, P = 0.593; Figure 3B). In line, in STEMI patients, there was no difference of AA‐induced platelet aggregation between prasugrel and ticagrelor (7 U, interquartile range IQR 2‐12 U vs 7 U, IQR 2‐14 U; respectively, P = 0.628; Figure 3B).
3.4. Distribution of ADP‐ and AA‐induced platelet aggregation
According to a predefined cut‐offs, only 3% of patients in the prasugrel group and 4% of study participants in the ticagrelor group had high on‐treatment platelet reactivity (HTPR; Figure 4). Moderate on‐treatment platelet reactivity (MTPR) was found in 27% prasugrel‐treated patients and in 31% of ticagrelor‐treated patients. The majority of patients displayed low on‐treatment platelet reactivity (LTPR; prasugrel: 70%; ticagrelor: 65%; Figure 4).
Figure 4.
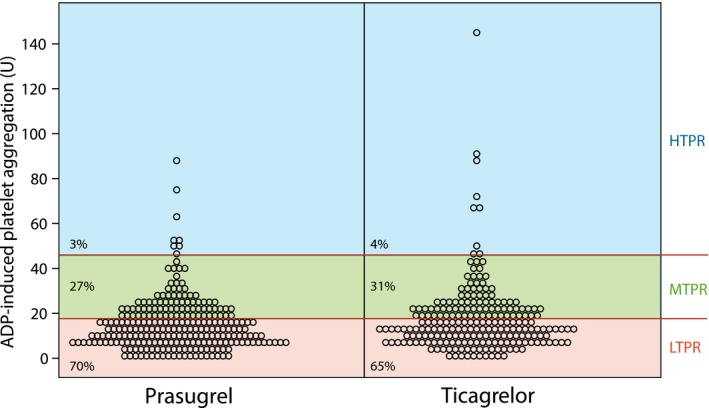
Scatter plot showing distribution of adenosine diphosphate (ADP)‐ induced platelet aggregation values in relation to high on‐treatment platelet reactivity (HTPR), moderate on treatment platelet reactivity (MTPR) and low on treatment platelet reactivity (LTPR) in patients treated with ticagrelor and prasugrel
According to a predefined cut‐off, 12% of patients had HTPR to aspirin treatment.
Whereas AA‐ and ADP‐induced platelet aggregation weakly correlated in ticagrelor‐treated patients (r 2 = 0.49; P < 0.001), this correlation was moderate for prasugrel (r 2 = 0.62; P < 0.001). About 1% of ticagrelor‐ and 2% of prasugrel‐treated patients were classified as displaying a HTPR phenotype to both agonists (right upper quadrant of Figure 5).
Figure 5.
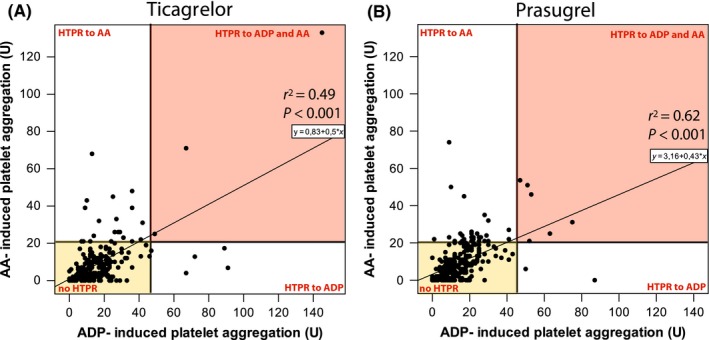
Scatter plot showing the correlation between arachidonic acid (AA) and adenosine diphosphate (ADP) – induced aggregation assessed by multiple electrode aggregometry (MEA) in ticagrelor (A) and prasugrel treated patients (B). HTPR: high on treatment platelet reactivity
3.5. Contribution of clinical characteristics to the phenotype of response to aspirin and ADP receptor blockers
Classification tree analysis (CHAID) was used to detect discriminators of the phenotype of response to antiplatelet agents. The analysis included common risk factors for coronary artery disease, past medical history, co‐morbidities, co‐medication, age, status at hospitalization (stable angina or acute coronary syndrome), laboratory parameters and sex. Age emerged as the strongest variable influencing aspirin response status (Figure 6): The mean AA‐induced platelet aggregation in those younger than 49 years of age was 49% higher as in older patients (13.1 U vs 8.8 U; P = 0.011). The second strongest discriminator was sex: Male patients had 40% higher AA‐induced platelet aggregation values than female patients (Figure 6: 9.5 U vs 6.8 U; P = 0.026).
Figure 6.
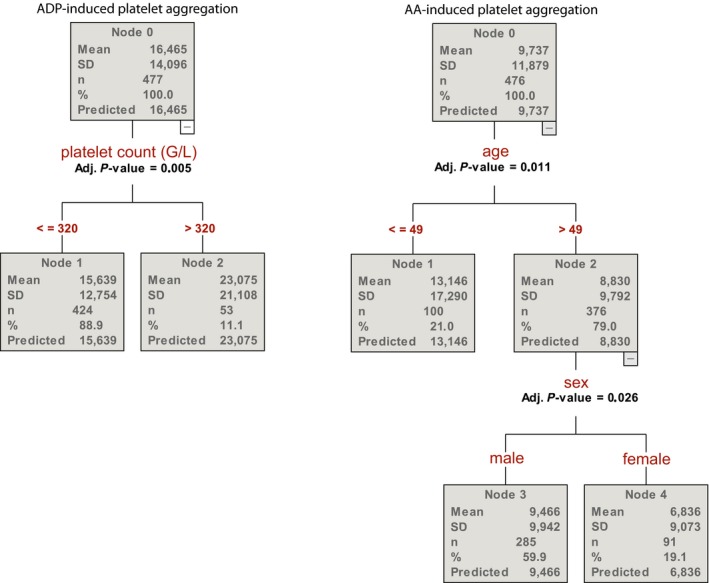
Chi‐squared automatic interaction detection (CHAID) analysis for the discriminators of arachidonic acid (AA) and adenosine diphosphate (ADP) – induced aggregation assessed by multiple electrode aggregometry (MEA)
Platelet count emerged as the only variable influencing ADP antagonists response status showing that those with higher platelet count (>320 g/L) had higher ADP‐induced platelet aggregation (Figure 6).
4. DISCUSSION
In the present study, we aimed to characterize platelet reactivity and discriminators of therapeutic response in ACS patients treated with dual antiplatelet therapy consisting of potent platelet inhibitors prasugrel or ticagrelor and aspirin. We found that both ADP‐blockers show a similar potency to inhibit platelet reactivity during the maintenance phase of treatment: We observed in both ACS entities NSTE‐ACS and STEMI that only 3% of all patients treated with prasugrel and 4% treated with ticagrelor exhibited HTPR. This frequency is somehow remarkable as previous reports stated up to 4‐fold higher incidence of HTPR in patients under prasugrel or ticagrelor treatment, with some reports even reporting 35%‐46% of those patients inadequately responding to the novel P2Y12 inhibitors.8, 21, 22 We assume that this difference might be due to the characteristics of our study cohort of ACS patients that have not been pre‐treated with clopidogrel. Previous studies that reported higher HTPR incidences in prasugrel‐ and ticagrelor‐treated patients have enrolled significant numbers of patients that displayed HTPR under clopidogrel.23, 24 Data from haemodialysis patients 25 or from randomized controlled trials have remarkably shown that despite the switch to a novel more potent P2Y12 inhibitor up to 20% of the patients continue to exhibit a HTPR phenotype if they have already been it on clopidogrel treatment.25, 26, 27 For this purpose, HTPR in clopidogrel can be assumed as an independent risk factor for HTPR in patients treated with novel platelet inhibitors. In our treatment naive study cohort, we found satisfying levels of platelet inhibition as consequence of treatment with novel P2Y12 inhibitors. In a next step, we aimed to characterize the response to aspirin treatment as previous observational studies have shown that HTPR to arachidonic acid (AA) which reflects low responsiveness to acetylsalicylic acid might occur in up to 45% of all patients.19, 28 In contrast HTPR to P2Y12 inhibitors, the pathomechanism of low responsiveness to aspirin is less well characterized. Current literature indicates that it is a consequence of pharmacodynamic characteristics (under dosing, poor absorption and noncompliance) and altered metabolism (stress‐induced generation of COX‐2 and polymorphisms in the COX‐1).19, 29 HTPR to aspirin translates into markedly increased risk of all course mortality and risk for stent thrombosis.10 In our study cohort, we found 12% of our patients to exhibit HTPR while on treatment with aspirin. Nevertheless, ACS patients are treated with DAPT within the highly vulnerable phase of 12 months post‐ACS, in which low response to one antiplatelet agent might be compensated by the second agent. Not surprisingly, dual nonresponsiveness to antiplatelet treatment has been shown to increase the risk for cardiovascular events.19, 30 Current literature shows that up to 9% of all patients treated with clopidogrel and aspirin exhibit this high‐risk phenotype. However, the prevalence of dual nonresponsiveness to novel P2Y12 inhibitors and aspirin in ACS patients was unknown. To the best of our knowledge, we are the first to investigate the incidence of dual nonresponsiveness when using ticagrelor or prasugrel in combination with aspirin. We observed that only 1% of ticagrelor‐ and 2% of prasugrel‐treated patients were classified as displaying a HTPR phenotype to both aspirin an ADP‐blockers, further reassuring the benefit of DAPT in those patients.
Regarding bleeding risk, the majority of our patients exhibited LTPR with 70% of the prasugrel‐ and 65% of the ticagrelor‐treated patients exhibiting this exaggerated treatment response. In line with our previous findings,24 we observed an skewed distribution of HTPR vs. LTPR for prasugrel (3% vs. 70%, respectively) and ticagrelor (4% vs. 64%; respectively) as compared to published data for clopidogrel (41% vs. 20%; respectively).24, 31 Overall, the proportion of patients with moderate on‐treatment reactivity (MTPR) was in line with previous observations (prasugrel: 27%, ticagrelor: 31%),24 which is comparable to MTPR rates in clopidogrel‐treated patients.20, 32 Bearing in mind that roughly only one third of all patients exhibit this phenotype with the most favourable net clinical benefit, we ought to identify discriminators of response phenotype to antiplatelet agents. The most potent discriminator of the response to aspirin was age, as those older than 49 years of age, especially female patients, displayed the lowest platelet aggregation values. This might explain why females, especially older ones, under treatment with aspirin might be at increased bleeding risk, as reported in several studies.33, 34 In opposite, younger males had less potent aspirin effect on average, which, however, was sufficient in the majority of them with only 12% HTPR.
For ADP antagonists, we identified platelet count (>320 g/L) as a risk factor for higher platelet reactivity, which might be only of minor clinical importance. Platelet count also emerged as a predictor of response to clopidogrel in several studies.35 Interestingly, and in contrary to clopidogrel,2, 7 no co‐morbidities or other clinical characteristic influenced the response to prasugrel or ticagrelor. Especially in diabetes mellitus, an attenuated response to clopidogrel and aspirin therapy has been shown.36, 37, 38, 39 However, in our study cohort we could not identify diabetes as discriminator in the chi‐squared automatic interaction detection analysis for inadequate treatment response, proving that the novel more potent antiplatelet agents ticagrelor and prasugrel seem to overcome this shortcoming.
4.1. Limitations
The main limitation of the study is lack of outcome data. Additionally, the time point of platelet function testing might have influenced our observations. Platelet function testing was performed within the hospital stay after the maintenance dose administration of P2Y12 receptor inhibitors, and not direct during the acute phase of infarction. Mean platelet volume has been shown to be an indicator of platelet activation and plays a pivotal role in the pathophysiology of atherosclerotic disease and is an independent risk factor for acute myocardial infarction.40, 41, 42 Subsequently, some observations proposed that MPV can predict nonresponsiveness to clopidogrel treatment.43, 44 Unfortunately, in the present study we did not record MPV or other platelet indices. However, previous studies have shown that MPV does not affect the response to novel antiplatelet therapies and does not influence the risk of HTPR with ticagrelor.45 For this purpose, we assume that the influence might be negligible in our study cohort treated with potent novel antiplatelet agents.
5. CONCLUSION
High on‐treatment reactivity to dual antiplatelet therapy is infrequent in patients concomitantly treated with potent P2Y12 inhibitors and aspirin in acute coronary syndrome. Age and gender were predictors of platelet reactivity to aspirin, which was lowest in patients older than 49 years of age, especially in female patients, suggesting that they might be at increased risk for bleeding. Whether such association exists, it must be explored in outcome studies.
CONFLICT OF INTEREST
DvL received honoraria for advisory boards from AstraZeneca and Daiichi Sankyo. JSM received lecture or consultant fees from Daaichi, Eli Lilly, Bayer, Roche and Astra Zeneca. All other authors report no conflict of interests.
Winter M‐P, Schneeweiss T, Cremer R, et al. Platelet reactivity patterns in patients treated with dual antiplatelet therapy. Eur J Clin Invest. 2019;49:e13102 10.1111/eci.13102
Funding information
The study was supported by a grant funded by the Austrian Society of Cardiology.
REFERENCES
- 1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070‐3078. [DOI] [PubMed] [Google Scholar]
- 2. Winter M‐P, Grove EL, De Caterina R, et al. Advocating cardiovascular precision medicine with P2Y12 receptor inhibitors. Eur Heart J Cardiovasc Pharmacother. 2017;3(4):221‐234. [DOI] [PubMed] [Google Scholar]
- 3. Selhorst G, Schmidtler F, Ott A, et al. Platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor in comparison to clopidogrel: a retrospective pharmacodynamic analysis. Platelets. 2018;1‐7. 10.1080/09537104.2018.1445836 [DOI] [PubMed] [Google Scholar]
- 4. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001‐2015. [DOI] [PubMed] [Google Scholar]
- 5. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045‐1057. [DOI] [PubMed] [Google Scholar]
- 6. Siller‐Matula JM, Hintermeier A, Kastner J, et al. Distribution of clinical events across platelet aggregation values in all‐comers treated with prasugrel and ticagrelor. Vascular pharmacology. 2016;79:6‐10. [DOI] [PubMed] [Google Scholar]
- 7. Winter MP, Kozinski M, Kubica J, Aradi D, Siller‐Matula JM. Personalized antiplatelet therapy with P2Y12 receptor inhibitors: benefits and pitfalls. Postepy Kardiol Interwencyjnej. 2015;11(4):259‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonello L, Pansieri M, Mancini J, et al. High on‐treatment platelet reactivity after prasugrel loading dose and cardiovascular events after percutaneous coronary intervention in acute coronary syndromes. J Am Coll Cardiol. 2011;58(5):467‐473. [DOI] [PubMed] [Google Scholar]
- 9. Parodi G, Valenti R, Bellandi B, et al. Comparison of prasugrel and ticagrelor loading doses in ST‐segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61(15):1601‐1606. [DOI] [PubMed] [Google Scholar]
- 10. Mayer K, Bernlochner I, Braun S, et al. Aspirin treatment and outcomes after percutaneous coronary intervention: results of the ISAR‐ASPI registry. J Am Coll Cardiol. 2014;64(9):863‐871. [DOI] [PubMed] [Google Scholar]
- 11. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119‐177. [DOI] [PubMed] [Google Scholar]
- 12. Neumann F‐J, Sousa‐Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87‐165. [DOI] [PubMed] [Google Scholar]
- 13. Christ G, Siller‐Matula JM, Francesconi M, Dechant C, Grohs K, Podczeck‐Schweighofer A. Individualising dual antiplatelet therapy after percutaneous coronary intervention: the IDEAL‐PCI registry. BMJ Open. 2014;4(10):e005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siller‐Matula JM, Lang IM, Neunteufl T, et al. Interplay between genetic and clinical variables affecting platelet reactivity and cardiac adverse events in patients undergoing percutaneous coronary intervention. PLoS One. 2014;9(7):e102701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siller‐Matula JM, Gouya G, Wolzt M, Jilma B. Cross validation of the multiple electrode aggregometry. A prospective trial in healthy volunteers. Thromb Haemost. 2009;102(2):397‐403. [DOI] [PubMed] [Google Scholar]
- 16. Komosa A, Siller‐Matula Jm, Kowal J, et al. Comparison of the antiplatelet effect of two clopidogrel bisulfate formulations: Plavix and generic‐Egitromb. Platelets. 2015;26(1):43‐47. [DOI] [PubMed] [Google Scholar]
- 17. Siller‐Matula JM, Christ G, Lang IM, Delle‐Karth G, Huber K, Jilma B. Multiple electrode aggregometry predicts stent thrombosis better than the VASP assay. J Thromb Haemost. 2010;8(2):351‐359. [DOI] [PubMed] [Google Scholar]
- 18. Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on‐treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261‐2273. [DOI] [PubMed] [Google Scholar]
- 19. Siller‐Matula JM, Delle‐Karth G, Christ G, et al. Dual non‐responsiveness to antiplatelet treatment is a stronger predictor of cardiac adverse events than isolated non‐responsiveness to clopidogrel or aspirin. Int J Cardiol. 2013;167(2):430‐435. [DOI] [PubMed] [Google Scholar]
- 20. Siller‐matula Jm, Delle‐karth G, Lang Im, et al. Phenotyping vs. genotyping for prediction of clopidogrel efficacy and safety: the PEGASUS‐PCI study. J Thromb Haemost. 2012;10(4):529‐542. [DOI] [PubMed] [Google Scholar]
- 21. Michelson AD, Frelinger AL, Braunwald E, et al. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON‐TIMI 38 trial. Eur Heart J. 2009;30(14):1753‐1763. [DOI] [PubMed] [Google Scholar]
- 22. Alexopoulos D, Xanthopoulou I, Gkizas V, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5(6):797‐804. [DOI] [PubMed] [Google Scholar]
- 23. Mayer K, Orban M, Bernlochner I, et al. Predictors of antiplatelet response to prasugrel during maintenance treatment. Platelets. 2015;26(1):53‐58. [DOI] [PubMed] [Google Scholar]
- 24. Siller‐Matula JM, Akca B, Neunteufl T, et al. Inter‐patient variability of platelet reactivity in patients treated with prasugrel and ticagrelor. Platelets. 2016;27(4):373‐377. [DOI] [PubMed] [Google Scholar]
- 25. Alexopoulos D, Panagiotou A, Xanthopoulou I, et al. Antiplatelet effects of prasugrel vs. double clopidogrel in patients on hemodialysis and with high on‐treatment platelet reactivity. J Thromb Haemost. 2011;9(12):2379‐2385. [DOI] [PubMed] [Google Scholar]
- 26. Alexopoulos D, Xanthopoulou I, Davlouros P, et al. Prasugrel overcomes high on‐clopidogrel platelet reactivity in chronic coronary artery disease patients more effectively than high dose (150 mg) clopidogrel. Am Heart J. 2011;162(4):733‐739. [DOI] [PubMed] [Google Scholar]
- 27. Dridi NP, Johansson PI, Clemmensen P, et al. Prasugrel or double‐dose clopidogrel to overcome clopidogrel low‐response–the TAILOR (Thrombocytes And IndividuaLization of ORal antiplatelet therapy in percutaneous coronary intervention) randomized trial. Platelets. 2014;25(7):506‐512. [DOI] [PubMed] [Google Scholar]
- 28. Crescente M, Di Castelnuovo A, Iacoviello L, Vermylen J, Cerletti C, de Gaetano G. Response variability to aspirin as assessed by the platelet function analyzer (PFA)‐100. A systematic review. Thromb Haemost. 2008;99(1):14‐26. [DOI] [PubMed] [Google Scholar]
- 29. Michelson Ad, Cattaneo M, Eikelboom Jw, et al. Aspirin resistance: position paper of the Working Group on Aspirin Resistance. J Thromb Haemost. 2005;3(6):1309‐1311. [DOI] [PubMed] [Google Scholar]
- 30. Eshtehardi P, Windecker S, Cook S, et al. Dual low response to acetylsalicylic acid and clopidogrel is associated with myonecrosis and stent thrombosis after coronary stent implantation. Am Heart J. 2010;159(5):891‐898.e1. [DOI] [PubMed] [Google Scholar]
- 31. Aradi D, Kirtane A, Bonello L, et al. Bleeding and stent thrombosis on P2Y12‐inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 2015;36(27):1762‐1771. [DOI] [PubMed] [Google Scholar]
- 32. Siller‐Matula JM, Trenk D, Schrör K, et al. Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv. 2013;6(11):1111‐1128. [DOI] [PubMed] [Google Scholar]
- 33. Verdoia M, Pergolini P, Rolla R, et al. Advanced age and high‐residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J Thromb Haemost. 2016;14(1):57‐64. [DOI] [PubMed] [Google Scholar]
- 34. Horyniecki M, Lacka‐Gazdzik B, Niewiadomska E, Mazur B, Snit M, Labuz‐Roszak B. Prevalence of high on‐treatment platelet reactivity in patients with chronic kidney disease treated with acetylsalicylic acid for stroke prevention. Pol. Arch Intern Med. 2018;128(11):667‐676. [DOI] [PubMed] [Google Scholar]
- 35. Giustino G, Kirtane AJ, Généreux P, et al. Relation between platelet count and platelet reactivity to thrombotic and bleeding risk: from the assessment of dual antiplatelet therapy with drug‐eluting stents study. Am J Cardiol. 2016;117(11):1703‐1713. [DOI] [PubMed] [Google Scholar]
- 36. Schuette C, Steffens D, Witkowski M, et al. The effect of clopidogrel on platelet activity in patients with and without type‐2 diabetes mellitus: a comparative study. Cardiovasc Diabetol. 2015;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kakouros N, Rade JJ, Kourliouros A, Resar JR. Platelet function in patients with diabetes mellitus: from a theoretical to a practical perspective. Int J Endocrinol. 2011;2011:742719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheen AJ, Legrand D. Aspirin and clopidogrel resistance in patients with diabetes mellitus. Eur Heart J. 2006;27(23):2900; author reply ‐1. [DOI] [PubMed] [Google Scholar]
- 39. Bonaventura A, Liberale L, Montecucco F. Aspirin in primary prevention for patients with diabetes: Still a matter of debate. Eur J Clin Invest. 2018;48(10):e13001. [DOI] [PubMed] [Google Scholar]
- 40. Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. 2003;9(3):177‐190. [DOI] [PubMed] [Google Scholar]
- 41. Pal R, Bagarhatta R, Gulati S, Rathore M, Sharma N. Mean platelet volume in patients with acute coronary syndromes: a supportive diagnostic predictor. J Clin Diagn Res. 2014;8(8):MC01‐MC04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta‐analysis. J Thromb Haemost. 2010;8(1):148‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Asher E, Fefer P, Shechter M, et al. Increased mean platelet volume is associated with non‐responsiveness to clopidogrel. Thromb Haemost. 2014;112(1):137‐141. [DOI] [PubMed] [Google Scholar]
- 44. Koh YY, Kim HH, Choi DH, et al. Relation between the change in mean platelet volume and clopidogrel resistance in patients undergoing percutaneous coronary intervention. Curr Vasc Pharmacol. 2015;13(5):687‐693. [DOI] [PubMed] [Google Scholar]
- 45. Verdoia M, Pergolini P, Rolla R, et al. Mean platelet volume and high‐residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Expert Opin Pharmacother. 2015;16(12):1739‐1747. [DOI] [PubMed] [Google Scholar]


