Abstract
Objective
Tofacitinib is an oral JAK inhibitor for the treatment of rheumatoid arthritis (RA). Altered lymphocyte cell counts and a potential association with increased infection rates have been reported in RA patients treated with JAK inhibitors. This analysis was undertaken to evaluate the short‐, mid‐, and long‐term effects of tofacitinib on lymphocytes and infection rates in patients with RA.
Methods
In this post hoc analysis, absolute lymphocyte counts (ALCs) were obtained from phase III studies (12–24 months; n = 717–958) and phase I/II/III/long‐term extension studies of tofacitinib (≤117 months) (All RA population; n = 7,061); lymphocyte subset counts (LSCs) were from phase II studies (1.5–6 months’ exposure; n = 236–486), an ORAL Sequel vaccine substudy (~22 months; n = 198), and an ORAL Sequel lymphocyte substudy (~50 months; n = 55–1,035) of tofacitinib. The reversibility of ALC/LSC changes was evaluated. The relationship of ALC and LSC to infections was analyzed in the All RA population. The value of monitoring ALC alone was assessed by examining correlations between ALCs and LSCs.
Results
Tofacitinib treatment resulted in an initial increase in ALC versus pretreatment baseline, which gradually declined to steady state by ~48 months. CD4+ and CD8+ T cell counts decreased over long‐term treatment, and ALC and LSC changes were reversible upon treatment cessation. Patients with ALCs of <500 cells/mm3 had an increased risk of serious infections. There was no strong association between CD4+ T cell, CD8+ T cell, B cell, or natural killer cell counts and serious infection incidence rates. ALC and CD4+ or CD8+ T cell counts correlated well (R = 0.65–0.86).
Conclusion
Our findings indicate that monitoring of ALC alone appears to be adequate to assess infection risk in tofacitinib‐treated patients with RA.
Introduction
Tofacitinib is an oral JAK inhibitor for the treatment of rheumatoid arthritis (RA). The efficacy and safety of tofacitinib 5 mg and 10 mg twice daily, administered as monotherapy or in combination with conventional synthetic disease‐modifying antirheumatic drugs (DMARDs) (mainly methotrexate [MTX]), in patients with moderately to severely active RA, have been demonstrated in phase II 1, 2, 3, 4, 5 and phase III 6, 7, 8, 9, 10, 11 studies of up to 24 months’ duration and in long‐term extension (LTE) studies with up to 114 months of observation 12, 13, 14.
Tofacitinib partially and reversibly inhibits signaling of multiple cytokines via the JAK/STAT pathway 15, 16. Members of the common γ‐chain family of cytokines, including interleukin‐2 (IL‐2), IL‐4, IL‐7, IL‐9, IL‐15, and IL‐21, signal through JAK1/JAK3 and are important for the development and proliferation of T cells, natural killer (NK) cells, and B cells 17, 18, 19; therefore, the modulation of cytokine signaling by tofacitinib might be expected to change immune cell counts and function over time, resulting in immune response suppression. Several studies within the tofacitinib development program have evaluated cell‐mediated immunity and humoral‐mediated immunity 20, 21, 22, 23.
Treatment with tofacitinib is associated with increased infections, likely related to immunomodulation, relative to findings in patients treated with placebo. In a meta‐analysis of interventional studies, rates of serious infections associated with tofacitinib in patients with moderately to severely active RA were similar to those reported with biologic DMARDs 24. A recent report noted a trend toward increasing risk of serious infection with lower lymphocyte counts 25. Confirmed decreases in absolute lymphocyte counts (ALCs) to <500 cells/mm3 occur during the first 3 months of exposure in ~0.04% of patients receiving tofacitinib at 5 mg or 10 mg twice daily; recommendations state that these patients should discontinue treatment if this threshold is reached, due to an increased risk of serious infection 26, 27.
Here, we evaluate the effects of tofacitinib on ALCs, lymphocyte subset counts (LSCs), and infection rates in patients with RA. The data are discussed in the context of immune function. Our primary objective was to characterize the short‐, mid‐, and long‐term effects of tofacitinib treatment on ALCs and LSCs in patients with RA. Additional objectives were 1) to assess whether ALC and LSC changes observed with long‐term tofacitinib treatment are reversible upon treatment cessation, 2) to evaluate the association of infection rates in patients receiving tofacitinib with ALCs or LSCs, and 3) to assess the value of monitoring LSCs in addition to ALCs to mitigate the risk of infection.
Patients and Methods
Patient populations
Patient data were derived from relevant phase I, II, and III and LTE studies from the tofacitinib development program. Patients were age ≥18 years and fulfilled the American College of Rheumatology 1987 revised criteria for classification of RA 28. Patients in LTE studies had previously participated in a qualifying phase I, II, or III index study of tofacitinib 12. An overview of the patient populations evaluated in this study is presented in Figure 1.
Figure 1.
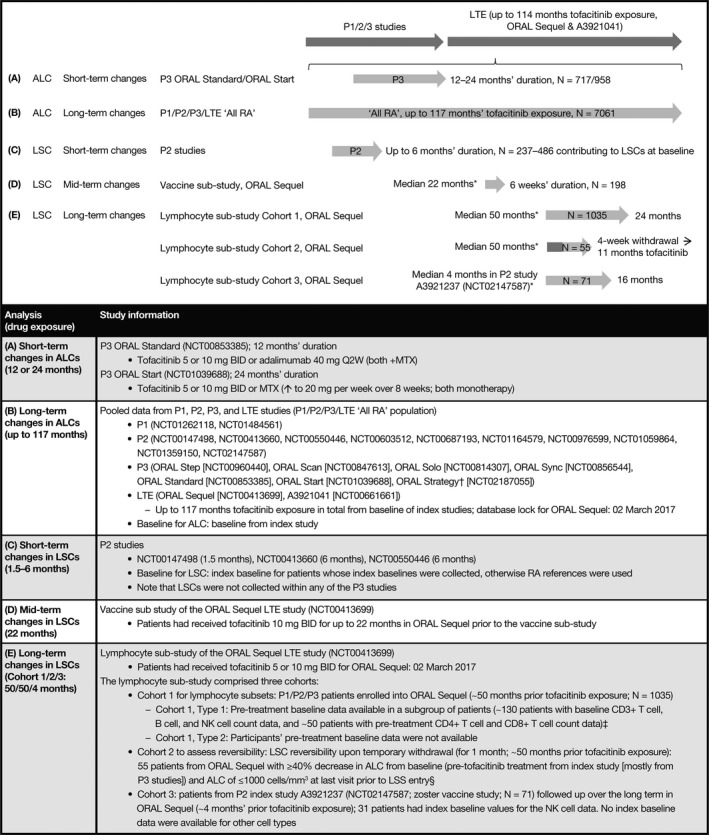
Overview of the study and analysis populations. * = Prior tofacitinib exposure. † = ORAL Strategy is a phase IIIb/IV study. ‡ = Power calculations indicated that these sample sizes (patients with available pretreatment baseline data) were adequate to detect long‐term changes of ≥20% in each of the lymphocyte subset counts (LSCs) with at least 80% probability. § = Nearly all patients were originally participants in phase III studies and thus did not have pretreatment baseline LSC data. P = phase; LTE = long‐term extension; ALC = absolute lymphocyte count; RA = rheumatoid arthritis; BID = twice daily; Q2W = every 2 weeks; MTX = methotrexate; NK = natural killer; LSS = lymphocyte substudy.
ALC assessments
ALCs were recorded as part of safety monitoring procedures throughout the tofacitinib RA clinical program.
Short‐term changes
Short‐term changes in ALCs were assessed in 2 phase III studies: ORAL Standard (NCT00853385) and ORAL Start (NCT01039688). ORAL Standard was a study to evaluate tofacitinib plus MTX versus adalimumab plus MTX over 12 months in 717 patients with RA and prior inadequate response to MTX (MTX‐IR) 11. ORAL Start (NCT01039688) evaluated tofacitinib versus MTX over 24 months in 958 MTX‐naive patients with RA 9.
Long‐term changes
Long‐term changes in ALCs were evaluated using pooled data from studies of tofacitinib, across the entire duration of tofacitinib exposure (phases I, II, and III and LTE “All RA” population [n = 7,061], up to 117 months of treatment [median ~36 months] overall; ORAL Sequel lymphocyte substudy database lock March 02, 2017). A detailed study list is provided in Figure 1.
LSC assessments
Short‐term changes
Short‐term changes in LSCs were assessed using data from 3 phase II studies (NCT00147498, NCT00413660, and NCT00550446) of 1.5–6 months’ duration (n = 236–486 contributing to each LSC subtype at baseline). LSCs were not collected within any of the phase III or IIIb/IV studies.
Mid‐term changes
Mid‐term changes in LSCs were assessed using data from a vaccine substudy of ORAL Sequel (NCT00413699) in patients who had received tofacitinib 10 mg twice daily for a median of 22 months (up to 1,632 days) before enrollment into the vaccine substudy (n = 198).
Long‐term changes
Long‐term changes in LSCs were assessed using data from a lymphocyte substudy of ORAL Sequel (NCT00413699) in patients who had previously received tofacitinib 5 mg or 10 mg twice daily for a median of ~50 months before enrollment into the lymphocyte substudy. The lymphocyte substudy comprised 3 cohorts (Figure 1). Cohort 1 was investigated to ascertain the effects of long‐term treatment with tofacitinib for a further 2 years in 1,035 patients (~50 months prior tofacitinib exposure). Cohort 2 evaluated whether the effects of tofacitinib on LSC were reversible upon temporary withdrawal (for 4 weeks) after long‐term treatment in 55 patients (~50 months’ prior exposure). In cohort 3, the long‐term effects of tofacitinib in patients enrolling only from the qualifying zoster vaccine study (A3921237) were evaluated (~4 months’ prior exposure; n = 71) (NCT02147587).
Analyses
ALCs and LSCs were ascertained and described by dose and visit over time, via descriptive statistics, line graphs, and box plots. For evaluations of LSC stability during long‐term treatment, reference ranges were derived from pretreatment baseline values (5th–95th percentile) in the tofacitinib development program in RA, and verified against values reported in literature (see Supplementary Table 1, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40780/abstract). Lymphocyte subsets were evaluated by flow cytometric analysis at a central laboratory, and included the following: total T cells (CD3+), CD4+ T helper cells (CD3+CD4+), CD8+ cytotoxic T cells (CD3+CD8+), NK cells (CD3−CD16+CD56+), and B cells (CD3−CD19+).
The reversibility of long‐term changes in ALC was investigated during follow‐up of patients in the All RA population who permanently discontinued tofacitinib treatment due to confirmed ALC of <500 cells/mm3. The reversibility of short‐term changes in LSC was evaluated using data from phase II study NCT00147498, in which patients received tofacitinib for 6 weeks, followed by treatment withdrawal for 6 weeks. The reversibility of long‐term changes in LSCs was evaluated in cohort 2 of the lymphocyte substudy through a 4‐week temporary withdrawal phase in patients previously treated with tofacitinib for a median of ~50 months. Patients in cohort 2 had a ≥40% reduction in ALC from the index study baseline and an ALC of ≤1,000 cells/mm3 at the last visit prior to entry into the lymphocyte substudy.
The clinical effect of changes in ALC and LSC on risk of infections was analyzed using the All RA population. To ascertain whether modifications to current monitoring and discontinuation recommendations (ALC <500 cells/mm3) are warranted, incidence rates of infections were calculated by ALC categories and by patient groups, defined by quartiles of nadir LSC values for each patient.
To evaluate the value of monitoring LSCs in addition to ALC to minimize risk of infection, the relationship between LSCs and ALC was assessed by examining their correlation before and after tofacitinib treatment. The maintenance of a correlation between ALC and LSC at low cell counts was also evaluated. Scatterplots for each pair of observations (ALC, LSC) and estimated Pearson correlation coefficients were generated at pretreatment baseline and after tofacitinib exposure.
A Cox regression model was used to identify and assess factors associated with time to confirmed lymphopenia (ALC <500 cells/mm3). Each factor was assessed individually, and a multivariable model was developed using an automated, backward elimination procedure.
Results
Changes in ALC over time
Short‐term changes in ALC: data from ORAL Standard and ORAL Start
In the ORAL Standard study (MTX‐IR), increases in ALC were observed at month 1 in both tofacitinib plus MTX treatment groups and the adalimumab plus MTX treatment group, but these did not persist in the tofacitinib plus MTX groups (Figure 2A). At month 12, median decreases in ALC from baseline were −190 cells/mm3 with tofacitinib 5 mg twice daily plus MTX and −310 cells/mm3 with tofacitinib 10 mg twice daily plus MTX. In ORAL Start (MTX‐naive), increases in ALC from baseline were observed from month 1 to month 3, followed by decreases to month 24; at month 12, the median change in ALC was −110 cells/mm3 with tofacitinib 5 mg twice daily and –150 cells/mm3 with tofacitinib 10 mg twice daily; at month 24, decreases were numerically greater with tofacitinib 5 mg twice daily (–330 cells/mm3) and tofacitinib 10 mg twice daily (–430 cells/mm3) as monotherapy compared to MTX monotherapy (−170 cells/mm3) (Figure 2B).
Figure 2.
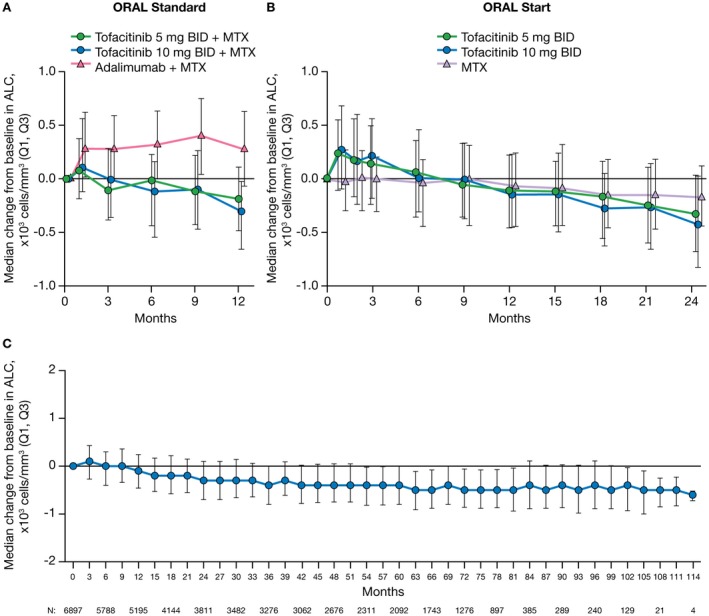
Median change from baseline in absolute lymphocyte counts (ALCs) in rheumatoid arthritis (RA) patients from the phase III ORAL Standard study (0–12 months) (A), the phase III ORAL Start study (0–24 months) (B), and the phase I/phase II/phase III/long‐term extension All RA population (0–114 months) (C). Data reported here for the All RA population include patients experiencing up to 114 months of exposure to tofacitinib; however, due to limited numbers of patients with data after month 102, interpretations should be made with caution. Only 1 patient had data at month 117, and that data point was therefore removed. Q1, Q3 = first through third quartiles; BID = twice daily; MTX = methotrexate.
Long‐term changes in ALC: data from the All RA population
In the All RA population, the median ALC in tofacitinib‐treated patients declined by ~24% from pretreatment baseline to 48 months, stabilizing at approximately –400 cells/mm3 (Figure 2C). Despite stable median ALC values after 48 months, 80 patients (1.2%; 25 of 2,983 patients in the tofacitinib 5 mg twice daily group and 55 of 3,914 patients in the tofacitinib 10 mg twice daily group) experienced a confirmed (2 sequential measurements) ALC of <500 cells/mm3. Fifty‐eight of these 80 patients were from the LTE studies. At the time of the event, 64 of the 80 patients were receiving combination therapy (tofacitinib plus background treatment) and 16 patients were receiving tofacitinib monotherapy.
Changes in LSCs over time
Short‐term, mid‐term, and long‐term changes in LSCs are shown in Figure 3. In the phase II studies of tofacitinib in RA, treatment with tofacitinib 5 mg twice daily for up to 6 months (short‐term) was associated with a minimal decrease from baseline in CD3+, CD4+, and CD8+ T cell counts; B cell counts increased from baseline, while NK cell counts decreased. At study entry in the ORAL Sequel vaccine substudy, treatment with tofacitinib for a median of 22 months (mid‐term) was associated with a decrease from baseline in CD3+, CD4+, and CD8+ T cell counts and a slight decrease in B cell and NK cell counts. Treatment with tofacitinib for ~50 months from index study baseline to entry into the ORAL Sequel lymphocyte substudy (long‐term) was associated with a decrease in CD3+, CD4+, and CD8+ T cell counts, a minimal change in B cell counts, and a large increase in NK cell counts in the subset of patients with available pretreatment baseline data. Short‐, mid‐, and long‐term changes in CD4+ T cell counts and in CD8+ T cell counts were observed to be similar.
Figure 3.
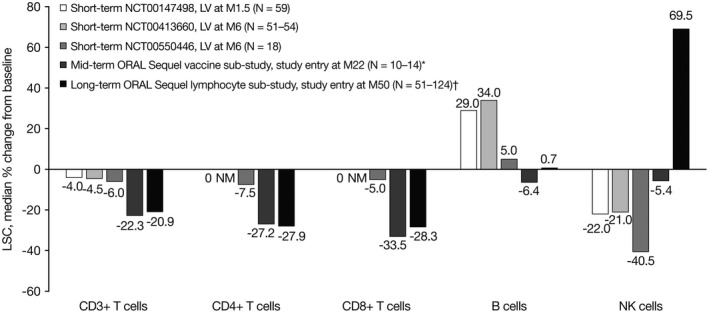
Median percentage change from baseline in lymphocyte subset counts (LSCs) in rheumatoid arthritis patients from phase II studies of tofacitinib 5 mg twice daily (short‐term), from the ORAL Sequel vaccine substudy of tofacitinib 10 mg twice daily (mid‐term), and at entry into the ORAL Sequel lymphocyte substudy (tofacitinib 5 mg or 10 mg twice daily) (long‐term). * = Includes only the subgroup of patients with pretreatment baseline LSC data (for CD3+ T cells, B cells, and natural killer [NK] cells, n = 14; for CD4+ and CD8+ T cells, n = 10). † = Cohort 1 type 1, i.e., the subgroup of patients in cohort 1 who originally participated in the phase II program and had pretreatment baseline data (for CD3+ T cells and B cells, n = 124; for CD4+ and CD8+ T cells, n = 51; for NK cells, n = 121). LV = last visit; M = month; NM = not measured.
Stability of LSCs during extended long‐term treatment
Patients in cohort 1 entered the lymphocyte substudy after a median of ~50 months of treatment in ORAL Sequel. During the next 27 months, there was no change in CD3+ T cell counts (Supplementary Figure 1A, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40780/abstract), CD4+ or CD8+ T cell counts (data not shown; results similar to CD3+ T cell results), NK cell counts (Supplementary Figure 1B), or B cell counts (Supplementary Figure 1C), and the long‐term effects of tofacitinib in this study were reflective of steady state.
The proportion of patients with LSC values below RA reference ranges for T cells and NK cells, and above the RA reference range for B cells in the lymphocyte substudy (cohort 1) is shown in Supplementary Table 2 (http://onlinelibrary.wiley.com/doi/10.1002/art.40780/abstract). Across all visits, despite stable mean changes over time, CD3+, CD4+, and CD8+ T cell counts were below the RA reference range in ~13–18%, 17–21%, and 12–19% of the patients, respectively, after long‐term tofacitinib treatment. B cell counts above the RA reference range were recorded in ~2–6% of the patients, and NK cell counts were below the RA reference range in ≤0.9% of the patients. These proportions were consistent across visits.
Reversibility of changes in ALC and LSC values over time
Reversibility of changes in ALCs after long‐term treatment
Of 80 patients in the All RA population with a confirmed ALC of <500 cells/mm3 during treatment with tofacitinib, 88% (n = 70) had ALCs in the lymphopenic range (<1,500 cells/mm3) prior to tofacitinib initiation (Table 1). In 70 of 75 patients (93%) with follow‐up ALC data after the confirmed ALC of <500 cells/mm3 during tofacitinib treatment, the ALC increased to ≥500 cells/mm3 (median 3–6 weeks following tofacitinib discontinuation) (Table 1). In the remaining 5 patients, the ALC of <500 cells/mm3 persisted for the entire duration of their follow‐up (0.5–6 months). One patient had a diagnosis of lymphoma, and another had a diagnosis of acute promyelocytic leukemia, when low ALCs occurred. The remaining 3 patients discontinued from the study due to lymphopenia, per protocol requirement. Of the 5 patients who had no follow‐up ALC data after the confirmed ALC of <500 cells/mm3, 4 either discontinued or were hospitalized for a serious adverse event, and 1 completed the study with no occurrence of an adverse event associated with the lymphopenia event.
Table 1.
Baseline ALC categories patients in the All RA population who developed an ALC of <500 cells/mm3 during tofacitinib treatment, and time to first ALC of ≥500 cells/mm3 in patients in the All RA population who had a confirmed ALC of <500 cells/mm3 during tofacitinib treatment and discontinued treatment at any time during the studya
| No. (%) of patients/median time to first observation | |
|---|---|
| Baseline ALC, cells/mm3 | |
| <500 | 4 (5.0) |
| ≥500 to <750 | 22 (27.5) |
| ≥750 to <1,000 | 19 (23.8) |
| ≥1,000 to <1,500 | 25 (31.3) |
| ≥1,500 | 8 (10.0) |
| Missing data | 2 (2.5) |
| Total | 80 (100) |
| First ALC of ≥500 cells/mm3 after tofacitinib discontinuation, cells/mm3 | |
| ≥500 to <1,000 | 61 (87.1)/6.0 weeks |
| ≥1,000 to <1,500 | 8 (11.4)/4.4 weeks |
| ≥1,500 | 1 (1.4)/2.9 weeks |
| Total | 70 (100)b |
Baseline data on absolute lymphocyte counts (ALCs) were obtained from the index study. All RA = rheumatoid arthritis patients from the tofacitinib phases I, II, and III and long‐term extension studies.
Five patients whose ALC never reached ≥500 cells/mm3 after confirmed ALC <500 cells/mm3 during tofacitinib treatment, as well as 5 patients without available follow‐up ALC data, were not included.
Reversibility of changes in LSCs after short‐term treatment
The reversibility of LSCs (toward baseline values) was evaluated in a 6‐week withdrawal phase following a 6‐week treatment phase in the phase II study A3921019/NCT00147498. Observed changes in T cell counts in patients treated with tofacitinib 5 mg twice daily were small and similar to changes with placebo treatment. This resulted in similar distributions of T cell counts during tofacitinib treatment and following treatment withdrawal, compared to placebo (data not shown). At week 6 of treatment, a 29% median increase from baseline in B cell counts was observed with tofacitinib 5 mg twice daily; after the treatment withdrawal phase, B cell counts decreased toward baseline levels and were similar to those in the placebo group. A 22% median decrease in NK cells was observed at week 6 of treatment with tofacitinib 5 mg twice daily; after the treatment withdrawal phase, NK cell counts increased toward baseline levels and were similar to those in patients who received placebo. For both NK cells and B cells, the median percentage change from pretreatment baseline at week 12 was close to 0 with tofacitinib 5 mg twice daily, indicating complete reversibility of the observed changes.
Reversibility of changes in LSCs after long‐term treatment
The lymphocyte substudy (cohort 2) assessed reversibility of changes in LSCs after temporary withdrawal of tofacitinib treatment for 4 weeks, following treatment for ~50 months. In 12 of 22 patients (54.5%), 11 of 26 patients (42.3%), and 12 of 17 patients (70.6%) who had low CD3+, CD4+, or CD8+ T cell counts, respectively, values returned to within the RA reference range after the 4‐week withdrawal (for LSC cutoff values, see Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.40780/abstract).
After the 4‐week withdrawal, B cell and NK cell counts remained within the RA reference range in the majority of patients; no patient had an NK cell value below the reference range or a B cell value above the reference range. Of 2 patients who had high NK cell values, the level returned to within the RA reference range in 1 but remained above the reference range in the other. Three patients had low B cell values prior to tofacitinib withdrawal; the level had returned to within the RA reference range at week 4 in 1 but remained below the reference range in the other 2.
Association of ALC and LSC with infection rates following long‐term treatment
Incidence rates of serious infections and herpes zoster in the All RA safety population (n = 7,061) are presented in Table 2. The incidence rates of serious infections following a confirmed ALC of <500 cells/mm3 were higher versus ALC categories ≥500 cells/mm3, and herpes zoster generally showed an increasing risk with lower ALC values. The rate of opportunistic infections also exhibited a trend toward an increase with decreasing ALC (data not shown), but there were too few cases to draw meaningful conclusions.
Table 2.
Incidence rates of serious infections and herpes zoster by confirmed ALC category in the All RA populationa
| Confirmed ALC, cells/mm3 | Serious infection | Herpes zoster | ||||
|---|---|---|---|---|---|---|
| n | No. (%) of patients with event | IR (95% CI) following confirmed ALCb | n | No. (%) of patients with event | IR (95% CI) following confirmed ALCb | |
| Missing data | 35 | 0 (0) | 35 | 1 (2.9) | ||
| ≥2,000 | 6,092 | 94 (1.5) | 2.4 (1.9–2.9) | 6,092 | 113 (1.9) | 2.9 (2.4–3.5) |
| ≥1,500 to <2,000 | 4,477 | 130 (2.9) | 2.3 (2.0–2.8) | 4,427 | 177 (4.0) | 3.3 (2.9–3.9) |
| ≥1,000 to <1,500 | 4,271 | 215 (5.0) | 2.4 (2.1–2.7) | 4,154 | 311 (7.5) | 3.7 (3.3–4.2) |
| ≥750 to <1,000 | 1,706 | 83 (4.9) | 2.6 (2.0–3.2) | 1,589 | 122 (7.7) | 4.3 (3.6–5.1) |
| ≥500 to <750 | 614 | 48 (7.8) | 4.0 (2.9–5.3) | 568 | 55 (9.7) | 5.3 (4.0–6.9) |
| <500 | 76 | 6 (7.9) | 7.1 (2.6–15.5) | 67 | 3 (4.5) | 4.2 (0.9–12.2) |
| Overall | 7,061 | 576 (8.2) | 2.5 (2.3–2.7) | 7,061 | 782 (11.1) | 3.6 (3.4–3.9) |
Only patients with at least 2 visits at which absolute lymphocyte counts (ALCs) were obtained after the tofacitinib start date were included in the analysis. ALC was monitored independently of infection events. Patients with an ALC of <500 cells/mm3 discontinued tofacitinib treatment and continued to be followed up to resolution or until the ALC was determined by the investigator to be stabilized. All RA = rheumatoid arthritis patients from the tofacitinib phases I, II, and III and long‐term extension (LTE) studies; 95% CI = 95% confidence interval.
Incidence rates (IRs) are the number of patients with events per 100 patient‐years and are based on events occurring after the confirmed ALC value was reached, i.e., an event is counted in a category if the event occurred only after the patient reached that category and did not occur while the patient was in any of the previous categories. For patient‐years, the total follow‐up time was calculated up to the day of the first event, subject to a risk period of 28 days beyond the last dose or to the data cutoff date. Gaps in dosing between index and LTE studies are included up to 28 days or to the data cutoff date.
Table 3 shows the relationship between LSCs and the risk of serious infections and herpes zoster. Overall, there were generally no strong associations between LSCs (CD3+ T cells, CD4+ T cells, CD8+ T cells, NK cells, and B cells) and serious infections or herpes zoster.
Table 3.
Incidence rates (95% CI) of serious infections and herpes zoster by quartile of nadir CD3+ T cell, CD4+ T cell, CD8+ T cell, and NK cell counts, and zenith B cell counts, in the All RA populationa
| LSC, quartile, ×1,000 cells/mm3 | Serious infection | Herpes zoster | ||
|---|---|---|---|---|
| n/no. with event | IR (95% CI) | n/no. with event | IR (95% CI) | |
| CD3+ T cells | ||||
| Q1, <0.62 | 531/25 | 0.85 (0.55–1.26) | 533/109 | 4.17 (3.42–5.03) |
| Q2, 0.62 to <0.90 | 533/28 | 1.07 (0.71–1.55) | 534/82 | 3.42 (2.72–4.25) |
| Q3, 0.90 to <1.26 | 533/30 | 1.37 (0.92–1.96) | 539/73 | 3.54 (2.78–4.45) |
| Q4, ≥1.26 | 534/40 | 2.04 (1.46–2.78) | 536/61 | 3.31 (2.53–4.25) |
| CD3+CD4+ T cells | ||||
| Q1, <0.39 | 353/11 | 0.53 (0.26–0.95) | 355/76 | 4.12 (3.25–5.16) |
| Q2, 0.39 to <0.55 | 354/11 | 0.58 (0.29–1.04) | 356/53 | 3.05 (2.28–3.99) |
| Q3, 0.55 to <0.76 | 353/12 | 0.71 (0.37–1.24) | 357/45 | 2.83 (2.07–3.79) |
| Q4, ≥0.76 | 354/15 | 1.15 (0.64–1.90) | 357/28 | 2.26 (1.50–3.27) |
| CD3+CD8+ T cells | ||||
| Q1, <0.13 | 351/12 | 0.60 (0.31–1.04) | 353/75 | 4.25 (3.35–5.33) |
| Q2, 0.13 to <0.21 | 356/9 | 0.50 (0.23–0.94) | 359/49 | 2.90 (2.14–3.83) |
| Q3, 0.21 to <0.31 | 353/13 | 0.75 (0.40–1.28) | 356/43 | 2.64 (1.91–3.56) |
| Q4, ≥0.31 | 354/15 | 1.07 (0.60–1.77) | 357/35 | 2.64 (1.84–3.68) |
| B cells (CD3−CD19+) | ||||
| Q1, <0.14 | 532/46 | 2.01 (1.47–2.68) | 535/101 | 4.87 (3.97–5.92) |
| Q2, 0.14 to <0.22 | 534/27 | 1.09 (0.72–1.58) | 536/83 | 3.65 (2.90–4.52) |
| Q3, 0.22 to <0.33 | 539/26 | 1.05 (0.69–1.54) | 541/74 | 3.23 (2.54–4.05) |
| Q4, ≥0.33 | 536/24 | 0.97 (0.62–1.45) | 540/67 | 2.92 (2.26–3.71) |
| NK cells (CD3−CD16+CD56+) | ||||
| Q1, <0.07 | 427/34 | 1.70 (1.18–2.37) | 429/58 | 3.16 (2.40–4.08) |
| Q2, 0.07 to <0.12 | 426/14 | 0.69 (0.38–1.17) | 429/61 | 3.27 (2.50–4.20) |
| Q3, 0.12 to <0.18 | 429/18 | 0.85 (0.50–1.34) | 432/62 | 3.19 (2.44–4.08) |
| Q4, ≥0.18 | 428/15 | 0.71 (0.40–1.17) | 431/55 | 2.78 (2.10–3.62) |
Incidence rates (IRs) are the number of patients with events per 100 patient‐years. Events are counted up to 28 days beyond the last dose or to the data cutoff date. 95% CI = 95% confidence interval; NK = natural killer; All RA = rheumatoid arthritis patients from the tofacitinib phases I, II, and III and long‐term extension studies; LSC = lymphocyte subset count; Q = quartile.
A Cox regression analysis was performed to identify and assess potential risk factors for lymphopenia (i.e., ALC <500 cells/mm3). The results showed that patients with lower ALCs at baseline (P < 0.0001), older age (P = 0.0012), higher tofacitinib dose (P = 0.0071), and those receiving background MTX (P = 0.0068) were at increased risk.
Correlation between ALCs and LSCs: utility of LSC monitoring
LSCs did not appear to be strongly associated with infection events; hence, if subset counts correlate well with ALCs, additional measurement of LSCs would not add value over and above the ALC to minimize risk of infection. The relationship between CD3+ T cell counts and ALCs was evaluated by estimating Pearson correlation coefficients (R) at pretreatment baseline (phase II baseline) and after short‐term (phase II postbaseline), mid‐term (ORAL Sequel vaccine substudy entry), and long‐term (ORAL Sequel lymphocyte substudy entry) treatment with tofacitinib. A high degree of correlation was seen at baseline (R = 0.79), which increased with long‐term tofacitinib treatment (R = 0.89), indicating that changes in ALCs reflect changes in CD3+ T cell counts. This correlation was also observed at low values, i.e., low ALCs were associated with low CD3+ T cell counts (data not shown). Comparable results were observed for CD4+ T cell counts and ALCs (R = 0.82–0.86; Supplementary Figure 2A, http://onlinelibrary.wiley.com/doi/10.1002/art.40780/abstract), likely because CD4+ T cells constituted ~50% of the ALC at baseline. The correlation between CD8+ T cell counts and ALCs was only slightly lower at baseline and after tofacitinib treatment (R = 0.65–0.70) versus that observed with CD4+ T cells (Supplementary Figure 2B).
In contrast to CD4+ and CD8+ T cell counts, a poor correlation between ALC and NK cell counts was noted at baseline (R = 0.27), which improved with long‐term tofacitinib treatment (R = 0.47). B cell counts and ALCs were moderately correlated at baseline (R = 0.61), with the correlation slightly decreasing following long‐term tofacitinib treatment (R = 0.55).
Discussion
This post hoc pooled analysis of tofacitinib therapy in patients with RA evaluated the short‐, mid‐, and long‐term effects of tofacitinib on ALCs and LSCs, the reversibility of changes in ALCs or LSCs, the association between ALCs/LSCs and infections, and the value of monitoring LSCs in addition to ALCs. A summary of the effects of tofacitinib on immune cells is presented in Supplementary Table 3, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40780/abstract.
Tofacitinib treatment initially resulted in a transient increase in ALC, followed by a gradual decline to steady state by ~48 months. In contrast, adalimumab‐treated patients in the ORAL Standard trial experienced a sustained increase in ALC over 12 months, and MTX‐treated patients in ORAL Start showed smaller decreases compared to tofacitinib‐treated patients over 24 months. The different populations evaluated in these studies (MTX‐naive versus MTX‐IR) should, however, be acknowledged. Approximately 1% of patients in the All RA population experienced a confirmed ALC of <500 cells/mm3, and 80% of these patients were receiving tofacitinib plus background therapy at the time of the event. In most patients who permanently discontinued tofacitinib treatment due to an ALC of <500 cells/mm3, the ALC reverted to ≥500 cells/mm3 between 3 and 6 weeks after discontinuation. Patients receiving tofacitinib with a confirmed nadir ALC of <500 cells/mm3 had an increased risk of serious infection, while herpes zoster showed a trend toward an increasing risk with lower ALC values. The rate of opportunistic infections also showed a trend toward increases with decreasing ALC, but there were too few cases to draw meaningful conclusions. Given that lymphopenia is associated with an increased risk of serious infections during tofacitinib treatment 29, ALC evaluation at baseline and monitoring every 3 months is recommended.
Initiation of tofacitinib is not recommended in patients with an ALC of <500 cells/mm3, and therapy should be discontinued in those developing a confirmed ALC of <500 cells/mm3 during treatment 26. Patients receiving tofacitinib with a confirmed nadir ALC of between 500 and <750 cells/mm3 showed a trend toward increased risk of serious infection and herpes zoster, which is relevant to the recommendation in the European Union label to interrupt dosing within this range until ALC returns to >750 cells/mm3, due to increased risk of infection 27. A higher threshold has the potential to avoid more infections, but may also disproportionately exclude patients who could benefit from tofacitinib and not experience a serious infection 30.
Regarding LSCs, tofacitinib treatment resulted in 1) a slight initial decrease in T cell counts compared to pretreatment baseline, which decreased further with mid‐ to long‐term treatment, 2) an initial decrease in NK cell counts, followed by a shift to increased NK cell counts with mid‐ to long‐term treatment, and 3) an initial increase in B cell counts, which returned to baseline with long‐term treatment. Reversible decreases in NK cells and increases in B cells with tofacitinib have been reported previously 20. For all cell types evaluated, no further progressive decline in LSCs occurred in the lymphocyte substudy. Withdrawal of tofacitinib for 4 weeks in patients previously treated with tofacitinib for ~50 months demonstrated that changes in T cell and B cell levels are reversible, although the increase in NK cells upon treatment withdrawal is somewhat counterintuitive given the observed increases in NK cell counts over long‐term tofacitinib treatment. Potential mechanisms for this phenomenon are discussed below. Also, there were no strong associations between LSCs and serious infections. ALCs correlated well with CD4+ T cell/CD8+ T cell counts; hence, specific CD4+ T cell or CD8+ T cell monitoring would be unlikely to further minimize infection risk. CD4+ and CD8+ T cells together constitute ~70% of ALCs 31, 32, and changes in these subtypes therefore should be reflected in ALCs.
Similar early effects on NK, B, and T cells have been reported with other JAK inhibitors. In a 12‐week study of upadacitinib in RA, a dose‐dependent reduction in NK cell counts was reported, with a mean decrease of 18.3% and 28.0% with upadacitinib at 6 mg and 12 mg twice daily, respectively (33); the safety profiles were found to be comparable to those reported in a phase III study of upadacitinib in daily doses of 15 mg or 30 mg (34). The European Public Assessment Report for baricitinib describes a 20% decrease in NK cells, with recovery to near‐baseline levels at week 52 35. Also, early increases in B cell counts that were sustained through ≥24 weeks were reported for baricitinib 36. An early increase in lymphocyte counts followed by a gradual decrease to baseline by ~1 year has been reported for baricitinib as well 37; however, the effects beyond 1 year have not been published, and it is unclear whether the lymphocytes reached steady state.
The initial increase in ALC with both adalimumab and tofacitinib treatment in this study may be due to the initial amelioration of disease or, alternatively, to a direct/indirect effect of JAK inhibition on lymphocyte trafficking and margination. In the absence of foreign antigens, cytokines such as IL‐7/IL‐15 provide survival and/or proliferation signals to maintain the population of CD4+ and CD8+ T cells 38, 39, 40. IL‐15 is also the dominant cytokine for prolonging NK cell survival 40, 41. Therefore, long‐term modulation of cytokine signaling by tofacitinib, but not adalimumab or MTX, could impact the setpoint for steady‐state populations of CD4+ T, CD8+ T, and NK cells, which compete for a limited pool of IL‐7/IL‐15. With decreased IL‐7/IL‐15 signaling, a reduction in NK cell numbers may be observed earlier than CD4+ and CD8+ T cells, due to the relatively shorter half‐life of NK cells. With continued tofacitinib dosing, CD4+ and CD8+ T cell numbers decrease slowly to a new setpoint, reducing the competition for IL‐15. The increased availability of IL‐15, coupled with >10‐fold higher expression of the IL‐15 receptor (CD122, IL‐2/IL‐15Rβ) on NK cells, may lead to a compensatory increase in IL‐15 signaling, resulting in a higher NK cell number setpoint after long‐term dosing 40, 42. Upon treatment discontinuation, CD4+, CD8+, and NK cells all increase in response to increased cytokine signaling. The physiologic implications of the observed increase in circulating B cell numbers in response to JAK inhibition, followed by an eventual normalization after 4 years, are unclear. Overall, there is a lack of information concerning the mechanism of action for the effect of tofacitinib on ALC/LSC; hence, discussions can only be considered in hypothesis‐generating terms.
The impact of immunotherapy on the immune system cannot be understood without considering both numerical and functional changes in immune cells, and several studies have been conducted to characterize the potential effect of tofacitinib on immune function, i.e., cell‐mediated immunity (T cell and NK cell function) and humoral‐mediated immunity (B cell and vaccination responses). Cell‐mediated immunity data suggest that tofacitinib does not significantly impair T cell function assessed via non–antigen‐specific stimulation, nor does it affect the generation and maintenance of responses using antigens as varied as tetanus toxoid (study A3921061/NCT01163253) (ref. 21 and Supplementary Figure 3, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40780/abstract) and herpesviruses 23. A modest decrease in NK cell cytotoxic activity was observed in tofacitinib‐treated patients with RA (study A3921237/NCT02147587) (Supplementary Figure 4, http://onlinelibrary.wiley.com/doi/10.1002/art.40780/abstract); the clinical significance of these data is unclear, although the possibility of an impact on herpes zoster risk cannot be excluded. Similarly, studies measuring serum Ig levels and vaccination responses in tofacitinib‐treated patients with RA and psoriasis suggest that tofacitinib may not impair humoral‐mediated immunity 21, 22, 23.
This analysis had several limitations. Multiple patient populations (some small), from several different studies, were evaluated. Also, most patients did not have baseline index study values for LSCs. Data on the All RA population were not evaluated continuously, and the LTE population is a selected population of patients tolerant to treatment, potentially limiting the generalizability of results. Protocol amendments prevented the inclusion of patients with lymphopenia (ALC <500 cells/mm3) and patients who developed confirmed lymphopenia were withdrawn from the studies, making it difficult to capture information on serious infections after the occurrence of confirmed lymphopenia. Finally, no direct investigation of the relationship between the level and function of ALCs/LSCs was performed.
In conclusion, tofacitinib treatment results in a transient increase in ALC, followed by a gradual decline to reach steady state by ~48 months. Changes in both ALC and LSC are reversible upon treatment cessation. Although the overall effects of tofacitinib on cell‐mediated and humoral‐mediated immunity appear to be modest, risks of serious infections and herpes zoster were generally increased in the setting of confirmed low ALCs (<500 cells/mm3). From a clinical perspective, evaluation of ALC at baseline and monitoring every 3 months during tofacitinib treatment is recommended. Initiation of tofacitinib is not recommended in patients with an ALC of <500 cells/mm3, and if this develops, tofacitinib therapy should be discontinued 26. ALC and CD4+/CD8+ T cell counts correlated well, and there was no strong correlation between CD4+/CD8+ T cell counts and serious infections, suggesting that monitoring of these lymphocyte subsets may not provide any additional information. Thus, ALC monitoring alone appears to be adequate to minimize risk. Laboratory and safety outcomes related to immune function continue to be evaluated within the tofacitinib development program.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. van Vollenhoven had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design
Valdez, Krishnaswami, Biswas, Lazariciu, Hodge, Wang, Choy.
Acquisition of data
Van Vollenhoven, Lee, Biswas, Lazariciu, Hodge, Wang.
Analysis and interpretation of data
Van Vollenhoven, Lee, Strengholt, Mojcik, Valdez, Krishnaswami, Biswas, Lazariciu, Hazra, Clark, Hodge, Wang, Choy.
Role of the Study Sponsor
Medical writing support, under the guidance of the authors, was provided by Paul Scutt, PhD, at CMC Connect, a division of McCann Health Medical Communications Ltd, Manchester, UK, and was funded by Pfizer Inc, New York, NY, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461–4). The authors had the final decision to submit the manuscript for publication.
Supporting information
Acknowledgments
The authors would like to acknowledge the support of all the study patients and investigators.
Supported by Pfizer Inc.
Dr. van Vollenhoven has received consulting fees, speaking fees, and/or honoraria from AbbVie, AstraZeneca, Biotest, Bristol‐Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer Inc, and UCB (less than $10,000 each) and research support from AbbVie, Bristol‐Myers Squibb, GlaxoSmithKline, Pfizer Inc, and UCB. Dr. Lee has received consulting fees from Pfizer Inc (less than $10,000) and research support from Green Cross Co. and Hanmi Pharmaceutical. Mr. Strengholt and Drs. Valdez, Krishnaswasmi, Biswas, Hodge, and Wang own stock or stock options in Pfizer Inc. Drs. Mojcik, Hazra, and Clark owned stock in Pfizer Inc at the time of the analysis. Ms Lazariciu is a consultant for Pfizer Inc through her employment with IQVIA Canada. Dr. Choy has received consulting fees, speaking fees, and/or honoraria from Amgen, Biogen, Chugai Pharma, Eli Lilly, Janssen, Novartis, Pfizer Inc, Regeneron, Roche, R‐Pharm, Sanofi‐Aventis, Bristol‐Myers Squibb, Boehringer Ingelheim, Hospira, MSD, and UCB (less than $10,000 each) and research support from BioCancer, Pfizer Inc, Roche, and UCB.
References
- 1. Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease‐modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. [DOI] [PubMed] [Google Scholar]
- 2. Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double‐blind, placebo‐controlled phase IIa trial of three dosage levels of CP‐690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. [DOI] [PubMed] [Google Scholar]
- 3. Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez‐Reino J, et al. A phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. [DOI] [PubMed] [Google Scholar]
- 4. Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators . Phase II study of tofacitinib (CP‐690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63:1150–8. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12‐week, randomized, phase 2 study. Mod Rheumatol 2015;25:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burmester GR, Blanco R, Charles‐Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP‐690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 7. Fleischmann R, Kremer J, Cush J, Schulze‐Koops H, Connell CA, Bradley JD, et al. Placebo‐controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 8. Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin‐Mola E, et al. Tofacitinib in combination with nonbiologic disease‐modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 9. Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley J, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 10. Van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP‐690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four–month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 11. Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 12. Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open‐label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 13. Wollenhaupt J, Silverfield J, Lee EB, Terry K, Kwok K, Strengholt S, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open‐label, long‐term extension studies over 9 years [abstract]. Arthritis Rheumatol 2017;69 Suppl 10 URL: https://acrabstracts.org/abstract/tofacitinib-an-oral-janus-kinase-inhibitor-in-the-treatment-of-rheumatoid-arthritis-safety-and-efficacy-in-open-label-long-term-extension-studies-over-9-years/. [Google Scholar]
- 14. Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open‐label, long‐term extension study. Arthritis Res Ther 2016;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyle DL, Wei N, Singhal AK, Rosengren S, Kaplan I, Soma K, et al. The JAK inhibitor tofacitinib suppresses synovial JAK1‐STAT1 signalling in rheumatoid arthritis [abstract]. Ann Rheum Dis 2013;72 Suppl 3 URL: http://scientific.sparx-ip.net/archiveeular/?c=a&view=4&-searchfor=Boyle&item=2013OP0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cutolo M. The kinase inhibitor tofacitinib in patients with rheumatoid arthritis: latest findings and clinical potential. Ther Adv Musculoskelet Dis 2013;5:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arend WP. Physiology of cytokine pathways in rheumatoid arthritis [review]. Arthritis Rheum 2001;45:101–6. [DOI] [PubMed] [Google Scholar]
- 18. Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP‐690,550). J Immunol 2011;186:4234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by γc family cytokines. Nat Rev Immunol 2009;9:480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, et al. The mechanism of action of tofacitinib: an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2016;34:318–28. [PubMed] [Google Scholar]
- 21. Winthrop K, Korman N, Abramovits W, Rottinghaus ST, Tan H, Gardner A, et al. T‐cell‐mediated immune response to pneumococcal conjugate vaccine (PCV‐13) and tetanus toxoid vaccine in patients with moderate‐to‐severe psoriasis during tofacitinib treatment. J Am Acad Dermatol 2018;78:1149–55. [DOI] [PubMed] [Google Scholar]
- 22. Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016;75:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winthrop KL, Wouters AG, Choy EH, Soma K, Hodge JA, Nduaka CI, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol 2017;69:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strand V, Ahadieh S, French J, Geier J, Krishnaswami S, Menon S, et al. Systematic review and meta‐analysis of serious infections with tofacitinib and biologic disease‐modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015;17:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long‐term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xeljanz (tofacitinib) prescribing information. New York (NY): Pfizer Inc; 2017. URL: http://labeling.pfizer.com/ShowLabeling.aspx?id=959. [Google Scholar]
- 27. Xeljanz (tofacitinib citrate) prescribing information. Sandwich (UK): Pfizer Ltd; 2017. URL: https://www.medicines.org.uk/emc/medicine/33167. [Google Scholar]
- 28. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 29. Van Vollenhoven RF, Riese R, Krishnaswami S, Kawabata T, Fosser C, Rottinghaus S, et al. Relationship between lymphocyte count and risk of infection in rheumatoid arthritis patients treated with tofacitinib [abstract]. Arthritis Rheum 2013;65 Suppl 10 URL: https://acrabstracts.org/abstract/relationship-between-lymphocyte-count-and-risk-of-infection-in-rheumatoid-arthritis-patients-treated-with-tofacitinib/. [Google Scholar]
- 30. Burmester GR, Szekanecz Z, Biswas P, Krishnaswami S, Mojcik CF, Valdez H, et al. Monitoring of absolute lymphocyte count in patients with rheumatoid arthritis treated with tofacitinib [abstract]. Arthritis Rheumatol 2017;69 Suppl 10 URL: https://acrabstracts.org/abstract/monitoring-of-absolute-lymphocyte-count-in-patients-with-rheumatoid-arthritis-treated-with-tofacitinib/. [Google Scholar]
- 31. Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol 2004;72:203–12. [DOI] [PubMed] [Google Scholar]
- 32. Jentsch‐Ullrich K, Koenigsmann M, Mohren M, Franke A. Lymphocyte subsets’ reference ranges in an age‐ and gender‐balanced population of 100 healthy adults: a monocentric German study. Clin Immunol 2005;116:192–7. [DOI] [PubMed] [Google Scholar]
- 33. Kremer JM, Emery P, Camp HS, Friedman A, Wang L, Othman AA, et al. A phase IIb study of ABT‐494, a selective JAK‐1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti–tumor necrosis factor therapy. Arthritis Rheumatol 2016;68:2867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burmester GR, Kremer J, van Den Bosch F, Li Y, Zhou Y, Othman AA, et al. A phase 3 randomized, placebo‐controlled, double‐blind study of upadacitinib (ABT‐494), a selective JAK‐1 inhibitor, in patients with active rheumatoid arthritis with inadequate response to conventional synthetic DMARDs [abstract]. Arthritis Rheumatol 2017;69 Suppl 10 URL: https://acrabstracts.org/abstract/a-phase-3-randomized-placebo-controlled-double-blind-study-of-upadacitinib-abt-494-a-selective-jak-1-inhibitor-in-patients-with-active-rheumatoid-arthritis-with-inadequate-response-to-convention/. [Google Scholar]
- 35. European Medicines Agency . European Public Assessment Report for Olumiant–international non‐proprietary name: baricitinib. 2016. URL: https://www.ema.europa.eu/documents/assessment-report/olumiant-epar-public-assessment-report_en.pdf.
- 36. Emery P, McInnes I, Genovese MC, Smolen JS, Kremer J, Dougados M, et al. Characterization of changes in lymphocyte subsets in baricitinib‐treated patients with rheumatoid arthritis in two phase 3 studies [abstract]. Arthritis Rheumatol 2015;67 Suppl 10 URL: https://acrabstracts.org/abstract/characterization-of-changes-in-lymphocyte-subsets-in-baricitinib-treated-patients-with-rheumatoid-arthritis-in-two-phase-3-studies/. [Google Scholar]
- 37. Kremer J, Huizinga TW, Chen L, Saifan CG, Issa M, Witt SL, et al. Analysis of neutrophils, lymphocytes, and platelets in pooled phase 2 and phase 3 studies of baricitinib for rheumatoid arthritis [abstract]. Ann Rheum Dis 2017;76 Suppl 2 URL: http://scientific.sparx-ip.net/archiveeular/?searchfor=Kremer&c=a&view=4&item=2017FRI0090. [Google Scholar]
- 38. Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol 2009;39:2088–94. [DOI] [PubMed] [Google Scholar]
- 39. Boyman O, Krieg C, Homann D, Sprent J. Homeostatic maintenance of T cells and natural killer cells. Cell Mol Life Sci 2012;69:1597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma A, Koka R, Burkett P. Diverse functions of IL‐2, IL‐15, and IL‐7 in lymphoid homeostasis. Annu Rev Immunol 2006;24:657–79. [DOI] [PubMed] [Google Scholar]
- 41. Ali AK, Nandagopal N, Lee SH. IL‐15‐PI3K‐AKT‐mTOR: a critical pathway in the life journey of natural killer cells. Front Immunol 2015;6:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bielekova B, Catalfamo M, Reichert‐Scrivner S, Packer A, Cerna M, Waldmann TA, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL‐2Rα‐targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A 2006;103:5941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


