Abstract
Background and Aims
The impact of tobacco control on European older adults has not been studied, despite evidence that smoking cessation at old age can bring significant life expectancy gains. Our aim was to evaluate the impact of tobacco control policies on smoking among older adults in Europe from 2004 to 2013.
Design
We used longitudinal data from the Survey of Health, Ageing and Retirement in Europe (SHARE, aged 50+ years) from four waves from 2004 to 2013. We used logistic regression models with clustered standard errors to determine whether the implementation of tobacco control policies was associated with changes in smoking status. Furthermore, we studied whether these associations varied by socio‐demographic characteristics. Regression coefficients were converted to changes the probability of smoking [marginal effects (ME)].
Measurements
Smoking status was the dependent variable, and the Tobacco Control Scale (TCS) was the explanatory variable, overall and by its main policy components (pricing and smoke‐free policies). Covariates included age, sex, education and country and wave fixed‐effects.
Findings
A 10‐point increase in TCS was associated with a lower probability of smoking by 1.6 percentage points [95% confidence interval (CI) = −3.208, −0.056] for those aged 50–65, but not for older Europeans. Among those with primary school or no education, the associated drop was of 1.5 percentage points (95% CI = –2.751, −0.253). By contrast, no significant relation between TCS and smoking was observed among those with high education. Higher TCS scores for pricing (ME = –0.636, 95% CI = –0.998, −0.275) and smoke‐free policies (ME = –0.243, 95% CI = –0.445, −0.041) were associated with a significantly lower probability of smoking (P = 0.001 and P = 0.018, respectively).
Conclusion
Increases in tobacco taxes and smoke‐free policies are significantly related with a reduction in smoking among European older adults, suggesting potential health gains for this rising share of the population. These policies may be more effective among the lowest educated.
Keywords: Older adults, SHARE, smoke‐free policies, smoking, TCS, tobacco control policies, tobacco taxes
Introduction
Comprehensive tobacco control policies have been introduced in many European countries during the last 2 decades, especially since the World Health Organization (WHO) Framework Convention on Tobacco Control was signed in 2004 1, 2. Earlier studies suggested an important impact of these policies on smoking behaviours, both separately 2, 3, 4, 5 and as a comprehensive package 6, 7, 8, 9, 10, 11. Furthermore, some policies such as increasing tobacco prices were found to be more effective for low socio‐economic status (SES) individuals 12, 13, 14, hence potentially reducing socio‐economic inequalities in smoking. However, there are still important gaps in the measurement of tobacco control (TC) policies’ effectiveness.
First, most research has focused on adolescents and young adults or the overall adult population 6, 7, 8, 15, while little attention has been paid to the effect on older adults 16. In fact, doctors are less prone to advise older patients to quit smoking 17, perhaps because they feel that long‐term addiction is not reversible, or that benefits are not worth the effort. However, cessation at older ages can still bring significant gains in life expectancy and quality of life 18, 19, 20, 21 and a lower risk of disability 22, 23. Also, considering the ageing of the European population 24 , it is very important from a public health perspective to study the effectiveness of tobacco control policies among this increasing share of the population.
Secondly, most of the previous evidence is based on cross‐sectional 6, 8, 9, 15, 25 or repeated cross‐sectional samples 10, 11 . Cross‐sectional estimates may be affected by unobserved characteristics at country‐ and individual‐level that may influence smoking behaviour. In a cross‐sectional design, countries with a stricter TC policy may be those traditionally less tolerant towards tobacco and where tobacco prevalence has been lower before the onset of new policies. While repeated cross‐section designs take into account national levels of tobacco consumption prior to the implementation of new policies, the strength of their evidence is limited, because they study changes in smoking behaviour over time at the population‐level but not at individual‐level 26. On the contrary, with a longitudinal sample we can include country and individual fixed‐effects to control for time‐invariant characteristics that may affect the probability of smoking. This enables us to compare the evolution of smoking among ‘exposed’ individuals [those living in a country where the Tobacco Control Scale (TCS) has increased] with the evolution of smoking among ‘non‐exposed’ or ‘control’ individuals (those living in a country where TCS did not increase or increased less).
Our aim was to evaluate the impact of TC policies on smoking among older adults in Europe from 2004 to 2013. To do so, we used SHARE (Survey of Health, Ageing and Retirement in Europe), a longitudinal data set that followed Europeans older than 50 years. The data set covers a time‐period (2004–13) during which major tobacco control laws were introduced in the countries under study (TCS increased by 50% in our sample during that period). This allowed us to exploit the trends in tobacco control policies within countries and to examine its association with longitudinal changes in smoking status at individual‐level. In addition, we tested whether tobacco control policies are associated differently with smoking among socio‐demographic groups and which type of tobacco control policies are most strongly associated with changes in smoking prevalence.
Methods
Data
SHARE is a cohort of individuals aged 50 years and older from 20 European countries. So far, five waves have been collected (the first in 2004 and the last in 2013). We used data from waves 1 (2004–05), 2 (2006–07), 4 (2011) and 5 (2013) for the countries that participated in the four waves: Austria, Belgium, Denmark, France, Germany, Italy, Netherlands, Spain, Sweden and Switzerland. Wave 3 was excluded from our analysis because it did not include information about smoking. Individuals were randomly selected from national or regional population registries. In the latter case, two‐ or multi‐stage designs were used, in which regions were sampled first and then individuals randomly selected within regions 27. The original sample was formed by 25 320 individuals at wave 1, 7923 of them being followed‐up in waves 2, 4 and 5, making a total of 31 692 observations. These individuals who were followed across waves formed the balanced longitudinal sample, which we used in our analysis 28, 29. The difference between 31 692 and the total number of observations in each model is due to missing values in some of the covariates; namely, 960 observations presented missing values in the weights, 540 in education and 60 in smoking status.
Dependent variable
The dependent variable was smoking status, defined as a binary variable based on the question: ‘Do you smoke at the present time?’, taking the value 1 for ‘yes’ and 0 for ‘no’.
Explanatory variable
The explanatory variable was the TCS, which was first introduced by Joossens & Raw 30. The TCS is an indicator that evaluates the TC policies at country‐level every year. It ranges from 0 to 100. TC policies are categorized as follows: pricing, smoke‐free policies, information campaigns, bans on advertising, health warning labels and treatment to smokers. As described in the Supporting information, Table S1, more points are allocated to policies regarded by experts as more effective. The TCS includes policies that were already set at the beginning of each year. For example, if a new policy is introduced in a certain country by May 2008, it is only included in the TCS of 2009. This variable has been used previously in similar research 6, 8, 11. We have re‐calculated the scores based on the score allocation of 2013, using the underlying data from the earlier reports 31 in order to make it comparable over the years.
When we make use of longitudinal data, we look at whether changes in smoking status are associated with changes in TC policies within countries (as measured by TCS). However, when we observe an individual who changes their smoking status between two waves (e.g. from smoker to non‐smoker), we do not know at exactly in which year they did so: it could have been at any year between that wave (e.g. wave 4, 2011) and the previous one (e.g. wave 3, 2007). Therefore, we assigned that person‐wave observation the average TCS score of the years between those two waves (e.g. 2008–11). That average TCS score would be measuring the average state of TC policies in the country during the period when they changed their smoking status (e.g. they quit smoking). The years when every wave was carried out as well as its associated TCS score are presented in the Supporting information, Table S3.
We divided the TCS into three categories: pricing policies, smoke‐free policies and other TC policies (information campaigns, bans on advertising, health warning labels and treatment to smokers). We focused mainly on the two types of policies that have contributed the most to the increase in TCS over the period: pricing and smoke‐free policies (Supporting information, Table S2). Furthermore, these two polices have been discussed as some of the most effective against tobacco consumption 5.
Socio‐demographic characteristics
Age, sex and SES were used as potential confounders. Age was used as continuous variable and as a dummy variable for those older than 65 in the analysis by socio‐demographic categories. Furthermore, the SES was measured by the highest level of education completed in three categories: none or primary, secondary and tertiary.
Statistical analysis
As descriptive analysis, we explored the correlation between the variation in TCS and the variation of smoking per country during the period under analysis. We did so by calculating Pearson's correlation coefficients and through scattergraphs. In the multivariate analysis, we used a weighted logistic regression model with smoking status as the outcome and TCS, age (as continuous variable), sex, education, country and time as fixed‐effects. Education was included as a categorical variable with three categories: (i) tertiary, (ii) secondary and (iii) primary or non‐education (reference category). Male was the reference category for sex. Time fixed‐effects (i.e. wave binary variables) were included to control for determinants of smoking that may vary uniformly across European countries over time (e.g. economic cycles). Country fixed‐effects (i.e. country binary variables) controlled for those country characteristics that were invariant during the period under analysis, such as culture, institutions or other life‐style aspects. By using country and time fixed‐effects, we exploited country‐specific changes in TCS. ‘Cluster‐robust’ standard errors clustered at individual‐level were used to account for the longitudinal structure of the data by using the ‘cluster‐robust’ variance matrix estimator following Wooldridge (2006) 32. Coefficients were reported as marginal effects, which indicate how much the probability of smoking varies (in percentage points) when the explanatory variable increases by one unit, while setting the rest of the control variables constant at their average values.
To investigate whether the association of TCS with smoking was different by socio‐demographic group, we interacted the TCS with socio‐demographic categorical variables. Sex and education were initially measured by categorical variables, as explained above. Regarding age, we created a dummy variable for those older than 65 at baseline. As sensitivity analysis, we further carried out two more models: first, a logistic model clustering standard errors at country‐level to account for within‐country correlation of the error term 33; and secondly, a linear probability model with individual fixed‐effects to account for individual characteristics that are fixed over time 34. All analyses were carried out using Stata/MP version 13.0. We used the ‘Logit’ command, with ‘VCE cluster’ to indicate cluster‐robust standard errors, except for the linear probability model, which used ‘xtreg’.
Furthermore, we checked for multi‐collinearity using the variance inflation factor (VIF) and exploring changes in standard errors 35. We found a risk of multi‐collinearity (VIF for the TCS variables between 9 and 12) when including country dummies, but little variation in standard errors arose, so we kept the model with country dummies as our main model in order to control for non‐observable country characteristics.
Due to the hierarchical structure of the data, multi‐level appeared as the ideal model to account for time and country data clustering 36. However, this model presented two limitations 37: (i) due to the small sample size at the higher unit‐level (10 countries), the estimation of the random components and random slopes relied on a low number of degrees of freedom and (ii) the sample of higher unit‐level was not random and, therefore, confounding variables at country‐level might bias the results. Instead, we opted to cluster standard errors at individual‐level and include country fixed‐effects, which are applicable to a small number of countries and control for invariable country characteristics.
Treatment of attrition
Longitudinal surveys are affected by attrition due to death, moving away or non‐response. If there are systematic differences between those who form the balanced panel and those who were sampled at wave 1, our results may be biased. To test for attrition bias, we carried out the variable addition test 38, which led to a rejection of the null hypothesis of non‐attrition bias (t = −8.35, P‐value < 0.01).
To deal with this bias, we constructed inverse probability weights (IPW), following Jones (2005) 39. IPWs give more weight to observations that have a higher probability of dropping out. To construct the IPW, we estimated the probability of responding in all waves as a function of observable variables at first wave: age, smoking status, initial TCS, self‐reported health status, number of limitations and chronic conditions. IPWs were then formed by the inverse of the predicted probability of responding in the balanced longitudinal sample. Lastly, we multiplied these weights by the cross‐sectional weights from the first wave provided by SHARE, which aim to make the sample representative of the 50+ population at wave 1 for each country 40. The weights were added to the regression as probability weights.
Results
Descriptive analysis
Smoking prevalence at baseline ranged from 15.1% in Belgium to 28.7% in Denmark, with an average of 20.2% (Table 1). Smoking in our sample decreased by 5 percentage points (pp) on average from 2004 to 2013, with Spain showing the highest decrease (−8.6 pp). The data on TCS by country and year reflect a significant effort from most countries in implementing TC policies (Table 2). Overall, the average TCS for these countries has increased by 20 points from 2004 to 2013.
Table 1.
Smoking prevalence of the longitudinal sample per country and wave.
| Smoking prevalence (%) | Absolute variation | ||||
|---|---|---|---|---|---|
| Wave 1 | Wave 2 | Wave 4 | Wave 5 | Wave 5–wave 1 | |
| (2004–2005) | 2007 | 2011 | 2013 | ||
| Austria | 21.3 | 19.7 | 21.2 | 15.8 | −5.5 |
| (16.5, 26.0) | (15.1, 24.2) | (16.4, 25.8) | (11.5, 20.1) | ||
| Germany | 18.8 | 16.6 | 16.9 | 16.7 | −2.1 |
| (14.9, 22.6) | (12.9, 20.2) | (13.3, 20.4) | (13.1, 20.1) | ||
| Sweden | 16.6 | 13.4 | 14.7 | 11.2 | −5.4 |
| (13.6, 19.6) | (10.5, 16.2) | (11.8, 17.5) | (8.7, 13.6) | ||
| Netherlands | 26.6 | 24.9 | 20.1 | 18.3 | −8.2 |
| (23.1, 30.0) | (21.4, 28.3) | (16.8, 23.2) | (15.2, 21.4) | ||
| Spain | 20.6 | 16.9 | 13.4 | 11.9 | −8.6 |
| (16.9, 24.2) | (13.4, 20.3) | (10.1, 16.5) | (8.9, 15.0) | ||
| Italy | 20.0 | 17.7 | 17.3 | 13.6 | −6.4 |
| (17.2, 22.8) | (14.9, 20.3) | (14.6, 20.1) | (11.1, 16.1) | ||
| France | 16.2 | 14.8 | 12.1 | 13.2 | −3.1 |
| (13.4, 19.0) | (12.1, 17.3) | (9.7, 14.4) | (10.7, 15.6) | ||
| Denmark | 28.7 | 24.4 | 23.5 | 20.0 | −8.7 |
| (25.1, 32.3) | (20.9, 27.8) | (20.1, 26.9) | (16.8, 23.2) | ||
| Switzerland | 18.4 | 19.3 | 22.0 | 18.7 | 0.2 |
| (14.3, 22.6) | (15.1, 23.5) | (17.7, 26.3) | (14.5, 22.7) | ||
| Belgium | 15.1 | 14.6 | 14.1 | 13.4 | −1.7 |
| (13.1, 17.1) | (12.6, 16.5) | (12.1, 16.0) | (11.5, 15.3) | ||
| Averagea | 20.2 | 18.2 | 17.5 | 15.3 | −5.0 |
Smoking prevalence was calculated using the weighted longitudinal sample; 95 confidence intervals in brackets.
Unweighted average of countries’ prevalence.
Table 2.
Evolution of Tobacco Control Scale (TCS) by country.
| Year | Variation | |||
|---|---|---|---|---|
| TCS | 2004 | 2013 | Absolute | % |
| Austria | 25 | 44 | 19 | 76 |
| Germany | 28 | 46 | 18 | 64 |
| Sweden | 51 | 64 | 13 | 25 |
| Netherlands | 47 | 58 | 11 | 23 |
| Spain | 33 | 71 | 38 | 115 |
| Italy | 42 | 64 | 22 | 52 |
| France | 54 | 73 | 19 | 35 |
| Denmark | 41 | 59 | 18 | 44 |
| Switzerland | 31 | 57 | 26 | 85% |
| Belgium | 46 | 62 | 16 | 34 |
| Averagea | 39.7 | 59.65 | 20 | 50 |
Unweighted average of countries’ scores.
The association between the variation in smoking prevalence and variation in TCS between 2004 and 2013 presents a Pearson's correlation coefficient of ρ = −0.09. The negative correlation is higher for variation in TCS of price policies (ρ = −0.46), and to a lesser extent for smoke‐free policies (ρ = −0.21), whereas other policies are positively correlated (ρ = 0.25) (Fig. 1).
Figure 1.
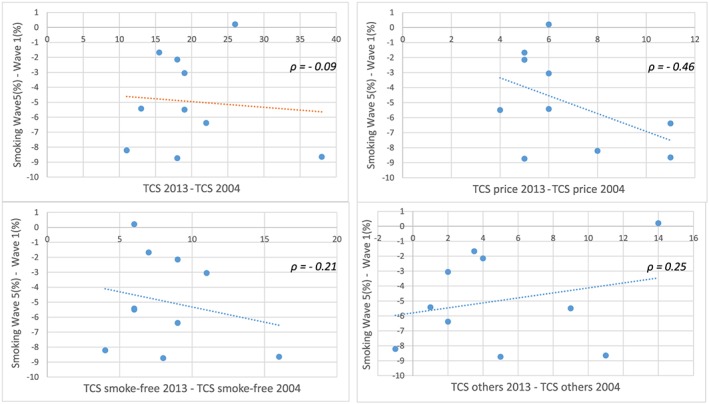
Scattergraph: change (in percentage points) in smoking prevalence (wave 5–wave 1) versus change in Tobacco Control Scale (TCS) (2013–2004). [Colour figure can be viewed at wileyonlinelibrary.com]
Multivariable analysis
We observed a negative association between TCS and smoking likelihood (Table 3, column 3), although not significant at 5%. A 10‐point rise in TCS was associated with a 1.1 pp [marginal effects (ME) = –0.112, 95% confidence interval (CI) = –0.228, 0.003)] drop in the risk of smoking (P‐value = 0.057). Distinguishing by type of tobacco control policy, increases in prices (ME = –0.636, 5% CI = –0.998, −0.275) and smoke‐free policies (ME = –0.243, 95% CI = –0.445, −0.041) were significantly associated with lower probability of smoking. Conversely, the rest of the tobacco control policies showed no clear association (ME = 0.094, 95% CI = –0.099, 0.289, P‐value = 0.338) (Table 4). Sensitivity analyses with respect to statistical modelling reported very similar results (Supporting information, Tables S5–S8).
Table 3.
Logistic model for the probability of smoking: marginal effects (ME).a
| (1) | (2) | (3) | |
|---|---|---|---|
| Variables | ME (95% CI) | ME (95% CI) | ME (95% CI) |
| TCS | −0.164** (−0.252, −0.070) | −0.043 (−0.165, 0.078) | −0.112+ (−0.228, 0.003) |
| Age (years) | −0.926** (−1.050, −0.802) | −0.909** (−1.034, −0.784) | |
| Female | −4.999** (−7.156, −2.842) | −4.880** (−7.036, −2.723) | |
| Educational level | |||
| Base category: none or primary | |||
| Secondary | 2.369+ (−0.292, 5.031) | 3.368+ (0.592, 6.143) | |
| Tertiary | −2.279+ (−4.857, 0.298) | −1.399 (−4.198, 1.398) | |
| Wave fixed‐effects | Included | Included | |
| Country fixed‐effects | Included | ||
| Observations | 30 682 | 30 176 | 30 176 |
95% Confidence intervals (CI) in brackets. TCS = Tobacco Control Scale.
P < 0.01,
P < 0.05,
P < 0.1. Clustered standard errors at individual‐level.
Marginal effects can be interpreted as how much the probability of smoking varies (in percentage points) when the explanatory variable increases by 1 unit.
Table 4.
Logistic model of the probability of smoking with type of policies: marginal effects (ME).a
| (1) | (2) | (3) | |
|---|---|---|---|
| Variables | ME (95% CI) | ME (95% CI) | ME (95% CI) |
| TCS price | −0.419** (−0.657, −0.180) | −0.463** (−0.725, −0.201) | −0.636** (−0.998, −0.275) |
| TCS smoke‐free | −0.275** (−0.469, −0.081) | −0.270* (−0.513, −0.026) | −0.243* (−0.445, −0.041) |
| TCS other | 0.057 (−0.212, 0.327) | 0.198+ (−0.029, 0.426) | 0.094 (−0.099, 0.289) |
| Age (years) | −0.914**(−1.038, −0.789) | −0.908** (−1.037, −0.784) | |
| Female | −4.873**(−7.029, −2.716) | −4.878** (−7.033, −2.722) | |
| Educational level | |||
| Base category: none or primary | |||
| Secondary | 3.449* (0.742, 6.157) | 3.368* (0.593, 6.143) | |
| Tertiary | −1.363 (−4.007, 1.280) | −1.396 (−4.192, 1.400) | |
| Wave fixed‐effects | Included | Included | |
| Country fixed‐effects | Included | ||
| Observations | 30 682 | 30 176 | 30 176 |
95% Confidence intervals (CI) in brackets. TCS = Tobacco Control Scale.
P < 0.01,
P < 0.05,
P < 0.1. Clustered standard errors at individual‐level.
Marginal effects can be interpreted as how much the probability of smoking varies (in percentage points) when the explanatory variable increases by 1 unit.
The TCS coefficient was not significantly different by sex (Table 5). On the contrary, a 10‐point rise in the TCS significantly decreased the probability of smoking by 1.6 pp (95% CI = –3.208, −0.056) for those younger than 65 years, whereas the association was not significant among those older than 65 (ME = 0.340 pp, 95% CI = –1.475, 2.155, P‐value = 0.714). When taking into account prevalence at baseline, relative differences were not significant at 5% [odds ratio (OR) = 1.015, 95% CI = 1.000, 1.030]. A 10‐point higher TCS was associated with a 1.5 pp (95% CI = –2.751, −0.253) lower probability of smoking for those with no or primary education, whereas no significant association was found for the highest educated (ME = 1.826 pp, 95% CI = –0.461, 4.114, P‐value = 0.118). In this case, both absolute and relative differences were significant.
Table 5.
Marginal effects of a 10 point‐increase in TCS on the probability of smoking (in percentage points), by socio‐demographic category.
| Marginal effect on the probability of smokinga | Absolute difference | Relative difference | |||
|---|---|---|---|---|---|
| (in percentage points) | (ME interaction)b | (OR interaction)c | |||
| Educational level | |||||
| (1) None or primary | −1.502* (−2.751, −0.253) | ||||
| (2) Secondary | −2.212+ (−4.441, 0.016) | (2) versus (1) | −7.103 (−2.743, 1.325) | 0.996 (0.982, 1.010) | |
| (3) Tertiary | 1.826 (−0.461, 4.114) | (3) versus (1) | 3.332** (1.147, 5.509) | 1.027** (1.009, 1.046) | |
| Age (years) | |||||
| (4) 50–65 at baseline | −1.632* (−3.208, −0.056) | ||||
| (5) 65+ at baseline | 0.340 (−1.475, 2.155) | (5) versus (4) | 1.972* (0.152, 3.792) | 1.015+ (1.000, 1.030) | |
| Sex | |||||
| (6) Males | −1.227 (−3.028, 0.573) | ||||
| (7) Females | −0.891 (−2.444, 0.659) | (7) versus (6) | 0.335 (−1.458, 2.130) | 1.001 (0.988, 1.015) | |
95% Confidence Intervals in brackets.
P < 0.01,
P < 0.05,
P < 0.1.
Marginal Effect indicate how much the probability of smoking change (in percentage points) with a 10‐point increase in TCS. They come from the logistic model including simultaneously interactions between TCS and each sociodemographic category (education, age and sex); and were calculated following Karaka‐Mandic et al (2011) 49.
Marginal Effects (M.E.) of the interactions represent the absolute difference (in percentage points) of the TCS marginal effect over sociodemographic category (Full results in Table S9, Supporting Information).
Odds Ratio (O.R.) of the interactions represent the relative difference (in percentage points) of the TCS marginal effect over sociodemographic category (Full results in Table S9, Supporting Information). TCS = Tobacco Control Scale.
Pricing policies were related to lower smoking only among those with secondary (ME = −6.480 pp, 95% CI = –10.155, −2.805) or lower levels of education (ME = −6.306 pp, 95% CI = –12.623, −0.010), and not among the more highly educated individuals (ME = 0.908 pp, 95% CI = –6.114, −7.931) (Table 6). They also affected more men than women, although the relative difference was not significant (OR = 1.03, 95% CI = 0.999, 1.063). No significant absolute or relative difference was observed by age group. Lastly, smoking‐free policies also significantly affected the lower‐educated more in absolute and relative differences: ME = −3.338 pp, 95% CI = –5.771, −0.906; tertiary: ME = 1.524 pp, 95% CI = –2.237, 5.286), whereas no consistent differences were found among age group and sex (Table 7).
Table 6.
Marginal effects of a 10 point‐increase in TCS price on the probability of smoking (in percentage points) by socio‐demographic category.
| ME on the probability of smokinga | Absolute Differenceb | Relative Differencec | |||
|---|---|---|---|---|---|
| (in percentage points) | (ME interaction) | (OR interaction) | |||
| Educational level | |||||
| (1) None or primary | −6.480** (−10.155, −2.805) | ||||
| (2) Secondary | −6.306* (−12.623, 0.010) | (2) versus (1) | −0.173 (−4.906, 5.253) | 1.008 (0.973, 1.045) | |
| (3) Tertiary | 0.908 (−6.114, 7.931) | (3) versus (1) | 7.388* (1.128, 13.648) | 1.062* (1.005, 1.122) | |
| Age (years) | |||||
| (4) 50–65 at baseline | −4.833* (−9.588, −0.079) | ||||
| (5) 65+ at baseline | −5.034+ (−0.0103, 0.0002) | (5) versus (4) | 0.200 (−4.698, 4.297) | 0.991 (0.955, 1.029) | |
| Sex | |||||
| (6) Males | −7.610** (−12.773, −2.448) | ||||
| (7) Females | −2.530 (−7.115, 2.054) | (7) versus (6) | 5.080* (1.052, 9.107) | 1.030+ (0.999, 1.063) | |
95% Confidence Intervals in brackets.
P < 0.01,
P < 0.05,
P < 0.1.
Marginal Effect indicate how much the probability of smoking change (in percentage points) with a 10‐point increase in TCS price. They come from the logistic model including simultaneously interactions between TCS price and each sociodemographic category (education, age and sex); and were calculated following Karaka‐Mandic et al (2011) 49.
Marginal Effects (M.E.) of the interactions represent the absolute difference (in percentage points) of the TCS price marginal effect over sociodemographic category (Full results in Table S10, Supporting Information).
Odds Ratio (O.R.) of the interactions represent the relative difference (in percentage points) of the TCS price marginal effect over sociodemographic category (Full results in Table S10, Supporting Information). TCS = Tobacco Control Scale.
Table 7.
Marginal effects (ME) of a 10 point‐increase in Tobacco Control Scale (TCS) smoke‐free on the probability of smoking (in percentage points) by socio‐demographic category.
| ME on the probability of smoking a | Absolute difference | Relative difference | |||
|---|---|---|---|---|---|
| (in percentage points) | (ME interaction) b | (OR interaction) c | |||
| Educational level | |||||
| (1) None or primary | −3.338** (−5.771, −0.906) | ||||
| (2) Secondary | −4.274* (−8.017, 0.528) | (2) versus (1) | −0.935 (−4.935, 3.064) | 0.996 (0.968, 1.025) | |
| (3) Tertiary | 1.524 (−2.237, 5.286) | (3) versus (1) | 4.863** (0.815, 8.911) | 1.040** (1.006, 1.075) | |
| Age (years) | |||||
| (4) 50–65 at baseline | −3.550* (−6.153, −0.947) | ||||
| (5) 65+ at baseline | −0.480 (−3.534, 2.573) | (5) versus (4) | 3.069+ (−0.402, 6.542) | 1.022 (0.994, 1.051) | |
| Sex | |||||
| (6) Males | −3.151*(−6.305, 0.001) | ||||
| (7) Females | −2.179+ (−4.644, 0.307) | (7) versus (6) | 0.972 (−2.494, 4.439) | 1.003 (0.978, 1.030) | |
95% Confidence Intervals in brackets.
P < 0.01,
P < 0.05,
P < 0.1.
Marginal Effect indicate how much the probability of smoking change (in percentage points) with a 10‐point increase in TCS smoke‐free. They come from the logistic model including simultaneously interactions between TCS price and each sociodemographic category (education, age and sex); and were calculated following Karaka‐Mandic et al (2011) 49.
Marginal Effects (M.E.) of the interactions represent the absolute difference (in percentage points) of the TCS smoke‐free marginal effect over sociodemographic category (Full results in Table S11, Supporting Information).
Odds Ratio (O.R.) of the interactions represent the relative difference (in percentage points) of the TCS smoke‐free marginal effect over sociodemographic category (Full results in Table S11, Supporting Information). TCS = Tobacco Control Scale.
Discussion
Summary of findings
A 10‐point increase in TCS was associated with a drop in the probability of smoking by 1.1 pp, although not significant at 5% (P‐value = 0.057). By contrast, pricing (P‐value = 0.001) and smoke‐free policies (P‐value = 0.018) were significantly and negatively associated with smoking. The negative association between TCS and smoking was observed especially among those aged between 50 and 65 years and among those with lower levels of education (none, primary or secondary). By contrast, no relationship between TCS and smoking was found among those older than 65 and among those with higher education. Furthermore, the association of TCS with smoking was not found to be different between men and women.
Interpretation of the results
Increases in prices and smoke‐free policies were significantly associated with a reduction in smoking, in line with previous evidence 5. In particular, our research is among the first to verify this association for a population aged 50 years and older.
An increase in TCS diminished the probability of smoking for those aged younger than 65 years but not for those aged 65 or more. We suggest two explanations for this differential effect. First, the lack of effect of TCS on those older than 65 is possibly explained by them having a larger share of the so‐called ‘hardcore’ smokers, compared to those younger than 65 years. Hardcore smokers, defined as those unwilling or unable to quit 41, may be less responsive to current tobacco control policies. Studies in different countries found that hardcore smokers are more prevalent among older‐aged smokers 42, 43, 44. Causes behind the increased number of hardcore smokers include increased nicotine dependence and lower motivation to quit 45.
Secondly, the absolute difference in the TCS effect might be due to differences in initial smoking prevalence between age groups. Smoking prevalence among those aged 65+ years was already low at baseline (9.1%), and after 10 years it decreased by less than 2 pp, to 7.4%. In contrast, prevalence among those younger than 65 fell by more than 6 pp, from 27 to 20.2% over the same period. When we take into account the baseline odds of smoking, the relative difference in TCS effect between age groups was not significant at 5%.
A 10‐point increase in TCS decreased the probability of smoking by 1.5 pp for those with none or primary education, whereas no association was found for those with higher education. This suggests that the increase in TCS altered the socio‐economic pattern of smoking. It is expectable that low‐SES people reacted more to increases in prices, as they have a lower disposable income. In fact, pricing policies have been consistently identified as more effective for low SES groups 12, 14. For example, Hu et al. 46 found that TC policies during the period 1990–2007, and in particular increases in prices, were related to smoking more among low‐SES groups. However, another study by Bosdriesz et al. 11, using more recent data (from 2006 to 2012) did not find that raising taxes was more effective among the lower‐educated. It is worth noting that we focused on people older than 50 years, whereas these European studies address the entire adult population (20 years and older). Smoke‐free policies were also associated with lower smoking prevalence only among the lower‐educated, unlike previous studies which found no differential effect over SES 1, 2.
Limitations
Although our study design can be considered as a ‘quasi‐random’ experiment, TC policies are not randomly implemented across countries. The implementation of policies responds to a political process that may be influenced by changes in societal attitudes towards tobacco. A country‐specific short‐term decrease in societal tolerance towards smoking could simultaneously lead to the implementation of TC policies and to a drop in smoking 47.
Nevertheless, for national societal attitudes towards smoking being the driver of the implementation of TC policies, the political process should have followed a ‘bottom‐up’ path, with citizens encouraging national governments to take action against tobacco. Societal preferences for TC policies are expected to be channelled through voting for parties with different ideologies and policy proposals, at least to a certain extent. However, previous research found that the implementation of TC policies in Europe did not vary with the ideology of the political party in power 48. Nevertheless, politicians may follow public opinion on TC once they are in power, regardless of their ideological background. In such a case, implementation of TC policies could still derive from changes in societal attitudes towards smoking.
Therefore, our data did not permit us to draw definite conclusions about causal relationships. However, we consider that causality is likely, because the longitudinal nature of the data allowed us to relate country‐specific changes of TC policies with longitudinal changes in smoking behaviour.
Our results may be affected by attrition and non‐response bias. Our sample represents 31.3% of the initial sample at wave 1. We aimed to reduce the potential bias by using survey weights. Furthermore, contrary to the longitudinal weights supplied by SHARE, our IPWs are designed for the outcome of interest, smoking; and they also account for other relevant variables not included in the SHARE weights such as health conditions or TCS. There may also be other factors related with non‐response that may affect smoking behaviour, which are excluded from our weighting procedure.
Conclusions and policy implications
Our results show that tobacco control policies in general, and more specifically price increases and smoke‐free policies, are significantly related to a reduced smoking prevalence among European older adults from 2004, suggesting potential health gains among this rising share of the population. The greater relationship observed among lower‐educated people suggests that tobacco control policies may also contribute to decreasing socio‐economic inequalities.
Declaration of interests
None.
Supporting information
Table S1 Description of Tobacco Control Scale (TCS).
Table S2 Evolution of Tobacco Control Scale by policy dimension.
Table S3 Waves information and corresponding TCS score of the longitudinal sample.
Table S4 Descriptive characteristics of the longitudinal balanced sample at baseline.
Table S5 Sensitivity analysis of Table 3 (I): Linear model with individual fixed effects.
Table S6 Sensitivity analysis of Table 3 (II): Logistic model with errors clustered at country level, Marginal Effects.
Table S7 Sensitivity analysis of Table 4 (I): Coefficients from linear model with individual fixed effects.
Table S8 Sensitivity analysis of Table 4 (II): Logistic model with errors clustered at country level, Marginal effects.
Table S9 Logistic model with interactions of TCS per socioeconomic group.
Table S10 Logistic model with interactions of TCS price per socioeconomic group.
Table S11 Logistic model with interactions of TCS smoke‐free per socioeconomic group.
Acknowledgements
We thank all members of the Nova Healthcare Initiative research group for their comments and suggestions. This paper uses data from SHARE waves 1, 2 (SHARELIFE), 4, 5 and 6 (doi numbers: 10.6103/SHARE.w1.610, 10.6103/SHARE.w2.610 10.6103/SHARE.w4.610, 10.6103/SHARE.w5.610, 10.6103/SHARE.w6.610), see Börsch‐Supan et al. (2013) for methodological details. The SHARE data collection has been primarily funded by the European Commission through FP5 (QLK6‐CT‐2001‐00360), FP6 (SHARE‐I3: RII‐CT‐2006‐062193, COMPARE: CIT5‐CT‐2005‐028857, SHARELIFE: CIT4‐CT‐2006‐028812) and FP7 (SHARE‐PREP: no. 211909, SHARE‐LEAP: no. 227822, SHARE M4: no. 261982). Additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the US National Institute on Aging (U01_AG09740‐13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1‐AG‐4553‐01, IAG_BSR06‐11, OGHA_04‐064, HHSN271201300071C) and from various national funding sources is gratefully acknowledged (see www.share‐project.org). The first author was supported by a PhD research Grant from Fundação para a Ciência Tecnologia. This work was also supported by the SILNE‐R project, financed by the European Union's Horizon 2020 research and innovation programme (Grant Agreement number 635056).
Serrano‐Alarcón, M. , Kunst, A. E. , Bosdriesz, J. R. , and Perelman, J. (2019) Tobacco control policies and smoking among older adults: a longitudinal analysis of 10 European countries. Addiction, 114: 1076–1085. 10.1111/add.14577.
Footnotes
When scores of information campaigns, bans on advertising, health warning labels and treatment to smokers are accounted separately, they remain constant in most of the country‐wave observations. However, when we add up these categories into a single aggregated category (other TC policies), the score varies for every country‐wave observation. In this way we ensure having enough variability in our explanatory variable of interest.
(10‐point rise in TCS) × (−0.11) = −1.1 percentage points.
References
- 1. World Health Organization (WHO) . Who Framework Convention on Tobacco Control. Geneva: WHO 2003; Available at: http://whqlibdoc.who.int/publications/2003/9241591013.pdf (accessed 25 February 2019) (Archived at http://www.webcitation.org/76SBtM2Nb on 25 February 2019).
- 2. Levy D. T., Chaloupka F., Gitchell J. The effects of tobacco control policies on smoking rates: a tobacco control scorecard. J Public Heal Manag Pract 2004; 10: 338–353. [DOI] [PubMed] [Google Scholar]
- 3. Fichtenberg C. M., Glantz S. A., Repace J., Lowrey A., Glantz S., Parmley W. et al Effect of smoke‐free workplaces on smoking behaviour: systematic review. BMJ 2002; 325: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertollini R., Ribeiro S., Mauer‐Stender K., Galea G. Tobacco control in Europe: a policy review. Eur Respir Rev 2016; 25: 151–157. 10.1183/16000617.0021-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson L. M., Avila Tang E., Chander G., Hutton H. E., Odelola O. A., Elf J. L. et al Impact of tobacco control interventions on smoking initiation, cessation, and prevalence: a systematic review. J Environ Public Health 2012; 2012: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaap M. M., Kunst A. E., Leinsalu M., Regidor E., Ekholm O., Dzurova D. et al Effect of nationwide tobacco control policies on smoking cessation in high and low educated groups in 18 European countries. Tob Control 2008; 17: 248. [DOI] [PubMed] [Google Scholar]
- 7. Kuipers M. A. G., Monshouwer K., Van Laar M., Kunst A. E. Tobacco control and socioeconomic inequalities in adolescent smoking in Europe. Am J Prev Med 2015; 49: e64–e72. 10.1016/j.amepre.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 8. Hublet A., Schmid H., Clays E., Godeau E., Gabhainn S. N., Joossens L. et al Association between tobacco control policies and smoking behaviour among adolescents in 29 European countries. Addiction 2009; 104: 1918–1926. [DOI] [PubMed] [Google Scholar]
- 9. Gallus S., Lugo A., La Vecchia C., Boffetta P., Chaloupka F. J., Colombo P. et al Pricing policies and control of tobacco in Europe (PPACTE) project: cross‐national comparison of smoking prevalence in 18 European countries. Eur J Cancer Prev 2014; 23: 177–185. [DOI] [PubMed] [Google Scholar]
- 10. Farrelly M. C., Pechacek T. F., Thomas K. Y., Nelson D. The impact of tobacco control programs on adult smoking. Am J Public Health 2008; 98: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosdriesz J. R., Willemsen M. C., Stronks K., Kunst A. E. Tobacco control policy and socio‐economic inequalities in smoking in 27 European countries. Drug Alcohol Depend 2016; 165: 79–86. 10.1016/j.drugalcdep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 12. Hill S., Amos A., Clifford D., Platt S. Impact of tobacco control interventions on socioeconomic inequalities in smoking: review of the evidence. Tob Control 2014; 23: e89–e97. [DOI] [PubMed] [Google Scholar]
- 13. Hiscock R., Bauld L., Amos A., Fidler J. A., Munafò M. Socioeconomic status and smoking: a review. Ann NY Acad Sci 2012; 1248: 107–123. [DOI] [PubMed] [Google Scholar]
- 14. Brown T., Platt S., Amos A. Equity impact of population‐level interventions and policies to reduce smoking in adults: a systematic review. Drug Alcohol Depend 2014; 138: 7–16. [DOI] [PubMed] [Google Scholar]
- 15. ENSP (2015). SILNE ‐ Tackling socio‐economic inequalities in smoking: learning from natural experiments by time trend analyses and cross-national comparisons. Information Release 4. Available at: http://silne.ensp.org/wp-content/uploads/2015/03/SILNE_info_release_4_201412.pdf (accessed 25 February 2019) (Archived at http://www.webcitation.org/76SGNpshR on 25 February 2019).
- 16. Lugo A., La Vecchia C., Boccia S., Murisic B., Gallus S. Patterns of smoking prevalence among the elderly in Europe. Int J Environ Res Public Health 2013; 10: 4418–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Connolly M. J. Smoking cessation in old age: closing the stable door? Age Ageing 2000; 29: 193–195. [DOI] [PubMed] [Google Scholar]
- 18. Taylor D. H., Hasselblad V., Henley S. J., Thun M. J., Sloan F. A. Benefits of smoking cessation for longevity. Am J Public Health 2002; 92: 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LaCroix A. Z., Lang J., Scherr P., Wallace R. B., Cornoni‐Huntley J., Berkman L. et al Smoking and mortality among older men and women in three communities. N Engl J Med 1991; 324: 1619–1625. [DOI] [PubMed] [Google Scholar]
- 20. Doll R., Peto R., Boreham J., Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004; 328: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirdes J. P., Maxwell C. J. Smoking cessation and quality of life outcomes among older adults in the Campbell's survey on well‐being. Can J Public Health/Rev Can sante publique 1994; 85: 99–102. [PubMed] [Google Scholar]
- 22. Friedman R. J., Sengupta N., Lees M. Economic impact of venous thromboembolism after hip and knee arthroplasty: potential impact of rivaroxaban. Expert Rev Pharmacoecon Outcomes Res 2011; 11: 299–306. [DOI] [PubMed] [Google Scholar]
- 23. Timmermans E. J., Huisman M., Kok A. A. L., Kunst A. E. Smoking cessation and 16‐year trajectories of functional limitations among Dutch older adults: results from the Longitudinal Aging Study, Amsterdam. J Gerontol Ser A 2018; 73: 1722–1728. [DOI] [PubMed] [Google Scholar]
- 24. Statistical Office of the European Union (EUROSTAT) . People in the EU: Who Are We and How Do We Live? Luxembourg: Eurostat; 2015.
- 25. Martínez‐Sánchez J. M., Fernández E., Fu M., Gallus S., Martínez C., Sureda X. et al Smoking behaviour, involuntary smoking, attitudes towards smoke‐free legislations, and tobacco control activities in the European Union. PLOS ONE 2010; 5: 16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rafferty A, Walthery P, King‐Hele S. Analysing change over time: repeated cross sectional and longitudinal survey data. UK Data Service, University of Essex and University of Manchester. Swindon: Economic and Social Research Council; 2015. Available at: https://www.ukdataservice.ac.uk/media/455362/changeovertime.pdf (accessed 25 February 2019) (Archived at http://www.webcitation.org/76SGXrGPn on 25 February 2019).
- 27. Malter F, Börsch‐Supan A. SHARE Wave 4, Innovations and Methodology. Munich: MEA, Max Planck Institute for Social Law and Social Policy; 2013. Available at: http://www.share-project.org/uploads/tx_sharepublications/Method_FRB_FINAL.pdf (accessed 25 February 2019) (Archived at http://www.webcitation.org/76SGeuZyp on 25 February 2019).
- 28. Hunkler C, Gruber S, Orban A, Stuck S, Brandt M. Guide to easy SHARE. Munich: MEA, Max Planck Institute for Social Law and Social Policy; 2013, pp. 1–39.
- 29. Verropoulou G. Determinants of change in self‐rated health among older adults in Europe: a longitudinal perspective based on SHARE data. Eur J Ageing 2012; 9: 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joossens L., Raw M. The tobacco control scale: a new scale to measure country activity. Tob Control 2006; 15: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joossens L, Raw M. The Tobacco Control Scale 2013 in Europe.Louvain, Belgium: Association of European Cancer Leagues; 2014.
- 32. Wooldridge J. M. Cluster‐sample methods in applied econometrics: an extended analysis. Michigan State University; 2006. Available at: https://pdfs.semanticscholar.org/ebcb/e37f03a030e63828bf0b761f9ef957d9fbb8.pdf (accessed 25 February 2019) (Archived at http://www.webcitation.org/76SHgG3KK on 25 February 2019).
- 33. Colin Cameron A., Miller D. L. A Practitioner's guide to cluster‐robust inference. J Hum Resource 2015; 50: 317–372. 10.3368/jhr.50.2.317. [DOI] [Google Scholar]
- 34. Cameron A. C., Trivedi P. K. Microeconometrics using Stata, vol. 5, Stata Press Books. 2009706 pp. Available at: http://stata.biz/news/statanews.23.4.pdf
- 35. Williams R. Multicollinearity 2015;1–14. Available at: https://www3.nd.edu/~rwilliam/stats2/l11.pdf
- 36. Cheah B. C. Clustering Standard Errors Or Modeling Multilevel Data. New York, NY: University of Columbia; 2009, pp. 2–4. [Google Scholar]
- 37. Möring K. The fixed effects approach as an alternative to multilevel analysis for cross‐national analyses. Köln, Germany: GK SOCLIFE Working Paper Series; 2012;, pp. 1–19. [Google Scholar]
- 38. Verbeek M., Nijman T. Testing for selectivity bias in panel data models. Int Econ Rev (Phil) 1992; 681–703. [Google Scholar]
- 39. Jones A. M. ‘Attrition bias’ in applied econometrics for health economists. Health Economics. Oxford: Radcliffe Publishing 2005; 1–182.15386673 [Google Scholar]
- 40. De Luca G, Rossetti C, Malter F. Sample design and weighting strategies in SHARE Wave 5. In: Malter F, Borsch‐Supan A, editors. SHARE Wave 5: Innovations and Methodology. Munich: MEA, Max Planck Institute for Social Law and Social Policy; 2015, pp. 75–84.
- 41. Warner K. E., Burns D. M. Hardening and the hard‐core smoker: concepts, evidence, and implications. Nicotine Tob Res 2003; 5: 37–48. [DOI] [PubMed] [Google Scholar]
- 42. Emery S., Gilpin E. A., Ake C., Farkas A. J., Pierce J. P. Characterizing and identifying ‘hard‐core’ smokers: implications for further reducing smoking prevalence. Am J Public Health 2000; 90: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lund M., Lund K. E., Kvaavik E. Hardcore smokers in Norway 1996–2009. Nicotine Tob Res 2011; 13: 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jarvis M. J., Wardle J., Waller J., Owen L. Prevalence of hardcore smoking in England, and associated attitudes and beliefs: cross sectional study. BMJ 2003; 326: 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hughes J. R. The hardening hypothesis: is the ability to quit decreasing due to increasing nicotine dependence? A review and commentary. Drug Alcohol Depend 2011; 117: 111–117. 10.1016/j.drugalcdep.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hu Y., van Lenthe F. J., Platt S., Bosdriesz J. R., Lahelma E., Menvielle G. et al The impact of tobacco control policies on smoking among socioeconomic groups in nine European countries, 1990–2007. Nicotine Tob Res 2016; 19: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 47. Decicca P., Kenkel D., Mathios A., Shin Y. J., Lim J. Y. Youth smoking, cigarette prices, and anti‐smoking sentiment. Health Econ 2008; 17: 733–749. [DOI] [PubMed] [Google Scholar]
- 48. Bosdriesz J. R., Willemsen M. C., Stronks K., Kunst A. E. Tobacco control policy development in the European Union: do political factors matter? Eur J Public Health 2014; 25: 190–194. [DOI] [PubMed] [Google Scholar]
- 49. Karaca‐Mandic P., Norton E. C., Dowd B. Interaction terms in nonlinear models. Health Serv Res 2012; 47: 255–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Description of Tobacco Control Scale (TCS).
Table S2 Evolution of Tobacco Control Scale by policy dimension.
Table S3 Waves information and corresponding TCS score of the longitudinal sample.
Table S4 Descriptive characteristics of the longitudinal balanced sample at baseline.
Table S5 Sensitivity analysis of Table 3 (I): Linear model with individual fixed effects.
Table S6 Sensitivity analysis of Table 3 (II): Logistic model with errors clustered at country level, Marginal Effects.
Table S7 Sensitivity analysis of Table 4 (I): Coefficients from linear model with individual fixed effects.
Table S8 Sensitivity analysis of Table 4 (II): Logistic model with errors clustered at country level, Marginal effects.
Table S9 Logistic model with interactions of TCS per socioeconomic group.
Table S10 Logistic model with interactions of TCS price per socioeconomic group.
Table S11 Logistic model with interactions of TCS smoke‐free per socioeconomic group.


